SUMMARY
The promiscuous expression of tissue-restricted antigens in the thymus, driven in part by Autoimmune Regulator (Aire), is critical for the protection of peripheral tissues from autoimmune attack. Aire-dependent processes are thought to promote both clonal deletion and the development of Foxp3+ regulatory T (Treg) cells, suggesting that autoimmunity associated with Aire deficiency results from two failed tolerance mechanisms. Here, examination of autoimmune lesions in Aire−/− mice revealed an unexpected third possibility. We found that the predominant conventional T cell clonotypes infiltrating target lesions express antigen receptors that were preferentially expressed by Foxp3+ Treg cells in Aire+/+ mice. Thus, Aire enforces immune tolerance by ensuring that distinct autoreactive T cell specificities differentiate into the Treg cell lineage; dysregulation of this process results in the diversion of Treg cell-biased clonotypes into pathogenic conventional T cells.
INTRODUCTION
The immune system has evolved to protect the host from pathogens, while limiting collateral damage to host tissues. This latter process, termed immune tolerance, is enforced by multiple mechanisms. “Recessive” tolerance is imparted by the deletion or functional inactivation of lymphocytes exhibiting excessive reactivity to self antigens, whereas “dominant” tolerance is enforced by suppressor cells, such as Foxp3+ regulatory T (Treg) cells, which act in trans to suppress autoreactive lymphocytes. Breakdown of immune tolerance is associated with numerous autoimmune diseases, such as diabetes, lupus, and rheumatoid arthritis. Furthermore, immune tolerance promotes the acceptance of allogeneic transplants, and limits the efficacy of anti-tumor immune therapies. Therefore, there is great interest in defining the fundamental mechanisms imparting dominant and recessive immune tolerance, in the hopes that these processes can be manipulated for clinical benefit.
The induction of tolerance to peripheral organs in the thymus requires presentation of tissue-restricted antigens (TRAs) to developing thymocytes. Proposed mechanisms include the cellular transport of TRAs to the thymus, or the promiscuous expression of TRAs in situ by medullary thymic epithelial cells (mTECs) (Klein et al., 2009). Autoimmune regulator (Aire) is a transcription factor expressed by mTECs that promotes the ectopic expression of TRAs (Anderson et al., 2002; Derbinski et al., 2005; Sansom et al., 2014), the induction of genes involved in antigen processing and presentation (Anderson et al., 2005), and the production of chemokines that impact the density of dendritic cells in the medulla (Lei et al., 2011). Loss-of-function mutations in AIRE are associated with the human autoimmune syndrome APECED (also called APS-1), which is characterized by mucocutaneous candidiasis, autoimmune destruction of the parathyroid and adrenal glands, and hypogonadism (Aaltonen, 1997; Nagamine et al., 1997). In the mouse, loss-of-function Aire mutations result in multi-organ autoimmunity (Anderson et al., 2002; Hubert et al., 2009; Kuroda et al., 2005; Ramsey et al., 2002), the severity of which varies depending on genetic background (Jiang et al., 2005). Conceptually, Aire may prevent autoimmunity by promoting both recessive and dominant mechanisms of tolerance, driving the deletion of thymocytes reactive to promiscuously expressed TRAs, or by inducing the differentiation of such thymocytes into the Treg cell lineage (Malchow et al., 2013; Perry et al., 2014). In this study, we aimed to determine the functional contributions of these processes to the protection of peripheral organs from autoimmune attack.
A long-standing paradigm suggests that Aire enforces immune tolerance by driving the clonal deletion of autoreactive T cells (Mathis and Benoist, 2009; Metzger and Anderson, 2011). This paradigm is based in large part on data demonstrating that Aire is required for the thymic deletion of T cell receptor (TCR) transgenic T cells reactive to a model antigen expressed promiscuously under the dictates of the rat insulin promoter (Anderson et al., 2005; DeVoss et al., 2006; Liston et al., 2003; Taniguchi et al., 2012). More recently, a requirement for Aire has also been observed for the thymic deletion of TCR transgenic T cells reactive to a natural self antigen (Zhu et al., 2013). Beyond evidence from TCR transgenic systems, little is known about the impact of Aire on the clonal deletion of endogenous polyclonal T cell specificities. In this regard, Taniguchi et al. have demonstrated that the thymic frequency of endogenous CD4+ T cells specific for a peptide derived from the retinal antigen interphotoreceptor retinoid binding protein (IRBP) increases ~2-fold in Aire−/− mice (Taniguchi et al., 2012). However, the finding that measurable frequencies of IRBP-specific T cells are detected in the thymus and periphery of Aire+/+ mice (Taniguchi et al., 2012) indicates that the clonal deletion of IRBP-specific T cells is at best incomplete in a wild-type setting. Thus, the role of Aire in promoting the clonal deletion of T cells reactive to endogenous self antigens and the functional implications of this process for the prevention of autoimmunity remain unclear.
Several lines of evidence support the hypothesis that Aire enforces immune tolerance by promoting the thymic development of Treg cells. First, Treg cells isolated from APS-1 patients exhibit defects in suppressive capacity in vitro and diminished FOXP3 protein expression (Kekalainen et al., 2007; Laakso et al., 2010), demonstrating that loss-of-function AIRE mutations impact Treg cells in human subjects. Second, the ectopic expression of a model antigen by Aire-expressing cells can promote the thymic development of antigen-specific TCR transgenic Treg cells (Aschenbrenner et al., 2007). Third, Aire deficiency in mice is associated with a modest reduction in the number of thymic Foxp3+ cells (Anderson et al., 2005) and the density of these cells in the thymic medulla (Lei et al., 2011). Fourth, studies in mice provided direct evidence that Aire is critical for the thymic development of multiple naturally occurring Treg cell specificities (Malchow et al., 2013), and that Aire impacts the thymic repertoire of polyclonal Treg cells (Perry et al., 2014). Finally, recent work demonstrates that the transfer of perinatally-tagged Treg cells into NOD.Aire−/− neonates is sufficient to prevent the autoimmune manifestations of Aire deficiency, but only if donor Treg cells are obtained from Aire+/+ mice (Yang et al., 2015).
Thus, the mechanisms by which Aire enforces immune tolerance remain debated. Here, we directly demonstrated that CD4+ conventional T (Tconv) cells infiltrating the prostates of Aire−/− mice were dominated by TCR clonotypes that were preferentially expressed by Treg cells in an Aire+/+ (but not Aire−/−) setting. These findings suggest that a major mechanism by which Aire enforces immune tolerance is to direct distinct autoreactive T cell specificities into the Foxp3+ Treg cell lineage. When this process is dysregulated, Treg cell-biased clonotypes are mis-directed into the Tconv cell compartment and become pathogenic.
RESULTS
Treg Cells Are Abundant in the Prostatic Autoimmune Lesions of Aire-Deficient Mice
To enable an in-depth analysis of the mechanisms by which Aire functions to promote immune tolerance, we focused our studies on T cell auto-infiltration directed at the prostate, a target of autoimmunity in Aire−/− mice on a C57BL/6 (B6) background (Hou et al., 2009; Jiang et al., 2005; Setiady et al., 2006). B6.Aire−/− mice exhibit reduced fertility (Hubert et al., 2009; Kekalainen et al., 2015), suggesting that prostatic T cell infiltration in these mice impacts reproductive fitness. Moreover, loss-of-function mutations in AIRE in human subjects with APECED are also associated with infertility (Husebye et al., 2009), suggesting that AIRE is critical for maintaining reproductive fitness in humans.
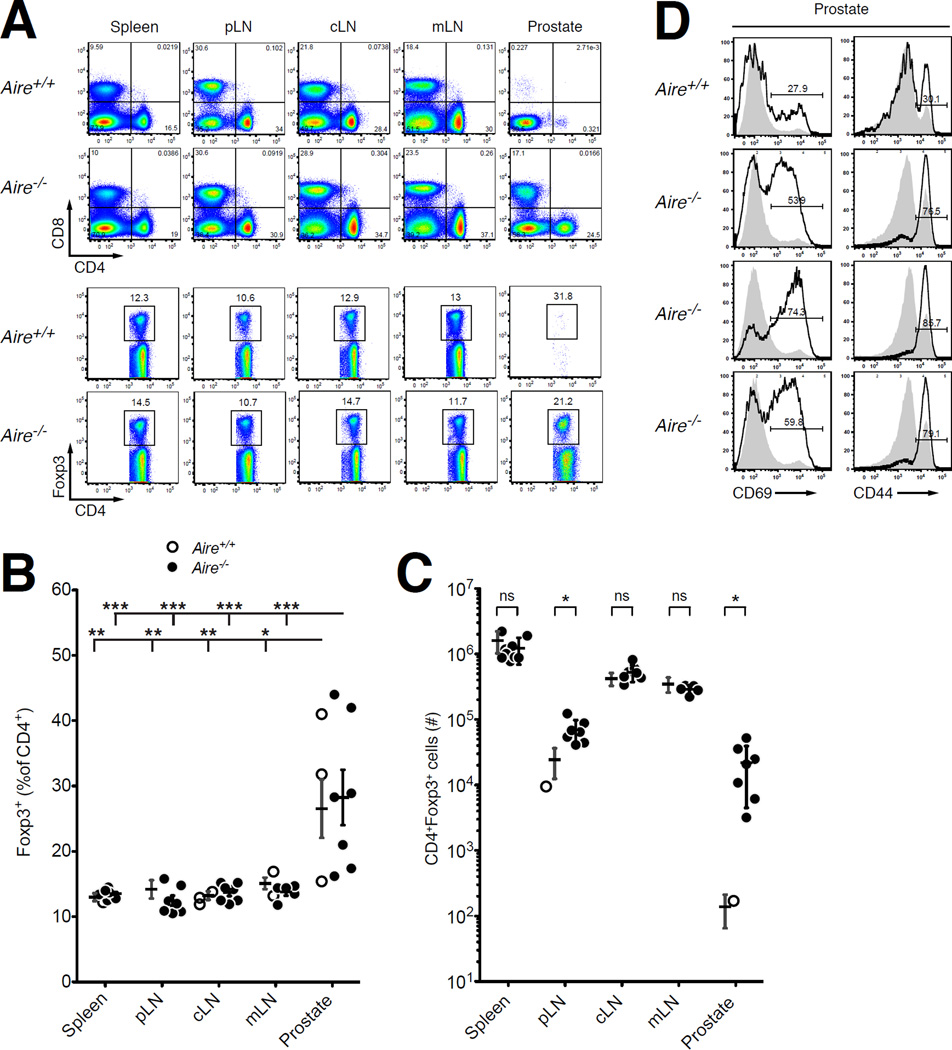
We first characterized the prostatic T cell infiltrate of 9-week-old male Aire−/− mice. Consistent with previous reports (Hou et al., 2009; Jiang et al., 2005; Setiady et al., 2006), we observed substantial infiltration of CD8+ and CD4+ T cells (Fig. 1A), including a prominent population of Foxp3+ Treg cells present at elevated percentages relative to the secondary lymphoid organs (Fig. 1B and C). A large fraction of CD4+ Tconv cells exhibited CD69 expression (Fig. 1D), indicative of recent TCR signaling within the prostate. These findings indicate that prostatic autoimmunity in Aire−/− mice is not associated with a deficit in Treg cell frequency. Importantly, these data also illustrate that the Treg cells present in Aire−/− mice are not able to prevent autoimmune prostatitis induced by the Tconv cells present in these mice. At least two possibilities can be invoked to explain this finding. First, Aire−/− mice may lack Aire-dependent Treg cell specificities required to prevent prostate-specific autoimmunity, enabling prostate-reactive Tconv cells in the repertoire to infiltrate the prostate. Alternatively, Aire−/− mice may contain new specificities of prostate-reactive Tconv cells that escape clonal deletion in the absence of Aire, specificities that cannot be suppressed by Treg cells present in these mice. In order to assess these possibilities, we performed a series of T cell transfer studies and TCR sequencing analyses to determine the impact of Aire on the functional potential and TCR repertoire diversity of the Treg and Tconv cell compartments in Aire+/+ and Aire−/− mice. Our studies focused exclusively on the CD4+ T cell compartment, for two reasons. First, recent studies demonstrate that Aire is critical for the development of some naturally occurring CD4+ Treg cell specificities (Malchow et al., 2013; Perry et al., 2014). Second, it has been demonstrated that autoimmunity in B6.Aire−/− mice is CD4+ T cell-dependent, with no requirement for CD8+ T cells (DeVoss et al., 2006; Devoss et al., 2008).
Figure 1. Treg Cells Are Present at Elevated Frequency in the Prostate of Aire−/− Mice.
(A) Representative flow cytometric analyses of 9-week-old Aire+/+ and Aire−/− males on the B6 background. Top, CD8 vs. CD4 expression by T cells isolated from the spleen, lymph nodes, and prostate. Bottom, for the CD4+CD8αneg cells in the top panels, plots of Foxp3 vs. CD4 expression. The percentage of cells falling within the denoted gates is indicated. pLN, periaortic lymph nodes; cLN, cervical lymph nodes; mLN, mesenteric lymph nodes.
(B and C) Summary plots of (B) the percentage of Foxp3+ cells among CD4+ T cells and (C) the total number of CD4+Foxp3+ T cells isolated from the indicated sites of Aire+/+ (open circles) or Aire−/− (closed circles) males depicted in panel A. N = 5 for Aire+/+ samples, N = 5–8 for Aire−/− samples. Data are pooled from N = 2 experiments. The mean +/− SEM is indicated. Asterisks denote P < 0.05. For (B), ANOVA and Tukey’s multiple comparison test were used to compare the different organ sites within Aire+/+ mice and the different organ sites within Aire−/− mice. For (C), t-tests were used to compare Aire+/+ vs. Aire−/− mice at each organ site.
(D) Representative flow cytometric analyses of CD69 and CD44 expression by CD4+ Tconv cells from >9- week-old Aire+/+ and Aire−/− males (indicated). Bold histograms denote CD4+ Tconv cells from the prostate, filled gray histograms denote CD4+ Tconv cells from the spleen. The percentage of cells expressing the indicated markers is shown.
Aire Impacts the Treg Cell TCR Repertoire in the Secondary Lymphoid Organs
To determine the impact of Aire-dependent processes on the selection of the CD4+ T cell repertoire, we analyzed the TCRs expressed by T cell populations isolated from different anatomical sites of 9-week-old male Aire+/+ or Aire−/− mice. We employed a well-established strategy in which mice express a fixed transgenic TCRβ chain (TCRβTg) (Hsieh et al., 2004; Malchow et al., 2013; Sant'Angelo et al., 1998) while maintaining a diverse repertoire of TCRα chains, thus enabling a survey of TCRαβ specificities via sequence analysis of TCRα transcripts (Malchow et al., 2013). All mice harbored a Foxp3GFP reporter allele (Haribhai et al., 2007), permitting isolation of viable Treg and Tconv cells for subsequent RNA isolation and sequence analysis. For repertoire analysis, we isolated T cells from the pooled spleen and lymph nodes and performed deep sequencing of the complete TCRα repertoire, regardless of variable-region usage, hereafter referred to as “complete TCRα sequencing” (Table S1). This approach yielded approximately 7.7×105 TCR sequence reads per sample (with an average complexity of 2.7×104 TCRs), providing a broad survey of the impact of Aire on the Treg and Tconv cell repertoires. In addition to providing insight regarding repertoire diversity, the complete TCRα sequencing data served as “catalogs” of the TCRs expressed by Treg and Tconv cells isolated from the pooled spleen and lymph nodes of Aire+/+ or Aire−/− mice. In some analyses, these catalogs served as a reference to determine whether particular T cell clonotypes were biased to the Treg cell lineage or the Tconv cell subset, and to determine the extent to which lineage bias was Aire-dependent.
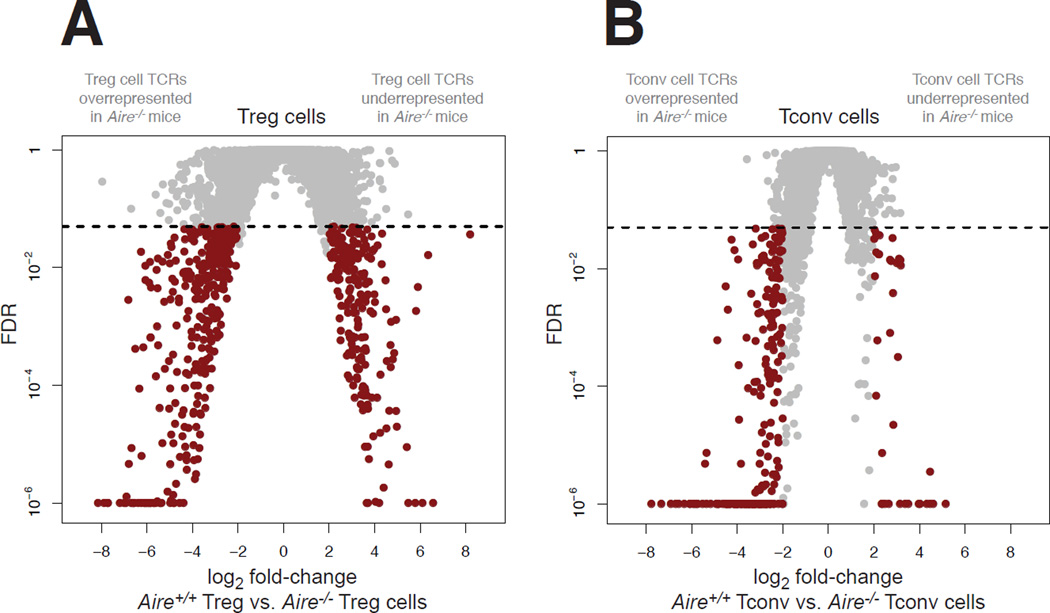
To determine the impact of Aire on the peripheral Treg cell repertoire, we purified Treg cells from the pooled spleen and lymph nodes of Aire+/+ and Aire−/− mice, and performed complete TCRα sequencing (Fig. 2A). Comparative analysis of TCR representation revealed that several of the most prevalent Treg cell TCRs expressed recurrently in the Aire+/+ catalog were absent or significantly reduced in frequency in Aire−/− mice (Fig. 2A, right arm of volcano plots), suggestive of Aire dependency of these Treg cell clonotypes. Quantification revealed that 215 of 7,955 recurrent Treg cell TCRs (2.7%) exhibited significantly reduced frequency in the Treg cell catalog of Aire−/− mice (Fig. 2A, right arm of volcano plot). Cumulatively, these TCRs comprised 5.4% (+/− 0.7% SD) of the peripheral Treg cell repertoire in Aire+/+ mice, providing a minimum estimate of the contribution of Aire-dependent Treg cells to the Treg cell repertoire in the secondary lymphoid organs. In addition, the analysis identified recurrent Treg cell TCRs that were significantly overrepresented in Aire−/− mice relative to Aire+/+ mice (Fig. 2A, left arm of volcano plots), suggestive of compensatory development, expansion, or escape from deletion in the absence of Aire. In this regard, 357 of 7,955 recurrent Treg cell TCRs (4.5%) exhibited significant overrepresentation in the Aire−/− catalog relative to the Aire+/+ catalog (Fig. 2A, left arm of volcano plot). Consistent with these comparative surveys, analysis using the Morisita-Horn similarity index (Magurran, 1988) revealed that the Treg cell repertoires of Aire−/− mice exhibited decreased similarity to the Treg cell repertoires of Aire+/+ mice (fig. S1). Taken together, these data demonstrate that Aire deficiency induces measurable perturbations in the Treg cell TCR repertoire in the spleen and lymph nodes.
Figure 2. Aire Impacts the CD4+ T Cell Repertoire in the Secondary Lymphoid Organs.
CD4+Foxp3+ Treg cells and CD4+Foxp3neg Tconv cells were purified by FACS from 9-week-old TCRβTg Foxp3GFP males on an Aire+/+ and Aire−/− background, and subjected to complete TCRα sequencing.
(A and B) The impact of Aire on the Treg cell repertoire (A) and Tconv cell repertoire (B) was analyzed via complete TCRα sequencing of T cell populations isolated from the pooled spleen and lymph nodes using the iRepertoire platform (see Experimental Procedures). For the TCRα chain sequences, volcano plots of false discovery rate (FDR) versus differential TCR representation (log2 fold-change) in Aire+/+ mice vs. Aire−/− mice are plotted for Treg cells (A) and Tconv cells (B). 7,955 TCRs are plotted in A, and 6,422 TCRs are plotted in B. Comparisons were made using EdgeR and were adjusted for multiple comparisons (see Experimental Procedures). Red dots denote TCRs with fold-change > 4 and FDR < 0.05. The arms of the volcano plots denoting TCRs that are overrepresented or underrepresented in Aire−/− mice are indicated in gray above each plot. Data points with FDR < 10−6 are plotted at 10−6. Horizontal dashed lines indicate FDR cutoff. N = 4 for Aire+/+ Treg cell samples, N = 5 for Aire−/− Treg cell samples, N = 4 for Aire+/+ Tconv cell samples, N = 5 for Aire−/− Tconv cell samples. See also Figure S1 and Table S1.
Next, we evaluated the impact of Aire on the peripheral Tconv cell repertoire. Complete TCRα sequencing of Tconv cells isolated from the pooled spleen and lymph nodes of Aire+/+ and Aire−/− mice revealed numerous TCRs (246 of 6,422 recurrent Tconv cell TCRs, 3.8%) that were significantly overrepresented in the Tconv cell catalog of Aire−/− mice relative to the Tconv cell catalog of Aire+/+ mice (Fig. 2B, left arm of volcano plot). On average, these Tconv cell clonotypes accounted for 4.0% (+/− 0.3% SD) of the Tconv cell repertoire in the pooled spleen and lymph nodes of Aire−/− mice. Congruent with this comparative analysis, the Tconv cell repertoires of Aire−/− mice exhibited reduced similarity to the Tconv cell repertoires of Aire+/+ mice, as assessed by the Morisita-Horn similarity index (fig. S1). These findings demonstrate that Aire deficiency is associated with the emergence of Tconv cell clonotypes that are typically not expressed by Tconv cells in an Aire+/+ setting.
The Autoimmune Defect in Aire−/− Mice is Intrinsic to the Tconv Cell Compartment
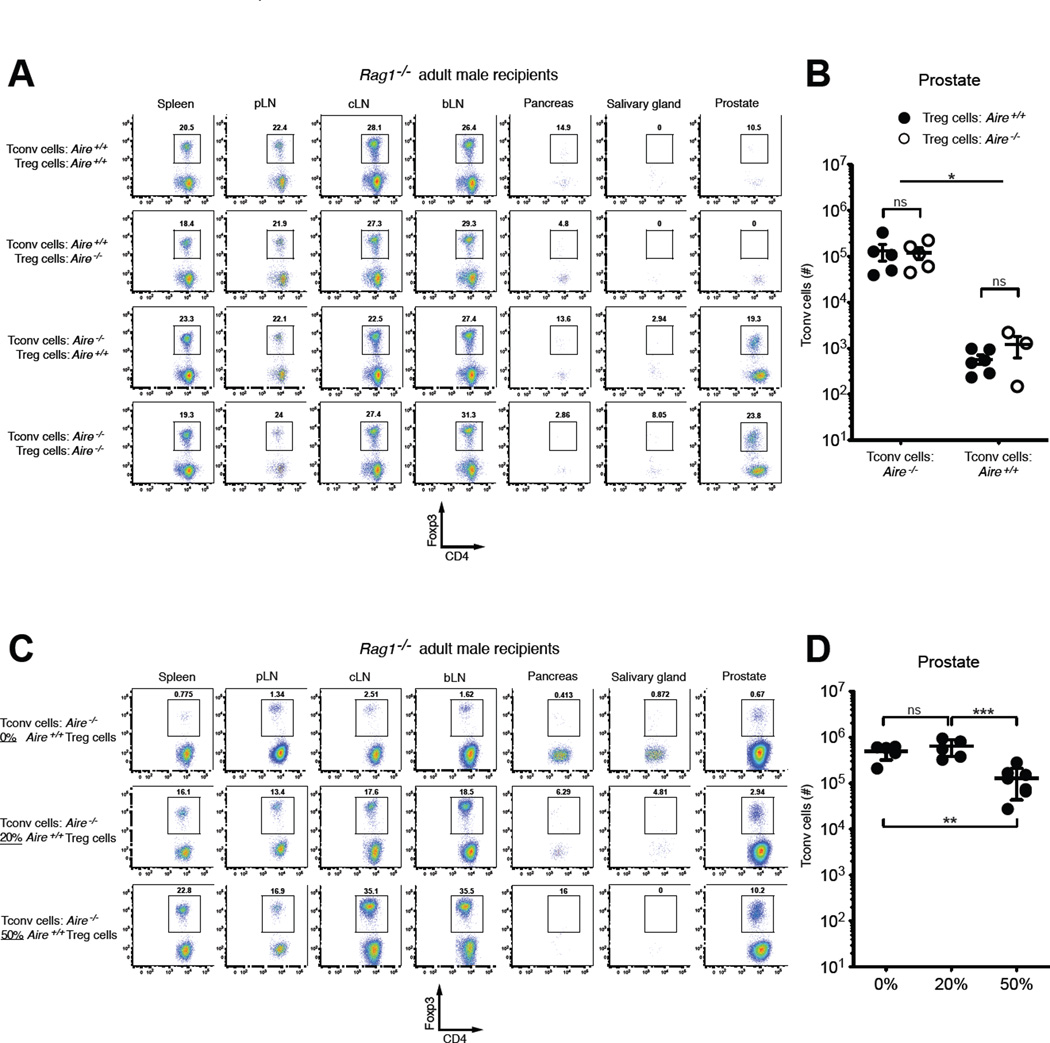
To examine the functional consequences of these repertoire perturbations on organ-specific autoimmunity, we sought to determine whether the autoimmune defects in Aire−/− mice map to alterations in the Treg cell compartment, the Tconv cell compartment, or both, by performing cell transfer experiments. We transferred different combinations of fluorescence activated cells sorting (FACS)-purified Treg cells and Tconv cells, isolated from 4–5-week-old male Aire+/+ or Aire−/− mice harboring a Foxp3GFP reporter allele (Haribhai et al., 2007), into 8–10-week-old adult male Rag1−/− mice lacking functional B and T cells (Fig. 3). In this way, the CD4+ T cell compartment of Rag1−/− mice was reconstituted with Treg and Tconv cells of defined origin, mixed at physiological ratios (12.5% Treg cells). At the experimental endpoint, the impact of cell transfer on prostatic T cell infiltration was determined. Since the pancreas is not a target organ of autoimmunity in B6.Aire−/− mice (Jiang et al., 2005), T cell infiltration in the pancreas was analyzed as a negative control. As shown in fig. S2, the transfer of Aire+/+ Tconv cells alone into Rag1−/− males induced prostatic T cell infiltration. Upon co-transfer with Aire+/+ Tconv cells, Aire−/− Treg cells were comparable to Aire+/+ Treg cells in the capacity to prevent prostatic T cell infiltration (Fig. 3A, compare rows 1 and 2), suggesting that Treg cells from Aire−/− mice do not exhibit a functional defect in this setting. In this regard, it is possible that the new Treg cell clonotypes that emerged in the absence of Aire (Fig. 2A, left arm of volcano plot) are able to functionally compensate for the loss of Aire-dependent Treg cell clonotypes (Fig. 2A, right arm of volcano plot). In contrast, when co-transferred with Aire+/+ Treg cells, the transfer of Aire−/− Tconv cells induced marked T cell infiltration of the prostate, whereas the transfer of Aire+/+ Tconv cells did not (Fig. 3A, compare rows 1 and 3). We observed identical results following the co-transfer of physiological ratios of Treg and Tconv cells into 3-day-old male Rag1−/− recipients (fig. S3), a setting that recapitulates the lymphopenic environment common in the neonatal period. These findings show that Tconv cells from Aire−/− mice exhibit enhanced autoimmune potential, and that when mixed at physiological ratios, co-transferred Treg cells from either Aire+/+ or Aire−/− backgrounds are not able to suppress prostatitis induced by Aire−/− Tconv cells. In additional experiments in which Aire−/− Tconv cells were transferred into adult male Rag1−/− mice, the co-transfer of supra-physiological percentages of Aire+/+ Treg cells (50%) significantly reduced prostatic infiltration, but ultimately failed to prevent prostatitis (Fig. 3C and D), further illustrating the potency of autoimmune potential of Tconv cells from Aire−/− mice. Taken together, our data indicate that although Aire deficiency impacts both the Treg cell and Tconv cell repertoires (Fig. 2), the autoimmune defect in Aire−/− mice functionally maps to the Tconv cell compartment (Fig. 3).
Figure 3. The Autoimmune Defect in Aire−/− Mice Maps to the Tconv Cell Compartment.
(A and B) A mixture of 7×106 CD4+Foxp3neg Tconv cells and 1×106 CD4+Foxp3+ Treg cells, isolated from the pooled spleen and lymph nodes of 4–5-week-old CD45.2+ Aire+/+ or Aire−/− males, as indicated, was transferred into 6–8-week-old male CD45.1+ Rag1−/− recipients. 3 weeks post-transfer, the fate of CD45.2+ donor cells was assessed at different sites. (A) Representative flow cytometric plots of Foxp3 vs. CD4 expression are shown for CD4+CD45.2+ donor T cells isolated from the indicated organs. The percentages of cells falling within the denoted gates are shown. pLN, periaortic lymph nodes; cLN, cervical lymph nodes; bLN, brachial lymph nodes. (B) Summary plot of the number of CD45.2+ donor Tconv cells recovered from the prostate. The source (Aire+/+ or Aire−/−) of donor Treg cells and Tconv cells is indicated. Data are pooled from N = 4 experiments. The mean +/− SEM is denoted. Asterisks indicate P < 0.05; ns, not significant. Statistical comparisons of all groups were made using ANOVA and Tukey’s multiple comparison test.
(C and D) A fixed number of CD4+Foxp3neg Tconv cells (1.5×106) from the pooled spleen and lymph nodes of 4–5-week-old CD45.2+ Aire−/− males, were transferred alone, or together with the indicated percentages of Treg cells from CD45.2+ Aire+/+ males, into 6–8-week-old male CD45.1+ Rag1−/− recipients. 3 weeks post-transfer, the fate of CD45.2+ donor cells was assessed at different sites. (C) Representative flow cytometric plots of Foxp3 vs. CD4 expression are shown for CD4+CD45.2+ donor T cells isolated from the indicated organs. (D) Summary plot of the number of CD45.2+ donor Tconv cells recovered from the prostate. Data are pooled from N > 3 experiments. The mean +/− SEM is denoted. Asterisks indicate P < 0.05; ns, not significant. Statistical comparisons of all groups were made using ANOVA and Tukey’s multiple comparison test. See also Figures S2 and S3.
In the Absence of Aire, Treg Cell-Biased Clonotypes are Diverted into the Tconv Cell Compartment
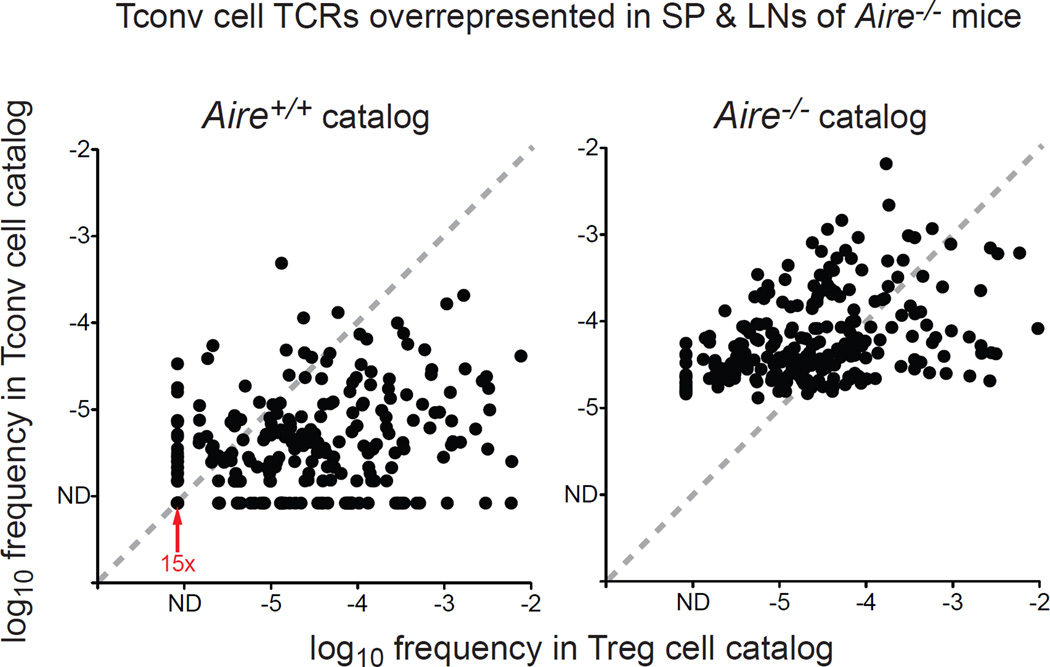
With this in mind, we focused our analysis on understanding the nature of the Tconv cell specificities that emerge in the absence of Aire (Fig. 2B, left arm of volcano plot). To do this, we plotted the relative frequencies of these TCRs in the Tconv cell and Treg cell catalogs from the pooled spleen and lymph nodes of Aire+/+ and Aire−/− mice (Fig. 4). Unexpectedly, this analysis revealed that the majority of the overrepresented Tconv cell TCRs (183 / 246, 74%) were preferentially expressed by Treg cells in Aire+/+ mice (Fig. 4, left plot, data points below the diagonal). In addition, many of these clonotypes exhibited a shift towards preferential representation in the Tconv cell subset in the Aire−/− setting (Fig. 4, right plot, data points above the diagonal). These data reveal that in the absence of Aire, lineage diversion of distinct Treg cell specificities contributes substantially to the emergence of new Tconv cell clonotypes in the secondary lymphoid organs. Further inspection revealed that of the TCRs that were overrepresented in the Tconv cell subset of Aire−/− mice, 6.1% (15 / 246) were not detected in the Tconv cell or Treg cell catalogs from Aire+/+ mice (Fig. 4, left plot, 15 data points indicated by red arrow), suggesting that these clonotypes are culled from the repertoire in Aire+/+ mice, but escape deletion in the Aire−/− background. Thus, clonotypes exhibiting patterns suggestive of deletional escape make minor contributions to the Tconv cell specificities that emerge in an Aire−/− setting.
Figure 4. A Large Proportion of Tconv Cell Clonotypes Overrepresented in Aire−/− Mice Are Preferentially Expressed by Treg Cells in Aire+/+ Mice.
For the TCR clonotypes that are recurrently overrepresented in the Tconv cell subset of Aire−/− mice relative to the Tconv cell subset of Aire+/+ mice (denoted in red in left arm of volcano plot in Fig. 2B), the mean frequencies of these clonotypes in the Tconv cell and Treg cell catalogs from Aire+/+ mice (left) and Aire−/− mice (right) are plotted. The shift of many TCRs from below the diagonal in the left plot to above the diagonal in the right plot reflects a shift from preferential representation in the Treg cell subset in Aire+/+ mice to preferential representation in the Tconv cell subset in Aire−/− mice. N = 246 TCRs. The red arrow denotes 15 superimposed data points, representing TCRs that are not detected (ND) in both the Tconv cell and Treg cell catalogs from Aire+/+ mice, suggestive of clonotypes that are deleted in Aire+/+ mice. Of the 246 TCRs shown, 183 TCRs (74%) are biased to the Treg cell lineage (i.e. lie below the diagonal) in the Aire+/+ catalogs. See also Table S1.
To determine whether diversion into the Tconv cell compartment in the absence of Aire was a universal feature of Aire-dependent Treg cell specificities, we cross-referenced the 215 T cell clonotypes that exhibited Aire-dependent selection into the Treg cell lineage with the 246 TCRs that exhibited overrepresentation in the Aire−/− Tconv cell catalog, identified in Fig. 2A and B. This analysis revealed that 12.6% (27 / 215) of the Aire-dependent Treg cell clonotypes exhibited diversion into the Tconv cell compartment in the absence of Aire, compared with 1.8% (139 / 7,740) of the recurrent Treg cell TCRs that did not exhibit Aire dependency.
Dominant Tconv Cell Clones Infiltrating the Aire−/− Prostate Are Skewed to the Treg Cell Lineage in Aire+/+ Mice
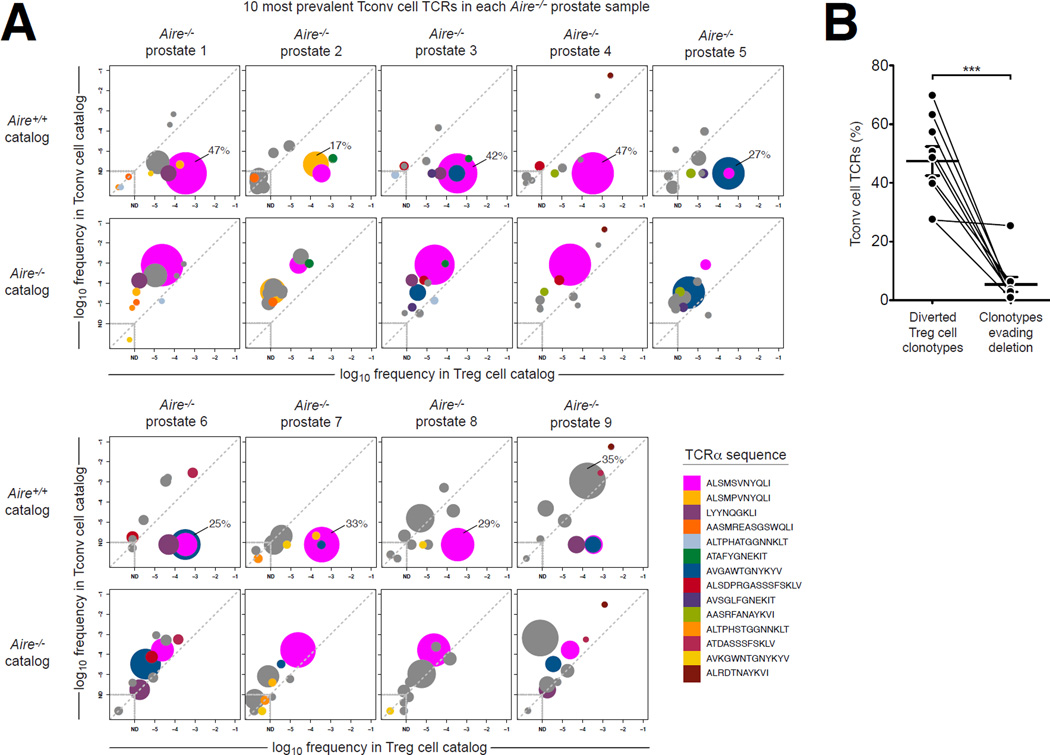
Thus far, our analysis of T cell populations from the spleen and lymph nodes revealed that Aire deficiency is associated with the emergence of new Tconv cell specificities with pathogenic potential, and that a large fraction of these Tconv cell specificities can be attributed to Treg cell-biased clonotypes that are diverted into the Tconv cell compartment in the absence of Aire. However, it remained unclear whether repertoire perturbations in the secondary lymphoid organs accurately reflected the specificities driving pathogenesis in the target organs. In this regard, we reasoned that characterization of the Tconv cell specificities infiltrating the autoimmune lesions of Aire−/− mice would reveal the prevailing mechanisms by which Aire enforces immune tolerance. Therefore, we performed complete TCRα sequencing of Tconv cells isolated directly from the prostatic lesions of Aire−/− mice (Figure 5 and Table S2). In this analysis, >25,000 CD4+ Tconv cells were sorted from each of the nine Aire−/− prostates analyzed (Fig. 5A). While each sample exhibited substantial repertoire complexity (>2,500 unique TCRα sequences per sample), each was dominated by a limited number of clonotypes, some of which were found recurrently in the mice analyzed (Fig. 5A). Of note, two TCRα chains of complementarity determining region three (CDR3) sequence ALSMSVNYQLI and ALSMPVNYQLI (indicated in magenta and peach, respectively), which differ by a single amino acid, together comprised 28% (+/− 17% SD) of the Tconv cell repertoire in the nine Aire−/− prostates analyzed (Fig. 5A). To characterize the nature of these and other dominant specificities, we identified the ten most prevalent Tconv cell clonotypes in each Aire−/− prostate sample, and similar to the analysis in Fig. 4, determined the extent to which these clonotypes were skewed to the Tconv cell subset or Treg cell subset in the Aire+/+ and Aire−/− catalogs derived from the pooled spleen and lymph nodes (Fig. 5A). In Fig. 5, TCRs that were among the ten most prevalent TCRs in more than one Aire−/− prostate sample are color-coded, and the size (area) of each data point is proportional to the frequency of this TCR in the Tconv cell subset of the Aire−/− prostate sample. Our data reveal that the predominant Tconv cell specificities infiltrating the Aire−/− prostates represented diverted Treg cell clonotypes, expressing TCRs that were preferentially expressed by Treg cells in the Aire+/+ catalog but predominantly associated with the Tconv cell compartment in the Aire−/− catalog (Fig. 5A, indicated by the shift of data points from below the diagonal in the Aire+/+ catalog plot to above the diagonal in the Aire−/− catalog plot). For example, in Aire−/− prostate sample 1, 47% of Tconv cell sequence reads encoded the ALSMSVNYQLI TCR (magenta), indicating predominance of this clonotype. Comparison with the reference catalogs revealed that this TCR was predominantly expressed by Treg cells in the Aire+/+ catalog (top row), but was skewed to the Tconv cell subset in the Aire−/− catalog (bottom row).
Figure 5. The Predominant TCR Clonotypes Expressed by Tconv Cells Infiltrating the Prostates of Aire−/− Mice are Preferentially Expressed by Treg Cells in Aire+/+ mice.
Tconv cells were FACS-purified from the prostates of nine 9-week-old Aire−/− TCRβTg Foxp3GFP males, and subjected to complete TCRα sequence analysis (see Experimental Procedures). These data were then compared to TCR frequency data from “catalogs” generated via complete TCRα sequencing of Tconv cell and Treg cell populations isolated from the pooled spleen and lymph nodes of Aire+/+ and Aire−/− mice, described in the text and depicted in Fig. 2.
(A) For the 10 most prevalent Tconv cell TCRs in each Aire−/− prostate sample, the average frequency (log10) of each TCR in the Tconv cell catalog is plotted vs. the average frequency (log10) in the Treg cell catalog for catalogs derived from Aire+/+ mice (top row) and Aire−/− mice (bottom row). The size (area) of each data point is proportional to the frequency of this TCR in the Tconv cell subset of the Aire−/− prostate sample. For each prostate sample, the frequency of the most prevalent TCR clonotype is indicated (as a percentage of sequence reads). For example, in Aire−/− prostate 1, 47% of Tconv cell TCR sequence reads encode the ALSMSVNYQLI TCR (magenta), indicating predominance of this clonotype. This TCR is preferentially expressed by Treg cells in the Aire+/+ catalog (top row), but is preferentially expressed by Tconv cells in the Aire−/− catalog (bottom row). TCRs that are found among the 10 most prevalent clonotypes in more than one Aire−/− prostate sample are color-coded as indicated in the legend. TCRs that are found only once among the 10 most prevalent Tconv cell TCRs in these nine samples are shown in gray. ND, not detected in catalog. Data points centered within the dashed boxes were not detected in both the Tconv cell and Treg cell catalogs for the indicated Aire genotype. See also Table S2.
(B) Summary of the data plotted in A. For each Aire−/− prostate sample, the cumulative percentage of Tconv cell clonotypes exhibiting representation patterns suggestive of “diverted Treg cell clonotypes” or “clonotypes evading deletion” are plotted. TCRs that fall below the diagonal in the Aire+/+ catalog and above the diagonal in the Aire−/− catalog are classified as “diverted Treg cell clonotypes”. TCRs that are not detected in the Aire+/+ catalog, but are observed in the Aire−/− catalog are classified as “clonotypes evading deletion”. Lines denote paired samples from a given mouse. The mean +/− SEM is indicated. Statistical analysis was performed using a paired t-test. Asterisks indicate P < 0.05.
We also noted that some of the sub-dominant Tconv cell clonotypes infiltrating Aire−/− prostates were not detected (ND) in the Tconv cell or Treg cell catalogs from Aire+/+ mice, but were observed in the Aire−/− catalogs, suggestive of clonotypes that escape deletion in an Aire−/− setting (Fig. 5A). For example, the AASMREASGSWQLI TCR (indicated in orange), which was expressed by 1.8% of the Tconv cells infiltrating Aire−/− prostate sample 2, was not detected in the Tconv cell and Treg cell catalogs from Aire+/+ mice, but was detected at low frequency in the catalogs from Aire−/− mice (Fig. 5A). However, quantification indicated that cumulatively, Tconv cell clonotypes that evade deletion in Aire−/− mice (“clonotypes evading deletion”) typically make only minor contributions to the prostatic Tconv cell infiltrate (mean frequency of 5% of Tconv cells, Fig. 5B), and are dwarved by clonotypes exhibiting diversion from the Treg cell lineage into the Tconv cell subset (“diverted Treg cell clonotypes”, mean frequency of 47% of Tconv cells, Fig. 5B).
Thus, the dominant Tconv cell clonotypes infiltrating the prostates of Aire−/− mice represent diverted Treg cell specificities, suggesting that a primary mechanism by which Aire enforces immune tolerance is to direct distinct autoreactive T cell clones into the Treg cell lineage.
MJ23 Tconv Cells Are Recurrently Found in Aire−/− Prostate Samples
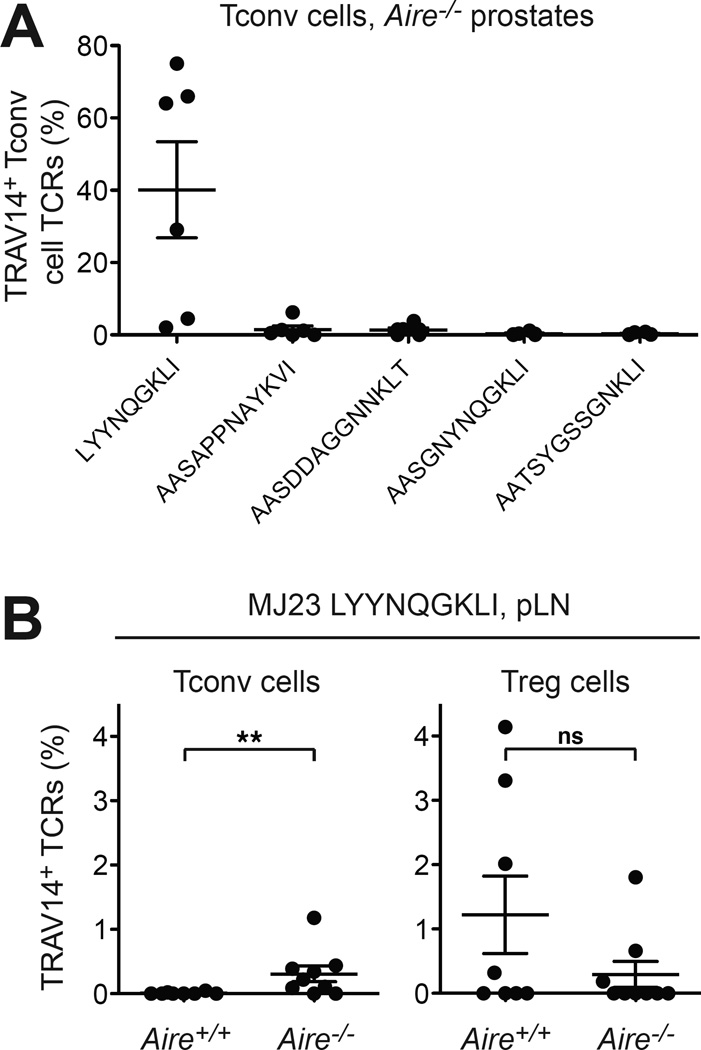
In a previous study employing TCRα repertoire analysis in fixed TCRβTg mice, we identified a canonical TCRα chain of sequence LYYNQGKLI that is recurrently expressed by Treg cells infiltrating oncogene-driven prostate tumors (Malchow et al., 2013). This clone, named “MJ23”, is reactive to a prostate-associated self antigen, is strongly biased to the Treg cell subset, and requires Aire for differentiation into the Treg cell lineage in the thymus (Malchow et al., 2013). Notably, the MJ23 TCR was also evident in our current analysis of the Tconv cell specificities infiltrating Aire−/− prostate samples (Fig. 5A, shown in purple), indicating that in the absence of Aire, prostate-specific MJ23 T cells emerge in the Tconv cell compartment and contribute to the Tconv cell specificities infiltrating the prostate of some Aire−/− mice. Given that the MJ23 TCRα chain utilizes a TRAV14 variable-region segment, we expanded our analysis to additional Aire−/− mice by sequencing TRAV14 TCRα chains expressed by prostate-infiltrating Tconv cells (Fig. 6). Our data reveal that in multiple cases, the dominant TCR expressed by TRAV14 Tconv cells at this site (up to 75%) was the MJ23 TCR (Fig. 6A). Consistent with this, TRAV14 TCRα sequencing of Treg cells and Tconv cells isolated from the prostate-draining lymph nodes (pLNs), a site where MJ23 Treg cells are recurrently enriched (Malchow et al., 2013), demonstrated that the MJ23 TCR was exclusively associated with the Treg cell lineage in Aire+/+ mice, but was found in both Treg cell and Tconv cell subsets in Aire−/− mice (Fig. 6B). Thus, in the absence of Aire, some MJ23 T cells are diverted from the Treg cell lineage into the Tconv cell subset and are recurrently enriched in prostatic autoimmune lesions, a finding congruent with the complete TCRα sequencing analyses presented above.
Figure 6. In the Absence of Aire, MJ23 T Cells Are Diverted Into the Tconv Cell Lineage And Dominate the Prostatic TRAV14+ Tconv Cell Infiltrate.
CD4+Foxp3neg Tconv cells and CD4+Foxp3+ Treg cells were FACS-purified from the prostate and prostatedraining lymph nodes (pLN) of 9-week-old TCRβTg Foxp3GFP males on the Aire+/+ or Aire−/− background. cDNA was then subjected to TRAV14 TCRα sequencing.
(A) In many mice, the prostate-specific MJ23 TCRα LYYNQGKLI is the predominant TCRα expressed by TRAV14+ Tconv cells infiltrating the Aire−/− prostate. For Tconv cells at this site, the percentages of TRAV14+ TCR encoding the indicated TCRα chains are plotted for each sample. The five most abundant TRAV14+ TCRs found in two or more samples are shown. The mean +/− SEM is indicated. N = 6 for Aire+/+ and Aire−/− groups.
(B) In the pLN, the MJ23 TCRα LYYNQGKLI is skewed to the Treg cell lineage in Aire+/+ mice, but found in both Tconv cell and Treg cell subsets in Aire−/− mice. For Tconv cells (left plot) or Treg cells (right plot) isolated from the pLN of Aire+/+ or Aire−/− mice, the percentage of TRAV14+ TCRs encoding the MJ23 TCRα is plotted. The mean +/− SEM is indicated. Asterisks indicate P < 0.05; ns, not significant. Statistical comparisons between Tconv cell and Treg cell subsets were made using the Mann-Whitney nonparametric test. N > 8 for Aire+/+ and Aire−/− groups.
Taken together, our results demonstrate that prostate-specific autoimmunity associated with Aire deficiency is dominated by Treg cell-biased specificities that are diverted into the Tconv cell compartment and become pathogenic in the absence of Aire. These findings suggest that a major mechanism by which Aire enforces immune tolerance is to ensure the proper differentiation of distinct autoreactive T cell clonotypes into the Treg cell lineage, thereby simultaneously removing these clonotypes from the Tconv cell repertoire.
DISCUSSION
A growing body of evidence indicates that Aire-dependent promiscuous gene expression can promote both clonal deletion and Treg cell development in the thymus. Here we sought to determine the relative contribution of these processes to the prevention of autoimmunity in the periphery. Our results revealed that Aire enforces immune tolerance by closely linking these two mechanisms, removing autoreactive clonotypes from the Tconv cell compartment and driving the differentiation of these cells into the Foxp3+ Treg cell lineage. When this process is dysregulated in Aire−/− mice, clonotypes that normally differentiate into the Treg cell pool are diverted into pathogenic Tconv cells that dominate the autoimmune T cell infiltrate in the prostate. Informally, we refer to these specificities as “Trogue" cells - Treg cell-biased clonotypes that “go rogue” in the absence of Aire.
Although our results do not exclude the possibility that T cell clonotypes that escape Aire-dependent clonal deletion contribute to autoimmune pathology in Aire−/− mice, our data indicate that these clonotypes make up only a minor fraction of the Tconv cells infiltrating the inflamed prostate (Fig. 5B). Thus, our data suggest that Aire-dependent clonal deletion, while evident in several TCR transgenic experimental systems (Metzger and Anderson, 2011), is not a primary mechanism by which Aire-dependent tolerance to TRAs is enforced. This idea is consistent with studies using peptide-MHC tetramers, which demonstrated that the clonal deletion of T cells specific for widespread self antigens and TRAs is incomplete (Moon et al., 2011; Taniguchi et al., 2012; Yu et al., 2015). Additionally, the fulminant systemic and organ-specific autoimmunity induced by sustained Treg cell ablation (Kim et al., 2007) demonstrates that the T cell repertoire contains autoreactive T cells with pathogenic potential, further illustrating the incomplete nature of deletional tolerance mechanisms.
Two lines of evidence are consistent with the idea that Trogue cells are "drivers" of prostatitis in Aire−/− mice, and are not “passenger” clones that are drawn into the prostate in a TCR-independent manner. First, the recurrent nature of the dominant Trogue cell clones, including ALSMSVNYQLI, AVGAWTGNYKYV, and LYYNQGKLI, strongly suggests that these cells are recruited and/or expanded in an antigen-driven manner. Second, the finding that a large percentage of Tconv cells infiltrating Aire−/− prostates exhibit CD69 expression indicates that the majority of these cells perceive TCR-dependent signals within the prostate.
At an early point in our studies, based on data demonstrating that Aire is required for the thymic development of multiple naturally occurring Treg cell specificities (Malchow et al., 2013; Perry et al., 2014), we had hypothesized that Aire−/− mice develop autoimmune pathology due to a functional deficiency in the Treg cell compartment, likely due to a lack of TRA-reactive specificities. However, data from co-transfer experiments demonstrated that following introduction into Rag1−/− males at physiological ratios, Treg cells from Aire−/− mice were fully capable of preventing prostatic infiltration by Aire+/+ Tconv cells. Moreover, this hypothesis would predict that the prostatic infiltrate in Aire−/− mice would be dominated by pre-existing Tconv cell clonotypes that are present in the repertoire of Aire+/+ mice, but become unrestrained due to a deficiency in the Treg cell compartment. However, we found that the predominant clonotypes infiltrating the Aire−/− prostate are typically not observed in the Aire+/+ Tconv cell catalog, but instead represent clonotypes that are preferentially expressed by Treg cells in Aire+/+ mice, a finding inconsistent with this hypothesis. Taken together, these lines of evidence indicate that the Treg cell pool in Aire−/− mice is not impaired by the loss of Aire-dependent Treg cell specificities and is capable of controlling Tconv cells produced in an Aire+/+ background. In this regard, it is possible that the new Treg cell clonotypes that emerge in the absence of Aire (denoted in the left arm of the Treg cell volcano plot in Fig. 2A) are able to functionally compensate for the loss of Aire-dependent Treg cell clonotypes.
Notably, the diversion of particular clonotypes into the Tconv cell subset is typically not associated with the complete loss of these specificities in the Treg cell compartment, potentially due to Aire-independent extrathymic Treg cell development following antigen encounter in the periphery. Thus, Aire deficiency establishes an arena in which Trogue cells and Treg cells with the same specificity may compete for antigenic niches. However, three lines of evidence suggest that in an Aire−/− setting, Trogue cells are able to overcome suppression by their Treg cell counterparts. First, the finding that multiple Trogue cell specificities are recurrently found in the Tconv cell infiltrate of Aire−/− prostates indicates that the Treg cells present in Aire−/− mice are not able to prevent prostate infiltration by these Trogue cells. Second, data from T cell transfer experiments demonstrate that even when Treg cells from Aire+/+ mice are transferred at supra-physiological percentages (50%), they are still unable to prevent prostatitis induced by the co-transfer of Tconv cells from Aire−/− mice. The concept of antigenic niche competition in an Aire−/− setting provides a rational basis for why a diverted Treg cell specificity may be more pathogenic than a non-Treg cell specificity that evades clonal deletion, as Trogue cell clonotypes may be harder to suppress due to their ability to directly out-compete their Treg cell counterparts for access to peptide-MHC ligands.
Our demonstration that Aire functions to direct distinct T cell clonotypes into the Treg cell lineage casts light on earlier observations that have been pivotal for our understanding of Aire-mediated immune tolerance. First, in experiments involving the grafting of thymi into athymic nude mice, Anderson et al. demonstrated that the grafting of one Aire+/+ thymus was unable to prevent autoimmunity induced by the simultaneous grafting of one Aire−/− thymus, whereas the grafting of four Aire+/+ thymi with one Aire−/− thymus was sufficient to suppress pathology (Anderson et al., 2005). Second, using mice in which the expression of Aire could be temporally regulated, it was demonstrated that Aire is dispensable after three weeks of age (Guerau-de-Arellano et al., 2009). Third, Yang et al. demonstrated that the transfer of perinatally-tagged Treg cells from Aire+/+ donors prevented autoimmunity in Aire−/− mice, but the transfer of Treg cells from Aire−/− mice was insufficient (Yang et al., 2015). These observations are consistent with our conclusion that autoimmune potential in an Aire−/− setting is associated with the emergence of Trogue cell specificities in the Tconv cell compartment. Specifically, we suggest that the experimental findings discussed above were the result of a “numbers game”, in which the early provision of an excess of Aire-dependent Treg cells educated in an Aire+/+ setting was sufficient to prevent autoimmunity induced by the Trogue cell doppelgangers that arise in the absence of Aire.
In a recent report, Takaba et al. demonstrated a critical role for Fezf2 in immune tolerance (Takaba et al., 2015). Fezf2 is a transcription factor that is preferentially expressed by mTECs and promotes the promiscuous thymic expression of a subset of TRA genes in an Aire-independent manner. In addition, Fezf2−/− mice exhibit reduced percentages of Treg cells in the thymus, and develop organ-specific autoimmunity characterized by immune cell infiltration and serum autoantibodies. Given the phenotypic parallels between Fezf2 deficiency and Aire deficiency, we hypothesize that Trogue cells may be key drivers of organ-specific autoimmunity in Fezf2−/− mice.
EXPERIMENTAL PROCEDURES
Mice
The following mice were purchased from the Jackson Laboratory, and maintained at the University of Chicago under specific-pathogen free conditions: C57BL/6J (B6) mice, CD45.1/.1 B6.SJL-Ptprca Pepcb/BoyJ mice, TRAMP C57BL/6-Tg(TRAMP)8247Ng/J mice, Rag1−/− B6.129S7-Rag1tm1Mom/J mice, Aire−/− B6.129S2-Airetm1.1Doi/J mice, and Foxp3GFP B6.Cg-Foxp3tm2Tch/J reporter mice. “TCRβTg” mice expressing a fixed TCRβ chain of sequence Vβ3(TRBV26)-ASSLGSSYEQY were generated as described previously (Malchow et al., 2013). Aire−/− mice, TCRβTg mice, and Foxp3GFP reporter mice were interbred to obtain Aire+/+ TCRβTg Foxp3GFP and Aire−/− TCRβTg Foxp3GFP littermates. CD45.1/.1 B6.SJL-Ptprca Pepcb/BoyJ mice and Rag1−/− mice were interbred to obtain CD45.1+ Rag1−/− mice. All mice were bred and maintained in accordance with the animal care and use regulations of the University of Chicago.
Antibodies, flow cytometry, and FACS
All antibodies used were purchased from eBioscience, Biolegend, and BD Biosciences. Typically, cells were stained for 20 minutes on ice in staining buffer (phosphate-buffered saline with 2% FCS, 0.1% NaN3, 5% normal rat serum, 5% normal mouse serum, 5% normal rabbit serum (all sera from Jackson Immunoresearch), and 10 µg/mL 2.4G2 FcR blocking antibody). Intracellular staining for Foxp3 was performed using antibody and fixation/permeabilization buffers from eBioscience. Flow cytometry was performed on an LSR-II or LSR-Fortessa flow cytometer (BD Biosciences), and analyzed using FlowJo data analysis software (Tree Star). FACS was performed using a FACSAria (BD Biosciences).
Lymphocyte isolation
Whole male genitourinary tracts were isolated and prostate lobes (anterior, dorsolateral, and ventral) were separated by mircodissection. Prostate lobes, salivary gland, and pancreas, were injected and digested with Liberase TL (10 mg/mL, Roche) and DNase (20 mg/mL, Roche) in RPMI for 30 min at 37°C. Digested tissue was mechanically disrupted with frosted microscope slides and viable lymphocytes were enriched using Histopaque 1119 (Sigma).
T cell transfer experiments
Polyclonal donor T cells: CD4+ T cells from pooled spleen and lymph nodes (axillary, brachial, cervical, inguinal, mesenteric, pancreatic, periaortic, and renal) from 4–5 week old male mice were enriched for CD4+ T cells by MACS (Miltenyi) and CD4+Foxp3neg Tconv cells and CD4+Foxp3+ Treg cells were sorted by FACS. CD45.1+ Rag1−/− recipients: For adult male recipients, a mixture of 7×106 CD4+Foxp3neg Tconv cells (Aire+/+ or Aire−/−) and 1×106 CD4+Foxp3+ Treg cells (Aire+/+ or Aire−/−) was transferred retro-orbitally into 6–8-week-old male CD45.1+ Rag1−/− recipients, and the fate of CD45.2+ donor cells was assessed by flow cytometry at 3 weeks post-transfer. For neonatal male recipients, a mixture of 7×106 CD4+Foxp3neg Tconv cells (Aire+/+ or Aire−/−) and 1×106 CD4+Foxp3+ Treg cells (Aire+/+ or Aire−/−) was transferred intraperitoneally into 3-day-old male CD45.1+ Rag1−/− recipients, and the fate of CD45.2+ donor cells was assessed by flow cytometry at 9 weeks post-transfer.
Statistical Analysis
Data were analyzed using Prism software (GraphPad). For the comparison of two groups, either the Student’s t-test (two-tailed) or the nonparametric Mann-Whitney test were used, depending on whether data were normally distributed. For comparison of multiple groups, one-way ANOVA couple with Tukey’s multiple comparison test were employed.
TCRα sequence analysis
CD4+Foxp3+ Treg cells and CD4+Foxp3neg Tconv cells were FACS-purified from different anatomical sites of 9-week-old TCRβTg Foxp3GFP males on an Aire+/+ and Aire−/− background, and cDNA was subjected to TCRα sequence analysis using either “TRAV14 TCRα sequencing” or “complete TCRα sequencing”, described in Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Aire impacts the peripheral repertoire of both Treg cells and Tconv cells
The autoimmune defect in Aire−/− mice maps to the Tconv cell compartment
Treg-biased clones are diverted into the Tconv cell subset in Aire−/− mice
Diverted clones dominate the Tconv cell infiltrate in prostatic lesions
Acknowledgments
We thank A. Bendelac, and R. Gottschalk for critical reading of the manuscript. This work was funded by the following sources (to P.A.S.): R01 (R01CA160371), R01 (R01AI110507), a Cancer Research Institute Investigator Award, and the University of Chicago Comprehensive Cancer Center. S.M. was supported by a Deutsche Forschungsgemeinschaft (DFG) Research Fellowship MA 6154/1-1. D.S.L. was supported by an NIH/NCI F31 predoctoral fellowship (CA183357). N.D.S. was supported by MSKCC Comprehensive Cancer Center (Support Grant #P30 CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
S.M., D.S.L., and P.A.S. conceived the project, designed experiments, and performed data analysis. S.M., D.S.L., V.L., and S.N. performed the experiments. N.D.S. and P.A.S. performed computational and statistical analysis of TCR sequence data. P.A.S. wrote the manuscript.
REFERENCES
- Aaltonen J. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- Daniely D, Kern J, Cebula A, Ignatowicz L. Diversity of TCRs on natural Foxp3+ T cells in mice lacking Aire expression. J Immunol. 2010;184:6865–6873. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, Chang H, Caspi RR, Fong L, Anderson MS. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoss JJ, Shum AK, Johannes KP, Lu W, Krawisz AK, Wang P, Yang T, Leclair NP, Austin C, Strauss EC, et al. Effector mechanisms of the autoimmune syndrome in the murine model of autoimmune polyglandular syndrome type 1. J Immunol. 2008;181:4072–4079. doi: 10.4049/jimmunol.181.6.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerau-de-Arellano M, Martinic M, Benoist C, Mathis D. Neonatal tolerance revisited: a perinatal window for Aire control of autoimmunity. J Exp Med. 2009;206:1245–1252. doi: 10.1084/jem.20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribhai D, Lin W, Relland LM, Truong N, Williams CB, Chatila TA. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- Hou Y, DeVoss J, Dao V, Kwek S, Simko JP, McNeel DG, Anderson MS, Fong L. An aberrant prostate antigen-specific immune response causes prostatitis in mice and is associated with chronic prostatitis in humans. J Clin Invest. 2009;119:2031–2041. doi: 10.1172/JCI38332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Hubert FX, Kinkel SA, Crewther PE, Cannon PZ, Webster KE, Link M, Uibo R, O'Bryan MK, Meager A, Forehan SP, et al. Aire-deficient C57BL/6 mice mimicking the common human 13-base pair deletion mutation present with only a mild autoimmune phenotype. J Immunol. 2009;182:3902–3918. doi: 10.4049/jimmunol.0802124. [DOI] [PubMed] [Google Scholar]
- Husebye ES, Perheentupa J, Rautemaa R, Kampe O. Clinical manifestations and management of patients with autoimmune polyendocrine syndrome type I. J Intern Med. 2009;265:514–529. doi: 10.1111/j.1365-2796.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- Jiang W, Anderson MS, Bronson R, Mathis D, Benoist C. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekalainen E, Pontynen N, Meri S, Arstila TP, Jarva H. Autoimmunity, Not a Developmental Defect, is the Cause for Subfertility of Autoimmune Regulator (Aire) Deficient Mice. Scand J Immunol. 2015;81:298–304. doi: 10.1111/sji.12280. [DOI] [PubMed] [Google Scholar]
- Kekalainen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pontynen N, Talvensaari K, Perheentupa J, Miettinen A, Arstila TP. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- Laakso SM, Laurinolli TT, Rossi LH, Lehtoviita A, Sairanen H, Perheentupa J, Kekalainen E, Arstila TP. Regulatory T cell defect in APECED patients is associated with loss of naive FOXP3(+) precursors and impaired activated population. J Autoimmun. 2010;35:351–357. doi: 10.1016/j.jaut.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS. Antigen-specific peripheral shaping of the natural regulatory T cell population. J Exp Med. 2008;205:3105–3117. doi: 10.1084/jem.20081359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- Magurran . Ecological Diversity and Its Measurement. Princeton, NJ: Princeton University Press; 1988. [Google Scholar]
- Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- Metzger TC, Anderson MS. Control of central and peripheral tolerance by Aire. Immunol Rev. 2011;241:89–103. doi: 10.1111/j.1600-065X.2011.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Dash P, Oguin TH, 3rd, McClaren JL, Chu HH, Thomas PG, Jenkins MK. Quantitative impact of thymic selection on Foxp3+ and Foxp3- subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc Natl Acad Sci U S A. 2011;108:14602–14607. doi: 10.1073/pnas.1109806108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis SE, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- Perry JS, Lio CW, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, Hsieh CS. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414–426. doi: 10.1016/j.immuni.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Human molecular genetics. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Shikama-Dorn N, Zhanybekova S, Nusspaumer G, Macaulay IC, Deadman ME, Heger A, Ponting CP, Hollander GA. Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia. Genome Res. 2014;24:1918–1931. doi: 10.1101/gr.171645.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant'Angelo DB, Lucas B, Waterbury PG, Cohen B, Brabb T, Goverman J, Germain RN, Janeway CA., Jr A molecular map of T cell development. Immunity. 1998;9:179–186. doi: 10.1016/s1074-7613(00)80600-7. [DOI] [PubMed] [Google Scholar]
- Setiady YY, Ohno K, Samy ET, Bagavant H, Qiao H, Sharp C, She JX, Tung KS. Physiologic self antigens rapidly capacitate autoimmune disease-specific polyclonal CD4+ CD25+ regulatory T cells. Blood. 2006;107:1056–1062. doi: 10.1182/blood-2005-08-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, Kodama T, Takayanagi H. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell. 2015;163:975–987. doi: 10.1016/j.cell.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, Jenkins MK, Anderson MS. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proc Natl Acad Sci U S A. 2012;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science. 2015;348:589–594. doi: 10.1126/science.aaa7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang N, Ebert PJ, Kidd BA, Muller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alphabeta CD8(+) T Lymphocytes. Immunity. 2015;42:929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ML, Nagavalli A, Su MA. Aire deficiency promotes TRP-1-specific immune rejection of melanoma. Cancer Res. 2013;73:2104–2116. doi: 10.1158/0008-5472.CAN-12-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.