Abstract
Gibberellins (GAs) are phytohormones controlling major aspects of plant growth and development. Although previous studies suggested the existence of a transport of GAs in plants, the nature and properties associated with this transport were unknown. We recently showed through micrografting and biochemical approaches that the GA12 precursor is the chemical form of GA undergoing long-distance transport across plant organs in Arabidopsis. Endogenous GA12 moves through the plant vascular system from production sites to recipient tissues, in which GA12 can be converted to bioactive forms to support growth via the activation of GA-dependent processes. GAs are also essential to promote seed germination; hence GA biosynthesis mutants do not germinate without exogenous GA treatment. Our results suggest that endogenous GAs are not (or not sufficiently) transmitted to the offspring to successfully complete the germination under permissive conditions.
Keywords: Arabidopsis thaliana, gibberellin, grafting, growth, offspring, seed germination, transport, vascular system
Abbreviations
- GA
gibberellin
- KAO
ent-kaurenoic acid oxidase
- GA20ox
GA20-oxidase
- NPF
NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER FAMILY
- DELLA
DELLA protein
- Col-0
Columbia-0
- ga1–3
ent-copalyl diphosphate synthase (CPS) mutant.
The gibberellins (GAs) are an important family of diterpenoid compounds, of which only a few members such as GA1 and GA4, actively regulate various growth processes throughout the plant life cycle, including seed germination, vegetative growth, flowering and fruit development.1 Hence, GA biosynthesis mutants are dwarfs and late flowering, whereas GA overdose causes exaggerated growth and sterility. Therefore it is essential that plants produce and accumulate appropriate levels of GAs to ensure normal growth. Biochemical and genetic approaches have led to the identification of the majority of GA biosynthesis genes and regulatory mechanisms controlling the optimal levels of bioactive GAs for plant growth.2 Moreover, the movement of GAs from production sites to tissues and organs that require GAs for growth may also represent a level of regulation. Strikingly, several studies support the idea of a local and long-distance transport of GAs in plants,3-8 however it remained unclear which form of endogenously made GA is mobile. In a recent publication, we addressed this question by performing a series of reciprocal micrograftings between hypocotyls of Arabidopsis wild-type and GA-deficient mutants altered at specific steps of the GA biosynthetic pathway.9 In this work we showed that wild-type rootstocks are able to restore the growth of kao1 kao2 mutant scions but not of triple ga20ox1 ga20ox2 ga20ox3 mutant scions, compared to respective self-grafted plants. Because the ent-kaurenoic acid oxidase (KAO) catalyzes the conversion of ent-kaurenoic acid into GA12, the immediate substrate for GA20-oxidases (GA20ox),10,11 our results indicated that GA12, the common precursor for all GAs,2 is the graft transmissible signal. This assumption was further supported by the fact that GA12 and all products of GA20ox activity accumulate to high levels in GA-deficient ga1–3 scions (mutant defective in the first committed step of GA biosynthesis)2 grafted onto wild-type rootstocks.9 Remarkably, using the same strategy, we found that GA12 can also move basipetally from shoot to root.9 Thus, endogenous GA12 has the capacity to move in both directions in Arabidopsis plants. Noteworthy, although micrografting procedure is an excellent approach to study long-range signaling in plants, this technique is inappropriate to monitor short-range cell-to-cell movement of GAs. Hence, previous works relying on exogenous GA feeding experiments have shown that other GAs, precursors and bioactive forms, can also move locally in plants.4,5,7,8
Plant hormones are small signaling compounds that often move throughout the body of the plant via the plant vascular system.12 Consistent with previous studies,13,14 our results revealed the presence of GA12 in xylem and phloem exudates, suggesting that GA12 is transported from root to shoot by the xylem and from photosynthetic source to sink tissues by the phloem.9 Recently, biochemical studies in heterologous systems allowed the identification of several GA transporters, belonging to the NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER FAMILY (NPF).15-17 The NPFs represent a large family of membrane proteins which transport a wide array of compounds including nitrate, peptides, glucosinolates and phytohormones.18 Although not demonstrated, it is tempting to speculate that these multifunctional transporters may contribute in the translocation of GA12 from parenchyma cells to the vessels. Major challenges will be to put these transporters into a more integrated picture, and to understand the molecular basis for their selectivity to so diverse variety of compounds.
GAs control a wide range of growth processes by stimulating the degradation of the DELLA proteins, a family of nuclear growth repressors.19 Accordingly, reduced bioactive GA levels cause an increase in DELLA abundance, which in turn restrains growth.19 Our results showed that DELLA accumulation is reduced in shoots of Col-0/kao1 kao2 grafts in comparison to Col-0/ga20ox1 ga20ox2 ga20ox3 grafts, hence correlating to some extend to their respective overall growth phenotype.9 Thus, GA12 is functional in recipient organs and drives growth via the activation of the GA-signaling pathway. Numerous studies emphasized the importance of GAs in the adaptation of plants to their surrounding growth conditions.20 We proposed that long-distance transport of GA12 across plant organs enables plants to adapt their growth and development in response to both endogenous and environmental inputs.
An interesting issue raised from the above results is whether endogenous GAs are transmitted to the offspring, especially in seeds. It has long been known that GAs act as positive regulators of seed germination as exemplified by the phenotype of severe GA-deficient mutant seeds (such as ga1–3 mutant) which fail to germinate in the absence of exogenous GAs.21 Remarkably, in contrast to ga1–3/ga1–3 grafts, the shoots of Col-0/ga1–3 grafts develop long and fertile siliques without GA treatment, thus indicating that wild-type rootstocks produce enough GAs to compensate the deficit in bioactive GAs in developing siliques of ga1–3 grafted scions (Fig. 1A,B). However, ga1–3 seeds collected from both Col-0/ga1–3 and GA-treated ga1–3/ga1–3 grafts (sprayed with 100 µM GA3 twice a week until flowering) failed to germinate under permissive conditions (although the seeds were morphologically normal), in contrast to wild-type seeds collected from Col-0/Col-0 grafts (Fig. 1C). Furthermore, exogenous application of bioactive GA3 equally rescued the germination defect of ga1–3 seeds collected from Col-0/ga1–3 and GA-treated ga1–3/ga1–3 grafts in a dose-dependent manner (Fig. 1C). Collectively, these results indicate that although endogenous GA12 easily move throughout the plant and promote growth of recipient organs, GAs produced by the plant fail to compensate the germination defect of progeny seeds deficient in GA synthesis, suggesting that endogenous GAs are not transmitted to the offspring. In this scenario, de novo synthesis of active GAs is necessary to stimulate seed germination under permissive conditions.2 On the other hand, we cannot exclude the possibility that small amounts of GAs transported from maternal tissues activate some regulatory steps during embryo development. Determination of endogenous GA contents in ga1–3 seeds collected from Col-0/ga1–3 grafts should allow us to address these possibilities.
Figure 1.
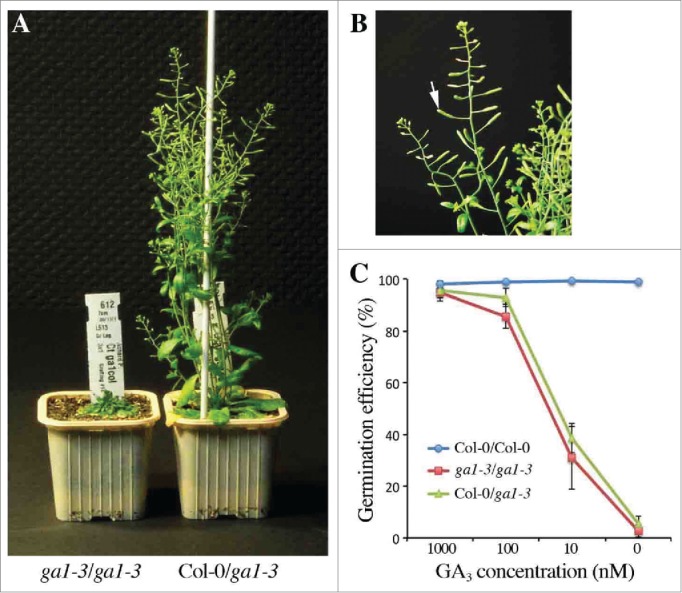
ga1–3 seeds collected from Col-0/ga1–3 grafts fail to germinate without exogenous GAs. (A) Overall shoot phenotypes of 5-week-old ga1–3/ga1–3 and Col-0/ga1–3 grafts. (B) Close-up of ga1–3 scion grafted onto wild-type (Col-0) rootstock. The arrow indicates a fertile silique. (C) Germination efficiency (%) of seeds collected from Col-0/Col-0, ga1–3/ga1–3 and Col-0/ga1–3 grafts. 150 to 200 seeds per genotype were imbibed in presence of GA3 at indicated concentrations, in the light at 22°C for 7 d. The values are the mean ±SD (n=3). Genotype notation is rootstock/grafted scion.
The recent period has seen many exciting advances in our understanding of the mechanisms governing GA transport. Still, multiple questions remain unaddressed, such as the dynamics of this process and the biological function associated with this transport. In this aim, the combination of micrografting and biochemistry provides a powerful tool to tackle long-distance signals in plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors' research group is currently supported by the Center National de la Recherche Scientifique.
References
- 1.Pimenta Lange MJ, Lange T. Gibberellin biosynthesis and the regulation of plant development. Plant Biol 2006; 90:281-90; http://dx.doi.org/ 10.1055/s-2006-923882 [DOI] [PubMed] [Google Scholar]
- 2.Hedden P, Thomas SG. Gibberellin biosynthesis and its regulation. Biochem J 2012; 444:11-25; PMID:22533671; http://dx.doi.org/ 10.1042/BJ20120245 [DOI] [PubMed] [Google Scholar]
- 3.Katsumi M, Foard DE, Phinney BO. Evidence for the translocation of gibberellin A3 and gibberellin-like substances in grafts between normal, dwarf1 and dwarf5 seedlings of Zea mays L. Plant & Cell Physiol 1983; 24:379-88 [Google Scholar]
- 4.Proebsting WM, Hedden P, Lewis MJ, Croker SJ, Proebsting LN. Gibberellin concentration and transport in genetic lines of pea. Plant Physiol 1992; 100:1354-60; PMID:16653128; http://dx.doi.org/ 10.1104/pp.100.3.1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksson S, Böhlenius H, Moritz T, Nilsson O. GA4 is the active gibberellin in the regulation of LEAFY transcription and Arabidopsis floral initiation. Plant Cell 2006; 18:2172-81; PMID:16920780; http://dx.doi.org/ 10.1105/tpc.106.042317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragni L, Nieminen K, Pacheco-Villalobos D, Sibout R, Schwechheimer C, Hardtke CS. Mobile gibberellin directly stimulates Arabidopsis hypocotyl xylem expansion. Plant Cell 2001; 23:1322-36; http://dx.doi.org/ 10.1105/tpc.111.084020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayan J, Voronin N, Gong F, Sun TP, Hedden P, Fromm H, Aloni R. Leaf-induced gibberellin signaling is essential for internode elongation, cambial activity, and fiber differentiation in tobacco stems. Plant Cell 2012; 24:66-79; PMID:22253226; http://dx.doi.org/ 10.1105/tpc.111.093096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shani E, Weinstain R, Zhang Y, Castillejo C, Kaiserli E, Chory J, Tsien RY, Estelle M. Gibberellins accumulate in the elongating endodermal cells of Arabidopsis root. Proc Natl Acad Sci USA 2013; 110:4834-39; PMID:23382232; http://dx.doi.org/ 10.1073/pnas.1300436110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnault T, Davière JM, Wild M, Sakvarelidze-Achard L, Heintz D, Carrera Bergua E, et al.. The gibberellin precursor GA12 acts as a long-distance growth signal in Arabidopsis. Nature Plants 2015; 1:15073; http://dx.doi.org/ 10.1038/nplants.2015.73 [DOI] [PubMed] [Google Scholar]
- 10.Regnault T, Davière JM, Heintz D, Lange T, Achard P. The gibberellin biosynthetic genes AtKAO1 and AtKAO2 have overlapping roles throughout Arabidopsis development. Plant J 2014; 80:462-74; PMID:25146977; http://dx.doi.org/ 10.1111/tpj.12648 [DOI] [PubMed] [Google Scholar]
- 11.Plackett ARG, Powers SJ, Fernandez-Garcia N, Urbanova T, Takebayashi Y, Seo M, Jikumaru Y, Benlloch R, Nilsson O, Ruiz-Rivero O, et al.. Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 2012; 24:941-60; PMID:22427334; http://dx.doi.org/ 10.1105/tpc.111.095109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robert HS, Friml J. Auxin and other signals on the move in plants. Nat Chem Biol 2009; 5:325-32; PMID:19377459; http://dx.doi.org/ 10.1038/nchembio.170 [DOI] [PubMed] [Google Scholar]
- 13.Hoad GV, Bowen MR. Evidence for gibberellin-like substances in phloem exudate of higher plants. Planta 1968; 82:22-32; PMID:24519793; http://dx.doi.org/ 10.1007/BF00384695 [DOI] [PubMed] [Google Scholar]
- 14.Lavender DP, Sweet GB, Zaerr JB, Hermann RK. Spring shoot growth in Douglas-fir may be initiated by gibberellins exported from the roots. Science 1973; 182:838-9; PMID:17772159; http://dx.doi.org/ 10.1126/science.182.4114.838 [DOI] [PubMed] [Google Scholar]
- 15.Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci USA 2012; 103:9653-58; http://dx.doi.org/ 10.1073/pnas.1203567109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito H, Oikawa T, Hamamoto S, Ishimaru Y, Kanamori-Sato M, Sasaki-Sekimoto Y, Utsumi T, Chen J, Kanno Y, Masuda S, et al.. The jasmonate-responsive GTR1 transporter is required for gibberellin-mediated stamen development in Arabidopsis. Nat Commun 2015; 6:6095; PMID:25648767; http://dx.doi.org/ 10.1038/ncomms7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba Y, Shimizu T, Miyakawa S, Kanno Y, Koshiba T, Kamiya Y, Seo M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J Plant Res 2015; 128:679-86; PMID:25801271; http://dx.doi.org/ 10.1007/s10265-015-0710-2 [DOI] [PubMed] [Google Scholar]
- 18.Leran S, Varala K, Boyer JC, Chiurazzi M, Crawford N, Daniel-Vedele F, David L, Dickstein R, Fernandez E, Forde B, et al.. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci 2014; 19:5-9; PMID:24055139; http://dx.doi.org/ 10.1016/j.tplants.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 19.Davière JM, Achard P. Gibberellin signaling in plants. Development 2013; 140:1147-51; http://dx.doi.org/ 10.1242/dev.087650 [DOI] [PubMed] [Google Scholar]
- 20.Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin in plant responses to abiotic stress. J Exp Bot 2014; 217:67-75; http://dx.doi.org/ 10.1242/jeb.089938 [DOI] [PubMed] [Google Scholar]
- 21.Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants of Arabidopsis thaliana. Theor Appl Genet 1980; 58:257-63; PMID:24301503; http://dx.doi.org/ 10.1007/BF00265176 [DOI] [PubMed] [Google Scholar]


