Abstract
Intensive rehabilitation interventions have been shown to be efficacious in improving upper extremity function in children with unilateral spastic cerebral palsy (USCP). These interventions are based on motor learning principles and engage children in skillful movements. Improvements in upper extremity function are believed to be associated with neuroplastic changes. However, these neuroplastic changes have not been well-described in children with cerebral palsy, likely due to challenges in defining and implementing the optimal tools and tests in children. Here we documented the implementation of three different neurological assessments (diffusion tensor imaging-DTI, transcranial magnetic stimulation-TMS and functional magnetic resonance imaging-fMRI) before and after a bimanual intensive treatment (HABIT-ILE) in two children with USCP presenting differential corticospinal developmental reorganization (ipsilateral and contralateral). The aim of the study was to capture neurophysiological changes and to document the complementary relationship between these measures, the potential measurable changes and the feasibility of applying these techniques in children with USCP.
Independent of cortical reorganization, both children showed increases in activation and size of the motor areas controlling the affected hand, quantified with different techniques. In addition, fMRI provided additional unexpected changes in the reward circuit while using the affected hand.
Keywords: Cerebral palsy, Bimanual training, fMRI, TMS, DTI
1. Introduction
Over the last decade, intensive rehabilitation interventions have been successfully developed for addressing upper extremity function of children with unilateral spastic cerebral palsy (USCP; e.g., Charles, Lavinder, & Gordon, 2001; Charles, Wolf, Schneider, & Gordon, 2006; de Brito Brandaõ, Gordon, & Mancini, 2012; DeLuca, Case-Smith, Stevenson, & Ramey, 2012; Deppe et al., 2013; Eliasson, Krumlinde-Sundholm, Shaw, & Wang, 2005; Fedrizzi et al., 2013; Gordon, Charles, & Steenbergen, 2006; Gordon, Schneider, Chinnan, & Charles, 2007; Gordon et al., 2008, 2011; Hoare et al., 2013; Sakzewski et al., 2011). These interventions are based on motor learning principles applied to intensive unimanual (constrained-induced movement therapy – CIMT) or bimanual (e.g., Hand and arm bimanual intensive therapy – HABIT) training. More recently, a combined bimanual and lower extremity stimulation has been proposed (Hand and arm bimanual intensive therapy including lower extremities: HABIT-ILE; Bleyenheuft & Gordon, 2014; Bleyenheuft, Arnould, Brandao, Bleyenheuft, & Gordon, 2014).
While principles of motor learning indicate that changes in motor skill are associated with neuroplastic changes in animal models (Kleim et al., 2004; Nudo, 2003), there is a lack of evidence for these neuroplastic changes in children with cerebral palsy. This lack of evidence is likely due to challenges in defining and implementing the optimal tools for measuring these changes.
Neuroplastic changes might be influenced by the basic corticospinal (CST) organization of the children with USCP. It is now well-established that children with USCP may present an atypical development of the descending motor pathways (Eyre et al., 2007; Staudt et al., 2004, for a review see Gordon, Bleyenheuft, & Steenbergen, 2013). From the original bilateral projections developed during embryogenesis, CST may either pursue a “classical” crossed (contralateral) development (with a natural pruning of the ipsilateral projections), or maintain and strengthen the ipsilateral projections from the unaffected hemisphere to the affected hand. The maintenance of such projections results in the affected hand being controlled either with projections from both hemispheres (bilateral organization), or with projections from the unaffected hemisphere exclusively (ipsilateral projections). It is generally accepted that children with ipsilateral reorganization present a more severely affected hand function, accompanied by mirror movements in upper extremities (Holmström et al., 2010; Staudt et al., 2004).
Since motor pathways can be reorganized in very different ways in children with USCP (Carr, Harrison, Evans, & Stephens, 1993; Guzzetta et al., 2007; Staudt et al., 2002, 2004), tracking of CST circuits with diffusion tensor imaging (DTI) is a key imaging approach to understand the connectivity of the motor system in children with USCP. DTI measurements of CST dysgenesis are strongly related to the manual dexterity of a child with USCP (Bleyenheuft, Grandin, Cosnard, Olivier, & Thonnard, 2007), but does not appear to be predictive of the potential for improvement in these children (Friel, Kuo, Carmel, Rowny, & Gordon, 2014). DTI is also hypothesized to be sensitive to changes after motor learning in children with USCP since changes in DTI have been detected after learning in different contexts (Imfeld, Oechslin, Meyer, Loenneker, & Jancke, 2009; Lazaridou et al., 2013; Li, Wang, Hu, Liang, & Chen, 2013).
Cortical changes that are linked to motor changes elicited by intensive therapy sessions can be captured by cortical mapping using single-pulse transcranial magnetic stimulation (TMS). TMS can assess the topography of the motor map (Vandermeeren, Davare, Duque, & Olivier, 2009) as well as the strength of the connections. Single-pulse TMS is also a safe procedure that is well-tolerated by children (Krishnan, Santos, Peterson, & Ehinger, 2015). Disadvantages of TMS include not being safe for use in people with seizure disorders and not being readily able to assess cortical areas that are not on the brain surface, such as the leg motor map. Functional magnetic resonance imaging (fMRI) is a key instrument to investigate plastic changes in the brain associated with motor learning (Ghilardi et al., 2000; Debas et al., 2010; Hardwick, Rottschy, Miall, & Eickhoff, 2013). However, application for tracking motor changes in children with spasticity may result in difficulties: First, the absolute necessity of keeping the head still while producing movements with upper extremities may be too difficult. Second, children with implanted metallic material could be automatically excluded due to the risks associated with the magnetic field.
The aim of this pilot study was to implement these three neurophysiological assessments (TMS, fMRI, DTI) in 2 children with USCP before and after a HABIT-ILE treatment. We aimed to capture neurophysiological changes and to document the complementary relationship between these measures, potential changes associated with therapy, and the feasibility of applying these techniques in children with USCP.
2. Material and methods
2.1. Participants
Two participants with different CST organization were included in this study.
Child 1 was a 6 year old girl presenting with a right hemiparesis consecutive to a left sylvian cerebral vascular accident (CVA) in perinatal period. The child was classified as level II on the Manual Ability Classification System (MACS, Eliasson et al., 2006) and presents a Gross Motor Function Classification System, (GMFCS, Palisano et al., 1997) of level I.
Child 2 was a 9 year old girl presenting with a left hemiparesis. The cerebral palsy is consecutive to a right sylvian CVA. Her MACS is classified as level II and her GMFCS as level I.
2.2. Intensive training
Participants were engaged in a HABIT-ILE treatment 9 h/day during 10 consecutive days (total amount of 90 hours) during school holidays. HABIT-ILE uses structured bimanual tasks that require simultaneous control and coordination of UE and LE movements (Bleyenheuft & Gordon, 2014; Bleyenheuft et al., 2014).
2.3. Functional/behavioral assessments
Before and after the HABIT-ILE session, upper and lower extremity changes were assessed.
On both hands, dexterity was measured with the Jebsen–Taylor test of hand function (JTTHF, Jebsen et al., 1969), pinch force with a pinch dynamometer (Mathiowetz, Wiemer, & Federman, 1986), Stereognosis with the Cooper test (Cooper, Majnemer, Rosenblatt, & Birnbaum, 1995). Manual ability was measured with (1) the ABILHAND-kids (logit) (Arnould, Penta, Renders, & Thonnard, 2004), (2) the Assisting Hand Assessment (AHA; Krumlinde-Sundholm & Eliasson, 2003; Krumlinde-Sundholm, Holmefur, Kottorp, & Eliasson, 2007) and (3) the Pediatric Evaluation of Disability Inventory (PEDI, Haley, Coster, Ludlow, Haltiwanger, & Andrellos, 1992; McCarthy et al., 2002; Vos-Vromans, Ketelaar and Gorter, 2005). Lower extremity assessment included the 6 minute walk test (6MWT), Enright (2003); Geiger et al. (2007); Li et al. (2007) and the ABILOCO-kids questionnaire (Caty, Arnould, Thonnard, & Lejeune, 2008). The Canadian Occupational Performance Measure (COPM) was used to measure changes in the functional goals (Carswell et al., 2004; Law, McColl, Opzoomer, Polatajko, & Pollock, 1990; Verkerk, Wolf, Louwers, Meester-Delver, & Nollet, 2006). More details are provided on these assessments in the supplementary material.
2.4. TMS motor mapping
Single-pulse TMS mapping of the motor cortex was performed before and after ninety hours of HABIT-ILE. The details of setup (Noirhomme et al., 2004) and MEP recording are available in the supplementary material.
In short, TMS pulses were delivered at a frequency of less than 0.1 Hz, with the coil being moved along the head in 2 cm increments between each pulse. Pulses were delivered until either a motor evoked potential (MEP) of the affected upper extremity (UE) was found, or until the hemisphere had been thoroughly mapped in 2 cm increments (<80% maximum stimulator output).
If an MEP of the affected FDI was found, the coil was held at that spot (“hotspot”) and the stimulator output was lowered until an MEP could no longer be elicited. The stimulator output at which an MEP of the affected FDI could be elicited from six of ten sequential pulses delivered at a frequency of 0.1 Hz was defined as the motor threshold (MT).
TMS mapping was subsequently performed by delivering single TMS pulses at a stimulus intensity of 110% the MT for the affected FDI. Three to six TMS pulses were delivered to each grid point. Both hemispheres were mapped.
After rehabilitation, TMS mapping was repeated using the same procedures as before rehabilitation. The MT of the affected FDI was found as described above. Three to six TMS pulses were delivered to each grid point on the skull cap, at a stimulus intensity of 110% the pre-rehabilitation MT of the affected FDI.
2.4.1. Data analyses
EMG data were exported from Signal into MATLAB (Mathworks). A MATLAB script was written to identify the onset time, offset time, and magnitude of the MEP for each muscle. Onset and offset time were determined relative to the time of TMS stimulation. The latency of MEP onset was defined as the duration between TMS stimulation and onset of the MEP. The strength of the MEP was defined as the peak-to-peak amplitude of the MEP.
For each grid point in the map, the average MEP strength and latency was calculated. Each grid point was categorized as a digit, wrist, or duel digit-wrist response site by the presence or absence of an MEP in the FDI or WF at that site. The number of digit and wrist sites were tallied. Differences in the number and MEP amplitude of digit and wrist responses before and after rehabilitation were calculated.
Similarly to TMS, children were assessed with imaging before and after intensive training. Imaging consisted of conventional MRI; functional magnetic resonance imaging (fMRI) and diffusion tensor images (DTI) performed at 3T (Achieva, Philips Healthcare, Eindhoven, The Netherlands.
2.5. fMRI
Functional MR images of brain activity were also collected using the same 3T head scanner (with repeated single-shot gradient-echo echo-planar imaging: echo time (TE) = 50 ms, flip angle (FA) = 908, Inplane Resolution = 1.964 mm × 1.964 mm, slice order descending and interleaved, slice thickness = 3 mm). Repetition time (TR) was 2250 ms, 38 slices (the whole brain is scanned 148 times per run, 6 times per condition per run).
2.5.1. fMRI paradigm
During the fMRI experiments, participants performed 3 runs of 5 min 33 s duration each sequence. There were 3×6 oral instructions (1500 ms) provided (“left hand”, “right hand”, “both hands”) per run. After the verbal command, the children performed the movement requested: grip and lift a cube (2 cm edge) with left hand, with right hand or grip two cubes simultaneously (one cube in each hand). Pretests showed that after 8 s all our participants finished the movements. The duration between trials was then chosen to be 16.5 s to ensure a comfortable baseline. During all experiments, one experienced physical therapist inside the scanning room checked all the movements and pressed four button boxes to record information about them (start and end of the left and right hand movement). This information was used to create the multiple regression model (General Linear Model; GLM) for the fMRI data analysis.
2.5.2. Data analysis: fMRI runs
The fMRI signal in the various conditions was compared using BrainVoyager QX (Version 2.3, Brain Innovation, Maastricht, The Netherlands). Prior to analysis, the functional data sets were subjected to a series of preprocessing operations. Preprocessing consisted of a linear trend removal for excluding scanner-related signal, a temporal high-pass filtering applied to remove temporal frequencies lower than 3 cycles per run, and a correction for small interscan head movements by a rigid body algorithm rotating and translating each functional volume in 3D space. Data were corrected for the difference between the scan times of the different slices. Data was smoothed in the spatial domain (Gaussian filter: Full Width at Half Maximum = 5 mm). Subsequently, the functional data were analyzed using a multiple regression model (General Linear Model; GLM) consisting of predictors, which corresponded to the particular experimental conditions of each experiment: movement of right hand condition (RC), left hand (LC) and both hands (BC) for the two sessions separately (6 conditions in all, RCs1 and RCs2 for session 1 and 2 respectively, etc.).1 The predictor time courses used were computed on the basis of a linear model of the relation between neural activity and hemodynamic response (Boynton, Engel, Glover, & Heeger, 1996). The statistical maps computed were overlaid to the 3D T1-weighted scans in the AC–PC native space. All coregistrations were corrected manually and the corrections of the movement were optimized (sinc interpolation).
2.5.3. fMRI statistical analyses and contrasts of interest
For each child, we performed first whole brain analysis using the following general contrast: [(RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest] aimed at isolating the areas responding to hand movements comparing to rest and found the sensori-motor cortex. Further, we computed ROI analysis in each area found with this contrast. Follow up contrasts [(RCs2 − RCs1), (LCs2 − LCs1), (BCs2 − BCs1), (LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1) or (RCs2 + BCs2) − (RCs1 + BCs1), (LCs2 − LCs1) − (RCs2 − RCs1) or (BCs2 − BCs1) − (RCs2 RCs1)2 ] were calculated at the peak of activation. These values were used to compare activation before and after the intensive training.
2.6. DTI
DTI images were acquired at the end of each fMRI session (details of the sequence provided in supplementary material). In order to determine FA of the CSTs, spheres of 3 mm (123 voxels) were created symmetrically in both tracts, their middle centered on the CS fibers at the level of the mid pons, as visualized in a transversal plane passing through the middle cerebellar peduncle (see Fig. 3). The number of voxels presenting a main z direction was then counted, and the value of the FA in z direction reported for the 123 voxels in each sphere. A determinist tracking was then made from the spheres. Only the fibers with FA > 0.15 and a deviation angle <50° were considered for this tracking.
Fig. 3.
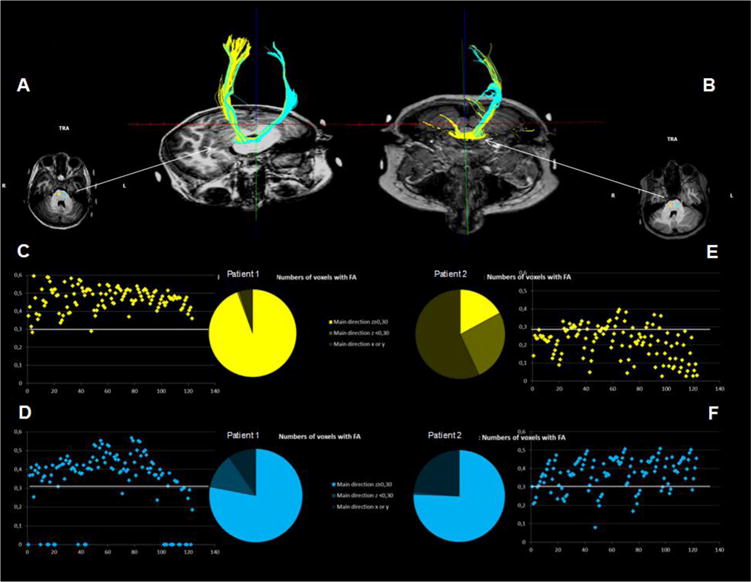
In order to determine FA of the CSTs, spheres of 3 mm (123 voxels) were created symmetrically in both tracts, their middle centered on the CS fibers as visualized in a transversal plane passing through the middle cerebellar peduncle (see picture in transversal plane). The number of voxels presenting a main z direction was then counted, and the value of the FA in z direction reported for the 123 voxels in each sphere. A determinist tracking was then made from the spheres. Only the fibers with FA > 0.15 were considered for this tracking and a deviation angular of less than 508 was required. The upper panels (A and B) represent the tracking for children 1 and 2 respectively. The lower panel delineates the fractional anisotropy of the fibers for right (yellow) and left (blue) CST respectively in both children. Fig. 4a: Child 2/at same q.
3. Results
3.1. Child 1
Child 1 showed improvements in upper and lower extremities in all functional assessments except the AHA (see Table 1).
Table 1.
Description of behavioral/functional changes.
| Child 1
|
Child 2
|
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| JTTHF MA (s) | 1080 | 915 | 275 | 84 |
| JTTHF LA (s) | 49 | 48 | 37 | 36 |
| Key pinch MA (kg) | 1.5 | 2.83 | 1.58 | 2.08 |
| Key pinch LA (kg) | 3.66 | 3.83 | 5.50 | 4.83 |
| ABILHAND-Kids (logits) | 0.34 | 2.15 | 2.89 | 6.68 |
| PEDI (self-care) | 38 | 58 | 51 | 63 |
| AHA (AHA-units) | 43 | 42 | 66 | 67 |
| Stereognisis MA (/10) | 2 | 5 | 3 | 4 |
| Stereognisis LA (/10) | 9 | 10 | 10 | 10 |
| 6MWT (m) | 390 | 480 | 374 | 520 |
| Abiloco-kids (logits) | 3.60 | 5.90 | 4.31 | 5.92 |
| COPM performance (/10) | 4.2 | 7.9 | 5.7 | 8.2 |
| COPM satisfaction (/10) | 7.8 | 8.8 | 5.0 | 8.8 |
JTTHF: Jebsen–Taylor Test of Hand Function; MA: more affected; LA: less affected; PEDI: pediatric evaluation of disability inventory; COPM: Canadian Occupational Performance Measure.
3.1.1. TMS
For child 1, the motor map of the affected hand was found in the contralateral, affected hemisphere.
After 90 hours of HABIT-ILE, substantial changes in the responsiveness and excitability of motor maps were found. In child 1 (contralateral map of the affected hand) there was a 50% increase in the number of digit and wrist responsive sites after rehabilitation. The average amplitude of the MEP of the affected FDI increased from 311.47 μV to 458.73 μV (47.3% increase). The average amplitude of the MEP of the affected WF increased from 262.73 μV to 428.37 μV (63.1% increase).
3.1.2. fMRI
3.1.2.1. Behavioral results
During the first fMRI assessment, over the 18 trials programmed with the right (affected) hand across the 3 runs, child 1 succeeded in only 11 trials. With the less-affected hand, 16 out of 18 trials were correctly performed. Finally, 12 out 18 bimanual trials were successful during this pre-training assessment.
At post-training assessment, with the same paradigm, all trials but 2 were completed with the right/more-affected hand (16 out of 18). All left hand (18/18) and bimanual trials (18/18) were correctly completed.
3.1.2.2. fMRI results
At q(False Discovery Rate) < 0.05 (t = 3.54) and cluster size >20 mm3, we reported 8 clusters for the contrast [(RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest] (see Table 2 and Fig. 1). We found clusters in the primary motor cortex (M1), primary sensory cortex (S1), the putamen and premotor cortex on the right hemisphere. On the right side we also found the left inferior and the superior frontal gyrus to be activated. The largest region was found in the supplementary motor area (SMA). On the left, we only discovered a cluster in the motor/premotor area.
Table 2.
Child 1 – Contrast: (RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest.
| Clusters* | Size (mm3) | t – Peak of activation | RCs2 − RCs1 | LCs2 − LCs1 | BCs2 − BCs1 | (RCs2 + LCs2 + BCs2) − (RCs1 + LCs1 + BCs1) | (RCs2 − RCs1) − (LCs2 − LCs1) |
|---|---|---|---|---|---|---|---|
| Right precentral gyrus, BA4 | 197 | 4.432 | 0.987 | 0.562 | 0.003 | 0.122 | 0.609 |
| Right postcentral and precentral gyri, BA3 and BA4 | 1102 | 4.858 | 0.278 | 0.858 | 0.288 | 0.398 | 0.247 |
| Left precentral gyrus, BA4–6 | 20 | 3.717 | 0.289 | 0.025 | 0.035 | 0.021 | 0.285 |
| SMA, BA6 | 1200 | 4.211 | 0.574 | 0.014 | 0.002 | 0.009 | 0.086 |
| Right superior frontal gyrus, BA6 | 33 | 3.793 | (−)0.063 | 0.437 | 0.298 | 0.979 | (−)0.016 |
| Right superior frontal gyrus, BA10 | 106 | 4.187 | 0.932 | 0.218 | 0.023 | 0.125 | 0.299 |
| Right inferior frontal gyrus, BA44 | 528 | 4.603 | 0.041 | 0.508 | 0.000 | 0.007 | 0.201 |
| Right putamen | 410 | 4.707 | 0.163652 | 0.254208 | 0.005 | 0.023 | 0.808 |
Found at q(FDR) < 0.05 and cluster size >20 mm3, columns 2–5 presenting p-values of a t-test. In case a contrast provided a decrease in activation, the p-value is preceded by a (−) sign
Fig. 1.
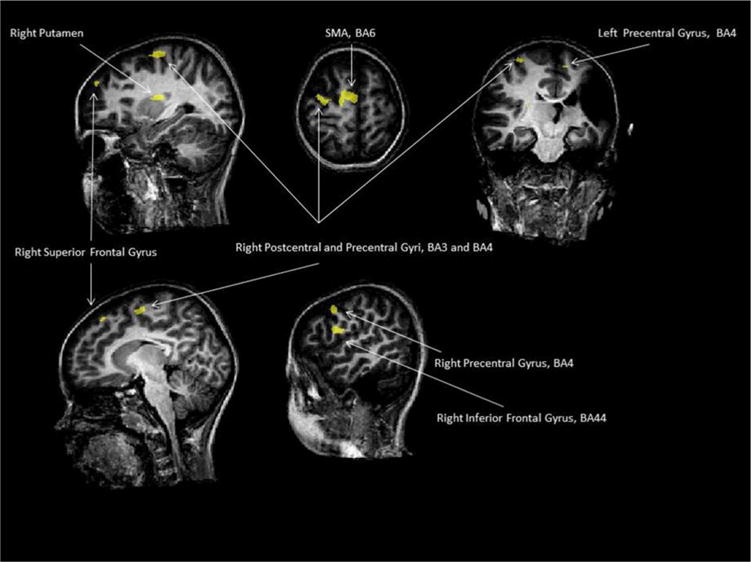
Child 1/at q(False Discovery Rate) < 0.05 (t = 3.54) and cluster size >20 mm3, the clusters for the contrast [(RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest].
Then, we computed ROI analysis in each area found. For the BC, we found an increase between before and after treatment in almost all clusters (all but right BA6 and one of the two cluster found in right M1). For the LC, the activation was significantly increased in the left motor/premotor cortex and in the SMA. Finally for the RC, we discovered an increase of activation in the right BA44. In the right BA6, the activity for the right hand is almost significantly higher in the first session than in the second (p = 0.063).
We did also the contrast (LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1) in a whole brain analysis with movement parameters used as confounds of non interest in the general linear model (see remark 1). At p < 0.0025 (t = 3.032) and cluster size >20 mm3, we found 5 clusters (see Table 3 and Fig. 2). Globally, the differences between before and after treatment were similar for all conditions showing an increase of activation.
Table 3.
Child 1 – Contrast: (LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1).
| Clusters* | Size (mm3) | t – Peak of activation | RCs2 − RCs1 | LCs2 − LCs1 | BCs2 − BCs1 | (RCs2 + LCs2 + BCs2) − (RCs1 +LCs1 +BCs1) | (RCs2 − RCs1) − (LCs2 − LCs1) |
|---|---|---|---|---|---|---|---|
| Right orbitofrontal cortex, BA 11 | 244 | 3.536 | 0.019 | 0.002 | 0.001 | 0.000 | 0.548 |
| Right orbitofrontal cortex, BA 11 | 125 | 3.437 | 0.086 | 0.000 | 0.001 | 0.000 | 0.101 |
| Right cingulate gyrus, BA32 | 261 | 3.829 | 0.015 | 0.000 | 0.002 | 0.000 | 0.315 |
| Left superior frontal gyrus, BA6 | 188 | 3.504 | 0.001 | 0.007 | 0.006 | 0.000 | 0.484 |
| Left superior frontal gyrus, BA6 | 117 | 3.279 | 0.000 | 0.005 | 0.31 | 0.000 | 0.276 |
Found at p < 0.0025 and cluster size >20 mm3, columns 2–5 presenting p-values of a t-test. In case a contrast provided a decrease in activation, the p-value is preceded by a (−) sign.
Fig. 2.
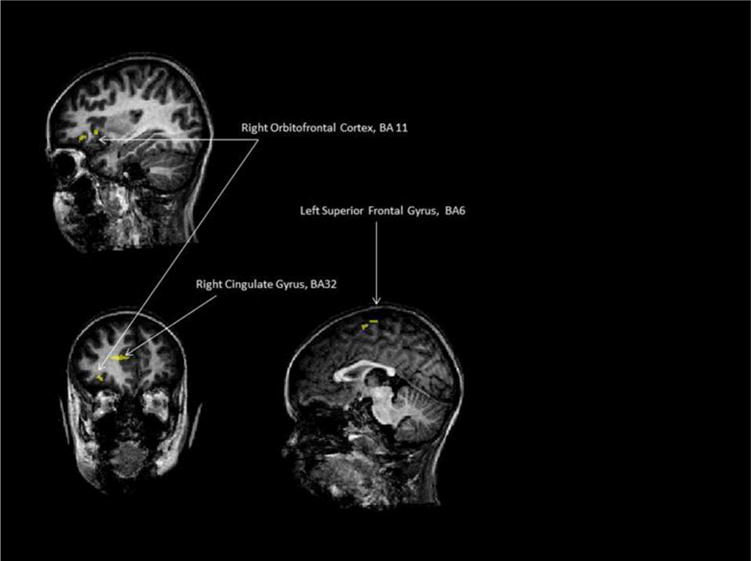
Child 1/clusters obtained with the contrast (LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1) in a whole brain analysis with movement parameters used as confounds of non interest in the general linear model, at p < 0.0025 (t = 3.032) and cluster size >20 mm3.
3.1.3. DTI
Over the 123 voxels of the left sphere, this child presented 94 voxels with a preferential z direction. Ninety-three of these 94 voxels presented a FA higher than 0.3 (see Fig. 3D).
Over the 123 voxels of the right sphere, 117 had a main direction in z. 116 out of 117 of these fibers presented a FA higher than 0.3 (see Fig. 3C).
Tracking from left and right spheres allowed the delineation of the respective left and right CST at the first iteration (see Fig. 3A).
Unfortunately, as this child’s pre-camp DTI was affected by movement artifacts that could not be corrected for, it was not possible to compare pre and post intensive rehabilitation DTI.
3.2. Child 2
Child 2 showed improvements in all functional assessments (see Table 1).
3.2.1. TMS
For child 2, no TMS-evoked responses could be found in the affected hemisphere, even at a stimulus intensity of 90% maximum stimulator output. However, when the contralesional (unaffected) hemisphere was stimulated, MEPs in both hands were evoked showing that the affected hand was controlled via ipsilateral connections from the unaffected motor cortex.
3.2.1.1. Changes in motor maps after rehabilitation
In this map (ipsilateral map of the affected hand), there was a 233% increase in the number of digit and wrist responsive sites after rehabilitation. The average amplitude of the MEP of the affected FDI increased from 482.04 μV to 2888.83 μV (499.3% increase). The average amplitude of the MEP of the affected WF increased from 309.04 μV to 3475.34 μV (1024.6% increase).
3.2.2. fMRI
3.2.2.1. Behavioral results
During the first assessment, child 2 succeeded in all trials (18/18) programmed for the right (less-affected) hand across the 3 runs. With the more-affected hand, only 7 out of 18 trials were correctly performed (the child systematically used the opposite hand to help during the task). Finally, 14 out 18 bimanual trials were successful during this pre-training assessment.
At post-training assessment, with the same paradigm, all trials were completed separately with the more-affected hand (left 18/18) and less-affected hand (right 18/18). In bimanual trials 16 out of 18 trials were correctly completed.
3.2.2.2. fMRI results
At same q(FDR) and same cluster size than child 1, we reported 35 clusters for the contrast [(RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest] but with large overlap (73,653 voxels activated in total; see Fig. 4a). Consequently3 we increased the statistical threshold to p(Bonf) < 0.001 and cluster size >20 mm3 allowing a separation between the sensori and motor regions. We defined 14 clusters (see Table 4 and Fig. 4b). S1 was found on both hemispheres contrary to M1 and premotor cortex only found on the left (healthy) hemisphere of the brain. SMA, left inferior and superior parietal lobules were also activated.
Fig. 4.
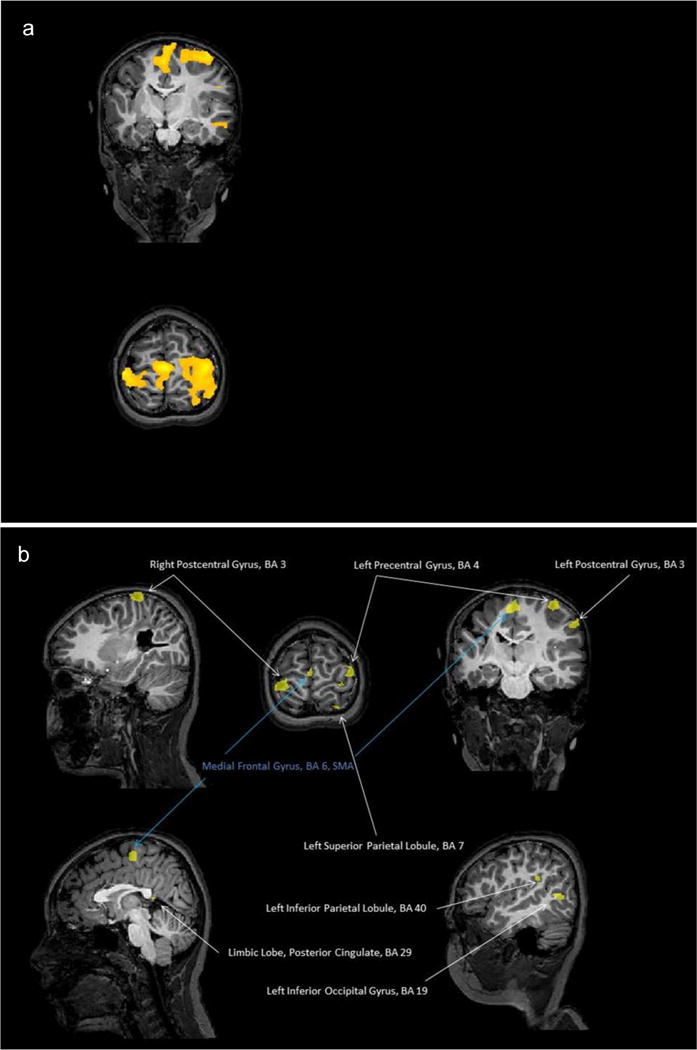
(a) Child 2/at same q(FDR) and same cluster size than child 1, we report 35 clusters for the contrast [(RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest] but with large overlappings (73,653 voxels activated in total). (b) Child 2/at statistical threshold to p(Bonf) < 0.001 and cluster size >20 mm3, 14 clusters were defined for the contrast [(RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest] (see Table 4).
Table 4.
Child 2 – Contrast: (RCs1 + RCs2 + LCs1 + LCs2 + BCs1 + BCs2) > rest.
| Clusters* | Size (mm3) | t – Peak of activation | RCs2 − RCs1 | BCs2 − BCs1 | (RCs2 + BCs2) − (RCs1 + BCs1) | (BCs2 − BCs1) − (RCs2 − RCs1) |
|---|---|---|---|---|---|---|
| Right postcentral gyrus, BA3 | 1635 | 9.116 | 0.287 | 0.747 | 0.407 | 0.492 |
| Right postcentral gyrus, BA3 | 26 | 6.557 | 0.588 | 0.041 | 0.124 | 0.169 |
| Left precentral gyrus, BA4 | 906 | 7.809 | 0.776 | 0.246 | 0.39 | 0.424 |
| Left precentral gyrus, BA6 | 117 | 7.095 | 0.28 | 0.742 | 0.651 | 0.195 |
| Left precentral gyrus, BA4 | 20 | 6.191 | 0.82 | 0.045 | 0.185 | 0.105 |
| Left postcentral gyrus, BA3 | 271 | 7.287 | 0.229 | 0.117 | 0.099 | 0.745 |
| Left postcentral gyrus, BA3 | 109 | 6.486 | 0.852 | 0.898 | 0.851 | 0.956 |
| Medial frontal gyrus, Brodmann area 6 – SMA | 905 | 8.769 | 0.664 | 0.273 | 0.363 | 0.547 |
| Left superior parietal lobule, BA7 | 99 | 6.642 | 0.333 | 0.049 | 0.08 | 0.364 |
| Left superior parietal lobule, BA7 | 140 | 6.433 | 0.007 | 0.040 | 0.005 | 0.541 |
| Left inferior parietal lobule, BA40 | 67 | 6.262 | 0.847 | 0.334 | 0.49 | 0.48 |
| Limbic lobe, cingulate gyrus, | 75 | 6.308 | 0.189 | 0.053 | 0.053 | 0.573 |
| Brodmann area 24 | ||||||
| Limbic lobe, posterior cingulate, | 36 | 6.602 | 0.405 | 0.545 | 0.391 | 0.83 |
| Brodmann area 29 | ||||||
| Left inferior occipital gyrus, BA19 | 262 | 6.731 | 0.399 | 0.397 | 0.317 | 1 |
Found at p(Bonf) < 0.001 and cluster size >20 mm3.
Further, we computed ROI analysis in each area found with this contrast. Due to the fact that this child did not succeed during the first session of the more-affected (left) hand task without help of the less-affected hand (for 11 on 18 trials), we decided to focus analysis on the right-hand and two-handed conditions. For the BC we found difference between activity after and before treatment in right S1, left M1 and in the superior parietal lobule (see Table 4). For the RC, a difference was observed in this last cluster only. No interaction was observed in any cluster.
Contrary to the first child, we didn’t find a difference with the contrast [(LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1)] at p < 0.0025 in a whole brain analysis with movement parameters used as confounds of non interest (see remark 1). Nevertheless we found 2 clusters (in BA11 and BA9) at p < 0.05 (t = 1.96) and cluster size >50 mm3 (see Table 5 and Fig. 5). The difference between before and after the treatment was mainly driven by the BC for the two clusters (without consideration of the LC) (Table 5).
Table 5.
Child 2 – Contrast: (LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1).
| Clusters* | Size (mm3) | t – Peak of activation | RCs2 − RCs1 | BCs2 − BCs1 | (RCs2 + BCs2) − (RCs1 + BCs1) | (BCs2 − BCs1) − (RCs2 − RCs1) |
|---|---|---|---|---|---|---|
| Right superior frontal gyrus, BA9 | 51 | 2.63 | 0.235 | 0.011 | 0.026 | 0.212 |
| Orbitofrontal cortex, BA 11 | 53 | 2.33 | 0.261 | 0.000 | 0.004 | 0.016 |
Found at p < 0.05 and cluster size >50 mm3
Fig. 5.
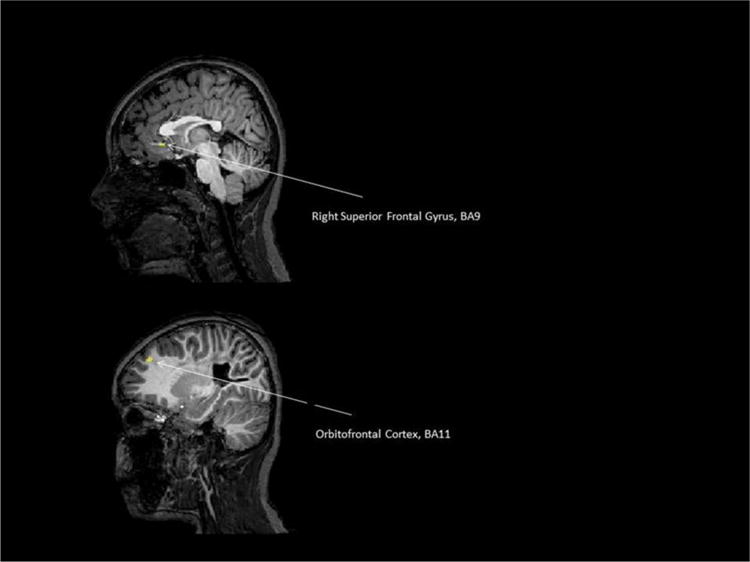
Child 2/the contrast [(LCs2 + RCs2 + BCs2) − (LCs1 + RCs1 + BCs1)] at p < 0.0025 in a whole brain analysis with movement parameters used as confounds of non interest.
3.2.3. DTI results
Over the 123 voxels of the right sphere, this child had 53 voxels with a preferential z direction. Twenty-one of these 53 voxels had a FA higher than 0.3 (see Fig. 3E).
Over the 123 voxels of the left sphere, 111 had a main direction in z. Ninety six out of 111 of these voxels had a FA higher than 0.3 (see Fig. 3F).
A mean FA was calculated in the z direction on all the voxels of the sphere. Before intensive training, the mean FA was of 0.385 in the z direction in the left sphere and of 0.345 in the right sphere. This value increased after intensive training in the left sphere (to 0.403) while it did not increase in the right sphere (0.245).
Tracking from the left sphere allowed the delineation of CST on the left side at the first iteration (see Fig. 3B). Tracking on the basis of the right sphere also allowed to track a projection to the left CST. Whatever the number of iterations of the program to track the fibers, no delineation of a CST on the right side was possible, as represented in the tracking image (Fig. 3B).
4. Discussion
Two children with USCP, presenting different CST post-lesion reorganization (child 1: contralateral connectivity, child 2: ipsilateral connectivity) were assessed before and after a 90 h HABIT-ILE intervention using three different neurophysiological assessments: DTI, fMRI, and TMS.
Child 1 had a motor map of the affected hand in the contralateral (affected) hemisphere, as attested by TMS and DTI investigations. DTI showed that while the CST was partly damaged, the remaining fibers were of good quality, allowing connectivity to the affected upper extremity muscles. TMS investigations showed an increase of both affected hand cortical map and cortical excitability of this map. fMRI presented changes consistent with TMS, but further allowed the demonstration of changes in sensory and associative areas.
Child 2 had a motor map of the affected hand in the unaffected motor cortex solely, as shown by the TMS cortical mapping and by the DTI investigations. Thus an ipsilateral projection was present from the unaffected hemisphere that controlled the affected hand. Despite partial results (due to inability to achieve the task with the affected hand at the start), fMRI highlighted an increase of activation in left motor (BA4) and visuo-motor (BA7) areas. In the ipsilesional side, solely BA3 presented an increased activation after HABIT-ILE. After the intervention, child 2 could perform the task easily with the more affected hand.
Interestingly, child 2 was undergone CST reorganization (i.e., ipsilateral innervation) achieved better functional scores than child 1 (preserved contralateral organization). This is not consistent with previous studies suggesting a better function of the affected hand when the motor map is located in the affected hemisphere (Holmström et al., 2010; Staudt et al., 2004). Furthermore, contrary to what has been previously observed with CIMT (Kuhnke et al., 2008), both children improved functionally and in the motor cortical areas controlling their affected hand. The two cases studied in this paper suggest thus that whatever the organization of the CST after the lesion, children encountered functional/behavioral changes after HABIT-ILE, and these improvements were mirrored by cortical changes.
The cortical organization of descending pathways could be identified both with DTI and TMS. Previous papers have shown that both conventional MRI and DTI could quantify indexes of symmetry estimating the CST dysgenesis, and that these indexes correlated with functional abilities of the paretic hand (Bleyenheuft et al., 2007; Bleyenheuft & Thonnard, 2010; Duque et al., 2003; Friel et al., 2014) but not change in hand function after intensive bimanual training (Friel et al., 2014). While conventional MRI systematically underestimates the CST dysgenesis, it might be considered in clinics as a good approximation of the consequence of the lesion on the CST (Bleyenheuft et al., 2007). However, an index of symmetry does not allow investigation of the quality of the fibers and the extent to which the motor pathways may have undergone reorganization post-lesion (Guzzetta et al., 2007; Staudt, 2010). As illustrated in our two cases, to innervate muscles of the more affected hand, the key may be not the quantity of CST fibers maintained, but the intrinsic quality of these fibers. To investigate quality of the fibers, a DTI analysis with a delineation of CST fibers can be useful. The interest of such information resides in the potential differential treatment that could be proposed as a function of the cortical organization.
In this pilot study, all assessments were performed pre- and post-intensive training. While comparison of DTI parameters could be done only in one child, due to technical reasons, some changes in FA were observed. Recent papers in the literature have pointed the potential changes in DTI parameters, interpreted as spontaneous recovery (Jang & Yeo, 2014) or due to intensive training (Kwon et al., 2014). However in the absence of data describing measurement variability between two measurements of DTI performed in the same subject at different time points (without treatment), we are cautious regarding this observation.
When DTI cannot be performed (due to the age of the child, inability to stay still, or some exclusion criteria) TMS can provide a good alternative to determine CST organization. While it should be verified in a larger sample, the matching between CST reconstruction with DTI and TMS data was very consistent in these children. More specific than the cortical organization, TMS allowed investigating changes in cortical excitability. In both children, the motor areas underwent a huge increase in cortical excitability. In the child with maintained crossed descending pathways, both the affected and unaffected motor areas controlling the hands had an increase of 50–60% in the average amplitude of the MEP. For the child with both motor areas located in the unaffected hemisphere, the average amplitude of the MEP was greatly increased in the area dedicated to the more affected (increase of 499%) and to the less affected hand (increase of 1025%).
TMS also allowed investigation of the number of digit and wrist responsive sites for the affected hand after rehabilitation. Paralleling the results of cortical excitability, the number of responsive sites increased after intervention, showing an expansion of the motor map related to the affected hand in both children. However, once again, this motor map extension was larger (233%) in the ipsilateral map of the affected hand of child 2, than in the contralateral map of the affected hand in child 1 (50% increase). This could be linked to a different potential of cortical changes depending on CS organization. After CIMT the difference highlighted in cortical modulation was already described as different (Juenger et al., 2013). However CIMT showed an increase of activity in motor areas controlling the affected hand when this area is contralateral to the affected hand and a decrease of activation when this area is ipsilateral to the affected hand (Juenger et al., 2013). TMS investigations also showed a decreased cortical excitability after CIMT in this last group. The authors suggested that this was probably linked to an inhibitory S1–M1 interaction: the activity in S1 (maintained in the impaired hemisphere) induced by the exercise would have an inhibitory action on the synaptic activity of M1 through inhibitory interhemispheric pathways. However, children improved in both cases. The authors suggested that the decrease of activation might correspond to a better refinement of the motor cortex. The contrast of the results we have in child 2 for bimanual training suggest a highly different neuromodulation in children with ipsilateral organization when treated with unimanual or bimanual intensive therapy. While in both interventions S1 of the affected hand (preserved in the ipsilesional hemisphere) presented more excitability after the treatment and a behavioral improvement was observed, the consequences of the treatments on ipsilateral motor maps were totally opposite. This raises the importance of comparing in ipsilateral children the amount of functional and cortical direct and long term benefit of CIMT versus bimanual intensive therapies.
Functional magnetic resonance imaging can be of great help in this context since it allows investigating the changes in other fields than the motor response. Especially of interest here are the sensory areas related to the affected hand and the associative areas and their neuromodulation after a bimanual intensive training. While the child 1 with a preserved contralateral organization did increase cortical activation after intervention for nearly all clusters evidenced at the first analysis in the “both hands” condition, both left and right cortical changes were separately observed. Interestingly, the motor changes of this child were consistent with TMS observation both for the affected and non-affected side, showing increases in activation. Changes were also noticed in the SMA for “both hands” and “left hand” use, as well as in BA10 (both hands condition), well known for being involved in executive functions and memory recall (Costa et al., 2011). In child 2, with “ipsilateral” reorganization, all changes were observed on the unaffected hemisphere, except for the modulation in BA3, with an increased activation of sensory area preserved in the lesional hemisphere. An increase in the M1 (BA4) of the unaffected hemisphere was observed consistently with TMS changes for the both hands condition. BA7, known to be related to visuo-motor coordination and especially to correction of the movement when grasping an object (Vingerhoets, 2014), presented also more activation after the intensive rehabilitation treatment in the “both hands” and “right hand” conditions. Unfortunately, the left hand condition could not be analyzed in this child since at pre-assessment, despite training, she was systematically using the less affected hand to give the object to the more affected and to take it out of the hand. This raises the one important problem of performing motor fMRI with children with CP: some of them have difficulty performing isolated movements with the hands, and therefore compromise the interpretability of fMRI data. In the future, devices allowing to grip and move objects without need of visual feedback and without risks of drop should be specifically designed.
If movements parameters are used as confounds of non-interest, as we presented in Tables 3 and 5 for children 1 and 2 respectively, some other higher level changes might be highlighted. In our 2 children, changes in activation were detected under these corrections when using both hands in BA11 added for child 1 to increased activations in BA32 and BA6. Child 2 showed in addition changes in BA9. All these areas are known to be connected to the concept of reward. BA11 has been described as having an important role in the reward of mediated behaviors, notably in psychiatry (Goldapple et al., 2004; Rogers et al., 1999). Furthermore its activation has been described as inversely proportional to the demanding aspect of the task (Pochon et al., 2002) suggesting here that the task being less demanding after the intensive training, more activation could be dedicated to emotional information. BA32 is described in the literature as activated by reward and reward anticipation (Knutson & Cooper, 2005; Pochon et al., 2002; for review see Haber & Knutson, 2010), especially in “self-related (personally relevant contextual value) processing along an affective dimension” (Lane et al., 1998; Posner & Rothbart, 1998). Finally BA9 has also been described as part of reward process, notably when some tasks are associated with high monetary reward (Pochon et al., 2002). Altogether these results suggest that after the intensive HABIT-ILE treatment, children felt rewarded when performing the task. Though this change in the reward circuit could be attributed either to the increased success performing the task or to the positive reinforcement implemented during the intensive intervention, this effect is of great interest because this change in the reward circuit activation may promote long-term use of both hands in everyday life activities. This unexpected finding illustrates the additional interest of fMRI for investigating changes in higher (and sometimes non-motor) level that may occur during intensive rehabilitation processes using motor learning.
In this pilot study, both imaging and TMS were applied in school age children who were awake and alert. This creates some difficulties specific to children that has to be considered for implementing studies on cortical changes. For imaging, the three main challenges are anxiety of the children, movement of the head and ability to perform the motor task. From 6 years old, with careful explanation to the children about what to expect, a visit to the MRI facility before participating, a short video to introduce children to the anticipated noise and training outside of the MRI prior to the actual imaging, children tolerated assessments without specific complaints. The task (picking a block) was too difficult to be performed by one child in the MRI during the first examination, resulting additionally in some head movements during the task. In our subsequent work a task was designed where objects were placed in the hand of the children, which solved the problem of inability to perform the task and drastically reduced associated head movements. Therefore the choice of the task and testing it prior to imaging appears to be crucial to obtain data of good quality. However, for younger children, the MRI and especially the fMRI is not likely to be easily performed. Therefore TMS might be more feasible. In our 2 children, no adverse events were reported during TMS. The main difficulty for the children was the length of the exam that had to be interrupted either for going to the toilet or drinking. Provided that no prior seizure history is reported, TMS thus would be easier to apply in younger and more affected children who have difficulty performing precise movements in the fMRI without associated head movements.
Supplementary Material
Acknowledgments
KF (NS K01062116). We thank children, families, and volunteers participating in this study. We thank Prof Etienne Olivier (IONS, UCL) for allowing us to use his TMS laboratory and equipment.
Abbreviations
- 3T
3 tesla
- 6MWT
Six-Minute Walk Test
- ACPC
anterior commissure posterior commissure
- AHA
Assisting Hand Assessment
- BA
Brodmann area
- CED
Cambridge Electronic Design
- CIMT
constraint-induced movement therapy
- COPM
Canadian Occupational Performance Measure
- CP
cerebral palsy
- CST
corticospinal tract
- CVA
cerebral vascular accident
- DTI
diffusion tensor imaging
- EMG
electromyography
- FDI
first dorsal interosseus
- fMRI
functional magnetic resonance imaging
- GMFCS
gross motor function classification system
- HABIT
Hand and arm bimanual intensive therapy
- HABIT-ILE
hand and arm bimanual intensive therapy including lower extremity
- JTTHF
Jebsen–Taylor test of hand function
- MACS
Manual Ability Classification System
- MEP
motor evoked potential
- MT
motor threshold
- PEDI
Pediatric Evaluation of Disability Inventory
- TE
echo time
- TFE
Turbo Field Echo
- TMS
transcranial magnetic stimulation
- TR
repetition time
- UE
upper extremity
- USCP
unilateral spastic cerebral palsy
- WF
wrist flexors
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ridd.2015.06.014.
Footnotes
We also created a second GLM where the movement parameters were used as confounds. However, it was a bit problematic because head movements were strongly related to hand movements required and we decided to use it only to explore potential areas outside sensori-motor cortex.
For the child 2 the conditions LC have not been used.
References
- Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND-Kids: A measure of manual ability in children with cerebral palsy. Neurology. 2004;63(6):1045–1052. doi: 10.1212/01.wnl.0000138423.77640.37. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Gordon AM. Hand-Arm Bimanual Intensive Therapy Including Lower Extremities (HABIT-ILE) for Children with Cerebral Palsy. Physical and Occupational Therapy in Pediatrics. 2014;34(4):390–403. doi: 10.3109/01942638.2014.932884. http://dx.doi.org/10.3109/01942638.2014.932884. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Arnould C, Brandao MB, Bleyenheuft C, Gordon AM. Hand and Arm Bimanual Intensive Therapy including lower extremity (HABIT-ILE) in children with unilateral spastic cerebral palsy: A randomized trial. Neurorehabilitation and Neural Repair. 2014 doi: 10.1177/1545968314562109. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Grandin CB, Cosnard G, Olivier E, Thonnard JL. Corticospinal dysgenesis and upper-limb deficits in congenital hemiplegia: A diffusion tensor imaging study. Pediatrics. 2007;120(6):e1502–e1511. doi: 10.1542/peds.2007-0394. [DOI] [PubMed] [Google Scholar]
- Bleyenheuft Y, Thonnard JL. Predictive and reactive control of precision grip in children with congenital hemiplegia. Neurorehabilitation and Neural Repair. 2010;24(4):318–327. doi: 10.1177/1545968309353327. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16(13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Carswell A, McColl MA, Baptiste S, Law M, Polatajko H, Pollock N. The Canadian Occupational Performance Measure: A research and clinical literature review. The Canadian Journal of Occupational Therapy. 2004;71(4):210–219. doi: 10.1177/000841740407100406. [DOI] [PubMed] [Google Scholar]
- Caty GD, Arnould C, Thonnard JL, Lejeune TM. ABILOCO-Kids: A Rasch-built 10-item questionnaire for assessing locomotion ability in children with cerebral palsy. Journal of Rehabilitation Medicine. 2008;40(10):823–830. doi: 10.2340/16501977-0267. http://dx.doi.org/10.2340/16501977-0267. [DOI] [PubMed] [Google Scholar]
- Charles J, Lavinder G, Gordon AM. Effects of constraint-induced therapy on hand function in children with hemiplegic cerebral palsy. Pediatric Physical Therapy. 2001;13(2):68–76. [PubMed] [Google Scholar]
- Charles JR, Wolf SL, Schneider JA, Gordon AM. Efficacy of a child-friendly form of constraint induced movement therapy in hemiplegic cerebral palsy: A randomized control trial. Developmental Medicine and Child Neurology. 2006;48:635–642. doi: 10.1017/S0012162206001356. [DOI] [PubMed] [Google Scholar]
- Cooper J, Majnemer A, Rosenblatt B, Birnbaum R. The determination of sensory deficits in children with hemiplegic cerebral palsy. Journal of Child Neurology. 1995;10(4):300–309. doi: 10.1177/088307389501000412. [DOI] [PubMed] [Google Scholar]
- Costa A, Oliveri M, Barban F, Torriero S, Salerno S, Lo Gerfo E, et al. Keeping memory for intentions: A cTBS investigation of the frontopolar cortex. Cerebral Cortex. 2011;21(12):2696–2703. doi: 10.1093/cercor/bhr052. http://dx.doi.org/10.1093/cercor/bhr052. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proceedings of the National Academy of Science U S A. 2010;107(41):17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito Brandaõ M, Gordon AM, Mancini MC. Functional impact of constraint therapy and bimanual training in children with cerebral palsy: A randomized controlled trial. American Journal of Occupational Therapy. 2012;66(6):672–681. doi: 10.5014/ajot.2012.004622. http://dx.doi.org/10.5014/ajot.2012.004622. [DOI] [PubMed] [Google Scholar]
- DeLuca SC, Case-Smith J, Stevenson R, Ramey SL. Constraint-induced movement therapy (CIMT) for young children with cerebral palsy: Effects of therapeutic dosage. Journal of Pediatric Rehabilitation Medicine. 2012;5(2):133–142. doi: 10.3233/PRM-2012-0206. http://dx.doi.org/10.3233/PRM-2012-0206. [DOI] [PubMed] [Google Scholar]
- Deppe W, Thuemmler K, Fleischer J, Berger C, Meyer S, Wiedemann B. Modified constraint-induced movement therapy versus intensive bimanual training for children with hemiplegia—A randomized controlled trial. Clinical Rehabilitation. 2013;27(10):909–920. doi: 10.1177/0269215513483764. http://dx.doi.org/10.1177/0269215513483764. [DOI] [PubMed] [Google Scholar]
- Duque J, Thonnard JL, Vandermeeren Y, Sebire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain. 2003;126:732–747. doi: 10.1093/brain/awg069. [DOI] [PubMed] [Google Scholar]
- Eliasson AC, Krumlinde-Sundholm L, Rösblad B, Beckung E, Arner M, Ohrvall AM, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: Scale development and evidence of validity and reliability. Developmental Medicine and Child Neurology. 2006;48(7):549–554. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- Eliasson AC, Krumlinde-Sundholm L, Shaw K, Wang C. Effects of constraint-induced movement therapy in young children with hemiplegic cerebral palsy: An adapted model. Developmental Medicine and Child Neurology. 2005;47(266):275. doi: 10.1017/s0012162205000502. [DOI] [PubMed] [Google Scholar]
- Enright PL. The Six-Minute Walk Test. Respiratory Care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Annals in Neurology. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- Fedrizzi E, Rosa-Rizzotto M, Turconi AC, Pagliano E, Fazzi E, Pozza LV, et al. Unimanual and bimanual intensive training in children with hemiplegic cerebral palsy and persistence in time of hand function improvement: 6-Month follow-up results of a multisite clinical trial. Journal of Child Neurology. 2013;28(2):161–175. doi: 10.1177/0883073812443004. http://dx.doi.org/10.1177/0883073812443004. [DOI] [PubMed] [Google Scholar]
- Friel KM, Kuo HC, Carmel JB, Rowny SB, Gordon AM. Improvements in hand function after intensive bimanual training are not associated with corticospinal tract dysgenesis in children with unilateral cerebral palsy. Experimental Brain Research. 2014;232(6):2001–2009. doi: 10.1007/s00221-014-3889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Strasak A, Treml B, Gasser K, Kleinsasser A, Fischer V, et al. Six minute walk test in children and adolescents. Journal of Pediatrics. 2007;150:395–399. doi: 10.1016/j.jpeds.2006.12.052. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Research. 2000;871(1):127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives in General Psychiatry. 2004;61(1):34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Charles J, Steenbergen B. Fingertip force planning during grasp is disrupted by impaired sensorimotor integration in children with hemiplegic cerebral palsy. Pediatric Research. 2006;60(5):587–591. doi: 10.1203/01.pdr.0000242370.41469.74. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: A randomized control trial. Developmental Medicine and Child Neurology. 2007;49(11):830–838. doi: 10.1111/j.1469-8749.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Chinnan A, Gill S, Petra E, Hung YC, Charles J. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Developmental Medicine and Child Neurology. 2008;50(12):957–958. doi: 10.1111/j.1469-8749.2008.03166.x. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Hung YC, Brandao M, Ferre CL, Kuo HC, Friel K, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: A randomized trial. Neurorehabilitation in Neural Repair. 2011;25(8):692–702. doi: 10.1177/1545968311402508. http://dx.doi.org/10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Bleyenheuft Y, Steenbergen B. Pathophysiology of impaired hand function in children with unilateral cerebral palsy. Developmental Medicine and Child Neurology. 2013;55(Suppl. 4):32–37. doi: 10.1111/dmcn.12304. http://dx.doi.org/10.1111/dmcn.12304. [DOI] [PubMed] [Google Scholar]
- Guzzetta A, Bonanni P, Biagi L, Tosetti M, Montanaro D, Guerrini R, et al. Reorganisation of the somatosensory system after early brain damage. Clinical Neurophysiology. 2007;118(5):1110–1121. doi: 10.1016/j.clinph.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. http://dx.doi.org/10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley SM, Coster W, Ludlow LH, Haltiwanger JT, Andrellos PJ. Pediatric evaluation of disability inventory: Development, standardization and administration manual. Boston: New England Medical Center; 1992. [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;15(67):283–297. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare B, Imms C, Villanueva E, Rawicki HB, Matyas T, Carey L. Intensive therapy following upper limb botulinum toxin A injection in young children with unilateral cerebral palsy: A randomized trial. Developmental Medicine and Child Neurology. 2013;55(3):238–247. doi: 10.1111/dmcn.12054. http://dx.doi.org/10.1111/dmcn. [DOI] [PubMed] [Google Scholar]
- Holmström L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Developmental Medicine and Child Neurology. 2010;52:145–152. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage. 2009;46(3):600–607. doi: 10.1016/j.neuroimage.2009.02.025. http://dx.doi.org/10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Jang SH, Yeo SS. Recovery of an injured corticoreticular pathway via transcallosal fibers in a patient with intracerebral hemorrhage. BMC Neurology. 2014;14:108. doi: 10.1186/1471-2377-14-108. http://dx.doi.org/10.1186/1471-2377-14-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Archives of Physical Medicine and Rehabilitation. 1969;50(6):311–319. http://dx.doi.org/10.1186/1471-2377-14-108. [PubMed] [Google Scholar]
- Juenger H, Kuhnke N, Braun C, Ummenhofer F, Wilke M, Walther M, et al. Two types of exercise-induced neuroplasticity in congenital hemiparesis: A transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Developmental Medicine and Child Neurology. 2013;55(10):941–951. doi: 10.1111/dmcn.12209. http://dx.doi.org/10.1111/dmcn.12209. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. Journal of Neuroscience. 2004;24:628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18(4):411–417. doi: 10.1097/01.wco.0000173463.24758.f6. Review. [DOI] [PubMed] [Google Scholar]
- Krishnan C, Santos L, Peterson MD, Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimulation. 2015;8(1):76–87. doi: 10.1016/j.brs.2014.10.012. http://dx.doi.org/10.1016/j.brs.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: Current evidence of validity, reliability, and responsiveness to change. Developmental Medicine and Child Neurology. 2007;49(4):259–264. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- Krumlinde-Sundholm L, Eliasson AC. Development of the Assisting Hand Assessment: A Rasch-built measure intended for children with unilateral upper limb impairments. Scandinavian Journal of Occupational Therapy. 2003;10:16–26. [Google Scholar]
- Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Developmental Medicine and Child Neurology. 2008;50(12):898–903. doi: 10.1111/j.1469-8749.2008.03119.x. http://dx.doi.org/10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Chang WH, Chang HJ, Yi SH, Kim MY, Kim EH, et al. Changes in diffusion tensor tractographic findings associated with constraint-induced movement therapy in young children with cerebral palsy. Clinical Neurophysiology. 2014;125(12):2397–2403. doi: 10.1016/j.clinph.2014.02.025. http://dx.doi.org/10.1016/j.clinph.2014.02.025. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Law M, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: An outcome measure for occupational therapy. Canadian Journal of Occupational Therapy. 1990;57:82–87. doi: 10.1177/000841749005700207. [DOI] [PubMed] [Google Scholar]
- Lazaridou A, Astrakas L, Mintzopoulos D, Khanicheh A, Singhal AB, Moskowitz MA, et al. Diffusion tensor and volumetric magnetic resonance imaging using an MR-compatible hand-induced robotic device suggests training-induced neuroplasticity in patients with chronic stroke. International Journal of Molecular Medicine. 2013;32(5):995–1000. doi: 10.3892/ijmm.2013.1476. http://dx.doi.org/10.3892/ijmm.2013.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AM, Lin J, Au JT, So HK, Tsang T, Wong E, et al. Standard references for the six minute walk in healthy children aged 7–16 years. American Journal of Respiratory and Critical Care Medicine. 2007;176:174–180. doi: 10.1164/rccm.200607-883OC. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Hu Y, Liang Y, Chen F. Structural changes in left fusiform areas and associated fiber connections in children with abacus training: Evidence from morphometry and tractography. Frontiers in Human Neuroscience. 2013;7:335. doi: 10.3389/fnhum.2013.00335. http://dx.doi.org/10.3389/fnhum.2013.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiowetz V, Wiemer DM, Federman SM. Grip and pinch strength: Norms for 6- to 19-year-olds. American Journal of Occupational Therapy. 1986;40(10):705–711. doi: 10.5014/ajot.40.10.705. [DOI] [PubMed] [Google Scholar]
- McCarthy ML, Silberstein CE, Atkins EA, Harryman SE, Sponseller PD, Hadley-Miller NA. Comparing reliability and validity of pediatric instruments for measuring health and well-being of children with spastic cerebral palsy. Developmental Medicine and Child Neurology. 2002;44(7):468–476. doi: 10.1017/s0012162201002377. [DOI] [PubMed] [Google Scholar]
- Noirhomme Q, Ferrant M, Vandermeeren Y, Olivier E, Macq B, Cuisenaire O. Registration and real-time visualization of transcranial magnetic stimulation with 3-D MR images. IEEE Transactions on Biomedical Engineering. 2004;51(11):1994–2005. doi: 10.1109/TBME.2004.834266. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Adaptive plasticity in motor cortex: Implications for rehabilitation after brain injury. Journal of Rehabilitation Medicine. 2003;41:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- Palisano RJ, Rosenbaum PL, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental Medicine and Child Neurology. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, et al. The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Science U S A. 2002;99(8):5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of Royal Society of London B. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19(20):9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Abbott DF, Macdonell RA, Jackson GD, Boyd RN. Equivalent retention of gains at 1 year after training with constraint-induced or bimanual therapy in children with unilateral cerebral palsy. Neurorehabilitation in Neural Repair. 2011;25(7):664–671. doi: 10.1177/1545968311400093. http://dx.doi.org/10.1177/1545968311400093. [DOI] [PubMed] [Google Scholar]
- Staudt M. Reorganization after pre- and perinatal brain lesions. Journal of Anatomy. 2010;217(4):469–474. doi: 10.1111/j.1469-7580.2010.01262.x. http://dx.doi.org/10.1111/j.1469-7580.2010.01262.x. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain. 2002;125(Pt 10):2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Annals in Neurology. 2004;56:854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y, Davare M, Duque J, Olivier E. Reorganization of cortical hand representation in congenital hemiplegia. European Journal of Neuroscience. 2009;29(4):845–854. doi: 10.1111/j.1460-9568.2009.06619.x. http://dx.doi.org/10.1111/j.1460-9568.2009.06619.x. [DOI] [PubMed] [Google Scholar]
- Verkerk GJ, Wolf MJ, Louwers AM, Meester-Delver A, Nollet F. The reproducibility and validity of the Canadian Occupational Performance Measure in parents of children with disabilities. Clinical Rehabilitation. 2006;20(11):980–988. doi: 10.1177/0269215506070703. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Frontiers in Psychology. 2014;5:151. doi: 10.3389/fpsyg.2014.00151. http://dx.doi.org/10.3389/fpsyg.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Vromans DC, Ketelaar M, Gorter JW. Responsiveness of evaluative measures for children with cerebral palsy: The Gross Motor Function Measure and the Pediatric Evaluation of Disability Inventory. Disability and Rehabilitation. 2005;27(20):1245–1252. doi: 10.1080/09638280500076178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


