Abstract
Direct detection of the TROSY component of proton-attached 15N nuclei (15N-detected TROSY) yields high quality spectra with high field magnets, by taking advantage of the slow 15N transverse relaxation. The slow transverse relaxation and narrow line width of the 15N-detected TROSY resonances are expected to compensate for the inherently low 15N sensitivity. However, the sensitivity of 15N-detected TROSY in a previous report was one-order of magnitude lower than in the conventional 1H-detected version. This could be due to the fact that the previous experiments were performed at low salt (0–50 mM), which is advantageous for 1H-detected experiments. Here, we show that the sensitivity gap between 15N and 1H becomes marginal for a non-deuterated, large protein (τc = 35 ns) at a physiological salt concertation (200 mM). This effect is due to the high salt tolerance of the 15N-detected TROSY. Together with the previously reported benefits of the 15N-detected TROSY, our results provide further support for the significance of this experiment for structural studies of macromolecules when using high field magnets near and above 1 GHz.
Keywords: Nitrogen detection, TROSY, High field magnet, protein NMR, salt concentration, sensitivity
Introduction
Heteronuclear NMR experiments that directly detect resonances of nuclei with a low gyromagnetic ratio (γ), such as 13C and 15N, have recently been proposed to expand the utility of solution NMR in structural and functional studies of macromolecules (Takeuchi et al., 2012). A variety of experiments have been proposed for NMR analyses of proteins, using 13C-direct detection (Arnesano et al., 2005; Bermel et al., 2003; Bermel et al., 2006a; Bermel et al., 2006b; Felli and Brutscher, 2009; Hsu et al., 2009; Lee et al., 2005; Serber et al., 2001; Takeuchi et al., 2010a; Takeuchi et al., 2008) and 15N-direct detection (Gal et al., 2011; Levy and Richter, 1979; Takeuchi et al., 2015; Takeuchi et al., 2012; Takeuchi et al., 2010b; Vasos et al., 2006).
Recently, we reported that direct detection of the TROSY (Pervushin, 2000; Pervushin et al., 1997) component of 15N (15N-detected TROSY) is advantageous for maximizing the benefit of a low γ-nuclei detection experiment at a magnetic field higher than 14.1 T, which corresponds to 600 MHz in proton frequency (Takeuchi et al., 2015). Henceforth, we will use the frequency of the proton resonance for the description of field strength. We found that the 15N-detected TROSY-HSQC spectrum of a protein with a 40 ns rotational correlation time (τc) can be recorded in a few hours, with additional resolution benefits. Unlike conventional 1H-detected TROSY, uniform deuteration is not necessary for the 15N-detected experiment. Thus, 15N-detected TROSY provides a previously unexplored opportunity for NMR studies of proteins that can only be expressed in mammalian or insect cells, or proteins that cannot be refolded for amide back exchange after expression in D2O media. Since 15N-detected TROSY is expected to yield the narrowest NMR resonances, this method will help to resolve the signal degeneracy problems experienced with high molecular weight or unstructured systems.
Although the slow transverse relaxation and the narrow line width of the 15N resonances are expected to compensate for the inherently low sensitivity of 15N, the 15N-detected TROSY still suffered from much lower sensitivity, as compared to the conventional 1H-detected TROSY version (Takeuchi et al., 2015). However, it should be noted that the previous study was conducted under experimental conditions that are advantageous for the 1H-detected experiments, including non-physiologically low salt concentrations (0–50 mM NaCl with less than 10 mM buffer). Here we show that, due to the significant tolerance of 15N-detection under high-salt conditions, the disadvantage of 15N-detected TROSY relative to 1H-detected TROSY in terms of the sensitivity becomes marginal, for a small protein in high-salt (1 M) conditions. In addition, for a non-deuterated, large protein (τc = 35 ns), the sensitivities of 15N- and 1H-detected TROSY experiments become comparable at a physiological salt concentration (200 mM). These results indicate a wide applicability of the 15N-detected TROSY for structural and functional studies of macromolecules under physiologically relevant conditions while not requiring sample deuteration.
Materials and methods
All chemicals were purchased from Sigma (St. Louis, MO) unless otherwise noted. All stable-isotope-labeled materials were acquired from Cambridge Isotope Laboratories (Cambridge, MA).
Expression and purification of the B domain of protein G (GB1)
The gene encoding His6-tagged GB1, consisting of 64 amino acid residues, was cloned into the pET9d vector (Novagen, San Diego, CA) as previously described (Frueh et al., 2005). GB1 was expressed in commercially available BL21 (DE3) E. coli cells (Novagen) at 37 °C, and protein expression was induced for 6 h. For the uniformly 15N13C labeled samples, the cells were cultured in 15N, 13C M9 media containing 8.5 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 2mM MgCl2, and 0.1 mM CaCl2 in H2O, which was supplemented with 3 g/L 13C glucose and 1 g/L of 15NH4Cl. The protein was purified by Ni-NTA affinity chromatography as previously described (Frueh et al., 2005).
Expression and purification of MBP
The 42-kDa maltose binding protein (MBP), which has been extensively studied in E. coli was used in this study (Gardner et al., 1998). MBP was expressed in commercially available BL21 (DE3) E. coli cells (Novagen) at 37 °C and protein expression was induced for overnight at the 30°C. To prepare the uniformly 15N13C labeled samples, the cells were cultured in 15N, 13C M9 media containing 8.5 g/L Na2HPO4, 3 g/L KH2PO4, 0.5 g/L NaCl, 2mM MgCl2, and 0.1 mM CaCl2 in H2O, which was supplemented with 3 g/L 13C glucose and 1 g/L of 15NH4Cl. MBP was purified in a buffer containing 25 mM Tris–HCl (pH 7.6), 300 mM NaCl, and 1 mM EDTA, and was then loaded onto amylose resin (3 ml, New England Biolabs, Beverly, MA, USA), which was equilibrated with the buffer described above. After washing the resin, MBP was eluted with the same buffer supplemented with 10 mM maltose.
NMR Experiments
NMR spectra were recorded on a Bruker (Billerica, MA) Avance III 800 spectrometer equipped with a triple-resonance cryogenic probe with carbon/nitrogen at the inner coil and the cryogenic preamplifiers (TCXI) that are designed for heteronuclear-detection experiments. For our TCXI probe, the 15N signal to noise ratio (SNR) for 90% formamide in DMSO-d6 is 138 against 2 ppm noise, which is slightly better than the manufacturer certified value for TCXI probes (SNR > 115). However, it should be noted that our TCXI probe shows unusual high sensitivity for 1H detected experiments. The SNR for 0.1% ethyl benzene in CDCl3 is 5800 against 200 Hz noise, which is more than two-fold greater than the manufacturer certified value for TCXI probes (SNR > 2300). The proton SNR value is almost comparable to the certified value for proton-optimized triple-resonance cryogenic probes with the proton inner coil and the cryogenic preamplifier (TCHI, SNR > 6000), which are designed for proton-detection experiments. Spectra of the uniformly labeled GB1 sample were recorded at 298 K, in a buffer containing 10 mM sodium phosphate (pH 6.8) and the indicated concentrations of NaCl. Spectra of the uniformly 15N13C labeled MBP sample were recorded at 283 K in buffer containing 10 mM HEPES-NaOH (pH 7.4), 1 mM EDTA, and 2 mM β-cyclodextrin. The 15N-detected and 1H-detected TROSY-HSQC experiments performed here are the same as those reported previously (Takeuchi et al., 2015). The 15N-detected TROSY-HSQC experiments were recorded with spectral widths of 40 ppm (15N, direct) and 6 ppm (1H, indirect). Acquisition times were set to 0.39 sec (15N, direct dimension) and 0.013 sec (1H, indirect dimension) for GB1. The 15N-detected TROSY-HSQC experiments of GB1 were recorded for 2.1 hr at 800 MHz. Acquisition times were set to 0.31 sec (15N, direct dimension) and 0.014 sec (1H, indirect dimension) for MBP. The 15N TRSOY-HSQC experiments of MBP were recorded for 8.5 hr at 800 MHz. The 1H-detected TROSY-HSQC experiments were recorded so they had the same acquisition times for both the 15N (indirect) and 1H (direct) dimensions, as in the 15N-detected experiments. The number of scans was set so that the 15N-detected and 1H-detected TROSY-HSQC have the same experimental time. The carbon decoupling was achieved by using a p5m4 supercycle (Fujiwara and Nagayama, 1988), with an adiabatic CHIRP pulse of 2.5 ms length and 25% smoothing (Bohlen and Bodenhausen, 1993). Zero filling was applied for each FID, but no apodization function was used for the direct dimension before Fourier transformation. For the indirect dimensions, zero filling and a cosine-apodization function were applied. The recycling delay was set to 1.0 s, for both the 15N-detected and 1H-detected TROSY-HSQC experiments. The tuning, matching, and 90° hard pulses were optimized for all of the 1H, 13C, and 15N channels without any problems under all conditions reported here. All spectra were processed with TOPSPIN (Bruker; Billerica, MA) and analyzed with the program Sparky (Goddard and Kneller).
Calculation of transverse relaxation rates
The transverse relaxations of the decoupled spin I (R2I) were calculated based on the standard expression, as follows:
| (1) |
| (2) |
| (3) |
where ΔσI is the difference between the axial and the perpendicular principal components of the axially symmetric chemical shift tensors. The spectrum density was estimated using equation (4)
| (4) |
where ω is the Larmor frequencies of the 1H and 15N spins, or their sum or difference.
The TROSY (R2IT) and anti-TROSY (R2IAT) transverse relaxations of spin I were calculated, as follows:
| (5) |
| (6) |
| (7) |
where Θ is the angle between the unique tensor axes of the dipole-dipole interactions and the chemical shift anisotropies (CSA) interaction. For the calculation of the transverse relaxation rates, the dipole-dipole interactions with directly bonded nuclei, as well as nuclei within defined distances, and the chemical shift anisotropies were taken into consideration, to mimic the typical proton density in a protein. For the estimation of the dipole-dipole interactions in a uniformly 15N-labeled protein, the typical distances in the α-helix region were used with the following distance cut-off criteria: < 4.5 Å for 1H-1H pairs and < 2.1 Å for 1H-15N pairs. These are the sequential HNi-HNi+1 distance (2.8 Å), HNi-HNi+2 distance (4.2 Å), intra residue HNi-Hαi distance (2.2 Å), sequential HNi-Hαi+1 distance (3.5 Å), HNi-Hαi+2 distance (4.4 Å), HNi-Hαi+3 distance (3.4 Å), HNi-Hαi+3 distance (4.2 Å), intra residue HNi-Hβi distance (2.5 Å), and sequential HNi-Hβi+1 distance (3.0 Å) for proton pairs. Contributions from remote protons that are more than 4 residues away in a.a. sequences are not considered. As for the estimation of the dipole-dipole interactions in a uniformly 2H15N-labeled protein, the same nuclei that were considered in the case of a uniformly 1H15N-labeled protein were used, while all non-exchangeable protons were replaced with deuterium. CSAs were also included in the calculation (Δσ15NH: −160ppm, Δσ1HN: 16 ppm). The angle between the unique tensor axes of the dipole-dipole interactions and the CSA interaction (Θ) was set to 17° for 15NH.
Results and discussion
Salt concentration dependence of 15N-detected and 1H-detected TROSY resonances
The sensitivity factor, L, of a cyrogenic probe can be expressed as
| (8) |
| (9) |
where Rs, Rc, a, b, l, n, μ, ω, and σ are the resistance of the sample, the resistance of the coil, the coil radius, the sample radius, the sample length, the number of coil turns, the permeability of free space, the Larmor frequency, and the sample conductivity, respectively (Kelly et al., 2002). Based on the relation, it has been shown that the effect of high ionic conductivity on the 13C-detecetd experiments is milder, as compared to that on the 1H-detected experiments (Shimba et al., 2004). Since the Larmor frequency of 15N is about one-tenth of that of 1H, the loss of sensitivities due to high ionic conductivity is expected to be even less in the 15N-detected experiments, as compared to both the 1H- and 13C-detected experiments. In theory, increasing the NaCl concentration from 0 mM to 200 mM would reduce the sensitivity of the 1H-detected experiment to less than 50%. However, the 15N-detected experiment is much less susceptive to the increase in salt concentration, and thus the sensitivity reduction induced by 200 mM NaCl is expected to be only about 5%. In addition to the reduction in sensitivity originating from the lower detection efficiency, the inhomogeneous excitation of nuclei in a sample would negatively affect the resultant sensitivity of the experiments. This effect is also much less detrimental in the 15N-detected experiment. The inhomogeneous excitation would also cause insufficient solvent suppression, but would only affect the 1H-detected experiment.
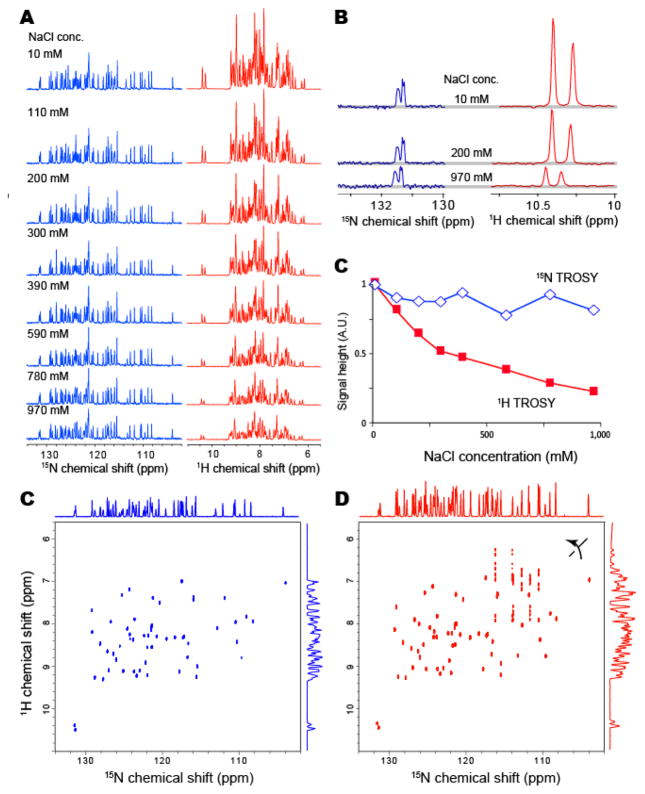
In order to experimentally confirm the effect of the salt concentration on the 15N-detected (Takeuchi et al., 2015) and 1H-detected (Pervushin, 2000; Pervushin et al., 1997) TROSY-HSQC experiments, the fixed concentration (1 mM) of uniformly 15N13C-labeled GB1 was dissolved in buffer with different salt concentrations, ranging from 10 mM to 1 M. All spectra were recorded with a triple-resonance cryogenic TCXI probe with cryogenic preamps and the inner coil for 15N and 13C, which are designed for heteronuclear-detection experiments. The salt concentration dependency of the resonances in the 15N-detected and 1H-detected TROSY-HSQC experiments is shown in Figure 1A and 1B. With the increase in NaCl concentration, the signal height of the 1H-detected resonances was reduced significantly, and the signal height was less than 25% with 1 M NaCl, as compared to the 10 mM NaCl condition (Figure 1C; red). In contrast, the signal height of the 15N-detected resonances was substantially less affected, even at a 1 M salt concentration (Figure 1C; blue). The signal reduction was only about 20% with 1 M NaCl, as compared to 10 mM NaCl. The signal reduction was slightly more than expected, however, this was mainly due to the inhomogeneous excitation and inversion of the 1H nuclei in the single transition-to-single transition polarization transfer (ST2PT) coherence transfer. In Figure 1D and 1E, the 2D 15N-detected and 1H-detected TROSY-HSQC experiments are compared under 1 M NaCl conditions. As shown in the figure, the disadvantage of the 15N-detected TROSY-HSQC experiment relative to the 1H-detected TROSY-HSQC experiment, in terms of the sensitivity, becomes marginal for this small protein (MW: 8K) under the high-salt conditions.
Figure 1. Salt concentration dependence of 15N-detected and 1H-detected TROSY resonances.
(A) Salt concentration dependence of one-dimensional 15N-detected (left) and 1H-detected (right) TROSY resonances of GB1 with indicated concentrations of NaCl. (B) Selected enlarged views of (A). The concentration of U-15N13C GB1 was 1 mM, and the protein was dissolved in 10 mM phosphate buffer (pH 6.8). The spectra were recorded at 298K and 800 MHz. (B) Salt concentration dependence of the signal height of the lower-field resonance in (A). (C and D) The two-dimensional (C) 15N-detected and (D) 1H-detected TROSY-HSQC spectra of U-15N13C GB1, in 10 mM phosphate buffer (pH 6.8) with 1 M NaCl. The spectra was recorded at 298K at 800 MHz.
If one assumes identical noise, efficiency in signal detection, and no B0/B1 field inhomogeneity, then the relative intensity (or peak area after FT) of the 1H-excited 15N-detected experiments (15N-detected TROSY-HSQC), as compared to that of the 1H-excited 1H-detected experiments (1H-detected TROSY-HSQC) becomes 0.032. Therefore, the marginal difference in the sensitivity of the 15N-detected TROSY-HSQC experiment relative to the 1H-detected TROSY-HSQC experiment under high salt conditions clearly indicates that the 15N-detection had an additional benefit, in that it avoided the detrimental effects of the high salt concentration on the sensitivity factor defined by the electric/thermal noise levels and the influence of the B0/B1 field inhomogeneity.
Management of 2J and 3J scalar couplings in 15N-detected TROSY-HSQC
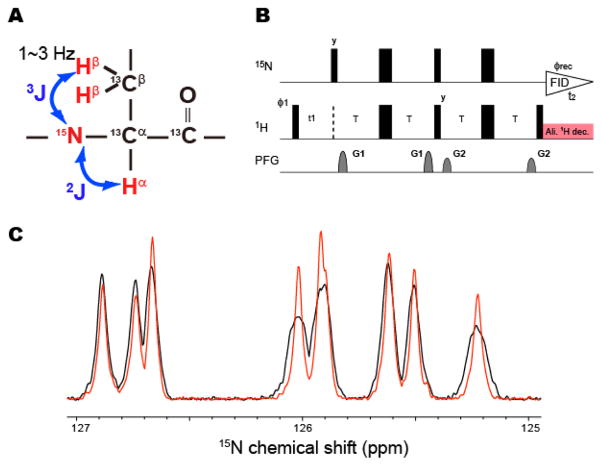
It should also be noted that the experiments were performed with non-deuterated GB1. Since the 15N-detected TROSY-HSQC experiment should be recorded without broad-band 1H decoupling in the detection period, the 15N-detected resonances are broadened (or split) due to the 2J and 3J coupling to Hα and Hβ, respectively, and their signal heights were reduced (Figure 2A). The 2J and 3J scalar coupling can be removed by the application of a proton composite decoupling that is selective to the aliphatic proton resonances in the 15N-detection period (Figure 2B). As shown in Figure 2C, the broadening (or splitting) of several resonances was significantly reduced and the signal heights become higher with the application of the selective 1H decoupling. Thus, the difference in the sensitivity between the 15N-detected and 1H-detected resonances would be even smaller with the removal of the 2J and 3J couplings. However, in most cases, the improvements in the signal height were not significant, as expected from the reduction of the line width. This appears to be due to the small sideband of the selective decoupling pulses, which affects the HN resonance. This effect causes the mixing of the sharp TROSY and the broad anti-TROSY coherences. Since the anti-TROSY resonances are expected to be 10-times broader, as compared to the TROSY resonances, even 1% of the mixing by the aliphatic proton decoupling would reduce the intensity of the TROSY resonances by 10%.
Figure 2. Application of selective composite decoupling to aliphatic proton resonances in the 15N-detected TROSY-HSQC experiment.
(A) The 2J and 3J couplings to Hα and Hβ, which cause the broadening (splitting) of the 15N-detected TROSY resonances. (B) The modified pulse sequence of the 15N-detected 2D TROSY-HSQC experiments. Narrow and wide rectangular black bars indicate non-selective π/2 and π pulses, respectively. All pulses were applied along the x-axis unless otherwise indicated. The delay T was set to 2.7 ms. The phase cycle employed was ϕ1 = (y -y x –x), and ϕrec = (x -x -y y). Phase sensitive spectra in the indirect 1H dimension (t1) were obtained by incrementing the phase ϕ1. The recycling delay was set to 1 s. The two sine-shaped pulsed field gradients were applied along the z-axis for 1.0 ms, with maximum intensities of G1 = 22.5 G/cm and G2 = 25 G/cm. The selective aliphatic decoupling was achieved by 0.1 kHz constant-adiabaticity WURST decoupling pulses centered at 1 ppm with a 4 kHz band width (Kupce and Freeman, 1996), which were applied in an MLEV manner (Levitt and Freeman, 1981). (C) One-dimensional 15N-detected TROSY resonances of U-15N13C GB1 with (red) and without (black) aliphatic proton selective composite decoupling. The spectra were recorded at 283K, in 10 mM phosphate buffer with 100 mM NaCl.
Sensitivity of 15N-detected TROSY for a non-deuterated, large protein under physiological salt conditions
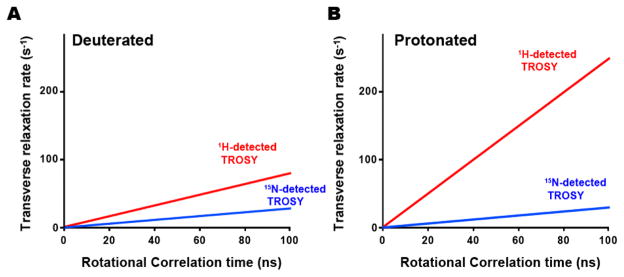
Figure 3 shows a comparison of the calculated transverse relaxation rates (R2) for the 15N-detected and 1H-detected TROSY experiments, for deuterated and non-deuterated proteins at 800 MHz. In a deuterated system, R2 of 15N-detected TROSY is 3-fold less, as compared to that of 1H-detected TROSY. However, the difference in R2 becomes much more substantial under non-deuterated conditions, and the R2 of 15N-detected TROSY is 10-fold slower than that of 1H-detected TROSY. As a result, the coherences loss during the pulse schemes are much less substantial for 15N-detected TROSY-HSQC, as compared to those for 1H-detected TROSY-HSQC, for a non-deuterated, large protein. Therefore, the disadvantage in the sensitivity of the 15N-detected TROSY-HSQC experiment relative to the 1H-detected TROSY-HSQC experiment would largely be resolved, for a non-deuterated, large protein at a physiological salt concentration.
Figure 3. Transverse relaxation rates of the 15N-detected (blue) and 1H-detected (red) TROSY resonances for a (A) deuterated and (B) non-deuterated protein, as a function of the rotational correlation time.
The transverse relaxation rates were calculated based on equation (5) in the supplemental materials and methods. The magnetic field strength was set to 800 MHz.
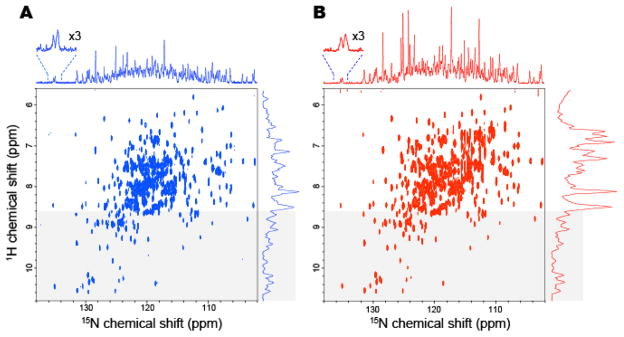
Figure 4 shows the 15N-detected TROSY-HSQC spectra (left) and the 1H-detected TROSY-HSQC spectra (right) of non-deuterated maltose binding protein (0.5 mM) in complex with 2 mM β-cyclodextrin, dissolved in 10 mM HEPES-NaOH buffer (pH 7.4) with 200 mM NaCl. Both spectra were recorded in 8.5 h at 283K, and the apparent τc of the system is 35 ns. All of the resonances that were observed in the 1H-detected TROSY-HSQC spectrum were also observed in the 15N-detected TROSY-HSQC spectrum, with the exception of the arginine side chains, which are folded in the indirect dimension of the 1H-detected spectrum but outside the spectrum width of the direct dimension in the 15N-detected spectrum, and the asparagine and glutamine sidechain resonances that are not efficiently refocused in the 15N-detected spectrum. As the result, only the mainchain resonances and tryptophan sidechain resonances are observed in the 15N-detected TROSY-HSQC spectrum, and the signal heights of the resonances varied less relative to each other. Furthermore, the results clearly indicated that the heights or the signal to noise ratios of the resonances from the structured region (shadowed) of the 15N-detected TROSY-HSQC experiment are now comparable to those of the 1H-detected TROSY-HSQC spectrum, and the sensitivity difference between these two experiments is now less than two-fold.
Figure 4. Comparison of (A) the 15N-detected TROSY-HSQC and (B) the 1H-detected TROSY-HSQC of 0.5 mM non-deuterated MBP in complex with 2 mM βCD at 283 K.
(A) The 15N-detected TROSY-HSQC was recorded in 8.5 hr, ns=176, F1=128 pts (14 ms), F2 = 2048 pts (315 ms). (B) The 1H-detected TROSY-HSQC was recorded in 8.5 hr, ns = 12, F1= 2048 pts (315 ms), F2=800 pts (18 ms). The 1H-detected TROSY-HSQC was transposed. 15N and 1H projections of the 2D spectra are indicated without any multiplication. The regions that were expected to contain mainly the resonances from the structured region of the proteins (> 8.6 ppm in 1H dimension) are indicated by shadowing. The spectra were recorded at 283 K and 800 MHz, and the apparent τc of the system deduced from the TROSY for rotational correlation times (TRACT) experiment was 35 ns (Lee et al., 2006).
Due to the significant tolerance of the 15N-detected TROSY-HSQC experiment to high salt conditions and with the benefit of the 15N-detected TROSY-HSQC under non-deuterated conditions, the sensitivity difference between the 15N-detected and 1H-detected experiments becomes marginal for a small protein in a buffer with 1M NaCl and a non-deuterated, large protein at a physiological salt concentration (200 mM NaCl). These results further assure the significance of the 15N-detected TROSY in exploitations of the high resolution and sensitivity offered by high field magnets above 1 GHz, as expected from the previous estimation (Takeuchi et al., 2015). It should be noted that the sensitivity for the 1H-detected experiments would be higher with a triple-resonance TCHI cryogenic probe with the proton inner coil and the cryogenic preamps under low salt conditions. However, for samples with physiological salt concentrations, there is no significant difference in the 1H sensitivity between the TCXI and TCHI probes.
The 1H sensitivity under high salt conditions can be improved by using shaped or smaller diameter tubes. For example, in the TCHI probes, the SNR per unit volume has reportedly been improved by 2.2-fold for a 1H-detected experiment by using the slot tube, as compared to the 5 mm round tube (Takeda et al., 2011). Thus, for 1H-detected experiments, the shaped or smaller diameter tubes would provide better sensitivity, especially for proteins that can be concentrated into a smaller volume without aggregation or precipitation. However, for proteins with limited solubility, the advantage of the using those tubes would be limited and may be worse for small diameter tubes (Takeda et al., 2011). It should also be noted that the sensitivity gain of the 1H-detected experiment with these tubes may actually be smaller than the reported value with the TCXI probes, since the 1H coil is farther away, as compared to the TCHI probes. Note that, due to the ongoing developments in hardware, the relative sensitivity of the 15N-detected experiments, as compared to the 1H-detected experiments, may vary depending on the different generations of the probe systems. Nevertheless, the higher resolutions and the dispensable deuteration remain as the clear advantages of the 15N-detected experiments over the 1H-detected experiments.
Although a lower concentration of salt (< 100 mM) is usually recommended for 1H-detected experiments, certain amounts of salts, including buffers etc, are often required to obtain a sample with a sufficient protein concentration for structural studies (Bagby et al., 2001; Golovanov et al., 2004). In addition, certain applications, such as residual dipole coupling measurements, structural analyses of membrane proteins in ionic detergents, and protein unfolding studies, require increased concentrations of salt and/or ionic materials, in order to perform the experiments under optimal conditions. Indeed, salt concentrations above 200 mM were used in 8 out of 46 cases (17%) in reports published in volume 9, issue 2 of Biomolecular NMR Assignment in 2015, and the average ± standard deviations of the ionic strength were 164 ± 116 mM. In addition, in 10 out of 46 cases (22%), the ionic strength was higher than that used for the MBP sample in this manuscript (218 mM). It should be noted that the conductivity of a sample is not only defined by the ionic strength but by the mobility in solution (Kelly et al., 2002), and the statistics clearly indicates that high ionic conditions are required in many cases. Furthermore, the susceptibility of 1H-detected experiments to salt is expected to be more severe at higher fields, where 15N-detected TROSY would have maximal benefits in resolution and sensitivity.
Since the relaxation of 15N TROSY is slower than that of 1H TROSY, the 15N-detection has the benefit that a longer acquisition in the indirect 15N dimension is avoided as would be needed in the 1H-detected experiment. This benefit is compounded by the use of a smaller spectral width (~6 ppm) in the indirect 1H dimension centered at the amide resonances. As a consequence, the chemical shift sampling of the indirect 1H dimension becomes more efficient in the 15N-detected experiment, and the number of scans per increment in a given total experimental time can be increased. These benefits would also contribute to closing the sensitivity gap of the 15N-detected TROSY-HSQC experiment, relative to that of the 1H-detected experiment. In addition, although the dispersion in the 15N dimension is almost invariant, the 1H dispersion is wider for structured proteins but is narrower for intrinsically disordered proteins (IDPs), and thus the sampling in the indirect dimension becomes even more efficient for this class of proteins.
Supplementary Material
Acknowledgments
This work was supported by METI (Grant name: development of core technologies for innovative drug development based upon IT) to IS and by NIH grants GM047467 and AI 37581 to GW. This work was also partly supported by JST, PRESTO to KT. Maintenance of NMR instruments was supported in part by NIH grant EB002026.
References
- Arnesano F, Banci L, Piccioli M. NMR structures of paramagnetic metalloproteins. Q Rev Biophys. 2005;38:167–219. doi: 10.1017/S0033583506004161. [DOI] [PubMed] [Google Scholar]
- Bagby S, Tong KI, Ikura M. [2] - Optimization of Protein Solubility and Stability for Protein Nuclear Magnetic Resonance. In: James Thomas VDaUSL., editor. Methods in Enzymology. Academic Press; 2001. pp. 20–41. [DOI] [PubMed] [Google Scholar]
- Bermel W, Bertini I, Felli IC, Kummerle R, Pierattelli R. 13C Direct detection experiments on the paramagnetic oxidized monomeric copper, zinc superoxide dismutase. J Am Chem Soc. 2003;125:16423–16429. doi: 10.1021/ja037676p. [DOI] [PubMed] [Google Scholar]
- Bermel W, Bertini I, Felli IC, Lee YM, Luchinat C, Pierattelli R. Protonless NMR experiments for sequence-specific assignment of backbone nuclei in unfolded proteins. J Am Chem Soc. 2006a;128:3918–3919. doi: 10.1021/ja0582206. [DOI] [PubMed] [Google Scholar]
- Bermel W, Bertini I, Felli IC, Piccioli M, Pierattelli R. 13C-detected protonless NMR spectroscopy of proteins in solution. Prog Nucl Magn Res Spec. 2006b;48:25–45. [Google Scholar]
- Bohlen JM, Bodenhausen G. Experimental Aspects of Chirp NMR Spectroscopy. Journal of Magnetic Resonance, Series A. 1993;102:293–301. [Google Scholar]
- Felli I, Brutscher B. Recent advances in solution NMR: fast methods and heteronuclear direct detection. ChemPhysChem. 2009;10:1356–1368. doi: 10.1002/cphc.200900133. [DOI] [PubMed] [Google Scholar]
- Frueh DP, Arthanari H, Wagner G. Unambiguous assignment of NMR protein backbone signals with a time-shared triple-resonance experiment. J Biomol NMR. 2005;33:187–196. doi: 10.1007/s10858-005-3204-z. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Nagayama K. Composite inversion pulses with frequency switching and their application to broadband decoupling. Journal of Magnetic Resonance (1969) 1988;77:53–63. [Google Scholar]
- Gal M, Edmonds KA, Milbradt AG, Takeuchi K, Wagner G. Speeding up direct (15)N detection: hCaN 2D NMR experiment. J Biomol NMR. 2011;51:497–504. doi: 10.1007/s10858-011-9580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KH, Zhang X, Gehring K, Kay LE. Solution NMR Studies of a 42 KDa Escherichia Coli Maltose Binding Protein/β-Cyclodextrin Complex: Chemical Shift Assignments and Analysis. Journal of the American Chemical Society. 1998;120:11738–11748. [Google Scholar]
- Goddard TD, Kneller DG. SPARKY. Vol. 3. University of California; San Francisco: [Google Scholar]
- Golovanov AP, Hautbergue GM, Wilson SA, Lian LY. A Simple Method for Improving Protein Solubility and Long-Term Stability. Journal of the American Chemical Society. 2004;126:8933–8939. doi: 10.1021/ja049297h. [DOI] [PubMed] [Google Scholar]
- Hsu STD, Bertoncini CW, Dobson CM. Use of protonless NMR spectroscopy to alleviate the loss of information resulting from exchange-broadening. J Am Chem Soc. 2009;131:7222–7223. doi: 10.1021/ja902307q. [DOI] [PubMed] [Google Scholar]
- Kelly AE, Ou HD, Withers R, Dötsch V. Low-Conductivity Buffers for High-Sensitivity NMR Measurements. Journal of the American Chemical Society. 2002;124:12013–12019. doi: 10.1021/ja026121b. [DOI] [PubMed] [Google Scholar]
- Kupce Ē, Freeman R. Optimized Adiabatic Pulses for Wideband Spin Inversion. Journal of Magnetic Resonance, Series A. 1996;118:299–303. [Google Scholar]
- Lee D, Hilty C, Wider G, Wüthrich K. Effective rotational correlation times of proteins from NMR relaxation interference. J Magn Reson. 2006;178:72–76. doi: 10.1016/j.jmr.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Lee D, Vögeli B, Pervushin K. Detection of C′,Cα correlations in proteins using a new time- and sensitivity-optimal experiment. J Biomol NMR. 2005;31:273–278. doi: 10.1007/s10858-005-2361-4. [DOI] [PubMed] [Google Scholar]
- Levitt MH, Freeman R. Composite pulse decoupling. Journal of Magnetic Resonance (1969) 1981;43:502–507. [Google Scholar]
- Levy G, Richter R. Nitrogen 15 Nuclear Magnetic Resonance Spectroscopy. John Wiley & Sons; 1979. [Google Scholar]
- Pervushin K. Impact of transverse relaxation optimized spectroscopy (TROSY) on NMR as a technique in structural biology. Q Rev Biophys. 2000;33:161–197. doi: 10.1017/s0033583500003619. [DOI] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc Natl Acad Sci U S A. 1997;94:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serber Z, Richter C, Dotsch V. Carbon-detected NMR experiments to investigate structure and dynamics of biological macromolecules. Chembiochem. 2001;2:247–251. doi: 10.1002/1439-7633(20010401)2:4<247::AID-CBIC247>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Shimba N, Kovacs H, Stern AS, Nomura AM, Shimada I, Hoch JC, Craik CS, Dötsch V. Optimization of 13C direct detection NMR methods. J Biomol NMR. 2004;30:175–179. doi: 10.1023/B:JNMR.0000048855.35771.11. [DOI] [PubMed] [Google Scholar]
- Takeda M, Hallenga K, Shigezane M, Waelchli M, Löhr F, Markley JL, Kainosho M. Construction and performance of an NMR tube with a sample cavity formed within magnetic susceptibility-matched glass. J Magn Reson. 2011;209:167–173. doi: 10.1016/j.jmr.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Arthanari H, Shimada I, Wagner G. Nitrogen detected TROSY at high field yields high resolution and sensitivity for protein NMR. J Biomol NMR. 2015 doi: 10.1007/s10858-015-9991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Frueh DP, Hyberts SG, Sun ZJ, Wagner G. High-resolution 3D CANCA NMR experiments for complete mainchain assignments using Ca direct-detection. J Am Chem Soc. 2010a doi: 10.1021/ja907717b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Gal M, Shimada I, Wagner G. Low γ-nuclei detection experiments for bimolecular NMR. Recent Developments in Biomolecular NMR. 2012:25–52. [Google Scholar]
- Takeuchi K, Heffron G, Sun ZY, Frueh DP, Wagner G. Nitrogen-detected CAN and CON experiments as alternative experiments for main chain NMR resonance assignments. J Biomol NMR. 2010b;47:271–282. doi: 10.1007/s10858-010-9430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Sun ZY, Wagner G. Alternate 13C-12C labeling for complete mainchain resonance assignments using Ca direct-detection with applicability toward fast relaxing protein systems. J Am Chem Soc. 2008;130:17210–17211. doi: 10.1021/ja806956p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasos PR, Hall JB, Kummerle R, Fushman D. Measurement of 15N relaxation in deuterated amide groups in proteins using direct nitrogen detection. J Biomol NMR. 2006;36:27–36. doi: 10.1007/s10858-006-9063-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.