ABSTRACT
Bloodstream infections (BSIs) represent a common complication among critically ill patients and a leading cause of morbidity and mortality. The prompt initiation of an effective antibiotic therapy is necessary in order to reduce mortality and to improve clinical outcomes. However, the choice of the empiric antibiotic regimen is often challenging, due to the worldwide spread of multi-drug resistant (MDR) organisms with reduced susceptibility to the available broad-spectrum antimicrobials. New therapeutic strategies are 5 to improve the effectiveness of antibiotic treatment while minimizing the risk of resistance selection.
Keywords: antimicrobial resistance, bloodstream infections, central venous catheter, combination therapy;daptomycin, de-escalation, empiric therapy, intensive care unit
Introduction
Bloodstream infections (BSIs) are a frequent and life-threatening condition in hospital settings.1-2 Critically ill patients are particularly predisposed to the acquisition of BSIs, which occur in approximately 7% of all patients within the first month of hospitalization in Intensive Care Unit (ICU).3 The Extended Prevalence of Infection in the ICU Study (EPIC II) conducted in 2007 showed that in ICU approximately 15% of patients had a BSI on the day of the study.4 In this context, BSIs are associated with particularly high mortality rates, ranging between 40% and 60%, with an overall 3-fold increase in the risk of hospital death.3,5-6 The acquisition of a BSI also results in increased length of ICU-stay and healthcare-related costs.7-8
The prompt initiation of an effective antibiotic treatment has demonstrated to reduce mortality and improve clinical outcomes, particularly when severe sepsis or septic shock are present.9-11 However, due to the widespread diffusion of multi-drug resistant (MDR) pathogens, the most commonly employed empiric regimens are often inappropriate, with an increased morbidity and mortality.12-13
In this article we will review the clinical and epidemiological characteristics of ICU-acquired BSIs (ICU-BSIs), with a specific focus on the problem of antimicrobial resistance and therapeutic strategies for empiric and targeted antibiotic therapy.
Epidemiology
ICU-BSIs present peculiar epidemiologic and microbiologic characteristics when compared with community-acquired- (CA) and hospital-acquired- (HA) BSIs.1,14-15 Critically ill patients are subjected to a specific spectrum of risk factors, including high illness severity at admission (APACHE III score), prolonged stay, need for mechanical ventilation, renal replacement therapy, recent surgery, and immunosuppression.2,5,16-18 The extensive use of intravascular catheters, however, is recognized as the most important factor contributing to the occurrence of BSI.19-20 Central venous catheters, in particular, represent the intravascular devices that are most frequently associated with the acquisition of a BSI, although arterial catheters can be also involved.2,5,21
Catheter-related BSIs (CR-BSIs) (defined as the growth of the same pathogen from catheter tip and peripheral blood culture), which represent up to 30% of cases, and primary BSIs, accounting for around 35% of cases, are the most common types of BSI in ICU.22-23 Ventilator-Associated-Pneumonia (VAP), which is a frequent complication when mechanical ventilation is required, is bacteraemic in around 15% of cases, and represent the most common source of secondary bacteraemia in critically ill patients.23-25 Secondary BSIs, mainly originating from lower respiratory tract and abdominal infections (including infections developing from urinary tract), account for the majority of BSI cases acquired in the community or in the hospital requiring ICU admission.16
Microbiology
In a recent point-prevalence survey, Magill et al found that Gram-positive pathogens were the most frequently isolated pathogens in HA-BSIs, followed by Candida spp.2
In the specific subset of ICU-BSIs, Gram-positives (mainly Staphylococcus aureus), represent the most commonly isolated organisms.3,6,23 Alarmingly, the isolation of S. aureus in blood cultures has been independently associated with increased mortality,5 even if CR-BSIs, which are a frequent source when infection is sustained by S. aureus, are generally characterized by better clinical outcomes compared with other sources of BSI and primary bacteraemia.3,5 VAP is also a common source of infection when BSIs are caused by S. aureus.24-25
Among Gram-negative pathogens, the most commonly isolates are Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumanii, and Pseudomonas aeruginosa, typically isolated from abdominal or urinary tract sites.4,26 CR- and primary BSIs can be occasionally caused by these pathogens.3
Candida spp plays also a major role in ICU, accounting for 8-15% of cases of BSIs,5,27 with a prevalence of 6.9 per 1000 patients.28 Moreover, patients with Candida BSIs had the greatest crude ICU mortality when compared with other organisms.28
Antibiotic resistance
During the last years, antimicrobial resistance has progressively increased worldwide, resulting in delays in the prescription of an effective antibiotic treatment and high mortality rates, non only in ICU setting.29-30 Tumbarello et al. reported that nearly 50% of patients with a BSI sustained by extended-spectrum β-lactamase (ESBL)-producing Enterobacteriacae did not receive an adequate antibiotic treatment within 72 hours from blood cultures, leading to a 3-fold increase in mortality.31 Similarly, in more than one third of BSIs caused by methicillin-resistant Staphylococcus aureus (MRSA), empiric antibiotic treatment was found not effective, and the inadequacy of antibiotic regimen represented an independent risk factor for mortality.32
Methicillin-resistance in S. aureus is one of the most important issue encountered in clinical practice, involving 18% of the isolates in Europe in 2013, with a wide variability between different countries and resistance rates ranging from 0% to 64.5%.33 Overall, higher rates of resistance have been reported in southern Europe (mainly Greece, Italy and Portugal), whereas in the Northern countries less than 1% of all isolates display resistance to methicillin.33 In the ICU setting, in particular, the problem of MRSA prevalence is even more alarming. The most impressive data comes from the recent EUROBACT study, encompassing 1,156 patients admitted to ICU with a new diagnosis of HA-BSI and reporting methicillin-resistance rates in up to 50% of isolates.26 For this reason, the prescription of vancomycin has progressively increased in recent years and still represents the most frequently used antimicrobial with activity against MRSA in critically ill patients.26,34 Nevertheless, a continuous elevation of minimum-inhibitory concentrations (MICs) for vancomycin (known as the “MIC-creep” phenomenon) has been observed worldwide, although with a wide variability between different countries and institution.35-36 The Centers for Disease Control (CDC) classified resistant bacteria as vancomycin-intermediate S. aureus (VISA) with MIC between 8 and 16 μg/mL, hetero-resistant VISA (h-VISA) with MIC between 1 and 4 μg/mL and vancomycin-resistant (VRSA) with MIC above 32 μg/mL. 37 As a result, the efficacy of vancomycin in MRSA bacteremia, which mainly depends on the MIC of the pathogen, has been questioned. In particular, MRSA bacteraemia treated with vancomycin has been associated with clinical failure and higher mortality if the strain displayed vancomycin MIC >1 mg/L.38-40 Furthermore, vancomycin through concentrations ≥15 mg/L, necessary to overcome the higher MIC values, increases the risk of nephrotoxicity, which represents a frequent adverse event also when standard doses are used.41
Regarding Gram-negative organisms, the EUROBACT study found that MDR Gram-negative pathogens play a role in more than half of cases in ICU.27 BSIs due to ESBL-producing Enterobacteriacae represent a challenge for clinicians, due to the resistance of the organisms to third generation cephalosporins. The effectiveness of antibiotic regimens including β-lactam/β-lactamase inhibitors has not been demonstrated in randomized clinical trials, in particular in the subset of critically ill patients, and carbapenems are often used as the first choice.42 As a consequence, carbapenem-resistance rates have progressively increased, with Acinetobacter spp., Klebsiella spp and Pseudomonas spp. showing carbapenem resistance in 69%, 37% and 5.7% of cases, respectively, in patients with HA-BSIs managed in ICU in Europe.27 However, carbapenem-resistance is widely jeopardized between different countries, with the higher reported rates in the southern European ICUs.43 Few treatment options for carbapenem-resistant pathogens are available so far, and the use of combination regimens including colistin and/or tigecycline in association with a carbapenem have been associated to a survival benefit when compared to a monotherapy in small observational studies.44-45. Unfortunately, MDR pathogens are often resistant to aminoglycosides, and cases of colistin resistance have been reported more and more frequently in areas where MDR Gram negatives are more common, with carbapenemase-producing Klebsiella pneumonia (KPC) showing colistin-resistance in up to 20% of isolates in Italian and Greek ICUs.46-49
Another emerging problem is fluconazole-resistance in Candida spp, which varies greatly by different countries and species, with the highest overall resistance rates reported in Denmark (33%) and the lowest in the Republic of Korea (0,9%).50 In Candida albicans, which is responsible for the great majority of cases of candidaemia, resistance to fluconazole involves up to 5% of isolates worldwide, as reported by recent studies.51-52
Empiric therapy
In critically ill patients, a delay in the prescription of an adequate empiric antibiotic therapy may result in increased mortality, whereas the early prescription of an effective antimicrobial treatment is linked to improved clinical outcomes.9-13 Also, the switch to an effective antimicrobial therapy upon availability of the susceptibility test is still associated with increased mortality compared with the prescription of an early effective regimen.31
For this reason, the empiric prescription of broad-spectrum antibiotics against the most likely involved pathogens, followed by de-escalation to a narrower spectrum therapy when patients' clinical conditions are stable and susceptibility tests are available, should be the most common and appropriate strategy. Nevertheless, due to the progressive increase in antimicrobial resistance, an empiric monotherapy with either piperacillin/tazobactam or a carbapenem can be inadequate in almost one third of cases in HA-BSIs managed in ICU.27
In order to provide an adequate empiric coverage, a thorough evaluation of the presence of risk factors for the acquisition of a BSI sustained by MDR pathogens is paramount. Specifically, the knowledge of the local epidemiology and resistance patterns are key, as a wide variability in resistance rates exists between different countries and institutions.32,43 Moreover, the eventual previous colonization with MDR pathogens should be considered, since it significantly increases the risk of acquisition of an infection sustained by the same pathogen.53-55 Risk factors for the acquisition of infections due to MDR bacteria are summarized in Table 1.32,55-60
Table 1.
Risk factors for BSIs due to MDR pathogens.
|
Another important element in the management of critically ill patients is the optimization of antimicrobial doses and ways of administration in order to achieve and maintain optimal plasmatic concentrations, finding the balance between the pharmacokinetic characteristics of each antimicrobial and the pathophysiological modifications occurring during sepsis.61 Suggested doses of the most common antimicrobials used in critically ill patients and administration schedules are reported in Table 2.61-70
Table 2.
suggested dosages of the most common antimicrobials used for the treatment of BSIs in critically ill patients.
| Antimicrobial | Dose |
|---|---|
| Ceftazidime | 15 mg/kg loading dose, then 6-8 g every 24 h c.i. |
| Cefepime | 15 mg/kg loading dose, then 2 g every 8 h c.i. |
| Piperacillin/tazobactam | 4.5 g (loading dose), then 18 g every 24 h c.i. |
| Meropenem | 1-2 g every 8 h e.i. |
| Ertapenem | 1 g every 12 h |
| Amikacin | 25-30 mg/kg every 24 h |
| Gentamicin | 7 mg/kg every 24 h |
| Vancomycin | 1 g loading dose, then 30 mg/kg every 24 h c.i. |
| Daptomycin | 8-10 mg/kg every 24 h |
| Tigecycline | 100-200 mg (loading dose), then 50-100 mg every 12 h |
| Colistin | 9 MU loading dose, 4.5 MU every 12 h |
| RifampinFosfomycin | 600-900 mg every 24 h4-6 g every 6 h c.i. |
| Caspofungin | 70 mg, then 50 mg every 24 h |
| Anidulafungin | 200 mg, then 100 mg every 24 h |
| Micafungin | 100 mg every 24 h |
Notes. c.i.: continuous infusion
e.i: extended infusion (6-8 hours)
Together with the prescription of antimicrobials, a prompt source control and the early removal of intravascular devices is mandatory, both in bacterial infections and candida infections.40,71
Providing an adequate empiric coverage, however, should not be the sole goal for the clinician, since the indiscriminate use of broad-spectrum antimicrobials is the main reason for the increasing selection of resistances.72-73 In particular, carbapenem resistance is a major concern, as underlined by a recent study by Armand-Lefevre et al. showing that even a brief exposure to imipenem (1-3 days) can be a risk factor for introducing imipenem-resistant gram-negative pathogens carriage status.74
The vicious circle of overuse of broad spectrum antimicrobials and selection of resistances has made necessary the introduction of the concept of “antibiotic stewardship.” The aim of antimicrobial stewardship is to optimize the use of antimicrobials by promoting the selection of the optimal antimicrobial regimen including dosing, duration of therapy and route of administration.75
Gram positive bacteria
Due to the “MIC-creep” phenomenon, alternatives to vancomycin should be considered in critically ill patients when bacteraemia is suspected, in particular when an empiric anti-MRSA regimen has to be initiated and an isolate with increased vancomycin MIC is suspected on the basis of local epidemiology. Daptomycin is a new lipopeptide characterized by a fast, concentration-dependent bactericidal activity against the majority of Gram-positive bacteria, including MRSA, hVISA, VISA and VRSA.76-77 Murray et al. compared daptomycin versus vancomycin for the treatment of bacteraemia sustained by MRSA with vancomycin MIC > 1 mg/L, and found that both 30-day mortality and persistent bacteraemia were significantly lower in patients treated with daptomycin.39 Therefore, daptomycin is currently the first choice in MRSA bacteraemia with vancomycin MIC >1 mg/L. Moreover, an empiric antibiotic treatment with high-dose daptomycin (8-10 mg/kg/die) may be more effective than an adequate empiric regimen with glycopeptides or betalactams when a S. aureus BSI is suspected, especially in a contest of high local prevalence of MRSA.78 Daptomycin is approved at a dose of 4 mg/kg for the treatment of complicated skin and soft-tissue infection (SSTI) and at 6 mg/kg for S. aureus BSIs, including treatment of right-sided endocarditis.40 However, the optimal dosages for daptomycin have not been yet well established. Daptomycin standard doses (4-6 mg/kg/day) have been questioned in favor of higher ones (8-10 mg/kg/day), reported to provide higher clinical and microbiological cure rates through the maximization of the concentration-dependent bactericidal activity, overcoming the augmented renal clearance in septic patients and minimizing the selection of resistant strains.79-80 Large randomized controlled trials evaluating high-dose daptomycin are lacking. Despite this, current guidelines suggest high-dose daptomycin for the treatment of infective endocarditis, based on expert opinion.40 Daptomycin also possess a good anti-biofilm activity, representing a possible advantage when catheters or other devices are the source of infection.81 Daptomycin is generally well tolerated and presents few side effects.82-83
Gram negative bacteria
The limited efficacy of available antimicrobial regimens is the major concern in the treatment of infections due to MDR Gram-negative pathogens, together with the lack of new effective antibiotic classes. In this setting, combination therapy approaches have been proposed in order to provide a better coverage including non-susceptible strains.84 The prescription of a combination therapy has been advocated to be effective in reducing mortality in patients presenting with severe sepsis or septic shock,85-86 and when the infection is sustained by P. aeruginosa,87 but results are controversial.88 The major advantages of combination regimens have been found in infections caused by MDR and, specifically, carbapenemase-producing organisms.89-90
For the treatment of BSIs sustained by KPC-producing Enterobacteriacae, which are the most common carbapenem-resistant nosocomial isolate, combination regimens including at least 2 active drugs have been associated with improved clinical outcomes and reduced mortality in comparison with the use of only one active drug, in particular when the combination includes a carbapenem.45,91-92
Tumbarello et al. analyzed a cohort of 125 patients with KPC-K. pneumoniae-BSIs and reported a significantly lower 30-day mortality in patients receiving a combination regimen compared with the ones treated with a monotherapy (34,1% vs 54,3%, P = 0,02). In particular, multivariate analysis found that a targeted therapy with meropenem in combination with colistin and tigecycline was independently associated with reduced mortality (OR:0.11; 95%CI: .02–.69; P = 0.01).44 Otherwise, inadequate initial antibiotic treatment resulted an independent risk factor for increased mortality.44 A recent study suggests that combination regimens including meropenem might be more effective when the isolate has meropenem MIC ≤ 8 mg/L.93 However, further evaluations are needed, in particular regarding the role of TDM, which may allow to achieve effective plasmatic concentrations also when meropenem MIC is > 8 mg/L.
For these reasons, a combination regimen including high-dose meropenem (1-2 g every 6-8 hours) together with high dose colistin (9 millions/daily) and/or high dose tigecycline (200 mg/daily) should be considered in critically ill patients, in particular when presenting with severe sepsis or septic shock and a carbapenem-resistant organism is suspected (i.e. previous colonization or infection due to a carbapenem-resistant strain, or when the risk of carbapenem-resistant pathogensis high on the basis of local epidemiology).
Candida
Patients with candidemia not receiving an adequate treatment within 12 hours after the collection of the blood cultures have been characterized as having an independent risk factor for increased mortality. Nevertheless, in only a minority of patients (less than 10%) the goal is achieved.94 A major problem is represented by a low sensitivity of blood culture, ranging from 50% and 75%, and by the length of Candida growth in vitro (frequently more than a week). Thus, blood cultures are not considered the optimal early detection method for the diagnosis of candidaemia, although still representing the gold standard.95 Both clinical scores based on the presence of well-defined risk factors for the development of invasive fungal infections and non-cultural biomarkers have been proposed in order to achieve an early diagnosis of candidaemia before the availability of blood cultures. Leon et al. proposed the “Candida score,” which includes previous surgery, multifocal colonization, total parenteral nutrition and severe sepsis, and, for a cut-off value of 3, might be helpful for the identification of critically ill non-neutropenic septic patients at high risk for having a candidaemia.96 However, into the wide clinical practice the “Candida score” have been associated with low sensitivity and specificity for the diagnosis of candidaemia, thus it should be used with caution.97 The detection of (1-3)-β-D-glucan, which is a pan-fungal marker, represents a recent and useful tool for the rapid diagnosis of candidaemia in adult patients, with a cut-off value of 80 pg/mL. Serial determinations (twice a week) clinically-driven should be performed.95 However, (1-3)-β-D-glucan does not represent a routine diagnostic method in standard practice so far, given that the test is available in only a minority of institutions. The use of procalcitonin (PCT) may also be useful when candidaemia is suspected, since, in a recent study by Martini et al., a PCT cut-off value of 2 ng/mL separated Candida sepsis from bacterial sepsis with a sensitivity of 92%, a specificity of 93%, and positive and negative predictive values of 94% in critically ill surgical patients with signs of sepsis and at high risk for fungal infection, helping to rule out bacteraemia.98 In order to achieve the goal of an early treatment of candidaemia, a pre-emptive antifungal therapy should be considered in critically ill patients with risk factors for candidaemia and positivity of (1-3)-β-D-glucan, when available.99 Current European Society of Clinical Microbiology and Infectious Disease (ESCMID) guidelines recommend the use of echinocandins, due to their rapid fungicidal activity, the optimal anti-biofilm activity, the broader spectrum of activity, the lower resistance rates and the favorable safety profile, characterized by low toxicity and low drug-drug interactions compared with azoles.99
Targeted therapy
De-escalation therapy and optimization of therapy duration are strongly recommended by current guidelines and are part of the majority of the stewardship programs.75,84 The main expected potential benefits are a reduction of antimicrobial resistance, lower antibiotic-related adverse events and overall decreased antimicrobial costs.100
De-escalation
Randomized controlled trials on de-escalation therapy in critically ill patients with BSIs are still lacking. However, 3 encouraging prospective observational studies supporting de-escalation have been recently published.101-103 All these studies suggest that, in patients with severe sepsis or septic shock, de-escalation, defined as either the withdrawal of one or more antimicrobials or the switch to a narrower spectrum therapy after the availability of susceptibility tests, does not affect mortality, which is at least not worse in de-escalated patients than in not-de-escalated ones. Nevertheless, in critically ill septic patients the goal of de-escalation is achieved in only approximately 50% of cases, even when the cultural results are available.102 The presence of many unsolved questions regarding de-escalation might be the reason for the decision of not to perform de-escalation into the wide clinical practice. In particular, the real effectiveness of de-escalation in reducing antimicrobial resistances have not been demonstrated so far, and studies specifically targeting severe infections sustained by MDR pathogens are lacking. Moreover, one study reported an increased number of superinfections and prolonged ICU-stay when de-escalation was performed.103 Nevertheless, in consideration of the available body of evidence showing that de-escalation do not affect mortality and the expected benefits on resistance selection and drug-related adverse events, de-escalation should be encouraged, when clinical setting allows it. Possible strategies for de-escalation in BSIs are listed in Table 3.
Table 3.
Possible strategies for de-escalation in BSIs.
| Pathogen | Antimicrobial options |
|---|---|
| MSSA or MSSE | Oxacillin (12-16 g every 24 h c.i.) or cefazolin (2-4 g every 8 h c.i) |
| Streptococci | Ampicillin (2 g every 4 h c.i.) or ceftriaxone (2 g every 24 h) |
| Enterococcus faecalis | Ampicillin (2 g every 4 h c.i.) |
| Non-ESBL Enterobacteriacae | Ceftriaxone (2 g every 24 h) |
| ESBL-Enterobacteriacae | Ertapenem (500 mg every 6 h, e.i. 4 h) ) |
| Susceptible P.aeruginosa | Piperacillin-tazobactam (4.5 g loading dose, then 18g q 24h c.i.) or antipseudomonal cephalosporin (ceftazidime 6 g every 24 h, c.i.or cefepime 6 g every 24 hours, c.i.) |
| Fluconazole-susceptible Candida spp | Fluconazole (loading dose 12 mg/kg every 12 h, then 400 mg every 24 h) |
Notes. MSSA: methicillin-susceptible Staphylococcus aureus
MSSE: methicillin-susceptible Staphylococcus epidermidis
Duration of therapy
The optimal duration of therapy for BSIs in critically ill patients is poorly defined and randomized controlled trials examining duration of therapy in the specific setting of severely ill bacteraemic patients are not available. In general, recommended treatment duration should be between 7 and 14 days for bacteraemia related to central venous catheters, pneumonia, urinary tract, skin and soft tissue and intra-abdominal infections.104 However, in recent years, groups of experts have suggested to keep antimicrobial therapy as short as possible, and the available body of evidence seems to support this concept.105-106 In a recent meta-analysis Havey et al. found no significant differences in clinical cure, microbiologic cure, and survival among bacteraemic patients receiving shorter (5-7 days) vs. longer (7-21 days) duration of therapy, irrespective of the source of infection. The major limitation of this systematic review was the lack of studies specifically targeting bacteraemic patients and critically ill ones.107 De Santis et al. in a retrospective study reported good clinical outcomes and low rates of clinical relapses in bacteraemic patients treated with short-course monotherapy (4-5 days) in ICU, in a clinical context characterized by the prevalence of Gram-positive pathogens [mainly coagulase-negative staphylococci (CoNS)] and low rates of MDR Gram-negative ones.108 General consensus exists regarding a longer duration of therapy (14 days) for S. aureus bacteremia, in order to avoid the risk of relapse.40 Moreover, in BSIs sustained by Candida spp. a duration of therapy of 14 days after the first negative blood culture is recommended by the guidelines.99 Procalcitonin (PCT)-based approaches can contribute in reducing the duration of antimicrobial therapy in patients with severe sepsis and septic shock managed in ICU, as suggested in a recent meta-analysis by Prkno et al.109 The authors analyzed 7 studies comprising a total of 1,075 patients with severe sepsis or septic shock and found that both hospital mortality and 28-day mortality were not different between PCT-guided therapy and standard treatment groups, whereas duration of antimicrobial therapy was significantly shorter when a PCT-guided therapy was applied. It is important to note that cut-off values of PCT levels to guide therapeutic decisions are not well established, varying between 0,25 ng/mL and 4 ng/mL in different studies and algorithms.109 Thus, further investigations are needed.
Preventive strategies
Due to the high mortality and the frequent isolation of difficult-to treat pathogens, efforts should be made in order to prevent the development of BSI in critically ill patients.
The systematic use of isolation precautions, including standard measures (hand hygiene and gloves, gowns, eye protection use) and contact-based ones, represent a key strategy for reducing the transmission of the majority of bacteria and to control outbreaks of MDR pathogens.
Moreover, infection control programs based on culture surveillance from nasal and rectal swabs, together with appropriate isolation precautions, are effective in reducing the incidence of infections due to MDR pathogens, and should be encouraged in patients coming from highly endemic settings or with epidemiologic links to MDR cases.110-112
During the past years effective measures have been put in place in order to improve standardized protocols dictating catheter insertion and management in ICU, and a structured training for healthcare workers has been encouraged, leading to a significant reduction in the incidence of CR-BSIs in ICU.113-116
Conclusions
Several strategies should be implemented in order to improve the clinical outcome of BSIs in the ICU setting. The key point still remains a prompt initiation of an effective antibiotic treatment, which should be tailored in each single patient on the basis of the infection source, the most frequent pathogens isolated and the risk of antibiotic resistances. Increased attention, however, should be paid to strategies that can limit the progressive increase of antimicrobial resistance, through a careful use of available broad-spectrum antimicrobials and the improvement of surveillance and preventive measures. New antimicrobials with activity against MDR Gram-negative pathogens are urgently needed.
Figure 1.
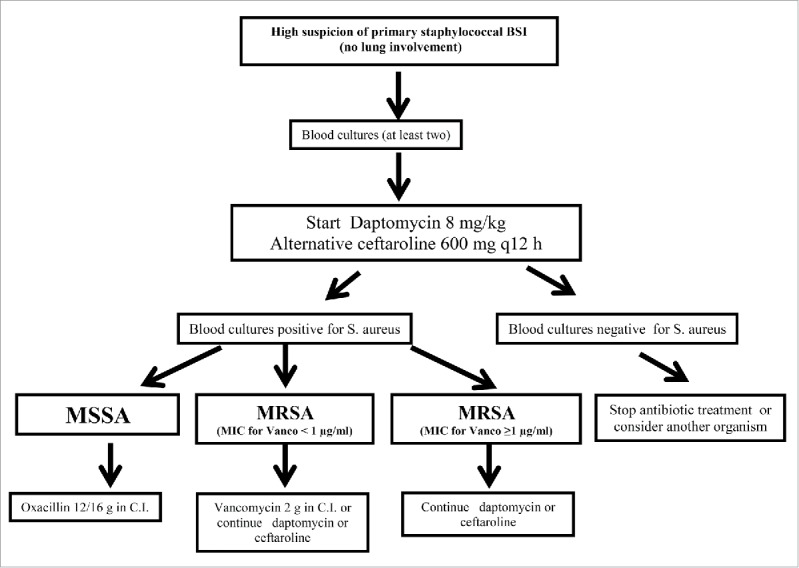
Treatment algorithm for empirical therapy of staphylococcal BSI
Abbreviations
- BSIs
Bloodstream infections
- ICU
Intensive Care Unit
- MDR
Multidrug-resistant
- ICU-BSIs
Intensive Care Unit-acquired bloodstream infections
- CA
Community-acquired
- HA
Hospital-acquired
- CR-BSIs
Catheter-related bloodstream infections
- VAP
Ventilator-associated pneumonia
- ESBL
Extended-spectrum β-lactamase
- MRSA
Methicillin-resistant Staphylococcus aureus
- MICs
Minimum inhibitory concentrations
- CDC
Centers for Disease Control
- VISA
Vancomycin-intermediate Staphylococcus aureus
- h-VISA
Heteroresistant vancomycin-intermediate Staphylococcus aureus
- VRSA
Vancomycin-resistant Staphylococcus aureus
- KPC
Carbapenemase-producing Klebsiella pneumoniae
- SSTI
Skin and soft-tissue infection
- CoNS
Coagulase-negative Staphylococci
- PCT
Procalcitonin
Disclosure of potential conflicts of interest
MB received honoraria from Pfizer, Novartis, MSD, Gilead, Astellas, Bayer, Angelini, Tetraphase, Achaogen. The other authors declare no conflict of interest.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39(3):309-17. Epub 2004 Jul 15. Erratum in: Clin Infect Dis. 2005. April 1; 40(7): 1077, Clin Infect Dis 2004; 39: 1093 [DOI] [PubMed] [Google Scholar]
- 2.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, et al.. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198-208; PMID:24670166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrouste-Orgeas M, Timsit JF, Tafflet M, Misset B, Zahar JR, Soufir L, Lazard T, Jamali S, Mourvillier B, Cohen Y, et al.. OUTCOMEREA Study Group. Excess risk of death from intensive care unit-acquired nosocomial bloodstream infections: A reappraisal. Clin Infect Dis 2006; 42:1118-26; PMID:16575729 [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, et al.. EPIC II Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323-9; PMID:19952319 [DOI] [PubMed] [Google Scholar]
- 5.Prowle JR, Echeverri JE, Ligabo EV, Sherry N, Taori GC, Crozier TM, Hart GK, Korman TM, Mayall BC, Johnson PD, et al.. Acquired bloodstream infection in the intensive care unit: Incidence and attributable mortality. Crit Care 2011; 15:R100; PMID:21418635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laupland KB, Zygun DA, Davies HD, Church DL, Louie TJ, Doig CJ. Population-based assessment of intensive care unit-acquired bloodstream infections in adults: Incidence, risk factors, and associated mortality rate. Crit Care Med 2002; 30:2462-7; PMID:12441755 [DOI] [PubMed] [Google Scholar]
- 7.Barnett AG, Page K, Campbell M, Martin E, Rashleigh-Rolls R, Halton K, Paterson DL, Hall L, Jimmieson N, White K, et al.. The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: A case-control study. BMJ Open 2013; 3:e003587; PMID:24176795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laupland KB, Lee H, Gregson DB, Manns BJ. Cost of intensive care unit-acquired bloodstream infections. J Hosp Infect 2006; 63:124-32; PMID:16621137 [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, Dodek P, Wood G, Kumar A, Simon D, et al.. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136:1237-48; PMID:19696123 [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al.. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589-96; PMID:16625125 [DOI] [PubMed] [Google Scholar]
- 11.Vallés J, Rello J, Ochagavía A, Garnacho J, Alcalá MA. Community-acquired bloodstream infection in critically ill adult patients: Impact of shock and inappropriate antibiotic therapy on survival. Chest 2003; 123:1615-24. [DOI] [PubMed] [Google Scholar]
- 12.Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit Care 2014; 18:596; PMID:25412897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, Hoppe-Bauer J, Dunne WM, Kollef MH. Resistance to empiric antimicrobial treatment predicts outcome in severe sepsis associated with Gram-negative bacteremia. J Hosp Med 2011; 6:405-10; PMID:21916003 [DOI] [PubMed] [Google Scholar]
- 14.Laupland KB, Church DL. Population-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev 2014; 27:647-64; PMID:25278570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenz R, Leal JR, Church DL, Gregson DB, Ross T, Laupland KB. The distinct category of healthcare associated bloodstream infections. BMC Infect Dis 2012; 12:85; PMID:22487002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallés J, Alvarez-Lerma F, Palomar M, Blanco A, Escoresca A, Armestar F, Sirvent JM, Balasini C, Zaragoza R, Marín M. Study Group of Infectious Diseases of the Spanish Society of Critical Care Medicine. Health-care-associated bloodstream infections at admission to the ICU. Chest 2011; 139:810-5; http://dx.doi.org/ 10.1378/chest.10-1715 [DOI] [PubMed] [Google Scholar]
- 17.Laupland KB, Gregson DB, Zygun DA, Doig CJ, Mortis G, Church DL. Severe bloodstream infections: A population-based assessment. Crit Care Med 2004; 32:992-7; PMID:15071391; http://dx.doi.org/ 10.1097/01.CCM.0000119424.31648.1E [DOI] [PubMed] [Google Scholar]
- 18.Laupland KB, Zygun DA, Davies HD, Church DL, Louie TJ, Doig CJ. Population-based assessment of intensive care unit acquired bloodstream infections in adults: Incidence, risk factors, and associated mortality rate. Crit Care Med 2002; 30:2462-7; PMID:12441755; http://dx.doi.org/ 10.1097/00003246-200211000-00010 [DOI] [PubMed] [Google Scholar]
- 19.Soufir L, Timsit JF, Mahe C, Carlet J, Regnier B, Chevret S. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: A matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol 1999; 20:396-401; PMID:10395140; http://dx.doi.org/ 10.1086/501639 [DOI] [PubMed] [Google Scholar]
- 20.Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter-related bloodstream infections. Int J Crit Illn Inj Sci 2014; 4:162-7; PMID:25024944; http://dx.doi.org/ 10.4103/2229-5151.134184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Horo JC, Maki DG, Krupp AE, Safdar N. Arterial catheters as a source of bloodstream infection: A systematic review and meta-analysis. Crit Care Med 2014; 42:1334-9; PMID:24413576; http://dx.doi.org/ 10.1097/CCM.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 22.Corona A, Bertolini G, Lipman J, Wilson AP, Singer M. Antibiotic use and impact on outcome from bacteraemic critical illness: The BActeraemia Study in Intensive Care (BASIC). J Antimicrob Chemother 2010; 65:1276-85; PMID:20335186; http://dx.doi.org/ 10.1093/jac/dkq088 [DOI] [PubMed] [Google Scholar]
- 23.Lim SJ, Choi JY, Lee SJ, Cho YJ, Jeong YY, Kim HC, Lee JD, Hwang YS. Intensive care unit-acquired blood stream infections: A 5-year retrospective analysis of a single tertiary care hospital in Korea. Infection 2014; 42:875-81; PMID:25030309; http://dx.doi.org/ 10.1007/s15010-014-0651-z [DOI] [PubMed] [Google Scholar]
- 24.Bassetti M, Taramasso L, Giacobbe DR, Pelosi P. Management of ventilator-associated pneumonia: Epidemiology, diagnosis and antimicrobial therapy. Expert Rev Anti Infect Ther 2012; 10:585-96; PMID:22702322; http://dx.doi.org/ 10.1586/eri.12.36 [DOI] [PubMed] [Google Scholar]
- 25.Kunac A, Sifri ZC, Mohr AM, Horng H, Lavery RF, Livingston DH. Bacteremia and ventilator-associated pneumonia: A marker for contemporaneous extra-pulmonic infection. Surg Infect (Larchmt) 2014; 15:77-83; PMID:24192306; http://dx.doi.org/ 10.1089/sur.2012.030 [DOI] [PubMed] [Google Scholar]
- 26.De Waele J, Lipman J, Sakr Y, Marshall JC, Vanhems P, Barrera Groba C, Leone M, Vincent JL. EPIC II Investigators. Abdominal infections in the intensive care unit: Characteristics, treatment and determinants of outcome. BMC Infect Dis. 2014; 14:420; PMID:25074742; http://dx.doi.org/ 10.1186/1471-2334-14-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabah A, Koulenti D, Laupland K, Misset B, Valles J, Bruzzi de Carvalho F, Paiva JA, Cakar N, Ma X, Eggimann P, et al.. Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: The EUROBACT International Cohort Study. Intensive Care Med 2012. December; 38:1930-45; http://dx.doi.org/ 10.1007/s00134-012-2695-9 [DOI] [PubMed] [Google Scholar]
- 28.Kett DH, Azoulay E, Echeverria PM, Vincent JL. Extended Prevalence of Infection in ICU Study (EPIC II) Group of Investigators. Candida bloodstream infections in intensive care units: Analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med 2011; 39:665-70; PMID:21169817; http://dx.doi.org/ 10.1097/CCM.0b013e318206c1ca [DOI] [PubMed] [Google Scholar]
- 29.Ammerlaan HS, Harbarth S, Buiting AG, Crook DW, Fitzpatrick F, Hanberger H, Herwaldt LA, van Keulen PH, Kluytmans JA, Kola A, et al.. Secular trends in nosocomial bloodstream infections: Antibiotic-resistant bacteria increase the total burden of infection. Clin Infect Dis 2013; 56:798-805; PMID:23223600; http://dx.doi.org/ 10.1093/cid/cis1006 [DOI] [PubMed] [Google Scholar]
- 30.de Kraker ME, Davey PG, Grundmann H. BURDEN study group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Med 2011; 8:e1001104; PMID:22022233; http://dx.doi.org/ 10.1371/journal.pmed.1001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D'Inzeo T, Fadda G, Cauda R, et al.. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: Importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother 2007; 51:1987-94. Erratum in: Antimicrob Agents Chemother 2007; 51: 3469; http://dx.doi.org/ 10.1128/AAC.01509-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassetti M, Trecarichi EM, Mesini A, Spanu T, Giacobbe DR, Rossi M, Shenone E, Pascale GD, Molinari MP, Cauda R, et al.. Risk factors and mortality of healthcare-associated and community-acquired Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2012; 18:862-9; PMID:21999245; http://dx.doi.org/ 10.1111/j.1469-0691.2011.03679.x [DOI] [PubMed] [Google Scholar]
- 33.European Antimicrobial Resistance Surveillance Network (EARS-Net) EARSS Annual Report. 2013. Available at: http://ecdc.europa.eu/en/activities/surveillance/EARS-Net. Last access 1September2015. [Google Scholar]
- 34.Bassetti M, De Gaudio R, Mazzei T, Morace G, Petrosillo N, Viale P, Bello G, La Face S, Antonelli M. A survey on infection management practices in Italian ICUs. Crit Care 2012; 16:R221; PMID:23151325; http://dx.doi.org/ 10.1186/cc11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sancak B, Yagci S, Mirza HC, Hasçelik G. Evaluation of vancomycin and daptomycin MIC trends for methicillin-resistant Staphylococcus aureus blood isolates over an 11 year period. J Antimicrob Chemother 2013; 68:2689-91; PMID:23788481; http://dx.doi.org/ 10.1093/jac/dkt247 [DOI] [PubMed] [Google Scholar]
- 36.Kehrmann J, Kaase M, Szabados F, Gatermann SG, Buer J, Rath PM, Steinmann J. Vancomycin MIC creep in MRSA blood culture isolates from Germany: a regional problem? Eur J Clin Microbiol Infect Dis 2011; 30:677-83; PMID:21229280; http://dx.doi.org/ 10.1007/s10096-010-1140-7 [DOI] [PubMed] [Google Scholar]
- 37.Vancomycin-intermediate Staphylococcus aureus (VISA), and Vancomycin-resistant Staphylococcus aureus (VRSA) 2007. Case definition. CSTE position Statement Number: 09-ID-58, 09-ID-59. Available at: http://www.cdc.gov/ncphi/disss/nndss/casedef/vancomycincurrent.htm. Last access 1September2015. [Google Scholar]
- 38.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis 2012; 54:51-8; PMID:22109947; http://dx.doi.org/ 10.1093/cid/cir764 [DOI] [PubMed] [Google Scholar]
- 39.Murray KP, Zhao JJ, Davis SL, Kullar R, Kaye KS, Lephart P, Rybak MJ. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis 2013; 56:1562-9; PMID:23449272; http://dx.doi.org/ 10.1093/cid/cit112 [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, et al.. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52:285-92; PMID:21217178; http://dx.doi.org/ 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 41.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009; 49:507-14; PMID:19586413; http://dx.doi.org/ 10.1086/600884 [DOI] [PubMed] [Google Scholar]
- 42.Harris PN, Tambyah PA, Paterson DL. β-lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: Time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis 2015; 15:475-85; PMID:25716293; http://dx.doi.org/ 10.1016/S1473-3099(14)70950-8 [DOI] [PubMed] [Google Scholar]
- 43.Dimopoulos G, Koulenti D, Tabah A, Poulakou G, Vesin A, Arvaniti K, Lathyris D, Matthaiou DK, Armaganidis A, Timsit JF. Bloodstream infections in ICU with increased resistance: Epidemiology and outcomes. Minerva Anestesiol 2015; 81:405-18; PMID:25220548 [PubMed] [Google Scholar]
- 44.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, et al.. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin Infect Dis 2012; 55:943-50; PMID:22752516; http://dx.doi.org/ 10.1093/cid/cis588 [DOI] [PubMed] [Google Scholar]
- 45.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E,Polsky B, Adams-Haduch JM, Doi Y. Treatment outcome of bacteremia due toKPC-producing Klebsiella pneumoniae: Superiority of combination antimicrobialregimens. Antimicrob Agents Chemother 2012; 56:2108-13; PMID:22252816; http://dx.doi.org/ 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parisi SG, Bartolini A, Santacatterina E, Castellani E, Ghirardo R, Berto A, Franchin E, Menegotto N, De Canale E, Tommasini T, et al.. Prevalence of Klebsiella pneumoniae strains producing carbapenemases and increase of resistance to colistin in an Italian teaching hospital from January 2012 To December 2014. BMC Infect Dis 2015; 15:244; PMID:26116560; http://dx.doi.org/ 10.1186/s12879-015-0996-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ah YM, Kim AJ, Lee JY. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents 2014; 44:8-15; PMID:24794735; http://dx.doi.org/ 10.1016/j.ijantimicag.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 48.Bassetti M, Righi E. SDD and colistin resistance: End of a dream? Intensive Care Med 2014; 40:1066-7; PMID:24861349; http://dx.doi.org/ 10.1007/s00134-014-3328-2 [DOI] [PubMed] [Google Scholar]
- 49.Kontopidou F, Giamarellou H, Katerelos P, Maragos A, Kioumis I, Trikka-Graphakos E, Valakis C, Maltezou HC; Group for the Study of KPC-producing Klebsiella pneumoniae infections in intensive care units. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: A multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect 2014; 20:O117-23; PMID:23992130; http://dx.doi.org/ 10.1111/1469-0691.12341 [DOI] [PubMed] [Google Scholar]
- 50.Antimicrobial resistance: global report on surveillance 2014. (WHO). Available at: http://www.who.int/drugresistance/en/. Last access 1 September 2015. [Google Scholar]
- 51.Pfaller MA, Messer SA, Moet GJ, Jones RN, Castanheira M. Candida bloodstream infections: Comparison of species distribution and resistance to echinocandin and azole antifungal agents in Intensive Care Unit (ICU) and non-ICU settings in the SENTRY Antimicrobial Surveillance Program (2008-2009). Int J Antimicrob Agents 2011; 38:65-9; PMID:21514797; http://dx.doi.org/ 10.1016/j.ijantimicag.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 52.Bassetti M, Merelli M, Righi E, Diaz-Martin A, Rosello EM, Luzzati R, Parra A, Trecarichi EM, Sanguinetti M, Posteraro B, et al.. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol 2013; 51:4167-72; PMID:24108614; http://dx.doi.org/ 10.1128/JCM.01998-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nesher L, Rolston KV, Shah DP, Tarrand JT, Mulanovich V, Ariza-Heredia EJ, Chemaly RF. Fecal colonization and infection with Pseudomonas aeruginosa in recipients of allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2015; 17:33-8; PMID:25546740; http://dx.doi.org/ 10.1111/tid.12323 [DOI] [PubMed] [Google Scholar]
- 54.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344:11-6; PMID:11136954; http://dx.doi.org/ 10.1056/NEJM200101043440102 [DOI] [PubMed] [Google Scholar]
- 55.Giannella M, Trecarichi EM, De Rosa FG, Del Bono V, Bassetti M, Lewis RE, Losito AR, Corcione S, Saffioti C, Bartoletti M, et al.. Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: A prospective observational multicentre study. Clin Microbiol Infect 2014; 20:1357-62; PMID:24980276; http://dx.doi.org/ 10.1111/1469-0691.12747 [DOI] [PubMed] [Google Scholar]
- 56.Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob Agents Chemother 2006; 50: 43-48; PMID:16377665; http://dx.doi.org/ 10.1128/AAC.50.1.43-48.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 2006; 42:692-699; PMID:16447117; http://dx.doi.org/ 10.1086/500202 [DOI] [PubMed] [Google Scholar]
- 58.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: Risk factors for acquisition, infection and their consequences. J Hosp Infect 2007; 65:204-211; PMID:17254667; http://dx.doi.org/ 10.1016/j.jhin.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 59.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. Carbapenem resistance among Klebsiella pneumoniae isolates: Risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 2009; 30: 666-671; PMID:19496647; http://dx.doi.org/ 10.1086/598244 [DOI] [PubMed] [Google Scholar]
- 60.Tumbarello M, Trecarichi EM, Tumietto F, Del Bono V, De Rosa FG, Bassetti M, Losito AR, Tedeschi S, Saffioti C, Corcione S, et al.. Predictive models for identification of hospitalized patients harboring KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2014; 58:3514-20; PMID:24733460; http://dx.doi.org/ 10.1128/AAC.02373-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med 2013; 39:2070-82; PMID:24045886; http://dx.doi.org/ 10.1007/s00134-013-3088-4 [DOI] [PubMed] [Google Scholar]
- 62.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, et al.. DALI Study. DALI: defining antibiotic levels in intensive care unit patients: Are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 2014; 58:1072-83; PMID:24429437; http://dx.doi.org/ 10.1093/cid/ciu027 [DOI] [PubMed] [Google Scholar]
- 63.Bauer KA, West JE, O'Brien JM, Goff DA. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother 2013; 57:2907-2912; PMID:23571547; http://dx.doi.org/ 10.1128/AAC.02365-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL, et al.. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin Infect Dis 2013; 56:236-44; PMID:23074313; http://dx.doi.org/ 10.1093/cid/cis856 [DOI] [PubMed] [Google Scholar]
- 65.Lodise TP Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: Clinical implications of an extended- infusion dosing strategy. Clin Infect Dis 2007; 44:357-363; PMID:17205441; http://dx.doi.org/ 10.1086/510590 [DOI] [PubMed] [Google Scholar]
- 66.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: A systematic review and meta-analysis. Clin Infect Dis 2013; 56:272-282. [DOI] [PubMed] [Google Scholar]
- 67.Taccone FS, Laterre PF, Spapen H, Dugernier T, Delattre I, Layeux B, De Backer D, Wittebole X, Wallemacq P, Vincent JL, Jacobs F. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit Care 2010; 14:R53; PMID:20370907; http://dx.doi.org/ 10.1186/cc8945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lorente L, Lorenzo L, Martín MM, Jiménez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother 2006; 40:219-23; PMID:16449546; http://dx.doi.org/ 10.1345/aph.1G467 [DOI] [PubMed] [Google Scholar]
- 69.Lorente L, Jiménez A, Martín MM, Iribarren JL, Jiménez JJ, Mora ML. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 2009; 33:464-8; PMID:19150225; http://dx.doi.org/ 10.1016/j.ijantimicag.2008.10.025 [DOI] [PubMed] [Google Scholar]
- 70.Patel GW, Patel N, Lat A, Trombley K, Enbawe S, Manor K, Smith R, Lodise TP Jr. Outcomes of extended infusion piperacillin/tazobactam for documented Gram-negative infections. Diagn Microbiol Infect Dis 2009; 64:236-40; PMID:19500529; http://dx.doi.org/ 10.1016/j.diagmicrobio.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 71.Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, Diaz-Martin A, Luzzati R, Rosin C, Lagunes L, et al.. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med 2014; 40:839-45. Erratum in: Intensive Care Med 2014; 40: 1186; http://dx.doi.org/ 10.1007/s00134-014-3310-z [DOI] [PubMed] [Google Scholar]
- 72.Chusri S, Silpapojakul K, McNeil E, Singkhamanan K, Chongsuvivatwong V. Impact of antibiotic exposure on occurrence of nosocomial carbapenem-resistant Acinetobacter baumannii infection: A case control study. J Infect Chemother 2015; 21:90-5; PMID:25454216; http://dx.doi.org/ 10.1016/j.jiac.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 73.Marchenay P, Blasco G, Navellou JC, Leroy J, Cholley P, Talon D, Bertrand X, Gbaguidi-Haore H. Acquisition of carbapenem-resistant Gram-negative bacilli in intensive care unit: Predictors and molecular epidemiology. Med Mal Infect 2015; 45:34-40; PMID:25640914; http://dx.doi.org/ 10.1016/j.medmal.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 74.Armand-Lefèvre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppé E, Bronchard R, Lepeule R, Lucet JC, El Mniai A, et al.. Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 2013; 57:1488-95. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y.. Multidrug-resistant Pseudomonas aeruginosa: Risk factors and clinical impact. Antimicrob Agents Chemother 2006; 50:43-8; http://dx.doi.org/ 10.1128/AAC.01823-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Society for Healthcare Epidemiology of America; Infectious Diseases Society of America; Pediatric Infectious Diseases Society . Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol 2012; 33:322-7; PMID:22418625; http://dx.doi.org/ 10.1086/665010 [DOI] [PubMed] [Google Scholar]
- 76.Barry AL, Fuchs PC, Brown SD. In vitro activities of daptomycin against 2,789 clinical isolates from 11 North American medical centers. Antimicrob Agents Chemother 2001; 45:1919-22; PMID:11353654; http://dx.doi.org/ 10.1128/AAC.45.6.1919-1922.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Streit JM, Jones RN, Sader HS. Daptomycin activity and spectrum: A worldwide sample of 6737 clinical Gram-positive organisms. J Antimicrob Chemother 2004; 53:669-74; PMID:14985278; http://dx.doi.org/ 10.1093/jac/dkh143 [DOI] [PubMed] [Google Scholar]
- 78.Bassetti M, Ansaldi F, De Florentiis D, Righi E, Pea F, Sartor A, Scarparo C, Carnelutti A. Is empiric daptomycin effective in reducing mortality in Staphylococcus aureus bacteraemia? A real-life experience. Intensive Care Med 2015; 41(11):2026-8 [DOI] [PubMed] [Google Scholar]
- 79.Bassetti M, Villa G, Ansaldi F, De Florentiis D, Tascini C, Cojutti P, Righi E, Sartor A, Crapis M, De Rosa FG, et al.. Risk factors associated with the onset of daptomycin non-susceptibility in Staphylococcus aureus infections in critically ill patients. Intensive Care Med 2015; 41:366-8; PMID:25447803; http://dx.doi.org/ 10.1007/s00134-014-3571-6 [DOI] [PubMed] [Google Scholar]
- 80.Bassetti M, Nicco E, Ginocchio F, Ansaldi F, de Florentiis D, Viscoli C. High-dose daptomycin in documented Staphylococcus aureus infections. Int J Antimicrob Agents 2010; 36:459-61; PMID:20846832; http://dx.doi.org/ 10.1016/j.ijantimicag.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 81.Bauer J, Siala W, Tulkens PM, Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother 2013; 57:2726-37; PMID:23571532; http://dx.doi.org/ 10.1128/AAC.00181-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moise PA, Hershberger E, Amodio-Groton MI, Lamp KC. Safety and clinical outcomes when utilizing high-dose (> or =8 mg/kg) daptomycin therapy. Ann Pharmacother 2009:43:1211-9; http://dx.doi.org/ 10.1345/aph.1M085 [DOI] [PubMed] [Google Scholar]
- 83.Figueroa DA, Mangini E, Amodio-Groton M, Vardianos B, Melchert A, Fana C, Wehbeh W, Urban CM, Segal-Maurer S. Safety of high-dose intravenous daptomycin treatment: Three-year cumulative experience in a clinical program. Clin Infect Dis 2009; 49:177-80; PMID:19500039; http://dx.doi.org/ 10.1086/600039 [DOI] [PubMed] [Google Scholar]
- 84.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, et al.. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165-228; PMID:23361625; http://dx.doi.org/ 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, et al.. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: A propensity-matched analysis. Crit Care Med 2010; 38:1773-85; PMID:20639750; http://dx.doi.org/ 10.1097/CCM.0b013e3181eb3ccd [DOI] [PubMed] [Google Scholar]
- 86.Díaz-Martín A, Martínez-González ML, Ferrer R, Ortiz-Leyba C, Piacentini E, Lopez-Pueyo MJ, Martín-Loeches I, Levy MM, Artigas A, Garnacho-Montero J; Edusepsis Study Group. Antibiotic prescription patterns in the empiric therapy of severe sepsis: Combination of antimicrobials with different mechanisms of action reduces mortality. Crit Care 2012; 16:R223; http://dx.doi.org/ 10.1186/cc11869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis 2004; 4:519-27; PMID:15288826; http://dx.doi.org/ 10.1016/S1473-3099(04)01108-9 [DOI] [PubMed] [Google Scholar]
- 88.Paul M, Lador A, Grozinsky-Glasberg S, Leibovici L. Beta lactam antibioticmonotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 2014; 1:CD003344; PMID:24395715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martínez JA, Cobos-Trigueros N, Soriano A, Almela M, Ortega M, Marco F, Pitart C, Sterzik H, Lopez J, Mensa J. Influence of empiric therapy with a beta-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob Agents Chemother. 2010. September; 54(9):3590; http://dx.doi.org/ 10.1128/AAC.00115-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y.Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56(4):2108-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798-803; PMID:21595793; http://dx.doi.org/ 10.1111/j.1469-0691.2011.03514.x [DOI] [PubMed] [Google Scholar]
- 92.Daikos GL, Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A, Stefanou I, Sypsa V, Miriagou V, Nepka M, et al.. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: Lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322-8; PMID:24514083; http://dx.doi.org/ 10.1128/AAC.02166-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tumbarello M, Trecarichi EM, De Rosa FG, Giannella M, Giacobbe DR, Bassetti M, Losito AR, Bartoletti M, Del Bono V, Corcione S, et al.. ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva). Infections caused by KPC-producing Klebsiella pneumoniae: Differences in therapy and mortality in a multicentre study. J Antimicrob Chemother. 2015; 70:2133-43; PMID:25900159; http://dx.doi.org/ 10.1093/jac/dkv200 [DOI] [PubMed] [Google Scholar]
- 94.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: A potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005; 49:3640-5; PMID:16127033; http://dx.doi.org/ 10.1128/AAC.49.9.3640-3645.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cuenca-Estrella M, Verweij PE, Arendrup MC, Arikan-Akdagli S, Bille J, Donnelly JP, Jensen HE, Lass-Flörl C, Richardson MD, Akova M, et al.. ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect 2012; 18 Suppl 7:9-18; PMID:23137134; http://dx.doi.org/ 10.1111/1469-0691.12038 [DOI] [PubMed] [Google Scholar]
- 96.León C, Ruiz-Santana S, Saavedra P, Galván B, Blanco A, Castro C, Balasini C, Utande-Vázquez A, González de Molina FJ, Blasco-Navalproto MA, et al.. Cava Study Group. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: A prospective multicenter study. Crit Care Med 2009; 37:1624-33; http://dx.doi.org/ 10.1097/CCM.0b013e31819daa14 [DOI] [PubMed] [Google Scholar]
- 97.Bruyère R, Quenot JP, Prin S, Dalle F, Vigneron C, Aho S, Leon C, Charles PE. Empirical antifungal therapy with an echinocandin in critically-ill patients: Prospective evaluation of a pragmatic Candida score-based strategy in one medical ICU. BMC Infect Dis 2014; 14:385; http://dx.doi.org/ 10.1186/1471-2334-14-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Martini A, Gottin L, Menestrina N, Schweiger V, Simion D, Vincent JL.Procalcitonin levels in surgical patients at risk of candidemia. J Infect 2010; 60:425-30; PMID:20226210; http://dx.doi.org/ 10.1016/j.jinf.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 99.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, et al.. ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18 Suppl 7:19-37; PMID:23137135; http://dx.doi.org/ 10.1111/1469-0691.12039 [DOI] [PubMed] [Google Scholar]
- 100.Masterton RG. Antibiotic de-escalation. Crit Care Clin 2011; 27:149-162; PMID:21144991; http://dx.doi.org/ 10.1016/j.ccc.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 101.Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, Corcia-Palomo Y, Fernández-Delgado E, Herrera-Melero I, Ortiz-Leyba C, Márquez-Vácaro JA. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 2014; 40:32-40; PMID:24026297; http://dx.doi.org/ 10.1007/s00134-013-3077-7 [DOI] [PubMed] [Google Scholar]
- 102.Mokart D, Slehofer G, Lambert J, Sannini A, Chow-Chine L, Brun JP, Berger P, Duran S, Faucher M, Blache JL, et al.. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: Results from an observational study. Intensive Care Med 2014; 40:41-9; PMID:24231857; http://dx.doi.org/ 10.1007/s00134-013-3148-9 [DOI] [PubMed] [Google Scholar]
- 103.Leone M, Bechis C, Baumstarck K, Lefrant JY, Albanèse J, Jaber S, Lepape A, Constantin JM, Papazian L, Bruder N, et al.. AZUREA Network Investigators. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: A multicenter non-blinded randomized noninferiority trial. Intensive Care Med 2014; 40:1399-408; PMID:25091790; http://dx.doi.org/ 10.1007/s00134-014-3411-8 [DOI] [PubMed] [Google Scholar]
- 104.Daneman N, Shore K, Pinto R, Fowler R. Antibiotic treatment duration for bloodstream infections in critically ill patients: A national survey of Canadian infectious diseases and critical care specialists. Int J Antimicrob Agents 2011; 38:480-5; PMID:21982833; http://dx.doi.org/ 10.1016/j.ijantimicag.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 105.Rubinstein E, Keynan Y. Short-course therapy for severe infections. Int J Antimicrob Agents 2013; 42 Suppl:S22-4; PMID:23706543; http://dx.doi.org/ 10.1016/j.ijantimicag.2013.04.013 [DOI] [PubMed] [Google Scholar]
- 106.Rubinstein E. Short antibiotic treatment courses or how short is short? Int J Antimicrob Agents 2007; 30 Suppl 1:S76-9; PMID:17826038; http://dx.doi.org/ 10.1016/j.ijantimicag.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 107.Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: A systematic review and meta-analysis. Crit Care 2011; 15:R267; PMID:22085732; http://dx.doi.org/ 10.1186/cc10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Santis V, Gresoiu M, Corona A, Wilson AP, Singer M. Bacteraemia incidence, causative organisms and resistance patterns, antibiotic strategies and outcomes in a single university hospital ICU: Continuing improvement between 2000 and 2013. J Antimicrob Chemother 2015; 70:273-8; PMID:25190722; http://dx.doi.org/ 10.1093/jac/dku338 [DOI] [PubMed] [Google Scholar]
- 109.Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock–a systematic review and meta-analysis. Crit Care 2013; 17:R291; PMID:24330744; http://dx.doi.org/ 10.1186/cc13157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ben-David D, Maor Y, Keller N, Regev-Yochay G, Tal I, Shachar D, Zlotkin A, Smollan G, Rahav G. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol 2010; 31:620-6; PMID:20370465; http://dx.doi.org/ 10.1086/652528 [DOI] [PubMed] [Google Scholar]
- 111.Kochar S, Sheard T, Sharma R, Hui A, Tolentino E, Allen G, Landman D, Bratu S, Augenbraun M, Quale J. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol 2009; 30:447-52; PMID:19301985; http://dx.doi.org/ 10.1086/596734 [DOI] [PubMed] [Google Scholar]
- 112.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: Epidemiology and prevention. Clin Infect Dis 2011; 53:60-7; PMID:21653305; http://dx.doi.org/ 10.1093/cid/cir202 [DOI] [PubMed] [Google Scholar]
- 113.Van der Kooi TI, Wille JC, van Benthem BH. Catheter application, insertion vein and length of ICU stay prior to insertion affect the risk of catheter-related bloodstream infection. J Hosp Infect 2012; 80:238-244; PMID:22243832; http://dx.doi.org/ 10.1016/j.jhin.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 114.Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: Epidemiologic and economic consequences. Am J Infect Control 2011; 39:640-646; PMID:21641088; http://dx.doi.org/ 10.1016/j.ajic.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 115.Herzer KR, Niessen L, Constenla DO, Ward WJ Jr, Pronovost PJ. Cost-effectiveness of a quality improvement programme to reduce central line-associated bloodstream infections in intensive care units in the USA. BMJ Open 2014; 4:e006065; PMID:25256190; http://dx.doi.org/ 10.1136/bmjopen-2014-006065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bassetti M, De Waele JJ, Eggimann P, Garnacho-Montero J, Kahlmeter G, Menichetti F, Nicolau DP, Paiva JA, Tumbarello M, Welte T, et al.. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensive Care Med 2015; 41:776-95; PMID:25792203; http://dx.doi.org/ 10.1007/s00134-015-3719-z [DOI] [PMC free article] [PubMed] [Google Scholar]


