Abstract
Background
While the inverse association between high-density lipoprotein cholesterol (HDL-C) and risk of (CVD) has been long established, it remains unclear whether low HDL-C remains a CVD risk factor when levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) are not elevated. This is a timely issue because recent studies have questioned whether HDL-C is truly an independent predictor of CVD.
Methods and Results
3590 men and women from the Framingham Heart Study offspring cohort without known CVD were followed between 1987 and 2011. Low HDL-C (<40 mg/dL in men and <50 mg/dL in women) was defined as “isolated” if TG and LDL-C were both low (<100 mg/dL). We also examined higher thresholds for TG (150 mg/dL) and LDL-C (130 mg/dL) and compared low versus high HDL-C phenotypes using logistic regression analysis to assess association with CVD. Compared to isolated low HDL-C, CVD risks were higher when low HDL-C was accompanied by LDL-C ≥100 mg/dL and TG <100 mg/dL (OR 1.3 [1.0, 1.6]), TG ≥100 mg/dL and LDL-C <100 mg/dL (OR 1.3 [1.1, 1.5]), or TG and LDL-C ≥ 100 mg/dL (OR 1.6, [1.2, 2.2]), after adjustment for covariates. When low HDL-C was analyzed with higher thresholds for TG (≥150 mg/dL) and/or LDL-C (≥130 mg/dL) results were essentially the same. In contrast, compared to isolated low HDL-C, high HDL-C was associated with 20-40% lower CVD risk except when TG and LDL-C were elevated.
Conclusions
CVD risk as a function of HDL-C phenotypes is modulated by other components of the lipid panel.
Keywords: triglycerides, coronary heart disease risk, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, epidemiology
Introduction
Low levels of high-density lipoprotein cholesterol (HDL-C) have long been associated with increased risk for cardiovascular disease (CVD)1 and have been documented as a critical risk factor for estimating 10-year risk of CVD2. This is biologically plausible since HDL particles drive reverse cholesterol transport and may exert a variety of anti-atherogenic effects3.
However, the relationships between HDL-C, triglycerides (TG) and LDL-C have complicated efforts toward establishing the extent to which low HDL-C independently increases CVD risk when other lipids are within the normal ranges4. Because TG levels were not routinely measured in the Framingham Heart Study until recently, the extent to which an “isolated low HDL-C” might associate with increased CVD risk could not have been accurately determined. This has now become a clinically relevant issue because outcome trials that have targeted HDL-C pharmacologically and have included patients with low HDL-C at baseline, have not demonstrated reduced CVD risk despite appreciable increases in HDL-C.5-10 Similarly, a Mendelian randomization study failed to identify polymorphisms associated with high HDL-C that correlate with reduced CVD risk.11
Therefore, to clarify the association between HDL-C and CVD risk, we compared HDL-C in isolation (i.e., low levels of HDL-C, TG and LDL-C) to low HDL-C with higher TG, higher LDL-C, or both. We also assessed the extent to which high HDL-C either in isolation or combined with higher TG, higher LDL-C, or both was associated with reduced CVD risk compared to isolated low HDL-C.
Methods
Study Participants
Participants were adult men and women from the Framingham Heart Study (FHS) Offspring Cohort (OC) whose baseline evaluation took place between 1987 and 1991 (Examination cycle 4). The development of new CVD events12 was monitored through 2011, as previously described.13
Of the initial 3925 participant samples available, 188 were excluded due to loss to follow-up, history of CVD at baseline, or TG >400 mg/dL. After excluding users of lipid lowering therapy (n=147), the final sample size available for analysis was 3590 men and women. Blood samples represent single measurements collected during examination cycle 4 and obtained following an overnight fast of at least 12 hours. Total cholesterol and TG were measured using commercial enzymatic assays, HDL-C was measured by enzymatic method following precipitation of apoB-containing lipoproteins, and LDL-C was calculated using the Friedewald formula.14 The study was approved by the institutional review boards at Vanderbilt University, Boston University, Dartmouth College and the National Institutes of Health. Data were accessed from Vanderbilt University and Dartmouth College.
Assessment of Risk Factors
We defined isolated low HDL-C consistent with guideline-endorsed thresholds (<40 mg/dL for men and <50 mg/dL for women) in the presence of optimal LDL-C and TG levels (both <100 mg/dL). Thresholds for higher LDL-C and TG were as previously defined, either ≥100 mg/dL for each or ≥130 mg/dL for LDL-C and ≥150 mg/dL for TG).15,16 The other phenotypes of interest were age, gender, BMI, smoking status, hypertension, and diabetes.
Outcome Events
Incident CVD was defined as an occurrence of fatal or nonfatal myocardial infarction (MI), stroke or CVD death. Cases were established as previously established by FHS.12,13
Statistical Methods
Logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI) of CVD risk, using lipid and lipoprotein measurements obtained at Examination 4. “Isolated” low HDL-C (referent) was defined as HDL-C <40 mg/dL (men) or <50 mg/dL (women), TG <100 mg/dL, and LDL-C <100 mg/dL. We also used alternative thresholds for LDL-C<130 mg/dL and TG < 150 mg/dL in all combinations to assess the independent effects of HDL-C in the presence of a variety of backgrounds. Analyses were adjusted for age at initial lipid profiling, gender, diabetes, hypertension, and smoking status. All analyses were performed using SAS (Cary, NC, version 9.3)
Results
Table 1 shows the baseline characteristics of our cohort, the isolated low HDL-C (referent) stratified by TG and LDL-C thresholds of 100 mg/dL. The group with isolated low HDL-C (mean: 35±4 mg/dL [men], 44±4 mg/dL [women]) was characterized by a relatively normal mean body mass index (BMI= 26.1±6.3 kg/m2), systolic blood pressure (SBP= 118±20 mmHg) and a low prevalence of diabetes (6%) in contrast to low HDL-C groups with a higher TG phenotype (≥ 100 mg/dL). Overall, the baseline characteristics of the isolated high and low HDL-C groups were similar regardless of TG and LDL-C measures (Supplementary Tables 1-3).
Table 1.
Baseline characteristics of the total cohort, isolated low HDL-C (Referent) and other HDL-C groups.
| Total Cohort | Low HDL-C | High HDL-C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Referent | |||||||||
| TG<100 | <100 | ≥100 | ≥100 | TG<100 | <100 | >100 | >100 | ||
| LDL<100 | ≥100 | <100 | ≥100 | LDL<100 | ≥100 | <100 | ≥100 | ||
| (n=3590) | (n= 84) | (n= 300) | (n=137) | (n=853) | (n=388) | (n=1098) | (n=72) | (n=658) | |
| Age | 41 (11) | 46(10) | 49(10) | 53(10) | 53(10) | 46(10) | 51(10) | 52(11) | 54(9) |
| Gender | 1871 (52%) | 57(68%) | 201(67%) | 66(48%) | 378(44%) | 253(65%) | 601(55%) | 38(53%) | 277(42%) |
| BMI | 26.8(4.8) | 26.1(6.3) | 26.5 (4.5) | 29.4(5.8) | 29.0(5.1) | 23.8(3.9) | 25.4(4.1) | 26.6(4.3) | 27.5(4.0) |
| SBP | 125 (34) | 118 (20) | 120 (38) | 125 (53) | 130 (38) | 118 (18) | 123(30) | 130(19) | 130(36) |
| Smokers | 883(25%) | 21 (25%) | 90 (30%) | 28 (20%) | 274 (32%) | 81(21%) | 235(21%) | 20(28%) | 134(20%) |
| Diabetes | 162 (5%) | 5 (6%) | 5 (2%) | 18(13%) | 70 (8%) | 6(2%) | 22(2%) | 4(6%) | 32(5%) |
| HDL-C, mean | |||||||||
| Men | 44 (11) | 35(4) | 35(3) | 31(4) | 33(4) | 56(13) | 51(10) | 48(8) | 47(7) |
| Women | 56(15) | 44(4) | 44(4) | 39(7) | 41(6) | 67(13) | 66(12) | 65(12) | 61(9) |
| LDL-C, mean | |||||||||
| Men | 135(34) | 83(15) | 136(21) | 86(12) | 145(31) | 86 (11) | 137(25) | 86(13) | 152(28) |
| Women | 128(36) | 86(12) | 139(30) | 84(14) | 151(33) | 83(12) | 132(25) | 85(13) | 147(33) |
| TG, median | |||||||||
| Men | 109 | 62 | 80 | 222 | 165 | 59 | 71 | 149 | 136 |
| Women | 87 | 65 | 75 | 163 | 154 | 56 | 67 | 127 | 128 |
CVD risk was associated with low HDL-C but this relationship was influenced by LDL-C and/or TG (Table 2). Compared to isolated low HDL-C, CVD was higher when low HDL-C was accompanied by LDL-C ≥100 mg/dL (OR 1.3 [1.0, 1.6], TG ≥100 mg/dL (OR 1.3 [1.1, 1.5]) or both (OR 1.6, [1.2, 2.2]). These ORs were all adjusted for covariates. In contrast, compared to isolated low HDL-C, isolated high HDL-C (low levels of TG and LDL-C in the presence of high HDL-C) was consistently associated with reduced CVD risk (OR = 0.6, [0.5,0.7]). This association persisted even when high HDL-C was accompanied by higher LDL-C (≥ 100 and ≥ 130 mg/dL) or higher TG (≥ 100 and ≥ 150 mg/dL), but was no longer significantly protective when both LDL-C and TG equaled or exceeded 100 mg/dL.
Table 2.
Effect sizes of low HDL-C and high HDL-C in conjunction with varying levels of TG and LDL-C*
| Low HDL-C | High HDL-C | ||||||
|---|---|---|---|---|---|---|---|
| N | OR | CI | N | OR | CI | ||
| TG<100, LDL<100 | 84 | TG<100, LDL<100 | 388 | 0.6 | (0.5-0.7) | ||
| TG<100, LDL>=100 | 300 | 1.3 | (1.0-1.6) | TG<100, LDL>=100 | 1098 | 0.7 | (0.5-1.0) |
| TG>=100,LDL<100 | 137 | 1.3 | (1.1-1.5) | TG>=100,LDL<100 | 72 | 0.7 | (0.6-1.0) |
| TG>=100,LDL>=100 | 853 | 1.6 | (1.2-2.2) | TG>=100,LDL>=100 | 658 | 0.9 | (0.7-1.4) |
| TG<100, LDL<130 | 213 | TG<100, LDL<130 | 929 | 0.6 | (0.5-0.7) | ||
| TG<100, LDL>=130 | 171 | 1.3 | (1.1-1.5) | TG<100, LDL>=130 | 557 | 0.7 | (0.6-1.0) |
| TG>=100,LDL<130 | 414 | 1.3 | (1.0-1.5) | TG>=100,LDL<130 | 255 | 0.7 | (0.5-1.0) |
| TG>=100,LDL>=130 | 576 | 1.6 | (1.3-2.0) | TG>=100,LDL>=130 | 475 | 0.9 | (0.7-1.3) |
| TG<150, LDL<100 | 133 | TG<150, LDL<100 | 434 | 0.6 | (0.5-0.7) | ||
| TG<150, LDL>=100 | 660 | 1.3 | (1.0-1.7) | TG<150, LDL>=100 | 1531 | 0.7 | (0.5-1.0) |
| TG>=150,LDL<100 | 88 | 1.2 | (1.0-1.5) | TG>=150,LDL<100 | 26 | 0.7 | (0.5-1.0) |
| TG>=150,LDL>=100 | 493 | 1.6 | (1.2-2.2) | TG>=150,LDL>=100 | 225 | 0.9 | (0.6-1.3) |
| TG<150, LDL<130 | 367 | TG<150, LDL<130 | 1095 | 0.6 | (0.5-0.7) | ||
| TG<150, LDL>=130 | 426 | 1.3 | (1.1-1.6) | TG<150, LDL>=130 | 870 | 0.8 | (0.6-1.0) |
| TG>=150,LDL<130 | 260 | 1.2 | (1.0-1.5 | TG>=150,LDL<130 | 89 | 0.7 | (0.5-1.0) |
| TG>=150,LDL>=130 | 321 | 1.6 | (1.2-2.1) | TG>=150,LDL>=130 | 162 | 0.9 | (0.6-1.3) |
adjusted for age, sex, T2DM, SBP, smoking status, menopausal status & LLT.
To further examine the association of TG on CVD risk, ORs were plotted against increasing TG stratified into 4 categories (low HDL-C and low LDL <100 or <130 mg/dL; 2) low HDL-C and LDL ≥100 or ≥ 130 mg/dL; 3) high HDL-C and LDL <100 or <130 mg/dL; and 4) high HDL-C and LDL ≥100 or ≥ 130 mg/dL) (Figure). As expected, the subgroups with LDL-C <100 mg/dL were generally at a lower risk of CVD than comparable groups with LDL-C ≥100 mg/dL, regardless of HDL-C phenotype. However, LDL-C or TG could affect the direction of the association depending on the actual levels.
Figure 1.
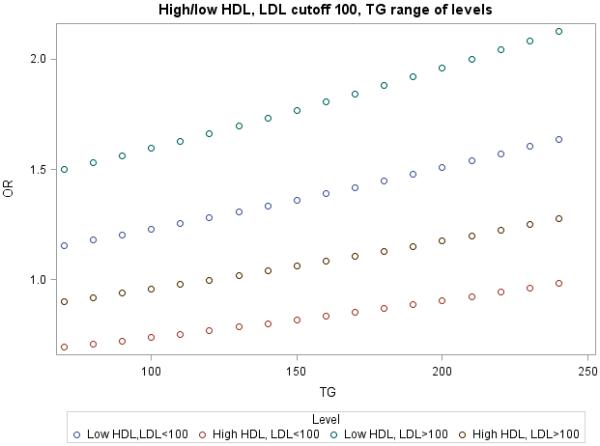
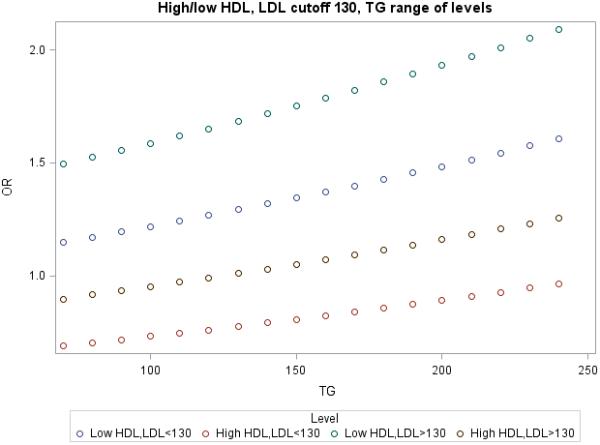
TG levels as a function of Incident CVD with low or higher HDL-C and a using an LDL-C cutpoint of 100 mg/dL or 130 mg/dL.
Discussion
The most novel finding in this study is that low HDL-C in isolation is considerably less predictive of CVD risk in the presence of high TG, high LDL-C, or both. We are aware of one study that attempted to evaluate isolated low HDL-C and found it to be associated with increased CVD risk17 but the LDL-C and TG thresholds used (160 and 200 mg/dL, respectively), may have been too high to evaluate the true risk of isolated HDL-C.
For many decades, a low level of HDL-C has been viewed as a robust and independent CVD risk factor1-4. In contrast, recent studies have failed to demonstrate that high HDL-C acquired naturally or pharmacologically, is associated with reduced CVD risk.5-11,18 The current study does not refute the potential clinical relevance of HDL-C, when direct comparison is made between isolated low and higher HDL-C levels. Within this context, higher HDL-C is associated with lower CVD risk, when other risk factors are constant.
The increased risk of CVD associated with low HDL-C is most evident in the presence of higher levels of other lipids or lipoproteins. This issue could not have been addressed in the original Framingham cohort because fasting TG levels were not routinely measured until approximately 2 decades ago. Consequently, the Framingham offspring cohort provides a unique opportunity to systematically investigate the effects of low HDL-C, both in isolation and within the framework of elevated TG and LDL-C. Whereas isolated low HDL-C using the lowest thresholds to define low TG and LDL-C (i.e., < 100 mg/dL) was uncommon (n=84, 2.3% of the study cohort) we observed similar trends with higher thresholds of the other risk factors that had larger cell sizes. We also found that increased TG and LDL-C appreciably raised CVD risk, consistent with prior studies demonstrating a 30-60% increase in CVD risk when LDL-C exceeded 130 mg/dL15 and an approximate 10-20% increase in CVD risk when TG exceeded 150 mg/dL compared to < 100 mg/dL.19
Recently, TG has gained traction as an important biomarker of CVD risk with some proposing that TG-rich lipoproteins (TRLs; e.g., very low density lipoprotein) and their cholesterol-enriched remnants play a causative role in disease.20,21 Specifically, TRLs have been implicated in pro-inflammatory signaling pathways, impairment of insulin sensitivity and upregulation of factors promoting thrombosis.22,23
Another novel finding in the current study was the association of TG levels on CVD risk across HDL-C and LDL-C subgroups (Figure). For example, at TG <100 mg/dL, CVD risk was low in the setting of high HDL-C and LDL-C <100 (or <130) mg/dL. However, the presence of higher TG (> 200 mg/dL) within this subgroup was associated with increased CVD risk to the level shown by the subgroup with higher LDL-C and lower TG (<100 mg/dL). A similar pattern emerged with low HDL-C. Therefore, TG levels essentially reclassify risk of CVD irrespective of HDL-C. This is consistent with studies that have supported a direct role for TG-rich lipoproteins in promoting atherothrombosis24 and more recently defects in genes controlling TG metabolism, including APOC3, ANGPTL3 and ANGPTL4 that have been linked to reduced TG and are associated with reduced coronary calcification25 or reduced CVD risk.26-30 Moreover, a recent genome-wide association study identified common polymorphisms associated with TG to strongly influence risk of coronary disease20 and post-hoc analyses found TG to be more accurate than HDL-C in predicting recurrent CVD events after an acute coronary syndrome.19,31
The Framingham Heart Study was the first large epidemiological study in the U.S to demonstrate an inverse association between low HDL-C and coronary heart disease32, although the isolated low HDL-C phenotype was not specifically examined. The most direct application of this relationship came from the National Cholesterol Education Program Adult Treatment Panel (ATP I) recommendations that HDL-C not be measured when total cholesterol is in the recommended range (less than 200 mg/dL).33 However, triglycerides were not adjusted for in these analyses and the prevalence of isolated low HDL-C was not reported. Another study of CVD survivors with desirable total cholesterol34 also found low HDL-C to be predictive of recurrent events but since mean LDL-C and TG levels were 115 ± 23 mg/dL and 124 ± 65 mg/dL, respectively, many subjects had concomitant dyslipidemia.
Our results are not intended to alter the large body of evidence supporting HDL as inversely related to CVD risk based on its role in mediating reverse cholesterol transport. Indeed recent studies suggest that HDL function may be more predictive of CVD risk compared with its cholesterol content35, as reflected by HDL-C levels. As such, functionality rather than the cholesterol content of HDL has been viewed as a better predictor of CVD risk based on the well-established anti-inflammatory, anti-oxidant and endothelial restorative properties of HDL.36 In this regard, elevated TG and insulin resistance may both impair HDL function37-39 and predate the development of diabetes.40 Indeed, the prevalence of diabetes in Framingham Offspring subjects with low HDL- C, LDL-C<100 and TG < 100 mg/dL was approximately 50% lower compared to higher TG (Table 1) and also corresponded to the lower CVD rates observed (Table 2).
Limitations of the current study include the lack of additional measures (e.g., apoB or LDL particle concentration) that may provide incremental CVD risk prediction beyond conventional lipids and lipoproteins.41 As noted above, there was also a relative paucity of cases with isolated low HDL-C using the stringent threshold (< 100 mg/dL) for TG and LDL-C, although these data are consistent with the National Health and Nutrition Examination Survey (NHANES) III, that reported isolated low HDL-C in only 4.8% in men and 8.7% of women over age 35 years.42
Conclusions
In the Framingham Offspring Study, low and high HDL-C phenotypes are not uniformly predictive of CVD risk. TG and LDL-C represent important modifiers of incident CVD risk at both ends of the HDL-C spectrum.
Supplementary Material
What is Known:
HDL-C is inversely associated with cardiovascular risk (CVD). However, the extent to which HDL-C remains an independent CVD risk factor when other lipids are normal is unclear.
What the Study Adds:
In the Framingham Offspring Study, HDL-C was not uniformly predictive of CVD risk.
Triglycerides and LDL-C were important modifiers of incident CVD at both ends of the HDL-C spectrum.
Compared to isolated low HDL-C, the risk of CVD was 30-60% higher when low HDL-C was accompanied by higher levels of TG, LDL-C or both.
High HDL-C was not associated with reduced CVD risk if accompanied by TG and LDL-C ≥ 100 mg/dL
Acknowledgments
Sources of Funding: This study was supported in part by NIH (HL094980) and a Veterans Affairs Merit Award (MM), Training Grant NIH (HL007751) (IMP), NIH (GM103534) (JB and SMW) and NIH (HL106845 and HL057986) (SF).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Miller GJ, Miller NE. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975;1:16–9. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- 2.Sheridan S, Pignone M, Mulrow C. Framingham-based tools to calculate the global risk of coronary heart disease: a systematic review of tools for clinicians. J Gen Intern Med. 2003;18:1039–52. doi: 10.1111/j.1525-1497.2003.30107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RB, Sr, Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ. HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation. 1977;55:767–72. doi: 10.1161/01.cir.55.5.767. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS. dal-OUTCOMES Investigators. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 7.AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 8.HPS2-THRIVE Collaborative Group HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 10.Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379. doi: 10.1136/bmj.g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 13.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.National Cholesterol Education Program Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 16.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S, American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 17.Huxley RR, Barzi F, Lam TH, Czernichow S, Fang X, Welborn T, Shaw J, Ueshima H, Zimmet P, Jee SH, Patel JV, Caterson I, Perkovic V, Woodward M. Asia Pacific Cohort Studies Collaboration and the Obesity in Asia Collaboration. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: an individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation. 2011;124:2056–64. doi: 10.1161/CIRCULATIONAHA.111.028373. [DOI] [PubMed] [Google Scholar]
- 18.Zanoni P, Khetarpal SA, Larach DB, Hancock-Cerutti WF, Millar JS, Cuchel M, DerOhannessian S, Kontush A, Surendran P, Saleheen D, Trompet S, Jukema JW, De Craen A, Deloukas P, Sattar N, Ford I, Packard C, Majumder Aa, Alam DS, Di Angelantonio E, Abecasis G, Chowdhury R, Erdmann J, Nordestgaard BG, Nielsen SF, Tybjærg-Hansen A, Schmidt RF, Kuulasmaa K, Liu DJ, Perola M, Blankenberg S, Salomaa V, Männistö S, Amouyel P, Arveiler D, Ferrieres J, Müller-Nurasyid M, Ferrario M, Kee F, Willer CJ, Samani N, Schunkert H, Butterworth AS, Howson JM, Peloso GM, Stitziel NO, Danesh J, Kathiresan S, Rader DJ. CHD Exome+ Consortium; CARDIoGRAM Exome Consortium; Global Lipids Genetics Consortium. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. doi: 10.1126/science.aad3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. PROVE IT-TIMI 22 Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 20.Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Döring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–52. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 22.Welty FK. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis? Curr Cardiol Rep. 2013;15:400. doi: 10.1007/s11886-013-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64:2535–2540. doi: 10.1016/j.jacc.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 24.Zilversmit DB. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem. 1995;41:153–8. [PubMed] [Google Scholar]
- 25.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O'Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–5. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 27.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Crosby J, Peloso GM, Auer PL, Crosslin DR, Stitziel NO, Lange LA, Lu Y, Tang ZZ, Zhang H, Hindy G, Masca N, Stirrups K, Kanoni S, Do R, Jun G, Hu Y, Kang HM, Xue C, Goel A, Farrall M, Duga S, Merlini PA, Asselta R, Girelli D, Olivieri O, Martinelli N, Yin W, Reilly D, Speliotes E, Fox CS, Hveem K, Holmen OL, Nikpay M, Farlow DN, Assimes TL, Franceschini N, Robinson J, North KE, Martin LW, DePristo M, Gupta N, Escher SA, Jansson JH, Van Zuydam N, Palmer CN, Wareham N, Koch W, Meitinger T, Peters A, Lieb W, Erbel R, Konig IR, Kruppa J, Degenhardt F, Gottesman O, Bottinger EP, O'Donnell CJ, Psaty BM, Ballantyne CM, Abecasis G, Ordovas JM, Melander O, Watkins H, Orho-Melander M, Ardissino D, Loos RJ, McPherson R, Willer CJ, Erdmann J, Hall AS, Samani NJ, Deloukas P, Schunkert H, Wilson JG, Kooperberg C, Rich SS, Tracy RP, Lin DY, Altshuler D, Gabriel S, Nickerson DA, Jarvik GP, Cupples LA, Reiner AP, Boerwinkle E, Kathiresan S. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, Garimella KV, Fisher S, Abreu J, Barry AJ, Fennell T, Banks E, Ambrogio L, Cibulskis K, Kernytsky A, Gonzalez E, Rudzicz N, Engert JC, DePristo MA, Daly MJ, Cohen JC, Hobbs HH, Altshuler D, Schonfeld G, Gabriel SB, Yue P, Kathiresan S, Exome sequencing. ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–7. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl J Med. 2016;374:1134–44. doi: 10.1056/NEJMoa1507652. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dewey FE, Gusarova V, O'Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai KV, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016;374:1123–33. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, Mundl H, Olsson AG. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65:2267–75. doi: 10.1016/j.jacc.2015.03.544. [DOI] [PubMed] [Google Scholar]
- 32.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 33.Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. The Expert Panel. Arch Intern Med. 1988;148:36–69. [PubMed] [Google Scholar]
- 34.Miller M, Seidler A, Kwiterovich PO, Pearson TA. Long-term predictors of subsequent cardiovascular events with coronary artery disease and 'desirable' levels of plasma total cholesterol. Circulation. 1992;86:1165–70. doi: 10.1161/01.cir.86.4.1165. [DOI] [PubMed] [Google Scholar]
- 35.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefer AM. Prostacyclin, high density lipoproteins, and myocardial ischemia. Circulation. 1990;81:2013–5. doi: 10.1161/01.cir.81.6.2013. [DOI] [PubMed] [Google Scholar]
- 37.Skeggs JW, Morton RE. LDL and HDL enriched in triglyceride promote abnormal cholesterol transport. J Lipid Res. 2002;43:1264–74. [PubMed] [Google Scholar]
- 38.Greene DJ, Skeggs JW, Morton RE. Elevated triglyceride content diminishes the capacity of high density lipoprotein to deliver cholesteryl esters via the scavenger receptor class B type I (SR-BI). J Biol Chem. 2001;276:4804–11. doi: 10.1074/jbc.M008725200. [DOI] [PubMed] [Google Scholar]
- 39.Barylski M, Toth PP, Nikolic D, Banach M, Rizzo M, Montalto G. Emerging therapies for raising high-density lipoprotein cholesterol (HDL-C) and augmenting HDL particle functionality. Best Pract Res Clin Endocrinol Metab. 2014;28:453–61. doi: 10.1016/j.beem.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–8. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 41.AACC Lipoproteins and Vascular Diseases Division Working Group on Best Practices. Cole TG, Contois JH, Csako G, McConnell JP, Remaley AT, Devaraj S, Hoefner DM, Mallory T, Sethi AA, Warnick GR. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC Lipoprotein and Vascular Diseases Division Working Group on Best Practices. Clin Chem. 2013;59:752–70. doi: 10.1373/clinchem.2012.196733. [DOI] [PubMed] [Google Scholar]
- 42.Miller M, Zhang M. Factors influencing coronary risk in low HDL syndromes. Atherosclerosis. 2003;169:347–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


