Abstract
Glucagon secretion dysregulation in diabetes fosters hyperglycemia. Recent studies report that mice lacking glucagon receptor (Gcgr-/-) do not develop diabetes following streptozotocin (STZ)-mediated ablation of insulin-producing β-cells. Here, we show that diabetes prevention in STZ-treated Gcgr-/- animals requires remnant insulin action originating from spared residual β-cells: these mice indeed became hyperglycemic after insulin receptor blockade. Accordingly, Gcgr-/- mice developed hyperglycemia after induction of a more complete, diphtheria toxin (DT)-induced β-cell loss, a situation of near-absolute insulin deficiency similar to type 1 diabetes. In addition, glucagon deficiency did not impair the natural capacity of α-cells to reprogram into insulin production after extreme β-cell loss. α-to-β-cell conversion was improved in Gcgr-/- mice as a consequence of α-cell hyperplasia. Collectively, these results indicate that glucagon antagonism could i) be a useful adjuvant therapy in diabetes only when residual insulin action persists, and ii) help devising future β-cell regeneration therapies relying upon α-cell reprogramming.
DOI: http://dx.doi.org/10.7554/eLife.13828.001
Research Organism: Mouse
eLife digest
After meals, digested food causes sugar to accumulate in the blood. This triggers the release of the hormone insulin from beta cells in the pancreas, which allows liver cells, muscle cells and fat cells to use and store the sugar for energy. Other cells in the pancreas, called alpha cells, release a hormone called glucagon that counteracts the effects of insulin by telling the liver to release sugar into the bloodstream. The balance between the activity of insulin and glucagon keeps blood sugar levels steady.
Diabetes results from the body being unable to produce enough insulin or respond to the insulin that is produced, which results in sugar accumulating in the blood. Diabetes also increases the production of glucagon, which further increases blood sugar levels. Recently, some researchers have reported that mice that lack the receptor proteins through which glucagon works do not develop diabetes, even when they are treated with a drug called streptozotocin that wipes out most of their beta cells. This suggests that the high blood sugar levels seen in diabetes result from an excess of glucagon, and not a lack of insulin.
Drugs that block the action of glucagon have been found to reduce the symptoms of mild diabetes in mice and are now being tested in humans. However, it is less clear whether this treatment has any benefits in animals with more severe diabetes.
Streptozotocin destroys most of a mouse’s beta cells but a significant fraction of them persist, while a different system relying on diphtheria toxin destroys more than 99% of these cells. Damond et al. have now found that treating mice that lack glucagon receptors with diphtheria toxin causes the mice to develop severe diabetes. Mice that lacked glucagon receptors that had been treated with streptozotocin also developed diabetes after they had been treated with an insulin-blocking drug. Further experiments showed that blocking glucagon receptors in typical mice with diabetes reduces blood sugar, but only if there is some insulin left in their bodies.
Damond et al. also found that the glucagon receptor-lacking mice have more alpha cells, which have the ability to convert into insulin-producing cells after the widespread destruction of beta cells. Together, the experiments suggest that blocking glucagon could be a useful treatment for diabetes, but only in individuals who still have some insulin-producing cells. Such treatment would help reduce the release of sugar from the liver and increase the production of insulin in converted alpha cells in the pancreas. Damond et al. are now investigating how alpha cells convert into beta cells, with the aim of learning how to make beta cells regenerate more efficiently.
Introduction
Glucagon, a 29-amino acid-long hormone synthetized in pancreatic α-cells through cleavage of its precursor, proglucagon, by prohormone convertase 2 (PC2), counterbalances the effects of insulin on blood glucose homeostasis by stimulating hepatic glycogenolysis and gluconeogenesis (Gromada et al., 2007). In addition, the two hormones act in a paracrine fashion to reciprocally regulate α- and β-cell function (Unger and Orci, 2010).
Hypersecretion of glucagon in diabetes exacerbates hepatic glucose output, thereby fostering hyperglycemia and ketogenesis (Unger et al., 1970; Unger, 1971; Sherwin et al., 1976; D'Alessio, 2011). In consequence, antagonists of glucagon signaling are currently being tested in clinical trials for diabetes (Campbell and Drucker, 2015). The importance of glucagon signaling in diabetes was recently highlighted in studies performed with glucagon receptor knockout (Gcgr-/-) mice and in animals lacking α-cells due to pancreatic aristaless-related homeobox (Arx) deficiency. Surprisingly, these animals did not exhibit the usual signs of diabetes, such as hyperglycemia or glucose intolerance, after streptozotocin (STZ)-mediated β-cell destruction (Conarello et al., 2006; Lee et al., 2011; 2012; Hancock et al., 2010). These findings lead to hypothesize that glucagon is responsible for the features of diabetes (Unger and Cherrington, 2012). Although suppression of glucagon action is likely to attenuate the consequences of insulin deficiency, its primary role in the hyperglycemia is uncertain. Indeed, because STZ causes an incomplete β-cell ablation due to variations in administration protocols and in genetic background-dependent sensitivity (Deeds et al., 2011; Cardinal et al., 1998; Gurley, 2006), it is possible that the “diabetes resistance” phenotype of Gcgr-/- mice relies on the action of insulin from residual β-cells. Thus, to determine whether lack of glucagon signaling would also prevent hyperglycemia and diabetes in the context of a more severe insulin deficiency, we used a transgenic model of diphtheria toxin (DT)-mediated β-cell ablation, termed RIP-DTR, which leads to an almost complete β-cell elimination (Thorel et al., 2010; Chera et al., 2014). Also, because adult RIP-DTR mice spontaneously reconstitute new insulin-producing cells by α-cell transdifferentiation in this condition of severe insulin insufficiency, we explored whether the compensatory α-cell hyperplasia due to glucagon signaling blockade (Furuta et al., 1997; Gelling et al., 2003; Longuet et al., 2013) influences the reprogramming of α-cells toward insulin production.
Here we show that near-total β-cell loss triggers severe hyperglycemia and all the metabolic features of type 1 diabetes (cachexia, glucose intolerance, and death) in mice with constitutive or induced glucagon signaling deficiency. We report that the absence of hyperglycemia observed in glucagon-deficient mice after STZ treatment can be explained through the persistence of a residual β-cell mass, which ensures a low level of insulin action.
Results
Near-total β-cell ablation leads to full-blown diabetes in mice lacking glucagon signaling
Recent reports indicate that Gcgr-/- mice do not develop hyperglycemia after STZ-mediated β-cell loss. Here we aimed at determining the effect of the absence of glucagon action in the context of a more extreme insulin deficiency. For this purpose, we crossed Gcgr-/- mutant animals (Gelling et al., 2003) with RIP-DTR mice, in which diphtheria toxin (DT) injection triggers the near-total (>99% ) β-cell loss (Thorel et al., 2010).
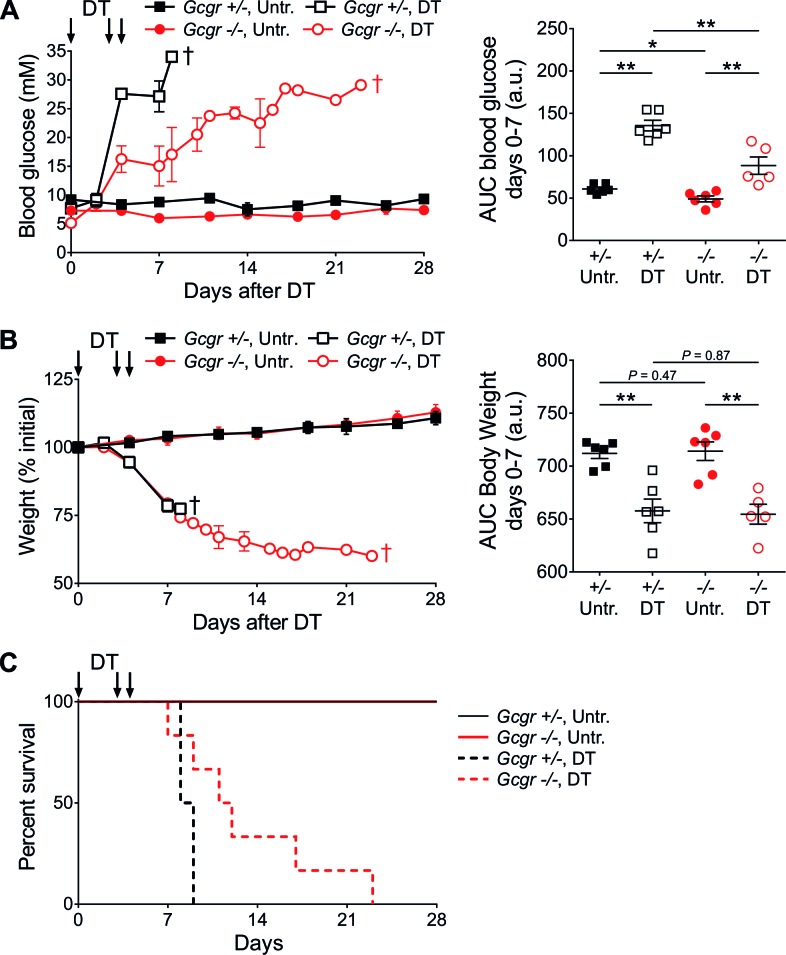
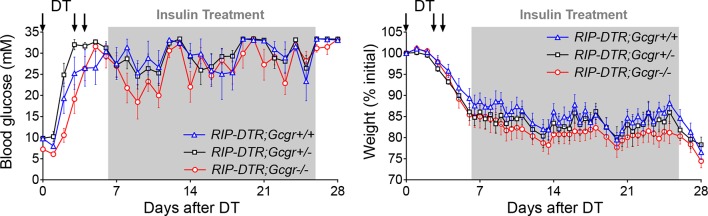
RIP-DTR;Gcgr-/- mice, like Gcgr-/- mice, displayed lower basal glucose levels than controls (RIP-DTR;Gcgr+/+ and RIP-DTR;Gcgr+/-; not shown) (Gelling et al., 2003). Upon DT-induced β-cell ablation, both control and knockout animals developed severe hyperglycemia, with a slower kinetics in RIP-DTR;Gcgr-/- mice (Figure 1A). Animals of both groups lost weight at similar rates (Figure 1B), and died in absence of exogenous insulin treatment (Figure 1C). By contrast, administration of long-acting insulin, although insufficient to normalize blood glucose levels, permitted survival and body weight maintenance (Figure 1—figure supplement 1). As soon as insulin treatment was discontinued, blood glucose levels and body weight quickly deteriorated in all groups. Altogether, these findings indicate that Gcgr-/- mice are not protected against hyperglycemia after near-total β-cell loss, but develop classical signs of type 1 diabetes and require insulin therapy.
Figure 1. Gcgr-/- mice become diabetic after massive β-cell ablation.
(A) Random-fed glycemia (left) and area under the glycemia curve (AUC) between days 0 and 7 after DT (right) in untreated (Untr.) and DT-treated RIP-DTR;Gcgr+/- and RIP-DTR;Gcgr-/- females. (B) Body weight (left) and AUC body weight (days 0–7 after DT; right). †, all mice of the group were dead at this time point (see Figure 1C). *p<0.05; **p<0.01; Mann-Whitney U test. C: Survival curve of RIP-DTR;Gcgr+/- and RIP-DTR;Gcgr-/- mice after DT treatment (N=5–6). Survival analysis of DT-treated animals (Gcgr+/- versus Gcgr-/-): p=0.044; Log-rank test.
Figure 1—figure supplement 1. Insulin administration stabilizes body weight and allows survival of DT-treated Gcgr-/-mice.
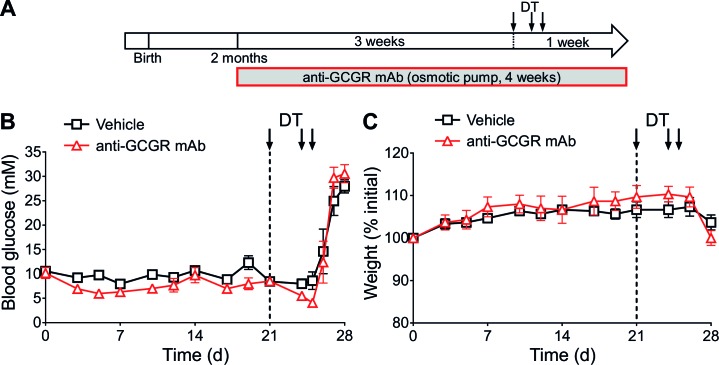
Constitutive Gcgr deletion leads to increased embryonic lethality, and defects in pancreatic development and islet-cell maturation (Vuguin et al., 2006; Vuguin and Charron, 2011; Ouhilal et al., 2012). Since these abnormalities may encompass long-lasting compensatory metabolic adaptations, we conditionally inhibited glucagon action in adult mice that had developed normally using a glucagon receptor antagonizing monoclonal antibody (anti-GCGR mAb). We first assessed its activity in C57BL/6 wild type mice (Figure 2—figure supplement 1A). In agreement with a previously described antibody (Gu et al., 2009; Yan et al., 2009), anti-GCGR treatment led to a reduction in basal glycemia (Figure 2—figure supplement 1B), and triggered α-cell hyperplasia and hypertrophy, as observed in Gcgr-/- animals (Figure 2—figure supplement 1C–D) (Gelling et al., 2003). In addition, antibody-treated Gcgr+/+ mice showed altered responses, like Gcgr-/- animals, to intraperitoneal glucose and insulin tolerance tests (Figure 2—figure supplement 1E–F). Anti-GCGR administration in Gcgr+/+ mice therefore phenocopies the main metabolic and cellular alterations of Gcgr-/- mice and thus represents a valuable tool for inducing glucagon signaling antagonism in vivo.
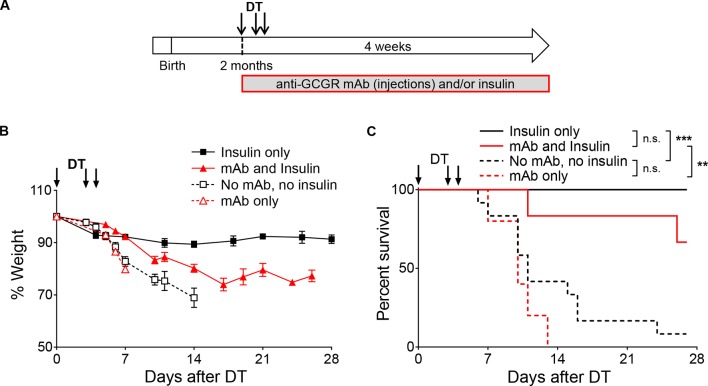
To assess whether induced glucagon receptor blockade prevents diabetes upon near-total β-cell ablation, we pre-treated adult RIP-DTR mice with the anti-GCGR mAb for 3 weeks, and then injected them with DT (Figure 2A). In agreement with the above results using RIP-DTR;Gcgr-/- animals, all mice became severely hyperglycemic and lost weight after DT, regardless of antibody treatment (Figure 2B–C). Moreover, only insulin administration allowed for survival following β-cell ablation, not glucagon receptor inhibition (Figure 2—figure supplement 2). Collectively, these observations indicate that the lack of glucagon signaling is not sufficient per se to prevent severe hyperglycemia and diabetes following extreme β-cell loss, and contrast with previous studies in which Gcgr-/-, or anti-GCGR-treated mice did not develop the metabolic manifestations of the disease when β-cell ablation was mediated by STZ (Conarello et al., 2006; Lee et al., 2011; 2012; Wang et al., 2015).
Figure 2. Anti-GCGR mAb-treated mice become diabetic after massive β-cell ablation.
(A) Experimental design. (B-C) Random-fed glycemia (B) and body weight (C) after DT in C57BL/6 males pre-treated with vehicle or mAb (N=3).
Figure 2—figure supplement 1. Anti-GCGR mAb administration recapitulates the metabolic and cellular phenotypes of Gcgr-/- mice.
Figure 2—figure supplement 2. Insulin administration is required to stabilize body weight and allow survival of anti-GCGR-treated mice after DT.
DT leads to a more complete β-cell ablation than STZ
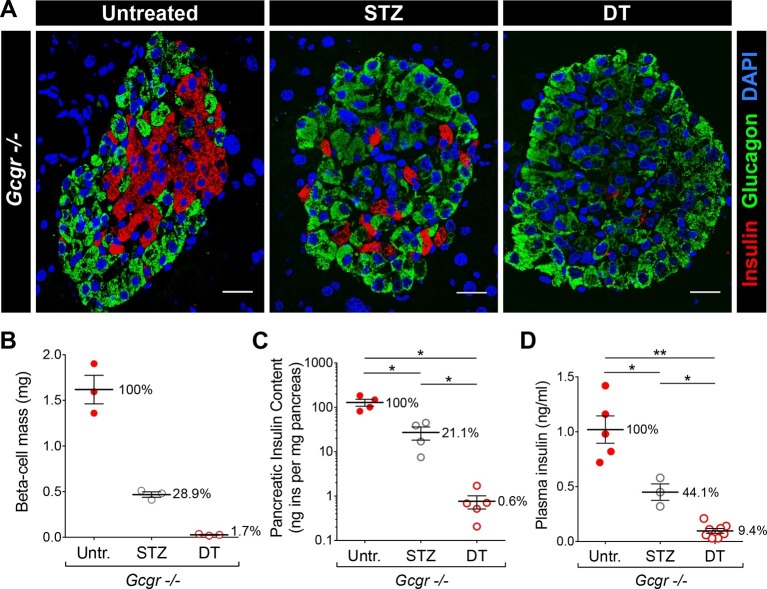
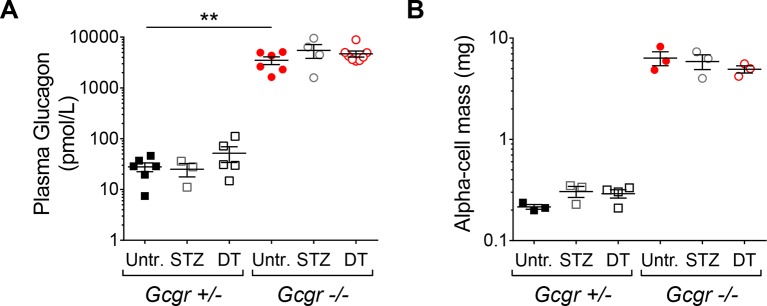
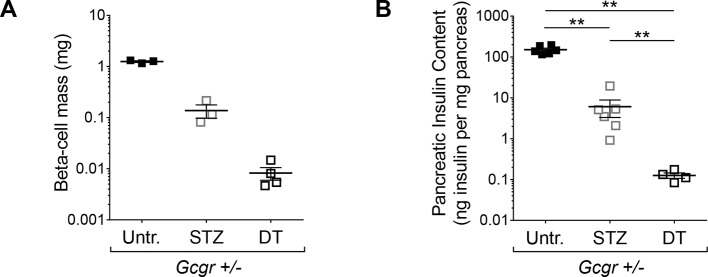
The different impact of STZ and DT treatments on glycemia in Gcgr-/- mice may result from a difference in completeness of β-cell destruction. To test this hypothesis, we compared the relative ablation efficiencies of these two methods. To maximize β-cell destruction, we treated Gcgr+/- and Gcgr-/- mice with two high doses of STZ (200 and 150 mg/kg, one week apart). Following the first injection, control mice became severely hyperglycemic. By contrast, Gcgr-/- animals remained normoglycemic even after the second STZ injection, as previously reported (not shown) (Lee et al., 2011; 2012). RIP-DTR;Gcgr-/- animals remained markedly hyperglucagonemic after STZ- or DT-mediated β-cell loss and α-cell mass was not affected (Figure 3—figure supplement 1A–B). Histologically, we observed that nearly 90% of islet sections were totally devoid of β-cells after DT, versus only 45% after STZ (Figure 3A). Accordingly, the β-cell mass and pancreatic insulin content were reduced by 98–99% after DT, but only by 70–80% after STZ (Figure 3B–C). In addition, plasma insulin levels were just above detection threshold after DT, but readily detectable after STZ (Figure 3D). We made similar observations in mice with normal glucagon signaling (Figure 3—figure supplement 2). Together, these results indicate that β-cell destruction is more complete after DT- than after STZ-treatment in Gcgr-/- mice.
Figure 3. DT administration leads to a more complete β-cell ablation than STZ.
(A) Islet sections stained for insulin (red) and glucagon (green) from untreated, STZ-, or DT-treated RIP-DTR;Gcgr-/- females, 6 days after the last STZ or DT injection. Scale bars: 20 μm. (B-D) β-cell mass (B), pancreatic insulin content (C) and fed plasma insulin levels (D) in untreated (Untr.), STZ-, or DT-treated RIP-DTR;Gcgr-/- males and females, 6 days after the last injection. STZ administration: two injections (200 and 150 mg/kg). *p<0.05; **p<0.01; Mann-Whitney U test.
Figure 3—figure supplement 1. RIP-DTR;Gcgr-/- mice remain hyperglucagonemic and α-cell mass is not affected after STZ- or DT-treatment.
Figure 3—figure supplement 2. Higher efficiency of β-cell ablation after DT- than after STZ-treatment in mice with normal glucagon signaling.
Residual insulin action protects STZ-treated Gcgr-/- mice from hyperglycemia
Because β-cell ablation was incomplete after STZ, we aimed at determining whether the action of residual circulating insulin might, in combination with glucagon signaling deficiency, protect Gcgr-/- mice from diabetes.
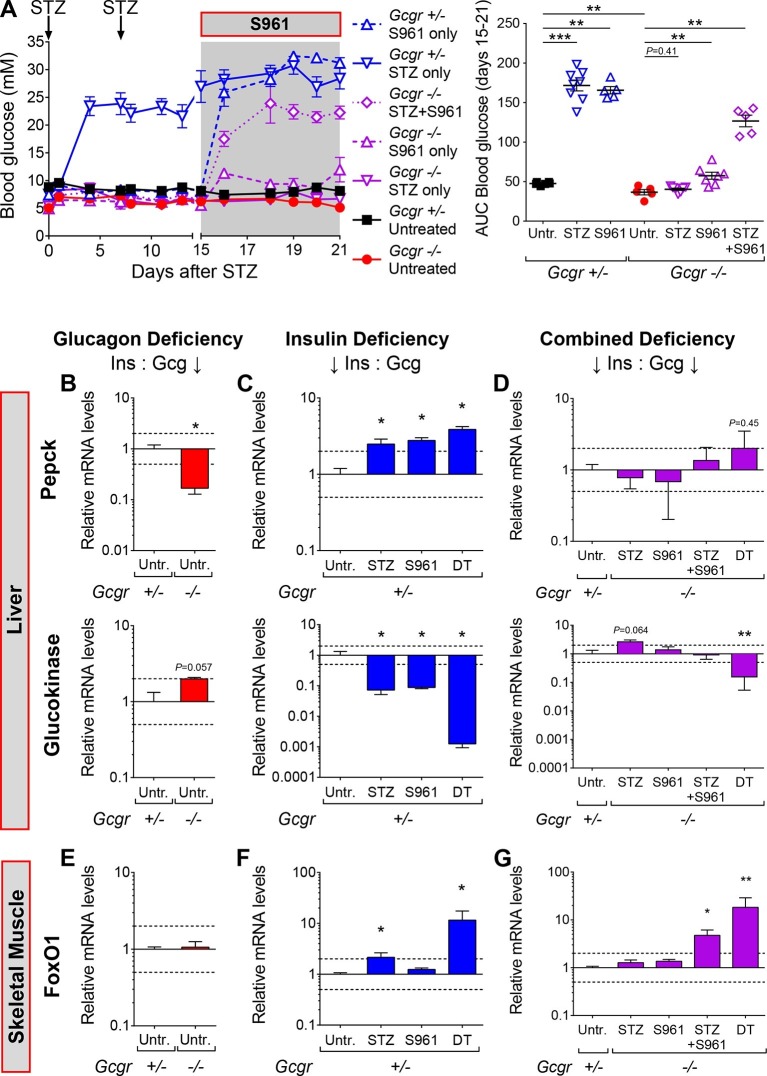
To test this hypothesis, we inhibited insulin action using the insulin receptor antagonist drug S961 (Schäffer et al., 2008). In vivo, S961 administration induces hyperglycemia in wild type animals and closely recapitulates the phenotype of mice with liver-specific insulin receptor deletion (Yi et al., 2013; Michael et al., 2000). In agreement with its previously reported action, S961 administration in Gcgr+/- mice triggered a strong increase in glycemia (Figure 4A; blue dashed vs black continuous line). Interestingly, Gcgr-/- animals exhibited a smaller but significant increase in glycemia, indicating that glucagon deficiency has a beneficial effect in this situation of relative insulin deficit (purple dashed vs red continuous line). Although STZ-treated Gcgr-/- mice remained normoglycemic, as previously reported (Conarello et al., 2006; Lee et al., 2011; 2012), they developed severe hyperglycemia after insulin receptor inhibition (continuous vs dotted purple line). This suggests that residual insulin action, likely originating from STZ-escaping β-cells, is still present after STZ administration in Gcgr-/- animals, and is necessary to prevent hyperglycemia and diabetes.
Figure 4. Inhibition of insulin action triggers hyperglycemia in STZ-treated Gcgr-/-mice.
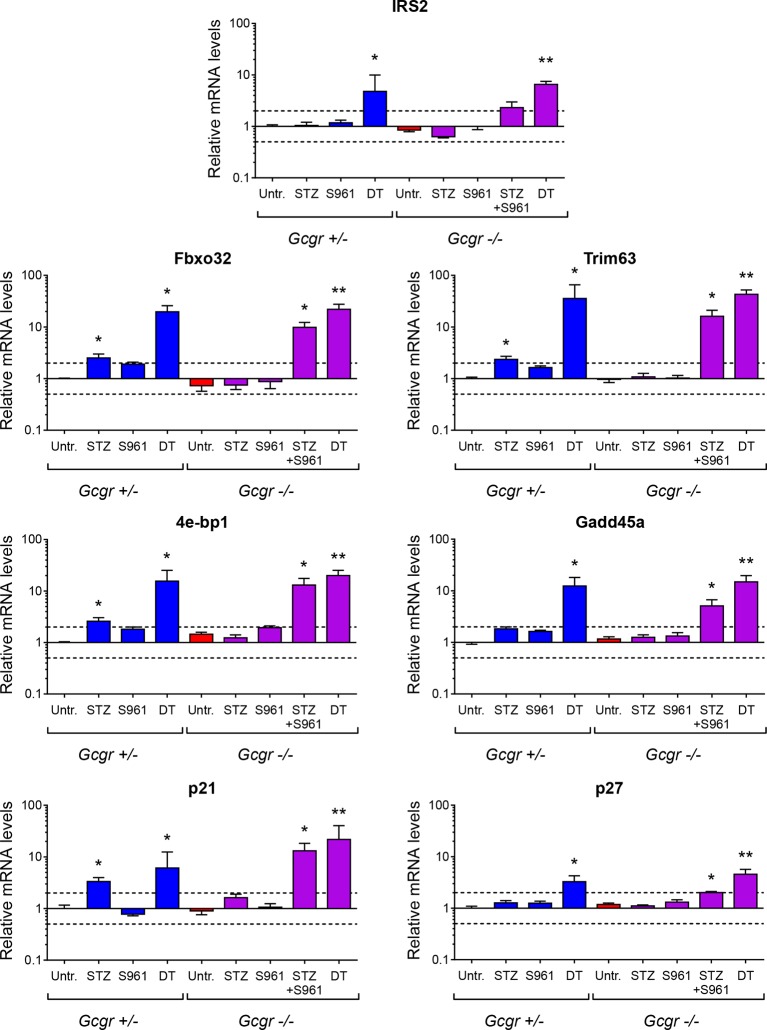
(A) Random-fed glycemia after STZ and/or S961 administration in Gcgr+/- and Gcgr-/- females (left), and area under the glycemia curve (AUC) during S961 treatment (right). (B-D) Hepatic Pepck (top) and Glucokinase (bottom) mRNA levels relative to those of untreated Gcgr+/- (control) mice (N=4–6). (B) Glucagon deficiency: Gcgr-/- background. (C) Insulin deficiency: β-cell ablation or insulin signaling inhibition. (D) Combined deficiency: β-cell ablation and/or insulin signaling inhibition in a Gcgr-/- background. (E-G) FoxO1 mRNA levels in skeletal muscle, relative to those of untreated Gcgr+/- mice (N=4–6). STZ administration: 200 mg/kg at day 0 and 150 mg/kg at day 7. S961 treatment: osmotic pump (days 15 to 21). *p<0.05; **p<0.01; Mann-Whitney U test. Only groups that exhibited a > twofold regulation as compared to controls (dashed lines) were tested.
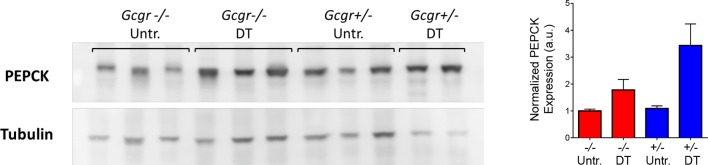
Figure 4—figure supplement 1. Higher hepatic PEPCK protein expression after DT in both Gcgr+/- and Gcgr-/- mice.
Figure 4—figure supplement 2. Liver glycogen concentration is reduced after DT-treatment in both RIP-DTR-Gcgr+/- and RIP-DTR-Gcgr-/- mice.
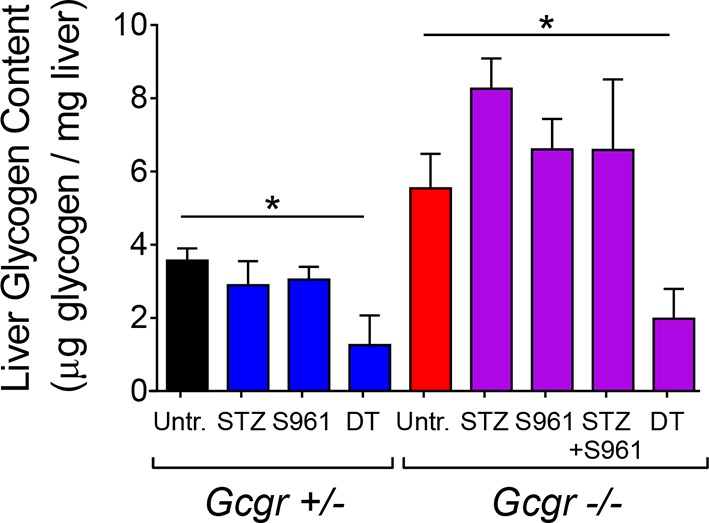
Figure 4—figure supplement 3. Expression of genes negatively regulated by insulin signaling in skeletal muscle.
To better characterize the effect of insulin insufficiency in a glucagon-deficient context, we evaluated hepatic transcript levels of Phosphoenolpyruvate carboxykinase (Pepck) and Glucokinase (Gck), two hormone-sensitive enzymes whose transcription is regulated by the relative levels of glucagon and insulin signaling (Rucktäschel et al., 2000; Chakravarty et al., 2005; Iynedjian et al., 1995). Liver is a relevant organ to assess the impact of insulin and glucagon deficiency because re-expression of the glucagon receptor in the liver of STZ-treated Gcgr-/- mice, and conditional inactivation of the insulin receptor in hepatocytes are both sufficient to trigger hyperglycemia (Lee et al., 2012; Michael et al., 2000). In conditions of glucagon deficiency (increased insulin/glucagon ratio; Gcgr-/- mice), we observed a decreased expression of the gluconeogenic enzyme Pepck and an increased expression of the glycolytic enzyme Gck as compared to Gcgr+/- controls (Figure 4B), which is consistent with a previous study (Yang et al., 2011). By contrast, upon induced insulin deficiency (decreased insulin/glucagon ratio), as in STZ-, S961-, or DT-treated Gcgr+/- animals, Pepck and Gck exhibited the opposite regulation (Figure 4C). We observed the strongest effect after DT, which caused a 1000-fold decrease in Gck expression, suggesting that it led to a more complete suppression of insulin action than STZ or S961. When inducing insulin insufficiency in a Gcgr-/- background, a situation of combined insulin and glucagon deficiency, we observed Pepck and Gck mRNA levels similar to those measured in untreated Gcgr+/- control mice, except after DT, which induced a strong downregulation of Gck expression in Gcgr-/- livers (Figure 4D). We also confirmed the increase in hepatic PEPCK expression after DT at the protein level (Figure 4—figure supplement 1). Similarly, DT-, but not STZ-treatment depleted liver glycogen stores in RIP-DTR;Gcgr-/- animals (Figure 4—figure supplement 2). These results suggest that lack of glucagon action can compensate for the effect of partial insulin insufficiency on the expression of rate-limiting enzymes and hepatic glycogen metabolism, but not after near-total β-cell loss, a situation where the effect of insulin deficiency outweighs that of glucagon deficiency.
We then assessed insulin signaling activity in skeletal muscle by measuring the expression of the transcription factor Forkhead box protein O1 (FoxO1) and of several of its target genes, such as Insulin receptor substrate 2 (Irs2), which are induced upon insulin insufficiency (Long et al., 2011). FoxO1 mRNA levels were similar in untreated Gcgr+/- and Gcgr-/- mice (Figure 4E). In Gcgr-/- animals, STZ or S961 administration did not significantly affect FoxO1 expression. By contrast, FoxO1 and its targets were strongly upregulated upon combined STZ and S961-, or DT-treatment, reflecting a more severe insulin insufficiency (Figure 4G and Figure 4—figure supplement 3).
Together, these results indicate that lack of glucagon signaling efficiently compensates for the consequences of insulin insufficiency only if residual insulin action persists after β-cell loss.
Glucagon signaling blockade attenuates hyperglycemia after STZ-mediated β-cell loss only when residual insulin production persists
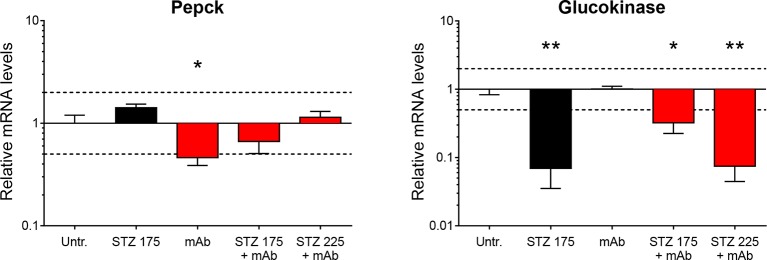
As Gcgr-/- mice exhibit resistance to STZ-induced hyperglycemia, we assessed the impact of glucagon signaling blockade on C57BL/6 mice made hyperglycemic with a single injection of either 175 or 225 mg/kg STZ. Once the animals were hyperglycemic, we implanted them with an osmotic pump containing the anti-GCGR mAb. In mice injected with 175 mg/kg STZ, antibody treatment strongly reduced, but did not completely normalize, blood glucose levels (Figure 5A and B). By contrast, animals that had received 225 mg/kg STZ remained severely hyperglycemic (>30 mM) after anti-GCGR mAb administration. As expected, residual pancreatic insulin content negatively correlated with the dose of STZ (Figure 5C). We thus observed beneficial effects of glucagon signaling inhibition only in diabetic mice that had retained a relatively higher pancreatic insulin after STZ-mediated β-cell loss. Strikingly, the impact of glucagon signaling inhibition on the glycemia of diabetic mice was dependent on very small measurable differences in residual pancreatic insulin, as seen after 175 and 225 mg/kg STZ (respectively 1.79% and 0.45% of the pancreatic insulin content of non-ablated controls). As seen in Gcgr-/- animals, anti-GCGR mAb administration resulted in a lower expression of hepatic Pepck (Figure 5—figure supplement 1). In addition, the highest STZ dose triggered a stronger glucokinase downregulation than the 175 mg/kg dose in mAb-treated mice.
Figure 5. Anti-GCGR mAb treatment does not normalize hyperglycemia after efficient STZ-mediated β-cell ablation.
(A) Random-fed glycemia in C57BL/6 males treated with STZ (single injection at day 0: 175 or 225 mg/kg) and/or anti-GCGR mAb (osmotic pump, days 6 to 14; N=3–6). (B) Area under the glycemia curves during mAb treatment. (C) Pancreatic insulin content. *p<0.05; **p<0.01; Mann Whitney U test.
Figure 5—figure supplement 1. Hepatic Pepck and Glucokinase expression after STZ and/or anti-GCGR mAb treatment.
Collectively, our findings support the notion that, regardless the method of β-cell ablation (STZ or DT), the beneficial effects of inhibiting glucagon action, either genetically or pharmacologically, rely upon residual insulin action.
Induction of insulin production in α-cells after β-cell ablation also occurs in absence of glucagon signaling
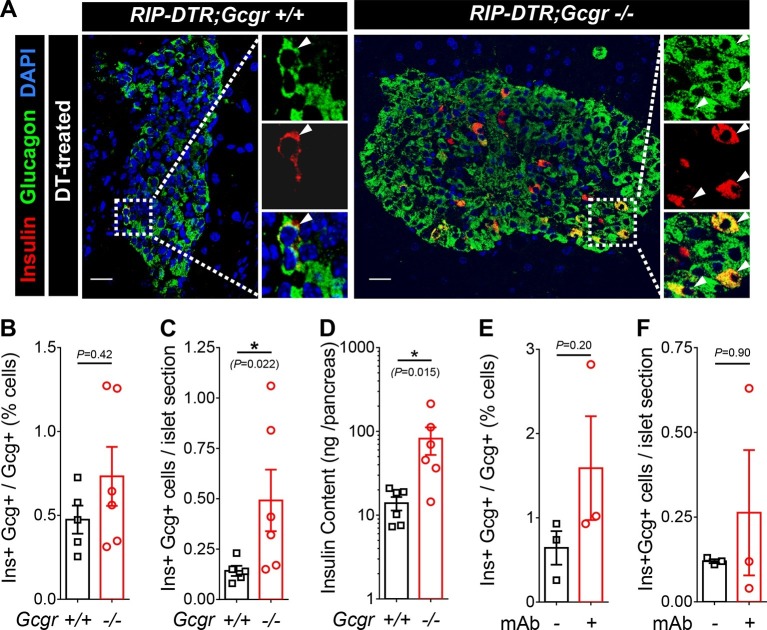
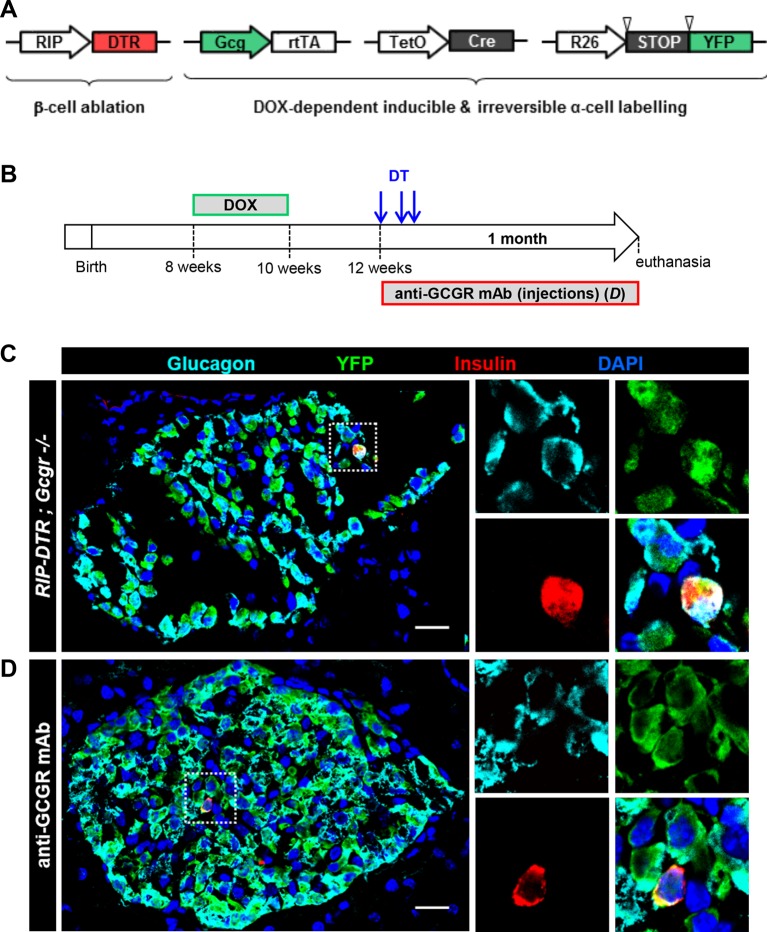
We have previously shown that massive β-cell ablation triggers insulin expression in a small fraction of the α-cell population, with the appearance of glucagon/insulin bihormonal cells (Thorel et al., 2010). We report above that in such a situation of near-total β-cell loss, lack of glucagon action fails to normalize glycemia. We then assessed whether the α-cell expansion triggered by glucagon signaling inhibition could have a beneficial effect on α-cell reprogramming. One month after DT-mediated β-cell ablation, we observed bihormonal cells in RIP-DTR;Gcgr+/+ and RIP-DTR;Gcgr-/- mice (Figure 6A). Because RIP-DTR;Gcgr-/- animals have α-cell hyperplasia (Gelling et al., 2003; Longuet et al., 2013) and the number of bihormonal cells was proportional to the number of α-cells in both groups (Figure 6B), we observed a significant increase in the absolute number of bihormonal cells in RIP-DTR;Gcgr-/- mice (Figure 6C). Consistent with these observations, they had a higher pancreatic insulin content (Figure 6D). These results indicate that there is an increased number of α-cells engaged into reprogramming in mice lacking glucagon signaling. We also observed the appearance of bihormonal cells in DT-treated adult RIP-DTR mice undergoing anti-GCGR mAb treatment (Figure 6E–F). We confirmed the α-cell origin of these newly formed bihormonal cells using a previously described tetracycline-activated system, which allows the specific and efficient doxycycline (DOX)-dependent irreversible tracing of α-cells with YFP (Figure 6—figure supplement 1A–B) (Thorel et al., 2010). One month after DT injection in Gcgr-/- mice, we observed that a significant fraction of insulin-producing cells were also YFP-positive and therefore derived from cells that had previously expressed glucagon (Figure 6—figure supplement 1C). We confirmed these observations in animals in which conditional GCGR inhibition was applied after DT-mediated β-cell ablation (Figure 6—figure supplement 1D).
Figure 6. Absence of glucagon signaling does not block the appearance of new glucagon-insulin bihormonal cells after β-cell ablation.
(A) Islet sections exhibiting glucagon-insulin co-expressing cells (arrowheads) from RIP-DTR;Gcgr+/+ and RIP-DTR;Gcgr-/- females (1 m after DT). Scale bars: 20 μm. (B-D) Percentage of glucagon+ cells that co-express insulin (B), bihormonal cells per islet section (C), and pancreatic insulin content (D) in RIP-DTR;Gcgr+/+ and RIP-DTR;Gcgr-/- females (1 m after DT, N=5–6). (E-F) Percentage of glucagon+ cells that co-express insulin (E), and bihormonal cells per islet section (F) in vehicle- or anti-GCGR mAb- treated RIP-DTR males (2 weeks after DT, N=3). *p<0.05; Mann-Whitney U test.
Figure 6—figure supplement 1. Newly formed bihormonal cells in Gcgr-/- mice are reprogrammed α-cells.
Together, these findings indicate that although glucagon signaling blockade does not prevent hyperglycemia in diabetic mice that exhibit extreme insulin deficiency, it results in enhanced formation of new insulin-producing cells by increasing the absolute number of converting α-cells.
Discussion
Glucagon receptor inhibition decreases hyperglycemia in various animal models of diabetes (Gu et al., 2009; Johnson et al., 1982; Brand et al., 1994; Sloop et al., 2004; Mu et al., 2011; Sorensen et al., 2006), as well as in patients with type 2 diabetes (Kelly et al., 2015). The extent of these benefits remains however disputed in situations where the β-cell population is nearly completely depleted, as in long-standing type 1 diabetes (Wang et al., 2012; Meier et al., 2005). Previous studies have shown that STZ-mediated β-cell ablation does not induce diabetes in the Gcgr-/- mouse model (Conarello et al., 2006; Lee et al., 2011; 2012), giving rise to the hypothesis that mice cannot develop hyperglycemia in absence of glucagon action (Unger and Cherrington, 2012). Here, we show that Gcgr-/- and anti-GCGR mAb-treated animals develop severe hyperglycemia after massive DT-mediated β-cell ablation (Figures 1 and 2). Our results suggest that the disparity in blood glucose levels observed between STZ- and DT-treated Gcgr-/- animals originate from a difference in β-cell destruction efficiency (Figure 3).
Recent studies reached conflicting conclusions regarding the beneficial effect of glucagon signaling blockade in severely diabetic mice: Wang et al reported that anti-GCGR mAb treatment was sufficient to normalize glycemia of STZ-treated BALB/c animals (Wang et al., 2015), whereas Steenberg et al did not observe improvements in glucose tolerance after GCGR antagonism or glucagon immunoneutralisation in C57BL/6 mice (Steenberg et al., 2016). These discrepancies may be explained by differences in completeness of β-cell ablation linked to the protocol of injection (single high dose versus multiple low doses) and/or to strain-dependent sensitivity; it was indeed reported that BALB/c mice are less sensitive to STZ than C57BL/6 animals (Cardinal et al., 1998; Gurley, 2006). Here, we injected C57BL/6 mice with two different high doses of STZ that triggered a severe hyperglycemia; after anti-GCGR mAb treatment, however, we observed a decrease in glycemia only in animals treated with the lowest STZ dose. These results indicate that a small difference in pancreatic insulin, such as that observed after 175 and 225 mg/kg STZ, can cause a major difference in glycemia in animals lacking glucagon signaling, thereby highlighting the importance of residual insulin action and providing a potential explanation for discrepancies between previous studies (Figure 5). Remarkably, STZ-treated Gcgr-/- mice became hyperglycemic upon S961-mediated insulin receptor antagonism, illustrating the requirement of residual insulin action for maintenance of normoglycemia in these animals (Figure 4). Collectively, these findings demonstrate that a total absence of glucagon action is not sufficient to prevent hyperglycemia in case of severe insulin deficiency.
Although Gcgr-/- mice developed diabetes upon massive β-cell ablation, lack of glucagon action reduced or normalized glycemia in conditions of less severe insulin deficiency. In particular we observed that i) anti-GCGR mAb administration reduced hyperglycemia in C57BL/6 mice treated with the lowest STZ dose (Figure 5) and ii) S961 treatment caused a less severe increase in glycemia in Gcgr-/- than in Gcgr+/- animals (Figure 4A). Our data on hepatic expression of Pepck and Gck, two rate-limiting enzymes of gluconeogenesis and glycolysis, respectively, suggest that lack of glucagon signaling counterbalances the effects of insulin insufficiency after STZ or S961. This would prevent, or limit, the rise in net hepatic glucose output by decreasing gluconeogenesis and glycogenolysis, and by increasing glycolysis and glycogenesis. Absence of glucagon action is however not sufficient to compensate severe insulin deficiency after DT, as reflected by Gck downregulation and reduced hepatic glycogen content, thereby contributing to the elevation of blood glucose. Interestingly, the mRNA levels of FoxO1 target genes in skeletal muscle were strongly upregulated, reflecting insulin signaling insufficiency, after DT and STZ+S961, the two conditions that led to hyperglycemia (Figure 4 and Figure 4—figure supplement 3). In addition, gonadal adipose tissue was markedly depleted in experimental conditions leading to hyperglycemia (in Gcgr-/- mice after STZ+S691 and DT; in Gcgr+/- mice after STZ and DT; not shown). Together, these findings provide new insights into the mechanisms by which lack of glucagon signaling protects against elevated blood glucose levels in situations of insulin insufficiency. Recent studies have shown that protection against STZ-mediated hyperglycemia also rely on the high levels of circulating glucagon-like peptide-1 (GLP-1) in Gcgr-/- animals (Gu et al., 2010; Ali et al., 2011; Jun, 2014; Omar et al., 2014). Yet, these high levels of GLP-1 combined with a lack of glucagon action were insufficient to maintain normoglycemia after near-total β-cell loss.
Finally, we report here that lack of glucagon signaling does not compromise the ability of α-cells to convert to insulin production after DT-mediated near-total β-cell loss. Indeed, YFP-traced α-cells become glucagon/insulin bihormonal cells after DT in RIP-DTR;Gcgr-/- mice and in animals treated with the anti-GCGR antibody (Figure 6 and Figure 6—figure supplement 1). The proportion of α-cells co-expressing insulin after DT is comparable between mice with either intact, reduced or absent glucagon signaling, indicating that glucagon does not play an essential role in the α-to-β transdifferentiation process. Interestingly, because glucagon signaling inhibition leads to a compensatory α-cell hyperplasia (Furuta et al., 1997; Gelling et al., 2003; Longuet et al., 2013), the absolute number of newly formed insulin-producing cells through α-cell conversion was augmented in RIP-DTR;Gcgr-/- mice. As previously described in adult mice (Chera et al., 2014), we also observed the δ-to-β conversion in β-cell-ablated RIP-DTR;Gcgr-/- mice (not shown).
In conclusion, although inhibition of glucagon action alone is insufficient to prevent diabetes in conditions of near-total insulin deficiency, it is beneficial when residual insulin action persists, as in STZ-treated Gcgr-/- animals. Combination of glucagon inhibition with insulin therapy may however increase the risk of hypoglycemia. We encountered this problem when using subcutaneous insulin pellets in DT-treated RIP-DTR;Gcgr-/- mice: they became hypoglycemic and died likely as a consequence of the constitutive insulin release from the pellets, which could not be compensated by glucagon action. Our findings suggest that diabetes therapy through glucagon suppression would be unsafe if exogenous insulin has to be supplemented, but may be beneficial in patients with sufficient residual insulin action. In case of near-total insulin deficiency, transient glucagon receptor blockade could also serve as a means to increase the α-cell mass before triggering insulin production in these cells, a strategy that might be envisioned as a novel therapy to treat diabetes.
Materials and methods
Mice
Gcgr-/- (Gelling et al., 2003), RIP-DTR (Rat insulin promoter - diphtheria toxin receptor) (Thorel et al., 2010), Gcg-rtTA (Glucagon promoter - reverse tetracycline transactivator) (Thorel et al., 2010), TetO-Cre (Tetracycline operator - Cre recombinase) (Perl et al., 2002), and R26-YFP (Rosa26 promoter - yellow fluorescent protein) (Srinivas et al., 2001) mice were described previously and bred on a C57BL/6-enriched mixed genetic background. As pups born from Gcgr-/- mothers die perinatally (Vuguin et al., 2006), Gcgr+/- females were used for breeding. C57BL/6 mice were purchased from Janvier Labs (France). All mice used in this study were adult (10–20 week old) males or females. They were housed and treated in accordance with the guidelines and regulations of the Direction Générale de la Santé, state of Geneva. Blood glucose was measured from tail blood using a handheld glucometer (detection range: 0.6 to 33.3 mM, values exceeding 33.3 mM were artificially set to 34 mM).
Diphtheria toxin (DT), Streptozotocin (STZ), and Doxycycline (DOX) treatments
For β-cell ablation in RIP-DTR mice, DT (D0564, Sigma, St. Louis, MO) was injected i.p. in 3 injections of 125 ng each, at days 0, 3, and 4. STZ (S0130, Sigma) was used as an alternative method of β-cell ablation. It was freshly diluted in citrate buffer and administered in 5-h fasted mice. Two different protocols were used depending on the genetic background: i) Gcgr+/- and Gcgr-/- mice: two i.p. injections of 200 and 150 mg/kg, one week apart; ii) C57BL/6 mice: single i.p. injection (175 or 225 mg/kg). For inducible α-cell labeling in Gcg-rtTA;TetO-Cre;R26-YFP mice, DOX (D9891, Sigma) was added to drinking water (1 mg/ml) for 2 weeks followed by at least 2 weeks of clearance before DT injection.
Anti-GCGR mAb
Anti-GCGR monoclonal antibody A-9 was generated at Eli Lilly and Company (Yan H, Hu S-FS, Boone TC, Lindberg RA, inventors; Amgen Inc., assignee. Compositions and methods relating to glucagon receptor antibodies. United States patent US 8158759 B2, 2012 Apr 17). It was delivered either via i.p. injections, thrice weekly (9 mg/kg per injection), or using a s.c. implanted osmotic pump (model 2002, Alzet, Cupertino, CA) containing 11 mg/ml of anti-GCGR mAb in PBS (estimated delivery rate: 5.5 μg/h for 2 weeks).
S961
The insulin receptor inhibitor S961 was a kind gift of Lauge Schäffer (Novo Nordisk, Denmark) (Schäffer et al., 2008). Mice were implanted s.c. with an osmotic pump (model 1007D, Alzet) loaded with 40 nmol S961 (estimated delivery rate: 0.25 nmol/h for 1 week).
Insulin
Long-acting insulin detemir (Levemir, Novo Nordisk) was freshly diluted in NaCl 0.9% and injected s.c. twice per day (1.7 U/kg in the morning, 3.3 U/kg in the evening). Insulin pellets (LinShin Canada Inc., Canada) were implanted s.c.
Intraperitoneal glucose tolerance test (ipGTT) and insulin tolerance test (ITT)
For the ipGTT, mice were fasted overnight (15 hr) and then injected i.p. with 2 mg/kg D-glucose. For the ITT, mice were fasted for 5 hr and injected i.p. with 0.7 U/kg insulin (Humalog, Eli Lilly).
Immunofluorescence
Following euthanasia, collected pancreata were processed as described (Desgraz and Herrera, 2009). Paraffin and cryostat sections were 5 and 10 μm-thick, respectively. Primary antibodies: guinea pig anti-insulin (1:400, Dako, Denmark), mouse anti-glucagon (1:250 to 1:1000, Sigma), and rabbit anti-GFP (1:200, Molecular Probes Inc., Eugene, OR). Secondary antibodies were coupled to Alexa Fluor dyes 488, 568, or 647 (1:500, Molecular Probes Inc.); or to FITC, Cy3, or Cy5 (1:500, Jackson ImmunoResearch, West Grove, PA). Images were acquired on a confocal microscope (TCS SPE, Leica Microsystems, Germany). For cell mass measurement, 8 to 12 equally spaced sections per pancreas were imaged on a Leica M205 FA stereo microscope. Islets were manually selected using ImageJ (NIH) and thresholding was applied to measure the insulin- and glucagon-positive areas.
RNA extraction and RT-qPCR
After dissection, liver and skeletal muscle (gastrocnemius) were immediately stored in RNAlater (Sigma). Tissues were homogenized with a Polytron and total RNA was extracted with the Qiagen (Germany) RNeasy mini kit (standard kit for liver, fibrous tissue kit for muscle). Reverse transcription was performed using the Qiagen QuantiTect RT kit. qPCR reactions and analyses were performed as described (Thorel et al., 2010); each sample was run in triplicate. For normalization, eight housekeeping genes were tested and the three more stable across our experimental conditions were defined using geNorm (Vandesompele et al., 2002): β-Glucuronidase (Gusb), Glyceraldehyde-3-phosphate dehydrogenase (Gapdh), and Non-POU-domain-containing, octamer binding protein (Nono) for liver; β-actin (Actb), Gapdh, and Gusb for skeletal muscle. Primer sequences are indicated in Supplementary file 1.
Hormone and glycogen measurements
Protein extracts from total pancreas were prepared as described (Strom et al., 2007). Blood samples were collected in EDTA-coated tubes and plasma was separated by centrifugation. Insulin and glucagon concentrations were measured using Ultrasensitive Mouse Insulin and Glucagon ELISA kits (Mercodia, Sweden), respectively. Glycogen concentration was measured from the supernatatant of homogenized liver tissue using a glycogen asssay kit (Sigma).
Immunoblotting
Liver samples were lyzed in radioimmumoprecipitation (RIPA) buffer with protease inhibitors (Thermo Fisher Scientific, Waltham, MA). Protein concentration was measured using a BCA assay (Thermo Fisher Scientific). Proteins were resolved on a TruPAGE gel (Sigma) and transferred to a PVDF membrane. The membrane was blocked in Tris-buffered saline with 0.1% Tween containing 5% bovine serum albumin. Primary antibodies were rabbit anti-PEPCK (1:1500, Abcam, UK) and mouse anti-tubulin (1:2500), both incubated overnight at 4°C; secondary antibodies were horseradish peroxidase-conjugated anti-rabbit (1:5000) and anti-mouse (1:5000). Proteins were detected using ECL plus substrate (Thermo Fisher Scientific) and images were acquired on a LAS-4000 imager (Fujifilm, Japan).
Statistical analyses
Data are presented as mean ± SEM. P values were calculated with GraphPad Prism 6 (GraphPad Software, La Jolla, CA). The following statistical tests were applied: unpaired, two-tailed, Mann-Whitney U test for two sample comparisons; one- or two-way ANOVA with post hoc Bonferroni correction for multiple comparisons; Log-rank (Mantel-Cox) test for survival analyses.
Acknowledgements
We thank Gissela Cabrera Gallardo, Carine Gysler and Muriel Urwyler for excellent technical help. We thank Rohn Millican and Paul Cain for generating Gcgr Ab reagent, and Lauge Schäffer (Novo Nordisk) for kindly providing S961. Work was funded by grants from the Department of Veterans Affairs, the NIH (DK66636, DK72473, DK89572, DK89538), the Vanderbilt Diabetes Research and Training Center (DK20593), and the Juvenile Diabetes Research Foundation (JDRF) (to ACP), as well as the Institute of Genomics and Genetics of Geneva (iGE3), the Swiss National Science Foundation (National Research Programme NRP63), the NIH (Beta Cell Biology Consortium), the JDRF, and the European Union (to PLH).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding Information
This paper was supported by the following grants:
Institute of Genomics and Genetics of Geneva to Nicolas Damond, Pedro L Herrera.
Juvenile Diabetes Research Foundation to Alvin C Powers, Pedro L Herrera.
U.S. Department of Veterans Affairs to Alvin C Powers.
National Institutes of Health DK66636 to Alvin C Powers.
Vanderbilt Diabetes Research and Training Center DK20593 to Alvin C Powers.
National Institutes of Health DK72473 to Alvin C Powers.
National Institutes of Health DK89572 to Alvin C Powers.
National Institutes of Health DK89538 to Alvin C Powers.
Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung NRP63 to Pedro L Herrera.
National Institutes of Health BCBC & HIRN to Pedro L Herrera.
European Union Imidia to Pedro L Herrera.
Additional information
Competing interests
The other authors declare that no competing interests exist.
JSM: Employee and shareholder of Eli Lilly and Company.
Author contributions
ND, Conceived and performed the experiments and analyses, Wrote the manuscript.
FT, Conceived the experiments, Analysis and interpretation of data, Wrote the manuscript.
JSM, Shared the anti-Gcgr mAb and contributed to discussion, Contributed unpublished essential data or reagents.
MJC, Generated and shared the Gcgr-/- mice, Contributed to the planning of experiments, Edited the manuscript, Contributed unpublished essential data or reagents.
PMV, Contributed unpublished essential data or reagents, Generated and shared the Gcgr-/- mice, contributed to the planning of experiments and edited the manuscript.
ACP, Generated and shared the Gcgr-/- mice, Contributed to the planning of experiments, Edited the manuscript, Conception and design, Contributed unpublished essential data or reagents.
PLH, Conceived the experiments, Analysis and interpretation of data, Wrote the manuscript.
Ethics
Animal experimentation: All mice were housed and treated in accordance with the guidelines and regulations of the Direction Générale de la Santé, state of Geneva (license number GE/103/14).
Additional files
References
- Ali S, Lamont BJ, Charron MJ, Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. The Journal of Clinical Investigation. 2011;121:1917–1929. doi: 10.1172/JCI43615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand CL, Rolin B, Jørgensen PN, Svendsen I, Kristensen JS, Holst JJ. Immunoneutralization of endogenous glucagon with monoclonal glucagon antibody normalizes hyperglycaemia in moderately streptozotocin-diabetic rats. Diabetologia. 1994;37:985–993. doi: 10.1007/bf00400461. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. Islet alpha cells and glucagon--critical regulators of energy homeostasis. Nature Reviews. Endocrinology. 2015;11:329–338. doi: 10.1038/nrendo.2015.51. [DOI] [PubMed] [Google Scholar]
- Cardinal JW, Allan DJ, Cameron DP. Differential metabolite accumulation may be the cause of strain differences in sensitivity to streptozotocin-induced beta cell death in inbred mice. Endocrinology. 1998;139:2885–2891. doi: 10.1210/endo.139.6.6048. [DOI] [PubMed] [Google Scholar]
- Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-c. Critical Reviews in Biochemistry and Molecular Biology. 2005;40:129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic delta cells into insulin producers. Nature. 2014;514:503–507. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, Ronan J, Liu F, Roy RS, Zhu L, Charron MJ, Zhang BB. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia. 2007;50:142–150. doi: 10.1007/s00125-006-0481-3. [DOI] [PubMed] [Google Scholar]
- D'Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes, Obesity and Metabolism. 2011;13:126–132. doi: 10.1111/j.1463-1326.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC. Single dose streptozotocin-induced diabetes: Considerations for study design in islet transplantation models. Laboratory Animals. 2011;45:131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgraz R, Herrera PL. Pancreatic neurogenin 3-expressing cells are unipotent islet precursors. Development. 2009;136:3567–3574. doi: 10.1242/dev.039214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, Johansen PB, Rossetti L, Jelicks LA, Serup P, Nishimura E, Charron MJ. Lower blood glucose, hyperglucagonemia, and pancreatic cell hyperplasia in glucagon receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocrine Reviews. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Gu W, Yan H, Winters KA, Komorowski R, Vonderfecht S, Atangan L, Sivits G, Hill D, Yang J, Bi V, Shen Y, Hu S, Boone T, Lindberg RA, Véniant MM. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. The Journal of Pharmacology and Experimental Therapeutics. 2009;331:871–881. doi: 10.1124/jpet.109.157685. [DOI] [PubMed] [Google Scholar]
- Gu W, Winters KA, Motani AS, Komorowski R, Zhang Y, Liu Q, Wu X, Rulifson IC, Sivits G, Graham M, Yan H, Wang P, Moore S, Meng T, Lindberg RA, Veniant MM. Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. AJP: Endocrinology and Metabolism. 2010;299:e13828. doi: 10.1152/ajpendo.00102.2010. [DOI] [PubMed] [Google Scholar]
- Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. AJP: Renal Physiology. 2006;290:F214–F222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Molecular Endocrinology. 2010;24:1605–1614. doi: 10.1210/me.2010-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iynedjian PB, Marie S, Gjinovci A, Genin B, Deng SP, Buhler L, Morel P, Mentha G. Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. regulation of gene expression in cultured hepatocytes. The Journal of Clinical Investigation. 1995;95:1966–1973. doi: 10.1172/JCI117880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science. 1982;215:1115–1116. doi: 10.1126/science.6278587. [DOI] [PubMed] [Google Scholar]
- Jun LS. Absence of glucagon and insulin action reveals a role for the glucagon-like peptide-1 receptor in endogenous glucose production. Diabetes. 2014 doi: 10.2337/db14-1052. [DOI] [PubMed] [Google Scholar]
- Kelly RP, Garhyan P, Raddad E, Fu H, Lim CN, Prince MJ, Pinaire JA, Loh MT, Deeg MA. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes, Obesity and Metabolism. 2015;17:414–422. doi: 10.1111/dom.12446. [DOI] [PubMed] [Google Scholar]
- Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Berglund ED, Wang M.-y., Fu X, Yu X, Charron MJ, Burgess SC, Unger RH. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proceedings of the National Academy of Science of the Uunited States of America. 2012;109:14972–14976. doi: 10.1073/pnas.1205983109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Cheng Z, Copps KD, White MF. Insulin receptor substrates irs1 and irs2 coordinate skeletal muscle growth and metabolism via the akt and AMPK pathways. Molecular and Cellular Biology. 2011;31:430–441. doi: 10.1128/MCB.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, de Chavez V, Vuguin PM, Charron MJ, Powers AC, Drucker DJ. Liver-specific disruption of the murine glucagon receptor produces alpha-cell hyperplasia: Evidence for a circulating alpha-cell growth factor. Diabetes. 2013;62:1196–1205. doi: 10.2337/db11-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: Indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–2228. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular Cell. 2000;6:87–97. doi: 10.1016/s1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- Mu J, Jiang G, Brady E, Dallas-Yang Q, Liu F, Woods J, Zycband E, Wright M, Li Z, Lu K, Zhu L, Shen X, Sinharoy R, Candelore ML, Qureshi SA, Shen DM, Zhang F, Parmee ER, Zhang BB. Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia. 2011;54:2381–2391. doi: 10.1007/s00125-011-2217-2. [DOI] [PubMed] [Google Scholar]
- Omar BA, Andersen B, Hald J, Raun K, Nishimura E, Ahrén B. Fibroblast growth factor 21 (FGF21) and glucagon-like peptide 1 contribute to diabetes resistance in glucagon receptor-deficient mice. Diabetes. 2014;63:101–110. doi: 10.2337/db13-0710. [DOI] [PubMed] [Google Scholar]
- Ouhilal S, Vuguin P, Cui L, Du X-Q, Gelling RW, Reznik SE, Russell R, Parlow AF, Karpovsky C, Santoro N, Charron MJ. Hypoglycemia, hyperglucagonemia, and fetoplacental defects in glucagon receptor knockout mice: A role for glucagon action in pregnancy maintenance. AJP: Endocrinology and Metabolism. 2012;302:e13828. doi: 10.1152/ajpendo.00420.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A-KT, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucktäschel AK, Granner DK, Christ B. Regulation by glucagon (camp) and insulin of the promoter of the human phosphoenolpyruvate carboxykinase gene (cytosolic) in cultured rat hepatocytes and in human hepatoblastoma cells. The Biochemical Journal. 2000;352 Pt 1:211–217. doi: 10.1042/bj3520211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffer L, Brand CL, Hansen BF, Ribel U, Shaw AC, Slaaby R, Sturis J. A novel high-affinity peptide antagonist to the insulin receptor. Biochemical and Biophysical Research Communications. 2008;376:380–383. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- Sherwin RS, Fisher M, Hendler R, Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. The New England Journal of Medicine. 1976;294:455–461. doi: 10.1056/NEJM197602262940901. [DOI] [PubMed] [Google Scholar]
- Sloop KW, Cao JX, Siesky AM, Zhang HY, Bodenmiller DM, Cox AL, Jacobs SJ, Moyers JS, Owens RA, Showalter AD, Brenner MB, Raap A, Gromada J, Berridge BR, Monteith DK, Porksen N, McKay RA, Monia BP, Bhanot S, Watts LM, Michael MD. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. The Journal of Clinical Investigation. 2004;113:1571–1581. doi: 10.1172/JCI20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen H, Brand CL, Neschen S, Holst JJ, Fosgerau K, Nishimura E, Shulman GI. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes. 2006;55:2843–2848. doi: 10.2337/db06-0222. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Developmental Biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenberg VR, Jensen SM, Pedersen J, Madsen AN, Windeløv JA, Holst B, Quistorff B, Poulsen SS, Holst JJ. Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetologia. 2016;59:363–370. doi: 10.1007/s00125-015-3794-2. [DOI] [PubMed] [Google Scholar]
- Strom A, Bonal C, Ashery-Padan R, Hashimoto N, Campos ML, Trumpp A, Noda T, Kido Y, Real FX, Thorel F, Herrera PL. Unique mechanisms of growth regulation and tumor suppression upon apc inactivation in the pancreas. Development. 2007;134:2719–2725. doi: 10.1242/dev.02875. [DOI] [PubMed] [Google Scholar]
- Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM. Studies of pancreatic alpha cell function in normal and diabetic subjects. The Journal of Clinical Investigation. 1970;49:837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Glucagon physiology and pathophysiology. The New England Journal of Medicine. 1971;285:443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- Unger RH, Orci L. Paracrinology of islets and the paracrinopathy of diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16009–16012. doi: 10.1073/pnas.1006639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. The Journal of Clinical Investigation. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:research0034.1–research0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, Nejathaim M, Charron MJ, Teitelman G. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology. 2006;147:3995–4006. doi: 10.1210/en.2005-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuguin PM, Charron MJ. Novel insight into glucagon receptor action: Lessons from knockout and transgenic mouse models. Diabetes, Obesity and Metabolism. 2011;13:144–150. doi: 10.1111/j.1463-1326.2011.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged c-peptide production in type 1 diabetes as measured with an ultrasensitive c-peptide assay. Diabetes Care. 2012;35:465–470. doi: 10.2337/dc11-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M-Y, Yan H, Shi Z, Evans MR, Yu X, Lee Y, Chen S, Williams A, Philippe J, Roth MG, Unger RH. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proceedings of the National Academy of Sciences of the United States of America . 2015;112:2503–2508. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Gu W, Yang J, Bi V, Shen Y, Lee E, Winters KA, Komorowski R, Zhang C, Patel JJ, Caughey D, Elliott GS, Lau YY, Wang J, Li YS, Boone T, Lindberg RA, Hu S, Véniant MM. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. The Journal of Pharmacology and Experimental Therapeutics. 2009;329:102–111. doi: 10.1124/jpet.108.147009. [DOI] [PubMed] [Google Scholar]
- Yang J, MacDougall ML, McDowell MT, Xi L, Wei R, Zavadoski WJ, Molloy MP, Baker JD, Kuhn M, Cabrera O, Treadway JL. Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor (GCGR) knockout mice: Implications on anti-glucagon therapies for diabetes. BMC Genomics. 2011;12:281. doi: 10.1186/1471-2164-12-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P, Park JS, Melton DA. Betatrophin: A hormone that controls pancreatic beta cell proliferation. Cell. 2013;153:747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]