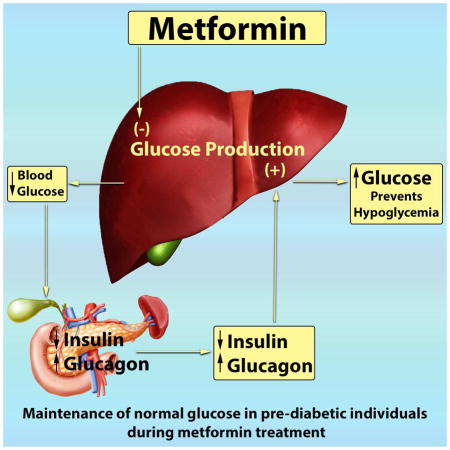
Summary
The therapeutic mechanism of metformin action remains incompletely understood. Whether metformin inhibits glucagon-stimulated endogenous glucose production (EGP), as in preclinical studies, is unclear in humans. To test this hypothesis, we studied 9 prediabetic individuals using a randomized, placebo-controlled, double-blinded, crossover study design. Metformin increased glucose tolerance, insulin sensitivity and plasma glucagon. Metformin did not alter average basal EGP, although individual variability in EGP correlated with plasma glucagon. Metformin increased basal EGP in individuals with severe hyperglucagonemia (>150pg/ml). Decreased fasting glucose after metformin treatment appears to increase glucagon to stimulate EGP and prevent further declines in glucose. Similarly, intravenous glucagon infusion elevated plasma glucagon (>150pg/ml) and stimulated a greater increase in EGP during metformin therapy. Metformin also counteracted the protein catabolic effect of glucagon. Collectively, these data indicate metformin does not inhibit glucagon-stimulated EGP, but hyperglucagonemia may decrease the ability of metformin to lower EGP in prediabetic individuals.
Graphical abstract
Introduction
The biguanide metformin is the most commonly prescribed oral anti-hyperglycemic agent, consumed annually by over 150 million people worldwide. Despite metformin’s efficacy in lowering blood glucose and decreasing the incidence of type 2 diabetes mellitus (T2DM) (Diabetes Prevention Program Research Group, 2002), its mechanisms of action remain incompletely understood. In type 2 diabetic (T2D) individuals, metformin lowers blood glucose by decreasing endogenous glucose production (EGP) (DeFronzo et al., 1991; Hundal et al., 2000; Musi et al., 2002; Stumvoll et al., 1995). Subsequent work demonstrated that metformin acted to inhibit EGP by activating AMP-activated protein kinase (AMPK) (Shaw et al., 2005; He et al., 2009). However, metformin reduced EGP in AMPK knockout mice, challenging the notion that AMPK is required for decreased EGP by metformin (Foretz et al., 2010). However, these authors utilized supra-pharmacologic doses of metformin, and Cao et al. (Cao et al., 2014) subsequently demonstrated that pharmacologic doses of metformin could indeed inhibit hepatic gluconeogenesis. Metformin was also recently discovered to decrease glucagon induced glucose production (Miller et al., 2013) and diminish the use of gluconeogenic metabolites for glucose production by altering mitochondrial glycerophosphate dehydrogenase and the cellular redox status in the liver (Madiraju et al., 2014). Moreover, metformin was recently shown to impart decreased fasting glucose and hepatic glucose production through the intestines (Duca et al., 2015; Buse et al., 2016). Therefore, several lines of evidence suggest that metformin lowers EGP by independent or perhaps combined mechanisms that 1) change levels of rate-limiting gluconeogenic enzyme levels (He et al., 2009; Foretz et al., 2010), 2) decrease glucagon action (Miller et al., 2013) or 3) limit the conversion of gluconeogenic substrates (e.g., lactate, alanine, amino acids [AAs]) to glucose (DeFronzo et al., 1991; Madiraju et al., 2014; Stumvoll et al., 1995).
Although preclinical models have provided clues on how metformin may elicit its therapeutic effect, translating these mechanisms to the clinical situation has been difficult because many studies have used supra-pharmacologic dosing schemes and biguanide derivatives contraindicated for human use (He et al., 2009; He and Wondisford, 2015). Furthermore, metformin may also influence glucogenic precursors and insulin sensitivity through its influence on amino acid kinetics; a possibility which has yet to be explored in humans. Therefore, we investigated if metformin at therapeutic doses would inhibit glucagon-stimulated EGP and amino acid (AA) kinetics in humans. We conducted a randomized, placebo-controlled, double-blinded, crossover study in prediabetic individuals and measured EGP and AA kinetics using stable-isotope methodology during basal, glucagon-deficient, and glucagon-stimulated conditions.
Results and Discussion
Nine participants completed the study with physical characteristics in Table S1; 7 had a family history of T2DM and 8 were metformin naïve. One participant had previously used metformin but discontinued more than 2 years before the study commenced. Some participants were taking antidepressant medications (n=5), statins (n=3), β-blocker (n=1), or diuretic (n=1) through the entire study and these participants did not differ in their response to metformin therapy.
Metformin and placebo were prescribed at a dose of 500 mg, twice daily during the first week and 1000 mg, twice daily during the second week. On the basis of returned pill counts, subjects adhered to the prescribed doses with a compliance rate 99% and 94% during week 1 and 96% and 94% during week 2 for metformin and placebo, respectively. Four participants reported gastrointestinal discomfort, 3 of which were during metformin. Body weight and composition remained unchanged during the two-week study (Table S1).
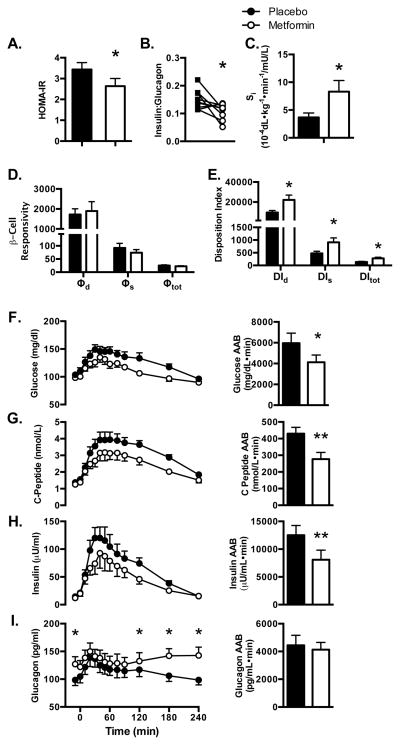
Compared with placebo, metformin treated patients had lower mean fasting plasma glucose, insulin, and c-peptide levels but markedly higher mean plasma glucagon levels (Figure 1, Table 1). The insulin to glucagon ratio and the homeostasis model assessment of insulin resistance (HOMA-IR) were decreased by metformin (Figure 1A and B).
Figure 1. Metformin improves fasting and postprandial indices of insulin sensitivity.
(A) HOMA-IR and (B) insulin to glucagon ratio. (C) Whole-body insulin sensitivity (Si) (D) β-cell responsivity and (E) Disposition index modeled after a mixed-meal challenge. Basal and postprandial plasma values and area above baseline (AAB) after two weeks of metformin compared to placebo for (F) Glucose (G) C-peptide (H) Insulin and (I) Glucagon. Postprandial glucagon was decreased with metformin at 120, 180 and 240 minutes. *P<0.05, **P<0.01, ***P<0.001 metformin vs. placebo; Data presented as mean ± SEM.
Table 1.
Hormones, Leucine Kinetics and Resting Energy Expenditure
| Placebo | Metformin | |||||
|---|---|---|---|---|---|---|
| Basal | Somatostatin | Glucagon | Basal | Somatostatin | Glucagon | |
| Glucose (mg/dL) | 108±3 | 103±7 b | 214±8 c | 93±3 a | 85±10 a, b | 212±10c |
| Glucagon (pg/mL) | 90.7±10.0 | 54.3±7.5 b | 157.1±7.7 c | 119.1±13.5 | 60.2±6.9 b | 156.6±11.5 c |
| Insulin (μU/mL) | 13.2±1.4 | 0.53±0.06 b | 5.4±1.4 c | 11.4±1.5 a | 0.46±0.05 b | 4.7±0.5 |
| C-peptide (nmol/L) | 1.31±0.09 | 0.33±0.02 b | 0.46±0.03 c | 1.13±0.08 a | 0.28±0.02a,b | 0.38±0.03a,c |
| Leucine C Flux | 95.0±4.2 | 89.6±3.5 b | 85.5±4.0 c | 93.0±1.7 | 90.2±2.0 b | 87.6±2.4 |
| Leucine Oxidation | 19.9±1.2 | 16.5±1.2 b | 23.7±1.7 c | 18.3±0.8 | 16.4±0.7 | 19.8±1.3a,c |
| Leu Protein Synthesis | 75.1±3.8 | 73.2±3.5 b | 61.8±3.5 c | 74.7±2.0 | 73.8±2.0 b | 67.8±2.0a,c |
| Leucine N Flux | 155.0±6.3 | 197.9±7.5 b | 215.1±12.5 c | 159.3±6.2 | 204.7±6.1 b | 230.4±9.4c |
| Leucine Transamination | 79.9±4.2 | 124.8±7.1 b | 149.8±10.2 c | 84.5±4.7 a | 130.9±4.6 b | 165.0±7.8 c |
| Leucine Reamination | 60.0±4.2 | 108.3±4.2 b | 126.1±10.0 c | 66.3±4.7 a | 114.5±4.6 b | 145.2±7.3a,c |
| % Flux Oxidized | 21.0±1.3 | 18.5±1.5 b | 27.9±2.0 c | 19.7±1.0 | 18.2±0.8 b | 22.5±1.1a,c |
| REE (kcals/day) | 1669±138 | 1682±122 | 1925±139 c | 1727±103 | 1719±93 | 1858±97 |
| RER | 0.82±0.01 | 0.75±0.02 | 0.78±0.02 | 0.82±0.01 | 0.74±0.02 | 0.75±0.02 |
p<0.05 metformin vs. placebo;
p<0.05 somatostatin vs. basal;
p<0.05 glucagon vs. somatostatin.
Data expressed as μmol/kgFFM/hr for whole-body leucine kinetics. Data are represented mean ± SE
Whole-body insulin sensitivity, β-cell responsivity and disposition index (DI) were modeled following consumption of a mixed-meal (600 kcal; 30% carbohydrate, 55% fat, 15% protein) (Figure S1). Insulin sensitivity (Si) was significantly improved by metformin (Figure 1C). Although, β-cell responsivity (Φ, ΦD, ΦS) was unaltered (Figure 1D), total, dynamic and static disposition indices (DI, DID, DIS - the product of β-cell responsivity and Si; Figure 1E), were significantly improved by metformin. The 2-fold increase in whole body insulin sensitivity was markedly greater than the decrease in HOMA-IR, which may suggest that effects of metformin are more robust in response to the physiological challenge of a high-fat mixed meal. After the meal, metformin decreased (P<0.01) the area above baseline for glucose, c-peptide, and insulin (Figure 1F, G, and H). In addition to increasing postabsorptive plasma glucagon, metformin increased (P<0.01) postprandial plasma glucagon at 120, 180, and 240 minutes (Figure 1I).
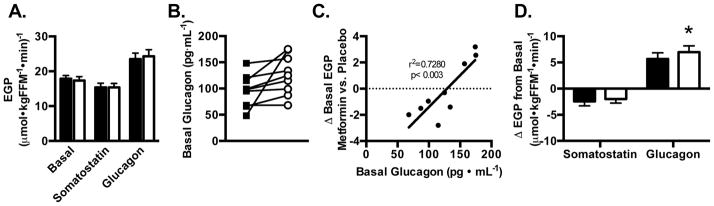
Although metformin improved glucose regulation and whole-body insulin sensitivity based on the oral minimal model, its effect on basal EGP was variable depending on the basal glucagon level (Figure 2A, B and C). Subtle changes in glucagon have been shown to have profound effects on hepatic glucose metabolism (Roden et al., 1996). Of interest, participants with basal glucagon levels <150 pg/mL (n=6 of 9 experienced a decrease (P=0.01, Cohen’s d=0.61) in EGP, while participants with basal glucagon levels >150 pg/mL (n=3 of 9) experienced an increase (P=0.02, Cohen’s d=1.26) in EGP after metformin treatment (Figure C). This accounts for the lack of overall change in mean EGP by metformin in our study (Figure 2A). It is possible that the improvement in glucose tolerance and insulin sensitivity occurred due to other mechanisms of action such as the influence of metformin in the gut (Duca et al., 2015; Buse et al., 2016) since the current study has not identified the mechanism of action by which metformin improves indices of insulin action. Previous reports that administered longer period have indicated that metformin lowers basal EGP in T2DM (Hundal et al., 2000; Stumvoll et al., 1995) but we found that in prediabetic humans the decline in EGP was prevented by compensatory increase in glucagon levels. These findings suggest that a compensatory increase in glucagon antagonizes metformin’s inhibitory effect on EGP and may be involved in the delayed or lack of response to metformin by some patients. Studies where combining metformin therapy with glucagon like peptide 1 (GLP1) agonists or dipeptidyl peptidase-4 (DPP4) inhibitors have demonstrated synergetic effect in achieving glycemic control (Zander et al., 2001; DeFronzo et al., 2009; Solis-Herrera et al., 2013) in T2DM but it remains to be determined whether such combination prevents the compensatory glucagon increase and increases the odds of hypoglycemic episodes in pre-diabetic individuals.
Figure 2. Endogenous glucose production after metformin treatment in prediabetic individuals.
(A) EGP during basal, glucagon-deficient (somatostatin), and glucagon-stimulated conditions (glucagon). (B) Fasted plasma glucagon. (C) Relationship between the change in basal EGP to basal, fasting glucagon after metformin. (C). The difference in EGP (Δ EGP) during glucagon-deficient (somatostatin) and glucagon-stimulated (glucagon) versus basal EGP. *P<0.05 metformin vs. placebo; Data presented as mean ± SEM.
We also examined the effect of metformin on whole body protein metabolism since amino acids and their metabolites may affect insulin sensitivity (Everett et al., 1981; Nair et al., 1992; Newgard, 2012) and play a key role in determining gluconeogenesis through regulating substrate availability (Felig et al., 1975). To study amino acid metabolism, we used di-labeled leucine (1-13C, 15N Leucine) to comprehensively assess leucine carbon and nitrogen flux, transamination, reamination, and oxidation (Table 1). During the basal period, metformin increased leucine transamination (6%; P=0.046) and reamination (15%; P=0.02) but did not alter leucine carbon flux (representing leucine appearance from endogenous protein degradation), nitrogen flux, or oxidation (Table 1). These results corroborate previous reports that leucine transamination is influenced by insulin treatment and glycemic control in diabetic individuals (Halvatsiotis et al., 2002; Nair et al., 1995). Such anabolic effects reduce the availability of amino moieties for synthesis of glucose precursors (e.g., alanine, glutamine) (Galim et al., 1980; Haymond and Miles, 1982). In addition to measuring amino acid kinetics, we quantitatively profiled systemic AA metabolites in plasma. There were 16 metabolites influenced by metformin, including elevated metabolites of the urea pathway (e.g., arginine, citrulline, P<0.001, ornithine, P=0.003). These results suggest that metformin treatment impacts amino acid metabolites involved in ammonia disposal. The targeted amino metabolite profiling also revealed a decline in gluconeogenic AAs (P=0.011) and specific metabolites such as glutamic acid, proline, alanine, isoleucine, alloisoleucine, and α-amino-N-butyric acid (P<0.05), which are all involved in subsequent transamination and gluconeogenic pathways (Table S2). We also noted that metformin did not affect concentrations of essential AA, which are derived from whole-body protein degradation in the fasted state. These findings are supported by our results showing that metformin did not affect leucine carbon flux derived from endogenous protein degradation. Previous findings in a pre-clinical model indicated that metformin lowers basal EGP by diminishing the use of gluconeogenic substrates (Madiraju et al., 2014). Conversely, we show that metformin decreases glucose precursors likely from increased utilization to preserve EGP in prediabetic individuals. An advantage of metformin over other glucose-lowering agents is the minimal risk of hypoglycemia. The prediabetic model allowed us to study individuals at high risk of developing T2D but without very high glucose levels as those with overt T2D. This allowed an opportunity identify decreased glucogenic precursors and increased plasma glucagon as likely mechanisms by which the glucose lowering effect of metformin does not result in hypoglycemia.
To further investigate the interaction between metformin treatment and glucagon, we continuously infused somatostatin for 2-hrs to suppress endogenous glucagon and insulin secretions (Figure S1). Following a period of low glucagon and insulin levels, we added a continuous infusion of glucagon to the somatostatin infusion for 3-hrs to raise plasma glucagon levels near those observed in participants with basal hyperglucagonemia (>150 pg/ml). Somatostatin was infused to prevent insulin secretion in response to glucagon administration. This study design allowed us to test the hypothesis that metformin antagonizes glucagon-mediated glucose production as reported in preclinical studies. Glucagon and insulin plasma concentrations during the somatostatin and somatostatin+glucagon infusions were not different between metformin and placebo treatments, whereas c-peptide levels were marginally lower (P<0.05) with metformin (Table 1). Plasma metformin concentration was 1.74±0.12 μM during glucagon infusions. By design, EGP was lower (P<0.05) during glucagon-deficient and higher (P<0.001) during glucagon-stimulated conditions when compared to the basal state (Figure 2A). EGP was not different with metformin during glucagon-deficient stages. The primary effect of metformin on EGP occurred during glucagon infusion. Specifically, the change in EGP from baseline was greater with metformin compared to placebo (1.3±0.4 μmol/kgFFM/min; P=0.017) (Figure 2D). A prevailing hypothesis is that metformin improves glucose regulation by inhibiting glucagon-stimulated glucose production. In support, studies in hepatocytes and mice indicate biguanides inhibit glucagon signaling to mitigate hepatic glucose production (Miller et al., 2013). Yet these preclinical models included phenformin and supratherapeutic doses of metformin to inhibit glucagon action. The current study included prediabetic adults consuming therapeutic doses of the only Federal Drug Administration approved biguanide. Our results indicate metformin does not inhibit glucagon-stimulated EGP in patients with prediabetes.
In addition to EGP, leucine AA kinetics were largely altered by somatostatin and further changed by the addition of glucagon infusion (Table 1). Importantly, somatostatin infusion with the accompanying decline in insulin and glucagon reduced leucine oxidation (absolute and % of leucine flux). However, increasing glucagon levels increased leucine oxidation, transamination, and reamination while decreasing protein synthesis. These effects of glucagon are congruent with our previous data in healthy individuals (Nair et al., 1987) and those with type 1 diabetes (Charlton and Nair, 1998). The glucagon-induced increase in leucine oxidation (P=0.01) and decrease in protein synthesis (P=0.03) were attenuated by metformin treatment (Table 1). During placebo treatment glucagon, as previously reported, increased resting energy expenditure (Nair et al., 1984) but the glucagon effect on resting energy expenditure was not observed during metformin (Table 1). These data suggest metformin counteracts the catabolic effects of glucagon by attenuating both the increase in leucine oxidation, energy expenditure and the decrease in protein synthesis. Glucagon secretion is well-known to increase to prevent hypoglycemia and plasma glucagon levels are high in the diabetic states (Felig et al., 1976). Thus, the current results show that the potential adverse catabolic effects of the increase in glucagon levels (Nair et al., 1987; Pain et al., 1983) seems to be mitigated by metformin.
We used a randomized, double-blind, crossover study design to test the prevailing hypothesis that metformin would inhibit glucagon-mediated substrate metabolism in individuals with prediabetes. Although a sample size of 9 prediabetic individuals not currently on glucose lowering medications may appear to be a limitation of the current study, this study design allowed detection of meaningful changes in glucose and glucagon while using gold-standard approaches to assess glucogenic metabolites and endogenous glucose production. Additional participants groups including obese, normoglycemic and overt T2D may have allowed further comparisons in people with normal glucose levels and severe hyperglycemia. Future studies might consider further addressing the relationship between metformin, glucagon and glycemic control using larger sample sizes and diverse participant populations.
Conclusion
With the prospect of developing alternative or improved therapies to counter T2D worldwide, understanding the therapeutic action of metformin in humans is critical. The current study offers insight into the effects of metformin in prediabetic individuals. We demonstrated that metformin may affect glucagon-mediated AA metabolism and energy expenditure, but in contrast to prevailing hypothesis we found no inhibitory effect of metformin on glucagon-induced EGP. In the current study, EGP during hyperglucagonemia was actually greater with metformin compared to placebo. Therefore, the effect of metformin on EGP depends on plasma glucagon levels such that any inhibitory effect of metformin on EGP was neutralized at high basal glucagon levels or during glucagon infusion studies. The current study provides insight in humans that high glucagon levels antagonize the ability of metformin to suppress EGP, disputing a previous report in preclinical models (Miller et al., 2013). These findings provide impetus for further study to determine if glucagon may be a biomarker used during metformin therapy to improve precision medicine to stop the progression of prediabetes to overt T2D.
Methods
This study was approved by the Mayo Clinic Institutional Review Board and all subjects gave written, informed consent. This study was conducted at Mayo Clinic, Rochester, MN from December 2013 to December 2014 and registered at ClinicalTrials.gov (NCT01956929).
Study Participants
Inclusion criteria were: obesity (body mass index >30 kg/m2), sedentary (<1 hour of structured activity per week), nonsmoking, and not taking any medication to control blood glucose. Qualifying participants (n=33) had a medical history and physical examination, including a blood test after an overnight fast to measure glucose, hemoglobin A1c, and chemistry profile. As recommended by the American Diabetes Association (ADA, 2014), participants with plasma glucose between 100–125 mg/dL or hemoglobin A1c of 5.7%–6.4% (5.93±1.16) were considered prediabetic and were eligible for the study.
Randomization and Study Intervention
The study was a randomized, double-blind, crossover trial with a >6-week washout period between interventions. Participants were randomized for a two-treatment, two-period crossover design using permuted block randomization. Block sizes of 2 and 8 were used to assign participants to a treatment sequence of either placebo followed by metformin or vice versa. Assignment of treatment sequences to participants was accomplished through the central research pharmacy to preserve the blind for study investigators. Participants were instructed to not change their diet, physical activity, or medications for the entirety of the study. Participants were directed to take the study medication with the morning and evening meals (week 1: 500mg, twice daily; week 2: 1,000mg, twice daily). The 1,000-mg, twice daily dose was continued during the inpatient study days (Figure S1). Metformin and placebo capsules were matched for size and color. Participants received extra capsules so that returned pills could be counted to determine compliance. Resting energy expenditure (REE) and body composition were measured during an outpatient visit before the 3 consecutive inpatient study days in the Clinical Research Unit (CRU). Inpatient visits were repeated after the second intervention.
Diet and Inpatient Study Preparation
Participants were provided a weight-maintaining diet with a standardized macronutrient composition (50% carbohydrate, 30% fat, 20% protein) for 3 days before and during the inpatient study days. Participants were admitted to the CRU the evening before each inpatient study day and consumed their evening meal and medication by 1900 (Figure S1).
Study Outcomes
The primary study outcomes were basal and glucagon-stimulated EGP. Secondary outcomes included AA kinetics and results from the mixed meal tolerance test.
Mixed-Meal Tolerance Test
Insulin sensitivity (SI); total, dynamic, and static β-cell responsivity (Φ, ΦD, ΦS); and disposition indices (DI, DID, DIS) were modeled after a mixed-meal (600 kcal; 30% carbohydrate, 55% fat, 15% protein) (Cobelli et al., 2014).
Glucose and AA Metabolism
EGP and whole-body AA kinetics (ie, flux, transamination, reamination, oxidation) were determined on day 2 during a 2-hour basal period, a 2-hour period of somatostatin infusion (93 ng/kg of fat-free mass [kg FFM]/min, to suppress pancreatic hormone secretion), and a 3-hour period of continuously glucagon (3 ng/kgFFM/min, to elevate plasma glucagon) plus somatostatin, as previously described (Charlton and Nair, 1998; Charlton et al., 1996; Nair et al., 1987). EGP and AA kinetics were measured by an isotope-dilution technique using a priming bolus of [13C]sodium bicarbonate concomitant with primed-continuous infusions of [6,6-2H2]glucose and [1-13C,15N]leucine (Charlton et al., 1996; Charlton and Nair, 1998; Nair et al., 1995). Whole-body REE and respiratory exchange ratio (RER) were assessed by indirect calorimetry for 20 minutes during each period. Arterialized venous blood and expired air were collected hourly and every 10 minutes during the last 30 minutes of each period.
Measurement of Hormones and Metabolites
Plasma glucose, insulin, C peptide, and glucagon concentrations were measured as previously described (Lalia et al., 2015). Plasma enrichment of the infused stable isotopes and [13C]α-ketoisocaproic acid, the transaminated product of leucine, was measured using gas chromatography–mass spectrometry (GC-MS). The isotope enrichment of 13CO2 in expired air was measured by isotope-ratio mass spectrometry (Charlton and Nair, 1998; Charlton et al., 1996; Nair et al., 1987; Nair et al., 1995). AA metabolites in plasma were analyzed using liquid chromatography–tandem mass spectrometry and GC-MS (Lanza et al., 2010).
Sample Size and Statistical Analysis
A modified intention-to-treat analysis was conducted for this study. While 12 participants were randomized, only 11 participants initiated the study. Two participants did not complete the crossover design and samples were not analyzed at time of the other participants due to cost constraints. To avoid assay batch effects, a decision was made to not include these data in the final analysis. As such, data on the 9 completing subjects were analyzed using SAS (version 9.4) according to the principles of a two-period, two-treatment crossover study with no assumed carryover effect. Models with period effect were found to not suggest a significant period effect, so final results without period effect are presented. In addition to test for differences at each of the three time points (basal, somatostatin infusion, and glucagon infusion), the incremental change in values (i.e., somatostatin – basal and glucagon – basal at each treatment visit) were tested to address change in baseline values during the two study visits. Data are presented as mean with 95% confidence intervals or standard error with P-values provided. Statistical significance was set at P<0.05.
Supplementary Material
Highlights.
Metformin does not inhibit glucagon-stimulated glucose production
Increased glucagon prevents hypoglycemia during metformin therapy
Metformin therapy reduces glucogenic precursors
Metformin counteracts the catabolic effects of glucagon
Acknowledgments
We are grateful for the expert technical assistance from Katherine Klaus, Dawn Morse, Roberta Soderberg, Antigoni Lalia, Daniel Jakaitis, Melissa Aakre, Andrew Lexvold and the staff of the Mayo Clinic Metabolomics Resource Core and CTSA and Adrian Vella for useful discussions. This publication was supported by NIH grants R01DK041973 (KSN), and T32DK007352 (ARK, MMR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional funding was provided by The Minnesota Obesity Center grant DK50456, and U24DK100469 from the NIDDK and originates from the NIH Director’s Common Fund. Dr. Nair is also supported by the David Murdock Professorship and is the guarantor of this work. Funding organizations had no role in any aspect of this study. The authors have no conflict of interest to disclose.
Footnotes
Clinical Trial Registration: Clinicaltrials.gov (NCT01956929).
Author Contributions
Conceptualization, ARK, RRE, MMR, MLJ, IRL, KSN; Methodology, ARK, MMR, MLJ, REC, IRL, KSN; Validation, ARK, RRE, IRL, KSN; Formal Analysis, ARK, REC, MS, CC; Investigation, ARK, RRE, MMR, MLJ; Resources, ARK, RRE, REC, CC, KSN; Data Curation, ARK, REC, MS, CC; Writing – Original Draft, ARK; Writing – Review & Editing, MMR, MLJ, REC, FEC, IRL, KSN; Visualization, ARK; Supervision, ARK, RRE, IRL, KSN, Project Administration, ARK, RRE, KSN; Funding Acquisition, KSN.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation. Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care. 2016;39:198–205. doi: 10.2337/dc15-0488. [DOI] [PubMed] [Google Scholar]
- Cao J, Meng S, Chang E, Beckwith-Fickas K, Xiong L, Cole RN, Radovick S, Wondisford FE, He L. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK) J Biol Chem. 2014;289:20435–20446. doi: 10.1074/jbc.M114.567271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton MR, Adey DB, Nair KS. Evidence for a catabolic role of glucagon during an amino acid load. J Clin Invest. 1996;98:90–99. doi: 10.1172/JCI118782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton MR, Nair KS. Role of hyperglucagonemia in catabolism associated with type 1 diabetes. Effects on leucine metabolism and the resting metabolic rate. Diabetes. 1998;47:1748–1756. doi: 10.2337/diabetes.47.11.1748. [DOI] [PubMed] [Google Scholar]
- Cobelli C, Dalla MC, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63:1203–1213. doi: 10.2337/db13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Barzilai N, Simonson DC. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1991;73:1294–1301. doi: 10.1210/jcem-73-6-1294. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Hissa MN, Garber AJ, Luiz GJ, Yuyan DR, Ravichandran S, Chen RS. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32:1649–1655. doi: 10.2337/dc08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015 doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett AW, Prior G, Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J. 1981;194:365–368. doi: 10.1042/bj1940365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P, Wahren J, Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Felig P, Wahren J, Sherwin R, Hendler R. Insulin, glucagon, and somatostatin in normal physiology and diabetes mellitus. Diabetes. 1976;25:1091–1099. doi: 10.2337/diab.25.12.1091. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galim EB, Hruska K, Bier DM, Matthews DE, Haymond MW. Branched-chain amino acid nitrogen transfer to alamine in vivo in dogs. Direct isotopic determination with [15N]leucine. J Clin Invest. 1980;66:1295–1304. doi: 10.1172/JCI109981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvatsiotis P, Short KR, Bigelow ML, Nair KS. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes. 2002;51:2395–2404. doi: 10.2337/diabetes.51.8.2395. [DOI] [PubMed] [Google Scholar]
- Haymond MW, Miles JM. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes. 1982;31:86–89. doi: 10.2337/diab.31.1.86. [DOI] [PubMed] [Google Scholar]
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wondisford FE. Metformin action: concentrations matter. Cell Metab. 2015;21:159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalia AZ, Johnson ML, Jensen MD, Hames KC, Port JD, Lanza IR. Effects of Dietary n-3 Fatty Acids on Hepatic and Peripheral Insulin Sensitivity in Insulin-Resistant Humans. Diabetes Care. 2015;38:1228–1237. doi: 10.2337/dc14-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Zhang S, Ward LE, Karakelides H, Raftery D, Nair KS. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS ONE. 2010;5:e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splachnic and leg tissues in type I diabetic patients. J Clin Invest. 1995;95:2926–2937. doi: 10.1172/JCI118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled type I (insulin- dependent) diabetic patients. Diabetologia. 1984;27:13–16. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- Nair KS, Halliday D, Matthews DE, Welle SL. Hyperglucagonemia during insulin deficiency accelerates protein catabolism. Am J Physiol Endocrinol Metab. 1987;253:E208–E213. doi: 10.1152/ajpendo.1987.253.2.E208. [DOI] [PubMed] [Google Scholar]
- Nair KS, Schwartz RG, Welle SL. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol Endocrinol Metab. 1992;263:E928–E934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain VM, Albertse EC, Garlick PJ. Protein metabolism in skeletal muscle,diaphragm, and heart of diabetic rats. Am J Physiol. 1983;245:E604–E610. doi: 10.1152/ajpendo.1983.245.6.E604. [DOI] [PubMed] [Google Scholar]
- Roden M, Perseghin G, Petersen KF, Hwang JH, Cline GW, Gerow K, Rothman DL, Shulman GI. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest. 1996;97:642–648. doi: 10.1172/JCI118460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Herrera C, Triplitt C, Garduno-Garcia JJ, Adams J, DeFronzo RA, Cersosimo E. Mechanisms of glucose lowering of dipeptidyl peptidase-4 inhibitor sitagliptin when used alone or with metformin in type 2 diabetes: a double-tracer study. Diabetes Care. 2013;36:2756–2762. doi: 10.2337/dc12-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- Zander M, Taskiran M, Toft-Nielsen MB, Madsbad S, Holst JJ. Additive glucose-lowering effects of glucagon-like peptide-1 and metformin in type 2 diabetes. Diabetes Care. 2001;24:720–725. doi: 10.2337/diacare.24.4.720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.