Abstract
Autism spectrum disorders (ASDs) affect approximately 1 in 110 children in the United States. This report profiles fetal-brain reactive autoantibodies of a large cohort of mothers of children with autism and controls, yielding significant associations between the presence of IgG reactivity to fetal brain proteins at 37 and 73 kDa and a childhood diagnosis of full autism (p = 0.0005), which also correlated with lower expressive language scores (p = 0.005). Additionally, we report on reactivity to proteins at 39 and 73 kDa, which correlated with the broader diagnosis of ASD (p = 0.0007) and increased irritability on the Aberrant Behavioral Checklist (p = 0.05). This study provides evidence of multiple patterns of reactivity to fetal brain proteins by maternal antibodies associated with ASD and specific childhood behavioral outcomes.
Keywords: Autism, Maternal antibodies, Autoantibodies, Fetal brain
Introduction
Autism spectrum disorders (ASDs) comprise a range of human behavioral disorders manifesting in childhood with a prevalence of approximately 1 in 110 children (Kogan et al. 2009). Individuals with ASDs are clinically characterized by a highly variable constellation of combined impairments in sociability, verbal and non-verbal communication, and repetitive, stereotypical behaviors and restricted interests (APA 2000). These symptoms are usually diagnosed in early childhood, supporting the current view of a pre-natal or early post-natal etiology.
The lack of identified causative factors in the majority of autism cases, coupled with the increase in reported incidence of ASDs over the past two decades, have broadened the scope of investigation for etiological mechanisms in ASDs. Twin studies demonstrating high heritability estimates of approximately 0.8 and strong concordance rates among monozygotic (mz) twins (Taniai et al. 2008) support a role for heritable genetic influences on autism development, but the inconsistency of findings in numerous independent studies (reviewed in (Abrahams and Geschwind 2008)) imply complex etiologies. An outcome of autism may therefore arise from a combination of genetic predisposition and some other neurodevelopmetal perturbation, i.e., a gene-by-environment (G × E) interaction. Maternal immune factors which cross the placenta during pregnancy have the potential to exert such an environmental effect.
Advances in the understanding of immune system involvement in neurodevelopment (Boulanger and Shatz 2004; Stevens et al. 2007), as well as findings of increased autoimmunity and altered immune regulation in patients with autism and their family members (Atladottir et al. 2009), have fostered considerable effort towards elucidating roles of immune system dysregulation in autism. The mechanism proposed by our group and others involves the gestational transfer of maternal IgG antibodies that bind to fetal brain proteins and may thus alter fetal neurodevelopment (Braunschweig et al. 2008). In previous reports, findings of autoantibodies recognizing fetal brain have been shown in subsets of asymptomatic mothers of children with ASDs (Singer et al. 2008), including a retrospective report identifying these autoantibodies in mid-pregnancy blood draws from women who went on to deliver children who were later diagnosed with ASDs (Croen et al. 2008). Furthermore, when purified IgG from mothers of children with autism was administered to pregnant Rhesus macaque monkeys in a pilot study, behavioral stereotypies reminiscent of those associated with autism were observed in the offspring (Martin et al. 2008).
An etiologic role for maternal antibodies in ASDs is plausible due to the dynamics of the gestational transfer of maternal IgG during pregnancy. In humans, maternal antibodies are detected in fetal circulation as early as 13 weeks of gestation, and their concentration increases to approximately 50% of maternal levels by 30 weeks of gestation (Simister 2003). Due to facilitated transport across the placenta by the neonatal Fc receptor, circulating IgG levels in full-term infants exceeds those in maternal circulation (Malek et al. 1996). The developing blood–brain barrier, which regulates passage of antibodies into the CNS, is actively changing during fetal neurodevelopment and is variably permissive to IgG molecules during gestation.
In this study, we sought to investigate the anti-fetal brain antibody profiles of an expanded cohort of 560 mothers of both cases and controls, and explore in greater detail the temporal expression of the antigens recognized by those antibodies in the Rhesus macaque. In addition, we examined a subset of these mothers who were analyzed previously for reactivity towards pooled human fetal brain protein, to confirm their reactivity to Rhesus brain as well as to proteins from several human fetal CNS regions and non-CNS tissue. Furthermore, we examined distinct maternal anti-fetal brain autoantibody profiles for their association with different developmental and behavioral presentations in affected children.
Methods
Study Subjects
This population-based case control study examined plasma from 560 mothers who have children with full autism (AU; n = 204), children with broader-phenotype autism spectrum disorders (ASD; n = 71), children with non-autism developmental delay (DD; n = 102) or typically developing children (TD; n = 183) (Table 1) enrolled through the Center for Children's Environmental Health (CCEH) in the CHARGE (Childhood Autism Risk from Genetics and Environment) study at the M.I.N.D. Institute at the University of California, Davis (Hertz-Picciotto et al. 2006). A sub-population of these mothers (AU; n = 61, DD; n = 40, TD; n = 62) were analyzed in a previous publication (Braunschweig et al. 2008), and were included for comparison of antibody reactivity between human fetal-brain protein and Rhesus macaque fetal brain protein. These mothers are referred to as the “original cohort”. Mothers recruited since our original study (AU; n = 143, ASD; n = 71, DD; n = 62, TD; n = 121) are referred to as the “new cohort.” Case and control mothers included in this study were matched for age and time since birth of the index child. Recruitment for the CHARGE study was unbiased towards any medical factors, and all children enrolled met the following criteria: (a) age 24–60 months at enrollment, (b) live with at least one biological parent, (c) have a parent who speaks either English or Spanish, (d) born in California, and (e) reside in the catchment areas of a specified list of regional centers in Northern California. Study subject demographics are shown in Table 1. This study followed the ethical guidelines of the most recent Declaration of Helsinki (Edinburgh 2000), and was approved by the institutional review boards at the University of California, Davis, and The State of California Department of Developmental Services. Informed consent was obtained prior to participation.
Table 1. Demographic factors for study population.
| Primary diagnosis | N | Maternal age (years)* | Child age (years)* | Mullens score* | Vineland score* | ABC score** | Parity** |
|---|---|---|---|---|---|---|---|
| Autism (AU) | 204 | 34.7 ± 5.6 | 3.7 (2.1–5.8) | 58.6 ± 16.3 | 63.1 ± 12.1 | 45.7 ± 27.4 | 1.8 ± 1.0 |
| ASD | 71 | 35.9 ± 4.9 | 3.8 (2.1–5.9) | 65.8 ± 17.2 | 68.7 ± 13.0 | 41.3 ± 31.3 | 2.1 ± 2.0 |
| Typically Developing (TD) | 183 | 34.7 ± 5.8 | 3.4 (2.2–4.9) | 103.3 ± 19.2 | 104.2 ± 16.3 | 7.6 ± 10.9 | 2.0 ± 1.1 |
| Developmental Delay (DD) | 102 | 33.1 ± 6.9 | 3.6 (2.0–4.9) | 58.0 ± 14.3 | 63.9 ± 14.6 | 31.3 ± 28.4 | 2.1 ± 1.3 |
Children classified with ASD did not meet full criteria for autism on either or both the ADI-R and ADOS instruments, but did meet criteria on either the communication or the social interaction domain of the ADI-R prior to 36 months, were within 2 points of the cut-off on the other domain, and were above the social + communication cutoff for ASD on the ADOS module 1 or 2. Mullen's score is the standardized composite score of the MSEL (mean = 100 and SD = 15). Vineland score is the standardized composite score from the VABS (mean = 100 and SD = 15)
mean ± range
mean ± SD
Expert diagnosis of all probands was carried out by clinicians at the University of California, Davis, M.I.N.D. Institute, to further detail the initial diagnoses of ASDs or to confirm typical development. Clinical assessments were carried out as reported previously (Hansen et al. 2008). The Social Communication Questionnaire (SCQ) was used to screen children of control mothers for behavioral and developmental symptoms characteristic of ASDs. Children who scored above the screening cut-off score of 15 underwent complete assessment with the ADI-R and the ADOS. Control children who met established study criteria for AU or ASD (see below) were classified as such for analysis.
All mothers of children enrolled in the present study completed the Aberrant Behavior Checklist. The ABC is a parental survey consisting of 58 questions designed to measure the severity of autism-associated behaviors, including irritability, lethargy, stereotypy, hyperactivity and inappropriate speech. The irritability subscale includes self-injurious and aggressive behavior. The total score range within the ABC is 0–174, with higher scores indicating more severely affected behavior.
All enrolled children were assessed for cognitive function using the Mullen Scales of Early Learning (MSEL) (Mullen 1995) and adaptive function through parental interview using the Vineland Adaptive Behavior Scales (VABS) (Sparrow et al. 1984). The MSEL is composed of 4 scales including Visual Reception, Fine Motor, Receptive Language and Expressive Language, each of which yields a T score with mean = 50 and SD = 10. The VABS is composed of 4 scales including Communication, Daily Living, Socialization and Motor Skills. Each of the VABS subscales yields a score from 20 to 160 with a mean among typically developing children of 100. Subscale scores on both instruments are combined to yield a composite score which is standardized to a mean of 100 among TD children; individuals not meeting AU or ASD criteria with a composite score below 70 were classified as DD.
Final autism diagnosis was confirmed by the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1997) and the Autism Diagnostic Observation Schedule (ADOS), modules 1 or 2 (Joseph et al. 2002). The ADI-R consists of a standardized, semi-structured interview and a diagnostic algorithm for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR) (APA 2000), with definitions of autism from the International Classification of Diseases, Tenth Revision (ICD-10) (WHO 1992) (Steinhausen and Erdin 1992). Administration of all diagnostic instruments was carried out by trained clinical personnel, and bilingual and bicultural staff aided in testing of Spanish speaking families.
Due to the observed variability in clinical presentation, we chose to sub-classify autism phenotypes into full Autism (AU) and the broader grouping of Autism Spectrum Disorder (ASD). This design permitted us to assess possible relationships between maternal autoantibody patterns and AU and ASD strata. Children in the AU group met criteria for autism in all three behavioral domains—reciprocal social interaction, communication and repetitive behaviors—on the ADI-R and the autism criteria for social interaction and communication on the ADOS; those included in the ASD group met such full criteria in only one or two of the domains on the ADI-R and were within two points on the other(s), and met ASD criteria on the ADOS. The term “ASDs” refers to the combined AU and ASD subgroups.
Plasma Collection
Blood was collected from enrolled mothers in yellow-top acid-citrate-dextrose tubes (BD Diagnostic, Franklin Lakes, NJ). Plasma was separated from cells, coded, aliquoted to minimize freeze/thaw cycles, and stored at −80°C until use. The samples used in this study had not previously undergone a freeze/thaw cycle.
Protein Preparation
Rhesus macaque brain tissue was placed in a tenfold greater volume of 20 mM HEPES-OH, pH 7.5 buffer supplemented with (in mM) 1 EDTA, 5 DTT, 1 PMSF, 0.2 Na2VO3, 1 NaF and one tablet Complete (Roche, per 50 ml Buffer). The tissue was then mechanically homogenized using a Polytron 3000 homogenizer (Brinkman) and sonicated for 3 min. The sample was then centrifuged 10 min at 3,000× g to remove insoluble material. The supernatant was concentrated fivefold by ultrafiltration (Mr cutoff 10,000) and diluted in 50 mM Tris–HCl pH 6.8 containing 25% glycerol and 1% LDS. The protein sample concentration was then adjusted to 3.5 mg/ml, as determined by BCA assay (Pierce). Human fetal and adult proteins were purchased from Abcam (Cambridge, MA) and were supplied as electrophoresis-ready purified protein samples. According to the manufacturer, the human fetal and adult proteins were prepared identically to the Rhesus samples described above.
Western Blotting
Western blotting was performed as previously described (Cabanlit et al. 2007), with modifications detailed below. Briefly, SDS-PAGE was performed using pre-cast 4–12% Prep-well or multi-well mini-gels (Invitrogen). Gels were loaded with 300 μg protein (or 10 μg/lane for multi-well mini-gels) in electrophoresis buffer containing 1× Nu-PAGE LDS sample buffer (Invitrogen) and 100 mM DTT. The sample was heated to 95°C for 10 min and centrifuged for 5 min at 10,000 × g. Six μl of MagicMark molecular weight standard (Invitrogen) was loaded into the marker well and protein sample(s) were loaded in the appropriate well(s). Electrophoresis was carried out at 150 V for 80 min. The gel was then transferred to 0.2 μm pore nitrocellulose membrane at 35 V for 16 h at 4°C. Protein quality and loading was confirmed by staining the membrane with Ponceau S (Sigma). Membranes from preparative well gels were cut into strips and all blots were blocked with Blocker Casein in PBS (Thermo Fisher) for 10 min and probed with maternal plasma diluted 1:400 for 2 h at room temperature. After washing, strips were incubated with goat anti-human secondary antibody (Invitrogen), diluted 1:20,000 in PBS/0.05% Tween20 for 1 h. Blots were then washed and incubated with Super Signal West Pico chemilluminescent substrate (Pierce) and imaged on a FluorChem 8900 imager (Alpha Innotech). Scoring for the presence and apparent molecular weight of a positive band was performed by individuals blinded to child diagnosis, using internal positive and negative controls on each blot.
Statistical Analysis
Statistical analysis of band prevalence was performed with SAS statistical analysis software (SAS Institute Inc). A logistic model building approach was employed to find the band or group of bands most strongly associated with AU or ASD. Comparisons between diagnostic groups were then made using a Fisher's exact test, applied to individual bands as well as combinations of bands described in the model, to determine associations with clinical diagnosis. Differences were considered significant at p < 0.05. For comparisons meeting applicability criteria, Mantel–Haenszel adjusted odds ratios were calculated. In order to identify all possible relationships between band pattern and AU or ASD diagnosis, statistical significance was evaluated without correction for multiple comparisons.
Results
Antibody Reactivity to Fetal Human and Fetal Rhesus Macaque Brain Proteins
Reactivity to Rhesus macaque fetal-brain protein (RFB) at three gestational ages (49 days, 100 days and 152 days) was assessed in plasma from a cohort (original cohort) of mothers of children with AU and mothers of TD and DD controls who were previously characterized for reactivity to commercially available human fetal-brain protein (HFB) (Clontech). The source of the HFB protein in that study was a pool of spontaneously aborted human fetuses spanning 20–40 weeks gestation (Braunschweig et al. 2008). A major limitation of the HFB sample is the unknown relative contributions of brain tissue within the above gestational range. In order to observe the timing of antigen expression, western blotting was performed with preparatory-well gels loaded with HFB or RFB of each of the three gestational ages and probed with diluted maternal plasma (Fig. 1).
Fig. 1.
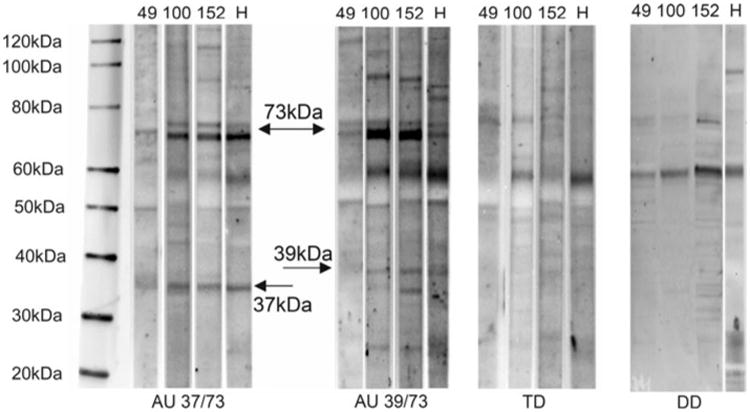
Representative western blot of maternal antibody reactivity to 37, 39 or 73 kDa antigens and non-reactive control. Reactivity of plasma IgG from two AU mothers one TD and one DD mother to fetal human (H) and fetal Rhesus macaque brain proteins of 152 day, 109 day and 49 day gestation are shown. Arrows indicate 37, 39 and 73 kDa bands. Among the Rhesus brains tested, antigen expression appears highest in late gestation (152 day sample)
Interestingly, the 73 kDa band appears as a doublet of two closely migrating bands in most of the protein samples analyzed. This may represent alternative forms of a single protein since reactivity to both members of the doublet are commonly observed together. The relative intensity of the reactive bands among all positive mothers was generally highest in the 152 day gestation Rhesus brain sample, leading us to proceed with screening all of the plasma from the previously uncharacterized mothers (new cohort) in this study using protein derived from this fetal brain.
Band Prevalence
All of the mothers from our previous study (original cohort) who displayed paired reactivity to the 37 and 73 kDa antigens continue to show reactivity using RFB, while the mothers in the TD group who did not have immunoreactive bands to HFB also lacked immunoreactivity towards RFB (Table 2). Furthermore, no significant differences were found in band prevalence between the original cohort and the new cohort, which includes mothers recruited since the original study.
Table 2. Comparison of reactivity to human and Rhesus brain protein.
| Study population (n) | Original cohort | New cohort | ||
|---|---|---|---|---|
|
|
|
|||
| HFB 37 and 73 kDa | RFB 37 and 73 kDa | Study population (n) | RFB 37 and 73 kDa | |
| AU (n = 61) | 7 (12%) | 7 (12%) | AU (n = 143) | 10 (7%) |
| TD (n = 62) | 0 (0%) | 0 (0%) | TD (n = 121) | 0 (0%) |
| DD (n = 40) | 0 (0%) | 0 (0%) | DD (n = 62) | 0 (0%) |
| Significance (p value) | ||||
| AU vs. TD | 0.0061* | 0.0061* | 0.0022* | |
| AU vs. DD | 0.0401* | 0.0401* | 0.0341* | |
| AU vs. Controls | 0.0008* | 0.0008* | 0.0002* | |
| Odds Ratios (OR ± 95% CI)** | ||||
| AU vs. TD | 17.2 (0.96–308) | 17.2 (0.96–308) | 17.8 (1.1–300) | |
| AU vs. DD | 9.9 (0.58–169) | 9.9 (0.58–169) | 9.2 (0.55–154) | |
| AU vs. Controls | 24.9 (1.45–429) | 24.9 (1.45–429) | 26.8 (1.6–454) | |
For group comparisons, differences are considered significant at p < 0.05
Significant differences yielded odds ratios and 95% CI
The current findings are consistent with our previous observation that immunoreactivity to bands at 37 and 73 kDa in combination, are highly significantly associated with a diagnosis of AU in the child (p = 0.0077), yielding an adjusted odds ratio of 39.4 (95% CI: 2.4–658). This paired antibody reactivity is thus the most specific maternal biomarker associated with autism described to date, while remaining absent in all control mothers studied.
Refinements to the immunoblotting protocol used in our lab since our original report have substantially increased the band resolution of our western blots. These improvements led to the observation of another immunoreactive band migrating near the 37 kDa band, at approximately 39 kDa. Interestingly, we observed the most highly significant association between maternal antibody reactivity against fetal brain proteins at 39 and 73 kDa in mothers whose children were diagnosed with ASD when compared with the TD control group (p = 0.000217; OR = 13.3, 95% CI 2.8–63), or the DD control group (p = 0.008; OR = 7.3, 95% CI 1.5–34.7) which was not observed when mothers of AU children were compared with controls (p = 0.241). Furthermore, significant differences in IgG reactivity between AU and ASD mothers to the 39 kDa band alone (p = 0.034) or the 39 and 73 kDa bands together (p = 0.0185) were observed (Table 3). Finally, reactivity to all three antigens was observed exclusively in a subset of AU mothers with a prevalence of approximately 3%. Because of the unknown effect of this triple reactivity, mothers positive for all three antigens were analyzed separately from the 37/73 and 39/73 kDa groups. Consistent with our previous findings, reactivity to the 37, 39 and 73 kDa, alone, or in combination, did not differ between DD and TD mothers.
Table 3. Statistical analysis of maternal antibody reactivity against fetal Rhesus macaque brain.
| Prevalence (n (%)) | 37 kDa | 39 kDa | 73 kDa | 37 and 73 kDa | 39 and 73 kDa | 37, 39 and 73 kDa |
|---|---|---|---|---|---|---|
| AU (n = 204) | 28 (14%) | 25 (12%) | 52 (25%) | 14 (7%) | 8 (4%) | 6 (3%) |
| AU reg (n = 100) | 12 (12%) | 16 (16%) | 31 (31%) | 8 (8%) | 6 (6%) | 4 (4%) |
| AU EO (n = 104) | 16 (15%) | 9 (9%) | 21 (20%) | 6 (6%) | 2 (2%) | 2 (2%) |
| ASD (n = 71) | 11 (16%) | 17 (24%) | 21 (30%) | 3 (4%) | 9 (13%) | 0 (0%) |
| ASD reg (n = 31) | 4 (13%) | 8 (26%) | 10 (32%) | 1 (3%) | 3 (10%) | 0 (0%) |
| ASD EO (n = 40) | 7 (18%) | 9 (23%) | 11 (28%) | 2 (5%) | 6 (15%) | 0 (0%) |
| AU and ASD (n = 275) | 39 (14%) | 42 (15%) | 73 (27%) | 17 (6%) | 17 (6%) | 6 (2%) |
| TD (n = 183) | 13 (7%) | 24 (13%) | 25 (14%) | 0 (0%) | 2 (1%) | 0 (0%) |
| DD (n = 102) | 3 (3%) | 12 (12%) | 16 (16%) | 0 (0%) | 2 (2%) | 0 (0%) |
| Significance (p value) | ||||||
| AU vs. TD | 0.0317* | 0.881 | 0.0032* | 0.0001* | 0.0376* | 0.03 |
| AU vs. DD | 0.0022* | 1.0 | 0.058 | 0.003* | 0.734 | 0.100 |
| AU vs. ASD | 0.844 | 0.034* | 0.537 | 0.572 | 0.0185* | 0.355 |
| AU vs. Controls** | 0.0047* | 0.766 | 0.0167* | 0.0077* | 0.241 | 0.005* |
| ASD vs. TD | 0.0533 | 0.0375* | 0.0057* | 0.0207* | 0.000217* | 1.0 |
| ASD vs. DD | 0.004* | 0.040* | 0.038* | 0.067 | 0.008* | 1.0 |
| ASD vs. Controls | 0.0118* | 0.0113* | 0.0157* | 0.0127* | 0.0005* | 1.0 |
| AU and ASD vs. TD | 0.0164* | 0.352 | 0.000066* | 0.00017* | 0.0025* | 0.085 |
| AU and ASD vs. DD | 0.0014* | 0.412 | 0.028* | 0.0049* | 0.114 | 0.195 |
| AU and ASD vs. Controls | 0.0018* | 0.192 | 0.0047* | 0.000026* | 0.0131* | 0.013* |
| TD vs. DD | 0.185 | 0.853 | 0.602 | 1.0 | 0.617 | 1.0 |
|
| ||||||
| Odds ratios (OR ± 95% CI) | 37 kDa | 39 kDa | 73 kDa | 37 and 73 kDa | 39 and 73 kDa | 37, 39 and 73 kDa |
|
| ||||||
| AU vs. TD | 2.0 (1.1–5.0) | 1.1 (0.6–2.0) | 2.6 (1.7–5.0) | 28.7 (1.7–485) | 4.8 (1–22.2) | 12.3 (0.7–220) |
| AU vs. DD | 5.34 (1.58–18) | 1.06 (1.5–2.2) | 1.87 (1.0–3.5) | 15.9 (0.9–268) | 1.5 (0.3–7.62) | 6.81 (1.4–122) |
| AU vs. Controls | 2.7 (1.4–5.4) | 1.1 (0.6–2.0) | 1.8 (1.1–2.9) | 39.4 (2.4–658) | 2.3 (0.7–7.9) | 19.1 (1.1–342) |
| ASD vs. TD | 2.4 (1.0–5.7) | 2.1 (1.1–4.2) | 2.7 (1.4–5.2) | 19 (1.0–370.5) | 13.3 (2.8–63) | NA |
| ASD vs. DD | 6.05 (1.62–23) | 2.36 (1.0–5.3) | 2.25 (1.1–4.7) | 10.5 (0.5–206) | 7.3 (1.5–34.7) | NA |
| ASD vs. Controls | 3.1 (1.3–7.2) | 2.5 (1.3–4.9) | 2.2 (1.2–4) | 23.6 (1.2–463) | 8.2 (2.4–27.8) | NA |
| AU and ASD vs. TD | 2.2 (1.1–4.3) | 1.3 (0.8–2.3) | 2.7 (1.6–4.4) | 25.4 (1.5–417) | 6.9 (1.6–29.9) | 9.0 (0.5–161) |
| AU and ASD vs. DD | 5.5 (1.6–18) | 1.3(0.7–2.7) | 1.9 (1.1–3.6) | 14.0 (0.8–235) | 3.3 (0.7–14.7) | 5.0 (0.3–89) |
| AU and ASD vs. Controls | 2.8 (1.5–5.4) | 1.4 (0.9–2.4) | 1.9 (1.2–3.0) | 31.5 (1.9–526) | 3.8 (1.3–11.4) | 14.0 (0.8–250) |
AU autism, ASD autism spectrum disorder, reg developmental regression onset, EO early onset, TD typically developing, DD developmental delay
significant difference between mothers of case and control children, p < 0.05
controls are combined TD and DD groups
Behavioral Analysis
The possibility that maternal reactivity to either the 37 and 73 kDa bands or the 39 and 73 kDa bands may correlate with specific behavior(s) within autism, led us to analyze scores on the ABC, ADI-R, ADOS, MSEL and VABS. Scores on several of these instruments among children diagnosed with AU or ASD were correlated with maternal band status and results are summarized in Table 4. Most significantly, the presence of reactivity to the 37 and 73 kDa bands associated with a lower score on the expressive language domain of the MSEL compared to those with no bands (p = 0.0045). Additionally, maternal reactivity to the 39 and 73 kDa bands was associated with increased irritability on the ABC (p = 0.0473) compared to children with ASDs of mothers with no bands.
Table 4. Behavioral and demographic characteristics of maternal antibody reactivity among mothers of children diagnosed with AU and ASD.
| Band status | N | Maternal age (years)* | Child age (years) | Mullens composite score | Mullens expressive language | Vineland composite score | ABC irritability score | ABC composite score |
|---|---|---|---|---|---|---|---|---|
| 37 and 73 kDa | 17 | 31.4 | 3.5 | 54.1 | 23.3*** | 61.7 | 10.5 | 45.1 |
| SD | 5.4 | 0.8 | 6.4 | 6.4 | 7.9 | 8.6 | 28.4 | |
| 39 and 73 kDa | 17 | 36.18 | 3.98 | 69.44 | 29.7 | 72.47 | 16.2** | 45.3 |
| SD | 4.9 | 0.8 | 22.7 | 10.5 | 16.9 | 10.7 | 35.3 | |
| 37, 39 and 73 kDa | 6 | 36.06 | 3.42 | 56.00 | 24.8*** | 63.80 | 12.2 | 52.6 |
| SD | 7.8 | 0.7 | 9.7 | 6.6 | 10.4 | 8.8 | 25.9 | |
| No Bands | 232 | 35.2 | 3.7 | 60.2 | 35.3 | 64.1 | 12.8 | 44.7 |
| SD | 5.4 | 0.9 | 16.7 | 16.1 | 12.3 | 8.3 | 27.8 |
scores are listed as mean ± SD
p < 0.05
p < 0.005
Antibody Titer and Blocking
To further understand the strength of reactivity between these maternal antibodies and their fetal targets, a titration experiment was undertaken. Samples from mothers displaying reactivity to fetal brain were diluted at 1:400, 1:800, 1:1,600 and 1:3,200 and used to probe a western blot of 152GD Rhesus brain (Fig. 2). Reactivity to all antigens was observed with plasma samples diluted at 1:1,600, but was undetectable at a dilution of 1:3,200.
Fig. 2.
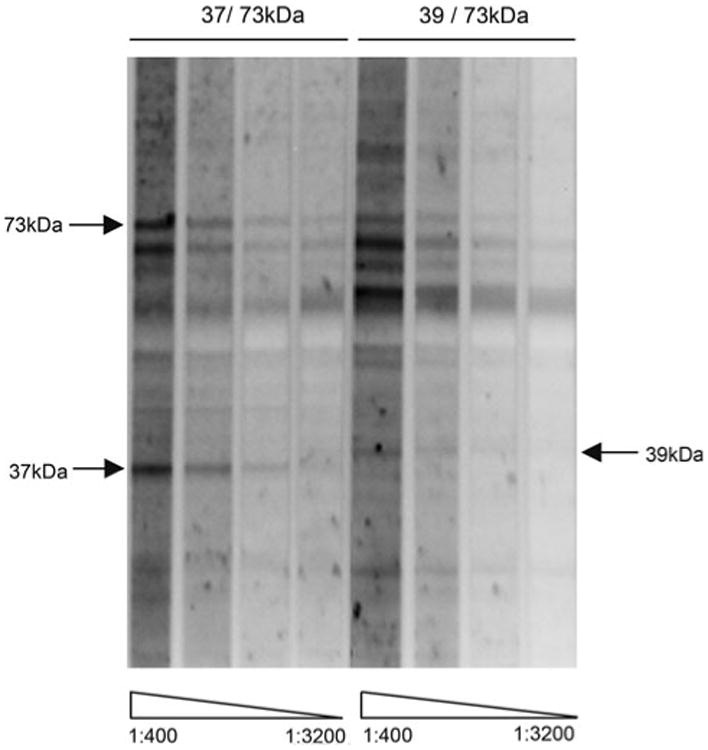
Serial dilution of maternal plasma samples. Representative maternal antibody samples shown react at 37 and 73 or 39 and 73 kDa were diluted at 1:400, 1:800, 1:1,600 and 1:3,200 and were used to probe a western blot of 152 day gestation fetal Rhesus brain protein. Reactivity was observed at 1:1,600 dilution for all antigens
Antigen Tissue Distribution
To determine the relative expression of the 37, 39 and 73 kDa antigens in several different human tissues, western blots were performed (Fig. 3). Consistent reactivity was observed between human fetal brain (hFB) and Rhesus fetal brain (rFB) protein for all antigens. Reactivity to the 37 kDa antigen was observed in all human proteins with highest relative expression in the small intestine (SI). Bands of reactivity to the 73 kDa antigen doublet was observed in all human and Rhesus fetal CNS protein samples analyzed, but was not in adult brain (AB) nor SI, demonstrating a CNS-developmental expression pattern. Reactivity to the 39 kDa antigen was variable between CNS samples analyzed and appears to display selective regional expression in the brain. Identical, tissue-specific patterns of reactivity were observed in all mothers identified as positive for the 37, 39 and 73 kDa bands.
Fig. 3.
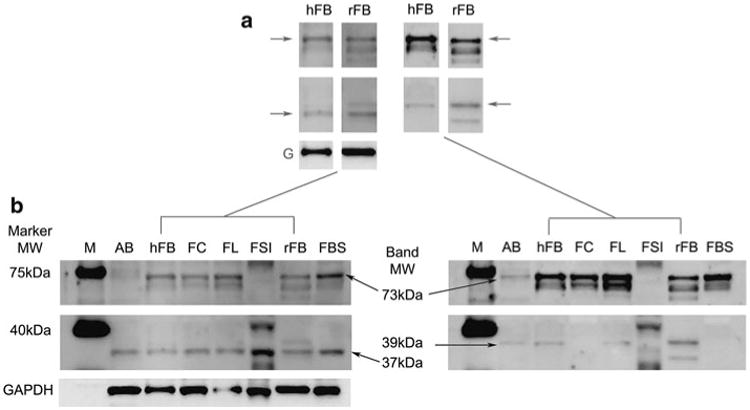
Maternal antibody reactivity to fetal and adult proteins. a Maternal antibody reactivity to the 37/73 kDa (arrows, left strips) and the 39/73 kDa (arrows, right strips) antigens in human fetal brain (hFB) and Rhesus macaque fetal brain (rFB) was highly consistent. Reactivity to the 37 kDa antigen was found in human adult brain (AB), human fetal tissues including brain (hFB), fetal cerebellum (FC), fetal frontal lobe (FFL), fetal brain stem (FBS) and fetal small intestine (FSI) as well as rhesus fetal brain (rFB). Reactivity to the 73 kDa antigen was faintly detectible in AB and was present in all fetal CNS-derived protein sources but not in SI. Reactivity to the 39 kDa antigen was observed in AB, hFB, FL and rFB but not in FC, FBS or FSI, suggesting a regional CNS expression of this antigen
Discussion
This large study of a well-characterized cohort of mothers provides additional support for a strong association between maternal anti-fetal brain antibody reactivity and the diagnosis of autism in the child. In agreement with earlier results, we continue to observe strikingly significant associations between maternal IgG reactivity to proteins at 37 and 73 kDa and a diagnosis of AU in the child, with reactivity to this pair of bands continuing to be noted exclusively in the AU and ASD groups. By expanding our original study, we were able determine that reactivity to the 37 and 73 kDa bands individually was also significantly, though not exclusively, associated with AU and ASD. This finding may reflect the additive effect of the different maternal antibody reactivity patterns on ASD symptoms in a subset of sensitive individuals. Interestingly, the mothers of children with AU or ASD were twice as likely to have antibodies to either the 37 kDa band or the 73 kDa band alone compared to the mothers of typically developing controls. Additionally, paired reactivity to bands at 39 and 73 kDa was seen at a significantly higher frequency in both the AU and ASD groups versus controls, although 2 (1%) of mothers of TD and 2 (2%) of mothers of DD children had this pattern of reactivity. Of additional interest was the finding that reactivity to the 39 kDa band alone, as well as in conjunction with the 73 kDa band, was seen in the plasma of mothers whose children were classified as ASD more often than in plasma from mothers of children with full AU, suggesting a differential impact on brain function and severity of associated behavioral symptoms of this pattern. No other measured maternal factors, including ethnicity, age or parity, were associated with antibody status. Taken together, these observations further support the concept of gestationally transferred maternal antibodies as a potential etiologic factor in some cases of autism.
In our previous report, we found evidence of an association between the presence of the 37 and 73 kDa bands with a diagnosis of regressive autism (Braunschweig et al. 2008). Within the larger cohort described in the present study, the proportion of subjects with regression was somewhat decreased, and an increased number of subjects with early-onset autism had maternal reactivity to these bands, leading to a roughly equal proportions of both onset classifications in the 37 and 73 kDa groups (data not shown).
In this study we attempted to further characterize the timing of expression and tissue distribution of the 37, 39 and 73 kDa antigens. The use of RFB protein has allowed us investigate the expression of these antigens at early, middle and late gestation time points, thus limiting potential dilution effects of a pooled sample spanning gestation. Also, by using freshly acquired tissue to prepare our protein extract, we observe higher band resolution than with the commercially prepared HFB protein. Additionally, we resolved the 73 kDa band to be composed of a closely migrating doublet of bands in nearly all mothers who were 73 kDa positive.
Analysis of the Expressive Language subscale of the MSEL among children with autism (37 and 73 kDa vs. no bands) demonstrated an association between impaired use of expressive language and the presence of these antibodies in maternal plasma. Impairments in language and communication are characteristic features of ASDs, and maternal antibody reactivity to the 37 and 73 kDa antigens may represent a biomarker of risk for increased severity of these deficits. A significant association with higher scores on the Irritability subscale of the ABC was observed among children of mothers who react to the 39 and 73 kDa antigens. The Irritability subscale of the ABC includes aggressive and self-injurious behaviors. Further work is required to determine how maternal IgG reactivity to these brain antigens is associated with different behavioral features of autism. However, we cannot yet rule out the possibility that these antibodies may merely represent biomarkers of an autism-associated maternal immune phenomenon, even if they are not pathologically significant.
In addition to expanded analysis of IgG from mothers of AU/ASD and TD children, we included a control group of mothers of children with non-autism developmental delay. As in our previous report, reactivity to the 37 and 73 kDa bands distinguishes between autism and DD, supporting a role for distinct etiologies in these disorders. Interestingly, there were additional reactivities that were significantly associated with the DD group (data not shown). Links between these additional maternal IgG bands and non-autism developmental delay in offspring will be explored in a separate report.
The dynamics of circulating levels of specific antibodies in humans depends on a variety of factors and is known to vary greatly from subject to subject. For this reason, we controlled for maternal age and time since birth of the index child in our study population. In this study, an average of 3.6 years elapsed between birth of the index child and the maternal blood draw, although we did not see an effect of time since birth on the presence of brain-reactive antibodies in any comparisons (data not shown). Interestingly, maternal antibodies specific for fetal brain have been observed up to 18 years after the birth of the child (Zimmerman et al. 2007), suggesting the existence of a pool of memory B-cells which serves to maintain these antibody levels. In future studies, we will attempt to characterize these cells and determine the factor(s) responsible for their persistence.
There is an expanding literature on maternal IgG reactivity to fetal proteins as a component of pathogenesis in autism. Early work by Warren et al. demonstrated the ability of maternal antibodies to induce complement-dependent cytotoxicity in the lymphocytes of their autistic children at significantly increased rates over controls (Warren et al. 1990). A role for neurodevelopmental pathogenesis by maternal antibody toxicity was implicated in a study which found behavioral alterations in rat pups whose mothers were injected with plasma from a mother of multiple children with autism (Dalton et al. 2003). A subsequent study demonstrated persistent reactivity to prenatal rat brain tissue in a cohort of mothers of children with autism compared with controls (Zimmerman et al. 2007). This report was followed by a recent study (Singer et al. 2009) describing behavioral alterations in the pups of pregnant mice that were administered human maternal IgG derived from mothers of children with autism during gestation. Martin et al. noted similar behavioral stereotypies in a small cohort of Rhesus macaque offspring whose mothers were injected with brain-reactive human maternal IgG during gestation (Martin et al. 2008). These observations continue to support the notion of an association between gestational exposure to brain-specific maternal antibodies and the subsequent development of behaviors that are commonly seen in individuals with an ASD.
Facilitated transfer of maternal IgG across the human placenta leads to levels detectable in fetal circulation by 13 weeks gestation, increasing during pregnancy to often exceed maternal levels at delivery in full-term infants (Garty et al. 1994). This system is thought to have evolved as a way of preparing the immunologically naïve fetus for postnatal exposures to which the mother has already mounted successful, protective responses (Harris et al. 2006). Interestingly, these maternal antibodies are often detectable in the infant's circulation beyond 6 months of age, suggesting that the protective role of these antibodies may continue until the infant is able to generate its own effective humoral immune response. Further, this window of exposure overlaps major processes in neurodevelopment, supporting the etiologic relevance of fetal brain reactive maternal antibodies.
The large majority of maternal antibody specificities acquired by the fetus are protective in nature, but strong evidence exists for pathogenic roles of autoreactive antibodies in some cases of maternal autoimmunity. In lupus, maternal anti-Ro/SS-A and anti-La/SS-B autoantibodies can cause a condition known as neonatal lupus syndrome. Interestingly, a subset of Lupus patients with CNS manifestations have autoantibodies which recognize the NMDA receptor and cross-react with DNA (Lee et al. 2009), raising the possibility that non-protein antigens may be relevant as well. Although these pathogenic maternal antibodies are metabolized from neonatal circulation by 9 months of age, they can sometimes cause permanent developmental defects (Tincani et al. 2006). In children born to mothers with autoimmune thyroid diseases such as Hashimoto's thyroiditis or Graves' disease, abnormal thyroid function is often observed at birth, but usually resolves within several weeks thereafter (Kvetny and Poulsen 2006).
The finely orchestrated events underlying neurodevel-opment rely on the correct timing and localization of gene expression and subsequent protein production. Slight alterations stemming from in utero exposure to disruptive factors, such as maternal antibodies, could lead to a temporary reduction in the availability or activity of important molecules for normal neurodevelopment and may precipitate the symptoms of autism. Interestingly, our observation of increased relative expression of the 37, 39 and 73 kDa antigens in late-gestation fetal brain suggests that the functions of the antigen proteins are more relevant to brain processes which arise later in neurodevelopment. Furthermore, the 39 and 73 kDa antigens appear to be expressed at higher levels in fetal CNS regions than adult brain which further supports the hypothesis of maternal antibody interference with important developmental pathways in autism.
The ontogeny of the maternal anti-fetal brain antibodies described in this study remains unknown. Environmental exposures, sub-clinical autoimmunity or defective down-regulation of immune activation all have the potential to underlie autoantibody formation. Further studies aimed at identifying risk factors for developing anti-brain antibodies are currently underway. Moreover, the identification of the target antigens of these autoantibodies is of urgent concern, as they may provide the ability to identify an elevated risk among mothers for delivering a child who will be diagnosed with an ASD. Work is presently ongoing to determine and verify the target antigens. Furthermore, such a disease mechanism involving gestational exposure to maternal autoantibodies would lend itself to medical intervention, which may provide the opportunity to reduce this risk as has been demonstrated in other maternal autoimmune conditions. The antigenic identities may also provide clues as to the developmental processes that may underlie some cases of autism.
Acknowledgments
The authors wish to gratefully acknowledge their funding sources for this work: NIEHS 1 P01 ES11269-01, the U.S. Environmental Protection Agency (U.S. EPA) through the Science to Achieve Results (STAR) program (Grant R829388), the UC Davis M.I.N.D. Institute.
Contributor Information
Daniel Braunschweig, Division of Rheumatology/Allergy and Clinical Immunology, University of California, Davis, 451 E. Health Sciences Drive, Suite 6510 GBSF, Davis, CA 95616, USA; The M.I.N.D. Institute, University of California, Davis, Davis, CA, USA; NIEHS Center for Children's Environmental Health, University of California, Davis, Davis, CA 95616, USA.
Paul Duncanson, Division of Rheumatology/Allergy and Clinical Immunology, University of California, Davis, 451 E. Health Sciences Drive, Suite 6510 GBSF, Davis, CA 95616, USA.
Robert Boyce, Division of Rheumatology/Allergy and Clinical Immunology, University of California, Davis, 451 E. Health Sciences Drive, Suite 6510 GBSF, Davis, CA 95616, USA.
Robin Hansen, The M.I.N.D. Institute, University of California, Davis, Davis, CA, USA; NIEHS Center for Children's Environmental Health, University of California, Davis, Davis, CA 95616, USA; Department of Pediatrics, University of California, Davis, Davis, CA, USA.
Paul Ashwood, The M.I.N.D. Institute, University of California, Davis, Davis, CA, USA; NIEHS Center for Children's Environmental Health, University of California, Davis, Davis, CA 95616, USA; Department of Medical Microbiology and Immunology, University of California, Davis, Davis, CA, USA.
Isaac N. Pessah, The M.I.N.D. Institute, University of California, Davis, Davis, CA, USA; NIEHS Center for Children's Environmental Health, University of California, Davis, Davis, CA 95616, USA; Department of Veterinary Molecular Biosciences, University of California, Davis, Davis, CA, USA
Irva Hertz-Picciotto, The M.I.N.D. Institute, University of California, Davis, Davis, CA, USA; NIEHS Center for Children's Environmental Health, University of California, Davis, Davis, CA 95616, USA; Department of Public Health Sciences, Division of Epidemiology, University of California, Davis, Davis, CA, USA.
Judy Van de Water, Email: javandewater@ucdavis.edu, Division of Rheumatology/Allergy and Clinical Immunology, University of California, Davis, 451 E. Health Sciences Drive, Suite 6510 GBSF, Davis, CA 95616, USA; The M.I.N.D. Institute, University of California, Davis, Davis, CA, USA; NIEHS Center for Children's Environmental Health, University of California, Davis, Davis, CA 95616, USA.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders: DSM-IV text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124(2):687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nature Reviews Neuroscience. 2004;5(7):521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: Maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Annals of the New York Academy of Sciences. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: The early markers for autism study. Biological Psychiatry. 2008;64(7):583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Annals of Neurology. 2003;53(4):533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- Edinburgh 2000 www.cgmh.org.tw/intr/intr1/c0040/web/C/Declaration%20of%20Helsinki.pdf.
- Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clinical and Diagnostic Laboratory Immunology. 1994;1(6):667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, Deprey LJ, et al. Regression in autism: Prevalence and associated factors in the CHARGE Study. Ambulatory Pediatrics. 2008;8(1):25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Harris NL, Spoerri I, Schopfer JF, Nembrini C, Merky P, Massacand J, et al. Mechanisms of neonatal mucosal antibody protection. Journal of Immunology. 2006;177(9):6256–6262. doi: 10.4049/jimmunol.177.9.6256. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2002;43(6):807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. Prevalence of Parent-Reported Diagnosis of Autism Spectrum Disorder Among Children in the US, 2007. Pediatrics. 2009 doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- Kvetny J, Poulsen H. Transient hyperthyroxinemia in newborns from women with autoimmune thyroid disease and raised levels of thyroid peroxidase antibodies. The Journal of Maternal-Fetal & Neonatal Medicine. 2006;19(12):817–822. doi: 10.1080/14767050600927304. [DOI] [PubMed] [Google Scholar]
- Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nature Medicine. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, et al. Diagnosing autism: Analyses of data from the autism diagnostic interview. Journal of Autism and Developmental Disorders. 1997;27(5):501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. American Journal of Reproductive Immunology. 1996;36(5):248–255. doi: 10.1111/j.1600-0897.1996.tb00172.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain, Behavior, and Immunity. 2008;22(6):806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E, editor. Mullen scale of early learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21(24):3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. Journal of Neuroimmunology. 2008;194(1–2):165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. Journal of Neuroimmunology. 2009;211(1–2):39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Sparrow SB, Balla DA, Cicchetti DV. Vineland adaptive behavior scales survey form manual. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Steinhausen HC, Erdin A. Abnormal psychosocial situations and ICD-10 diagnoses in children and adolescents attending a psychiatric service. Journal of Child Psychology and Psychiatry. 1992;33(4):731–740. doi: 10.1111/j.1469-7610.1992.tb00908.x. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Taniai H, Nishiyama T, Miyachi T, Imaeda M, Sumi S. Genetic influences on the broad spectrum of autism: Study of proband-ascertained twins. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008;147B(6):844–849. doi: 10.1002/ajmg.b.30740. [DOI] [PubMed] [Google Scholar]
- Tincani A, Rebaioli CB, Taglietti M, Shoenfeld Y. Heart involvement in systemic lupus erythematosus anti-phospholipid syndrome and neonatal lupus. Rheumatology (Oxford) 2006;454:iv8–iv13. doi: 10.1093/rheumatology/kel308. [DOI] [PubMed] [Google Scholar]
- Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, et al. Detection of maternal antibodies in infantile autism. Journal of the American Academy of Child and Adolescent Psychiatry. 1990;29(6):873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- WHO. ICD-10: International statistical calssification of diseases and related health problems, 10th Revision. Geneva: World Health Organization; 1992. [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, et al. Maternal antibrain antibodies in autism. Brain, Behavior, and Immunity. 2007;21(3):351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]


