Abstract
Crop vulnerability to multiple abiotic stresses is increasing at an alarming rate in the current global climate change scenario, especially drought. Crop improvement for adaptive adjustments to accomplish stress tolerance requires a comprehensive understanding of the key contributory processes. This requires the identification and careful analysis of the critical morpho-physiological plant attributes and their genetic control. In this review we try to discuss the crucial traits underlying drought tolerance and the various modes followed to understand their molecular level regulation. Plant stress biology is progressing into new dimensions and a conscious attempt has been made to traverse through the various approaches and checkpoints that would be relevant to tackle drought stress limitations for sustainable crop production.
Keywords: cellular tolerance, drought, gene regulation, omics, stress gene discovery
Introduction
Over 10,000 years ago humans initiated crop improvement through selective breeding to meet the increasing demand for food and fodder. Natural calamities and anthropogenic activities are posing serious challenges for sustained increase in global food production. Under present crop growing conditions, detrimental environmental factors, generally termed as abiotic stresses (drought, salinity, temperature extremes, nutrient deficiencies and toxicities) pose threat to normal growth and development of crop plants. These abiotic stresses can reduce average yield by more than 50%.1-2 In tropical countries, although we come across multiple stresses simultaneously in crop lands, drought is considered as the major one.3 One-third of the world's population dwells in water-stressed regions and global climate change will definitely increase the occurrence and severity of drought episodes. Under the conditions of climate change and diminishing water resources crop biologists have the challenging task to evolve crop types that can grow and produce sufficient biomass and yield. Although attempts have been made by agronomists to mitigate drought stress threats, plant biologists/physiologists are interested in adopting a holistic approach of maintaining major biological processes under drought or desiccation stress. The ultimate aim should be to develop a genotype with commendable survival and production capacity under drought, rather than just survival characters, characteristic of xeric habitat adaptability. For identifying such characters, we need to overlook the various acclimatory processes that the plant adopts in order to equilibriate with the environment to reach the meta stable state called homeostasis, under unfavourable conditions.4 This homeostatic state is attained through various time dependent adaptive responses which make certain hardy species to withstand harsh detrimental conditions. This helps them to integrate various physiological processes in a coordinated manner to contribute to the greatest plant attribute called environmental plasticity.
The physiological integration of various tolerance/ acclimation mechanisms is a resultant of altered gene expression and/or existing protein functions. These responses pave way for either avoidance which prevent the very exposure to stress itself or tolerance strategies which permits the plant to thrive through the stress. Multiple factors govern stress tolerance including stress characteristics (duration, rate, severity and combinatorial effects) as well as plant characters (variations at genotype, developmental stage and tissue levels) which ultimately deliver a tolerant or susceptible phenotype. It is highly challenging to tackle the multi-threat face of drought, how and where to begin to strategise to achieve the goal of attaining tangible levels of drought tolerance.
How to Unravel the Complexity of Drought Stress Response: Trait Based Approach?
Nature's way of response to stress in most biological systems is the 'fight' or 'flight' response for every 'fright'. As a result of this phenomenon, plants being sessile, try to maintain essential metabolic activity for their sustenance and the degree or extent of response depends on different inherent tolerance associated mechanisms. The adaptive strategies revolve around certain key attributory physiological traits.5 Analysis of adaptive mechanisms of plants will contribute to the knowledge, the requirement for targeted crop improvement toward drought resistance. The complex responses to drought, from perception to ultimate physiological changes, need to be considered at a global systems biology level to examine the multiple interactive components.6-7 Since drought tolerance is a complex trait, for targeted crop improvement, an understanding of the traits linked to plant water relations and cell tolerance to drought assumes significance and integrative traits are being used in high throughput phenotyping.5,8 A few critical traits related to water relations such as water mining and water conservation determining water use efficiency (WUE) and cellular tolerance (CT) to desiccation are considered to be decisive for drought adaptation. The efficiency of the system to combat stress effects relies on how best the water relations are maintained either by mining more available soil water or by conserving water or both, along with various CT mechanisms. Efforts have been made to dissect and manipulate some of these characters with varied degrees of success.9
Water mining forms an important strategy wherein in the root system architecture attributes to tolerate drought to a considerable extent. Crops such as wheat with a deeper root system can have higher yields in rain fed systems due to efficient water mining ability.10 Improvement in root branching and density can lead to drought tolerance as noticed in rice using transgenic approach.11 Many other root attributes have been reviewed extensively in the recent past.12-13 There are also recent evidences emphasizing the importance of combining water acquisition and CT traits for maintaining higher spikelet fertility in rice under drought stress thereby enhancing field level tolerance to water limitation.14 In addition to water extraction from drying soil, water conservation strategies to retain tissue water are crucial for drought adaptation. Water conservation depends upon stomatal and non-stomatal transpiration, among which the later seems to be the crucial component since cuticular transpiration can happen continuously during day and night under dry conditions where vapour pressure deficit (VPD) will be high. In higher plants, cuticular wax forms a hydrophobic layer covering aerial organs, which is deposited either outside of the cuticle (epicuticular wax), or within the cuticular matrix (intracuticular wax).15 Epidermal wax layer provides a protective barrier between the plant and its environment, which functions as a barrier to water loss and prevents dehydration of underlying cells.15 Therefore, an important morpho-physiological feature (dehydration avoidance mechanism), the deposition of epicuticular waxes, can enable the plant to maintain hydration under the conditions of low VPD.8 However, reduction in transpiration can affect the evaporative cooling which makes it essential for a tradeoff between water conservation and stomatal regulation to bestow a cooler canopy. The genomic regions for canopy temperature in wheat have been identified recently and their genetic associations with stomatal conductance and grain yield have also been evaluated.16 A considerable number of such component traits are responsible for the quantitative regulation of utilization of water, the most critical resource under moisture deficit stress conditions, which could vary across diverse plant species and types.17-18
Tissue water status determines the overall metabolic activity of the cells constituting it, which ultimately underlies the efficacy of various CT mechanisms. Cellular tolerance mechanisms equip each cell to build up a force against drought. Stress affects cellular energy status and induces energy saving responses resulting in low energy syndrome (LES). In general, LES includes transcriptional and translational reprogramming which is essential for stress acclimation.19 Cellular tolerance can be achieved by addressing key processes like transcriptional regulation, protein turnover, membrane stability attributory traits, active oxygen species scavenging (AOS) and so on.7,9 Intrinsic cellular tolerance mechanisms can significantly contribute for growth and yield under abiotic stresses. Multiple cellular pathways and genes regulate cellular tolerance thereby offering a multi- level protection umbrella by employing various mechanisms like AOS scavenging, protein protection and turnover and osmoregulation depending on the species and genotype.20 Rice plants overexpressing Arabidopsis homeodomain-leucine zipper transcription factor Enhanced Drought Tolerance/HOMEODOMAIN GLABROUS11 (EDT1/HDG11) had higher levels of abscisic acid, proline, soluble sugars and AOS-scavenging enzyme activities during stress which probably conferred drought tolerance and higher grain yield due to maintenance of pollen fertility.20 These findings suggest that manipulation of cellular tolerance can immensely benefit plant growth and productivity under stress. Similarly, numerous traits including primary, secondary, and integrative drought-resistance traits exist, whose significance needs to be identified through precise phenotyping and be validated for intervention towards crop improvement for attainment of drought tolerance.3,5,8 The molecular scrutiny of some of these traits is highly recommended. Hence the deployment of trait based gene prospecting assumes significance because of the fact that the current era is driven by 'gene revolution' for crop improvement after a yesteryear era of green revolution.
Prospecting the Trait Regulatory Genes
A wide array of gene discovery tools have helped to advance our understanding of stress signal perception and transduction and associated molecular regulatory networks.21-23 These tools have helped in the successful revelation of several stress-inducible genes and various transcription factors that regulate the drought-stress-inducible systems. Initially, gene transcript analysis from drought tolerant crop species was adopted as a good approach for gene discovery, which has been primarily achieved by general gene expression analysis that is clearly reflected even in recent studies.24-26 Later on, identification of the differentially expressed genes were successfully done by different approaches like subtractive hybridization, suppressive subtractive hybridization,27 differential display,28-29 cDNA-AFLP,30 microarray technology and other means.22,31 The microarray analysis of Arabidopsis thaliana genome has provided a powerful and widely used method to research the effects of various gene expressions which is also reflected in diverse plant species.32 Similarly, Targeting-Induced Local Lesions IN Genomes (TILLING), a reverse-genetic strategy for the discovery and mapping of induced mutations can also be attempted.33 Generation and characterization of stress specific ESTs was attempted by many when genome information was limited.34
Large-scale genome sequencing projects helped in the identification of important genes in certain plant types like Arabidopsis,35 rice,36 maize,37 poplar38 and other species, in the initial years. When the genome sequence is not available and genome size is significantly large, alternative approaches have been followed. Under such conditions, comparative genomics emerged as a viable attempt, which enables assessment of data from one species to investigate other incongruent species, which has already been achieved in case of annotating wheat sequences using information from E. coli, human, A. thaliana and rice.39 However, in the recent past, the unravelling of genome information of a few species like the chickpea,40 mulberry,41 soybean,42-43 Medicago,44 pigeon pea45-46 and peanut (www.peanutbioscience.com)47 has provided tremendous potential for targeted way of addressing various crop improvement issues. Over the years, technology advancement has led to a great leap in gene discovery from the mid 20th century to the late 2000s and till date (Fig. 1).
Figure 1.
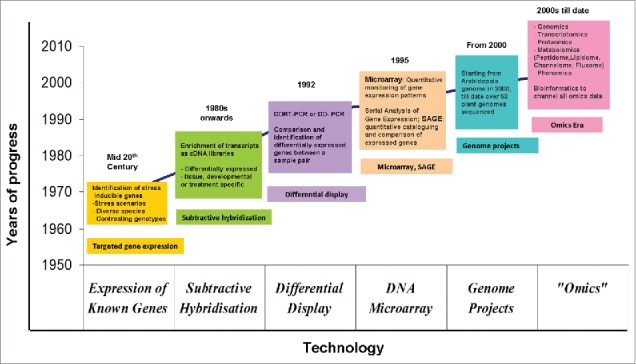
Schematic depiction of the evolution of stress responsive gene discovery technologies over the years
The molecular breeding research ventures, in parallel, has helped in the identification of key quantitative trait loci (QTLs) defining the genomic regions and the underlying genes that regulate the various morpho-physiological traits that confer drought tolerance to superior accessions across different plant species.48-54
Recently, a number of “omics” studies have reinforced the fact that the data resulting from these studies may promote our understanding of signaling pathways and will provide a new layer for analysis in systems biology.55 Transcriptome based gene prospecting approach is gaining momentum these days.56-57 Proteome and metabolome analyses in different plant species contributed to a better understanding of molecular mechanisms that are involved under drought.58-60 There are several omics data repositories which are publically available as listed in Table 1, which can hasten our access to data generated for multi- level analysis for a comprehensive understanding of the experimental scenario. Integration of multiple data sets from different omics technologies have to be materialized by the use of various in silico and bioinformatics tools owing to the pressure of the rapid pace at which crop improvement programmes are advancing.61-62 Whole plant tolerance attributes root deep within at individual cell/ tissue level which makes it highly significant to understand the cellular mechanisms of desiccation tolerance in diverse plants. This may eventually enable future molecular improvement for realizable levels of drought tolerance in crop plants.
Table 1.
List of publically available Omics Data Repositories
Target Genes to Manipulate Cellular Tolerance-Stress Responsive Upstream Regulatory and Downstream Functional Genes
Stress responsive genes have specific elucidated roles that classify them under 2 major categories namely upstream regulatory genes and downstream regulatory/ functional genes.22,63 However, the identity of the genes by proper annotation makes it meaningful in the context of its use for crop improvement. The rapid advancement in DNA sequencing technologies has accelerated plant biology research by unravelling the genomes of many important plant species keeping multiple ‘omics’ options open. This avenue is best realized when one gets to know the exact roles of the genes identified. The Gene Ontology (GO) concept has helped in the annotation of homologous gene and protein sequences in multiple organisms using a common platform which eases the query and retrieval of genes and proteins based on their shared biology.64 A number of genes have been identified to be involved in CT, and their functions were confirmed by transgenic approaches.2,7,11,65-67 It has also been reported recently that ectopic expression of stress specific transcription factors in combination aids in combating multiple abiotic stresses.68-70 Large numbers of review articles have compiled the relevance of multiple upstream regulatory as well as downstream functional genes underlying a wide array of drought tolerance traits in imparting abiotic stress tolerance (Table 2). There is a well defined and tightly regulated signaling network starting from stress signal perception up to downstream functional gene activation, and the genes which regulate their activity, whichever be the tolerance mechanism.71 There have been several reports on the roles of different members in the signaling cadre which stand critical in imparting plant abiotic stress tolerance which is consolidated in Table 3.
Table 2.
Recent reviews on abiotic stress tolerance trait regulation in plants
| Article content | Author and year of publication | Reference |
|---|---|---|
| Successful genetic engineering of drought-tolerant crops | Yang et al. 2010 | 81 |
| Progress studies of drought-responsive genes in rice | Hadiarto and Tran, 2011 | 82 |
| Targeting regulatory networks for abiotic stress tolerance | Reguera et al. 2012 | 67 |
| Stress-induced metabolic rearrangements and regulatory networks | Krazensky and Jonak, 2012 | 71 |
| Interaction of plant biotic and abiotic stresses | Atkinson and Urwin, 2012 | 83 |
| Genetic engineering: evaluation of achievements, limitations, and possibilities | Lawlor, 2013 | 7 |
| Recent advances in drought stress tolerance research in wheat | Rana et al. 2013 | 84 |
| Genetic engineering and breeding of drought-resistant crops | Hu and Xiong, 2014 | 9 |
| Metabolic adjustment and regulation of gene expression | Bhargava and Sawant, 2014 | 85 |
| Transcriptional regulatory network in the drought response and its crosstalk | Nakashima et al. 2014 | 63 |
| Safety aspects of genetically modified crops with abiotic stress tolerance | Liang et al. 2014 | 86 |
| Drought-stress regulatory networks and strategies for drought-tolerant transgenic rice development | Todaka et al. 2015 | 87 |
Table 3.
Signaling partners associated with diverse abiotic stress responses in plants.
| Signalling Cadre | Gene | Reference |
|---|---|---|
| Signal perception | ARCK1 (RLCK) | 88 |
| GbRLK (RLK) | 89 | |
| GUDK (RLCK) | 90 | |
| Signal transduction by phosphorelay | MAPKKK | 91 |
| PtMKK4 | 92 | |
| SpMPK3 | 93 | |
| AtMPK12 | 94 | |
| CBL-CIPK | 95 | |
| 96 | ||
| CDPK1 | 97 | |
| Upstream regulatory genes | ||
| Stress specific transcription factors | NAC | 98,105 |
| AP2/ERF | 99,105 | |
| WRKY | 100,105 | |
| HSFs | 101,105 | |
| bZIP | 102,105 | |
| Zinc fingers (ZF) | 103,105 | |
| 104,105 | ||
| Basal regulatory genes | BTF3 | 106 |
| NF-Ys | 65,107,108 | |
| 107 | ||
| 108 | ||
| TAFs-AtTAF10 | 109 | |
| Downstream regulatory/ functional genes | ||
| Water mining | GmNAC | 110 |
| V-H+Ppase- ThVPV-H+Ppase- TaVP | 111,112 | |
| Water conservation | EsWAX1 | 113 |
| DEWAX | 114 | |
| Water relations | OsLEA3-2 | 115 |
| HVA1 | 116 | |
| Cellular tolerance | ||
| Osmoregulation | ||
| Trehalose | OsTPS1 | 117 |
| AtTPPD | 118 | |
| Proline | P5CS | 119 |
| PvP5CS | 120 | |
| Mannitol | mtlD | 121 |
| Glycine betaine | codA | 66 |
| codA | 122 | |
| ROS scavenging | Ec-apx1 | 123 |
| mRNA and Protein turnover | OsRDCP1 | 124 |
| OSRIP18 | 125 | |
| CspA/B | 126 | |
| AtCSP3 | 127 | |
| OsSUV3 | 128 | |
| p68 helicase | 129 |
Insights into Drought Responsive Gene Regulation are Critical
Drought being an oligogenic trait, has to be addressed by encompassing multiple genes wherein understanding the regulatory attributes of the activity of the gene in question would be the most critical aspect. In this direction, regulon biology is catching up in the race as a new area in the field of plant molecular stress biology.72-73 Regulons are thought to be key regulators which have an influence on the expression of multiple effector genes. Regulons are a group of operons/genes spread around the chromosome but controlled by a common factor or stimulus and multiple regulons form a modulon. There are many transcriptional switches that regulate various plant processes at different developmental stages;74 however, the similar information under diverse environmental conditions is limited.75 There have been recent reports on this emerging concept. A regulon conserved in monocot and dicot plants defines a functional module in antifungal plant immunity.76 DUO1 regulon encompasses genes with a range of cellular functions, including transcription, protein fate, signaling, and transport in Arabidopsis. Hence, Arabidopsis DUO1 regulon has a major role in shaping the germline transcriptome and functions to commit progenitor germ cells to sperm cell differentiation.77 In this direction, a concerted effort in identification of DNA regulatory motifs assumes significance,78 since this will help in recognizing the crucial regulons contributing to stress responses. Hence, characterization of plant responses to multiple stress conditions and discovery of the common regulons activated under a variety of stress conditions is very vital. In addition to the knowledge gained on conserved regulatory motifs, it is very essential to understand the importance of certain domains of unknown function found in many novel proteins enriched under stress with respect to their binding specificity.79-80 This in turn will help in the unravelling of various interactive partners that aid specific proteins in their mission of stress protection under each stress.
Conclusion
Diverse genes linked to various cellular tolerance mechanisms activated under drought stress act in a concerted manner to bestow varying degrees of stress tolerance. It will be highly rewarding if we examine different pathway linked genes active in the stress scenario under scrutiny. Targeted genetic manipulation to enhance cellular tolerance under stress will be more economically viable if we combine multiple trait regulatory genes by using modern biotechnological tools. This approach will serve in managing drought tolerance which is a complex multi-trait faceted attribute, which is the key to higher marginal productivity under stress.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by University Grants Commission-Council of Scientific and Industrial Research, Government of India in the form of Junior Research Fellowship to PMS (File No. F.17–3/2002(SA-I) and partial funding from the Department of Biotechnology and Indian Council of Agricultural Research, Government of India to KNN.
References
- 1.Bray EA, Bailey Serres J, Weretilnyk E. Responses to abiotic stresses In: Gruissem W, Buchannan B, Jones R, editors. Biochemistry and Molecular Biology of Plants. Rockville, MD: American Society of Plant Biologists; 2000; p. 158. [Google Scholar]
- 2.Golldack D, Li C, Mohan H, Probst N. Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 2014; 5:151; PMID:24795738; http://dx.doi.org/ 10.3389/fpls.2014.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nouri MZ, Komatsu S. Subcellular protein overexpression to develop abiotic stress tolerant plants. Front Plant Sci 2013; 4:1-7; PMID:23346092; http://dx.doi.org/ 10.3389/fpls.2013.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins WG, Hüner NPA. Introduction to Plant Physiology 2008; 4th Edition John Wiley & Sons [Google Scholar]
- 5.Kamoshita A, Chandra Babu R, Boopathi NM, Fukai S. Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. Field Crops Res 2008; 109:1-23; http://dx.doi.org/ 10.1016/j.fcr.2008.06.010 [DOI] [Google Scholar]
- 6.Krishnan A, Pereira A. Integrative approaches for mining transcriptional regulatory programs in Arabidopsis. Brief Funct Genomics Proteomic 2008; 7(4):264-74; PMID:18632743; http://dx.doi.org/ 10.1093/bfgp/eln035 [DOI] [PubMed] [Google Scholar]
- 7.Lawlor DW. Genetic engineering to improve plant performance under drought: physiological evaluation of achievements, limitations, and possibilities. J Exp Bot 2013; 64(1):83-108; PMID:23162116; http://dx.doi.org/ 10.1093/jxb/ers326 [DOI] [PubMed] [Google Scholar]
- 8.Tuberosa R. Phenotyping for drought tolerance of crops in the genomics era. Front Physiol 2012; 3:1-26; PMID:22275902; http://dx.doi.org/ 10.3389/fphys.2012.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu H, Xiong L. Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol 2014; 65:715-41; PMID:24313844; http://dx.doi.org/ 10.1146/annurev-arplant-050213-040000 [DOI] [PubMed] [Google Scholar]
- 10.Wasson AP, Richards RA, Chatrath R, Misra SC, Sai Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 2012; 63(9):3485-98; http://dx.doi.org/ 10.1093/jxb/ers111; PMID:22553286 [DOI] [PubMed] [Google Scholar]
- 11.Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci USA 2007; 104:15270-5; PMID:17881564; http://dx.doi.org/ 10.1073/pnas.0707294104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA. Root traits contributing to plant productivity under drought. Front Plant Sci 2013; 4: 442; PMID:24204374; http://dx.doi.org/ 10.3389/fpls.2013.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvaraj MG, Ogawa S, Ishitani M. Root phenomics-New windows to understand plant performance and increase crop productivity. J Plant Biochem Physiol 2013; 1:4 [Google Scholar]
- 14.Raju BR, Narayanaswamy BR, Mohankumar MV, Sumanth KK, Rajanna MP, Mohanraju B, Udayakumar M, Sheshshayee MS. Root traits and cellular level tolerance hold the key in maintaining higher spikelet fertility of rice under water limited conditions. Functional Plant Biol 2014; 41: 930-9; http://dx.doi.org/ 10.1071/FP13291 [DOI] [PubMed] [Google Scholar]
- 15.Mamrutha HM, Mogili T, Jhansi Lakshmi K, Rama N, Kosma DK, Udaya Kumar M, Jenks MA, Nataraja KN. Involvement of leaf cuticular wax amount and crystal morphology in regulating post harvest water loss in mulberry (Morus species). Plant Physiol Biochem 2010; 48: 690-6; PMID:20580887; http://dx.doi.org/ 10.1016/j.plaphy.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 16.Rebetzke GJ, Rattey AR, Farquhar GD, Richards RA, Condon AG. Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Functional Plant Biol 2013; 40: 14-33; http://dx.doi.org/ 10.1071/FP12184 [DOI] [PubMed] [Google Scholar]
- 17.Sheshshayee MS, Abou-Kheir E, Sreevathsa R, Srivastava N, Mohanraju B, Nataraja KN, Prasad TG, Udayakumar M. Phenotyping for root traits and their improvement through biotechnological approaches to sustaining crop productivity In: de Oliveira AC, Varshney RK, editors. Root genomics Springer-Verlag: Berlin: 2011; pp. 205-32 [Google Scholar]
- 18.Kadam N, Yin X, Bindraban P, Struik PC, Jagadish KSV. Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water-deficit stress than rice? Plant Physiol 2015; 167(4):1389-401; PMID:25614066; http://dx.doi.org/ 10.1104/pp.114.253328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomé F, Nägele T, Adamo M, Garg A, Marco-llorca C, Nukarinen E, Pedrotti L, Peviani A, Simeunovic A, Tatkiewicz A, et al.. The low energy signaling network. Front Plant Sci 2014; 5:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Chen X, Wang Z, Wang S, Wang Y, Zhu Q, Li S, Xiang C. Arabidopsis EDT1/HDG11 confers drought tolerance in transgenic rice without yield penalty. Plant Physiol 2013; 162: 1378-91; PMID:23735506; http://dx.doi.org/ 10.1104/pp.113.217596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray EA. Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J Exp Bot 2004; 55: 2331-41; PMID:15448178; http://dx.doi.org/ 10.1093/jxb/erh270 [DOI] [PubMed] [Google Scholar]
- 22.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. J Exp Bot 2007; 58(2): 221-7; PMID:17075077; http://dx.doi.org/ 10.1093/jxb/erl164 [DOI] [PubMed] [Google Scholar]
- 23.Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought- tolerant transgenic rice plants. Front Plant Sci 2015; 6:84; PMID:25741357; http://dx.doi.org/ 10.3389/fpls.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruthvi V, Rama N, Govind G, Nataraja KN. Expression analysis of drought specific genes in peanut (Arachis hypogaea L.). Physiol Mol Biol Plants 2013; 19: 277-81; PMID:24431496; http://dx.doi.org/ 10.1007/s12298-012-0156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parvathi MS, Nataraja KN, Yashoda BK, Ramegowda HV, Mamrutha HM, Rama N. Expression analysis of stress responsive pathway genes linked to drought hardiness in an adapted crop, finger millet (Eleusine coracana). J Plant Biochem Biotech 2013; 22:193-201; http://dx.doi.org/ 10.1007/s13562-012-0135-0 [DOI] [Google Scholar]
- 26.Basu S, Roychoudhury A. Expression profiling of abiotic stress-inducible genes in response to multiple stresses in rice (Oryza sativa L.) varieties with contrasting level of stress tolerance. BioMed Res Int 2014; 706890: 12 pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diatchenko L, Lau YF, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Lukyanov K, Gurskaya N, Sverdlov ED, Siebert PD. Suppression subtractive hybridization: a method for generating differentially regulated or tissue specific cDNA probes and libraries. Proc Natl Acad Sci USA 1996; 93: 6025-30; PMID:8650213; http://dx.doi.org/ 10.1073/pnas.93.12.6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of polymerase chain reaction. Science 1992; 257: 967-71; PMID:1354393; http://dx.doi.org/ 10.1126/science.1354393 [DOI] [PubMed] [Google Scholar]
- 29.Cho YJ, Meade JD, Walden JC, Chen X, Guo Z, Liang P. Multicolor fluorescent differential display. Biotechniques 2001; 30: 562-72; PMID:11252792 [DOI] [PubMed] [Google Scholar]
- 30.Kivioja T, Arvas M, Saloheimo M, Penttilä M, Ukkonen E. Optimization of cDNA-AFLP experiments using genomic sequence data. Bioinformatics 2005; 21(11): 2573-9; PMID:15774551; http://dx.doi.org/ 10.1093/bioinformatics/bti393 [DOI] [PubMed] [Google Scholar]
- 31.Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 2002; 31: 279-92; PMID:12164808; http://dx.doi.org/ 10.1046/j.1365-313X.2002.01359.x [DOI] [PubMed] [Google Scholar]
- 32.Slonim DK, Yanai I. Getting started in gene expression microarray analysis. PLoS Comput Biol 2009; 5(10): e1000543; PMID:19876380; http://dx.doi.org/ 10.1371/journal.pcbi.1000543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Till BJ, Zerr T, Comai L, Henicoff S. A protocol for TILLING and Ecotilling in plants and animals. Nat Protocols 2006; 1: 2465-77; PMID:17406493; http://dx.doi.org/ 10.1038/nprot.2006.329 [DOI] [PubMed] [Google Scholar]
- 34.Govind G, Harshavardhan VT, Patricia JK, Dhanalakshmi R, Senthil-Kumar M, Sreenivasulu N, Udayakumar M. Identificationand functional validation of a unique set of drought induced genes preferentially expressed in a response to gradual water stress. Mol Genet Genomics 2009; 281(6):591-605; PMID:19224247; http://dx.doi.org/ 10.1007/s00438-009-0432-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Arabidopsis Genome Initiative . Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000; 408: 796-815; PMID:11130711; http://dx.doi.org/ 10.1038/35048692 [DOI] [PubMed] [Google Scholar]
- 36.International Rice Genome Sequencing Project . The map-based sequence of the rice genome. Nature 2005; 436: 793-800; PMID:16100779; http://dx.doi.org/ 10.1038/nature03895 [DOI] [PubMed] [Google Scholar]
- 37.Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, et al.. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009; 326(5956): 1112-5; PMID:19965430; http://dx.doi.org/ 10.1126/science.1178534 [DOI] [PubMed] [Google Scholar]
- 38.Tuskan GA, Difazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts S, Salamov A, et al.. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 2006; 313(5793): 1596-604; PMID:16973872; http://dx.doi.org/ 10.1126/science.1128691 [DOI] [PubMed] [Google Scholar]
- 39.Heslop-Harrison JS. Comparative genome organization in plants: from sequence and markers to chromatin and chromosomes. Plant Cell 2000; 12:617-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varshney RK, Song C, Saxena RK, Azam S, Yu S, Sharpe AG, Cannon S, Baek J, Rosen BD, Tar'an B, et al.. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat Biotechnol 2013; 31(3): 240-6; PMID:23354103; http://dx.doi.org/ 10.1038/nbt.2491 [DOI] [PubMed] [Google Scholar]
- 41.He N, Zhang C, Qi X, Zhao S, Tao Y, Yang G, Lee TH, Wang X, Cai Q, Li D, et al.. Draft genome sequence of the mulberry tree Morus notabilis. Nat Commun 2013; 4: 2445; PMID:24048436; http://dx.doi.org/ 10.1038/ncomms3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al.. Genome sequence of the palaeopolyploid soybean. Nature 2010; 463: 178-83; PMID:20075913; http://dx.doi.org/ 10.1038/nature08670 [DOI] [PubMed] [Google Scholar]
- 43.Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, et al. “Genome sequence of the palaeopolyploid soybean.” Nature 2010; 465: 120; http://dx.doi.org/ 10.1038/nature08957 [DOI] [PubMed] [Google Scholar]
- 44.Young ND, Debellé F, Oldroyd GE, Geurts R, Cannon SB, Udvardi MK, Benedito VA, Mayer KF, Gouzy J, Schoof H, et al.. The Medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011; 480: 520-4; PMID:22089132; http://dx.doi.org/ 10.1038/480162a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh NK, Gupta DK, Jayaswal PK, Mahato AK, Dutta S, Singh S, Bhutani S, Dogra V, Singh BP, Kumawat G, et al.. The first draft of the pigeonpea genome sequence. J Plant Biochem Biotechnol 2012; 21(1): 98-112; PMID:24431589; http://dx.doi.org/ 10.1007/s13562-011-0088-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varshney RK, Chen W, Li Y, Bharti AK, Saxena RK, Schlueter JA, Donoghue MT, Azam S, Fan G, Whaley AM, et al.. Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat Biotechnol 2012; 30(1): 83-9; http://dx.doi.org/ 10.1038/nbt.2022 [DOI] [PubMed] [Google Scholar]
- 47. http://www.peanutbioscience.com/ THE INTERNATIONAL PEANUT GENOME INITIATIVE (IPGI)
- 48.Lebreton C, Lazić-Jančić V, Steed A, Pekić S, Quarrie SA. Identification of QTL for drought responses in maize and their use in testing causal relationships between traits. J Exp Bot 1995; 46 (7): 853-65; http://dx.doi.org/ 10.1093/jxb/46.7.853 [DOI] [Google Scholar]
- 49.Kirigwi FM, Van Ginkel M, Brown-Guedira G, Gill BS, Paulsen GM, Fritz AK. Markers associated with a QTL for grain yield in wheat under drought. Mol Breeding 2007; 20: 401-13; http://dx.doi.org/ 10.1007/s11032-007-9100-3 [DOI] [Google Scholar]
- 50.Fleury D, Jefferies S, Kuchel H, Langridge P. Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot 2010; 61(12): 3211-22; PMID:20525798; http://dx.doi.org/ 10.1093/jxb/erq152 [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Cui F, Wang L, Li J, Ding A, Zhao C, Bao Y, Yang Q, Wang H. Conditional and unconditional QTL mapping of drought-tolerance-related traits of wheat seedling using two related RIL populations. J Genet 2013; 92: 213-31; PMID:23970077; http://dx.doi.org/ 10.1007/s12041-013-0253-z [DOI] [PubMed] [Google Scholar]
- 52.Kumar A, Dixit S, Henry A, Marker-Assisted introgression of major qtls for grain yield under drought in rice In: Varshney RK and Tuberosa R, editors. Translational Genomics for Crop Breeding: Abiotic Stress, Yield and Quality Volume 2, John Wiley & Sons Ltd, Chichester, UK: 2013; http://dx.doi.org/ 10.1002/9781118728482.c [DOI] [Google Scholar]
- 53.Dixit S, Singh A, Sta Cruz MT, Maturan PT, Amante M, Kumar A. Multiple major QTL lead to stable yield performance of rice cultivars across varying drought intensities. BMC Genetics 2014; 15:16; PMID:24491154; http://dx.doi.org/ 10.1186/1471-2156-15-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dixit S, Huang BE, Sta Cruz MT, Maturan PT, Ontoy JCE, Kumar A. QTLs for tolerance of drought and breeding for tolerance of abiotic and biotic stress: an integrated approach. PLoS ONE 2014; 9(10): e109574; PMID:25314587; http://dx.doi.org/ 10.1371/journal.pone.0109574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mochida K, Shinozaki K. Advances in omics and bioinformatics tools for systems analyses of plant functions. Plant Cell Physiol 2011; 52(12): 2017-38; PMID:22156726; http://dx.doi.org/ 10.1093/pcp/pcr153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasmussen S, Barah B, Suarez-Rodriguez MC, Bressendorff S, Friis P, Costantino P, Bones AM, Nielsen HB, Mundy J. Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol 2013; 161: 1783-94; PMID:23447525; http://dx.doi.org/ 10.1104/pp.112.210773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Wang Y, Tang Y, Kakani VG, Mahalingam R. Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L.). BMC Plant Biol 2013; 13:153; PMID:24093800; http://dx.doi.org/ 10.1186/1471-2229-13-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benesova M, Hola D, Fischer L, Jedelsky PL, Hnilicka F, Wilhelmová N, Rothová O, Kočová M, Procházková D, Honnerová J, et al.. The physiology and proteomics of drought tolerance in maize: Early stomatal closure as a cause of lower tolerance to short-term dehydration? PLoS ONE 2012; 7(6):e38017; PMID:22719860; http://dx.doi.org/ 10.1371/journal.pone.0038017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ngara R, Ndimba BK. Understanding the complex nature of salinity and drought-stress response in cereals using proteomics technologies. Proteomics 2014; 14:611-21; PMID:24339029; http://dx.doi.org/ 10.1002/pmic.201300351 [DOI] [PubMed] [Google Scholar]
- 60.Arbona V, Manzi M, de Ollas C, Gómez-Cadenas A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int J Mol Sci 2013; 14:4885-911; PMID:23455464; http://dx.doi.org/ 10.3390/ijms14034885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards D, Batley J. Plant bioinformatics: from genome to phenome. Trends Biotechnol 2004; 22(5):232-7; PMID:15109809; http://dx.doi.org/ 10.1016/j.tibtech.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 62.Bhuiya AI, Islam ABMMK . Distinct structural and functional characteristics of stress-related genes of different plants revealed by insilico analysis. Enliven: Bioinform 2014; 1(1):004 [Google Scholar]
- 63.Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci 2014; 5:170; PMID:24904597; http://dx.doi.org/ 10.3389/fpls.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The Gene Ontology Consortium . Gene ontology: tool for the unification of biology. Nat Genet 2000; 25(1):25-9; PMID:10802651; http://dx.doi.org/ 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC, Anstrom DC, Bensen RJ, Castiglioni PP, Donnarummo MG, et al.. Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA 2007; 104:16450-5; PMID:17923671; http://dx.doi.org/ 10.1073/pnas.0707193104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kathuria H, Giri J, Nataraja KN, Murata N, Udayakumar M, Tyagi AK. Glycine betaine-induced water-stress tolerance in codA -expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol J 2009; 7: 512-26; PMID:19490479; http://dx.doi.org/ 10.1111/j.1467-7652.2009.00420.x [DOI] [PubMed] [Google Scholar]
- 67.Reguera M, Peleg Z, Blumwald E. Targeting metabolic pathways for genetic engineering abiotic stress-tolerance in crops. Biochim Biophys Acta 2012; 1819:186-94; PMID:21867784; http://dx.doi.org/ 10.1016/j.bbagrm.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 68.Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi- Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003; 15:63-78; PMID:12509522; http://dx.doi.org/ 10.1105/tpc.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Babitha KC, Ramu SV, Pruthvi V, Mahesh P, Nataraja KN, Udayakumar M. Co-expression of AtbHLH17 and AtWRKY28 confers resistance to abiotic stress in Arabidopsis. Transgenic Res 2012; 22:327-41; PMID:22948308; http://dx.doi.org/ 10.1007/s11248-012-9645-8 [DOI] [PubMed] [Google Scholar]
- 70.Pruthvi V, Narasimhan R, Nataraja KN. Simultaneous expression of abiotic stress responsive transcription factors, AtDREB2A, AtHB7 and AtABF3 improves salinity and drought tolerance in peanut (Arachis hypogaea L.). PLoS ONE 2014; 9(12): e111152; PMID:25474740; http://dx.doi.org/ 10.1371/journal.pone.0111152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krasensky J, Jonak C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 2012; 63(4):1593-608; PMID:21926090; http://dx.doi.org/ 10.1093/jxb/err460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mentzen WI, Wurtele ES. Regulon organization of Arabidopsis. BMC Plant Biol 2008; 8:99; PMID:18826618; http://dx.doi.org/ 10.1186/1471-2229-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakashima K, Ito Y, Yamaguchi-Shinozaki K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 2009; 149:88-95; PMID:19126699; http://dx.doi.org/ 10.1104/pp.108.129791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno-Risueno MA, Van Norman JM, Benfey PN. Transcriptional switches direct plant organ formation and patterning. Curr Top Dev Biol 2012; 98:229-57; PMID:22305165; http://dx.doi.org/ 10.1016/B978-0-12-386499-4.00009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baena-González E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007; 448: 938-42; http://dx.doi.org/ 10.1038/nature06069 [DOI] [PubMed] [Google Scholar]
- 76.Humphry M, Bednarek P, Kemmerling B, Koh S, Stein M, Göbel U, Stüber K, Pislewska-Bednarek M, Loraine A, Schulze-Lefert P, et al.. A regulon conserved in monocot and dicot plants defines a functional module in antifungal plant immunity. Proc Natl Acad Sci USA 2010; 107(50): 21896-901; PMID:21098265; http://dx.doi.org/ 10.1073/pnas.1003619107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borg M, Brownfield L, Khatab H, Sidorova A, Lingaya M, Twell D. The R2R3 MYB transcription factor DUO1 activates a male germline-specific regulon essential for sperm cell differentiation in Arabidopsis. Plant Cell 2011; 23: 534-49; PMID:21285328; http://dx.doi.org/ 10.1105/tpc.110.081059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma S, Bachan S, Porto M, Bohnert HJ, Snyder M, Dinesh-Kumar SP. Discovery of stress responsive dna regulatory motifs in Arabidopsis. PLoS ONE 2012; 7(8):e43198; PMID:22912824; http://dx.doi.org/ 10.1371/journal.pone.0043198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luhua S, Hegie A, Suzuki N, Shulaev E, Luo X, Cenariu D, Ma V, Kao S, Lim J, Gunay MB, et al.. Linking genes of unknown function with abiotic stress responses by high-throughput phenotype screening. Physiol Plant 2013; 148(3):322-33; PMID:23517122; http://dx.doi.org/ 10.1111/ppl.12013 [DOI] [PubMed] [Google Scholar]
- 80.Franco-Zorrilla JM, López-Vidriero I, Carrasco JL, Godoya M, Vera P, Solano R, DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc Natl Acad Sci USA 2014; 111(6):2367-72; PMID:24477691; http://dx.doi.org/ 10.1073/pnas.1316278111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang S, Vanderbeld B, Wan J, Huang Y. Narrowing down the targets: Towards successful genetic engineering of drought-tolerant crops. Mol Plant 2010; 3(3): 469-90; PMID:20507936; http://dx.doi.org/ 10.1093/mp/ssq016 [DOI] [PubMed] [Google Scholar]
- 82.Hadiarto T, Tran LP. Progress studies of drought-responsive genes in rice. Plant Cell Rep 2011; 30: 297-310; PMID:21132431; http://dx.doi.org/ 10.1007/s00299-010-0956-z [DOI] [PubMed] [Google Scholar]
- 83.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 2012; 63(10):3523-43; http://dx.doi.org/ 10.1093/jxb/ers100 [DOI] [PubMed] [Google Scholar]
- 84.Rana RM, Rehman SU, Ahmed J, Bilal M. A comprehensive overview of recent advances in drought stress tolerance research in wheat (Triticum aestivum L.), Asian J Agri Biol 2013; 1(1):29-37 [Google Scholar]
- 85.Bhargava S, Sawant K, Drought stress adaptation: metabolic adjustment and regulation of gene expression. Plant Breed 2013; 132:21-32; http://dx.doi.org/ 10.1111/pbr.12004 [DOI] [Google Scholar]
- 86.Liang C, Prins TW, van de Wiel CCM, Kok EJ. Safety aspects of genetically modified crops with abiotic stress tolerance. Trends Food Sci Technol 2014; 40(1): 115-122; doi.org/ 10.1016/j.tifs.2014.08.005 [DOI] [Google Scholar]
- 87.Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci 2015; 6:84; PMID:25741357; http://dx.doi.org/ 10.3389/fpls.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka H, Osakabe Y, Katsura S, Mizuno S, Maruyama K, Kusakabe K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. Abiotic stress-inducible receptor-like kinases negatively control ABA signaling in Arabidopsis. Plant J 2012; 70: 599-613; PMID:22225700; http://dx.doi.org/ 10.1111/j.1365-313X.2012.04901.x [DOI] [PubMed] [Google Scholar]
- 89.Zhao J, Gao Y, Zhang Z, Chen T, Wangzhen Guo W, Zhang T. A receptor-like kinase gene (GbRLK) from Gossypium barbadense enhances salinity and drought-stress tolerance in Arabidopsis. BMC Plant Biol 2013; 13:110; PMID:23915077; http://dx.doi.org/ 10.1186/1471-2229-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venkategowda R, Basu S, Krishnan A, Pereira A. The rice receptor-like cytoplasmic kinase GUDK is required for drought tolerance and grain yield under normal and drought stress conditions. Plant Physiol 2014; 166(3): 1634-45; PMID:25209982; http://dx.doi.org/ 10.1104/pp.114.248203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang G, Lovato A, Polverari A, Wang M, Liang YH, Ma YC, Cheng ZM. Genome-wide identification and analysis of mitogen activated protein kinase kinase kinase gene family in grapevine (Vitis vinifera). BMC Plant Biol 2014; 14(1): 219; PMID:25158790; http://dx.doi.org/ 10.1186/s12870-014-0219-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang L, Su H, Han L, Wang C, Sun Y, Liu F. Differential expression profiles of poplar MAP kinase kinases in response to abiotic stresses and plant hormones, and overexpression of PtMKK4 improves the drought tolerance of poplar. Gene 2014; 545(1):141-8; PMID:24780863; http://dx.doi.org/ 10.1016/j.gene.2014.04.058 [DOI] [PubMed] [Google Scholar]
- 93.Li C, Chang PP, Ghebremariam KM, Qin L, Liang Y. Overexpression of tomato SpMPK3 gene in Arabidopsis enhances the osmotic tolerance. Biochem Biophys Res Commun 2014; 443(2): 357-62; PMID:24275141; http://dx.doi.org/ 10.1016/j.bbrc.2013.11.061 [DOI] [PubMed] [Google Scholar]
- 94.Marais DLD, Auchincloss LC, Sukamtoh E, McKay JK, Logan T, Richards JH, Juenger TE. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc Natl Acad Sci USA 2014; 111(7): 2836-41; PMID:24550314; http://dx.doi.org/ 10.1073/pnas.1321429111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sánchez-Barrena MJ, Martínez-Ripoll M, Albert A. Structural biology of a major signaling network that regulates plant abiotic stress: The CBL-CIPK mediated pathway. Int J Mol Sci 2013; 14: 5734-49; http://dx.doi.org/ 10.3390/ijms14035734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang H, Yang B, Liu W, Li H, Wang L, Wang B, Deng M, Liang W, Deyholos MK, Jiang Y. Identification and characterization of CBL and CIPK gene families in canola (Brassica napus L.). BMC Plant Biol 2014; 14:8; PMID:24397480; http://dx.doi.org/ 10.1186/1471-2229-14-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vivek PJ, Tuteja N, Soniya EV. CDPK1 from ginger promotes salinity and drought stress tolerance without yield penalty by improving growth and photosynthesis in Nicotiana tabacum. PLoS ONE 2013; 8(10): e76392; PMID:24194837; http://dx.doi.org/ 10.1371/journal.pone.0076392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K, NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta 2012; 1819: 97-103; PMID:22037288; http://dx.doi.org/ 10.1016/j.bbagrm.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 99.Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 2012; 1819: 86-96; PMID:21867785; http://dx.doi.org/ 10.1016/j.bbagrm.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 100.Chen L, Song Y, Li S, Zhang L, Zou C, Yu D. The role of WRKY transcription factors in plant abiotic stresses. Biochim Biophys Acta 2012; 1819: 120-8; PMID:21964328; http://dx.doi.org/ 10.1016/j.bbagrm.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 101.Scharf K, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim Biophys Acta 2012; 1819: 104-19; PMID:22033015; http://dx.doi.org/ 10.1016/j.bbagrm.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 102.E ZG, Zhang YP, Zhou JH, Wang L. Roles of the bZIP gene family in rice. Genetics Mol Res 2014; 13(2): 3025-36; PMID:24782137; http://dx.doi.org/ 10.4238/2014.April.16.11 [DOI] [PubMed] [Google Scholar]
- 103.Li W, He M, Wang J, Wang Y. Zinc finger protein (ZFP) in plants- A review. POJ 2013; 6(6):474-80 [Google Scholar]
- 104.Zhang H, Liu Y, Wen F, Yao D, Wang W, Guo J, Ni L, Zhang A, Tan M, Jiang M. A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defence and oxidative stress tolerance in rice. J Exp Bot 2015; 65(20):5795-809 http://dx.doi.org/ 10.1093/jxb/eru313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lindemose S, O'Shea C, Jensen MK, Skriver K. Structure, function and networks of transcription factors involved in abiotic stress responses. Int J Mol Sci 2013; 14: 5842-78; PMID:23485989; http://dx.doi.org/ 10.3390/ijms14035842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y, Zhang X, Lu S, Wang M, Wang L, Wang W, Cao F, Chen H, Wang J, Zhang J, Tu J. Inhibition of a basal transcription factor 3-like gene Osj10gBTF3 in rice results in significant plant miniaturization and typical pollen abortion. Plant Cell Physiol 2012; 53: 2073-89; PMID:23147221; http://dx.doi.org/ 10.1093/pcp/pcs146 [DOI] [PubMed] [Google Scholar]
- 107.Petroni K, Kumimoto RW, Gnesutta N, Calvenzani V, Fornari M, Tonelli C, Holt BF 3rd, Mantovani R. The promiscuous life of plant Nuclear Factor Y transcription factors. Plant Cell 2012; 24: 4777-92; PMID:23275578; http://dx.doi.org/ 10.1105/tpc.112.105734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laloum T, De Mita S, Gamas P, Baudin M, Niebel A. CCAAT-box binding transcription factors in plants: Y so many? Trends Plant Sci 2013; 18(3): 157-66; PMID:22939172; http://dx.doi.org/ 10.1016/j.tplants.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 109.Gao X, Ren F, Lu Y. The Arabidopsis mutant stg1 identifies a function for TBP-Associated Factor 10 in plant osmotic stress adaptation. Plant Cell Physiol 2006; 47(9): 1285-94; PMID:16945932; http://dx.doi.org/ 10.1093/pcp/pcj099 [DOI] [PubMed] [Google Scholar]
- 110.Liu G, Li X, Jin S, Liu X, Zhu L, Nie Y, Zhang X. Overexpression of rice NAC gene SNAC1 improves drought and salt tolerance by enhancing root development and reducing transpiration rate in transgenic cotton. PLoS ONE 2014; 9(1): e86895; PMID:24489802; http://dx.doi.org/ 10.1371/journal.pone.0086895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pei L, Wang J, Li K, Li Y, Li B, Gao F, Yang A. Overexpression of Thellungiella halophila H+-pyrophosphatase gene improves low phosphate tolerance in maize. PLoS One 2012; 7: e43501; PMID:22952696; http://dx.doi.org/ 10.1371/journal.pone.0043501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li X, Guo C, Gu J, Duan W, Zhao M, Ma C, Du X, Lu W, Xiao K. Overexpression of VP, a vacuolar H+-pyrophosphatase gene in wheat (Triticum aestivum L.), improves tobacco plant growth under Pi and N deprivation, high salinity, and drought. J Exp Bot 2014; 65(2): 683-96; PMID:24474810; http://dx.doi.org/ 10.1093/jxb/ert442 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Zhu L, Guo J, Zhu J, Zhou C. Enhanced expression of EsWAX1 improves drought tolerance with increased accumulation of cuticular wax and ascorbic acid in transgenic Arabidopsis. Plant Physiol Biochem 2014; 75: 24-35; PMID:24361507; http://dx.doi.org/ 10.1016/j.plaphy.2013.11.028 [DOI] [PubMed] [Google Scholar]
- 114.Go YS, Kim H, Kim HJ, Suh MC. Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-type transcription factor. Plant Cell 2014; 26: 1666-80; PMID:24692420; http://dx.doi.org/ 10.1105/tpc.114.123307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Duan J, Cai W. OsLEA3-2, an abiotic stress induced gene of rice plays a key role in salt and drought tolerance. PLoS ONE 2012; 7(9): e45117; PMID:23024799; http://dx.doi.org/ 10.1371/journal.pone.0045117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen YS, Lo SF, Sun PK, Lu CA, Ho TH, Yu SM. A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty. Plant Biotechnol J 2015; 13(1):105-16; http://dx.doi.org/ 10.1111/pbi.12241 [DOI] [PubMed] [Google Scholar]
- 117.Li H-W, Zang B-S, Deng X-W, Wang X-P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011; 234: 1007-18; PMID:21698458; http://dx.doi.org/ 10.1007/s00425-011-1458-0 [DOI] [PubMed] [Google Scholar]
- 118.Krasensky J, Broyart C, Rabanal FA, Jonak C. The redox-sensitive chloroplast trehalose-6-phosphate phosphatase AtTPPD regulates salt stress tolerance. Antioxid Redox Signal 2014; 21(9): 1289-304; PMID:24800789; http://dx.doi.org/ 10.1089/ars.2013.5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghanti KKS, Sujata KG, Vijay Kumar BM, Nataraja KN, Janardhan RK, Srinath RM, et al.. Heterologous expression of P5CS gene in chickpea enhances salt tolerance without affecting yield. Biol Plant 2011; 55: 634-40; http://dx.doi.org/ 10.1007/s10535-011-0161-0 [DOI] [Google Scholar]
- 120.Chen JB, Yang JW, Zhang ZY, Feng XF, Wang SM. Two P5CS genes from common bean exhibiting different tolerance to salt stress in transgenic Arabidopsis. J Genet 2013; 92(3): 461-9; PMID:24371167; http://dx.doi.org/ 10.1007/s12041-013-0292-5 [DOI] [PubMed] [Google Scholar]
- 121.Hema R, Vemanna RS, Sreeramulu S, Reddy CP, Senthil-Kumar M, Udayakumar M. Stable expression of mtlD Gene imparts multiple stress tolerance in finger millet. PLoS ONE 2014; 9(6): e99110; PMID:24922513; http://dx.doi.org/ 10.1371/journal.pone.0099110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goel D, Singh AK, Yadav V, Babbar SB, Murata N, Bansal KC. Transformation of tomato with a bacterial codA gene enhances tolerance to salt and water stresses. J Plant Physiol 2011; 168(11): 1286-94; PMID:21342716; http://dx.doi.org/ 10.1016/j.jplph.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 123.Bhatt D, Saxena SC, Jain S, Dobriyal AK, Majee M, Arora S. Cloning, expression and functional validation of drought inducible ascorbate peroxidase (Ec-apx1) from Eleusine coracana. Mol Biol Rep 2012; 40(2):1155-65; http://dx.doi.org/ 10.1007/s11033-012-2157-z [DOI] [PubMed] [Google Scholar]
- 124.Bae H, Kim SK, Cho SK, Kang BG, Kim WT. Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.). Plant Sci 2011; 180: 775-82; PMID:21497713; http://dx.doi.org/ 10.1016/j.plantsci.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 125.Jiang SY, Bhalla R, Ramamoorthy R, Luan HF, Venkatesh PN, Cai M, Ramachandran S. Over-expression of OSRIP18 increases drought and salt tolerance in transgenic rice plants. Transgenic Res 2012; 21(4): 785-95; PMID:22038450; http://dx.doi.org/ 10.1007/s11248-011-9568-9 [DOI] [PubMed] [Google Scholar]
- 126.Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoecker M, Abad M, Kumar G, Salvador S, D'Ordine R, et al.. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol 2008; 147: 446-55; PMID:18524876; http://dx.doi.org/ 10.1104/pp.108.118828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim M, Sato S, Sasaki K, Saburi S, Matsui H, Imai R, COLD SHOCK DOMAIN PROTEIN 3 is involved in salt and drought stress tolerance in Arabidopsis. FEBS Open Bio 2013; 3: 438-42; PMID:24251108; http://dx.doi.org/ 10.1016/j.fob.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tuteja N, Sahoo RK, Garg B, Tuteja R. OsSUV3 dual helicase functions in salinity stress tolerance by maintaining photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. IR64). Plant J 2013; 76(1): 115-27; PMID:23808500 [DOI] [PubMed] [Google Scholar]
- 129.Tuteja N, Banu MSA, Huda KMK, Gill SS, Jain P, Pham XH, Tuteja R. Pea p68, a DEAD-box helicase, provides salinity stress tolerance in transgenic tobacco by reducing oxidative stress and improving photosynthesis machinery. PLoS ONE 2014; 9(5): e98287; PMID:24879307; http://dx.doi.org/ 10.1371/journal.pone.0098287 [DOI] [PMC free article] [PubMed] [Google Scholar]


