Abstract
Introduction
Fluorescence anisotropy (FA) is one of the major established methods accepted by industry and regulatory agencies for understanding the mechanisms of drug action and selecting drug candidates utilizing a high-throughput format.
Areas covered
This review covers the basics of FA and complementary methods, such as fluorescence lifetime anisotropy and their roles in the drug discovery process. The authors highlight the factors affecting FA readouts, fluorophore selection, and instrumentation. Furthermore, the authors describe the recent development of a successful, commercially valuable FA assay for Long QT syndrome drug toxicity to illustrate the role that FA can play in the early stages of drug discovery.
Expert opinion
Despite the success in drug discovery, the FA-based technique experiences competitive pressure from other homogeneous assays. That being said, FA is an established yet rapidly developing technique, recognized by academic institutions, the pharmaceutical industry, and regulatory agencies across the globe. The technical problems encountered in working with small molecules in homogeneous assays are largely solved, and new challenges come from more complex biological molecules and nanoparticles. With that, FA will remain one of the major work-horse techniques leading to precision (personalized) medicine.
1. Introduction: screening methods in drug discovery
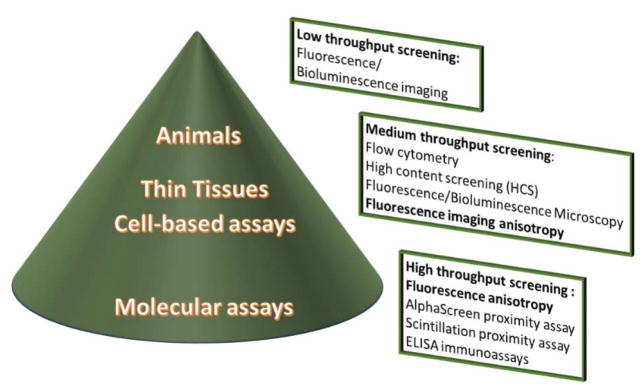
Drug discovery is a complex and exciting process with the ultimate goal of identifying a treatment for a disease, medical condition, or side effect of a therapy. In a biology-driven setting, drug discovery starts from validation of a biological target and goes through several steps of screening drug-like molecules, narrowing down the potential molecules to identify the lead candidate. Preclinical biological assays can be roughly characterized by the type of target complexity: molecular, cellular, tissue sections, and small animals (Figure 1).
Figure 1.
Optical techniques in the drug discovery process. The dimensions of the cone reflect the number of compounds tested during the screening process: a large number in the high-throughput molecular assays, in which FA plays a critical role; a relatively low number for cell studies and thin tissues, with limited involvement of FA; and a few selected compounds in animal testing.
The initial screening of drugs is conducted via molecular assays that focus on the interaction between a drug candidate and the isolated purified target. These assays are designed to test hundreds of thousands of drug-like compounds from commercially available or targeted libraries in a process known as high-throughput screening (HTS). In the next step, cell-based studies are conducted on a selected group of compounds. Traditionally, cell studies were less suitable for HTS,1 but the advance of high content screening (HCS) and the integration of flow cytometry with plate readers has increased the use of high-throughput cell assays in drug discovery.2–4 Finally, small mammals, such as rodents, as well as rabbits, dogs, and monkeys, that share a large number of genes with humans are used to finalize the drug screening process, providing a translation phase from in vitro assays to clinical studies. The in vivo evaluation relies on imaging and certain non-imaging techniques (i.e. LC-MS) that are commonly deployed to investigate biodistribution, pharmacokinetics, and biological activity of potential therapeutics, as well as optimize drug delivery.5, 6
Optical techniques utilizing fluorescently labeled molecules have become dominant in drug discovery, spanning from molecular assays to full body imaging, and almost completely replacing the radio-labeling that led drug discovery in the 20th century.7, 8 This is due to the superior sensitivity of fluorophores to environmental factors, as well as their multidimensionality, i.e., their ability to provide diverse simultaneous readouts, such as spectral characteristics, intensity, lifetime, and anisotropy. Despite some limitations as compared to radiolabeling, such as difficulties in quantitating the amount of fluorophore in a heterogeneous sample and structural alteration of the drug molecules after labeling, the rapidly growing variety of convenient, low cost commercial fluorophores make fluorescent molecules exciting for drug discovery studies.
One of the common techniques for testing fluorescently labeled compounds is fluorescence anisotropy (FA). Due to the versatility of FA and the availability of high quality polarizers, stable detectors, and excitation sources, this method has found widespread use in diverse biological applications, from probing the cellular microenvironment9, 10 and monitoring cell signaling pathways,11, 12 to 2D and 3D imaging,13–17 temperature mapping,18 and evaluation of drug delivery systems.19 Introduced in 1970s and with the first dedicated instruments in 1980s, FA has become a standard way of quantitatively measuring biomarkers (first clinical utility of FA), elucidating the mechanism of drug action, and screening potential drug candidates.20, 21 In the last decade, a variety of FA designs and formats have been utilized to study enzymes,22–24 as well as protein-protein25 and protein-DNA interactions, with the goal of developing drugs.26,27 A number of recent reviews cover different aspects of these applications in great detail.28–30
In this article, The authors first review and discuss basic, parameters of steady-state FA related to drug discovery that include polarization phenomena, fluorophore selection, factors affecting anisotropy, and instrumentation design. Second, with more than 15,000 references of FA in drug discovery as of April 2015, instead of reviewing numerous publications, the authors illustrate the application of steady-state FA to the FDA accepted hERG assay. Third, the article presents advanced concepts related to FA that include time-resolved fluorescence anisotropy (TR-FA) and two-photon (2P) excitation for imaging studies. Finally, the authors present novel directions in which FA plays or is expected to play an important role and compare the technique to other emerging platforms.
2. Steady-state fluorescence anisotropy
2.1. Principle of fluorescence anisotropy
In the FA technique, a fluorophore is irradiated with linearly polarized light. The resultant fluorescence intensity is measured through a polarization filter placed in front of the detector and oriented either parallel or perpendicular to the incident polarized light. The FA value (r) can then be determined from Eq. 131 in Table 1. Polarization represents the same phenomena, but is calculated with a different equation (Eq. 2.) Polarization and anisotropy are interrelated. Fluorescence polarization is more commonly used in clinical medicine for measurement of biomarkers, while fluorescence anisotropy is used in mechanistic studies and drug discovery. The instrument correction parameter, G, (known as a G-factor) reflects the sensitivity of the system to differently polarized light and is often measured automatically on modern instruments or manually for example by isotropic standards (r = 0).32
Table 1.
Equations used in steady-state fluorescence anisotropy measuremnts
| Measurement | Formula | Parameters | Eq. | |
|---|---|---|---|---|
| Anisotropy |
|
III – the intensity of fluorescence emission parallel to the vertically polarized light I⊥ – the intensity of fluorescence emission perpendicular to the vertically polarized light G – instrument correction factor |
1 | |
| Polarization |
|
2 | ||
| Perrin Equation |
|
ro – the limited (fundamental, maximum) fluorescence anisotropy, usually ro ≤0.4 τ – fluorescence lifetime of the fluorophore θrot – rotational correlation time of the macromolecule Dτ – diffusion coefficient |
3 | |
| Stokes-Einstein-Debye Equation |
|
η – viscosity, poise V – hydrodynamic volume kB = 1.38×10−23 J K−1, Boltzmann’s constant T – temperature, K v – specific volume MW – molecular weight h – hydration, typically 0.2 g H2O per gram of protein R = 8.31 J K−1 mol−1, universal constant |
4 |
On the molecular level, FA relates to the rotation of the fluorophore dipole. Anisotropy thus directly relates to the rotational correlation time (θrot) of the fluorophore, which is proportional to the hydrodynamic volume (V) of the molecule in solution according to the Perrin and Stokes-Einstein-Debye equations (Eqs 3 and 4)28, 33 that connect the measurable value r with the size of the target. Any change in the hydrodynamic volume (V) of the target (i.e. association, dissociation, cleavage, shape change, etc.) will have a direct effect on its anisotropy.
2.2. Factors affecting fluorescence anisotropy
The essence of FA in drug discovery is its ability to distinguish differences in the hydrodynamic radius of the fluorescent entity upon its interaction with a drug. Thus, almost all anisotropy assays are based on indirect measurement of the size change and cover association, dissociation, cleavage, binding, rearrangement, and many other types of reactions that accompany an interaction between the drug and the biological target. Although in most cases the fluorophore itself remains unaltered, its selection is critical for success of the assay. A suitable fluorophore for FA applications has to meet the following criteria: i) facile conjugation under conditions appropriate for attachment to biologically relevant ligands to form a tracer, ii) unperturbed biological activity of the target by the tracer, and iii) sufficient fluorescence lifetime to allow for appreciable rotation of the target. The following fluorophore- and condition-related factors are typically considered in designing the assay.
2.2.1. Range of the FA and shape of the fluorophore
A range of FA values (assay window) of the dyes indicate whether they might be suitable in anisotropy applications. The upper limit, known as the limiting anisotropy (ro), is 0.4 for one photon excitation and does not depend on nature of the flurophore.28 The lower limit of the anisotropy value (rmin) corresponds to the non-bound form and, all other conditions being equal, depends on the nature of the dye. The popularity of fluorescein-type molecules for anisotropy assays is due to the fact that they are spherical and show low anisotropy (rmin=0.021 for fluorescein). Therefore, fluoresceins have a large dynamic range, allowing macromolecules of a variety of molecular weights to be assayed. Molecules that are less symmetric than spheres exhibit multiple correlation times and complex time-dependent decays of anisotropy.31 For oblate molecules such as cyanine dyes, especially NIR cyanine dyes (see below), this lack of symmetry leads to larger initial anisotropies (rmin > 0.17) that limit the dynamic range.34 Large values for rmin is the major limitation to the use of near infrared (NIR) dyes, for example, in drug assays.35
2.2.2. Dyes for labeling
With the rise of biologics and other new classes of pharmaceuticals, their efficient fluorescent labeling has become a bottleneck. Currently, labeling is produced by individual researchers using ready-to-use kits with pre-measured quantities of standard dyes, buffers, and collection vials. This type of manual labeling leads to variability between the products and poor reproducibility of results. Automation of chemical labeling is expected to become a driving force behind this routine, error-prone synthetic procedure. In addition, biological molecules (i.e. proteins) and nanoparticles often present multiple labeling sites. The lack of tight control over the degree of labeling leads to quenching of the fluorophores and low sensitivity of the assay. A new generation of fluorophores that minimize this quenching effect, such as reported by Zhegalova et al,36 without affecting their biological targets will have to be developed.
2.2.3. Linker flexibility
Ideally, the fluorophore has to be tightly associated with the target in the bound state. However, this is difficult to achieve synthetically. Incorporation of a reactive group, such as N-hydroxysuccinimide (NHS) or maleimide, requires linkers that are often made from aliphatic chains. This leads to incomplete immobilization (wobbling) of the fluorophore on the target and, therefore, to higher local mobility of the fluorophore (“propeller effect”).28 This effect contributes to dynamic disorder of the complex and faster depolarization, complicating assay analysis.37
2.2.4. Brightness of the fluorophore
The FA value (r) of the mixture of bound and non-bound fluorophores is supposed to be directly related to its corresponding fractions (see a discussion of FA additivity in ref.28). However, the additivity is valid for fluorophores with significant brightness (defined as the product of molar absorptivity and quantum yield38) in both forms. Traditional probes with a rigid geometry in the excited state, such as fluoresceins, porphyrines, and some rhodamines, do not significantly change in brightness upon interaction with proteins, and therefore the additivity law can be safely applied. The situation is different, however, for fluorophores with flexible excited-state geometries, such as cyanine dyes that experience major changes in fluorescence behavior in response to the microenvironment,39 thus leading to potential error in calculating the fraction of bound ligand and, subsequently, Kd. The fraction has to be corrected using the following equation (Eq. 5):40
| Corrected fraction of bound ligand |
|
x – fraction of bound ligand rb – bound anisotropy rf – free anisotropy g – brightness enhancement factor (can be found from the total intensity of the emission, i.e., I// + 2I⊥ |
5 |
An exception is the cyanine dye Cy3B,41 which has a purposely rigidified skeleton geometry. Such rigidified dyes are not available in the NIR spectral range. For that, NIR fluorophores with low protein binding, i.e. highly water soluble NIR dyes with sufficient brightness in the free state, are preferred.34
2.2.5. Fluorescence lifetime
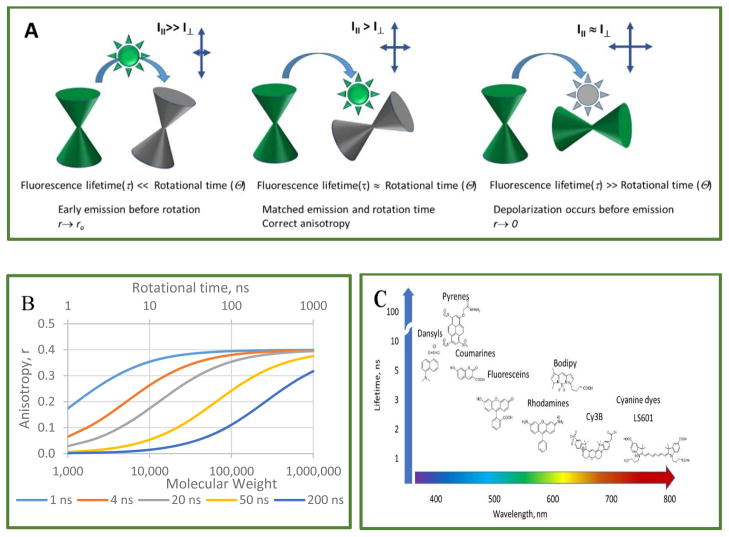
The fluorescence lifetime (FL) of a dye is another key parameter that needs to be considered in fluorophore selection. For the best sensitivity, FL of the dye has to match the rotational correlation time of the target. This time lies within a range of several hundred picoseconds for small molecules, such as free dyes in solution, to several tens of nanoseconds for large macrocomplexes. The effect of rotation on the r values is strongest when the fluorophore emits on the same time scale as the rotation. If the lifetime is much shorter than rotation (τ ≪ θrot), then fluorescence emission is over before the molecular rotation is complete, making determination of θrot and FA problematic. Slowly tumbling molecules in solution generate higher r values than molecules that are tumbling faster (Perrin equation, Eq. 3). Figure 2A illustrates this relationship. The shorter the lifetime, the less the fluorophore will rotate between the moments of absorption and emission, and the higher the r value will be, in extreme cases approaching fundamental anisotropy (r → r0). If FL is much longer than rotation time, τ ≫ θrot, then the fluorescent ligand will become completely depolarized before the emission ends, showing low anisotropy.
Figure 2.
Simulation of the relationship between molecular weight (MW), rotational time, and FA. A: Graphic interpretation. B: Simulations are shown for dyes with various fluorescence lifetimes: 1 ns (cyanine dyes), 4 ns (fluorescein and Alexa Fluor 488), 20 ns (dansyl dyes, pyrens) and a hypothetical dye at 200 ns. Simulations assume ro fundamental anisotropy = 0.4 and rigid attachment of dyes to spherical carriers. C: Common classes of fluorophores and specific examples used in FA assays.
The selection of a dye with the correct FL improves the sensitivity of the assay, which can be roughly defined as the range of FA values between the free and the bound fluorophore. Simulation of the relationship between r and the molecular weight of the target based on Eqs. 3 and 4 is shown in Figure 2B. Small round proteins (<10 kDa) with short rotational correlational times (θrot<10 ns), and fluorophores with low FL (1–4 ns) will work the best. For high molecular weight proteins (MW>100 kDa), fluorophores with a longer lifetime are preferable. Figure 2C shows the common classes of fluorophores used in FA assays for drug discovery. Long FL dyes, such as pyrenes,42, 43 emit in the UV spectral range. Longer wavelength fluorophores (visible, red, and NIR) tend to have shorter FLs. The recently emerged BODIPY fluorophores have unusually longer FLs than other dyes in the similar spectral range, making their anisotropy values sensitive to binding interactions over a larger molecular weight range. The search for efficient fluorophores with long (>20 ns) lifetimes and emitting in the red part of the spectrum has recently produced an interesting compound, ADOTA+, with a lifetime of 25 ns.44 Furthermore, for really large targets (MW>1,000 kDa), fluorophores with long lifetimes, such as Ru or Re-based emitters with lifetimes in the range of few hundred ns to microseconds, have been identified, thus extending the theoretically accessible molecular weight range.45
2.2.6. Light scattering
The FA of scattered light is equal to 1 and is the major source of false positives and false negatives in FA assays. Raman and Rayleigh scattering are two mechanisms responsible for the false results. The relative contribution of Raman scattering to the total fluorescence can be minimized by increasing the fluorophore concentration. In addition, increasing the fluorophore concentration can minimize the contribution of highly polarized stray light in the instrument that interferes with fluorescence anisotropy measurements. Rayleigh scattering becomes an issue if the buffer has insoluble particulates or when nanoparticles are involved. The best way to avoid both Raman and Rayleigh interference, and to increase the dynamic range of the assay, is to use longer wavelengths, as the intensities of both types of scattering are proportional to λ−4, where λ is the wavelength. To do that, additional testing might be necessary, such as r vs. the wavelength of emission (emission scan), which is implemented in most spectrophotometers to identify the conditions under which the r value is wavelength-independent. In the high-throughput experiments, examination of total fluorescence intensity (I// + 2I⊥) versus anisotropy plots should identify the scattering artifacts.
2.2.7. Spectral range, visible vs NIR
In typical FA experiments the tracer is kept at low nM, while the screening compounds are added at the μM range. At this level, even weakly absorbing and emitting compounds can easily overwhelm the signal from the tracer, resulting in false positives. Red-shifted tracers decrease false positives during the screening process, thus reducing interference from yellow/brown compounds. Although the vast majority of fluorescent probes utilized in FA tracers have excitation and emission in the visible range, 400–600 nm, fluorophores with longer wavelengths have been reported, but are largely limited to the Cy5 class of probes emitting in the red part of the spectra at 600–700 nm.12, 46, 47 Longer wavelength fluorophores may also help reduce light scattering caused by compound insolubility (aggregation) because the intensity of scattered light decreases with increasing wavelength.
NIR probes which absorb and emit light within the range of 700–900 nm have several benefits in biological studies, including negligible interference from endogenous fluorophores and lower hindrance from light scattering.48 However, the utility of NIR dyes in FA assays has been limited due to less than ideal anisotropy behavior stemming from the elongated structures of these dyes, as well as their short FLs (below 1.5 ns). These factors lead to a large anisotropy for free dyes (above 0.2 units),49 significantly reducing the dynamic range of an assay. Furthermore, commercially available NIR probes, such as Cy7 (General Electric), Alexa 750 (Life Technologies), and IR800CW (Li-COR), have relatively long linkers that further narrow the dynamic range. This leads to incomplete immobilization of the fluorescent probe on the macromolecule of interest and, therefore, to higher local mobility of the fluorophore via the propeller effect. To address these problems, Gustafson et al34 evaluated a number of NIR dyes with the goal of identifying a dye for FA in the NIR spectral range. The study identified a dye with low anisotropy in the free (non-bound) state and demonstrated the potential of NIR anisotropy in analytical applications.
2.2.8. Temperature
The major effect of temperature is on the rotational mobility of the fluorophore, where a higher temperature leads to a shorter rotational correlation time and, therefore, lower anisotropy (see Perrin eq. 3). An opposite effect of temperature on dyes is decreasing the fluorescence lifetime, especially in molecules with strong thermal sensitivity such as certain coumarines50 or cyanine dyes.51 Overall, the role of temperature is difficult predict,52 but needs to be controlled in order to generate reproducible results.
2.2.9. Polarity, pH, ionic strength
FA assays in drug discovery are generally considered to be homogenous. However, in any study the microenvironment around the fluorophore changes substantially. Association with or dissociation from a macromolecule results in an alteration of the microenvironment imposed by the nature of the binding site of the macromolecule or the interior of the nanoparticle. Local polarity, pH, and electrostatic interaction of the fluorophore with the macromolecular matrix all affect the FA values. Thus, the micropolarity of binding sites strongly affects the lifetime,39 creating multiple FLs that complicate anisotropy readings. Similarly, encapsulation of fluorophores in micelles, liposomes, or polymeric nanoparticles affects their lifetime and, hence, their anisotropy.53 Ionic strength does not directly affect the FA. However, very high affinity interactions (low-nM) are problematic for FA assays because they require the use of very low fluorophore concentrations, resulting in weak fluorescent signal. This problem can be alleviated if increasing the ionic strength can be used to reduce the affinity of the interaction.54
2.3. FA instrumentation
Current FA instrumentation can be divided into three types. Cuvette based instruments based on fluorescence spectrophotometers collect high-quality data, explore the properties of fluorophores, and test the design of the FA assay in homogeneous solution. Microplate readers, also based on a homogeneous format, are designed for HTS using validated assays. The newest class of the FA instruments are re-engineered scanning microscopes that are intended to collect high spatial data from heterogeneous systems, such as cells, thin tissues, or ultra-high density plates.
2.3.1. Spectrophotometer design
A standard configuration for instruments that measure FA includes an excitation source (Xe lamp, lasers), filters or monochromators, a set of rotatable polarizers, and detectors (photomultiplier tubes (PMT), CCD diode array cameras). A conventional geometry is based on an L-configuration. Measurement of FA in this geometry is a relatively slow process that is limited by rotation of the polarizers (a few seconds for a single-point measurement, up to a minute for several iterations). Higher end spectrophotometers offer a T-configuration that uses two emission polarizers with two identical detectors, thus avoiding rotation of the polarizers and speeding up data acquisition via simultaneous vertical and horizontal polarizer emission measurements.
Polarizers are optical devices that can isolate one direction of the electric vector and are the principal part of FA measurement that differentiates this method from other fluorescence-based techniques. The key parameter pertinent to FA is the extinction ratio, which is the ratio of the transmission of a linear polarized signal in the parallel configuration to the transmission intensity at the perpendicular orientation when the polarizer is rotated by 90°. Typical polarizers, such as film-based ones installed in many plate-readers, maintain a decent extinction ratio of 1000:1. Higher-end fluorimeters utilize polarizers made from calcite crystals (i.e. Glan-Thompson or Glan-Taylor) with a high 100,000:1 extinction ratio, suitable for high-precision anisotropy measurements. Calcite polarizers (in the uncoated version) also offer the broadest wavelength range from 350 nm to 2.3 micron, enabling measurements in the NIR region where (from the authors’ experience) most of the film polarizers are unsuitable. Calcite polarizers are also required for UV measurements.
2.3.2. High throughput plate readers
The geometry of the HTS systems is different from that of fluorimeters and reads from either from the top (more common) or bottom of the plate. Advanced plate readers, such as the Synergy Neo (BioTek), are equipped with a dual PMT read head (similar to a T- format) for ultra-fast polarization assays with no movement of the polarizers. Such a configuration enables the measurement of FA in 384-well plates in 11 seconds. A similar strategy is utilized in the PHERAstar (BMG Labtech), where the dual emission detection mode allows simultaneous recording of intensities that are parallel and perpendicular to the plane of excitation light.55 Instead of using a PMT as a detector, the ViewLux (PerkinElmer) high-speed plate reader is equipped with a top-of-the line EMCCD (Electron Multiplying Charge Coupled Device) camera. The advantage of this camera over PMT-based readers is that all of the wells of an entire plate are read simultaneously. A 1536-well plate can be read in almost the same time as a 96-well plate. Overall, the system is reported to read up to 200,000 samples per hour.
2.3.3. Z-factor
Few statistical tools are currently available to detect quality hits with a high degree of confidence.56, 57 A Z-factor,58 denoted as Z′, that reduces the amplitude and variability of the assay signal to only one parameter is commonly used in HTS, including FA, to judge the significance of the response. A Z′ between 0.5 and 1 corresponds to an excellent assay with no overlap between the negative and positive controls, while a Z′ between 0 and 0.5 denotes a marginal assay that might be improved by increasing the number of controls. The Z-factor parameter is used to compare different screening techniques in order to identify the best type of assay.59 In most published FA-based screenings Z′ lies in the range 0.50–0.70, although some can go to almost 0.8.60, 61 Assays with higher Z′ factors are preferable and it has been noted that taking the extra time and effort to optimize HTS assays for even slightly-improved Z′ factors reduces false positives and increases confidence in the screening data.62
2.4. Applications of steady-state FA: hERG cardiotoxicity primer
In the previous sections the authors provided background on steady-state FA, the most common and standard method of screening. In this section the authors will provide what they believe is an exemplary illustration of how this type of FA has changed the practice of today’s pharmaceutical industry. The following case demonstrates the rationale for and subsequent development of a steady-state FA assay for drugs causing Long QT Syndrome (LQTS), and describes different facets of the assay design.
LQTS is a cardiovascular disorder that causes fast, chaotic heartbeats, triggering seizures and in some cases sudden death. Many common drugs increase the occurrence of this condition, which has been the cause of several drugs being removed from the market (such as Vioxx in 2004 and Valdecoxib in 2005).63, 64 The hERG gene encodes a protein in the potassium ion channel that coordinates the heart’s beating. Strong binding of a drug to the hERG encoded protein (hERG blockade) poses potential risk to the patient. Because of this risk, hERG safety testing has been recommended by the FDA before drugs are administered in human trials.65
To avoid the significant cost associated with late stage drug development (a typical Phase I study for a single compound with a preclinical long QT signal was nearly US $1 million in 200364), pharmaceutical companies started prescreening drug-like libraries to eliminate LQTS inducing compounds at earlier stages. However, most of the established in vivo and in vitro techniques for evaluating drugs for hERG binding (such as the gold standard patch-clamp technique) were labor intensive and inadequate for screening the large number of compounds in libraries that emerged in the late 1990s and early 2000s.
With tighter regulation, rising costs, and frequent withdrawal of multibillion dollar drugs from the market, pharmaceutical companies started developing QT assays suitable for HTS. The first assays utilized membrane potential-sensitive dyes that rely on a change of fluorescence intensity,66 and were soon based on FA. In 2007, Pfizer reported the development of the first 384-well FA assay for high-throughput assessment of hERG biding during the lead optimization process.67
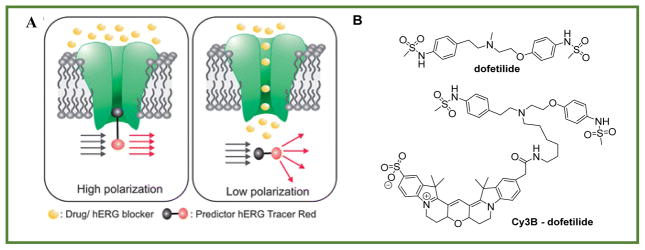
The principle of this assay, shown in Figure 3A, is that purified membrane expressing an hERG-encoded protein is first treated with a tracer composed of a ligand that binds tightly to the channel and a fluorescent tag connected to the ligand through a linker. Fluorophores associated with the large mass of membrane show high anisotropy values. The drugs of question are then added to the membrane, leading to displacement of the tracer from the membrane and a resulting in a decrease of the mass associated with the fluorophore, and therefore lower anisotropy values.
Figure 3.
FA based hERG assay. A: Principle of the assay (from ref.68 published by the Royal Society of Chemistry with permission), B: Structure of the tracer made from dofetilide, a drug with high affinity to hERG receptors, and a fluorescent dye, Cy3B.
The major challenge in the Pfizer study was to identify a fluorescent tracer. In displacement assays the tracer is a known, high-affinity ligand with a fluorescent tag that does not substantially disrupt the affinity of the receptor–ligand interaction. As mentioned earlier, the ideal dye should have negligible interaction with the receptor and provide a good dynamic range of anisotropy values. Screening a focus library of dyes and linkers, and performing computational studies, led to discovery of a novel fluorescent derivative of the drug dofetilide that has a high affinity to hERG channels. In the final tracer design, a fluorescent probe was attached through an aliphatic spacer to the central tertiary nitrogen of the dofetilide derivative (Figure 3B) that retained high affinity for the hERG channel. Electrophysiological patch clamp experiments confirmed that, similar to the drug itself, the tracer blocked the hERG channel current.69 Using this technique to screen the library for drugs with Z′>0.6 has been shown to be equivalent to more traditional radiometric methods and has the same predictive power as assessment by patch clamp technique.
Soon after the Pfizer study, Invitrogen (now Life Technologies) launched a Predictor hERG kit. This kit, with an optimized proprietary red dye,63 became the first commercial FA hERG assay, leading to the widespread acceptance of the method. Using this assay, several companies today (Life Technologies, Charles River Company, Cyprotex) provide HTS service to identify hERG incompatible compounds. More recently, in response to the insufficient amount of protein samples in the Predictor kit, Yildiz et al68 have developed a cell-free system using a biomimetic lipid membrane platform into which the hERG channel can be folded.
The hERG story is compelling on several levels. First, the hERG assay is an excellent example of how the pharmaceutical industry and academia have addressed the critical demand from medical practitioners and regulatory agencies. Second, understanding the mechanism of LQTS at the molecular level, as well as the reasons behind drug cardiotoxicity, and skillful application of synthetic chemistry in designing the tracer, led to the successful development and validation of the FA assay. Third, following commercialization of the assay, acceptance by regulatory agencies and continued improvement has made the anisotropy-based hERG assay a robust and reliable technique that set the standard for the future assay development.
3. Advanced concepts and applications
3.1. Time-resolved fluorescence anisotropy (TR-FA)
A combination of FL measurements with anisotropy provides an additional useful tool known as time-resolved fluorescence anisotropy (TR-FA) that can be described using Eq. 6 in Table 2. In TR-FA, the emission polarizer is toggled between vertical and horizontal positions to alternate the decay measurements. This method enables estimation of the rotational time (θrot) by determining the FA decay (Eq. 7) directly from the difference of the FL decays. These measurements are similar to recording fluorescence decays (either with the TCSPC (time correlated single photon counting) or frequency modulation, the two most common methods in FL measurements70). All rotational information of the fluorophore is found in the difference between the decays obtained at parallel and perpendicular orientations. When plotted, this difference provides an immediate visual check of the resulting r(t) and allows evaluation of the quality of the assay. For the best assay, the fluorescence lifetime decays and anisotropy decay should be on the same time scale. TR-FA is rather costly in terms of time, with currently limited potential in HTS (although strong efforts are being made to change this perception),71 and no commercial systems available for the screening, However, the method is indispensable in fluorophore selection to achieve the highest sensitivity in the developed assays, as well as in understanding the behavior of the fluorophores during cell imaging.72
Table 2.
Equations used in time-resolved fluorescence anisotropy measuremnts
| Measurement | Formula | Parameters | Eq. | |
|---|---|---|---|---|
| TR-FA decay |
|
Where t is the time after fluorophore excitation | 6 | |
| TR-FA fit (for one component) |
|
r∞ – anisotropy after complete depolarization (usually r∞ ≈ 0) | 7 |
3.2. FA and computational simulations of dye-conjugates motion
The data from FA and TR-FA experiments, similar to the established nuclear magnetic resonance, electron paramagnetic resonance, and neutron scattering methods, can be used to simulate and probe local protein and dye dynamics. These studies provide an insight into the motion of dye-conjugates, as well as predict the behavior of the fluorescently labeled macromolecules. For example, Schroder et al73 have applied molecular dynamic (MD) simulation to bacteriorhodopsin labeled with Alexa Fluor 488 to compare the dynamic of the labeled and non-labeled proteins. The perturbation caused by the dye on the dynamic of the proteins was found to be negligible. In a recent study, Kortkhonjia et al74 investigated MD of pyrene-labeled antibodies to probe their internal dynamics with the goal of identifying which amino acid residues contribute to the overall affinity of the antibody.
3.3. Two-photon excitation
Two-photon (2P) excitation is well-established for microscopy applications, although there are no commercially available plate readers capable of 2P excitation. The use of 2P excitation is expected to increase the polarization contrast of FA assays and provide a greater dynamic range for anisotropy. The limiting anisotropy r0 of 2P is higher than for one-photon (1P) excitation, reaching the level of 0.57 (instead of 0.4 for 1P).28 2P also enables a higher level of focusing on small volumes. Tirri et al75 illustrated the advantage of 2P excitation in an estrogen receptor ligand binding assay using a 1 μL volume without losing the sensitivity and quality of the assay, as judged by an almost identical and excellent Z′ = 0.85.
4. Novel applications: biologics, nanotechnology, and imaging for precision medicine
In the last decade, advances in recombinant technology have brought new biopharmaceuticals or biologics in an emerged class of drug-related compounds that were produced using biotechnology.76 Biologics are proteins (including antibodies) or nucleic acids (DNA, RNA, RNAi, or antisense oligonucleotides) that are used for therapeutic or in vivo diagnostic purposes. Similarly, new classes of nanoparticles with tunable properties have been developed for improved targeted treatments, novel imaging modalities, or both at the same time (theranostics).77 The rise of recombinant technology and nanotechnology thus opened the way for more effective personalized (precision) medicine78, 79 to diagnose earlier and cure rare genetic diseases and cancers, as well as Alzheimer’s and other diseases.
4.1. Aptamers
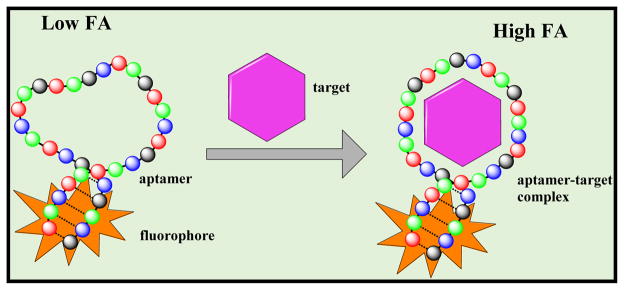
Among the new tools being developed by researchers is a class of nucleic acid probes called aptamers, which are ssDNA/RNA molecules selected to target a wide range of molecules.80 One special feature of aptamers is that they can be isolated by selection not only against individual disease-related proteins, but also against complex targets, such as whole cancer cells, without prior knowledge of the number and arrangement of proteins on the cellular surface. Aptamers of different classes (such as DNA, RNA, spiegelmers, and protein aptamers) produced via the synthetic process called SELEX (Systemic Evolution of Ligands by Exponential Enrichment) exhibit significant advantages relative to antibody therapeutics in terms of size, synthetic accessibility, and chemical modification.81 Because the specificity and binding affinity of aptamers can be modified, they are likely to replace antibodies in many biomedical, diagnostic, and imaging applications, although the current affinities of aptamers are typically orders of magnitude lower than that of antibodies. The first aptamer therapeutic was approved by the FDA in 2005 and by 2015 more than 25 aptamer-based therapeutics were undergoing clinical trials for treating macular degeneration, inflmmatory diseases, hematological disorders, cancers, and several other diseases.82 Reflecting the high potential for aptamers as therapeutics, several anisotropy assays for aptamer screening have been developed.83–85 One such approach is schematically illustrated in Figure 4.
Figure 4.
Schematic representation of aptamer-binding assay using FA. Fluorescently labeled aptamer using either an end labeled or intercalated fluorophore tumbles rapidly and shows low FA values. After binding the target, FA increases.
4.2. Nanoparticle in drug delivery, diagnostics and assay improvements
The increasing role of nanotechnology in drug delivery and diagnostics demands efficient methods for characterizing nanoparticles and optimizing nanoparticle design. FA is quite suitable for these studies and, in combination with FL, has often been used to measure the hydrodynamic size of nanoparticles, as well as their transformations, such as formation, degradation, and shape change.86, 87 FA has also been utilized to verify the conjugation efficiency of fluorophores to nanoparticles and as a quality control method to verify fluorescent labeling in diagnostic probes.34, 88 Using FA, Sahoo et al 89 confirmed the labeling of biocompatible magnetic nanoparticles with Rhodamine 110. In water this free rhodamine exhibited almost no FA (r ~ 0), but FA increased up to 0.4 when the dye was bound to nanoparticles. The effect of the magnetic field set an additional restriction on particle movement, leading to even higher anisotropy values, and further confirming the conjugation. Similarly, FA has validated the attachment of nanoparticles to fluorophore labeled DNA.90 Gustafson et al53 used FA to validate the encapsulation of an NIR imaging agent into porous nanocapsules designed for blood-triggered rapid release of cargo in emergency applications.
The low, close to zero initial anisotropy of quantum dots,91 long FL (10–100 ns),70 potentially low non-specific binding due to the surface modification and unmatched fluorescent brightness make quantum dots (QDs) very attractive for FA applications of drug screening. To develop anti-influenza small molecule drugs, an FA assay with targeted QDs that have high affinity for the viral protein hemagglutinin (HA) has been designed.92 In another study, Zhang et al93 developed FA sensitive QD based probes for H1N1 (swine flu) detection.
An interesting application of nanoparticles as enhancers of FA assays has recently attracted attention. There are a number of situations in which anisotropy values do not change significantly upon binding of the tracers to their targets. To enhance the sensitivity of the FA assay, nanomaterials (e.g. gold nanoparticles,94 graphene,95 and silica nanoparticles96) have been introduced into assay development in order to increase the binding-induced molecular weight change. For example, Liu et al97 have developed an FA signal amplification strategy by employing graphene oxide nanoparticles as the signal amplifier. Because of the large volume of the nanoparticle, the fluorophore exhibited high anisotropy values when bound to the graphene oxide surface. Conversely, low anisotropy values were observed when the fluorophore dissociated from the surface.
4.3. Fluorescence anisotropy with 2P imaging
One of the advantages of 2P excitation is minimal background and scattering. According to Rayleigh’s scattering law, the amount of scattering is proportional λ−4. If the wavelength is increased by a factor of 2 (i.e. from 500 nm to 1000 nm), the Rayleigh scattering (and Raman scattering, for that matter) is reduced by a factor of 16. Using 2P anisotropy imaging of actin-GFP, Vishwasrao et al98 have imaged the polymerization state of actin. The authors incorporated actin-GFP monomers into endogenous actin polymers and derived a relationship between the actin-GFP FA and the actin polymer fraction. This approach enabled quantitative monitoring of actin polymerization with high spatiotemporal resolution. In another 2P study, Dubach et al99 measured and mapped drug–target interaction at subcellular resolution in live cells and in real time. The authors measured intracellular distribution of a chemotherapeutic drug in live cells and within a tumor in living animals using BODIPY dye with a high 2P cross-section, thus enabling high-resolution spatial and temporal mapping of bound and unbound drug distribution.
Addressing the need for a super-resolution technique, Gould et al100 have modified fluorescence photoactivation localization microscopy (FPALM) with polarizers, (P-FPALM), for imaging single-molecule FA. This method allowed measurement of the orientation in biological specimens with a resolution below the diffraction limit.101 Similarly, Pavani et al102 used a super-resolution system to demonstrate 3D nanoscale imaging of the polarization-specific characteristics of single photoacivatable green fluorescent protein (PA-GFP) molecules within a cell. These and other super-resolution polarization techniques, (i.e. super resolution by polarization demodulation, SPoD103) in combination with HTS methods, will have a profound effect on live cell- and tissue-based assays in understanding drug mechanisms with higher precision.
5. Expert opinion
5.1. Current trend
FA is currently developing in several directions that can roughly be divided into three main areas: i) development of faster and more cost effective high-throughput systems, ii) integration with other modalities, and iii) design of novel assays for biologics and targeted nanoparticles. Advanced plate readers capable of screening large commercial libraries are steadily improving by having more flexibility, leading to better sensitivity and smaller reaction volumes. The latter is a key in improving cost-effectiveness. According to the instrument manufacturers, a typical price-tag for testing drug candidates decreases from $2.50 per well in 384-well plates to $0.40 in 1536 well and $0.10 in 3456 well plates.104 The current limit of commercial systems for FA applications is a 1536 well plate. Higher-density plates, with a working volume of less than 1 μL, require tight focusing to avoid interference from other wells. This interference prevents the widespread use of ultra-high density plates in FA applications, but can be minimized by using confocal or multiphoton excitation techniques.
Today, most commercial plate readers are equipped with millisecond time-gated capabilities to work with long-lived emitters, such as lanthanides. However, these systems are unsuitable to perform TR-FA on a nanosecond scale that reveals more about the mechanism of drug-target interaction. TR-FA is a promising, although slow, technique that is so far confined to a few relatively specialized laboratories focusing on photochemistry and biophysics, but not pharmaceuticals. Commercial implementation of fast, real-time TR-FA integrated high-throughput instrumentation, such as was recently developed by Alibha et al,71 will make TR-FA more accessible to pharmaceutical scientists.
Current HTS demands have placed more emphasis on cell-based assays. In response, a new breed of instrumentation combining FA and cell imaging using confocal point scanning systems, such as ImageXpress (Molecular Devices), and wide-field systems, such as the ScanR (Olympus), have emerged with high spatial (down to 100 nm) resolution. These are essentially confocal microscopes with polarizers which have been re-engineered to integrate with a plate reader.105 The utility of these tools, collectively known as high-content screening (HCS), goes beyond identification of the lead compound. In combination with many implemented features, the systems open up entire new possibilities all along the drug discovery pipeline, such as target validation, pathway prediction, toxicity, etc.106 The challenge in using these currently available medium-throughput systems lies in effective image management that includes image processing, feature extractions, and large data handling. Open-source software such as Fiji,107 several commercial packages, and newly developed algorithms108 that allow visualization of many variables and correlating FA data with other parameters are expected to be refined and optimized for HTS.
5.2. Comparison of FA to competing platforms in HTS
Despite the success in drug discovery, the FA-based technique experiences competitive pressure from other homogeneous assays, especially in high-throughput settings. Industry laboratories and academia core facilities are equipped today with multi-mode robotic plate readers featuring Fluorescence Anisotropy/Polarization, Time-Resolved Fluorescent Energy Transfer (TR-FRET), Enzyme Fragment Complementation (EFC), Electrochemical Luminescence (ECL), and other techniques. Selection of the right technology requires evaluations of many parameters, such as availability of the instrumentation, detection limits, dynamic range, scalability, cost, number of addition steps, robustness, and level of false positives and negatives. For example, assays based on chemiluminescent readouts (i.e. EFC), with virtually no signal background from the tested compounds, has much lower interference compared to FA. Similarly, the long fluorescence lifetime of the emitter (europium) in TR-FRET eliminates the compound interference, providing significant advantages over the FA technology.
Notable examples that gained popularity in the last decade include a re-engineered radiolabeling Scintillation Proximity Assay (SPA)109, 110 and the AlphaScreen/AlphaLisa platform111, 112 (where Alpha stands for amplified luminescent proximity homogeneous assay). Both platforms are highly sensitive, amenable to HTS, and often show extremely high Z-factors in the range of 0.8–0.95, while most of the values for the FA assays are within 0.5–0.7. The advantage of SPA over FA is that the radioligands more closely mimic the natural ligand than fluorescent labels. In some cases, for example in an androgen receptor assay, SPA has been shown to overcome the performance of FA (Z′ >0.85 for SPA and 0.6 for FA).59 However, having radioisotopes carry an additional cost relating to safety and waste disposal. The last two factors make the method difficult to implement in academia with open access core facilities. The advantage of a rapidly emerging Alpha technology is a high dynamic range (>200-fold),113 an order of magnitude higher than FA. Another benefit of Alpha is that the method can be used to assay large biocomplexes, due to the long distance travelled by singlet oxygen (up to 200 nm) without being consumed by the assay buffer. However, the large advantage of FA over this techniques is its ratiometric nature that minimizes artifacts due liquid handling.
Direct comparisons of the drug screening platforms are rare and often centered on a particular assay, such as cAMP114 or GCPR targets.115, 116 Based on the published results, information obtained from manufacturer web sites, and product brochures, as well as the references cited in the text, the differences are summarized in Table 3.
Table 3.
Summary of parameters to consider in selection of an assay technology
| FA | TR-FRET | EFC | AlphaLisa/AlphaScreen | SPA | |
|---|---|---|---|---|---|
| Radiation | No | No | No | No | Yes |
| Read-out | Fluorescence | Time gated emission | Chemilunescence | Fluorescence | Scintillation |
| Scalability, max | 1536 | 1536 | 1536 | 1536 | 384 |
| Standard plate readers | Yes | Yes | Yes | Yes | No |
| Compound interference | High, low with red dyes | Low | Low | Medium | High, low with red beads and plates |
| Sensitivity | Medium | Medium | High | High | High |
| Robustness, Z-factor | 0.5–0.75 | 0.7–0.8 | >0.7 | >0.8 | 0.5–0.8 |
5.3. Role of FA in complementary HTS studies
The evolution of drug discovery over the last 15 years puts a strong demand toward quality and reliability of HTS data,117 especially with the increase in size of the libraries (often to >1 million compounds), and cost associated with the screening. Such demand requires an integrated knowledge consisting of the assay design, diverse HTS instrumentation, chemistry, and chemometrics with the major goal of identifying the lead compound, and eliminating false positives (such as recently pointed PAINs that appear in every HTS assay118 or aggregates that might constitute up to 95% (!) of the active compounds119). As an established procedure, FA is expected to play an increasing role in validating the results from other assays, in a process known as orthogonal screening. Among recent studies, FA with SPA has been employed to identify antivirals for dengue virus120 and AlphaScreen was complemented with FA to find selective inhibitors for β-Catenin/Tcf.121
5.4. Outlook
Overall, FA is an established yet rapidly developing technique, recognized by academic institutions, the pharmaceutical industry, and regulatory agencies across the globe. The basic principles behind the technique are well understood. The technical problems encountered in working with small molecules in homogeneous assays such as insoluble compounds that scatter light, autofluorescence, and signal quenching from impurities are largely solved, and new challenges come from more complex biological molecules and nanoparticles. With that, FA will remain one of the major work-horse techniques in traditional drug discovery and will play an important role in the discovery and development of new generations of drugs and diagnostic agents. Based on the availability of the FA option in most plate readers, the wide variety of commercial assays, and relatively low cost, it is the authors’ opinion that FA should be a preferable choice for small substrates. The future of FA lies in new applications, in which biologics and targeted nanoparticles designed for early diagnostics and advanced treatments will play an increasing role in the pharmaceutical industry. The growth of biosimilars, biological products that are highly similar to other previously approved biological products, demands high level analytical tools, including FA, to confirm their identical structures and mode of action.122
ARTICLE HIGHLIGHTS BOX.
Fluorescence anisotropy (FA) is one of the major established methods accepted by industry and regulatory agencies for understanding the mechanisms of drug action and selecting drug candidates.
In the FA technique, an irradiated fluorophore is measured through a set of polarizers that are placed in parallel and perpendicular orientation to the incident light and in front of the detector.
To date, three main types of FA instrumentation exist, including cuvette based fluorescence spectrophotometers, microplate readers, and recently emerged scanning microscopes that often incorporate two-photon excitation to minimize background and increase the dynamic range of measurement.
A suitable fluorophore for FA applications should be absorbing and emitting in the red or even in near-infrared part of the spectra to avoid compound interference, have facile conjugation with the target without perturbing the target’s biological activity, as well as sufficient fluorescent lifetime to allow measurable rotation.
Identification of drugs using FA requires a well-designed fluorescent tracer. Its design is the most challenging step in drug discovery. Screening of drugs causing Long QT Syndrome (LQTS) illustrates the utility of FA in drug discovery.
The rise of biologics, nanotechnology, and imaging for precision (personalized) medicine are some of the new areas in which FA technology has potential applications.
Acknowledgments
The authors thank Sharon Bloch for helping with editing of the review.
Footnotes
FINANCIAL AND COMPETING INTEREST DISCLOSURE
This work was in part supported by National Cancer Institute (NCI)/National Institutes of Health (NIH) grant 1R21CA198419 and the Missouri EPSCor Program #IIA-1355406. The authors also acknowledge the Washington University Optical Spectroscopy Core Facility through NIH grant 1S10RR031621-01 for spectroscopy measurements and discussion. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Feng Y, Mitchison TJ, Bender A, et al. Multi-parameter phenotypic profiling: using cellular effects to characterize small-molecule compounds. Nat Rev Drug Discov. 2009;8(7):567–78. doi: 10.1038/nrd2876. [DOI] [PubMed] [Google Scholar]
- 2.Pomper MG, Lee JS. Small animal imaging in drug development. Curr Pharm Des. 2005;11(25):3247–72. doi: 10.2174/138161205774424681. [DOI] [PubMed] [Google Scholar]
- 3.Krutzik PO, Nolan GP. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat Methods. 2006;3(5):361–8. doi: 10.1038/nmeth872. [DOI] [PubMed] [Google Scholar]
- 4.Litwin VM, Marder P. Flow cytometry in drug discovery and development. Hoboken, N.J: Wiley; 2011. [Google Scholar]
- 5.Dufort S, Sancey L, Wenk C, et al. Optical small animal imaging in the drug discovery process. Biochim Biophys Acta Biomembr. 2010;1798(12):2266–73. doi: 10.1016/j.bbamem.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Bednar B, Ntziachristos V. Opto-acoustic imaging of drug discovery biomarkers. Curr Pharm Biotechnol. 2012;13(11):2117–27. doi: 10.2174/138920112802502079. [DOI] [PubMed] [Google Scholar]
- 7.Toseland CP. Fluorescent labeling and modification of proteins. J Chem Biol. 2013;6(3):85–95. doi: 10.1007/s12154-013-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinkeldam RW, Greco NJ, Tor Y. Fluorescent Analogs of Biomolecular Building Blocks: Design, Properties, and Applications. Chem Rev. 2010;110(5):2579–619. doi: 10.1021/cr900301e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeigler MB, Allen PB, Chiu DT. Probing rotational viscosity in synaptic vesicles. Biophys J. 2011;100(11):2846–51. doi: 10.1016/j.bpj.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt JA, Chung P-H, Kuimova MK, et al. Fluorescence Anisotropy of Molecular Rotors. Chemphyschem. 2011;12(3):662–72. doi: 10.1002/cphc.201000782. [DOI] [PubMed] [Google Scholar]
- 11.Newman M, Josiah S. Utilization of fluorescence polarization and time resolved fluorescence resonance energy transfer assay formats for SAR studies: Src kinase as a model system. J Biomol Screen. 2004;9(6):525–32. doi: 10.1177/1087057104264597. [DOI] [PubMed] [Google Scholar]
- 12.Turek-Etienne TC, Lei M, Terracciano JS, et al. Use of Red-Shifted Dyes in a Fluorescence Polarization AKT Kinase Assay for Detection of Biological Activity in Natural Product Extracts. J Biomol Screen. 2004;9(1):52–61. doi: 10.1177/1087057103259346. [DOI] [PubMed] [Google Scholar]
- 13.Davey AM, Walvick RP, Liu Y, et al. Membrane order and molecular dynamics associated with IgE receptor cross-linking in mast cells. Biophys J. 2007;92(1):343–55. doi: 10.1529/biophysj.106.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gough AH, Taylor DL. Fluorescence anisotropy imaging microscopy maps calmodulin binding during cellular contraction and locomotion. J Cell Biol. 1993;121(5):1095–107. doi: 10.1083/jcb.121.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitt JA, Matthews DR, Ameer-Beg SM, et al. Fluorescence lifetime and polarization-resolved imaging in cell biology. Curr Opin Biotechnol. 2009;20(1):28–36. doi: 10.1016/j.copbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Luchowski R, Sarkar P, Bharill S, et al. Fluorescence polarization standard for near infrared spectroscopy and microscopy. Appl Opt. 2008;47(33):6257–65. doi: 10.1364/ao.47.006257. [DOI] [PubMed] [Google Scholar]
- 17.Pu Y, Wang WB, Das BB, et al. Time-resolved fluorescence polarization dynamics and optical imaging of Cytate: a prostate cancer receptor-targeted contrast agent. Appl Opt. 2008;47(13):2281–9. doi: 10.1364/ao.47.002281. [DOI] [PubMed] [Google Scholar]
- 18.Baffou G, Kreuzer MP, Kulzer F, et al. Temperature mapping near plasmonic nanostructures using fluorescence polarization anisotropy. Opt Express. 2009;17(5):3291–98. doi: 10.1364/oe.17.003291. [DOI] [PubMed] [Google Scholar]
- 19.Bertz A, Ehlers J-E, Wöhl-Bruhn S, et al. Mobility of Green Fluorescent Protein in Hydrogel-Based Drug-Delivery Systems Studied by Anisotropy and Fluorescence Recovery After Photobleaching. Macromol Biosci. 2013;13(2):215–26. doi: 10.1002/mabi.201200325. [DOI] [PubMed] [Google Scholar]
- 20.Owicki JC. Fluorescence polarization and anisotropy in high throughput screening: perspectives and primer. J Biomol Screen. 2000;5(5):297–306. doi: 10.1177/108705710000500501. [DOI] [PubMed] [Google Scholar]
- 21.Smith DS, Eremin SA. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal Bioanal Chem. 2008;391(5):1499–507. doi: 10.1007/s00216-008-1897-z. [DOI] [PubMed] [Google Scholar]
- 22.Vickers CJ, González-Páez GE, Umotoy JC, et al. Small-Molecule Procaspase Activators Identified Using Fluorescence Polarization. Chem biochem. 2013;14(12):1419–22. doi: 10.1002/cbic.201300315. [DOI] [PubMed] [Google Scholar]
- 23.Baughman BM, Jake Slavish P, DuBois RM, et al. Identification of Influenza Endonuclease Inhibitors Using a Novel Fluorescence Polarization Assay. ACS Chem Biol. 2012;7(3):526–34. doi: 10.1021/cb200439z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haus P, Korbus M, Schröder M, et al. Identification of Selective Class II Histone Deacetylase Inhibitors Using a Novel Dual-Parameter Binding Assay Based on Fluorescence Anisotropy and Lifetime. J Biomol Screen. 2011;16(10):1206–16. doi: 10.1177/1087057111424605. [DOI] [PubMed] [Google Scholar]
- 25.Alontaga AY, li Y, Chen C-H, et al. Design of High Throughput Screening Assays and Identification of a SUMO1-Specific Small Molecule Chemotype Targeting the SUMO-Interacting Motif-Binding Surface. ACS Comb Sci. 2015;17(4):239–246. doi: 10.1021/co500181b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen B, McMurray C. Rapid method for measuring DNA binding to protein using fluorescence anisotropy. Protocol Exchange. 2009 [Google Scholar]
- 27.LiCata VJ, Wowor AJ. Applications of Fluorescence Anisotropy to the Study of Protein–DNA Interactions. In: John JC, William Detrich H III, editors. Methods in Cell Biology. Academic Press; 2008. pp. 243–62. [DOI] [PubMed] [Google Scholar]
- 28**.Jameson DM, Ross JA. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem Rev. 2010;110(5):2685–708. doi: 10.1021/cr900267p. The review provides detailed theory and historical overview of fluorescence anisotropy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yengo CM, Berger CL. Fluorescence Anisotropy and Resonance Energy Transfer: Powerful Tools for Measuring Real Time Protein Dynamics in a Physiological Environment. Curr Opin Pharmacol. 2010;10(6):731–37. doi: 10.1016/j.coph.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lea WA, Simeonov A. Fluorescence Polarization Assays in Small Molecule Screening. Expert Opin Drug Discov. 2011;6(1):17–32. doi: 10.1517/17460441.2011.537322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakowicz JR. Principles of fluorescence spectroscopy. 3. New York: Springer; 2006. p. 954. [Google Scholar]
- 32.DeRose PC, Resch-Genger U. Recommendations for fluorescence instrument qualification: the new ASTM Standard Guide. Anal Chem. 2010;82(5):2129–33. doi: 10.1021/ac902507p. [DOI] [PubMed] [Google Scholar]
- 33.Perrin F. The fluorescence of solutions: Molecular induction, polarization and duration of emission and photochemistry. Ann Phys. 1929;12:169–275. [Google Scholar]
- 34*.Gustafson TP, Cao Q, Achilefu S, et al. Defining a polymethine dye for fluorescence anisotropy applications in the near-infrared spectral range. Chemphyschem. 2012;13(3):716–23. doi: 10.1002/cphc.201100916. first NIR dye for anisotropy/polarization studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szmacinski H, Lakowicz JR. Depolarization of surface-enhanced fluorescence: an approach to fluorescence polarization assays. Anal Chem. 2008;80(16):6260–6. doi: 10.1021/ac8003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhegalova NG, He S, Zhou H, et al. Minimization of self-quenching fluorescence on dyes conjugated to biomolecules with multiple labeling sites via asymmetrically charged NIR fluorophores. Contrast Media Mol Imaging. 2014;9(5):355–62. doi: 10.1002/cmmi.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irving M. Steady-state polarization from cylindrically symmetric fluorophores undergoing rapid restricted motion. Biophys J. 1996;70(4):1830–5. doi: 10.1016/S0006-3495(96)79748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurth C, Pauli J, Lochmann C, et al. Integrating sphere setup for the traceable measurement of absolute photoluminescence quantum yields in the near infrared. Anal Chem. 2012;84(3):1345–52. doi: 10.1021/ac2021954. [DOI] [PubMed] [Google Scholar]
- 39.Berezin MY, Lee H, Akers W, et al. Ratiometric analysis of fluorescence lifetime for probing binding sites in albumin with near-infrared fluorescent molecular probes. Photochem Photobiol. 2007;83(6):1371–8. doi: 10.1111/j.1751-1097.2007.00173.x. [DOI] [PubMed] [Google Scholar]
- 40.Jameson D, Mocz G. Fluorescence Polarization/Anisotropy Approaches to Study Protein-Ligand Interactions. In: Ulrich Nienhaus G, editor. Protein-Ligand Interactions. Humana Press; 2005. pp. 301–22. [DOI] [PubMed] [Google Scholar]
- 41.Cooper M, Ebner A, Briggs M, et al. Cy3B: improving the performance of cyanine dyes. J Fluoresc. 2004;14(2):145–50. doi: 10.1023/b:jofl.0000016286.62641.59. [DOI] [PubMed] [Google Scholar]
- 42.Araya Y, Kasuga J-i, Toyota K, et al. Structure-Based Design and synthesis of fluorescent PPARalpha/delta co-agonist and its application as a probe for fluorescent polarization pssay of PPARdelta ligands. Chem Pharm Bull (Tokyo) 2008;56(9):1357–59. doi: 10.1248/cpb.56.1357. [DOI] [PubMed] [Google Scholar]
- 43.Matveeva EG, Rudolph A, Moll JR, et al. Structure-selective anisotropy assay for amyloid Beta oligomers. ACS Chem Neurosci. 2012;3(11):982–7. doi: 10.1021/cn3001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sørensen TJ, Thyrhaug E, Szabelski M, et al. Azadioxatriangulenium (ADOTA(+)): A long fluorescence lifetime fluorophore for large biomolecule binding assay. Methods Appl Fluoresc. 2013;1(2):025001. doi: 10.1088/2050-6120/1/2/025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo X-Q, Castellano FN, Li, et al. Use of a Long-Lifetime Re(I) Complex in Fluorescence Polarization Immunoassays of High-Molecular-Weight Analytes. Anal Chem. 1998;70(3):632–37. doi: 10.1021/ac970827k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turek-Etienne TC, Small EC, Soh SC, et al. Evaluation of fluorescent compound interference in 4 fluorescence polarization assays: 2 kinases, 1 protease, and 1 phosphatase. J Biomol Screen. 2003;8(2):176–84. doi: 10.1177/1087057103252304. [DOI] [PubMed] [Google Scholar]
- 47.Gerega A, Zolek N, Soltysinski T, et al. Wavelength-resolved measurements of fluorescence lifetime of indocyanine green. J Biomed Opt. 2011;16(6):067010. doi: 10.1117/1.3593386. [DOI] [PubMed] [Google Scholar]
- 48.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol. 2010;14(1):71–9. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Rurack K, Spieles M. Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600–1000 nm. Anal Chem. 2011;83(4):1232–42. doi: 10.1021/ac101329h. [DOI] [PubMed] [Google Scholar]
- 50.Zhegalova NG, Aydt A, Wang ST, et al. Molecular thermometers for potential applications in thermal ablation procedures. Proc SPIE. 2013 p. 85960I-60I-9. [Google Scholar]
- 51.Zhegalova NG, Dergunov SA, Wang ST, et al. Design of Fluorescent Nanocapsules as Ratiometric Nanothermometers. Chem Eur J. 2014;20(33):10292–97. doi: 10.1002/chem.201402828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumbhakar M, Mukerjee T, Pal H. Temperature Effect on the Fluroescence Anisotropy Decay Dynamics of Coumarin-153 Dye in Triton-X-100 and Brij-35 Miscellar Solutions. Photochem Photobiol. 2005;81(3):588–94. doi: 10.1562/2004-10-12-RA-341. [DOI] [PubMed] [Google Scholar]
- 53.Gustafson TP, Dergunov SA, Akers WJ, et al. Blood triggered rapid release porous nanocapsules. RSC Advances. 2013;3(16):5547–5555. doi: 10.1039/C3RA22693J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson BJ, Larkin C, Guja K, et al. Using Fluorophore-labeled Oligonucleotides to Measure Affinities of Protein-DNA Interactions. Meth Enzymol. 2008;450:253–72. doi: 10.1016/S0076-6879(08)03412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veiksina S, Kopanchuk S, Rinken A. Budded baculoviruses as a tool for a homogeneous fluorescence anisotropy-based assay of ligand binding to G protein-coupled receptors: The case of melanocortin 4 receptors. Biochim Biophys Acta Biomembr. 2014;1838(1 Part B):372–81. doi: 10.1016/j.bbamem.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Malo N, Hanley JA, Cerquozzi S, et al. Statistical practice in high-throughput screening data analysis. Nat Biotech. 2006;24(2):167–75. doi: 10.1038/nbt1186. [DOI] [PubMed] [Google Scholar]
- 57.Sui Y, Wu Z. Alternative Statistical Parameter for High-Throughput Screening Assay Quality Assessment. J Biomol Screen. 2007;12(2):229–34. doi: 10.1177/1087057106296498. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J-H, Chung TDY, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 59.Feau C, Arnold LA, Kosinski A, et al. A high-throughput ligand competition binding assay for the androgen receptor and other nuclear receptors. J Biomol Screen. 2009;14(1):43–8. doi: 10.1177/1087057108326662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souza-Fagundes EM, Frank AO, Feldkamp MD, et al. A high-throughput fluorescence polarization anisotropy assay for the 70N domain of replication protein A. Anal Biochem. 2012;421(2):742–9. doi: 10.1016/j.ab.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahapatra L, Mao C, Andruska N, et al. High-throughput fluorescence anisotropy screen for inhibitors of the oncogenic mRNA binding protein, IMP-1. J Biomol Screen. 2014;19(3):427–36. doi: 10.1177/1087057113499633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahlin JL, Walters MA. The essential roles of chemistry in high-throughput screening triage. Future Med Chem. 2014;6(11):1265–90. doi: 10.4155/fmc.14.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63**.Piper DR, Duff SR, Eliason HC, et al. Development of the Predictor hERG Fluorescence Polarization Assay Using a Membrane Protein Enrichment Approach. Assay Drug Dev Technol. 2008;6(2):213–23. doi: 10.1089/adt.2008.137. describes design and validation of current commercially available and commonly used FA assays. [DOI] [PubMed] [Google Scholar]
- 64.Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat Rev Drug Discov. 2003;2(6):439–47. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 65.US Food and Drug Administration. Guidance for industry: S7B nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals, 2005. 2006 [PubMed] [Google Scholar]
- 66.Dorn A, Hermann F, Ebneth A, et al. Evaluation of a High-Throughput Fluorescence Assay Method for hERG Potassium Channel Inhibition. J Biomol Screen. 2005;10(4):339–47. doi: 10.1177/1087057104272045. [DOI] [PubMed] [Google Scholar]
- 67.Deacon M, Singleton D, Szalkai N, et al. Early evaluation of compound QT prolongation effects: a predictive 384-well fluorescence polarization binding assay for measuring hERG blockade. J Pharmacol Toxicol Methods. 2007;55(3):238–47. doi: 10.1016/j.vascn.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Arslan Yildiz A, Kang C, Sinner EK. Biomimetic membrane platform containing hERG potassium channel and its application to drug screening. Analyst. 2013;138(7):2007–12. doi: 10.1039/c3an36159d. [DOI] [PubMed] [Google Scholar]
- 69**.Singleton DH, Boyd H, Steidl-Nichols JV, et al. Fluorescently Labeled Analogues of Dofetilide as High-Affinity Fluorescence Polarization Ligands for the Human Ether-a-go-go-Related Gene (hERG) Channel. J Med Chem. 2007;50(13):2931–41. doi: 10.1021/jm0700565. first application of the FA in QT drug toxicity developed by Pfizer. [DOI] [PubMed] [Google Scholar]
- 70**.Berezin MY, Achilefu S. Fluorescence lifetime measurements and biological imaging. Chem Rev. 2010;110(5):2641–84. doi: 10.1021/cr900343z. a comprehensive review of fluorescence lifetime measurements and imaging techniques in biological applications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alibhai D, Kelly DJ, Warren S, et al. Automated fluorescence lifetime imaging plate reader and its application to Förster resonant energy transfer readout of Gag protein aggregation. J Biophotonics. 2013;6(5):398–408. doi: 10.1002/jbio.201200185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suhling K, Siegel J, Lanigan PMP, et al. Time-resolved fluorescence anisotropy imaging appliedto live cells. Optics Letters. 2004;29(6):584–86. doi: 10.1364/ol.29.000584. excellent application of fluorescence lifetime anisotropy. [DOI] [PubMed] [Google Scholar]
- 73.Schroder GF, Alexiev U, Grubmuller H. Simulation of fluorescence anisotropy experiments: probing protein dynamics. Biophys J. 2005;89(6):3757–70. doi: 10.1529/biophysj.105.069500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kortkhonjia E, Brandman R, Zhou JZ, et al. Probing antibody internal dynamics with fluorescence anisotropy and molecular dynamics simulations. MAbs. 2013;5(2):306–22. doi: 10.4161/mabs.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tirri ME, Huttunen RJ, Toivonen J, et al. Two-Photon Excitation in Fluorescence Polarization Receptor-Ligand Binding Assay. J Biomol Screen. 2005;10(4):314–19. doi: 10.1177/1087057104273334. [DOI] [PubMed] [Google Scholar]
- 76.Walsh G. Biopharmaceuticals: biochemistry and biotechnology. John Wiley & Sons; 2013. [Google Scholar]
- 77.Berezin MY. Nanotechnology for biomedical imaging and diagnostics : from nanoparticle design to clinical applications. Hoboken, New Jersey: Wiley; 2015. [Google Scholar]
- 78.Katsnelson A. Momentum grows to make ‘personalized’ medicine more ‘precise’. Nat Med. 2013;19(3):249–49. doi: 10.1038/nm0313-249. [DOI] [PubMed] [Google Scholar]
- 79.Dolsten M, Søgaard M. Precision medicine: an approach to R&D for delivering superior medicines to patients. Clin Transl Med. 2012;1:7–7. doi: 10.1186/2001-1326-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang X, Tan W. Aptamers Generated from Cell-SELEX for Molecular Medicine : A Chemical Biology Approach. Acc Chem Res. 2010;43(1):48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9(7):537–50. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sundaram P, Kurniawan H, Byrne ME, et al. Therapeutic RNA aptamers in clinical trials. Eur J Pharm Sci. 2013;48(1–2):259–71. doi: 10.1016/j.ejps.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Q, Lv Q, Wang H. Aptamer fluorescence anisotropy sensors for adenosine triphosphate by comprehensive screening tetramethylrhodamine labeled nucleotides. Biosensors and Bioelectronics. 2015;70(0):188–93. doi: 10.1016/j.bios.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 84.Zhu Z, Ravelet C, Perrier S, et al. Single-Stranded DNA Binding Protein-Assisted Fluorescence Polarization Aptamer Assay for Detection of Small Molecules. Anal Chem. 2012;84(16):7203–11. doi: 10.1021/ac301552e. [DOI] [PubMed] [Google Scholar]
- 85.Gokulrangan G, Unruh JR, Holub DF, et al. DNA Aptamer-Based Bioanalysis of IgE by Fluorescence Anisotropy. Anal Chem. 2005;77(7):1963–70. doi: 10.1021/ac0483926. [DOI] [PubMed] [Google Scholar]
- 86.Apperson K, Karolin J, Martin RW, et al. Nanoparticle metrology standards based on the time-resolved fluorescence anisotropy of silica colloids. Meas Sci Technol. 2009;20(2):025310. [Google Scholar]
- 87.Schwartz S, Fixler D, Popovtzer R, et al. Fluorescence life-time imaging and steady state polarization for examining binding of fluorophores to gold nanoparticles. J Biophotonics. 2015 doi: 10.1002/jbio.201400136. [DOI] [PubMed] [Google Scholar]
- 88.Kumar R, Roy I, Ohulchanskyy TY, et al. Covalently Dye-Linked, Surface-Controlled, and Bioconjugated Organically Modified Silica Nanoparticles as Targeted Probes for Optical Imaging. ACS nano. 2008;2(3):449–56. doi: 10.1021/nn700370b. [DOI] [PubMed] [Google Scholar]
- 89.Sahoo Y, Goodarzi A, Swihart MT, et al. Aqueous Ferrofluid of Magnetite Nanoparticles: Fluorescence Labeling and Magnetophoretic Control. J Phys Chem B. 2005;109(9):3879–85. doi: 10.1021/jp045402y. [DOI] [PubMed] [Google Scholar]
- 90.Yang R, Jin J, Chen Y, et al. Carbon Nanotube-Quenched Fluorescent Oligonucleotides: Probes that Fluoresce upon Hybridization. J Am Chem Soc. 2008;130(26):8351–58. doi: 10.1021/ja800604z. [DOI] [PubMed] [Google Scholar]
- 91.Nelson K, Winter P, Shokeen M, et al. Nanotechnology for Biomedical Imaging and Diagnostics. John Wiley & Sons, Inc; 2014. Nanoparticles for Bioimaging; pp. 151–92. [Google Scholar]
- 92.Okamatsu M, Feng F, Ohyanagi T, et al. Fluorescence polarization-based assay using N-glycan-conjugated quantum dots for screening in hemagglutinin blockers for influenza A viruses. J Virol Methods. 2013;187(2):390–94. doi: 10.1016/j.jviromet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J, Tian J, He Y, et al. Protein-binding aptamer assisted signal amplification for the detection of influenza A (H1N1) DNA sequences based on quantum dot fluorescence polarization analysis. Analyst. 2013;138(17):4722–27. doi: 10.1039/c3an00830d. [DOI] [PubMed] [Google Scholar]
- 94.Ye B-C, Yin B-C. Highly Sensitive Detection of Mercury(II) Ions by Fluorescence Polarization Enhanced by Gold Nanoparticles. Angew Chem Int Ed Engl. 2008;47(44):8386–89. doi: 10.1002/anie.200803069. [DOI] [PubMed] [Google Scholar]
- 95.Huang Y, Liu X, Zhang L, et al. Nicking enzyme and graphene oxide-based dual signal amplification for ultrasensitive aptamer-based fluorescence polarization assays. Biosensors and Bioelectronics. 2015;63(0):178–84. doi: 10.1016/j.bios.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 96.Yue Q, Shen T, Wang L, et al. A convenient sandwich assay of thrombin in biological media using nanoparticle-enhanced fluorescence polarization. Biosensors and Bioelectronics. 2014;56(0):231–36. doi: 10.1016/j.bios.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 97.Liu J, Wang C, Jiang Y, et al. Graphene Signal Amplification for Sensitive and Real-Time Fluorescence Anisotropy Detection of Small Molecules. Anal Chem. 2013;85(3):1424–30. doi: 10.1021/ac3023982. [DOI] [PubMed] [Google Scholar]
- 98.Vishwasrao HD, Trifilieff P, Kandel ER. In vivo imaging of the actin polymerization state with two-photon fluorescence anisotropy. Biophys J. 2012;102(5):1204–14. doi: 10.1016/j.bpj.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dubach JM, Vinegoni C, Mazitschek R, et al. In vivo imaging of specific drug–target binding at subcellular resolution. Nat Commun. 2014;5:3946. doi: 10.1038/ncomms4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hess ST, Girirajan TPK, Mason MD. Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys J. 2006;91(11):4258–72. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gould TJ, Gunewardene MS, Gudheti MV, et al. Nanoscale imaging of molecular positions and anisotropies. Nat Meth. 2008;5(12):1027–30. doi: 10.1038/nmeth.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavani SRP, DeLuca JG, Piestun R. Polarization sensitive, three-dimensional, single-molecule imaging of cells with a double-helix system. Opt Express. 2009;17(22):19644–55. doi: 10.1364/OE.17.019644. [DOI] [PubMed] [Google Scholar]
- 103.Hafi N, Grunwald M, van den Heuvel LS, et al. Fluorescence nanoscopy by polarization modulation and polarization angle narrowing. Nat Methods. 2014;11(5):579–84. doi: 10.1038/nmeth.2919. [DOI] [PubMed] [Google Scholar]
- 104.Aurora High Performance Microplates. Website of Brooks Automation, Inc. http://www.brooks.com/products/life-science-systems/consumables/aurora-high-performance-microplates.
- 105.Matthews DR, Fruhwirth GO, Weitsman G, et al. A Multi-Functional Imaging Approach to High-Content Protein Interaction Screening. PloS One. 2012;7(4):e33231. doi: 10.1371/journal.pone.0033231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buchser W, Collins M, Garyantes T, et al. Assay Development Guidelines for Image-Based High Content Screening, High Content Analysis and High Content Imaging. In: Sittampalam GS, Gal-Edd N, Arkin M, et al., editors. Assay Guidance Manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2012. [PubMed] [Google Scholar]
- 107.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Meth. 2012;9(7):676–82. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sailem HZ, Sero JE, Bakal C. Visualizing cellular imaging data using PhenoPlot. Nat Commun. 2015;6 doi: 10.1038/ncomms6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bosworth N, Towers P. Scintillation proximity assay. Nature. 1989;341(6238):167–68. doi: 10.1038/341167a0. [DOI] [PubMed] [Google Scholar]
- 110.Glickman JF, Schmid A, Ferrand S. Scintillation Proximity Assays in High-Throughput Screening. Assay Drug Dev Technol. 2008;6(3):433–55. doi: 10.1089/adt.2008.135. [DOI] [PubMed] [Google Scholar]
- 111.Eglen RM, Reisine T, Roby P, et al. The Use of AlphaScreen Technology in HTS: Current Status. Curr Chem Genomics. 2008;1:2–10. doi: 10.2174/1875397300801010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cochran JN, Diggs PV, Nebane NM, et al. AlphaScreen HTS and live-cell bioluminescence resonance energy transfer (BRET) assays for identification of Tau-Fyn SH3 interaction inhibitors for Alzheimer disease. J Biomol Screen. 2014;19(10):1338–49. doi: 10.1177/1087057114547232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ungermannova D, Lee J, Zhang G, et al. High-throughput screening AlphaScreen assay for identification of small-molecule inhibitors of ubiquitin E3 ligase SCFSkp2-Cks1. J Biomol Screen. 2013;18(8):910–20. doi: 10.1177/1087057113485789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams C. cAMP detection methods in HTS: selecting the best from the rest. Nat Rev Drug Discov. 2004;3(2):125–35. doi: 10.1038/nrd1306. [DOI] [PubMed] [Google Scholar]
- 115.Thomsen W, Frazer J, Unett D. Functional assays for screening GPCR targets. Curr Opin Biotechnol. 2005;16(6):655–65. doi: 10.1016/j.copbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 116.Zhang R, Xie X. Tools for GPCR drug discovery. Acta Pharm Sinic. 2012;33(3):372–84. doi: 10.1038/aps.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mayr LM, Bojanic D. Novel trends in high-throughput screening. Curr Opin Pharmacol. 2009;9(5):580–88. doi: 10.1016/j.coph.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 118**.Baell J, Walters MA. Chemistry: Chemical con artists foil drug discovery. Nature. 2014;513(7519):481–3. doi: 10.1038/513481a. describes important and often overlooked limitations of high-throughput screening. [DOI] [PubMed] [Google Scholar]
- 119.Feng BY, Simeonov A, Jadhav A, et al. A High-Throughput Screen for Aggregation-Based Inhibition in a Large Compound Library. J Med Chem. 2007;50(10):2385–90. doi: 10.1021/jm061317y. [DOI] [PubMed] [Google Scholar]
- 120.Lim SP, Wang Q-Y, Noble CG, et al. Ten years of dengue drug discovery: Progress and prospects. Antiviral Res. 2013;100(2):500–19. doi: 10.1016/j.antiviral.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 121.Catrow JL, Zhang Y, Zhang M, et al. Discovery of Selective Small-Molecule Inhibitors for the beta-Catenin/T-Cell Factor Protein-Protein Interaction through the Optimization of the Acyl Hydrazone Moiety. J Med Chem. 2015 doi: 10.1021/acs.jmedchem.5b00223. [DOI] [PubMed] [Google Scholar]
- 122.Møller EH, Fano M, van de Weert M, et al. Comparability of protein pharmaceuticals and the suitability of methods for secondary structure analysis. Pharmaceutical Technology Europe. 2006 Jul;:38–42. [Google Scholar]