Abstract
Children with unilateral spastic cerebral palsy (USCP) often have tactile impairments. Intensive bimanual training improves the motor abilities, but the effects on the sensory system have not been studied. Here we compare the effects of bimanual training with and without tactile training on tactile impairments. Twenty children with USCP (6–15.5 years; MACS: I–III) were randomized to receive either bimanual therapy (HABIT) or HABIT + tactile training (HABIT + T). All participants received 82 h of standardized HABIT. In addition 8 sessions of 1 h were provided to both groups. The HABIT + T group received tactile training (without vision) using materials of varied shapes and textures. The HABIT group received training with the same materials without tactile directed training (full vision). Primary outcomes included grating orientation task/GOT and stereognosis. Secondary outcomes included two-point discrimination/TPD, Semmes-Weinstein monofilaments/SWM. The GOT improved in both groups after training, while stereognosis of the more-affected hand tended to improve (but p = 0.063). No changes were found in the TPD and the SWM. There were no group × test interactions for any measure. We conclude tactile spatial resolution can improve after bimanual training. Either intensive bimanual training alone or incorporation of materials with a diversity of shapes/textures may drive these changes.
Keywords: Sensory function, Tactile impairments, Tactile training, Bimanual training, Cerebral palsy, Hemiplegia, Hand, Intensive rehabilitation
1. Introduction
Impaired function in children with unilateral spastic cerebral palsy (USCP) does not purely result from motor impairments, but is also affected by concomitant sensory impairments. Tactile registration, tactile perception, and sensorimotor integration are essential for grasping and releasing objects (Gordon & Duff, 1999a, 1999b), dexterous manipulation (Bleyenheuft & Gordon, 2013), and activities of daily living (Auld, Boyd, Moseley, Ware, & Johnston, 2012). There have been studies investigating sensory contribution to motor control in children with USCP (Auld et al., 2012; Auld, Russo, Moseley, & Johnston, 2014; Gordon & Duff, 1999a). However, whether intensive bimanual training or tactile training is effective in modifying tactile impairments in children with USCP has never been investigated.
Early animal studies directly tested how sensory deprivation affected the motor system. Mott and Sherrington (1895) showed that deafferentation, an abolition of sensory input, impaired the performance of skilled movements in monkeys. Although the underlying pathophysiology is different in deafferented monkeys and in USCP, these studies highlighted the contribution of sensory input to fine motor control. Recently, neuroplastic changes were demonstrated in rats’ sensory cortex (S1) after enhanced environmental or motor training. Joo et al. (2012) showed increased somatosensory evoked potentials that paralleled motor recovery. Alwis and Rajan (2013) showed that 8 weeks of environment enrichment increased electrophysiological responses to tactile stimuli. These studies highlight the interaction between sensory and motor systems.
Studies investigating effects of somatosensory training programs on modifying sensorimotor functions in adult stroke are emerging (Carey, Macdonell, & Matyas, 2011). While it has been acknowledged that the sensory impairment is a major contribution to motor impairments in children with USCP (Bleyenheuft & Gordon, 2013), effective therapy in improving tactile impairment is lacking (Auld et al., 2014). The study by Charles, Lavinder, and Gordon (2001) is the only study using intensive hand therapy which reported an improvement in tactile discrimination in 3 children after constraint-induced movement therapy (CIMT). They attributed the improvement in tactile discrimination to an increase in tactile input and its subsequent change in cortical receptor fields for the fingers. More recently, studies investigating neuroplastic changes associated with intensive hand therapy using functional Magnetic Resonance Imaging (fMRI) or magnetoencephalography (MEG) demonstrated increased activation associated with CIMT (Juenger et al., 2013) or HABIT (Bleyenheuft et al., 2015) in S1 or M1 in USCP and in adult stroke (Laible et al., 2012). In summary, tactile function could be improved after intensive hand therapy in children with USCP, and neurophysiological changes associated with intensive therapy may be found in S1 or M1. These studies prompted us to probe deeper into the relationship among intensive hand training, tactile training, and their impact on tactile function in children with USCP.
Both unimanual and bimanual intensive therapy have been shown to improve hand motor function in children with USCP (Gordon et al., 2011, 2008; Sakzewski, 2012; Sakzewski et al., 2011). Bimanual intensive therapy improved self-determined goals more than unimanual therapy as bimanual training allows use of both hands (e.g., tying shoes) (Brandao, Gordon, & Mancini, 2012; Gordon et al., 2011). In addition, bimanual training improved bimanual coordination more than unimanual training (Hung, Casertano, Hillman, & Gordon, 2011). Consequently, we adopted bimanual training as a common ingredient in our study. We further aimed to compare the efficacy of two interventions in this pilot study: intensive bimanual training (hand–arm bimanual intensive therapy, HABIT) vs. intensive bimanual training that includes tactile training (HABIT + T) on modifying tactile function in children with USCP. We hypothesized that tactile function could be enhanced after HABIT due to the enriched environment created by exposure to objects of varied textures, and tactile function could be further enhanced with additional tactile training.
2. Methods
2.1. Participants
Participants included a sample of convenience that was recruited from a subset of two ongoing trials (Bleyenheuft, Arnould, Brandao, Bleyenheuft, & Gordon, 2015; Brandao et al., 2013). The inclusion criteria were established based on prior HABIT trials (Brandao et al., 2013; Gordon et al., 2011; Gordon, Schneider, Chinnan, & Charles, 2007): (1) age 6 to 18, diagnosed with congenital USCP, (2) the ability to lift the more-affected arm 15 cm above a table surface and grasp light objects, (3) cognition level defined as mainstreamed in school (Kaufman Brief Intelligence test score >70), (4) demonstrated ability to follow instructions and complete testing. Exclusion criteria included: (1) health problems unrelated to USCP, (2) uncontrolled seizures, (3) visual problems interfering with intervention/testing, (4) severe muscle tone at any joint (Modified Ashworth score >3.5), (5) orthopedic surgery on the more-affected hand within one year, and 6) botulinum toxin therapy in the upper limb within the last 6 months or intended treatment within the study period. Informed assent/consent forms were obtained from participants and caregivers. This study was approved by the respective Universities’ Institutional Review Boards.
2.2. Procedures
2.2.1. General intervention procedures
One bimanual training camp was conducted at Teachers College in New York City in early July 2012. Another bimanual training camp was conducted at Université Catholique de Louvain in Brussels, Belgium in late July 2012. In each site, participants were randomized offsite using concealed allocation stratified by their baseline tactile discrimination threshold (measured by Grating Orientation Task) and baseline unilateral dexterity (measured by Jebsen–Taylor Test of Hand Function) of the more-affected hand. Twenty participants were randomly assigned to a (1) HABIT including tactile training group (HABIT + T, n = 4 in New York, n = 6 in Brussels) or (2) HABIT group (HABIT, n = 4 in New York, n = 6 in Brussels).
HABIT is a form of intensive bimanual training for children with USCP using motor learning principles (Charles & Gordon, 2006). Children are engaged in using both hands in bimanual play and functional activities. The more-affected hand is treated as the assisting hand (active assist or stabilizer) in the context of task practice. Motor learning principles of whole-task and part-task practice are applied. Clinical trials of HABIT have shown efficacy in improving children’s manual dexterity, bimanual hand use, and performance of functional goals (Brandao et al., 2013, 2012; Gordon et al., 2011, 2007).
2.2.2. Intervention details
All participants received 82 h of standardized intensive bimanual training within 3 weeks by trained interventionists. In both sites, an additional 8 h of treatment was provided in a separate room with a different interventionist (specifically trained). Children’s regular interventionists were not allowed in this training room. During that time, the HABIT + T group received tactile training using tactile stimulating materials (without vision, described in Section 2.2.3). The HABIT group received the same dosage/schedule of controlled training with the same material but without specific tactile-directed training (standardized HABIT-full vision, see Section 2.2.3). Apart from the specially trained interventionist for those 8 h of tactile/control training, regular interventionists (for the 82 h of standardized HABIT) were trained at a pre-intervention session on procedures common to the 2 groups, such as strategies to engage children actively involving the use of both hands and safety. Daily team meetings reinforced individual treatment plans. The 2 camps had the same supervisor to ensure the uniformity of intervention.
2.2.3. Tactile and control training
The 8 h of specific tactile intervention or control training was conducted systematically by the same interventionists at both sites. During those 8 h, children received either tactile training or control training (1 h/session × 8 camp days, 8 sessions in total).
Children in the HABIT + T group received 8 sessions of 1 h tactile training. Specific training components encompassed (a) tactile discrimination and matching: training modalities included texture (e.g., fur and plastic), shape (e.g., circle and square), and size, (b) finger resistance discrimination and matching (cylinder blocks with different resistance for fingers to push in, see Fig. 1A and B). Training was primarily administered with the child blindfolded or exploring objects in bags (i.e., not visible). Yet, instructions and knowledge of results were given with vision. Both hands were required to engage in the tasks. Skill progression and engagement of both hands for bimanual manipulation were ensured. An example for a texture-matching task is that a child would first touch/feel an object with one hand (e.g., a sand-paper-top cylinder, see Fig. 1A). The child would be asked to identify the same object among various objects (e.g., fur-top cylinder, spiky-plastic-top cylinder, plastic alphabets) by exploring with the other hand. If children were not able to explore objects due to their motor impairments, interventionists supported the objects in children’s hand to allow exploration. Children received knowledge of results after each trial by visual/verbal feedback from the interventionists. Positive reinforcement was always provided.
Fig. 1.

(A) An example of an object-matching task during tactile training in the HABIT + T group. A child was first exposed to a sand-paper-top cylinder with the less-affected hand (on the right side of the picture), and was required to search for the same object with the more-affected hand (on the left side of the picture). The child’s vision was occluded by a screen during the discrimination training. (B) An example of the materials used during tactile training.
Children in the HABIT group did not receive tactile training. During the control training, they received standardized HABIT by playing with the same materials (full vision) in the same environment (same room/interventionist) as those provided to the HABIT + T group. Participants in this group received control training with the same schedule and frequency as those in the HABIT + T group (1hr/session on 8 camp days). Intervention materials were applied in the context of play and functional activities in this group. This design controlled for confounds of the differential effects by exposing children to different materials and different interventionists.
2.3. Measurements
Participants were evaluated directly prior to treatment (pre-test) and within 2 days after treatment (post-test) by one physical therapist blinded to group allocation. Primary outcomes included grating orientation task/GOT and stereognosis. Secondary outcomes included two-point discrimination/TPD, Semmes-Weinstein monofilaments/SWM, the Jebsen–Taylor Test of Hand Function/JTTHF and the Assisting Hand Assessment/AHA. The details of each assessment are described below.
The tactile spatial resolution was measured by the GOT using the JVP domes (Stoelting Co., Wood Dale, IL, USA). It was validated in children with USCP (Bleyenheuft & Thonnard, 2011). During testing, children had their palmer side of the index finger tip exposed and resting on the table. They were first given 4 practice trials with vision/verbal feedback, followed by 4 practice trials without vision and with verbal feedback. We used 11 domes presenting gratings with equal distances of bar & groove widths (0.35, 0.5, 0.75, 1.0, 1.2, 1.5, 2.0, 3.0, 3.5, 4.0, 4.5 mm). The domes were perpendicularly applied to the index finger pad for 1–2 s, which resulted in ~2 mm of deformation on the skin (a procedure validated in children, see Bleyenheuft, Cols, Arnould, & Thonnard, 2006; Bleyenheuft & Thonnard, 2007). Children determined the grating orientation (along or across the finger). Two-alternative orientations (forced-choice) were used for each trial. Each dome was tested in 10 trials using a pseudo-random presentation order of 5 trials with the gratings along horizontal axis and 5 trials with the gratings across the longitudinal axis of the finger. We started with the 3.0 mm dome. Children were tested with the next smaller width when the correct response rate of the current dome was ≥60%. They were tested with the next larger width when the correct response rate of the current dome was ≤50%. Threshold search stopped when the child consistently failed to discriminate (i.e., ≥2 domes showing the correct response rate ≤50%). A final threshold was calculated based on an estimate of 75% correct width (Van Boven & Johnson, 1994). A default threshold of 4.5 mm was assigned when they were unable to reach a correct response rate of 70% even when tested with the widest dome (4.5 mm).
Stereognosis was measured with the Manual Form Perception Test (Cooper, Majnemer, Rosenblatt, & Birnbaum, 1995). Children were asked to identify 10 objects by touching/feeling them. Five objects of daily use (toothbrush, tennis ball, comb, large cup, and candy-in-wrapper) and five shapes (circle, triangle, square, diamond, and octagon) were randomly presented to children. The total number of correctly identified items was the final score.
Static TPD was performed by using Disk-criminator® (Mackinnon & Dellon, 1985). Children were first instructed with the testing procedure of differentiating between 1-point and 2-points on the less-affected hand with vision. They were tested on thumb, index, and middle finger pads of both hands. The evaluator randomly assigned 1- or 2-point stimuli. Each finger was tested with 10 random trials at each distance. When a child achieved 7 correct responses at the distance tested, evaluator tested with the next smaller distance. When a child failed to achieve 7 correct responses at the distance tested, evaluator tested with the next larger distance. The minimal distance children were able to distinguish two discrete points, ranging from 2–15 mm, was recorded for each finger as the final score. If a child could not discriminate 1- or 2-point stimuli with a distance of 15 mm, a default threshold of 15 mm was assigned.
Tactile perception was tested with Semmes-Weinstein monofilaments (SWM) (Smith & Nepher Roylan Inc. Germantown, WI, USA) (Weinstein, 1993). We used a 20 monofilaments kit. Monofilaments were applied to the index finger pad. Children said “touch” or “yes” when they felt the filament. A few practice trials were given on the less-affected hand until they understood the procedure. We started with the 4.31 filament and searched for the threshold.
The Jebsen–Taylor Test of Hand Function (JTTHF) (Jebsen, Taylor, Trieschmann, Trotter, & Howard, 1969) is a standardized test quantifying unilateral dexterity as the movement time (in seconds) to complete motor tasks. It consists of subtests including card flipping, small objects manipulation and placement, simulated eating, checker stacking, and empty and full can manipulation. The maximum completion time of each subtest was 180 s, and if it was clear a child could not complete the items, the test was stopped to prevent frustration and fatigue and the maximum time was recorded. The total score (in seconds) is an addition of the 6 subtests time.
The Assisting Hand Assessment (AHA, version 4.3) (Krumlinde-Sundholm & Eliasson, 2003) quantifies the effectiveness of the more-affected hand use in bimanual activities (in 0–100 AHA-unit). The test was videotaped and scored off-site by an evaluator blinded to group allocation.
2.4. Statistical design
Statistical analyses were performed using SPSS (Version 21). A 2 (group) × 2 (test session) ANOVA with repeated measures on test sessions was performed on each measure for the more- and the less-affected hand. This design was to test efficacy of training on tactile and motor function and to examine if treatment efficacy differed depending on group assignment. As many of the measures violated assumptions of normal distributions, we logarithm-transformed the raw data using log base10. Given that the ANOVA results on raw data and logarithm-transformed data were qualitatively similar, we thus reported the statistical results on the log-transformed data below. Figures show the raw values however. t-Tests were performed to test group differences at baseline. Pearson coefficient correlations were performed to examine the predictors of changes in function. p-Values under 0.05 were set as statistical significance.
3. Results
Patient flow is shown in the flow diagram (Fig. 2). Twenty children with USCP (ages 6–15.5 years; MACS level: I–III) met the inclusion criteria and were randomized to receive either HABIT or HABIT + T. One participant in the HABIT group in Brussels dropped out of the study. A total of 19 participants (ages 6–15.5 years; MACS level: I–III) completed 90 h of training. Clinical characteristics of participants are described in Table 1. There were no significant differences in the primary measures or secondary measures between the two groups at the baseline (all p > 0.15).
Fig. 2.
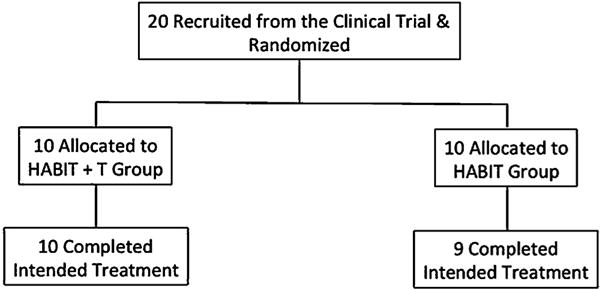
Patient flow chart.
Table 1.
Baseline participant characteristics.
| Characteristics | HABIT + T (n = 10) | HABIT (n = 9) | Control group |
|---|---|---|---|
| Mean age (SD), years, months | 8.9 (2.6) | 8 (1.1) | 8.2 (2.3) |
| Gender | |||
| Male | 4 | 6 | 4 |
| Female | 6 | 3 | 6 |
| Paretic hand | |||
| Right | 6 | 5 | 4 |
| Left | 4 | 4 | 6 |
| MACS | |||
| I | 2 | 0 | 3 |
| II | 6 | 8 | 6 |
| III | 2 | 1 | 1 |
| Baseline tactile discrimination threshold, mean (SD), mm | 4.23 (0.58) | 4.35 (0.42) | 4.11 (0.70) |
| Baseline stereognosis, mean (SD), n | 6.5 (3.63) | 5.22 (3.08) | 4.4 (3.75) |
| Baseline TPD-thumb, mean (SD), mm | 8.9 (5.36) | 9.22 (5.41) | NA |
| Baseline SWM, mean (SD) | 6.3 (2.5) | 5.78 (2.74) | NA |
| Baseline JTTHF, mean (SD),s | 368.06 (280.42) | 389.52 (308.25) | 364.69 (305.60) |
| Lesion type | |||
| CM | 1 | 0 | 0 |
| PV | 4 | 4 | 2 |
| C/SC | 2 | 4 | 7 |
| Unavailable | 3 | 1 | 1 |
Abbreviations: HABIT, hand–arm bimanual intensive therapy; HABIT + T, HABIT with additional tactile training, SD, standard deviation; MACS, manual ability classification system; TPD, two-point discrimination, SWM, Semmes-Weinstein monofilament; JTTHF, Jabsen–Taylor test of hand function, CM, cortical malformation; PV, periventricular injury; C/SC, cortical/subcortical lesion.
3.1. Changes in tactile spatial resolution & stereognosis after training
Fig. 3 shows the means of the more-affected hand for the HABIT + T and HABIT groups at pre-test and post-test for the GOT, stereognosis, the JTTHF, and the AHA. For the GOT, there was a 0.36 mm and a 0.82 mm improvement in the discrimination threshold in the more-affected hand for the HABIT + T and HABIT groups, respectively (Fig. 3A). There was a 1 mm and a 1.05 mm improvement in the discrimination threshold in the less-affected hand for the HABIT + T and HABIT groups, respectively (Fig. 3B). A test session effect showed that the improvement in the GOT was significant (Table 2, more-affected hand, p = 0.028; less-affected hand, p = 0.002). For the stereognosis, there was a 0.5 object and >1 object (1.7) improvement in the more-affected hand for the HABIT + T and HABIT groups, respectively (Fig. 3C). There was a trend of improvement in the stereognosis in the more-affected hand (p = 0.063). There was no significant Group × Test session interaction effect in the stereognosis in the more-affected hand. Finally, there were no significant changes in the stereognosis in the less-affected hand after training, (0.1 and 0.2 object more for the HABIT + T & HABIT groups, Fig. 3D). Nor did we find a significant Group × Test session interaction effect.
Fig. 3.
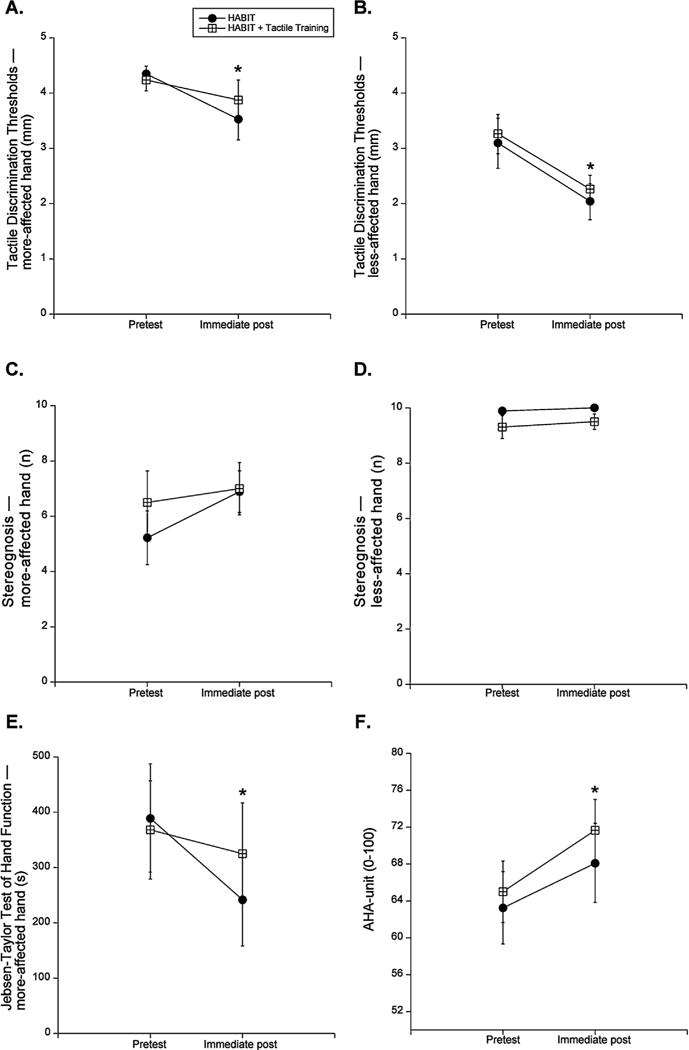
(A) Mean ± standard error of the mean (SEM) threshold of grating orientation task (GOT) of the more-affected hand. Solid circle represents HABIT group. Open rectangle represents HABIT + T group. (* p < 0.05 for the main effect of test session). (B) Mean ± SEM threshold of grating orientation task (GOT) of the less-affected hand. (C) Mean ± SEM numbers of identified objects of stereognosis of the more-affected hand. (D) Mean ± SEM numbers of identified objects of stereognosis of the less-affected hand. (E) Mean ± SEM time to complete JTTHF of the more-affected hand. ± (F) Mean ± SEM AHA-unit measured by the AHA.
Table 2.
Results for the more-affected hand.
| Pretest (95% CI) | Immediate posttest (95% CI) | Change score (pretest to immediate posttest) (95% CI) | Test session effect p value (partial η2)a | Interaction p value (partial η2)a | Power (1 − β)b | |
|---|---|---|---|---|---|---|
| Threshold of GOT (mm) | ||||||
| HABIT + T | 4.23 (3.89, 4.58) | 3.87 (3.06, 4.68) | −0.36 (−0.99, 0.27) | – | – | – |
| HABIT | 4.35 (3.99, 4.72) | 3.53 (2.68, 4.38) | −0.82 (−1.48, −0.16) | – | – | – |
| Mean | 4.29 (4.04, 4.54) | 3.70 (3.11, 4.29) | −0.59 (−1.05, −0.14) | 0.028 (0.253) | 0.501 (0.027) | 1.00 |
| Stereognosis (n) | ||||||
| HABIT + T | 6.5 (4.19, 8.81) | 7.00 (5.14, 8.87) | 0.50 (−0.88, 1.88) | – | – | – |
| HABIT | 5.22 (2.79, 7.66) | 6.89 (4.92, 8.86) | 1.67 (0.21, 3.12) | – | – | – |
| Mean | 5.86 (4.18, 7,54) | 6.94 (5.59, 8.30) | 1.08 (0.08, 2.08) | 0.063 (0.188) | 0.522 (0.025) | 0.99 |
| TPD thumb (mm) | ||||||
| HABIT + T | 8.9 (5.2, 12.60) | 8.6 (5.02, 12.18) | −0.30 (−1.40, 0.80) | – | – | – |
| HABIT | 9.22 (5.32, 13.12) | 8.89 (5.12, 12.66) | −0.33 (−0.80, 0.13) | – | – | – |
| Mean | 9.06 (6.37, 11.75) | 8.74 (6.14, 11.35) | −0.32 (−0.98, 0.35) | 0.413 (0.04) | 0.479 (0.03) | 0.99 |
| SWM | ||||||
| HABIT + T | 6.30 (4.50, 8.10) | 5.40 (3.50, 7.30) | −0.90 (−2.16, 0.36) | – | – | – |
| HABIT | 5.78 (3.88, 7.68) | 6.00 (3.99, 8.00) | 0.22 (−0.85, 1.29) | – | – | – |
| Mean | 6.04 (4.73, 7.35) | 5.70 (4.32, 7.08) | −0.34 (−1.24, 0.56) | 0.228 (0.084) | 0.106 (0.146) | 0.97 |
Abbreviations: GOT, grating orientation task; HABIT, hand–arm intensive bimanual therapy; HABIT + T, HABIT with additional tactile training; TPD, two-point discrimination; SWM, Semmes-Weinstein monofilaments; Mean, represents the average for the HABIT + T and HABIT groups.
Statistical results obtained from using ANOVA with repeated measure.
Power was calculated for the test session effect.
3.2. Changes in TPD and SWM after training
There were no significant changes after training in either hand in the TPD and SWM in either group (TPD all fingers, more-affected hand, p > 0.16; TPD all fingers, less-affected hand, p > 0.57; SWM more-affected hand, p = 0.23; SWM less-affected hand, p = 0.74). There were no Group × Test session interaction effects for either measure.
3.3. Changes in hand function after training
For the JTTHF, there was a 42s (19.7%) and a 148s (39.1%) decrease for the HABIT + T and the HABIT group, respectively (Fig. 3E, test session, p < 0.001). There was no significant Group × Test session interaction effect, indicating both groups did not improved in a significantly different way (p = 0.053). For the AHA, there was a 6.7 and a 4.9 AHA-unit improvement for the HABIT + T and the HABIT group, respectively (Fig. 3F, test session, p = 0.002). There was no Group × Test session interaction effect for the AHA, indicating both groups improved similarly (p = 0.56). These improvements were clinically meaningful (defined as a 5 AHA units improvement) for the HABIT + T group, and borderline clinically meaningful for the HABIT group.
3.4. Stability of measures in a control group without training
Since there were no differences between the two groups in the primary measures, we considered whether there was a learning effect in the participants simply by being tested twice across a 3-week period of time without training. We thereafter recruited an age-matched control group of 10 participants with USCP (see Table 3). They were tested two times with three-weeks in between on the primary and one secondary measure without any treatment. Paired t-tests confirmed stability of these measures across the two testing sessions in the control group (Table 3, no significant differences for any measure).
Table 3.
Outcomes remain stable over time in the control group.
| Outcome | Test 1 | Test 2a | t-Test p valueb |
|---|---|---|---|
| Tactile discrimination threshold (mm) | 4.11 (0.7) | 3.95 (0.73) | 0.42 |
| Stereognosis (n) | 4.4 (3.75) | 4.4 (3.57) | 0.95 |
| JTTHF (s) | 364.69 (305.6) | 352.56 (232.59) | 0.31 |
Values for Test 1 and Test 2 represents mean (SD).
Test 2 was performed 3 weeks after Test 1.
Statistical results obtained from using paired t-test.
3.5. Predictors of functional outcome
Due to the heterogeneity of the lesion and functions in USCP, we tested whether individual differences, including initial severity in tactile & motor function and age, would impact changes in function. Given no group differences were found in previous analyses, we used all participants (n = 19) to explore correlations between individual differences and changes in function. We found that participants with worse unilateral dexterity (baseline JTTHF) had a trend to improve more in stereognosis after training in the more-affected hand (R = 0.45, p = 0.052). Baseline unilateral dexterity was not correlated with improvements in threshold of discrimination in the more-affected hand (R = 0.3, p = 0.22). Baseline tactile function was not correlated with percentage improvements in the AHA (GOT & AHA % change, p = 0.88; stereognosis & AHA % change, p = 0.25). No relation was found between age and improvements in threshold of discrimination (p = 0.65), or between age and improvements in stereognosis (p = 0.95).
4. Discussion
This study aimed to investigate (1) changes in tactile function associated with intensive bimanual training in USCP, (2) the effects of additional tactile training to HABIT in enhancing tactile function. We hypothesized that tactile function would be improved after HABIT, and be further improved with added tactile training. Our results demonstrated that tactile function, specifically tactile spatial discrimination was modifiable immediately after intensive bimanual training. These improvements were not an effect of repeated testing across a 3-week period of time. Contrary to our hypothesis, both groups improved similarly. Although the improvements in the stereognosis did not reach statistical significance across the two groups (p = 0.063), it is worthwhile noticing that 31.6% (4 in the HABIT + T group, 2 in the HABIT group) of participants achieved the highest value (10 objects) at baseline in the stereognosis in the more-affected hand. This ceiling effect in the stereognosis at baseline may not leave sufficient room for change.
4.1. Enriched environment may improve tactile function in both groups
The comparable results in the improvements associated with either training group in the GOT could primarily be explained by an enriched environment in both groups. Animal studies (Alwis & Rajan, 2013) showed that enriched environment induced neural responses to discriminative whisker behaviors. Studies in healthy adults demonstrated that perceptual learning occurred after one to several sessions of tactile training (Harris, Harris, & Diamond, 2001) and was transferred to the contralateral and adjacent fingers (Kaas, van de Ven, Reithler, & Goebel, 2013).
Two possible types of enriched environments have been applied to both groups in our study. First, both groups received 82 h of standardized HABIT. The increased amount of tactile stimulation during those 82 h of intensive bimanual manipulation may have created an enriched environment and may explain comparable improvements in the GOT. Second, the novel training materials with different textures, shapes, and sizes used during the 8 h of tactile and controlled training could also have enriched the environment. Thus 8 h of specific tactile training may not be needed to enhance tactile function in children with USCP on top of the intensive bimanual therapy they already received. Instead, either intensive tactile input during standardized HABIT or an introduction of new materials during tactile/controlled training might drive tactile improvement.
4.2. Insufficient dose of tactile training may cause similar findings
The similar findings in the two groups might also be explained by insufficient tactile training dose in the HABIT + T group. Carey et al. (2011) showed that 10 h (60 mins/session, 3 sessions/week) of somatosensory discrimination training effectively improved tactile discrimination capacity in adult stroke patients. It is possible that 8 h of tactile training was insufficient to drive differential effects of perceptual learning between the two groups in children with USCP, especially when cognitive capacity was required in perceptual learning. It is important to note that children with USCP were learning new skills whereas adult stroke patients were re-learning the function they lost. Hence a more intensive training might be needed in children with USCP. Future studies should test an intensive dose of tactile training in a randomized controlled trial (RCT) or a cross-over study.
4.3. Improvements in spatial tactile discrimination in the both hands after intensive bimanual therapy
It is important to note that children with USCP had tactile impairments in both hands. In fact, the threshold of tactile discrimination of the less-affected hand is higher (worse acuity) than the dominant hand of typically developing (TD) children. The mean threshold of less-affected hand in USCP was 3.2 mm at baseline, while mean threshold in typically developing children varies from 1.37 (interquartile range = 1.12–1.83) at 6 years old to 1.10 (interquartile range = 0.71–1.28) at 16 years old (Bleyenheuft et al., 2006). Improvements were observed in both hands after training in our study. To our knowledge, this is the first study that reports tactile function can be improved in both hands after intensive bimanual training in children with USCP. This finding suggests that, when taking both hands into consideration, HABIT not only improves spatial-temporal control of the two hands (Hung et al., 2011), but also strengthens the tactile function in both hands. This may be an important consideration in choosing whether to do unimanual (CIMT) versus bimanual training.
4.4. HABIT and/or tactile training may improve tactile function involving reconstruction mental images of the stimuli
Despite the changes observed in the tactile spatial discrimination and the stereognosis, we did not find any significant changes in tactile pressure detection (SWM) and two-point discrimination. It has been previously shown that these sensory modalities are less affected than the others in children with CP (Arnould, Penta, & Thonnard, 2007; Cooper et al., 1995; Krumlinde-Sundholm & Eliasson, 2002; Van Heest, House, & Putnam, 1993). This may be related to the differential mechanisms associated with tactile spatial discrimination/stereognosis and pressure discrimination/TPD. Specifically, skills associated with spatial discrimination and stereognosis require a reconstruction of the mental image of the stimulus in the CNS, whereas skills associated with tactile pressure detection and two-point discrimination mainly require tactile input detection using non-spatial cues in the receptor population (Van Boven & Johnson, 1994). Thus neuroplastic changes after tactile training are more likely to be associated with changes in the mental representations of the tactile stimuli in the somatosensory cortex.
4.5. Correlation between baseline dexterity (JTTHF) and stereognosis
A moderate, albeit non-significant correlation between baseline JTTHF and stereognosis was found, whereby children with worse dexterity improved more in stereognosis in the more-affected hand after training. In two studies, researchers highlighted that stereognosis was correlated with manual ability in children with USCP (Arnould, Bleyenheuft, & Thonnard, 2014; Klingels et al., 2012). The association was possibly due to the requirement of active manipulation for allowing successful object identification. Children with the worst baseline dexterity are also more likely to change from a passive to an active exploration mode after training, due to their improvements in motor function. This probably accounts for improvement in stereognosis, whereas this association was not found between dexterity and tactile spatial discrimination in the more-affected hand (no active manipulation).
5. Limitations
Our study has a small sample size, which may render insufficient power to determine differences between groups, and the results may not be generalizable. However, our study is the first study to examine the effect of HABIT with or without tactile training in modifying tactile function in children with USCP, and no comparable studies using same measures were conducted in the literature. Therefore it is difficult to estimate an appropriate sample size a priori. We acknowledge that replication with a larger study is needed.
In this study, improvements in tactile function were measured following intensive bimanual training. Enriched environments may play a major role in the changes observed in both groups. Our study does not allow us to directly discriminate the effect of the amount of manipulative tasks (i.e. standardized HABIT) versus the manipulation of specific materials during the 8 h of specific tactile/control training as we collected our subjects from a sample of convenience. Future randomized controlled trials or cross-over studies should test the efficacy of these components in isolation and collect retention data several months after training.
We used a JVP dome-width of 4.5 mm as the widest bar width for testing based on a previous study (Bleyenheuft & Thonnard, 2011). We conservatively assigned 4.5 mm as the cap threshold for those who did not reach 70% of the correct rate when tested with 4.5 mm. In fact, 8 children in either group (16 out of 19 children, 84.2%) failed to reach 70% of the correct rate at baseline. Three out of 8 children in the HABIT + T group and 4 out of 8 children in the HABIT group improved from 4.5 mm to a measurable threshold after training. Conceivably, we could have underestimated improvements in these 7 children by capping their baseline threshold at 4.5 mm. Future studies using the GOT as an outcome measure may consider including a wider range of domes for allowing a more accurate threshold measure.
6. Conclusions
Our study is the first to report that tactile function can be enhanced in both hands after intensive bimanual training. Importantly, the improvements in outcome did not differ between the HABIT + T and the HABIT groups, suggesting the environment is driving the changes rather than specific tactile training. Our study suggests a window of opportunity for modifying tactile function by providing an enriched environment. The take-home message of our study is that tactile impairments can be improved when the tactile input is structured in the environment.
What this paper adds.
This is the first study in USCP to test the effect of systematic tactile training.
Tactile spatial discrimination was improved following intensive bimanual training.
HABIT with and without tactile training improved tactile function similarly.
Acknowledgments
HK & KF (NIH grant: NS K01062116). AH received a student scholarship from the Université catholique de Louvain. We thank children, families, and volunteers participating in our study.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Hsing-Ching Kuo, Email: hk2455@tc.columbia.edu.
Andrew M. Gordon, Email: ag275@tc.columbia.edu.
Aline Henrionnet, Email: aline.henrionnet@gmail.com.
Sylvie Hautfenne, Email: sylvie.hautfenne@outlook.com.
Kathleen M. Friel, Email: kaf3001@med.cornell.edu.
References
- Alwis DS, Rajan R. Environmental enrichment causes a global potentiation of neuronal responses across stimulus complexity and lamina of sensory cortex. Frontiers in Cellular Neuroscience. 2013;7:124. doi: 10.3389/fncel.2013.00124. http://dx.doi.org/10.3389/fncel.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnould C, Bleyenheuft Y, Thonnard JL. Hand functioning in children with cerebral palsy. Frontiers in Neurology. 2014;5:48. doi: 10.3389/fneur.2014.00048. http://dx.doi.org/10.3389/fneur.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnould C, Penta M, Thonnard JL. Hand impairments and their relationship with manual ability in children with cerebral palsy. Journal of Rehabilitation Medicine. 2007;39(9):708–714. doi: 10.2340/16151977-0111. http://dx.doi.org/10.2340/16151977-0111. [DOI] [PubMed] [Google Scholar]
- Auld ML, Boyd RN, Moseley GL, Ware RS, Johnston LM. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Archives of Physical Medicine and Rehabilitation. 2012;93(4):696–702. doi: 10.1016/j.apmr.2011.10.025. http://dx.doi.org/10.1016/j.apmr.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Auld ML, Russo R, Moseley GL, Johnston LM. Determination of interventions for upper extremity tactile impairment in children with cerebral palsy: A systematic review. Developmental Medicine & Child Neurology. 2014;56(9):815–832. doi: 10.1111/dmcn.12439. http://dx.doi.org/10.1111/dmcn.12439. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Arnould C, Brandao MB, Bleyenheuft C, Gordon AM. Hand and arm bimanual intensive therapy including lower extremity (HABIT-ILE) in children with unilateral spastic cerebral palsy: A randomized trial. Neurorehabilitation and Neural Repair. 2015;29(7):645–657. doi: 10.1177/1545968314562109. http://dx.doi.org/10.1177/1545968314562109. Epub 2014 December 19. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Cols C, Arnould C, Thonnard JL. Age-related changes in tactile spatial resolution from 6 to 16 years old. Somatosensory & Motor Research. 2006;23(3–4):83–87. doi: 10.1080/08990220600816440. http://dx.doi.org/10.1080/08990220600816440. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Dricot L, Gilis N, Kuo HC, Grandin C, Bleyenheuft C, et al. Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study. Research in Developmental Disabilities. 2015;43–44:136–149. doi: 10.1016/j.ridd.2015.06.014. http://dx.doi.org/10.1016/j.ridd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyenheuft Y, Gordon AM. Precision grip control, sensory impairments and their interactions in children with hemiplegic cerebral palsy: A systematic review. Research in Developmental Disabilities. 2013;34(9):3014–3028. doi: 10.1016/j.ridd.2013.05.047. http://dx.doi.org/10.1016/j.ridd.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Thonnard JL. Tactile spatial resolution measured manually: A validation study. Somatosensory & Motor Research. 2007;24(3):111–114. doi: 10.1080/08990220701496639. http://dx.doi.org/10.1080/08990220701496639. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Thonnard JL. Tactile spatial resolution in unilateral brain lesions and its correlation with digital dexterity. Journal of Rehabilitation Medicine. 2011;43(3):251–256. doi: 10.2340/16501977-0651. http://dx.doi.org/10.2340/16501977-0651. [DOI] [PubMed] [Google Scholar]
- Brandao MB, Ferre C, Kuo HC, Rameckers EA, Bleyenheuft Y, Hung YC, et al. Comparison of structured skill and unstructured practice during intensive bimanual training in children with unilateral spastic cerebral palsy. Neurorehabilitation and Neural Repair. 2013;28(5):452–461. doi: 10.1177/1545968313516871. http://dx.doi.org/10.1177/1545968313516871. [DOI] [PubMed] [Google Scholar]
- Brandao MB, Gordon AM, Mancini MC. Functional impact of constraint therapy and bimanual training in children with cerebral palsy: A randomized controlled trial. American Journal of Occupational Therapy. 2012;66(6):672–681. doi: 10.5014/ajot.2012.004622. http://dx.doi.org/10.5014/ajot.2012.004622. [DOI] [PubMed] [Google Scholar]
- Carey L, Macdonell R, Matyas TA. SENSe: Study of the effectiveness of neurorehabilitation on sensation: A randomized controlled trial. Neurorehabilitation and Neural Repair. 2011;25(4):304–313. doi: 10.1177/1545968310397705. http://dx.doi.org/10.1177/1545968310397705. [DOI] [PubMed] [Google Scholar]
- Charles J, Gordon AM. Development of hand–arm bimanual intensive training (HABIT) for improving bimanual coordination in children with hemiplegic cerebral palsy. Developmental Medicine & Child Neurology. 2006;48(11):931–936. doi: 10.1017/S0012162206002039. http://dx.doi.org/10.1017/s0012162206002039. [DOI] [PubMed] [Google Scholar]
- Charles J, Lavinder G, Gordon AM. Effects of constraint-induced therapy on hand function in children with hemiplegic cerebral palsy. Pediatric Physical Therapy. 2001;13(2):68–76. [PubMed] [Google Scholar]
- Cooper J, Majnemer A, Rosenblatt B, Birnbaum R. The determination of sensory deficits in children with hemiplegic cerebral palsy. Journal of Child Neurology. 1995;10(4):300–309. doi: 10.1177/088307389501000412. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Chinnan A, Gill S, Petra E, Hung YC, Charles J. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Developmental Medicine & Child Neurology. 2008;50(12):957–958. doi: 10.1111/j.1469-8749.2008.03166.x. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Duff SV. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. I: Anticipatory scaling. Developmental Medicine & Child Neurology. 1999a;41(3):166–175. doi: 10.1017/s0012162299000353. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Developmental Medicine & Child Neurology. 1999b;41(9):586–591. doi: 10.1017/s0012162299001231. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Hung YC, Brandao M, Ferre CL, Kuo HC, Friel K, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: A randomized trial. Neurorehabilitation and Neural Repair. 2011;25(8):692–702. doi: 10.1177/1545968311402508. http://dx.doi.org/10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand–arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: A randomized control trial. Developmental Medicine & Child Neurology. 2007;49(11):830–838. doi: 10.1111/j.1469-8749.2007.00830.x. http://dx.doi.org/10.1111/j.1469-8749.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME. The topography of tactile learning in humans. The Journal of Neuroscience. 2001;21(3):1056–1061. doi: 10.1523/JNEUROSCI.21-03-01056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YC, Casertano L, Hillman A, Gordon AM. The effect of intensive bimanual training on coordination of the hands in children with congenital hemiplegia. Research in Developmental Disabilities. 2011;32(6):2724–2731. doi: 10.1016/j.ridd.2011.05.038. http://dx.doi.org/10.1016/j.ridd.2011.05038. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation. 1969;50(6):311–319. [PubMed] [Google Scholar]
- Joo HW, Hyun JK, Kim TU, Chae SH, Lee YI, Lee SJ. Influence of constraint-induced movement therapy upon evoked potentials in rats with cerebral infarction. European Journal of Neuroscience. 2012;36(12):3691–3697. doi: 10.1111/ejn.12014. http://dx.doi.org/10.1111/ejn.12014. [DOI] [PubMed] [Google Scholar]
- Juenger H, Kuhnke N, Braun C, Ummenhofer F, Wilke M, Walther M, et al. Two types of exercise-induced neuroplasticity in congenital hemiparesis: A transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Developmental Medicine & Child Neurology. 2013;55(10):941–951. doi: 10.1111/dmcn.12209. http://dx.doi.org/10.1111/dmcn.12209. [DOI] [PubMed] [Google Scholar]
- Kaas AL, van de Ven V, Reithler J, Goebel R. Tactile perceptual learning: Learning curves and transfer to the contralateral finger. Experimental Brain Research. 2013;224(3):477–488. doi: 10.1007/s00221-012-3329-8. http://dx.doi.org/10.1007/s00221-012-3329-8. [DOI] [PubMed] [Google Scholar]
- Klingels K, Demeyere I, Jaspers E, De Cock P, Molenaers G, Boyd R, et al. Upper limb impairments and their impact on activity measures in children with unilateral cerebral palsy. European Journal of Paediatric Neurology. 2012;16(5):475–484. doi: 10.1016/j.ejpn.2011.12.008. http://dx.doi.org/10.1016/j.ejpn.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L, Eliasson AC. Comparing tests of tactile sensibility: Aspects relevant to testing children with spastic hemiplegia. Developmental Medicine & Child Neurology. 2002;44(9):604–612. doi: 10.1017/s001216220100264x. [DOI] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L, Eliasson AC. Development of the assisting hand assessment: A Rasch-built measure intended for children with unilateral upper limb impairments. Scandinavian Journal of Occupational Therapy. 2003;10:16–26. [Google Scholar]
- Laible M, Grieshammer S, Seidel G, Rijntjes M, Weiller C, Hamzei F. Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabilitation and Neural Repair. 2012;26(7):881–888. doi: 10.1177/1545968312437939. http://dx.doi.org/10.1177/1545968312437939. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. Two-point discrimination tester. Journal of Hand Surgery (American Volume) 1985;10(6 Pt 1):906–907. doi: 10.1016/s0363-5023(85)80173-8. [DOI] [PubMed] [Google Scholar]
- Mott FW, Sherrington CS. Experiments upon the influence of sensory nerves upon movement and nutrition of the limbs. Preliminary communication. Proceedings of the Royal Society of London. 1895;57:481–488. [Google Scholar]
- Sakzewski L. Bimanual therapy and constraint-induced movement therapy are equally effective in improving hand function in children with congenital hemiplegia. Journal of Physiotherapy. 2012;58(1):59. doi: 10.1016/S1836-9553(12)70075-9. http://dx.doi.org/10.1016/s1836-9553(12)70075-9. [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Equivalent retention of gains at 1 year after training with constraint-induced or bimanual therapy in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair. 2011;25(7):664–671. doi: 10.1177/1545968311400093. http://dx.doi.org/10.1177/1545968311400093. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO. A psychophysical study of the mechanisms of sensory recovery following nerve injury in humans. Brain. 1994;117(Pt 1):149–167. doi: 10.1093/brain/117.1.149. [DOI] [PubMed] [Google Scholar]
- Van Heest AE, House J, Putnam M. Sensibility deficiencies in the hands of children with spastic hemiplegia. Journal of Hand Surgery (American Volume) 1993;18(2):278–281. doi: 10.1016/0363-5023(93)90361-6. http://dx.doi.org/10.1016/0363-5023(93).90361-6. [DOI] [PubMed] [Google Scholar]
- Weinstein S. Fifty years of somatosensory research: From the Semmes-Weinstein monofilaments to the Weinstein Enhanced Sensory Test. Journal of Hand Therapy. 1993;6(1):11–22. discussion 50. [PubMed] [Google Scholar]


