Abstract
The first, highly anticipated randomized trial of adjuvant antiangiogenic therapy in renal cancer was recently reported. Although far from assuring, data from the adjuvant sorafenib or sunitinib for unfavorable renal carcinoma (ASSURE) trial offer a wealth of insights into the disease, treatments, and biological considerations for studies aimed at risk reduction.
In 2004, the FDA approved sorafenib, a vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor, for advanced renal cell carcinoma (RCC) based on 5.5 months improvement in progression free survival (PFS) compared to those treated with placebo. Soon after, sunitinib was also approved for advanced RCC based on eliciting an improvement in PFS1. This success heralded a revolution in the treatment of RCC, and coupled with the strong biological rationale of VEGF pathway dysregulation associated with von Hippel–Lindau tumor suppressor mutation in the clear cell renal carcinoma subtype, adjuvant studies were vigorously pursued. In the ASSURE study2, 1,943 patients with completely resected RCC were stratified by the UCLA international staging system and assigned 1:1:1 to sorafenib, sunitinib, or placebo for 54 weeks. The study was reported early on the advice of the Data, Safety, and Monitoring Committee, when the interim evaluation revealed low conditional power for the primary endpoint to be met2. No significant difference in disease-free survival (DFS) for either sorafenib (median, 6.1 versus 6.6 years, hazard ratio (HR) 0.97, 97.5% CI 0.80–1.17) or sunitinib (median, 5.8 years versus 6.6 years, HR 1.02, 97.5% CI 0.85–1.23) was observed when compared to placebo. In addition to the important but disappointing message to the RCC community that adjuvant therapy for risk reduction remains confined to clinical trials, this study revealed valuable information regarding three aspects of adjuvant therapy: agent specific toxicities and acceptable toxicity burden in the adjuvant setting, insights into the biological processes that govern micrometastasis, and the need for accurate risk assessment, which as a result, might directly impact patient care.
First, it is important to note that the adverse effects of these antiangiogenic agents are not trivial. In this first, randomized comparison of sunitinib and sorafenib, the expected differences in toxicity profile between the two agents were observed — notably the higher prevalence rash and hand–foot syndrome with sorafenib, and fatigue with sunitinib. Strikingly, this study revealed the difference in what toxicities will be tolerated when a patient is combating metastatic disease, where these adverse effects are considered quite manageable, in contrast with the setting where treatment intent is risk reduction. The result was a mid-study dose adjustment, where the starting dose of both drugs was lowered and the overall number of patients was expanded in order to account for a very high level of discontinuation in both treatment arms. These observations are key to consider when designing future adjuvant therapy studies in RCC.
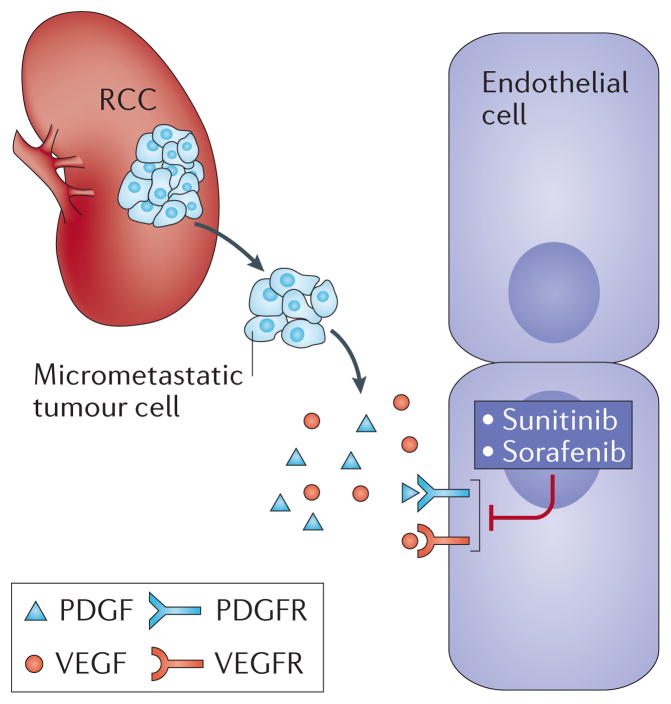
Second, targeting the VEGF pathway in the adjuvant setting for micro-metastatic disease is a treacherous enterprise, owing to the target molecule being located on the endothelium, and a long literature starting with Judah Folkman supporting the angiogenic switch being critical to transitions from dormancy to angiogenic growth3. One might reasonably assume that clear cell RCC falls outside this model, having inherently been angiogenically “switched on”. However, VEGF-targeted therapy has failed to achieve expectations in other tumor types in previous trials. Colon cancer adjuvant studies using chemotherapy plus bevacizumab — a recombinant humanized monoclonal antibody against VEGF-A — were also disappointing. In the National Surgical Adjuvant Breast and Bowel Project C-08 phase III trial that included 2,672 patients with stage II or III disease, bevacizumab was administered with FOLFOX (a chemotherapy regimen for treatment of colorectal cancer, composed of folinic acid, fluorouracil, and oxaliplatin) for 6 months followed by 6 months of monotherapy, compared to 6 months of FOLFOX alone4. Like the ASSURE trial, no difference in DFS or overall survival was observed and the treatment came at the cost of high toxicity4. Adjuvant chemotherapy plus bevacizumab also failed to demonstrate a benefit in invasive DFS in triple negative breast cancer5, and in overall survival for both non-small cell lung cancer6 and melanoma7. Randomized trials testing other VEGF receptor inhibitors, such as pazopanib (NCT01235962) and axitinib (NCT01599754) in RCC as adjuvant therapy are ongoing. To date, anti-angiogenesis therapies, despite being effective for metastatic disease, have yielded no successes in the adjuvant setting. One of the theories behind their failure is that anti-angiogenesis is a cytostatic process rather than cytotoxic and thereby allows for micro-metastatic adaptation and ultimately, evasion. In some sense this finding lends further support to Folkman’s model, which might predict in the adjuvant setting that antiangiogenic therapy is unlikely to eradicate micrometastases, as these cells reside in a state that may not require the support of tumor angiogenesis (Figure 1).
Figure 1.
Renal cell carcinoma (RCC) and Angiogenesis. The factors supporting micrometastatic disease, and early progression may be independent of the angiogenesis that high tumor burden metastatic disease requires for support. VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; VEGFR, vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor.
Finally, the enrollment for this study included patients with stage T1b disease (tumor size >4 cm but <7 cm, and confined to the kidney), and grade 3 or 4 histology, as well patients with higher stage. All histological types of renal cell carcinomas were included. The stratification on clinical risk features, and other parameters, failed to reveal any specific quality that identified patients who might benefit from the intervention, or who might be best served by avoiding the treatment. What seem to be clearly needed are more substantive and meaningful strategies for biological classification of tumors. Ideally, this would involve identifying robust predictive markers. To date, the search for a predictive feature indicative of a response to antiangiogenic therapy has been elusive in the metastatic disease setting, where these drugs are used commonly. However, using biological signatures to stratify patients into clear risk groups can also allow adjuvant therapies to be applied more strategically. For example, in estrogen-receptor positive breast cancer, the 21-gene assay based on rapid-polymerase chain reaction OncotypeDx® (Genomic Health, USA), is used to predict recurrence risk and select which patients should receive adjuvant endocrine or chemotherapy regardless of lymph node positivity8. In renal cell carcinoma, several expression tools have been integrated with clinical features and demonstrated to assign risk more accurately than clinical algorithms alone. Our group demonstrated a 34-gene subtype predictor (known as ClearCode34), to classify clear cell RCC patients into low risk or (ccA) or high risk (ccB) for disease recurrence9. Several other expression-based scoring algorithms have also been developed that accurately predict risk of recurrence in RCC10. Tissue collection was a central feature of the ASSURE study, and exploration of one or more of the established risk assessment classifying tools should be examined to determine if a subgroup can be defined that benefitted from the intervention.
In summary, negative trials such as ASSURE are equally important as positive trials, and provide us with valuable lessons to take forward into future studies. Data currently do not support a role for anti-angiogenesis therapy as adjuvant therapy for unfavorable kidney cancer, and in a majority of cases this treatment strategy leads to unwanted toxicity. The micro-metastatic niche in RCC and the role that angiogenesis has in promoting metastasis formation is peculiar and cannot be extrapolated from the macro-metastatic environment. This segment of the pathophysiology of RCC requires careful exploration. Recurrence score models based on molecular characteristics combined with clinicopathologic markers might better refine high risk RCC individuals.
Acknowledgments
WKR receives support from the NIH, K24CA172355.
Biographies
Dr. David D. Chism is an Assistant Professor of Medicine within the Division of Hematology Oncology at Vanderbilt University Medical Center. As a clinical researcher, he focuses on the development of novel therapeutics for patients with genitourinary malignancies.
Dr. W. Kimryn Rathmell is the Division Chief of Hematology Oncology at Vanderbilt University Medical Center. She is a physician-scientist with an expertise in genitourinary oncology, genetics and molecular biology. Her renal cell carcinoma research has used genetic techniques to study tumor initiating events and events that promote the development of invasive or metastatic features using in vitro, animal, and human systems.
Footnotes
Competing interests
The authors declare no conflicts of interest.
References
- 1.Coppin C, Kollmannsberger C, Le L, et al. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomized trials. BJU International. 2011;108(10):1556–1563. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 2.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. The Lancet. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5(16):1779–87. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 4.Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II–III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol. 2013;31:359–64. doi: 10.1200/JCO.2012.44.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative beast cancer (BEATRICE): primary results of a randomized, phase 3 trial. Lancet Oncol. 2013;14(10):933–42. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 6.Wakelee HA, Dahlberg SE, Keller SM, et al. Randomized phase III trial of adjuvant chemotherapy with or without bevacizumab in resected non-small cell lung cancer (NSCLC): Results of E1505. Presented at: 16th World Conference on Lung Cancer; September 6–9; Denver, CO. Abstract 1608. [Google Scholar]
- 7.Corrie PG, Marshall A, Dunn JA, et al. Adjuvant bevacizumab in patients with melanoma at high risk of recurrence (AVAST-M): preplanned interim results form a multicenter, open-label, randomized controlled phase study. Lancet Oncol. 2014;15(6):620–630. doi: 10.1016/S1470-2045(14)70110-X. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, estrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks SA, Brannon AR, Parker JS, et al. ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol. 2014;66(1):77–84. doi: 10.1016/j.eururo.2014.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rini B, Goddard A, Knezevic D, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: Development and validation studies. Lancet Oncol. 2015;16:676–685. doi: 10.1016/S1470-2045(15)70167-1. [DOI] [PubMed] [Google Scholar]