Abstract
Antiretroviral therapy has led to increased survival of HIV-infected patients but also increased prevalence of HIV-associated neurocognitive disorders. We previously identified YKL40 as a potential cerebrospinal fluid (CSF) biomarker of lentiviral central nervous system (CNS) disease in HIV-infected patients and in the macaque model of HIV encephalitis. The aim of this study was to define the specificity and sensitivity along with the predictive value of YKL40 as a biomarker of encephalitis and to assess its relationship to CSF viral load. CSF YKL40 and SIV RNA concentrations were analyzed over the course of infection in 19 SIV-infected pigtailed macaques and statistical analyses were performed to evaluate the relationship to encephalitis. Using these relationships, CSF alterations of 31 neuroimmune markers were studied pre-infection, during acute and asymptomatic infection, at the onset of encephalitis, and at necropsy. YKL40 CSF concentrations above 1122 ng/ml were found to be a specific and sensitive biomarker for the presence of encephalitis and were highly correlated with CSF viral load. Macaques that developed encephalitis had evidence of chronic CNS immune activation during early, asymptomatic, and end stages of infection. At the onset of encephalitis, CSF demonstrated a rise of neuroimmune markers associated with macrophage recruitment, activation and interferon response. CSF YKL40 concentration and viral load are valuable biomarkers to define the onset of encephalitis. Chronic CNS immune activation precedes the development of encephalitis while some responses suggest protection from CNS lentiviral disease.
Keywords: Human immunodeficiency virus, Simian immunodeficiency virus, Biomarker, Cerebrospinal fluid, YKL40, Encephalitis
INTRODUCTION
HIV-infected individuals are at high risk of developing a spectrum of neurological deficits collectively termed HIV-associated neurocognitive disorders (HAND). Approximately one of four terminally ill AIDS patients develops a severe neurodegenerative disorder associated with chronic HIV encephalitis (McArthur, 1997; Dore et al., 1999). Lentiviral encephalitis is characterized by an abundance of activated and infected macrophages, microglial nodules, and multinucleated giant cells. In the era of highly active antiretroviral treatment (HAART), HIV-infected individuals live longer and thus have developed an increased prevalence of neurological disease (Maschke et al., 2000; Sacktor et al., 2002; Gray et al., 2003; McArthur et al., 2003). Since development of lentiviral encephalitis occurs in only a fraction of infected individuals at variable time points post-infection, it is important to find biomarkers that indicate the onset of neurological disease. Knowing when the onset of encephalitis occurs is crucial to developing and instituting neuroprotective therapies (Kolson, 2008). Over the past decade a myriad of potential CSF biomarkers for encephalitis have been examined including CCL2 (MCP-1) (Zink et al., 2001; Mankowski et al., 2004), IL-6 (Mankowski et al., 2004), SIV RNA (Mankowski et al., 2004), osteopontin (Burdo et al., 2007), 14-3-3 (Helke et al., 2005), neurofilament light chain (Gisslen et al., 2007), and many others (Price et al., 2007; Kolson, 2008; Pendyala et al., 2009).
We recently discovered increased CSF YKL40 concentrations in both human and nonhuman primates with lentiviral encephalitis (Bonneh-Barkay et al., 2008). YKL40 is a member of the glycosyl hydrolase family 18 that lacks hydrolytic activity and is expressed by synovial cells, neutrophils, and macrophages. While its biological function is unknown, it has been shown to be especially upregulated in inflamed tissues of various origins. In lentiviral encephalitis, astrocytes are the predominant cell type expressing YKL40 (Bonneh-Barkay et al., 2010a) and are presumably the cellular source of increased CSF YKL40 concentrations. We have observed in vitro that YKL40 can bind to the extracellular matrix and displace basic fibroblast growth factor suggesting a potential role in mediating lentiviral-associated neurodegeneration.
To define the specificity and sensitivity along with the predictive value of YKL40 as a biomarker of lentiviral encephalitis, we analyzed CSF YKL40 concentrations over the course of infection in a historic cohort of 19 SIV-infected pigtailed macaques that have been fully characterized histopathologically. These analyses confirmed that YKL40 is a specific and sensitive biomarker for the presence of encephalitis at a concentration of 1122 ng/ml and is highly correlated with CSF SIV RNA concentrations. Retrospective analysis of CSF showed that macaques that developed encephalitis had evidence of chronic CNS immune activation throughout infection. Elevation of macrophage recruitment markers preceded or coincided the development of encephalitis and macrophage activation and interferon response proteins appeared later. CSF from terminally ill macaques that did not develop encephalitis showed evidence of a protective neuroimmune response.
MATERIALS AND METHODS
Animals
This study utilized 19 Macaca nemestrina (pigtailed macaques) inoculated with SIVDeltaB670 viral swarm by intravenous injection and necropsied between January 2004 and April 2009. Animals were euthanized upon development of clinical signs of AIDS. Macaques were housed and maintained according to the standards of the American Association of Laboratory Animal Care and the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Tissues
CSF draws were attempted prior to inoculation and every two weeks post-infection. CSF was aliquoted and stored at −80°C.
Brains were removed immediately after euthanasia and processed for neuropathological analysis as described previously (Bissel et al., 2008). Briefly, brains were bisected sagittally. The left half was fixed in 10% formalin and embedded in paraffin while the right half was microdissected and snap-frozen. Sections of brain were stained with hematoxylin & eosin or immunostained for macrophage/microglia-associated protein CD68 (clone KP1; Dako, Carpenteria, CA), Iba-1 (Wako Chemicals USA, Richmond, VA), or a polyclonal antibody against the SIV envelope gp110 (generously provided by Kelly Stefano Cole and Ronald Montelaro, University of Pittsburgh). SIVE (SIV encephalitis) was empirically defined as the presence of SIV+ microglial nodules, multinucleated giant cells, and profuse perivascular mononuclear infiltrates.
In Situ Hybridization (ISH)
ISH for YKL40 was performed as previously described (Bonneh-Barkay et al., 2010a; Bonneh-Barkay et al., 2012).
Protein Extraction from Brain Tissue
Approximately 100 mg frozen frontal cortical brain tissue was homogenized in ice cold extraction buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA (pH 7.5), 0.5% NP-40) containing 100 μl/ml protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 1X phosSTOP inhibitor (Roche, Indianapolis, IN) using a gentleMACS dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). Samples were sonicated 3 × 6 seconds using a Fisher Scientific Sonic Dismembrator (Model 101). Tissue extracts were centrifuged at 32,000 x g, 30 minutes, 4°C. Supernatants were collected and protein quantified using Pierce BCA Protein Assay (Thermo Fisher Scientific, Pittsburgh, PA).
Quantification of SIV in CSF and Brain Tissue
Virions from 500 μl of CSF were pelleted by centrifugation at 17,000 rpm for 1 hour. Total RNA was extracted from the viral pellet using Trizol (Invitrogen, Carlsbad, CA). Real-time reverse transcriptase PCR was performed with 20 μl of each RNA sample as previously described (Bissel et al., 2002; Fuller et al., 2002). Primers and probes were specific for the SIV U5/long terminal repeat region.
For brain tissue quantification, the number of SIV RNA copies were determined with modifications to previously published protocols (Cline et al., 2005; Venneti et al., 2008). Briefly, approximately 100 mg of frozen frontal cortical brain tissue was disrupted in Trizol reagent using small glass beads (0.5 mm). RNA isolation was performed according to Trizol reagent manufacturer's recommendations. Pelleted RNA was dissolved in molecular grade water and SIV gag RNA copy numbers were determined in a two-step PCR procedure. Duplicate test reactions per sample were prepared with 10 μl of a mix so that the final reaction concentrations contained 1X Reverse Transcriptase Buffer (10X M-MuLV Reverse Transcriptase Buffer, New England Biolabs, Ipswich, MA), 5 μM random nonamers (Sigma-Aldrich), 0.5 mM deoxynucleotide mix (Sigma-Aldrich), 40 Units RNase Inhibitor, Murine (New England Biolabs), and 100 Units M-MuLV Reverse Transcriptase (New England Biolabs). Viral cDNA was synthesized as described. The reactions were then subjected to a second Taqman PCR step by adding a cocktail so that the final concentrations contained 1X PCR Buffer (Taqman Universal PCR Master Mix, Applied Biosystems, Foster City, CA), 600 nM of both forward and reverse primers and 100 nM of probe. Run conditions were as described and nominal copy numbers for each sample were determined by interpolation onto a standard curve of SIV gag RNA standards.
YKL40 and Osteopontin ELISA
CSF YKL40 concentrations were measured in duplicate using the MicroVue YKL40 ELISA kit (Quidel Corporation, San Diego, CA) according to the manufacturer's protocol. Brain tissue protein extracts isolated from frontal cortical tissue were measured in duplicate using the Human Chitinase 3-like 1 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to manufacture's recommendations. CSF osteopontin concentrations were measured using the human osteopontin ELISA kit (Immuno-Biological Laboratories America, Minneapolis, MN) according to the manufacturer's recommendations. CSF neurofilament light chain concentrations were measured in duplicate using the Human neurofilament, light polypeptide ELISA kit (MyBioSource, Inc., San Diego, CA). Optical density was measured using the ELx800 Absorbance Microplate Reader (BioTek, Winooski, VT).
Multiplex Analysis of Neuroimmune Markers
Procarta Cytokine Assay Kits (Panomics, Fremont, CA) were used to simultaneously detect 30 nonhuman primate specific proteins and one human cytokine (M-CSF) per reaction in macaque CSF. The analytes are listed in Supplemental Table 1. These assays are based on Luminex xMAP detection technology that uses beads to quantitatively measure multiple cytokines in a small amount of sample. Cytokines were quantitated at the following time points: baseline (day 0 post-infection (pi)), during acute infection (days 10 and 14 pi), asymptomatic infection (days 28 and 42 pi), at the onset of encephalitis (as defined by elevation of CSF YKL40 concentrations over 1122 ng/ml), and at necropsy. For non-encephalitic macaques, day 70 pi was used in place of the development of encephalitis measurements since day 70 was the average time point YKL40 concentrations increased above 1122 ng/ml in encephalitic animals. CSF samples were diluted 1:2 in Nonhuman Primate Bodily Fluid Buffer (Panomics) and processed according to manufacturer's recommendations. The plate was read by the University of Pittsburgh Cancer Institute LUMINEX Facility using the Bio-Plex reader (Luminex 100) (Luminex Corporation, Austin, TX). Analyte concentrations then were calculated based on the respective standard curve for each analyte.
A subset of analytes shown to be differentially expressed in the CSF of SIV-infected macaques with encephalitis compared to those without encephalitis were evaluated in brain tissue protein extracts isolated from frontal cortical tissue. Samples were processed and read as described for the CSF.
Statistical Analyses
Mann-Whitney U tests were used to compare levels of CSF YKL40 and CSF viral loads between SIV-infected encephalitic macaques and SIV-infected macaques without encephalitis. Odds ratios generated to estimate the association of peak CSF YKL40 and CSF viral loads with the development of encephalitis. The optimal cut points for peak YKL40 and SIV RNA were determined by measuring all possible cut points and calculating the sensitivity and specificity at each point and generating a receiver of operator characteristic (ROC) curve. Results from correlation analyses are represented by r, the Spearman rank correlation coefficient.
RESULTS
Increased CSF YKL40 concentrations are associated with the development of SIV encephalitis
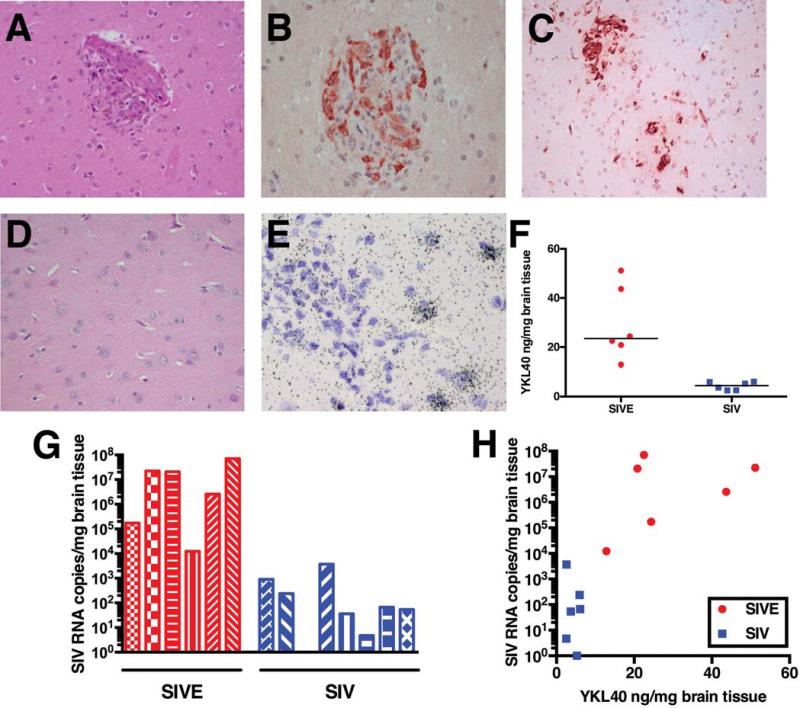
In order to determine the value of using CSF YKL40 as a marker for the presence of encephalitis, we performed a retrospective analysis of 19 pigtailed macaques that were infected with SIV Delta B670. SIV encephalitis (SIVE) was empirically defined histopathologically as the presence of SIV+ microglial nodules, multinucleated giant cells, and profuse perivascular mononuclear infiltrates. Ten macaques had histological presence of SIVE as illustrated in Fig. 1 a-c, while nine macaques were histologically normal (illustrated in Fig. 1d). The histological analysis was confirmed by quantification of SIV RNA copies in frontal cortical tissue (median of macaques with SIVE = 1.16 × 107 copies/mg brain tissue; median of SIV-infected macaques without encephalitis = 60 copies/mg brain tissue; P = 0.0007) (Fig. 1g). In macaques with SIVE, YKL40 expression was observed in astrocytes, and YKL40 expressing cells were most abundant in the vicinity of microglial nodules (Fig. 1e). SIV-infected macaques without encephalitis did not show YKL40 expressing cells by either immunohistochemistry or ISH for YKL40. Analysis of brain tissue protein extracts from frontal cortex contained significantly higher YKL40 concentrations (median = 23.5 ng/mg brain tissue; range 12.9 – 51.2 ng/mg brain tissue) than macaques without encephalitis (median = 4.5 ng/mg brain tissue; range 2.5 – 6.1 ng/mg brain tissue) (P = 0.002) (Fig. 1f). Analysis of a subset of macaques showed a correlation between YKL40 concentrations and SIV viral load in frontal cortical tissue (Fig. 1h).
Fig. 1. Macaques with SIV encephalitis show SIV-infected microglial nodules and increased SIV viral load and YKL40 expression in frontal cortical tissue.
Macaques were defined as having SIV encephalitis (SIVE) by histological examination of brain sections. Hematoxylin and eosin (H&E) stains show the presence of perivascular cuffing, a microglial nodule and multinuclear giant cells (a). Many cells in microglial nodules stain with an antibody against the SIV envelope protein (b). Microglial nodules and macrophage infiltrates can be observed with CD68 immunostains (c). SIV-infected macaques without encephalitis show no infiltrate (H&E) (d). In situ hybridization for YKL40 shows cells expressing YKL40 RNA surrounding a microglial nodule (e). YKL40 concentrations in frontal cortical tissue extracts of macaques with SIVE (red) are significantly elevated compared to SIV-infected macaques without encephalitis (blue), P = 0.002 (f). SIV RNA viral loads in frontal cortical tissue from macaques with SIVE (red) are higher than SIV-infected macaques without encephalitis (blue), P = 0.0007 (g). Correlation between frontal cortical SIV RNA viral load and frontal cortical YKL40 levels (h).
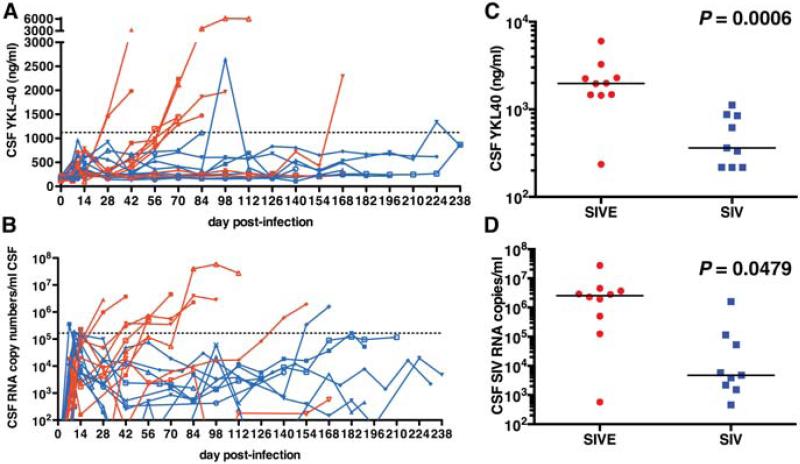
As we reported previously (Bonneh-Barkay et al., 2008), CSF YKL40 concentrations were elevated in macaques that on post-mortem examination showed evidence of SIV encephalitis (Fig. 2a). Retrospective analysis showed that the increase in CSF YKL40 concentrations was observed approximately 2-6 weeks prior to necropsy suggesting that the macaques were encephalitic for this length of time. At necropsy, the difference between CSF YKL40 concentrations in macaques with encephalitis (median = 1975 ng/ml) was significantly higher than macaques without encephalitis (median = 362 ng/ml) with little overlap (p = 0.0006) (Fig. 2c). Next, we performed a case control analysis with repeated observations to examine the association between CSF YKL concentrations and the presence of encephalitis. The risk of developing encephalitis was associated with a one standard deviation increase (SD=1412 ng/ml) in peak CSF YKL40 concentration (odds ratio 10.4 (p = 0.0387)). Using receiver operating characteristic (ROC) analysis we identified the optimal cut point for CSF YKL40 concentration to be associated with the presence of encephalitis by measuring all possible cut points and calculating the sensitivity and specificity at each point. It was determined that CSF YKL40 concentrations above 1122 ng/ml have a strong likelihood of being associated with encephalitis with a sensitivity of 0.9 and specificity of 0.889 (area under the curve = 0.822).
Fig. 2. Macaques that develop encephalitis have increased CSF YKL40 and SIV RNA concentrations.
Preserved CSF samples obtained from 19 macaques from prior studies were analyzed for YKL40 concentrations (a) and SIV viral load (b). Macaques that were determined to have presence of SIVE based on post-mortem histological findings are shown in red while SIV-infected non-encephalitic macaques are depicted in blue. The dashed lines (a and b) represent the concentration determined statistically likely to be associated with encephalitis, 1122 ng/ml for CSF YKL40 concentrations and 1.65 × 105 copies/ml CSF for SIV CSF viral load. Necropsy analyses of terminally ill SIV-infected non-encephalitic macaques (blue) show a significantly lower CSF YKL40, P = 0.0006 (c) and CSF SIV viral load, P = 0.0479 (d) than macaques with SIVE. The black lines represent the median.
CSF viral load becomes elevated in the majority of macaques that develop encephalitis
CSF viral loads have long been used as a measurement indicating the presence of lentiviral encephalitis (McArthur et al., 1997; Cinque et al., 1998a; Zink et al., 1999). Retrospective analysis of the same 19 macaques confirmed that the majority of macaques with post-mortem evidence of encephalitis have increased CSF viral load (Fig. 2b). However, some SIV-infected macaques without evidence of encephalitis exhibited elevated CSF viral loads; and although the CSF SIV RNA concentrations were significantly higher in terminally ill macaques with encephalitis (median = 2.55 × 106 SIV RNA copies/ml) (p = 0.0479) (Fig. 2d), there was overlap with a few SIV-infected non-encephalitic macaques (median = 4750 SIV RNA copies/ml). Performing similar statistical analyses as for YKL40 showed that the risk of developing encephalitis was associated with a one standard deviation (4.53 × 106 copies/ml) increase in CSF SIV RNA copies (odds ratio 12.27 (p = 0.0479)). The optimal cut point for CSF SIV RNA load to be linked to the presence of encephalitis was 1.65 × 105 copies/ml with a sensitivity of 0.9 and a specificity of 0.889.
Increases in SIV CSF viral load and CSF YKL40 concentrations are parallel during the development of encephalitis
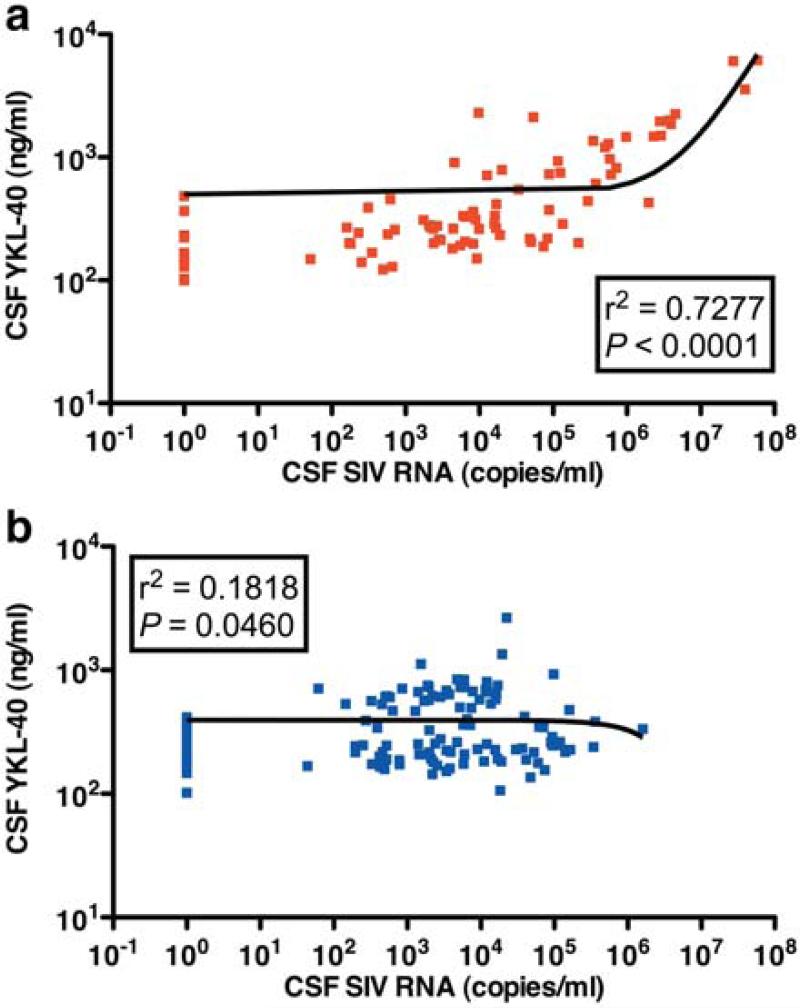
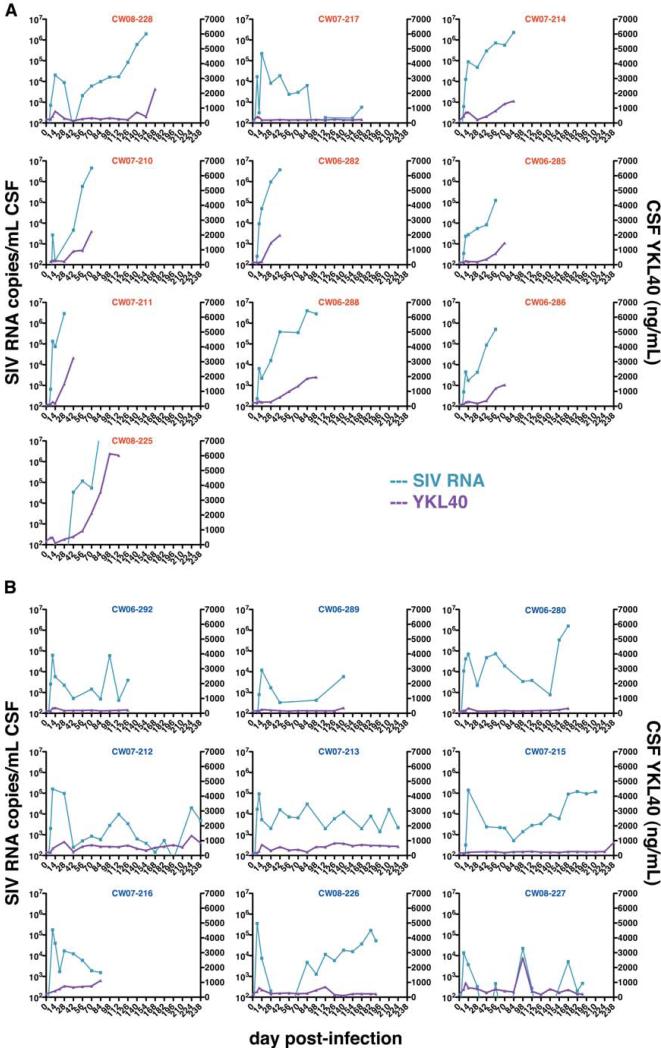
Looking at the relationship of CSF SIV viral load and YKL40 concentrations in all animals showed a Spearman rank correlation coefficient of 0.514 (p = 0.024). Linear regression analysis of SIV-encephalitic macaques alone showed a strong association (r2 = 0.728, p < 0.0001) (Fig. 3a), while macaques that did not develop encephalitis showed little association (r2 = 0.1818, p = 0.0460) (Fig. 3b). In order to verify the relationship of CSF SIV viral load and YKL40 concentrations, we plotted the two variables for each individual macaque over the course of infection (Fig. 4). In macaques that developed encephalitis, CSF YKL40 concentrations generally rise in parallel to CSF SIV RNA concentrations (Fig. 4a). Compared to CSF SIV viral load, it usually took one to two weeks longer for CSF YKL40 concentrations to reach the optimal cut point to be linked to the presence of encephalitis. Macaques that did not develop encephalitis showed little variation or elevation in CSF YKL40 concentrations even when CSF SIV RNA concentrations became elevated (Fig. 4b).
Fig. 3. CSF YKL40 and SIV RNA concentrations are tightly correlated in macaques that develop encephalitis.
Spearman rank correlation of CSF YKL40 concentrations and CSF SIV viral load in PTM that develop encephalitis (a) (red) and SIVE-infected non-encephalitic PTM (b) (blue).
Fig. 4. CSF YKL40 concentrations increase concomitantly with CSF viral load in macaques that developed SIV encephalitis.
CSF SIV RNA concentrations (blue, left axis) are graphed separately for each macaque to show the relationship with CSF YKL40 concentrations (purple, right axis) over the course of infection. 10 macaques that developed encephalitis (a) showed YKL40 increases concomitant with elevated CSF viral load while SIV-infected terminally ill non-encephalitic macaques (b) generally maintain similar CSF YKL40 levels throughout infection.
Macaques that develop encephalitis exhibit chronic CNS immune activation
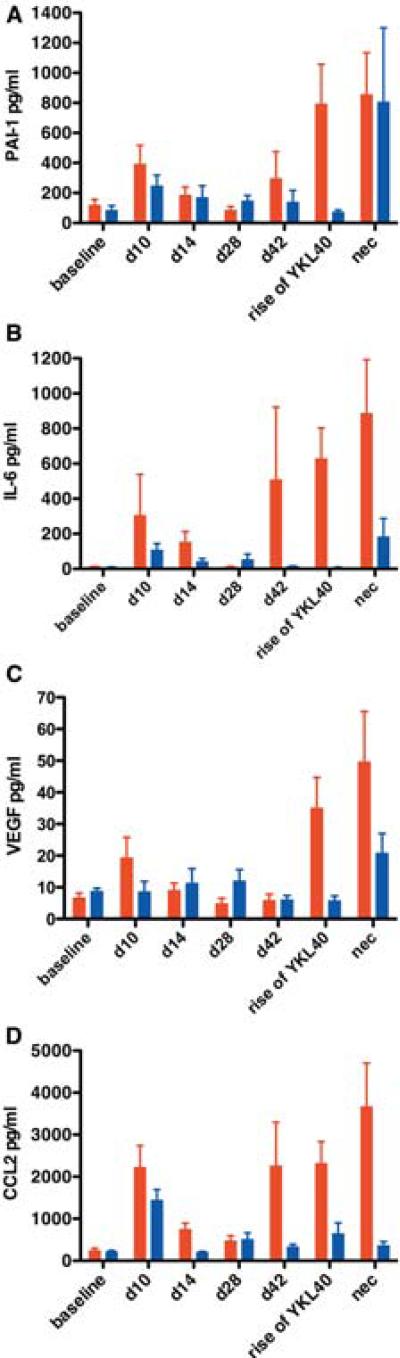
With the capacity to assess onset of neurological infection, we quantified other immune markers in serially sampled CSF. We examined 31 CSF analytes (Supplemental Table 1) using Luminex multiplex protein analysis in 10 macaques that developed SIVE and 8 macaques that did not develop encephalitis. We measured seven time points: preinfection, acute infection (days 10 and 14 pi), asymptomatic infection (days 28 and 42 pi), at the time point YKL40 concentrations rose (for non-encephalitic macaques we used day 70 pi, which was the average time of YKL40 increase in encephalitic macaques), and at necropsy. A few analytes were not detectable at any time point post-infection, while some were not affected by the disease process (Table 1 and 2). The remaining analytes could be divided into three broad categories: chronic immune activation, CNS macrophage activation and recruitment, and protective response. In macaques that developed SIVE, PAI-1, IL-6, VEGF, CCL2, and CCL11 were increased at acute infection and then again at the time YKL40 rose indicating chronic immune activation in macaques that developed SIVE (Fig. 5, Supplemental Fig. 2a, Supplemental Table 2). Some proteins, PAI-1, IL-6, and CCL2, were also increased at the later time point of asymptomatic infection. Macaques that did not develop encephalitis showed increased levels of these chronic activation markers compared to baseline but at a lower level.
Table 1.
Analyte was detected in 1 SIVnoE sample at day 42 (2.84 pg/ml).
Analyte was detected in 1 SIVnoE sample at necropsy (42.40 pg/ml).
Analyte detected in two macaques at necropsy. SIVE (43.34 pg/ml) and SIVnoE (24.24 pg/ml).
Table 2.
| Analytes unaffected by disease |
| GM-CSF |
| bFGF# |
| (IL-10) detected in SIVnoE |
| IL-12p70# |
| IL-23# |
| MCP-3 |
| PDGF-bb |
| Detected in some macaques during infection regardless of encephalitis |
| IP-10 |
Analyte detected randomly in both SIVE and SIVnoE.
Fig. 5. Some neuroimmune markers indicate chronic immune activation at all stages of infection in macaques that develop encephalitis.
Multiplex quantitation of 31 cytokines present in the CSF was performed on samples from baseline (d0), acute infection (d10 and d14), asymptomatic infection (d28 and d42), development of encephalitis (rise of YKL40), and at necropsy (nec). Compared to SIV-infected non-encephalitic macaques (blue), PAI-1 (a), IL-6 (b), VEGF (c), and CCL2 (d) were elevated in macaques that developed SIVE (red) during acute infection (except VEGF), and when encephalitis developed. Bars represent median concentrations of the indicated cytokine.
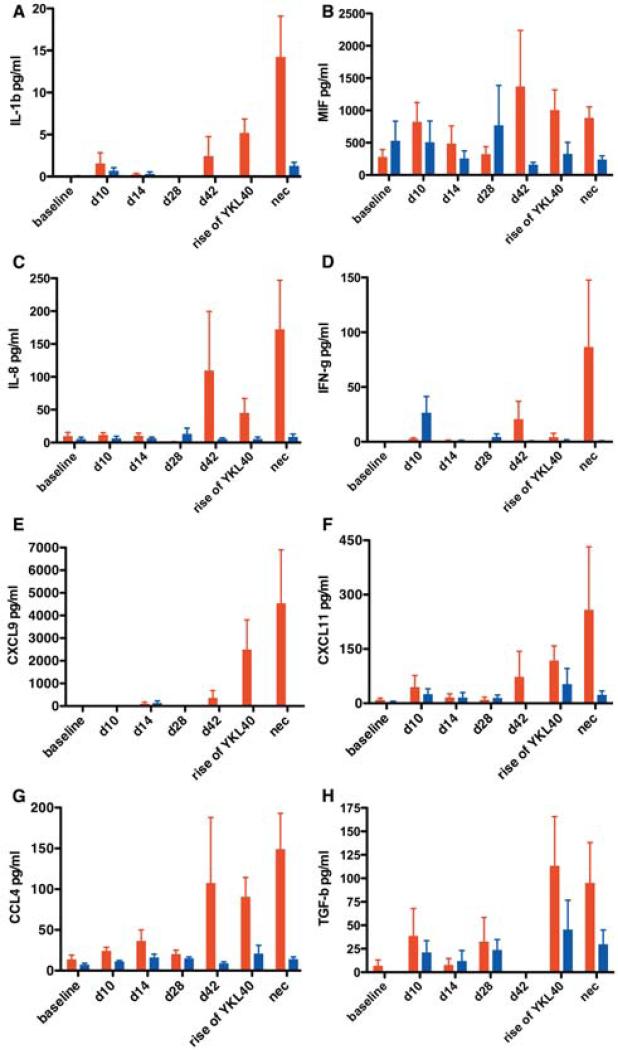
The second category of response included proteins that were elevated only in macaques that developed encephalitis at the time YKL40 rose or slightly before (day 42 pi) (Fig. 6). These proteins can be functionally classified as involved in CNS macrophage activation and recruitment and at least some of them are known to be induced by interferon-γ which is increased in macaques with encephalitis at day 42 pi, time of YKL40 rise, and at necropsy (Supplemental Table 2).
Fig. 6. The majority of elevated neuroimmune markers became elevated as encephalitis developed and were associated with macrophage recruitment and activation.
Multiplex quantitation of 31 cytokines present in the CSF was performed on samples from baseline (d0), acute infection (d10 and d14), asymptomatic infection (d28 and d42), development of encephalitis (rise of YKL40), and at necropsy (nec). IL-1β (a), MIF (b), IL-8 (c), IFN-γ (d), CXCL9 (e), CXCL11 (f), CCL4 (g), TGF-β (h) were elevated when encephalitis (red) developed or shortly before. SIV-infected non-encephalitic macaques (blue) show little elevation of these markers. Bars represent median concentrations of the indicated cytokine.
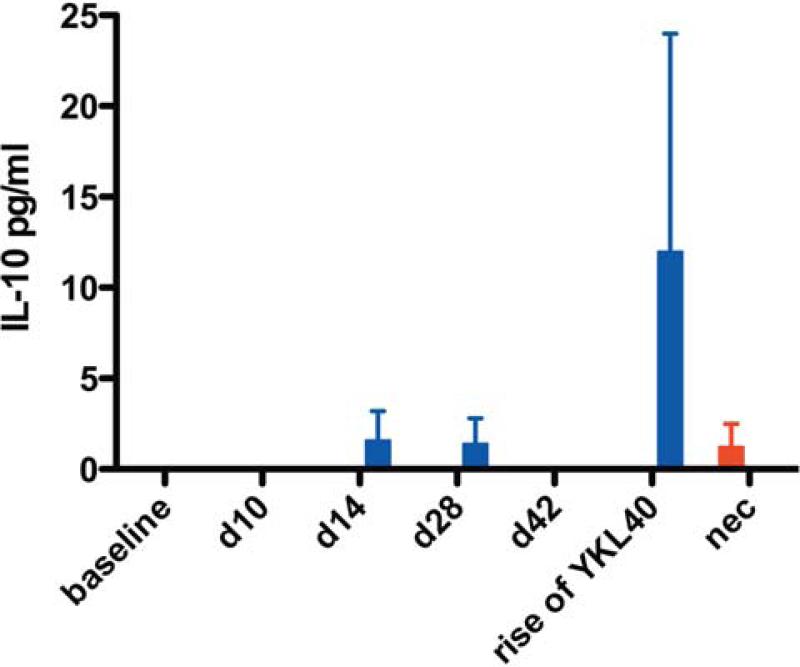
The final category of response was identified in macaques that did not develop encephalitis. The most prominent of these proteins was IL10 (Fig. 7) elevated during all stages of infection (acute, asymptomatic, and at the average time SIVE macaques developed encephalitis) (Supplemental Table 3). IFN-γ was increased in some animals during the acute stage of infection (Fig. 6d). PDGF-bb and RANTES were also more prevalent in the CSF of macaques that did not develop encephalitis (Supplemental Fig. 1b and 1c). These cytokines may be the signature of a protective response to the development of CNS lentiviral disease.
Fig. 7. Non-encephalitic SIV-infected macaques have detectable CSF IL-10 suggesting a protective response.
Multiplex quantitation of 31 cytokines present in the CSF was performed on samples from baseline (d0), acute infection (d10 and d14), asymptomatic infection (d28 and d42), development of encephalitis (rise of YKL40), and at necropsy (nec). Bars represent median concentrations of IL-10.
Relationship between CSF biomarkers of neuronal damage and CSF YKL40
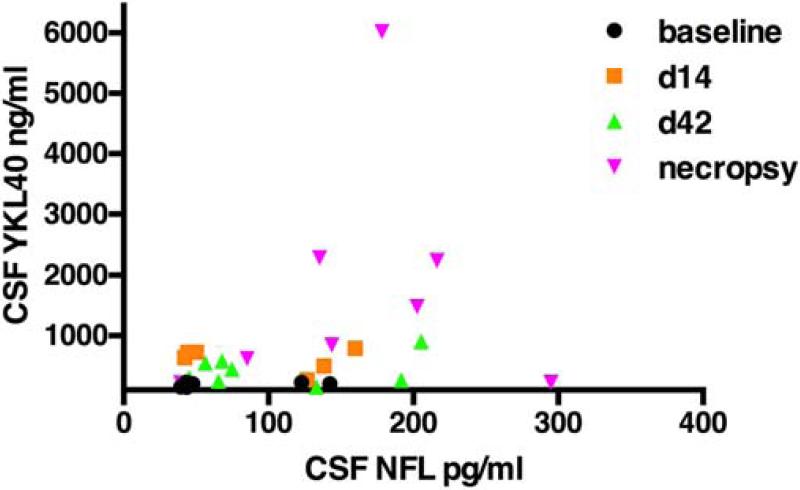
CSF biomarkers of neuronal damage include neurofilament light chain (NFL), amyloid beta fragments (Aβ42 and Aβ40), soluble amyloid precursor proteins, and total and phosphorylated tau (Sjogren et al., 2001; Andreasson et al., 2007; Gisslen et al., 2009; Hampel et al., 2010; Gunnarsson et al., 2011; Landqvist Waldo et al., 2013; Jessen Krut et al., 2014; Peterson et al., 2014). To explore the relationship between neuronal injury and YKL40 in the CSF, we longitudinally measured the CSF concentration of NFL in a subset of SIV-infected macaques with and without encephalitis. NFL has been shown to be a sensitive biomarker of active CNS infection during HIV infection (Peterson et al., 2014). Comparison of CSF YKL40 and NFL concentrations over the time course of SIV infection shows that higher YKL40 concentrations tended to correlate with higher NFL concentrations (Fig. 8). However, one SIV-infected macaque with low CSF YKL40 concentrations exhibited high concentrations of NFL. This macaque showed corticospinal tract Wallerian degeneration with minimal immune activation. This suggests YKL40 is linked with neuronal injury in the presence of inflammation.
Fig. 8. Macaques with elevated CSF YKL40 concentrations have elevated CSF neurofilament light chain concentrations.
CSF neurofilament light chain concentrations (NFL) were measured by ELISA at baseline (d0), day 14 post-infection (d14), day 42 post-infection (d42) and necropsy. Results were compared to CSF YKL40 concentrations in the same samples.
Brain protein extracts and CSF exhibit similar differences in neuroimmune marker concentrations
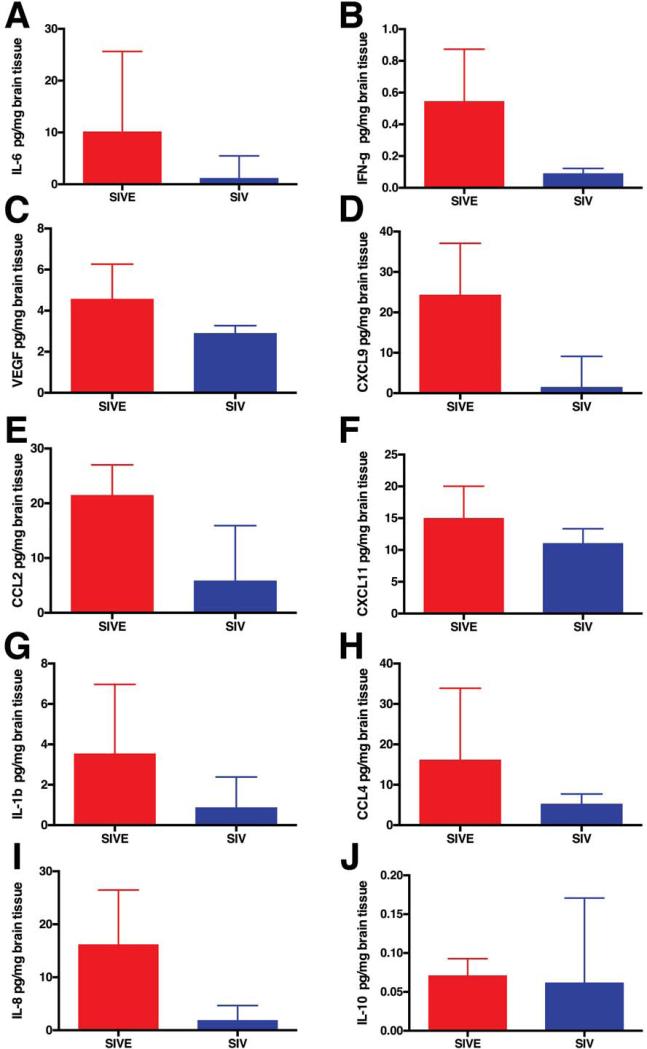
Since the CSF only partially reflects the environment of the CNS, we sought to examine the brain tissue concentrations of the neuroimmune markers that showed differential concentrations in the CSF of SIV-infected macaques with and without encephalitis. We used Luminex multiplex protein analysis to examine protein extracts from midfrontal cortical brain tissue in 5 macaques that developed SIVE and 7 SIV-infected macaques that did not develop encephalitis. Concentrations of IL-1β, IL-6, IL-8, IL-10, CCL2, CCL4, CXCL9, CXCL11, IFN-γ, MIF, and VEGF were measured. With the exception of MIF, which was not detected in these brain tissue lysates, the concentrations of neuroimmune markers in the brain reflected that observed in the CSF (Figs. 5,6,7 and 9). This suggests that use of CSF to reflect the environment of the brain parenchyma is valid for YKL40 (Fig. 1f) and the majority of neuroimmune markers examined.
Fig. 9. Neuroimmune markers in frontal cortical tissue protein extracts.
Multiplex quantitation of 10 cytokines in frontal cortical tissue protein extracts was performed. Compared to SIV-infected macaques without encephalitis (blue), IL-6 (a), IFN-γ (b), VEGF (c), CXCL9 (d), CCL2 (e), CXCL11 (f), IL-1β (g), CCL4 (h), and IL-8 (i) were elevated in macaques that developed SIVE (red). IL-10 was similar in SIV-infected macaques with and without encephalitis (j). Bars represent median concentration of indicated cytokine.
DISCUSSION
We have shown that CSF YKL40 concentration is a specific and sensitive indicator of encephalitis in SIV-infected macaques. Statistical modeling predicts that macaques with CSF YKL40 concentrations in excess of 1122 ng/ml have a 10-fold increased probability of having encephalitis. Retrospective analysis of serial CSF samples convey that elevated YKL40 concentrations and CNS viral replication occur two to eight weeks before animals require humane sacrifice. Although YKL-40 is not disease-specific, the unique macrophage-rich neuroinflammatory environment associated with infected macrophages once encephalitis develops is thought to initiate YKL-40 production in astrocytes. Monitoring viral replication and host response in order to follow the onset and course of CNS disease enables definition of pathological and immunological events during the progression of lentiviral encephalitis. Upon retrospective review, two interesting CSF expression profiles were detected in macaques that did versus those that did not develop encephalitis.
Acute increases in CSF cytokines and chemokines in SIV-infected macaques
Acute systemic primary infection is noted by a sharp rise in HIV RNA in the plasma (Lindback et al., 2000), induction of chronic immune activation (Douek et al., 2009), and clinical symptoms in the majority of individuals (80-90%) (Schacker et al., 1996; Hecht et al., 2002). Acute immune activation is associated with striking increases in the plasma levels of several cytokines and chemokines including IFNα, IFNγ, IL6, IL8, IL10, IL15, CXCL10, TNFα and CCL2 (Stacey et al., 2009). The kinetics of the appearance of these cytokines varies and some are transiently increased while others such as CCL2 and TNFα are more sustained. Of note, alterations in plasma cytokine levels are either not observed or are delayed and of lesser magnitude in acute hepatitis B and hepatitis C viral infection. Elevation of peripheral immune responses is communicated to the brain through a variety of pathways, leading to production of proinflammatory cytokines by microglia (Perry, 2004; Dantzer et al., 2008). We observe detectable acute increases of CCL2, IL6, IL1b, CXCL11 and others in the CSF of SIV-infected animals independent of development of encephalitis, although the magnitude of the increase is greater in animals that develop encephalitis. In addition to peripheral cytokine storms altering CSF cytokine concentrations, entry of HIV or SIV into the CNS can also occur during acute infection (Chakrabarti et al., 1991; Davis et al., 1992; Gray et al., 1993; Lackner et al., 1994; An et al., 1999; Witwer et al., 2009), thereby triggering proinflammatory cytokine responses in the CNS. Acute increases in TNFα, CCL2, and IL10 levels in the brain have been seen in SIV-infected pigtail macaques by others (Witwer et al., 2009). Interestingly, acute increases in YKL40 CSF levels are not identified, suggesting YKL40 expression is truly a marker of encephalitis and not induced by neuroimmune activation during acute infection. Together, these observations suggest that activation of brain immune responses during acute HIV or SIV infection might be predictive of the risk of developing encephalitis as observed with morbidity and mortality in the peripheral system (Deeks et al., 2013).
Macaques destined to develop encephalitis showed evidence of chronic neuroimmune activation
This acute CSF expression profile of macaques that subsequently developed encephalitis suggests may contribute to chronic neuroimmune activation. PAI-1, IL-6, VEGF, CCL2, and CCL11 were elevated throughout infection. Elevated expression of these cytokines in the CNS is thought to be associated with neurological diseases such as multiple sclerosis, bacterial meningitis, leukemia, and encephalitidies (Laurenzi et al., 1990; Perrella et al., 1992a; Rieckmann et al., 1995; Akenami et al., 1997; Zink et al., 2001; Kirk and Karlik, 2003; Mankowski et al., 2004; Roberts et al., 2004; Roscoe et al., 2009). Increased PAI-1 is suggestive of endothelial cell dysfunction but also might prevent neuronal apoptosis (Soeda et al., 2008). During inflammation, neuronal VEGF expression may lead to increased vascular permeability (Suidan et al., 2010). CCL2, a noted macrophage attractant is well known to be associated with lentiviral encephalitis (Cinque et al., 1998b; Zink et al., 2001). Elevated CNS IL6 may eventually promote expression of neuroinflammatory cytokines observed in the CNS of encephalitic animals.
Encephalitic macaques had a CSF neuroimmune profile consistent with macrophage activation and recruitment
The second CSF profile observed in macaques that develop encephalitis is concurrent with the development of CNS disease, i.e. rise in CSF YKL40 concentrations. Many of these neuroimmune markers are indicative of macrophage recruitment (IL-8) and activation (IL-1β, CCL4), while others are associated with a failed response to viral infection (IFNγ, CXCL11, CXCL9). We were unable to detect the presence of TNFα in any of the CSF samples regardless of the presence of encephalitis despite acute TNFα mRNA being readily detectable in brain tissue of SIV-infected macaques (Witwer et al., 2009). The lower limit of the multiplex assay used here was 4 pg/ml. Unfortunately, this assay may not be sensitive enough to detect changes in TNFα levels during lentiviral infection since CSF samples from patients with HIV encephalitis had a median concentration of 11.3 pg/ml (Monno et al., 1999). Increased MIF concentrations in plasma are thought to promote HIV transcription and replication (Regis et al., 2010; Delaloye et al., 2012) suggesting the increased CSF MIF levels seen at 42 days post-infection in macaques that go on to develop SIVE may promote an environment conducive to viral replication and development of encephalitis. TGF-β is an interesting cytokine with both regulatory and inflammatory activity depending on context (Li and Flavell, 2008). Since TGF-β is elevated in macaques that do not develop encephalitis, albeit at lower level compared to macaques with encephalitis, it is intriguing to hypothesize that in the right cellular and environmental context TGF-β may be protective. In addition, we also compared CSF YKL40 concentrations to osteopontin - a recently reported CSF biomarker of SIV encephalitis that is associated with macrophage survival and accumulation in the CNS (Burdo et al., 2007). YKL40 and osteopontin CSF concentrations had similar patterns of increase in macaques that developed encephalitis (Supplemental Fig. 2).
YKL40 levels are increased in diseases characterized by inflammation
Plasma levels of YKL40 are increased in a wide variety of conditions associated with acute and chronic inflammation and/or tissue remodeling such as infectious diseases (hepatitis c, pneumonia), autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease), remodeling diseases (osteoarthritis, liver cirrhosis), cancer (osteosarcomas, adenocarcinomas, breast, lung, melanoma), and other inflammatory conditions (asthma, pulmonary disease) (Johansen et al., 1997; Nordenbaek et al., 1999; Johansen et al., 2000; Vos et al., 2000; Jensen et al., 2003; Vind et al., 2003; Saitou et al., 2005; Johansen et al., 2006; Chupp et al., 2007; Letuve et al., 2008). In the CNS, YKL40 is significantly elevated in the CSF of acute and chronic neuroinflammatory conditions including lentiviral encephalitis, meningitis, traumatic brain injury, infarcts, early Alzheimer's disease, and glioblastoma (Junker et al., 2005; Hormigo et al., 2006; Bonneh-Barkay et al., 2008; Bonneh-Barkay et al., 2010a; Bonneh-Barkay et al., 2010b; Craig-Schapiro et al., 2010). While the functions of YKL40 in the CNS are poorly understood, YKL40 can act as a regulator of several biologic processes such as apoptosis, MAPK and Akt signaling, Th1/Th2 balance, oxidant injury, and TGFβ1 production (Lee et al., 2009; Chen et al., 2011; Dela Cruz et al., 2012). We observed that YKL40 CSF levels correlate significantly with CSF cytokines such as IL1β, IL6 and osteopontin during the development of SIV encephalitis. Increases in these inflammatory mediators and others such as TNFα and C-reactive protein have been shown to correlate with CSF and CNS tissue YKL40 expression in other CNS inflammation models (Bonneh-Barkay et al., 2010b; Bhardwaj et al., 2015). The proinflammatory cytokines IL1β, IL6, oncostatin M, TNFα, and factors released from differentiating monocyte-derived macrophages have been shown to induce YKL40 expression in astrocytes (Singh et al., 2011; Bonneh-Barkay et al., 2012; Bhardwaj et al., 2015). In the CNS, YKL40 induction is mostly restricted to astrocytes (Bonneh-Barkay et al., 2010a; Craig-Schapiro et al., 2010; Bonneh-Barkay et al., 2012) and is regulated by STAT3 and RelB/p50 complexes of NF-κB (Bhardwaj et al., 2015). Thus, during development of lentiviral encephalitis, activated and infected macrophage/microglia likely promote YKL40 expression in astrocytes.
Macaques destined to escape encephalitis showed a unique CSF profile
Identification of a CNS cytokine response associated with protection from encephalitis has been elusive. It might be that there is no single protective response and that complex interactions in an individual dictate whether there is CNS disease progression. We observed that SIV-infected non-encephalitic macaques have elevated CSF IL-10, CCL5, and PDGF-bb during the course of infection. The role of IL-10 during viral infection has been controversial despite its known immunosuppressive effects. Even though it has been suggested that IL-10 expression leads to chronic viral infection (Blackburn and Wherry, 2007), IL-10 is thought to suppress HIV-1 replication in macrophages (Ancuta et al., 2001). Polymorphisms in the il10 gene may also influence disease progression (Carrington et al., 2001; O'Brien and Nelson, 2004). CCL5 and CCL4 were early promising HIV-suppressive factors (Cocchi et al., 1995) and their immunohistochemical expression has been shown to be increased during lentiviral encephalitis (Sasseville et al., 1996; Sanders et al., 1998). Expression of unique combinations of these cytokines and other factors in balance may protect the host from CNS lentiviral disease.
Does the analysis of pigtailed macaque CSF presented here reflect the human disease or that of other species? YKL40 concentrations were significantly elevated in CSF of patients with an elevated CSF HIV viral load (>10,000 copies/ml), and there was a significant elevation of CSF YKL40 in patients with HIV encephalitis compared to patients without encephalitis (Bonneh-Barkay et al., 2008). YKL40 CSF concentrations are also increased in the CSF of rhesus macaques with SIVE (unpublished observations). Higher concentrations of IL-6, IL-8 and CCL4 in the CSF of HIV subjects with advanced stages of infection, HIV dementia or milder forms of HIV-associated neurocognitive disorder (HAND) have been observed (Laurenzi et al., 1990; Perrella et al., 1992b; Airoldi et al., 2012; Yuan et al., 2015). It will be interesting to examine YKL40 and other neuroimmune markers in longitudinal CSF samples from HIV-infected patients with HAND.
In summary, these data suggest that chronic CNS immune activation antedates the development of frank encephalitis. The next step is to determine how these findings translate to the development and presence of HAND in HIV-infected patients with or without antiretroviral treatment. We have already observed that YKL40 is increased in HIV-infected patients with high CSF viral loads (Bonneh-Barkay et al., 2008). Whether specific cytokines inhibit viral infection/replication, affect macrophage activation/recruitment, are neuroprotective, or some combination of all of these remains to be determined.
Supplementary Material
Acknowledgements
We thank Mark Stauffer, Jonette Werley and Heather Michaels for valuable technical assistance; Dawn L. McClemens-McBride and Premeela Rajakuman for assistance in obtaining clinical data for macaques; Holly Casamassa and Heather Dipietro for valuable veterinary assistance.
This work has been supported by National Institutes of Health grants MH071151 and MH01717 to C.A.W. and MH097476 to S.J.B. and C.A.W. This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory that is supported in part by award P30CA047904.
Footnotes
Ethical approval:
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Pittsburgh and their Institutional Animal Care and Use Committee.
Conflict of Interest:
The authors declare that they have no conflict of interest.
REFERENCES
- Airoldi M, Bandera A, Trabattoni D, Tagliabue B, Arosio B, Soria A, Rainone V, Lapadula G, Annoni G, Clerici M, Gori A. Neurocognitive impairment in HIV-infected naive patients with advanced disease: the role of virus and intrathecal immune activation. Clin Dev Immunol. 2012;2012:467154. doi: 10.1155/2012/467154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akenami FO, Koskiniemi M, Farkkila M, Vaheri A. Cerebrospinal fluid plasminogen activator inhibitor-1 in patients with neurological disease. J Clin Pathol. 1997;50:157–160. doi: 10.1136/jcp.50.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Bakri Y, Chomont N, Hocini H, Gabuzda D, Haeffner-Cavaillon N. Opposite effects of IL-10 on the ability of dendritic cells and macrophages to replicate primary CXCR4-dependent HIV-1 strains. J Immunol. 2001;166:4244–4253. doi: 10.4049/jimmunol.166.6.4244. [DOI] [PubMed] [Google Scholar]
- Andreasson U, Portelius E, Andersson ME, Blennow K, Zetterberg H. Aspects of beta-amyloid as a biomarker for Alzheimer's disease. Biomark Med. 2007;1:59–78. doi: 10.2217/17520363.1.1.59. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R, Yester JW, Singh SK, Biswas DD, Surace MJ, Waters MR, Hauser KF, Yao Z, Boyce BF, Kordula T. RelB/p50 complexes regulate cytokine-induced YKL-40 expression. J Immunol. 2015;194:2862–2870. doi: 10.4049/jimmunol.1400874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Bonneh-Barkay D, Starkey A, Trichel AM, Murphey-Corb M, Wiley CA. Systemic and brain macrophage infections in relation to the development of simian immunodeficiency virus encephalitis. J Virol. 2008;82:5031–5042. doi: 10.1128/JVI.02069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissel SJ, Wang G, Ghosh M, Reinhart TA, Capuano S, 3rd, Stefano Cole K, Murphey-Corb M, Piatak Jr M, Lifson JD, Jr., Wiley CA. Macrophages relate presynaptic and postsynaptic damage in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;160:927–941. doi: 10.1016/S0002-9440(10)64915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143–146. doi: 10.1016/j.tim.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wang G, Starkey A, Hamilton RL, Wiley CA. In vivo CHI3L1 (YKL-40) expression in astrocytes in acute and chronic neurological diseases. Journal of neuroinflammation. 2010a;7:34. doi: 10.1186/1742-2094-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and Macrophage Regulation of YKL-40 Expression and Cellular Response in Neuroinflammation. Brain Pathol. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Zagadailov P, Zou H, Niyonkuru C, Figley M, Starkey A, Wang G, Bissel SJ, Wiley CA, Wagner AK. YKL-40 expression in traumatic brain injury: an initial analysis. J Neurotrauma. 2010b;27:1215–1223. doi: 10.1089/neu.2010.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, Medina-Flores R, Murphey-Corb M, Rajakumar PA, Nyaundi J, Mellors JW, Bowser R, Wiley CA. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. Am J Pathol. 2008;173:130–143. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. J Leukoc Biol. 2007;81:1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M, Nelson G, O'Brien SJ. Considering genetic profiles in functional studies of immune responsiveness to HIV-1. Immunol Lett. 2001;79:131–140. doi: 10.1016/s0165-2478(01)00275-9. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L, Hurtrel M, Maire MA, Vazeux R, Dormont D, Montagnier L, Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Llado V, Eurich K, Tran HT, Mizoguchi E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin Immunol. 2011;140:268–275. doi: 10.1016/j.clim.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, Dziura JD, Reed J, Coyle AJ, Kiener P, Cullen M, Grandsaigne M, Dombret MC, Aubier M, Pretolani M, Elias JA. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Ceresa D, Mainini F, Terreni MR, Vagani A, Torri W, Bossolasco S, Lazzarin A. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998a;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998b;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Cline AN, Bess JW, Piatak M, Jr., Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Cruz CS, Liu W, He CH, Jacoby A, Gornitzky A, Ma B, Flavell R, Lee CG, Elias JA. Chitinase 3-like-1 promotes Streptococcus pneumoniae killing and augments host tolerance to lung antibacterial responses. Cell Host Microbe. 2012;12:34–46. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye J, De Bruin IJ, Darling KE, Reymond MK, Sweep FC, Roger T, Calandra T, Cavassini M. Increased macrophage migration inhibitory factor (MIF) plasma levels in acute HIV-1 infection. Cytokine. 2012;60:338–340. doi: 10.1016/j.cyto.2012.07.027. [DOI] [PubMed] [Google Scholar]
- Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, Wu MS, Weis K, Rinaldo CR, Haynes JR, Murphey-Corb M. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J Virol. 2002;76:3309–3317. doi: 10.1128/JVI.76.7.3309-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslen M, Hagberg L, Brew BJ, Cinque P, Price RW, Rosengren L. Elevated cerebrospinal fluid neurofilament light protein concentrations predict the development of AIDS dementia complex. J Infect Dis. 2007;195:1774–1778. doi: 10.1086/518043. [DOI] [PubMed] [Google Scholar]
- Gisslen M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, Spudich S, Hagberg L, Rosengren L, Price RW, Zetterberg H. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC neurology. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Hurtrel M, Hurtrel B. Early central nervous system changes in human immunodeficiency virus (HIV)-infection. Neuropathol Appl Neurobiol. 1993;19:3–9. doi: 10.1111/j.1365-2990.1993.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F. The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol. 2003;62:429–440. doi: 10.1093/jnen/62.5.429. [DOI] [PubMed] [Google Scholar]
- Gunnarsson M, Malmestrom C, Axelsson M, Sundstrom P, Dahle C, Vrethem M, Olsson T, Piehl F, Norgren N, Rosengren L, Svenningsson A, Lycke J. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69:83–89. doi: 10.1002/ana.22247. [DOI] [PubMed] [Google Scholar]
- Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol. 2010;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht FM, Busch MP, Rawal B, Webb M, Rosenberg E, Swanson M, Chesney M, Anderson J, Levy J, Kahn JO. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–1129. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- Helke KL, Queen SE, Tarwater PM, Turchan-Cholewo J, Nath A, Zink MC, Irani DN, Mankowski JL. 14-3-3 protein in CSF: an early predictor of SIV CNS disease. J Neuropathol Exp Neurol. 2005;64:202–208. doi: 10.1093/jnen/64.3.202. [DOI] [PubMed] [Google Scholar]
- Hormigo A, Gu B, Karimi S, Riedel E, Panageas KS, Edgar MA, Tanwar MK, Rao JS, Fleisher M, DeAngelis LM, Holland EC. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin Cancer Res. 2006;12:5698–5704. doi: 10.1158/1078-0432.CCR-06-0181. [DOI] [PubMed] [Google Scholar]
- Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]
- Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, Nilsson S, Zetterberg H, Gisslen M. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PloS one. 2014;9:e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- Johansen JS, Moller S, Price PA, Bendtsen F, Junge J, Garbarsch C, Henriksen JH. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scand J Gastroenterol. 1997;32:582–590. doi: 10.3109/00365529709025104. [DOI] [PubMed] [Google Scholar]
- Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, Garbarsch C, Bendtsen F. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/s0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- Junker N, Johansen JS, Hansen LT, Lund EL, Kristjansen PE. Regulation of YKL-40 expression during genotoxic or microenvironmental stress in human glioblastoma cells. Cancer Sci. 2005;96:183–190. doi: 10.1111/j.1349-7006.2005.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk SL, Karlik SJ. VEGF and vascular changes in chronic neuroinflammation. J Autoimmun. 2003;21:353–363. doi: 10.1016/s0896-8411(03)00139-2. [DOI] [PubMed] [Google Scholar]
- Kolson DL. YKL-40: a candidate biomarker for simian immunodeficiency virus and human immunodeficiency virus encephalitis? Am J Pathol. 2008;173:25–29. doi: 10.2353/ajpath.2008.080389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994;145:428–439. [PMC free article] [PubMed] [Google Scholar]
- Landqvist Waldo M, Frizell Santillo A, Passant U, Zetterberg H, Rosengren L, Nilsson C, Englund E. Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC neurology. 2013;13:54. doi: 10.1186/1471-2377-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenzi MA, Siden A, Persson MA, Norkrans G, Hagberg L, Chiodi F. Cerebrospinal fluid interleukin-6 activity in HIV infection and inflammatory and noninflammatory diseases of the nervous system. Clin Immunol Immunopathol. 1990;57:233–241. doi: 10.1016/0090-1229(90)90037-q. [DOI] [PubMed] [Google Scholar]
- Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, Coyle A, Chupp G, Reed J, Flavell RA, Elias JA. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ, Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Lindback S, Karlsson AC, Mittler J, Blaxhult A, Carlsson M, Briheim G, Sonnerborg A, Gaines H. Viral dynamics in primary HIV-1 infection. Karolinska Institutet Primary HIV Infection Study Group. AIDS. 2000;14:2283–2291. doi: 10.1097/00002030-200010200-00009. [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Clements JE, Zink MC. Cerebrospinal fluid markers that predict SIV CNS disease. J Neuroimmunol. 2004;157:66–70. doi: 10.1016/j.jneuroim.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Maschke M, Kastrup O, Esser S, Ross B, Hengge U, Hufnagel A. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC. NeuroAIDS: diagnosis and management. Hosp Pract (Minneap) 1997;32:73–74. 77–79, 84. doi: 10.1080/21548331.1997.11443542. passim. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- Monno L, Zimatore GB, Di Stefano M, Appice A, Livrea P, Angarano G. Reduced concentrations of HIV-RNA and TNF-alpha coexist in CSF of AIDS patients with progressive multifocal leukoencephalopathy. J Neurol Neurosurg Psychiatry. 1999;67:369–373. doi: 10.1136/jnnp.67.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sorensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis. 1999;180:1722–1726. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- O'Brien SJ, Nelson GW. Human genes that limit AIDS. Nat Genet. 2004;36:565–574. doi: 10.1038/ng1369. [DOI] [PubMed] [Google Scholar]
- Pendyala G, Trauger SA, Kalisiak E, Ellis RJ, Siuzdak G, Fox HS. Cerebrospinal fluid proteomics reveals potential pathogenic changes in the brains of SIV-infected monkeys. J Proteome Res. 2009;8:2253–2260. doi: 10.1021/pr800854t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella O, Carrieri PB, Guarnaccia D, Soscia M. Cerebrospinal fluid cytokines in AIDS dementia complex. J Neurol. 1992a;239:387–388. doi: 10.1007/BF00812156. [DOI] [PubMed] [Google Scholar]
- Perrella O, Guerriero M, Izzo E, Soscia M, Carrieri PB. Interleukin-6 and granulocyte macrophage-CSF in the cerebrospinal fluid from HIV infected subjects with involvement of the central nervous system. Arq Neuropsiquiatr. 1992b;50:180–182. doi: 10.1590/s0004-282x1992000200008. [DOI] [PubMed] [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, Yiannoutsos CT, Spudich SS, Price RW. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PloS one. 2014;9:e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RW, Epstein LG, Becker JT, Cinque P, Gisslen M, Pulliam L, McArthur JC. Biomarkers of HIV-1 CNS infection and injury. Neurology. 2007;69:1781–1788. doi: 10.1212/01.wnl.0000278457.55877.eb. [DOI] [PubMed] [Google Scholar]
- Regis EG, Barreto-de-Souza V, Morgado MG, Bozza MT, Leng L, Bucala R, Bou-Habib DC. Elevated levels of macrophage migration inhibitory factor (MIF) in the plasma of HIV-1-infected patients and in HIV-1-infected cell cultures: a relevant role on viral replication. Virology. 2010;399:31–38. doi: 10.1016/j.virol.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P, Albrecht M, Ehrenreich H, Weber T, Michel U. Semi-quantitative analysis of cytokine gene expression in blood and cerebrospinal fluid cells by reverse transcriptase polymerase chain reaction. Res Exp Med (Berl) 1995;195:17–29. doi: 10.1007/BF02576770. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Burudi EM, Flynn C, Madden LJ, Roinick KL, Watry DD, Zandonatti MA, Taffe MA, Fox HS. Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. J Neuroimmunol. 2004;157:81–92. doi: 10.1016/j.jneuroim.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Roscoe WA, Welsh ME, Carter DE, Karlik SJ. VEGF and angiogenesis in acute and chronic MOG((35-55)) peptide induced EAE. J Neuroimmunol. 2009;209:6–15. doi: 10.1016/j.jneuroim.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, Sugimoto K, Murata K, Nakano T. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476–481. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998;12:1021–1026. [PubMed] [Google Scholar]
- Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- Singh SK, Bhardwaj R, Wilczynska KM, Dumur CI, Kordula T. A complex of nuclear factor I-X3 and STAT3 regulates astrocyte and glioma migration through the secreted glycoprotein YKL-40. J Biol Chem. 2011;286:39893–39903. doi: 10.1074/jbc.M111.257451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C, Granerus AK, Vanderstichele H, Vanmechelen E, Blennow K. Both total and phosphorylated tau are increased in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:624–630. doi: 10.1136/jnnp.70.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, Hayashida S, Shimeno H. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb Haemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, Self SG, Borrow P. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suidan GL, Dickerson JW, Chen Y, McDole JR, Tripathi P, Pirko I, Seroogy KB, Johnson AJ. CD8 T cell-initiated vascular endothelial growth factor expression promotes central nervous system vascular permeability under neuroinflammatory conditions. J Immunol. 2010;184:1031–1040. doi: 10.4049/jimmunol.0902773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Bonneh-Barkay D, Lopresti BJ, Bissel SJ, Wang G, Mathis CA, Piatak M, Jr., Lifson JD, Nyaundi JO, Murphey-Corb M, Wiley CA. Longitudinal in vivo positron emission tomography imaging of infected and activated brain macrophages in a macaque model of human immunodeficiency virus encephalitis correlates with central and peripheral markers of encephalitis and areas of synaptic degeneration. Am J Pathol. 2008;172:1603–1616. doi: 10.2353/ajpath.2008.070967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003;38:599–605. doi: 10.1080/00365520310000537. [DOI] [PubMed] [Google Scholar]
- Vos K, Steenbakkers P, Miltenburg AM, Bos E, van Den Heuvel MW, van Hogezand RA, de Vries RR, Breedveld FC, Boots AM. Raised human cartilage glycoprotein-39 plasma levels in patients with rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis. 2000;59:544–548. doi: 10.1136/ard.59.7.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PloS one. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. Biomed Res Int. 2015;2015:506872. doi: 10.1155/2015/506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr., Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.