Abstract
Although it is known that dysphagia contributes to significant malnutrition, pneumonia, and mortality in amyotrophic lateral sclerosis (ALS), it remains unclear how swallowing impairment impacts quality of life in this vulnerable patient population. The aim of the current study was to (1) delineate swallow-related quality of life (SR-QOL) profiles in individuals with ALS and (2) evaluate relationships between SR-QOL, degree of swallowing impairment, and ALS global disease progression. Eighty-one ALS patients underwent a standardized videofluoroscopic swallow study and completed the swallowing quality of life (SWAL-QOL) instrument and ALS functional rating scale-revised (ALSFRS-R). Penetration Aspiration Scale (PAS) scores were derived by a blinded rater. Correlation analyses and a between groups ANOVA (safe vs. penetrators vs. aspirators) were performed. Mean SWAL-QOL score for this cohort was 75.94 indicating a moderate degree of SR-QOL impairment with fatigue, eating duration, and communication representing the most affected domains. Correlations were revealed between the SWAL-QOL and (1) PAS (r = −0.39, p < 0.001) and (2) ALSFRS-R (r = 0.23, p < 0.05). Mean (SD) SWAL-QOL scores for safe versus penetrator versus aspirator groups were 81.2 (2.3) versus 77 (3.4) versus 58.7 (5.9), respectively, with a main effect observed [F(2,78) = 9.71, p < 0.001]. Post hoc testing revealed lower SWAL-QOL scores for aspirators versus safe swallowers (p < 0.001) and aspirators versus penetrators (p < 0.001). Overall, SR-QOL was moderately reduced in this cohort of ALS patients and profoundly impacted in ALS aspirators and individuals with advanced disease. These findings highlight the importance of early multidisciplinary intervention to not only avoid malnutrition, weight loss, and pulmonary sequelae but also the associated reduced QOL seen in these individuals.
Keywords: Amyotrophic lateral sclerosis, Quality of life, Dysphagia, Deglutition, Deglutition disorders
Background
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive and incurable neurodegenerative disease affecting both upper and lower motor neurons [1]. Regardless of onset type, swallowing impairment is reported to occur in 85 % of ALS individuals at some point throughout the disease process [2, 3]. Treatment of ALS is primarily composed of symptom management and palliative care provided through a multidisciplinary care team [4–6]. Within the multidisciplinary care ALS clinic, the dysphagia management team includes a speech-language pathologist (SLP), dietician, respiratory therapist, and neurologist [4, 7, 8]. Typical dysphagia management in ALS includes early identification of swallowing impairment, dietary modifications, postural adjustments, mealtime compensations, and implementation of prophylactic percutaneous endoscopic gastronomy (PEG) tube placement [8–12].
Swallowing and feeding impairments are prevalent in ALS and may occur during any stage of the swallowing process due to muscle weakness or rigidity of the bulbar, respiratory, and limb musculature [4, 8]. Oral stage swallowing impairment in ALS includes prolonged mastication, mandible rigidity, difficulty manipulating the bolus due to lingual weakness, delayed swallow initiation, anterior spillage, and reduced tongue base retraction [3, 13–16]. Pharyngeal stage impairments include significant vallecular and/or pyriform sinus residue, incomplete velopharyngeal closure, reduced pharyngeal contraction, and incomplete laryngeal vestibule closure leading to penetration and aspiration [13, 15, 16]. Difficulties feeding are also noted in ALS due to limb involvement.
Swallowing impairment has been documented to adversely affect quality of life, mental well being, and psychosocial function in various patient populations including Parkinson’s disease [17], multiple sclerosis [14, 18], and head and neck cancer [19, 20]. Although the physiologic impact of dysphagia in ALS has been described in the literature [2–4, 13], its impact on quality of life and mental well being is yet to be as well defined. Only one published report exists documenting the impact of dysphagia on swallowing-related quality of life (SR-QOL) in ALS that concluded that ALS individuals with swallowing impairment experienced significant burden, increased eating duration, and decreased eating desire [21]. While this preliminary investigation in a small group of ALS patients (n = 30) provides some important preliminary data, the specific impact of global disease progression and dysphagia on SR-QOL has yet to be investigated in this patient population. Therefore, the aims of the current investigation were to: (1) Delineate SR-QOL profiles in individuals with ALS and (2) Determine the impact of swallowing impairment and ALS global disease progression on SR-QOL. We hypothesized that SR-QOL would be reduced in individuals with ALS, and that the presence of a swallowing impairment and more severe global disease progression would adversely affect SR-QOL.
Methods
Participants
Eighty-one individuals with a diagnosis of probable or definite ALS (Revised El-Escorial Criteria) were recruited and included in this study. Mean age was 61.5 years (SD: 10.3) and 58.9 % (n = 53) of participants were male. Mean disease duration from symptom onset was 21.2 months (SD: 14.8) and mean ALS functional rating scale-revised (ALSFRS-R) score was 34.43 (SD: 7.6), with a mean bulbar sub-score of 9.7 (SD: 2.4). Seventy-three percent of individuals demonstrated a spinal disease onset type. Inclusion/Exclusion criteria included: (1) diagnosis of ALS (Revised El-Escorial Criteria) by a certified neuromuscular neurologist, (2) no other neurological disease, (3) preserved cognition as evidenced by >24 on the Mini Mental Status Exam [22], and (4) no tracheotomy or mechanical invasive ventilation.
Procedures
This study was approved by the university institutional review board. All participants met inclusion criteria and provided written consent to participate. Following consent, participants attended a single testing session and underwent a standardized videofluoroscopic swallow study (VFSS), completed the validated Swallowing Quality of Life instrument (SWAL-QOL) [23–26], and the ALSFRS-R [27] survey.
For the standardized VFSS, participants were seated upright in a lateral viewing plane using a properly collimated Phillips BV Endura fluoroscopic C-arm unit (GE OEC 8800 Digital Mobile C-Arm system type 718074), while a Digital Swallow Workstation unit (Kay Pentax, Lincoln Park, NJ) digitally recorded the fluoroscopic images at 29.97 frames per second. As part of a standardized protocol, each patient completed the following trials: 1, 3, 20, and 90 cc thin liquid barium (Varibar Thin, EZ-EM, Inc., Westbury, NY) and 3 cc paste consistency barium (EZ-pudding, EZ-EM, Inc.) administered via syringe or medicine cup. Bailout criterion required the use of thickened liquids following two episodes of aspiration during the study. Images were recorded for subsequent analysis.
For completion of surveys, participants were seated comfortably in a quiet room and provided a pen, the SWAL-QOL, and ALSFRS-R surveys and given a brief explanation of each survey. In the event that a patient did not have adequate limb movement to grip a pen and circle responses, the clinician assisted by circling the responses the patient verbalized or pointed to. Participants were instructed to ask for clarification if they were unsure of any items on either instrument. Each participant completed the survey from their own point of view without verbal input or bias from the clinician regardless of the degree of physical assistance from the clinician.
A blinded rater with 25 years of clinical experience scored each swallow trial using the validated Penetration Aspiration scale (PAS) [28] to rate degree of airway protection. The worst PAS score across all trials was used for data analysis purposes and was used to determine the presence of dysphagia based on degree of airway safety. A total SWAL-QOL score was derived for each participant ranging from 0–100 (worst-best), as well as domain-specific scores for burden, eating duration, eating desire, food selection, communication, fear, mental health, social, sleep, and fatigue. A total ALSFRS-R score was derived ranging from 0 (severe global impairment) to 48 (no impairment) in addition to the ALSFRS-R bulbar sub-score that comprised the sum of questions 1–3 (speech, salivation and swallowing ability) ranging from 0 (total impairment) to 12 (no impairment).
Statistical Analyses
Descriptive statistics (mean, SD) were performed to delineate overall SR-QOL profiles in this group of ALS patients (Aim 1). In this study, PAS scores were utilized as an indicator of swallowing impairment. Spearman’s rho correlation analyses were performed between total SWAL-QOL scores and (1) PAS scores and (2) ALSFRS-R scores to determine relationships between swallowing impairment, global disease progression, bulbar sub-scores, and SR-QOL (Aim 2). Participants were then divided into airway safety groups that included: safe swallowers (PAS ≤ 2), penetrators (PAS: 3–5), and aspirators (PAS ≥ 6). To determine the impact of swallowing impairment on SR-QOL, a between groups analysis of variance (ANOVA) was performed on both the total SWAL-QOL score and individual domain scores across airway safety groups (Aim 2). LSD post hoc testing was performed when a main effect was observed. Statistical analyses were completed using SPSS (Version 22.0.0) and alpha was set at 0.05.
Results
Swallowing-Related Quality of Life Profiles in ALS
Overall mean SWAL-QOL score for the entire cohort was 75.9 (SD = 19.4, 95 % CI 71.76, 80.12), indicating a moderate degree of patient reported SR-QOL impairment. Table 1 delineates mean domain scores for this cohort. Fatigue (mean domain score: 59.5), eating duration (mean: 67.7), communication (mean: 67.9), and sleep (mean: 69.8) represent the most affected QOL domains in the entire cohort of ALS patients with eating desire (mean: 82.1) and food selection (mean: 81.8) representing the least impacted domains. Results are presented as mean ± standard deviation (SD) unless otherwise specified.
Table 1.
Mean (SD) swallowing-related quality of life (SWAL-QOL) scores for the entire cohort (overall) and for safe, penetrator, and aspirator ALS swallowing groups
| SWAL-QOL Domain |
Overall Mean (SD) |
Safe Mean (SD) |
Penetrator Mean (SD) |
Aspirators Mean (SD) |
p value |
|---|---|---|---|---|---|
| SWAL-QOL | 75.9 (19.4) | 81.2 (15.6) | 77.0 (15.6) | 58.7 (22.9) | p < .001 |
| Fatigue | 59.5 (21.6) | 90.7 (22.7) | 77.6 (18.9) | 66.9 (16.5) | p < 0.001a, 0.013c |
| Eating duration | 67.7 (31.3) | 89.7 (27.2) | 82.5 (28.1) | 68.5 (31.7) | p < 0.001a |
| Communication | 67.9 (28.7) | 77.0 (27.1) | 72.4 (24,8) | 45.4 (24.3) | p < 0.001a, 0.006b |
| Sleep | 69.9 (27.5) | 89.0 (27.5) | 83.8 (22.9) | 68.9 (29.8) | p < 0.003a, 0.049b |
| Fear | 75.7 (25.4) | 76.9 (22.9) | 68.6 (16.2) | 52.7 (29.9) | p < 0.003a |
| Mental | 77.1 (29.3) | 83.9 (25.4) | 77.6 (23.5) | 55.3 (33.1) | p < 0.001a, 0.005b |
| Social | 79.8 (26.8) | 85.4 (24.7) | 79.8 (20.7) | 57.9 (28.7) | p < 0.001a, 0.017b |
| Burden | 81.6 (21.9) | 87.4 (15.7) | 81.9 (21.4) | 64.8 (25.9) | p < 0.003a, 0.042b |
| Food selection | 81.8 (24.6) | 69.0 (20.5) | 80.5 (21.6) | 59.5 (27.2) | p < 0.023a |
| Eating desire | 82.1 (23.6) | 60.9 (18.7) | 65.7 (17.4) | 47.6 (32.5) | p < 0.034a, 0.012b |
| Symptoms | 79.5 (19.2) | 86.5 (11.9) | 77.4 (14.6) | 61.5 (27.5) | p < 0.001a, 0.003b, 0.025c |
Significant difference between safe swallowers and aspirators (p < 0.05)
Significant difference between penetrator and aspirator airway safety groups (p < 0.05)
Significant difference between safe swallowers and penetrators (p < 0.05)
Relationship Between SR-QOL, Swallowing Impairment, and Global Disease Progression
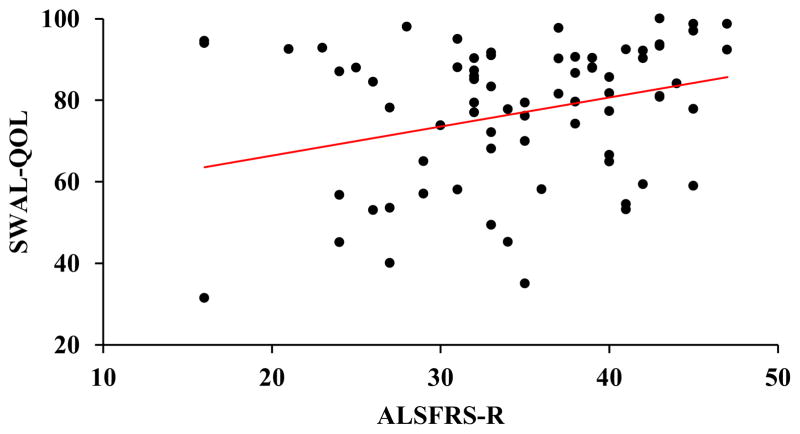
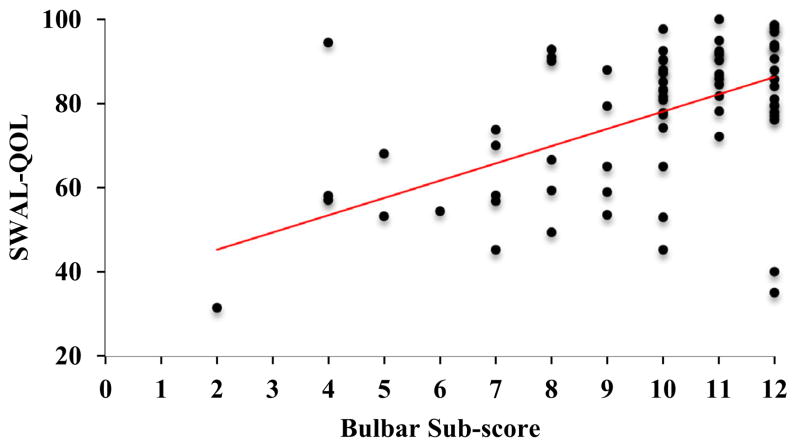
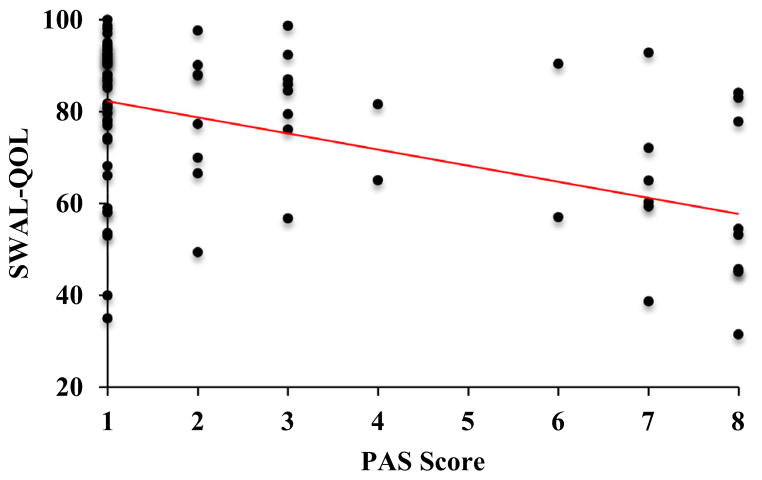
A positive correlation was revealed between the SWAL-QOL and ALSFRS-R (r = 0.23, p < 0.05). Higher (better) ALSFRS-R scores were associated with higher (better) SR-QOL total scores (Fig. 1). A positive correlation was also revealed between the SWAL-QOL and bulbar sub-scores of the ALSFRS-R (r = 0.46, p < 0.001). Higher (better) SR-QOL total scores were associated with higher (better) bulbar sub-scores (Fig. 2). A negative correlation was revealed between SWAL-QOL and PAS scores (r = −0.39, p < 0.001). Lower (worse) SWAL-QOL scores were associated with higher (worse) PAS scores (Fig. 3).
Fig. 1.
Scatterplot depicting a positive correlation between swallowing-related quality of life (total SWAL-QOL score) and global disease progression (ALSFRS-R score), r = 0.23, p < 0.05
Fig. 2.
Scatterplot depicting a positive correlation between swallowing-related quality of life (total SWAL-QOL score) and degree of bulbar ALS disease involvement (bulbar sub-scores of the ALSFRS-R), r = 0.46, p < 0.001
Fig. 3.
Scatterplot depicting a negative correlation between swallowing-related quality of life (total SWAL-QOL score) and degree of airway invasion during swallowing (Penetration Aspiration scale score), r = −0.39, p < 0.001
Impact of Swallowing Impairment on SR-QOL
VFSS analysis revealed 45 safe swallowers (55 %), 21 penetrators (26 %), and 15 (19 %) aspirators. Mean (SD) SWAL-QOL scores for safe versus penetrators versus aspirators were 81.2 (15.6) versus 77.0 (15.6) versus 58.7 (22.9), respectively. A significant main effect was observed [F(2,78) = 9.71, p < 0.001]. Post hoc testing revealed significant differences between safe swallowers and aspirators (mean difference: 22.44, 95 % CI 12.27, 32,61, p < 0.001) and between penetrators and aspirators (mean difference: 18.31, 95 % CI 6.78, 29.84, p < 0.001).
Individual domain data for the entire cohort and by swallow safety group are displayed in Table 1. Eating duration and fatigue represented the most impacted QOL domains among the penetrator and aspirator groups. All SWAL-QOL domains were significantly lower (worse) for the ALS aspirator group compared to the safe swallower group (p < 0.001). Domains of fatigue (p < 0.01) and symptoms (p < 0.02) were significantly worse in individuals who penetrated during swallowing compared to safe swallowers. When compared to the penetrator group, all individual domains except eating duration, fatigue, fear, and food selection were significantly lower for aspirators (p < 0.003).
Discussion
In this study, we demonstrated that SR-QOL is moderately reduced (global scores ~ 65–80) in individuals with ALS and that fatigue and eating duration represented the most affected domains in a general group of ALS patients. Further, we observed a relationship between airway protection during swallowing (as indexed by the PAS), disease progression, and SR-QOL. As hypothesized, the more advanced the global ALS disease progression and the more severe the airway invasion during swallowing, the worse the patient reported SR-QOL.
Given the physiologic nature of ALS, it is not surprising that fatigue and eating duration were the most impacted domains for the overall group of patients. Weakness and exhaustion are hallmark symptoms in ALS. These symptoms combined with limited functional reserve within the motor system [29] likely lead to increased meal times. These factors have also been noted to contribute to decreased oral intake because ALS patients are too weak or tired to get through prolonged mealtimes with limited functional reserve [10, 30]. Paris and colleagues [21] also noted altered QOL in individuals with ALS. Specifically, in their group of 30 individuals with ALS, results indicated that those with dysphagia reported increased eating duration in addition to increased burden and fear [21]. These results highlight the importance of adaptive feeding utensils to minimize the effort required during the oral preparatory phase (i.e., self-feeding). Recommending altered food consistencies that require less effort to consume (i.e., mechanical soft) to avoid prolonged meal times, fatigue, and subsequent weight loss may be beneficial even in ALS patients without dysphagia [10, 31]. Further, increased weakness and exhaustion resulting in prolonged meal time duration and decreased oral intake, in combination with the noted increased resting metabolic rate of individuals with ALS [30] have been coined the “perfect storm” for the development of malnutrition and further muscle wasting [8]. Therefore, introducing calorically dense alternatives (such as supplemental nutrition, homemade shakes) that require minimal effort to consume are highly advantageous in this patient population to minimize effort and exhaustion and reduce meal time durations while maximizing nutritional intake [10, 30, 32]. Additionally, alternative feeding methods including prophylactic PEG tube placement have been shown to prevent malnutrition and weight loss through supplementation and eventual replacement of oral intake [10, 30].
SR-QOL was associated with degree of airway safety during swallowing. The greater the degree of airway invasion, the worse the patient reported SR-QOL. Further, ALS aspirators reported significantly worse SR-QOL than both safe swallowers and penetrators. ALS aspirators also rated each SWAL-QOL domain significantly more impaired than safe swallowers and domains of communication, sleep, mental, social, burden, and eating desire significantly worse than ALS penetrators. In ALS patients who aspirated, severe impairments (< 60) were noted for specific QOL domains of fear, mental, social, eating desire, and communication. Preliminary normative data indicate that “normal swallowers” scored >93 and 96 in domains of eating desire and fear, respectively, indicating essentially no impairment in these SR-QOL domains [24]. Dysarthria was likely a comorbid bulbar symptom in individuals who were aspirators, as dysarthria and dysphagia often co-occur in bulbar and spinal onset ALS [3, 33]. Therefore, it is not surprising that patients with the worst airway protection during swallowing rated this domain lower than those with safe swallowing. In addition, it would be expected that eating desire would be reduced in these individuals due to the presence of swallowing difficulties, limb weakness affecting self-feeding, and increased fatigue during meal times. The finding that fear, mental, and social domains were severely affected in the presence of swallowing impairment is an important finding. These domains encompass feelings of anxiety, fear of pneumonia, depression, feelings of frustration, and the ability to actively and meaningfully engage in social activities. Paris and colleagues reported similar findings for the domains of burden, mental, communication, and food selection in ALS individuals with dysphagia [21]. Our findings also indicate an increased level of fear and reduced socialization in ALS aspirators, which lead to withdrawal in social settings, increased isolation, abandonment, and feelings of helplessness [34, 35]. These factors can contribute to decreased appetite, increased anxiety, and depression, which further exacerbate social isolation [10, 36]. In order to minimize these negative swallowing-related psychosocial sequelae, appropriate clinical care is paramount to identify symptoms and implement dysphagia management strategies and patient education in individuals with ALS and their caregivers.
As hypothesized, SR-QOL was also associated with global disease progression. Individuals with more advanced global disease progression were associated with worse SR-QOL. Additionally, SR-QOL was associated with bulbar sub-scores of global disease progression, with more advanced bulbar involvement (speech, salivation, and swallowing) being associated with poorer SR-QOL. Although Paris and colleagues did not find associations between QOL and global disease progression, they did report significant differences in speech, salivation, and swallowing impairment between their dysphagic and non-dysphagic groups [21]. This finding may be explained by the fact that our cohort of patients included 21 penetrators and 15 aspirators, whereas only 2 aspirators were identified in the previous study. Of note is the fact that dysphagia has been previously noted to rise by 9 % for every one-point decrease in the ALSFRS [37]. Since we have reported a relationship between SR-QOL and dysphagia, there is likely some co-linearity impacting these results. While this study incorporated the gold standard and validated measure of global disease progression (ALSFRS-R), this outcome is also a patient-report scale and this fact may have also impacted the obtained results.
It must be highlighted that in the current investigation swallowing impairment was based solely on a patient’s PAS status. We acknowledge that this represents only one aspect of swallowing impairment and may not be entirely representative of an individual’s swallowing ability and therefore may underestimate the number of dysphagic individuals in this cohort. Although aspiration is not the only etiology of aspiration pneumonia, aspiration pneumonia is a major cause of mortality in this particular patient population, therefore, it was chosen as our swallowing outcome [38]. However, future work could include a more comprehensive method to determine swallowing impairment in addition to airway safety. Future work investigating the relationship between SR–QOL with timing of PEG tube placement and degree of swallowing impairment would be beneficial in determining important outcomes for dysphagia management and the utility of prophylactic PEG tube placement in preserving SR-QOL in individuals with ALS. Future work could also utilize a validated depression scale to more comprehensively examine the impact swallowing impairment has on this domain.
Conclusions
The current findings add to a growing body of literature that emphasize the serious impact of dysphagia on psychosocial well being and quality of life [17–19, 39] and expands upon preliminary findings from Paris and colleagues [21] in a larger group of individuals with ALS. These results provide objective support to advocate for multidisciplinary dysphagia management to adequately address the multitude of physiologic, functional, and psychosocial sequelae surrounding dysphagia in ALS individuals and their caregivers. Our findings that fatigue and increased mealtimes are impacted in all ALS patients regardless of airway protection profiles also reinforce early patient education for the use of mealtime compensations, alternative feeding strategies, and adequate oral intake to minimize weight loss, dehydration, malnutrition, and further muscle wasting. Finally, these data provide much needed documentation of the significant impact of swallowing impairment on psychosocial function and quality of life in individuals with ALS.
Acknowledgments
The authors thank all of the participants who offered their time toward this publication. This study was funded, in part, by Grant R21HD075327 from the National Institute of Child Health and Development and a National Center for Medical Rehabilitation Research T32 Neuromuscular Plasticity Training Program.
Footnotes
Compliance with Ethical Standards
Conflict of Interest None.
References
- 1.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter RJ, 3rd, McDonald TJ, Howard FM., Jr The otolaryngologic presentation of amyotrophic lateral sclerosis. Otolaryngology. 1978;86(3 Pt 1):ORL479–84. doi: 10.1177/019459987808600319. [DOI] [PubMed] [Google Scholar]
- 3.Chen A, Garrett CG. Original articles: otolaryngologic presentations of amyotrophic lateral sclerosis. Otolaryngol Head Neck Surg. 2005;132:500–4. doi: 10.1016/j.otohns.2004.09.092. [DOI] [PubMed] [Google Scholar]
- 4.Kühnlein P, Gdynia H-J, Sperfeld A-D, Lindner-Pfleghar B, Ludolph AC, Prosiegel M, Riecker A. Diagnosis and treatment of bulbar symptoms in amyotrophic lateral sclerosis. Nat Clin Pract Neurol. 2008;4(7):366–75. doi: 10.1038/ncpneuro0853. [DOI] [PubMed] [Google Scholar]
- 5.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, Kalra S, Katz JS, Mitsumoto H, Rosenfeld J, Shoesmith C, Strong MJ, Woolley SC. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multi-disciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73(15):1227–33. doi: 10.1212/WNL.0b013e3181bc01a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Berg JP, Kalmijn S, Lindeman E, Veldink JH, de Visser M, Van der Graaff MM, Wokke JH, Van den Berg LH. Multidisciplinary ALS care improves quality of life in patients with ALS. Neurology. 2005;65(8):1264–7. doi: 10.1212/01.wnl.0000180717.29273.12. [DOI] [PubMed] [Google Scholar]
- 7.Goyal N, Mozaffar T. Respiratory and nutritional support in amyotrophic lateral sclerosis. Curr Treat Options Neurol. 2014;16(2):1. doi: 10.1007/s11940-013-0270-5. [DOI] [PubMed] [Google Scholar]
- 8.Plowman EK. Nutrition and Feeding Tube Placement for People with ALS: Best Practice in Clinical Decision Making. Dysphagia Cafe 2014 [Google Scholar]
- 9.Solazzo A, Vecchio L, Reginelli A, Monaco L, Sagnelli A, Monsorrò M, Martino N, Tedeschi G, Grassi R. Search for compensation postures with videofluoromanometric investigation in dysphagic patients affected by amyotrophic lateral sclerosis [Ricerca delle posture di compenso della disfagia con videofluoromanometria nella sclerosi laterale amiotrofica] Radiol Med. 2011;116(7):1083. doi: 10.1007/s11547-011-0698-1. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood DI. Nutrition management of amyotrophic lateral sclerosis. Nutr Clin Pract. 2013;28(3):392–9. doi: 10.1177/0884533613476554. [DOI] [PubMed] [Google Scholar]
- 11.Plowman EK. Pecutaneous gastronomy tube placemet increases survival in amyotrophic lateral sclerosis. Dysphagia Research Society 2015 [Google Scholar]
- 12.Braun MM, Osecheck M, Joyce NC. Nutrition assessment and management in amyotrophic lateral sclerosis. Phys Med Rehabil Clin N Am. 2012;23:751–71. doi: 10.1016/j.pmr.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Yorkston KM, Miller RM, Strand EA. Management of speech and swallowing in degenerative diseases. Tucson: Communication Skill Builders; 1995. [Google Scholar]
- 14.Miller RM, Britton D. Clinical dysphagia series. San Diego: Plural Publishing Inc; 2011. Dysphagia in neuromuscular diseases. [Google Scholar]
- 15.Logemann JA. Evaluation and treatment of swallowing disorders. Vol. 2. Austin: Pro-Ed; 1998. [Google Scholar]
- 16.Groher M, Crary M. Dysphagia: clinical management in adults and children. Vol. 1. Maryland Heights: Mosby Elsevier; 2010. [Google Scholar]
- 17.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, Rosenbek JC. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. 2009;24(9):1352–8. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugman TM, Ross E. Perceptions of the impact of speech, language, swallowing, and hearing difficulties on quality of life of a group of South African persons with multiple sclerosis. Folia Phoniatrica et Logopaedica. 2002;54(4):201. doi: 10.1159/000063194. [DOI] [PubMed] [Google Scholar]
- 19.Lin BM, Starmer HM, Gourin CG. The relationship between depressive symptoms, quality of life, and swallowing function in head and neck cancer patients 1 year after definitive therapy. Laryngoscope. 2012;122(7):1518–25. doi: 10.1002/lary.23312. [DOI] [PubMed] [Google Scholar]
- 20.Lee L-Y, Chen S-C, Chen W-C, Huang B-S, Lin C-Y. Oral medicine: postradiation trismus and its impact on quality of life in patients with head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:187–95. doi: 10.1016/j.oooo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Paris G, Martinaud O, Petit A, Cuvelier A, Hannequin D, Roppeneck P, Verin E. Oropharyngeal dysphagia in amyotrophic lateral sclerosis alters quality of life. J Oral Rehabil. 2013;40(3):199. doi: 10.1111/joor.12019. [DOI] [PubMed] [Google Scholar]
- 22.Rovner BW, Folstein MF. Mini-mental state exam in clinical practice. Hosp Pract. 1987;22(1):99, 103. 106 passim. [PubMed] [Google Scholar]
- 23.McHorney CA, Bricker DE, Kramer AE, Rosenbek JC, Robbins J, Chignell KA, Logemann JA, Clarke C. The SWAL-QOL outcomes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. 2000;15(3):115–21. doi: 10.1007/s004550010012. [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, Bricker DE. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17(2):97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 25.McHorney CA, Martin-Harris B, Robbins J, Rosenbek J. Clinical validity of the SWAL-QOL and SWAL-CARE outcome tools with respect to bolus flow measures. Dysphagia. 2006;21(3):141–8. doi: 10.1007/s00455-005-0026-9. [DOI] [PubMed] [Google Scholar]
- 26.Vanderwegen J, Nuffelen G, Bodt M. The validation and psychometric properties of the Dutch version of the Swallowing Quality-of-Life Questionnaire (DSWAL-QOL) Dysphagia. 2013;28(1):11. doi: 10.1007/s00455-012-9408-y. [DOI] [PubMed] [Google Scholar]
- 27.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. doi: 10.1016/S0022-510X(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11(2):93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 29.Talbot K. Amyotrophic lateral sclerosis: cell vulnerability or system vulnerability? J Anat. 2014;224(1):45–51. doi: 10.1111/joa.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desport JC, Preux PM, Truong CT, Courat L, Vallat JM, Couratier P. Nutritional assessment and survival in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(2):91–6. doi: 10.1080/14660820050515386. [DOI] [PubMed] [Google Scholar]
- 31.Körner S, Hendricks M, Kollewe K, Zapf A, Dengler R, Silani V, Petri S. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 2013;13(1):1–9. doi: 10.1186/1471-2377-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo ST, Steyn FJ, McCombe PA. Review article: body mass index and dietary intervention: implications for prognosis of amyotrophic lateral sclerosis. J Neurol Sci. 2014;340:5–12. doi: 10.1016/j.jns.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 33.da Costa Franceschini A, Mourao LF. Dysarthria and dysphagia in amyotrophic lateral sclerosis with spinal onset: a study of quality of life related to swallowing. NeuroRehabilitation. 2015;36(1):127–34. doi: 10.3233/nre-141200. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen NP, Frank C, Moltz CC, Vos P, Smith HJ, Karlsson U, Dutta S, Midyett A, Barloon J, Sallah S. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772–8. doi: 10.1016/j.ijrobp.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, Ortega P. Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia. 2002;17(2):139–46. doi: 10.1007/s00455-001-0113-5. [DOI] [PubMed] [Google Scholar]
- 36.McDonald ER, Wiedenfeld SA, Hillel A, Carpenter CL, Walter RA. Survival in amyotrophic lateral sclerosis: the role of psychological factors. Arch Neurol. 1994;51(1):17–23. doi: 10.1001/archneur.1994.00540130027010. [DOI] [PubMed] [Google Scholar]
- 37.Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, Traynor BG, Eurals C. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10(5–6):310–23. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langmore SE, Terpenning MS, Schork A, Chen Y, Murray JT, Lopatin D, Loesche WJ. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia. 1998;13(2):69–81. doi: 10.1007/pl00009559. [DOI] [PubMed] [Google Scholar]
- 39.Leow L, Huckabee M-L, Anderson T, Beckert L. The impact of dysphagia on quality of life in ageing and Parkinson’s disease as measured by the swallowing quality of life (SWAL-QOL) questionnaire. Dysphagia. 2010;25(3):216–20. doi: 10.1007/s00455-009-9245-9. [DOI] [PubMed] [Google Scholar]