ABSTRACT
Iron (Fe) is an essential microelement but is highly toxic when in excess. To cope with Fe excess, plants have evolved complex adaptive responses that include morphological and physiological modifications. The highly dynamic adjustments in overall root system architecture (RSA) determine root plasticity and allow plants to efficiently adapt to environmental constraints. However, the effects of Fe excess on RSA are poorly understood. Recently, we showed that excess Fe treatment in Arabidopsis not only directly impairs primary root (PR) growth but also arrests lateral root (LR) formation by acting at the tip of the growing primary root. Such a change is believed to help RSA adjust and restrict excessive Fe absorption in the part of the rhizosphere subject to acute toxicity while maintaining the absorption of other nutrients in the less stressed components of the root system. We further showed that the suppression of PR growth and LR formation under excess Fe is alleviated by K+ addition, providing useful insight into the effectiveness of nutrient management to improve RSA and alleviate Fe toxicity symptoms in the field.
Keywords: Adaptation, Fe toxicity, nutrient management, root system architecture
Iron (Fe) is an essential microelement but is highly toxic when in excess. Classic symptoms of Fe toxicity are leaf discoloration (bronzing) and a stunted root system.1 To cope with, and survive, adverse iron-toxic soil conditions and excessive iron accumulation in tissue, plants have evolved morphological and physiological avoidance and/or tolerance strategies. These include restricting excessive Fe absorption at the root level,2 immobilization of active iron that entered the tissues in “dumping sites,” e.g., old leaves or leaf-sheath tissue,3 and inclusion and tolerance via increased thresholds to elevated levels of Fe2+ within cells, such as through enzymatic detoxification.4 Among these strategies, restricting excessive Fe absorption is one of the most important, by “engaging the enemy outside the gates.” Highly dynamic changes in the overall root system architecture (RSA) determine root plasticity and allow plants to efficiently acclimate to environmental constraints and restrict the excessive accumulation of nutrients and toxicants. In fact, plants can respond to the heterogeneous availability of nutrient resources by flexibly, and relatively rapidly, allocating carbon flow to facilitate directional root growth to patches where the most favorable conditions are found.5-7 Excess Fe has been shown to inhibit LR initiation but not subsequent LR development,8,9 and these inhibitory effects are only seen in newly grown roots that are engaged in the elongation process for the duration of exposure to excess Fe and are not seen in the proximal root portions.8,9 Moreover, physical contact of the PR tip with excess Fe is necessary, and indeed sufficient, for LR formation inhibition in the newly grown roots.8 Excess Fe also arrests PR growth by decreasing both cell elongation and division,1,9,10 and principally results from direct contact of the root tip with external Fe.10,11 Concentrations of the main toxic (ferrous) form, Fe2+, tend to increase in vertically lower soil strata, where low pH and/or anoxic conditions prevail.12 Thus, we propose the purpose of the observed RSA adjustment to be the restriction of excessive Fe absorption, which also occurs predominantly in the Fe2+ form, and prevent serious Fe toxicity. Meanwhile, the relatively stable LR number and length in the proximal root portions may permit the maintenance of the absorption of other nutrients in the less stressed areas.
Additional to the above, a significant shift in plant tissue cation homeostasis, especially that of potassium (K), has been noted under Fe toxicity.1,13 Although the previous reports by our group and others laboratory had shown that potassium plays a critical role in regulating root development under Fe toxicity,10,14 the detailed morphological and physiological targets were not identified. Here, our data show that the suppression of PR growth and LR formation in the newly developed roots (the distal portion of the root system) under excess Fe is significantly alleviated by K+ addition (Fig. 1). This rescue effect from Fe toxicity is strongly reminiscent of K+'s alleviatory effects under the cation stress brought about by the NH4+ ion,15,16 an ion whose toxicity is also sensed at the root tip,17 and K+ amendment, thus, offers itself as a practical agricultural strategy to reduce the manifestation of cation toxicity in the field.18 Although the mechanism of alleviation remains as yet poorly understood, there are several plausible hypotheses, 1) K+ may reduce the activity and availability of Fe2+ in the root medium, thus facilitating maintenance of root development; 2) K+ may reduce the transport of Fe2+ into root cells to limit toxicity; 3) K+ may act on the target of Fe-mediated root development or the enzymatic systems that control Fe2+ immobilization and detoxification. These hypotheses require testing to identify the precise targets of K+ action, but the positive effects on RSA, and the alleviation of Fe toxicity symptoms in general by K+, may constitute a reasonable nutrient management approach to combating Fe toxicity in real-life agricultural settings.
Figure 1.
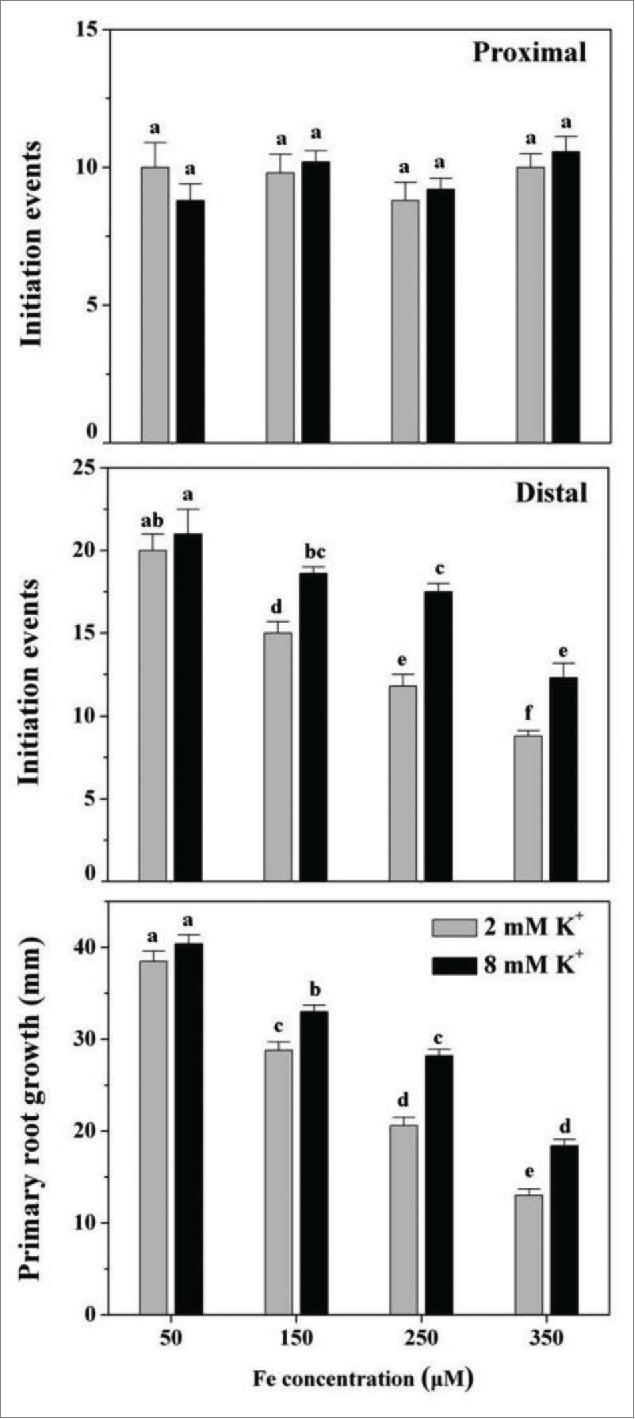
The effect of exogenous K+ on primary root growth and lateral root formation in Arabidopsis under excess Fe. Five-day-old Arabidopsis (Col-0) seedlings were transferred to medium supplemented with various concentrations of Fe (provided as Fe-EDTA; FeSO4.7H2O plus EDTA) plus various concentrations of K+ (provided as K2SO4) for an additional 5 days. Values are the means ± SE, n ≥ 4. Different letters represent means statistically different at the 0.05 level (one-way ANOVA with Duncan post-hoc test). Distal: distal root portion; Proximal: proximal root portion.
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31300210 and 41171234) and the Natural Sciences and Engineering Research Council of Canada (NSERC, Discovery Grant 217277-2009).
References
- 1. Becker M, Asch F. Iron toxicity in rice-conditions and management concepts. J Plant Nutr Soil Sci 2005; 168:558-73; http://dx.doi.org/ 10.1002/jpln.200520504 [DOI] [Google Scholar]
- 2. Chen CC, Dixon JB, Turner FT. Iron coatings on rice roots: morphology and models of development. Soil Sci Soc Am J 1980; 44:1113-9; http://dx.doi.org/ 10.2136/sssaj1980.03615995004400050046x [DOI] [Google Scholar]
- 3. Audebert A, Sahrawat KL. Mechanisms for iron toxicity tolerance in lowland rice. J Plant Nutr 2000; 23:1877-85; http://dx.doi.org/ 10.1080/01904160009382150 [DOI] [Google Scholar]
- 4. Majerus V, Bertin P, Swenden V, Fortemps A, Lobréaux S, Lutts S. Organ-dependent responses of the African rice to short-term iron toxicity: ferritin regulation and antioxidative responses. Biol Plant 2007; 51:303-31; http://dx.doi.org/ 10.1007/s10535-007-0060-6 [DOI] [Google Scholar]
- 5. Zhang H, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 1998; 279:407-8.; PMID:9430595; http://dx.doi.org/ 10.1126/science.279.5349.407 [DOI] [PubMed] [Google Scholar]
- 6. Giehl RF, Lima JE, von Wiren N. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. Plant Cell 2012; 24:33-49; PMID:22234997; http://dx.doi.org/ 10.1105/tpc.111.092973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gruber BD, Giehl RF, Friedel S, von Wiren N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol 2013; 163:161-79; PMID:23852440; 10.1104/pp.113.218453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li GJ, Song HY, Li BH, Kronzucker HJ, Shi WM. AUX1 and PIN2 Protect Lateral Root Formation in Arabidopsis under Fe Stress. Plant Physiol 2015; 169:2608-2623; http://dx.doi.org/ 10.1104/pp.15.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reyt G, Boudouf S, Boucherez J, Gaymard F, Briat JF. Iron and ferritin dependent ROS distribution impact Arabidopsis root system architecture. Mol Plant 2015; 8:439-53; PMID:25624148; http://dx.doi.org/ 10.1016/j.molp.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 10. Li GJ, Xu WF, Kronzucker HJ, Shi WM. Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis. J Exp Bot 2015; 66:2041-54; PMID:25711703; http://dx.doi.org/ 10.1093/jxb/erv005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Wang YP, Liu P, Song JM, Xu GD, Zheng GH. Effect of toxic Fe2+ level on the biological characteristics of rice root border cell. Russ J Plant Physiol 2012; 59:766-71; http://dx.doi.org/ 10.1134/S1021443712060209 [DOI] [Google Scholar]
- 12. Ratering S, Schnell S. Localization of iron-reducing activity in paddy soil by profile studies. Biochem 2000; 48:341-65; http://dx.doi.org/ 10.1023/A:1006252315427 [DOI] [Google Scholar]
- 13. Çelik H, Asik BB, Gürel S, Katkat AV. Potassium as an intensifying factor for iron chlorosis. Int J Agric Biol 2010; 12:359-64 [Google Scholar]
- 14. Li H, Yang X, Luo AC. Ameliorating effect of potassium on iron toxicity in hybrid rice. J Plant Nutr 2001; 24:1849-60; http://dx.doi.org/ 10.1081/PLN-100107598 [DOI] [Google Scholar]
- 15. Szczerba MW, Britto DT, Balkos Kronzucker HJ. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. J Exp Bot 2008; 59:303-13; PMID:18203690; http://dx.doi.org/ 10.1093/jxb/erm309 [DOI] [PubMed] [Google Scholar]
- 16. Balkos KD, Britto DT, Kronzucker HJ. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ 2010; 33:23-34; PMID:19781010; http://dx.doi.org/ 10.1111/j.1365-3040.2009.02046.x [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Li BH, Kronzucker HJ, Shi WM. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ 2010; 33:1529-42; PMID:20444215; http://dx.doi.org/ 10.1111/j.1365-3040.2009.02080.x [DOI] [PubMed] [Google Scholar]
- 18. Britto DT, Balkos KD, Becker A, Coskun D, Huynh WQ, Kronzucker HJ. Potassium and nitrogen poising: physiological changes and biomass gains in rice and barley. Can J Plant Sci 2014; 94:1085-9; http://dx.doi.org/ 10.4141/cjps2013-143 [DOI] [Google Scholar]


