Abstract
Bone marrow-derived mesenchymal stromal stem cells (BMSCs) are a promising cell source for treating articular cartilage defects (Bornes et al., 2014). BMSCs can be seeded within porous biomaterial scaffolds that support three-dimensional cell organization, chondrogenic differentiation and extracellular matrix deposition for the creation of engineered cartilage. This protocol describes our defined methods for isolation and expansion of human and ovine BMSCs, seeding of BMSCs within porous scaffolds and in vitro chondrogenic differentiation (Adesida et al., 2012; Bornes et al., 2015).
Materials and Reagents
-
Culture plate, 24 wells (Becton Dickinson Labware, catalog number: 353047)
Note: Currently, it is “Corning, Falcon®, catalog number: 353047”.
Pipette tips, 1,000 μl, 200 μl and 10 μl volumes (Corning, DeckWorks™, catalog numbers: 4124, 4121 and 4120)
-
Pasteur pipettes, 230 mm length (WHEATON, catalog number: 4500448667)
Note: Currently, it is “WHEATON, catalog number: 357335”.
Conical tube, 50 ml volume (Corning, Falcon®, catalog number: 352070)
Cell strainer, nylon with 100 μm pores (Corning, BD Biosciences, catalog number: 352360)
Tissue-culture flask, 150 cm2 surface area (T150) (Corning, Falcon®, catalog number: 355000)
Conical microtube, 1.5 ml volume (Bio Basic Canada, catalog number: TC152SN)
Biopsy punch, circular with 6 mm diameter ( Southern Anesthesia & Surgical, Miltex, catalog number: 3336)
Bone marrow aspirate, human or ovine, collected through needle aspiration at the iliac crest
Crystal violet solution (Sigma-Aldrich, catalog number: HT90132)
Alpha minimal essential medium (αMEM), containing Earle’s salts, ribonucleosides, deoxyribonucleosides and L-glutamine (Thermo Fisher Scientific, Corning, Mediatech, catalog number: 10022CV)
-
Fetal bovine serum (FBS), heat inactivated at 56 °C in the laboratory (Life Technologies, Gibco®, catalog number: 12483)
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 12483”.
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (1 M) (Life Technologies, Gibco®, catalog number: 15630)
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 15630”.
-
Sodium pyruvate, 100 mM (Life Technologies, Gibco®, catalog number: 11360)
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 11360”.
Fibroblast growth factor-two (FGF-2), human recombinant (Neuromics, catalog number: PR80001)
Dulbecco’s phosphate buffered saline (PBS), sterile filtered (Sigma-Aldrich, catalog number: D8537)
Human serum albumin (Sigma-Aldrich, catalog number: A4327)
Transforming growth factor-beta three (TGF-β3), human recombinant, HEK (Prospecbio, ProSpec, catalog number: CYT-113)
L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate (Sigma-Aldrich, catalog number: A8960)
Dexamethasone-Water Soluble (Sigma-Aldrich, catalog number: D2915)
L-proline (Sigma-Aldrich, catalog number: P5607)
Trypan blue solution, 0.4% (Sigma-Aldrich, catalog number: T8154)
Porous scaffolds, collagen I or esterified hyaluronic acid (described in detail in Bornes et al., 2015)
-
Penicillin-streptomycin-glutamine (Life Technologies, Gibco®, catalog number: 1248310378) (see Recipes)
Note: Currently, it is “Thermo Fisher Scientific, Gibco™, catalog number: 1248310378”.
Trypsin-ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, Corning, catalog number: 25052) (see Recipes)
Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich, catalog number: D6429) (see Recipes)
Insulin-transferrin-selenium (ITS+) premix (Cornig, BD Biosciences, catalog number: 354352) (see Recipes)
Expansion medium (see Recipes)
Serum-free medium (see Recipes)
TGF-β3 working solution (see Recipes)
Chondrogenic medium (see Recipes)
Equipment
Biosafety cabinet (Microzone Corporation, catalog number: BK-2-6 A2)
Incubator, containing humidified air at 37 °C with 5% carbon dioxide, and 3% oxygen (Thermo Fisher Scientific, catalog number: Forma Series II Water Jacket CO2)
Centrifuge, 1,500 revolutions per min (rpm) (Beckman Coulter, Allegra™, catalog number: X-22R)
Light microscope (Microscope, Omano, catalog number: OM159T)
Pipette (Drummond Scientific Company, catalog number: Pipet Aid XP)
Micropipettes, volumes of 100–1,000 μl, 20–200 μl, 2–20 μl, and 0.5–10 μl (Bio-Rad Laboratories, catalog numbers: 1660508, 1660507, 1660506, and 1660505)
Suction source
Forceps
Water bath, set to 37 °C (VWR International, catalog number: 89501)
Neubauer hemacytometer, 0.1 mm deep (Reichert Bright-Line) (Sigma-Aldrich, catalog number: Z359629)
Procedure
Methods involving manipulation of bone marrow aspirate, cells and porous scaffolds must be performed within a biosafety cabinet. Instruments, solutions and media in contact with bone marrow aspirate, cells and porous scaffolds must be sterile. Solutions and medium should be preheated to 37 °C in a closed container submersed in a water bath.
A. Isolation and expansion of BMSCs
Obtain a sterile bone marrow aspirate. Collection of heparinized human and ovine iliac crest aspirates has been described in detail previously (Buda et al., 2010; Bornes et al., 2015). Aspirate volumes of 15–60 ml and 20–40 ml may be obtained from human and ovine donors, respectively (Wakitani et al., 2007; Buda et al., 2010; Nejadnik et al., 2010; Zscharnack et al., 2010; Bornes et al., 2015). Aspirates should be taken to the laboratory directly following collection for processing in order to avoid cell death.
Filter the bone marrow aspirate using a 100 μm cell strainer to remove clots and tissue. Collect the filtrate in a sterile 50 ml conical tube.
For mononucleated cell counting, micropipette 50 μl of diluted bone marrow aspirate filtrate (1:10 in PBS; dilution might have to be increased to 1:50 if cells are highly concentrated) and 50 μl of diluted crystal violet solution (1:50 in PBS) into a 1.5 ml conical microtube and mix thoroughly. Micropipette 20 μl of this mixture into a hemacytometer. Count the number of mononucleated cells within the four grid squares of the hemacytometer using a light microscope. Divide the number of cells counted by four (number of grid squares in hemacytometer), multiply by 20 (dilution of aspirate filtrate) and multiply by 10,000 (hemacytometer factor) to calculate the concentration of mononucleated cells per ml of aspirate filtrate. The total number of mononucleated cells is then determined my multiplying the concentration by the total volume (in ml) of the aspirate filtrate.
Calculate the volume of bone marrow aspirate filtrate containing 15 million human mononucleated cells (Adesida et al., 2012) or 80 million ovine mononucleated cells (Bornes et al., 2015) and pipette this volume into a sterile 50 ml conical tube. Pipette 20 ml of expansion medium into the 50 ml conical tube and mix with the aspirate filtrate. Transfer the aspirate filtrate-medium mixture into a T150 flask (passage 0). If large numbers of mononucleated cells are present in the aspirate filtrate, multiple T150 flasks may be seeded.
Statically incubate each T150 flask for seven days undisturbed at 37 °C. No media changes should be performed during this period.
After seven days, aspirate off medium and wash adherent BMSCs with 10 ml of PBS. Pipette 20 ml of fresh expansion medium into each T150 flask.
Statically incubate each T150 flask at 37 °C, and change the medium twice per week until 80% confluence is obtained based on microscopy.
Once 80% confluence has been reached, aspirate off medium and wash cells with 10 ml of PBS.
Pipette 6 ml of trypsin-EDTA into each T150 flask and incubate at 37 °C for 5 min. Agitate the flask to promote detachment of BMSCs from the flask surface. Microscopy may be used to confirm detachment of cells.
Pipette the BMSC-trypsin-EDTA mixture into a 50-ml conical tube and add 2 ml of serum-containing medium (expansion medium or αMEM with FBS) to deactivate trypsin.
Centrifuge the resulting mixture for 10 min (1,500 rpm) and aspirate off the liquid component. The BMSC collection will be present at the bottom of the 50 ml conical tube.
For BMSC counting, re-suspend the BMSC collection within a known quantity of expansion medium (e.g. 10 ml). Micropipette 50 μl of BMSC collection and 50 μl of trypan blue into a 1.5-ml conical microtube and mix thoroughly. Micropipette 20 μl of this mixture into a hemacytometer. Count the number of BMSCs within the four grid squares of the hemacytometer using a light microscope. Divide the number of cells by four (number of grid squares), multiply by 2 (dilution of BMSC suspension) and multiply by 10,000 (hemacytometer factor) to calculate the concentration of cells (BMSCs per ml of suspension). The total number of BMSCs is then determined by multiplying this concentration by total volume (in ml) of the BMSC suspension. Each T150 flask can be expected to yield 1–3 million BMSCs for human donors and 3–10 million BMSCs for ovine donors.
Add expansion medium to the BMSCs and re-suspend. BMSCs derived from one T150 flask during passage 0 should be re-suspended in 40 ml of expansion medium and divided into two T150 flasks for passage 1. There are a total of two T150 flasks during passage 1 per one T150 flask during passage 0.
Repeat steps A7-12 for passage 1.
Add expansion medium to the BMSCs and re-suspend. BMSCs derived from each T150 flask during passage 1 should be re-suspended in 40 ml of expansion medium and divided into two T150 flasks for passage 2. There are a total of four T150 flasks during passage 2 per one T150 flask during passage 0.
Repeat steps A7-12 for passage 2 BMSCs (Figures 1A–1B). Subsequent passaging may be performed beyond passage 2 to increase the number of BMSCs available for use, although prolonged BMSC expansion has been shown to result in de-differentiation and loss of multipotent differentiation capacity of BMSCs (Wagner et al., 2008; Tsai et al., 2011). Therefore, the authors recommend using passage 2 BMSCs for use in chondrogenic differentiation.
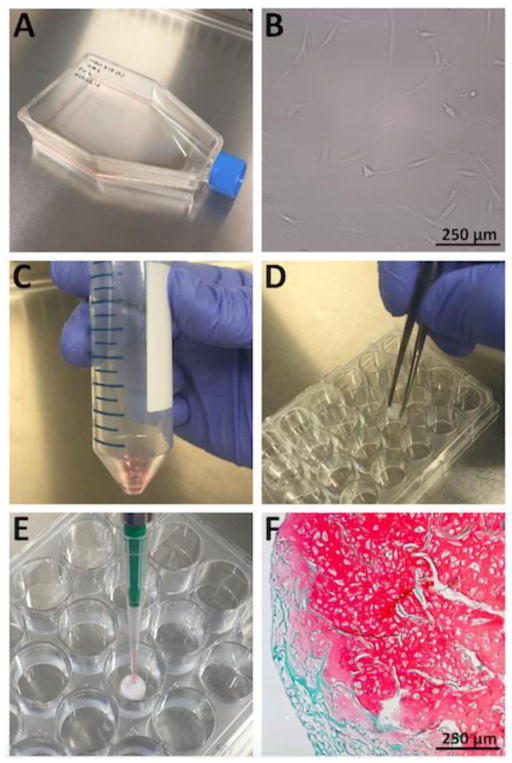
Figure 1. Isolation, expansion, seeding, and chondrogenic differentiation of BMSCs.
A. A tissue-culture flask (T150) containing isolated BMSCs and expansion medium. B. Adherent, human BMSCs on the surface of a tissue-culture flask demonstrating a characteristic spindle-shaped morphology during expansion (10× magnification). C. Re-suspension of BMSCs in chondrogenic medium within a 50 ml conical tube prior to scaffold seeding. D. Collagen I scaffold placement into the empty well of a 24 well culture plate using forceps. E. Micropipetting of a BMSC-chondrogenic medium suspension onto the central area of a collagen I scaffold. F. Extracellular cartilaginous proteoglycans stained with safranin O following three weeks of chondrogenic differentiation of human BMSCs seeded within a collagen I scaffold. Remnant collagen I scaffold is stained with fast green counterstain [staining protocol described in detail by Bornes et al. (2015); 10× magnification].
B. Seeding of porous biomaterial scaffolds with BMSCs and chondrogenic differentiation
Porous scaffold composition and size should be based on the goals of the study. For in vitro assessment of chondrogenesis, porous scaffold sheets composed of collagen I sponge or esterified hyaluronic acid mesh (Bornes et al., 2015) may be cut into 6 mm-diameter cylinders using a biopsy punch. Dimensions of the scaffold of choice must be known to calculate BMSC seeding density.
Calculate the number of BMSCs required to create a seeding density of 10 million BMSCs per cm3 of scaffold. Other densities may be considered, although the authors recommend a seeding density of 5–10 million BMSCs per cm3 of scaffold to be used in chondrogenic differentiation. For cylindrical collagen I scaffolds with a diameter of 6 mm and height of 3.5 mm, 989,602 BMSCs are required per scaffold for a density of 10 million BMSCs per cm3. For cylindrical esterified hyaluronic acid scaffolds with a diameter of 6 mm and height of 2 mm, 565,487 BMSCs are required per scaffold.
Following counting of passage 2 BMSCs, centrifuge the resulting mixture for 10 min (1,500 rpm) and aspirate off the liquid component. The BMSC collection will be present at the bottom of the 50 ml conical tube.
Re-suspend BMSCs in chondrogenic medium with a total volume dependent on the number of scaffolds to be seeded (Figure 1C). For each 6 mm diameter scaffold, BMSCs (number calculated in step B2) should be re-suspended in 20 μl of chondrogenic medium.
Place scaffolds within empty wells of a 24 well culture plate using forceps (Figure 1D).
Micropipette the 20 μl BMSC-medium suspension onto the central area of the flat surface of each scaffold (Figure 1E). If the suspension does not spread throughout the entirety of the surface of the scaffold, pre-soaking the scaffold with 20 μl of cell-free chondrogenic medium may be required to promote full dispersion of the BMSC-medium suspension over the scaffold. If a larger scaffold is to be used, multiple BMSC-medium suspensions may be micropipetted onto different areas of the scaffold to promote uniform seeding.
Incubate BMSC-seeded scaffolds at 37 °C for 15 min.
Micropipette 100 μl of chondrogenic medium onto the base of each BMSC-seeded scaffold.
Incubate BMSC-seeded scaffolds at 37 °C for 30 min.
Micropipette 1 ml of chondrogenic medium into each well to submerse the BMSC-seeded scaffolds.
Statically incubate 24 well plates at 37 °C for 2–3 weeks. Change the chondrogenic medium twice per week. BMSCs will differentiate into cells capable of producing cartilaginous extracellular matrix (Figure 1F).
Recipes
-
Penicillin-streptomycin-glutamine
10,000 units/ml penicillin
10 mg/ml streptomycin
29.2 mg/ml L-glutamine
-
Trypsin-ethylenediaminetetraacetic acid (EDTA)
0.05% trypsin
0.53 mM EDTA without sodium bicarbonate
Calcium and magnesium
-
Dulbecco’s modified Eagle’s medium (DMEM)
4.5 mg/ml glucose
110 μg/ml sodium pyruvate
L-glutamine
-
Insulin-transferrin-selenium (ITS+) premix
625 μg/ml insulin
625 μg/ml transferrin
625 μg/ml selenium
125 μg/ml bovine serum albumin
535 μg/ml linoleic acid
-
Expansion medium, 565 ml total volume with final concentrations listed
500 ml αMEM
50 ml FBS [8.8% volume/volume (v/v)]
-
5 ml penicillin-streptomycin-glutamine
88.5 units/ml penicillin
88.5 μg/ml streptomycin
258.4 μg/ml L-glutamine
5 ml HEPES (8.8 mM)
5 ml sodium pyruvate (885.0 μM)
282.5 μl FGF-2, 10 μg/ml stock solution (5 ng/ml)
-
Serum-free medium, 182 ml total volume with final concentrations listed
166 ml DMEM
2 ml HEPES (11.0 mM)
-
2 ml penicillin-streptomycin-glutamine
109.9 units/ml penicillin
109.9 μg/ml streptomycin
320.9 μg/ml L-glutamine
2 ml ITS+ premix
10 ml human serum albumin, 25 mg/ml stock solution (1.4 mg/ml)
-
TGF-β3 working solution, 10 ml total volume with final concentrations listed
9.4 ml DMEM
100 μl TGF-β3, 10 μg/ml stock solution (100 ng/ml)
500 μl human serum albumin, 25 mg/ml stock solution (1.3 mg/ml)
-
Chondrogenic medium, 100 ml total volume with final concentrations listed
-
87 ml serum-free medium
DMEM
9.6 mM HEPES
95.6 units/ml penicillin
95.6 μg/ml streptomycin
279.2 μg/ml L-glutamine
1 ml ITS+ premix
-
10 ml TGF-β3 working solution
DMEM
10.0 ng/ml TGF-β3
125 μg/ml human serum albumin
1 ml ascorbic acid 2-phosphate, 3.65 mg/ml stock solution (365 μg/ml)
1 ml dexamethasone, 10 μM stock solution (100 nM)
1 ml L-proline, 4 mg/ml stock solution (40 μg/ml)
-
Acknowledgments
Research related to this protocol was funded by the University Hospital Foundation at the University of Alberta Hospital, Canadian Institutes of Health Research and the Edmonton Orthopaedic Research Committee. Integra LifeSciences Corp. and Anika Therapeutics Inc. have generously provided in-kind biomaterials for our laboratory. Stipend support for Troy Bornes was provided by Alberta Innovates - Health Solutions and Canadian Institutes of Health Research.
References
- 1.Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3(2):9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res Ther. 2014;16(5):432. doi: 10.1186/s13075-014-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornes TD, Jomha NM, Mulet-Sierra A, Adesida AB. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther. 2015;6:84. doi: 10.1186/s13287-015-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 5.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117(2):459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 7.Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3(5):e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1(1):74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 9.Zscharnack M, Hepp P, Richter R, Aigner T, Schulz R, Somerson J, Josten C, Bader A, Marquass B. Repair of chronic osteochondral defects using predifferentiated mesenchymal stem cells in an ovine model. Am J Sports Med. 2010;38(9):1857–1869. doi: 10.1177/0363546510365296. [DOI] [PubMed] [Google Scholar]