Abstract
The suppression of inappropriate actions is critical for flexible behavior. Cortical-basal ganglia networks provide key gating mechanisms for action suppression, yet the specific roles of neuronal subpopulations are poorly understood. Here, we examine Arkypallidal (“Arky”) and Prototypical (“Proto”) globus pallidus neurons during a Stop task, which requires abrupt cancellation of an imminent action. We first establish that Arky neurons can be identified by their firing properties across the natural sleep/wake cycle. We then show that Stop responses are earlier and stronger in the Arky, compared to the Proto, subpopulation. In contrast to other basal ganglia neurons, pallidal Stop responses are selective to Stop, rather than Go, cues. Furthermore, the timing of these Stop responses matches the suppression of developing striatal Go-related activity. Our results support a two-step model of action suppression: actions-in-preparation are first paused via a subthalamic-nigral pathway, then cancelled via Arky GABAergic projections to striatum.
Keywords: basal ganglia, action inhibition, globus pallidus, stop-signal, impulsivity
Introduction
To achieve adaptive behavior we often need to suppress impulses to act (Bari and Robbins, 2013), and difficulty with action suppression is central to many human disorders. One of the most fruitful paradigms for investigating action suppression is the Stop-signal task (e.g. Hanes and Schall, 1996; Leventhal et al., 2012). Subjects are prompted to move by a Go! cue, but on some trials a subsequent Stop! cue signals that no movement should be made after all. Behavioral data in this task is fit well by models involving a race between Go and Stop processes, with the winner determining whether the action is successfully canceled (Logan et al., 1984). Race models also allow calculation of “stop-signal reaction time” (SSRT), an estimate of how long it takes subjects to inhibit actions. SSRTs are longer (i.e. stopping is slower) in clinical populations with impulsive behavior, particularly in ADHD (Lipszyc & Schachar 2010).
Basal ganglia circuits are centrally involved in adaptive behavioral control, and using single-unit electrophysiology we reported direct evidence that competing Go and Stop processes race along distinct basal ganglia pathways (Schmidt et al., 2013). Go-related activity develops in striatum (Str), while the subthalamic nucleus (STN) responds to the Stop cue; both converge onto individual neurons in substantia nigra pars reticulata (SNr), a basal ganglia output nucleus that gates the release of actions (Hikosaka et al., 2006). On trials with successful action suppression, Stop information reaches SNr before this gate can be opened by Str.
However, several aspects of our data suggested that this simple race is an incomplete account of basal ganglia involvement in stopping. First, the STN-SNr response to the Stop! cue is fast enough to account for behavioral inhibition, but is also very brief. It is therefore unlikely to block the more prolonged wave of Str activity that accompanies action initiation. Second, successful action cancellation was also associated with a sudden reduction in this Str Go-related activity, suggesting that mechanisms within the Str also contribute to stopping. However, this Str change did not consistently precede the SSRT, so Str-based mechanisms alone appear too slow to account for the speed of stopping. Finally, the fast STN-SNr response is not selective to the Stop! cue, but also occurs for the Go! cue that prompts contralateral movements.
To account for these observations we tentatively proposed (Schmidt et al., 2013) that stopping involves (at least) two complementary aspects: a fast, non-selective motor pause (mediated via the STN-SNr pathway), that buys time to allow complete cancellation in Str via a second, slower mechanism. Such a “two-step” arrangement could enable stopping to be both fast and selective, a key advantage over a single stop process.
We hypothesized that this second mechanism might involve the recently-characterized Arkypallidal neurons (Mallet et al., 2012). This subpopulation of globus pallidus pars externa (GPe) cells makes massive GABAergic projections solely to Str, and is thus well-placed to suppress Str activity if required. Here we show that, consistent with this hypothesis, Arky cell activity is selectively enhanced by the Stop! cue, at just the right time to be responsible for Str suppression on correct Stop trials. We thus describe the first distinct behavioral function of Arkypallidal neurons, while providing new insights into fundamental mechanisms of adaptive behavioral control.
Results
Activity across the sleep-wake cycle distinguishes GPe Arky and Proto neurons
We first needed to establish a clear electrophysiological signature of Arky cells, to discriminate them from the larger Proto population. Based on prior studies in anaesthetized rats (Mallet et al., 2008, 2012; Abdi et al. 2015) we hypothesized that identified Arkys would reliably show reduced activity during natural slow-wave sleep (SWS) compared to awake states, while Protos would not. We could then use this signature to analyze neurons during performance of the Stop-signal task, by recording cells during both task performance and natural sleep.
We habituated 8 rats to head-fixation (Fig. 1A), then performed juxtacellular recording electrodes during natural sleep and wakefulness. A total of 135 neurons were recorded from the GPe (also called simply GP in rodents) during both sleep and wake states in head-fixed animals (average recording duration > 8min, Fig. 1B). Arkys specifically express preproenkephalin (PPE) and the transcription factor FoxP2, whereas Protos instead express the transcription factor Nkx2-1 (Abdi et al., 2015). We successfully labeled 6 Arkys (PPE+ and FoxP2+) and 15 Protos (n=6 PPE-/FoxP2-; n=9 Nkx2-1+/FoxP2-), and these subpopulations showed no particular spatial distribution within the GPe (Fig. 1C). The properties of each labeled cell are in Fig. S1.
Figure 1. Properties of identified Arky and Proto neurons.
(A) Schematic of bilateral electrocorticograms (ECoG) and craniotomies above GPe (B) Head-fixed recording durations for all neurons (n=135, in 8 rats). (C) Location of each labeled neuron, represented on sagittal atlas sections at the indicated medio-lateral levels. (D) An identified Proto neuron during the awake state (left) and natural slow-wave sleep (SWS). Vertical bars, 1mV (units) and 0.5 mV (ECoG); horizontal bars, 1s. Images at right show the cell body labeled with neurobiotin (Nb; red), and co-expressing Nkx2-1 (cyan), but not FoxP2 (gray). Insets show a FoxP2 positive/Nkx2-1 negative GPe neuron located in the same focal plane (positive control for FoxP2 labeling). Scale bars, 10 μm. (E) An identified Arky neuron, co-expressing PPE (green), and FoxP2 (grey). (F) Identified Protos (n=15) sho and SWS states. (G) Average firing rates for all identified neurons, and rate change during SWS. Box-and-whisker plots show lowest sample value, first quartile, median, third quartile, maximum value.
On average, identified Protos in awake rats fired regularly (Fig. 1D; mean coefficient of variation, CV = 0.58 ± 0.08, range: 0.30 – 1.57; Fig. 1F) at high rates (47.3 ± 6.1 Hz, mean ± SEM; range: 10.8 – 82.2 Hz; Fig. 1G). This is consistent with labeled Protos in awake mice (Dodson et al., 2015), and with the majority subpopulation in many GPe studies in awake monkeys and rats (DeLong, 1971; Raz et al., 2000; Gage et al., 2010; Benhamou et al., 2012). Identified Arkys were more irregularly active (Fig. 1E; CV 1.86 ± 0.29; range: 1.1 – 2.84) with lower awake firing rates (8.9 ± 1.9 Hz; range: 4.8 – 18.1 Hz). Each of these firing properties was significantly different between Protos and Arkys (p<0.001, Mann-Whitney tests). Nonetheless, some Protos had a relatively low firing rate, within the Arky range (Fig. 1G). A subset of identified Protos showed clear pauses in activity from a high baseline (not shown), consistent with prior descriptions of high-frequency discharging GPe neurons (DeLong, 1971, Elias 2007). However, as this property was not observed in all identified Protos, we did not use it for classification purposes.
Upon transition from wakefulness to SWS, all labeled Arkys showed a strong, significant decrease in their firing rate (Fig. 1G; −62.0 ± 7.0 %; range: −48 to −88 %; p<0.05, Mann-Whitney tests), even as their firing remained irregular (CV = 1.93 ± 0.16; range 1.42 – 2.55). By contrast Protos were not obviously affected by the state change. There was no consistent direction of Proto firing rate change (mean 6.0 ± 7.9 %), and no individual Protos showed a substantial decrease in firing (range, −22.7 to +109.0 %), or a change in firing regularity (CV = 0.59 ± 0.06; range 0.31 – 1.21). The measures of 1) SWS firing rate, 2) CV during SWS, and 3) awake to SWS rate change were all significantly different between the two cell classes (p<0.001, Mann-Whitney tests for firing rate and CV; t-test for rate change).
Classification of Arky and Proto cells in freely-moving rats
By combining these measures, labeled Arky and Proto subpopulations formed two clearly distinct clusters (see the 3-D plot in Fig. 2A), and these distinct clusters were also apparent for the unlabeled cells. We used the first principal component of the combined measures to classify unlabeled cells into putative Arky and Proto cells. For all electrophysiological measures, there were no significant group differences between labeled and putative cells within each subpopulation, but significant differences between putative Arky and putative Proto cells (p<0.001, Mann-Whitney tests).
Figure 2. Classification of Arky and Proto neurons predicts oscillatory entrainment.
(A) Properties of individual GPe neurons in head-restrained rats. CV was measured during SWS. Cells form two distinct clusters, as seen in the 3D-plot and the projection onto the first principal component (far right). (B) Same analysis for freely-moving GPe units also reveals 2 clusters. The bimodal principal component projection was used to classify neurons into putative Arky (light blue) and putative Proto (dark blue), with units near threshold left unclassified (grey). (C,D) Spike timing of putative Proto and Arky neurons during sleep spindle oscillations for the head-fixed (C) and freely-moving (D) datasets. Phase histograms on the left sides show mean spike phases, with solid bars indicating units with significant phase-locking. Arrows show population average phase for these entrained units. Empty bars show mean phases of the other units within a subpopulation. Dashed sine waves indicate phase of the idealized frontal ECoG rhythm, i.e. peaks are maximal positive voltage at brain surface. Right plots show entrainment strength as measured by the mean resultant length (arrows indicate overall population average). Arkys are more strongly entrained to natural sleep spindle oscillations than Protos, and show distinct phase preference.
We then applied this same classification scheme to GPe cells recorded in freely-moving rats (n=4) trained to perform a Stop-signal task. Recording sessions included task performance followed by a period of rest in an adjacent quiet, dim location. Of 398 GPe cells, 266 were both active during task performance and recorded during natural wake/sleep transitions. Electrophysiological measures for this data set appeared very similar or identical to those obtained in head-fixed animals (Fig. 2B). We therefore used the strongly bimodal principal component distribution to divide unrestrained rat GPe neurons into putative Protos (more regular spiking at higher rates, unaffected by SWS; n=149) and Arkys (less regular spiking at lower rates that became even lower during SWS; n=108).
To assess this distinction we examined single-unit participation in brain rhythms. In both head-fixed and freely-moving datasets, entrainment to sleep spindle oscillations was more likely, and stronger, for neurons classified as Arky than those classified as Proto (Fig. 2C, D; for freely-moving data: 49% Arky entrained vs. 12% Proto, p= 9.2x10−6, binomial test; Arky mean resultant length greater than Proto, p=8.0x10−14, two-sided Kolmogorov-Smirnov test). Furthermore, Arkys preferentially fired on the descending phase of the spindles, whereas Protos preferentially fired on the ascending phase (Fig. 2D). In awake rats both Arkys and Protos showed similar population-level entrainment to LFP beta oscillations, with few individual cells strongly entrained (Fig. S2). However, the Arkys were much more likely than Protos to be entrained to high-voltage spindles (Fig. S2), consistent with prior reports of distinctive entrainment in (unidentified) GPe subpopulations (Paz et al., 2005). Thus, putative Arkys and Protos clearly participate differently in large-scale network dynamics, confirming that our classification scheme indeed divides GPe cells into functionally-distinct groups.
Stop-related activity of Arky and Proto neurons
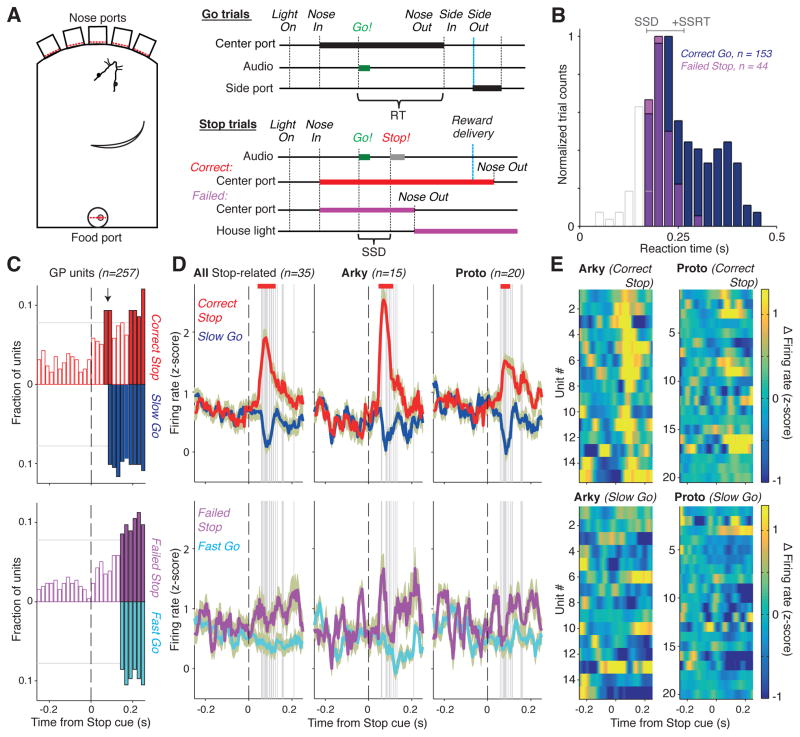
To assess the contributions of Arkys and Protos to action inhibition we examined their activity in the Stop task (Fig. 3A). Rats maintained their nose in a central port, then a brief tone instructed a rapid leftward or rightward movement. On 30% of trials this Go cue was swiftly followed by a Stop cue (white noise burst) signaling that they should cancel the imminent movement and instead maintain “fixation” in the central port. The delay between Go and Stop cue onset (stop-signal delay, SSD) was set such that rats were often successful at stopping, but failed often as well.
Figure 3. GPe Stop responses.
(A) Task setup. Reaction time (RT) is measured between Go cue onset and Nose Out. For Stop trials, the time between the onsets of Go and Stop cues is the Stop-signal delay (SSD). (B) RT distributions from a single session. White bars show trials with an RT faster than the SSD (so no Stop cue was presented). Grey line at the top indicates SSD (170ms; left end) and SSRT (95ms; line length) for this session, which also included 64 Correct Stop trials. (C) Fraction of units with significant trial-type-dependent firing at each moment (red, Correct Stop > Slow Go; blue, Slow Go > Correct Stop; magenta, Failed Stop > Fast Go; cyan, Fast Go > Failed Stop). Filled bars denote significant population differences (thin horizontal line shows significance threshold). A GPe subset had short-latency responses to the Stop signal (top, two filled red bars soon after the vertical dashed line); these cells are examined further in panels D,E. This short-latency GPe response was not seen on Failed Stop trials (bottom). (D) Activity time courses (± SEM) for Stop-responsive GPe neurons. Vertical grey lines indicate SSRT in the corresponding sessions. Bars mark significant differences in firing rate between the shown trial types (shuffle tests, corrected for multiple comparisons). Separating Stop-related neurons into Arkys and Protos (2nd and 3rd columns) reveals stronger Correct Stop responses for Arkys. (E) Individual unit activity showing Stop responses (top). Units are sorted by time of peak response (from 50–150ms after Stop cue). Bottom panels show the same units, in the same order, during Slow Go trials (i.e. without Stop cue; alignment made to SSD).
Reaction times (RTs) on Go trials were highly variable, showing the broad, skewed distribution that is typical for RTs. Failed Stop trial RTs were short, and resembled the early part of the Go trial distribution (Figs. 3B, S3). This observation is standard for Stop-signal tasks, and consistent with a race between independent Go and Stop processes (Logan et al., 1984). That is, the variable success in stopping may be accounted for by the variable relative timing of separate Go and Stop mechanisms, with Failed Stop trials being predominantly those for which movement preparation (Going) was quicker than usual, and so won the race. In this conceptualization, the Stop process begins at the Stop cue (when movement preparation may already be well advanced) and must be fast to successfully cancel actions on a sizable fraction of trials.
To screen for neurons that may participate in stopping we focused on firing rate changes shortly after Stop cue onset. We minimized the contribution of the variable timing of movement preparation, by comparing activity between trial subtypes for which the timing of movement preparation is similar (“latency-matching”). Specifically, Fast Go trials and Failed Stop trials have similar RT distributions, so neural differences between them are more likely to reflect Stop cue processing. Following the same logic, activity differences between Slow Go and Correct Stop trials soon after the Stop cue are more likely to be related to Stop cue processing, rather than the timing of movement preparation. Although behavioral (Boucher et al. 2007) and electrophysiological (Schmidt et al. 2013) data support the idea that Go and Stop processes initially evolve independently, this remains an assumption of the latency-matching analysis. Nonetheless, it is a useful approach to identifying candidate Stop-related neurons for more detailed examination.
Among all recorded GPe neurons (i.e. ignoring Proto versus Arky distinctions), a significant fraction showed preferential firing on Correct Stop trials, around 60–80ms after the Stop cue (Fig. 3C, top). This change was observed for Correct Stop, but not Failed Stop trials (Fig. 3C, bottom). Examination of the firing rate time course in this subset of cells (n=35) confirmed a clear reactive increase in spiking following the Stop signal (Fig. 3D). Within this subset of Stop-related GPe units, Arkys (n=15) had a significantly stronger response to the Stop signal compared to Protos (n=20; p=0.007 and p=0.0001, shuffle tests of the normalized response amplitudes in the response windows of 50–90 and 70–110ms, respectively). Protos found in our screen for Stop-related activity had a variety of responses, including both increases on Correct Stop trials and decreases for Slow Go trials (Figs. 3D, 3E). By contrast, Stop-related Arkys had a more consistent firing rate increase after the Stop cue (Figs. 3D, 3E).
Selectivity and timing of the Arky Stop response
We next assessed the specificity of this firing rate increase to Stop cue processing (Fig. 4A,B). We previously reported (Schmidt et al., 2013) that the very fast response of STN and SNr neurons was similar for Stop and contralateral Go cues. By contrast both Arkys and Protos responded significantly more to Stop rather than Go cues, whether assessed neuron-by-neuron (Fig. 4A), or by average responses (Fig. 4B). This suggests that GPe is involved specifically in stopping, rather than the global motor pause that accompanies abrupt salient events (Sharp et al., 2010; Wessel and Aron, 2013). It has been suggested that GPe helps stop specific movements (Aron, 2011), so we next assessed whether the Arky Stop response was significantly different when the preceding Go cue instructed contralateral versus ipsilateral movements. It was not (Fig. S4), possibly because we used the same Stop cue in both cases.
Figure 4. Multiple basal ganglia circuits for action suppression.
(A) Single-unit responses to Stop compared to Go cues. Selective responses to the Stop cue are seen only for GPe subpopulations (p-values, significance from shuffle tests). STN, SNr, Str data are from Schmidt et al., 2013). (B) Mean firing rate time courses for Stop-related units. Solid lines, normalized Stop responses; dotted lines Go responses; shading indicates ± SEM). Colored bars at the top indicate significant differences. (C) Comparison of Stop response timing between basal ganglia. (Left) Mean firing rates (z-scores, shifted to a common baseline) of units with short-latency Stop responses in STN, SNr and GPe. (Right) Cumulative distributions of single-unit Stop response latencies. (D) Relative timing of GPe and Str activity. On Correct Stop trials Str activity (red) shows an abrupt decrease after the Stop cue, compared to Slow Go trials (green). The prominent Arky Stop response occurs just before this decrease, consistent with a role for Arky neurons in suppressing Str movement-related activity (E,F) Two-step race model. (E) Left, variable Go process timing (green lines) leads to variable RTs. Salient sensory cues (Go or Stop) evoke a rapid Pause process (orange), that transiently elevates Go threshold (black dotted line). In Correct Stop trials (right) this buys additional time for the Stop process to finish before the Go process. (F) Left, Go process completion likely involves increased activity in the Str direct pathway to SNr. Middle, salient cues cause a rapid, less-selective and transient pause in movement initiation via PPN, STN and SNr. Right,Arky GABAergic projections to Str abolish movement preparation. Abbreviations: Ctx, cortex; Thal, thalamus; SC, PPN, pedunculopontine nucleus; superior colliculus.
GPe responses to the Stop cue were slower than STN and SNr responses (Fig. 4C), as expected given that these cells were identified from later bins in the latency-matching procedure (Fig. 3C). However, Stop-related Arkys showed significantly faster responses to the Stop cue than Stop-related Protos (two-sided Kolmogorov-Smirnov test on distributions of peak response time, p=0.04). Consistent with Arky-mediated action suppression, the Arky Stop response occurred just before the divergence of movement-related Str activity between Correct Stop and Slow Go trials (Fig. 4D). We conclude that the timing and selectivity of the Arky Stop response, and the specific, massive GABAergic projections of Arkys to Str, together provide strong evidence that Arkys have an important functional role in the cancellation of imminent actions.
Discussion
From the first recordings in behaving animals (DeLong, 1971) it has been noted that most GPe neurons fire near-continuously at a high rate while others are quieter and more bursty (Benhamou et al., 2012; Joshua et al., 2009; Paz et al., 2005). However, only recently has it become possible to characterize the developmental origins, chemical phenotypes and connectivity of distinct GPe subpopulations (Mallet et al., 2012; Mastro et al., 2014; Nobrega-Pereira et al., 2010; Abdi et al., 2015). Unlike other GPe subsets, Arkys derive from the lateral ganglionic eminence, selectively express preproenkephalin, FoxP2 and Meis2, and project solely to striatum. The present work builds on these GPe characterizations by examining Arky activity during a precisely-defined behavioral task. Prior recordings of (unidentified) GPe neurons found evidence for their involvement in suppressing movements that are currently inappropriate (Yoshida and Tanaka, 2015). By recording across the natural sleep/wake cycle to distinguish neuronal subpopulations (Berke, 2008), we showed here that the Arky subpopulation has just the right properties to serve as a specialized Stop process in the brain. Nonetheless, some Proto cells also had Stop responses, and it will be important to examine Arky and Proto activity under a range of conditions.
Race models of stop-signal performance provide a powerful, quantitative framework for interpreting neurophysiological data. Yet despite many single-unit studies, the neural basis of stopping has been hard to pin down. In non-human primates the best-understood potential Stop mechanism comes from saccade countermanding: successful cancellation is associated with increased activity of fixation-related neurons in superior colliculus and the frontal eye field shortly before saccades would have been initiated (Pare and Hanes, 2003). The steps preceding this change were hypothesized to involve the basal ganglia, and human imaging studies observed Stop-related activation of the cortical-STN (hyperdirect) pathway (Aron and Poldrack, 2006). We found that the STN-SNr pathway responds to a Stop cue very quickly, well in advance of SSRT (Schmidt et al., 2013). However, this STN-SNr response also occurs for Go cues, and also seems too transient to be solely responsible for Stopping. We therefore hypothesized that STN-SNr activation provides a very fast Pause signal (Frank, 2006), buying time for a distinct Cancel process acting within striatum (Fig. 4E). Additional evidence for a separate Pause mechanism comes from a task in which Go cues are sometimes followed by Stop cues, but sometimes by Continue cues; the Continue cues cause a slowing of reaction times without complete cancellation (Sharp et al., 2010).
Our new results support this two-step model, and demonstrate that Pause and Cancel involve distinct basal ganglia pathways (Fig. 4F). One interesting implication is that prior neurophysiological data may need to be reconsidered. The SSRT is an inferred measure of the stopping speed, and neural events occurring after the SSRT have been considered too slow to be involved in canceling actions. This has led to some hard-to-reconcile results. For example, microstimulation of the supplementary eye fields (SEF) improves Stop-signal performance (Stuphorn and Schall, 2006), but SEF neurons normally respond to Stop cues just after the SSRT (Stuphorn et al., 2000). In our two-step model, events occurring just after the SSRT can still play a causal role in canceling actions, as the SSRT is dominated by the speed of the initial Pause event. We therefore hypothesize that key frontal cortical regions provide essential information processing for the decision to stop, even if their neuronal responses seem slightly too slow when assuming a single, unified Stop process.
We do not know whether Arkys relay Stop decisions from elsewhere, or are actively involved in forming those decisions. This is in part because the input pathways to Arkys remain to be determined. The striatopallidal (indirect) pathway is often described as having a “NoGo” function, and has been implicated in action suppression (e.g. Freeze et al. 2013) and stopping (Aron, 2011; Jahfari et al., 2011; Wiecki and Frank, 2013). However, current theories of indirect pathway NoGo function involve restraining unrewarded actions (Collins & Frank 2014), rather than cancellation of imminent movements. Furthermore, we have not observed activity patterns in sensorimotor striatum that could drive the Stop-selective Arky response (though more “cognitive” striatal subregions may provide relevant signals; Eagle et al., 2011). We showed here that Arkys have a more specific Stop response than the most rapidly activated STN neurons, but more slowly activated STN neurons are potential contributors to Arky activity, as are neurons in the parafascicular nucleus (Kimura et al., 2004; Yasukawa et al., 2004). Finally, there are also direct cortical inputs to GPe (Naito and Kita, 1994; Milardi et al., 2015) to which no function has yet been ascribed, and this pathway might help tie together the cortex-focused literature on human stopping with our basal ganglia results.
Experimental Procedures
Please see Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by NIH (MH101697, NS078435), Agence Nationale de la Recherche (g14-CE13-0024-01), CNRS PEPS Idex Bordeaux (UB101 CR-2014R), and Deutsche Forschungsgemeinschaft BrainLinks-BrainTools Cluster of Excellence (EXC 1086). We thank P. Apicella for equipment loan, J. Baufreton and F. Georges for discussions, and J. Pettibone, K. Demarco and V. Hetrick for technical assistance. Microscopy was performed with the help of Stéphanie Morin in the Bordeaux Imaging Center of CNRS-INSERM and Bordeaux University, a component of France BioImaging.
Footnotes
The authors declare no competing financial interests.
Author Contributions: Conceptualization, N.M., R.S and J.D.B; Methodology, N.M. and J.D.B.; Formal Analysis and Software, R.S.; Investigation, N.M., D.L., F.C., and N.A.; Writing – Original Draft, N.M., R.S., and J.D.B.; Writing – Review and Editing, J.B.; Funding Acquisition, J.D.B., R.S., N.M, and T.B.; Resources and Supervision, J.D.B. and T.B.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J, Magill PJ. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J Neurosci. 2015;35:6667–6688. doi: 10.1523/JNEUROSCI.4662-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69:e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Benhamou L, Bronfeld M, Bar-Gad I, Cohen D. Globus Pallidus external segment neuron classification in freely moving rats: a comparison to primates. PLoS One. 2012;7:e45421. doi: 10.1371/journal.pone.0045421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD. Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J Neurosci. 2008;28:10075–10080. doi: 10.1523/JNEUROSCI.2192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev. 2007;114:376–397. doi: 10.1037/0033-295X.114.2.376. [DOI] [PubMed] [Google Scholar]
- Collins AGE, Frank MJ. Opponent Actor Learning (OpAL): Modeling Interactive Effects of Striatal Dopamine on Reinforcement Learning and Choice Incentive. Psychol Rev. 2014;121:337–366. doi: 10.1037/a0037015. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Larvin JT, Duffell JM, Garas FN, Doig NM, Kessaris N, Duguid IC, Bogacz R, Butt SJ, Magill PJ. Distinct Developmental Origins Manifest in the Specialized Encoding of Movement by Adult Neurons of the External Globus Pallidus. Neuron. 2015;86:501–513. doi: 10.1016/j.neuron.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Wong JCK, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. The Journal of Neuroscience. 2011;31:7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S, Joshua M, Goldberg JA, Heimer G, Arkadir D, Morris G, Bergman H. Statistical Properties of Pauses of the High-Frequency Discharge Neurons in the External Segment of the Globus Pallidus. J Neurosci. 2007;27:2525–2538. doi: 10.1523/JNEUROSCI.4156-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projections neurons. J Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage GJ, Stoetzner CR, Wiltschko AB, Berke JD. Selective activation of striatal fast-spiking interneurons during choice execution. Neuron. 2010;67:466–479. doi: 10.1016/j.neuron.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31:6891–6899. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Adler A, Rosin B, Vaadia E, Bergman H. Encoding of probabilistic rewarding and aversive events by pallidal and nigral neurons. J Neurophysiol. 2009;101:758–772. doi: 10.1152/jn.90764.2008. [DOI] [PubMed] [Google Scholar]
- Kimura M, Minamimoto T, Matsumoto N, Hori Y. Monitoring and switching of cortico-basal ganglia loop functions by the thalamo-striatal system. Neurosci Res. 2004;48:355–360. doi: 10.1016/j.neures.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Leventhal DK, Gage GJ, Schmidt R, Pettibone JR, Case AC, Berke JD. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73:523–536. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipszyc J, Schachar R. Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. J Int Neuropsych Soc. 2010;16:1064–1076. doi: 10.1017/S1355617710000895. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Mallet N, Micklem BR, Henny P, Brown MT, Williams C, Bolam JP, Nakamura KC, Magill PJ. Dichotomous organization of the external globus pallidus. Neuron. 2012;74:1075–1086. doi: 10.1016/j.neuron.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Marton LF, Bolam JP, Brown P, Magill PJ. Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity. J Neurosci. 2008;28:14245–14258. doi: 10.1523/JNEUROSCI.4199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastro KJ, Bouchard RS, Holt HA, Gittis AH. Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways. J Neurosci. 2014;34:2087–2099. doi: 10.1523/JNEUROSCI.4646-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milardi D, Gaeta M, Marino S, Arrigo A, Vaccarino G, Mormina E, Rizzo G, Milazzo C, Finocchio G, Baglieri A, Anastasi G, Quartarone A. Basal ganglia network by constrained spherical deconvolution: A possible cortico-pallidal pathway? Mov Disord. 2015;30:342–349. doi: 10.1002/mds.25995. [DOI] [PubMed] [Google Scholar]
- Naito A, Kita H. The cortico-pallidal projection in the rat: an anterograde tracing study with biotinylated dextran amine. Brain Res. 1994;653:251–257. doi: 10.1016/0006-8993(94)90397-2. [DOI] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Gelman D, Bartolini G, Pla R, Pierani A, Marin O. Origin and molecular specification of globus pallidus neurons. J Neurosci. 2010;30:2824–2834. doi: 10.1523/JNEUROSCI.4023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare M, Hanes DP. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J Neurosci. 2003;23:6480–6489. doi: 10.1523/JNEUROSCI.23-16-06480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Deniau JM, Charpier S. Rhythmic bursting in the cortico-subthalamo-pallidal network during spontaneous genetically determined spike and wave discharges. J Neurosci. 2005;25:2092–2101. doi: 10.1523/JNEUROSCI.4689-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–871. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16:1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- Stuphorn V, Taylor TL, Schall JD. Performance monitoring by the supplementary eye field. Nature. 2000;408:857–860. doi: 10.1038/35048576. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Aron AR. Unexpected Events Induce Motor Slowing via a Brain Mechanism for Action-Stopping with Global Suppressive Effects. J Neurosci. 2013;33:18481–18491. doi: 10.1523/JNEUROSCI.3456-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120:329–355. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- Yasukawa T, Kita T, Xue Y, Kita H. Rat intralaminar thalamic nuclei projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study. J Comp Neurol. 2004;471:153–167. doi: 10.1002/cne.20029. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Tanaka M. Two Types of Neurons in the Primate Globus Pallidus External Segment Play Distinct Roles in Antisaccade Generation. Cereb Cortex. 2015 doi: 10.1093/cercor/bhu308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.