Abstract
IMPORTANCE
Acute kidney injury (AKI) affects as many as 40% of patients undergoing surgery and is associated with increased all-cause mortality. Chronic kidney disease (CKD) is a well-known risk factor for cardiovascular mortality.
OBJECTIVE
To determine the association between kidney disease and long-term cardiovascular-specific mortality after vascular surgery.
DESIGN, SETTING, AND PARTICIPANTS
A single-center cohort of 3646 patients underwent inpatient vascular surgery from January 1, 2000, to November 30, 2010, at a tertiary care teaching hospital. To determine cause-specific mortality for patients undergoing vascular surgery, a proportional subdistribution hazards regression analysis was used to model long-term cardiovascular-specific mortality while treating any other cause of death as a competing risk. Kidney disease constituted the main covariate after adjusting for baseline patient characteristics, surgery type, and admission hemoglobin level. Final follow-up was completed July 2014 to assess survival through January 31, 2014, and data were analyzed from June 1, 2014, to September 7, 2015.
MAIN OUTCOMES AND MEASURES
Perioperative AKI, presence of CKD, and overall and cause-specific mortality.
RESULTS
Among the 3646 patients undergoing vascular surgery, perioperative AKI occurred in 1801 (49.4%) and CKD was present in 496 (13.6%). The top 2 causes among the 1577 deaths in our cohort were cardiovascular disease (845 of 1577 [53.6%]) and cancer (173 of 1577 [11.0%]). Adjusted cardiovascular mortality estimates at 10 years were 17%, 31%, 30%, and 41%, respectively, for patients with no kidney disease, AKI without CKD, CKD without AKI, and AKI with CKD. Adjusted hazard ratios (95%CIs) for cardiovascular mortality were significantly elevated among patients with AKI without CKD (2.07 [1.74–2.45]), CKD without AKI (2.01 [1.46–2.78]), and AKI with CKD (2.99 [2.37–3.78]) and were higher than those for other risk factors, including increasing age (1.03 per 1-year increase; 1.02–1.04), emergent surgery (1.47; 1.27–1.71), and admission hemoglobin levels lower than 10 g/dL (1.39; 1.14–1.69) compared with a hemoglobin level of 12 g/dL or higher.
CONCLUSIONS AND RELEVANCE
Perioperative AKI is common in patients undergoing vascular surgery and is associated with a high risk for cardiovascular-specific mortality comparable to that seen with CKD. These findings reinforce the importance of preoperative and postoperative risk stratification for kidney disease and the implementation of strategies now available to help prevent perioperative AKI.
Chronic kidney disease (CKD) is associated with increased cardiovascular-specific mortality in the general population, and this risk increases with progressive decline in the estimated glomerular filtration rate (eGFR) to dialysis dependence and end-stage renal disease (ESRD).1,2 Chronic kidney disease is also a well-recognized risk factor for adverse short- and long-term outcomes after vascular surgery. Chronic kidney disease is independently associated with excess 30-day mortality, with as much as 4.7 times the risk for postoperative death in patients undergoing thoracoabdominal and abdominal aortic repairs compared with patients with no kidney disease.3,4 Chronic kidney disease is a risk factor for 1-year mortality after peripheral vascular interventions and abdominal aortic aneurysm repair and for the combined outcome of stroke and all-cause mortality after carotid endarterectomy procedures.5–7
Acute kidney injury (AKI) is a common postoperative complication, and Huber et al8 recently demonstrated that small and often disregarded changes in serum creatinine levels are independently associated with higher hospitaland90-daymortality, increased use of health care resources, and increased hospital cost of care after a broad range of vascular surgical procedures, even after adjusting for preoperative variables and other postoperative complications. Several recent studies using contemporary consensus definitions for AKI have suggested that postoperative AKI is an important risk factor for long-term mortality after major vascular surgery.9–14 Unfortunately, these studies included only small patient cohorts and selected surgical procedures and largely failed to examine cause-specific mortality. In a large, single-center cohort of patients undergoing major vascular surgery, we examined the associations between cardiovascular-specific mortality and kidney disease while adjusting for demographic variables, comorbidities, hemoglobin levels, and other competing causes of death.
Methods
Data Source and Participants
We queried the University of Florida Integrated Data Repository to assemble a single-center cohort of perioperative patients 18 years or older who were admitted to the hospital for longer than 24 hours after any type of major vascular surgery procedure from January 1, 2000, to November 30, 2010.15 Final follow-up was completed July 2014 to assess survival through January 31, 2014. For patients undergoing multiple operations, we analyzed only the index procedure. We identified study participants as patients in whom the primary admission or discharge service was vascular or cardiothoracic surgery and who received a primary or secondary procedure code from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) for vascular surgery (eTable 1 in the Supplement).16 We subdivided procedure codes into categories as described previously16 and excluded patients receiving dialysis access procedures and other procedures specified in eTable 1 in the Supplement. The final cohort consisted of 3646 patients. The study was approved by the institutional review board and privacy office of the University of Florida, which determined that informed consent was not needed for this study.
Mortality
The main outcome of the study was cardiovascular-specific mortality, with any other cause of death treated as a competing risk. For the secondary analysis, cancer-specific mortality was treated as the main cause of death in a competing risk model. The date of death was determined using hospital records, the Social Security Death Index, and data from the Florida Bureau of Vital Statistics (detailed in the eMethods in the Supplement). Using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, we classified the primary cause of death as cardiovascular specific (codes I00-I99, Q20-Q28, E10-E14, N00-N08, N10-N16, and N17-N19), cancer specific (codes C00-C97), and all other causes. We included diabetes mellitus and kidney disease in the expanded cardiovascular disease category to capture additional deaths that were related to CKD but traditionally not classified under cardiovascular diseases, as previously reported by Gansevoort et al.1 For the sensitivity analysis, we used an alternative approach that was reported for the general population.17,18
Kidney Disease and Other Covariates
The main covariate was the presence of kidney disease during the index hospitalization. For all patients we calculated a preoperative reference eGFR by applying the CKD epidemiology collaboration equation using standardized reference serum creatinine levels, sex, race, and age.19 For the reference serum creatinine level, we used the minimum of all values available within the 6 months before admission or the minimum and mean of the creatinine values available within the 7 days before admission (used for sensitivity analyses).20 Patients with CKD who did not require renal replacement therapy and patients with ESRD who required renal replacement therapy before admission were identified by the previously validated combination of ICD-9-CM diagnostic and procedure codes (eMethods in the Supplement).21 Patients with CKD were stratified using reference eGFR levels without criteria for albuminuria as having mild(eGFR, ≥60mL/min/1.73m2),moderate (eGFR,30to<60mL/min/1.73m2), and severe (eGFR, <30 mL/min/1.73m2)CKD according to guidelines from Kidney Disease: Improving Global Outcomes (KDIGO).22
We defined AKI using the consensus KDIGO criteria as at least a 50% and/or a 0.3-mg/dL increase in serum creatinine level (to convert to micromoles per liter, multiply by 88.4) relative to the preoperative reference value.23 Patients with AKI were stratified according to the maximum change in serum creatinine level during the hospital admission in 3 stages. Stage 1 corresponded to a 50%change in serum creatinine level; stage 2, to a doubling in serum creatinine level; and stage 3, to a tripling or increase in serum creatinine level to 4.0 mg/dL or higher or the initiation of renal replacement therapy.
The presence of underlying comorbidities was identified by ICD-9-CM codes based on previously validated criteria,24 and we calculated the Charlson-Deyo comorbidity index and grouped patients into score categories of 0, 1, 2, and 3 or higher.25 Aspirin, statins, angiotensin-converting enzyme inhibitors, and β-blockers dispensed on the day of admission were extracted from the pharmacy database. We stratified hospital admission hemoglobin values into missing and 4 other categories (<8.0, 8.0–9.9, 10.0–11.9, and ≥12 g/dL [to convert to grams per liter, multiply by 10.0]).26 The thresholds were developed after constructing the spline function of the hemoglobin values and the risk for mortality in univariate analysis and are similar to previously used values in patients with kidney disease.26 Using primary admission service and primary and secondary procedure codes, we classified all surgical procedures as peripheral vascular procedures (consisting of open carotid, open peripheral, and endovascular peripheral procedures), endovascular thoracic and abdominal aortic procedures, open thoracic and abdominal procedures, and lower extremity amputations.
Statistical Analysis
Data were analyzed from June 1, 2014, to September 7, 2015. The analytical plan followed the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations for observational cohort studies.27 We used Kaplan-Meier estimates to calculate cumulative survival probabilities for all-cause mortality. We used the Fine and Gray proportional subdistribution hazards regression analysis to model cardiovascular- and cancer-specific mortality while treating any other cause of death as a competing risk.28 We first modeled cardiovascular-specific mortality using the occurrence of kidney disease during the index hospitalization as the primary covariate of interest and adjusted for preoperative covariates, including age, sex, ethnicity, comorbidities via the Charlson-Deyo comorbidity index,29 admission hemoglobin levels, and emergent surgery status. To better examine the effect of the renal insult on outcomes, we constructed a second model stratifying the AKI covariate into stages 1 through 3 and the CKD covariate into mild, moderate, and severe disease while adjusting for the same covariates as the first model. We developed a third model for cardiovascular-specific mortality that accounted for individual comorbidities; admission medications; surgery type; the preoperative covariates age, sex, ethnicity, and admission hemoglobin level; and emergent surgery status. The occurrence of kidney disease was not stratified according to the severity of the insult in this model. Adjusted hazards ratios (HRs) with 95% CIs were reported for each covariate in the model.
For internal validation and to assess prediction accuracy of competing risk models, we created independent validation data sets using a bootstrap cross-validation method. The prediction models were trained on 100 bootstrap samples that were drawn with replacement from the original data, and the models were assessed in the observations that were not in the bootstrap sample. Discriminative power was compared using the Harrell C index,30 and sensitivity analyses were performed using alternative classifications for cardiovascular and cancer causes of death and using different methods for missing hemoglobin values and reference serum creatinine level (eMethods in the Supplement). The Bonferroni correction was used whenever more than 2 groups were compared. All significance tests were 2 sided, with P < .05 considered statistically significant. Fine and Gray modeling was performed using R, version 3.2.0,with the cmprsk package,31 and all other statistical analyses were performed with SAS software (version 9.3; SAS Institute Inc).
Results
Baseline Characteristics and All-Cause Mortality
Baseline characteristics stratified by the occurrence of kidney disease and comorbidities are presented in Table 1 and eTable 2 in the Supplement. Overall, 2089 of the 3646 patients undergoing major vascular surgery (57.3%) had evidence of kidney disease during hospitalization. At the time of hospitalization, 128 patients (3.5%) had ESRD and 496 (13.6%) had CKD that did not require renal replacement therapy. Among the 496 patients with CKD, 115 (23.2%) presented with severe disease (eGFR, <30 mL/min/1.73m2). During hospitalization, 1801 patients (49.4%) developed AKI, and 1465 (81.3%) of these did not have underlying CKD. Patients with severe CKD before admission were more likely to develop AKI during hospitalization than patients with mild or moderate CKD. Patients with any form of kidney disease were more likely to be older, be of African American ethnicity, have comorbid congestive heart failure, and have an admission hemoglobin level lower than 10 g/dL. The presence of 3 or more comorbidities at admission was significantly more common in patients with ESRD and CKD regardless of whether their stay was complicated by AKI. Emergent surgery was common (1408 [38.60%] of all patients), which reflected the tertiary care referral patterns at the University of Florida and was associated with AKI and CKD. Patients who underwent open carotid and peripheral vascular procedures were less likely to develop perioperative AKI regardless of underlying comorbid CKD.
Table 1.
Clinical Characteristics for All Patients Stratified by Kidney Disease
| Variables | Patient Groupsa | ||||
|---|---|---|---|---|---|
| No Known Kidney Disease (n = 1557) | AKI | CKD Without AKI (n = 160) | ESRD (n = 128) | ||
| Without CKD (n = 1465) | With CKD (n = 336) | ||||
| Age, mean (SD), y | 62 (14) | 66 (14)b | 68 (12)b | 69 (12)b | 64 (12) |
| Age ≥65 y, No. (%) | 741 (47.6) | 895 (61.1)b | 234 (69.6)b | 112 (70.0)b | 62 (48.4) |
| Female, No. (%) | 575 (36.9) | 555 (37.9) | 94 (28.0)b | 58 (36.3) | 45 (35.2) |
| African American ethnicity, No. (%) | 116 (7.5) | 157 (10.7)b | 53 (15.8)b | 25 (15.6)b | 33 (25.8)b |
| Emergent surgery, No. (%) | 498 (32.0) | 607 (41.4)b | 156 (46.4)b | 65 (40.6) | 82 (64.1)b |
| Weekend admission, No. (%) | 139 (8.9) | 172 (11.7)b | 39 (11.6) | 16 (10.0) | 25 (19.5)b |
| Charlson-Deyo comorbidity index, No. (%) | |||||
| 0 | 86 (5.5) | 55 (3.8) | 1 (0.3)b | 3 (1.9) | 0b |
| 1 | 591 (38.0) | 540 (36.9) | 56 (16.7)b | 19 (11.9)b | 1 (0.8)b |
| 2 | 484 (31.1) | 458 (31.3) | 54 (16.1)b | 26 (16.3)b | 8 (6.3)b |
| ≥3 | 396 (25.4) | 412 (28.1) | 225 (67.0)b | 112 (70.0)b | 119 (93.0)b |
| Comorbidities, No. (%) | |||||
| Myocardial infarction | 205 (13.2) | 208 (14.2) | 62 (18.5)b | 28 (17.5) | 15 (11.7) |
| Congestive heart failure | 115 (7.4) | 177 (12.1)b | 75 (22.3)b | 39 (24.4)b | 32 (25.0)b |
| Chronic obstructive pulmonary disease | 450 (28.9) | 429 (29.3) | 112 (33.3) | 58 (36.3) | 24 (18.8) |
| Diabetes mellitus | 336 (21.6) | 281 (19.2) | 76 (22.6) | 49 (30.6)b | 54 (42.2)b |
| Surgery type, No. (%) | |||||
| Endovascular thoracic and abdominal | 240 (15.4) | 200 (13.7) | 60 (17.9) | 32 (20.0) | 13 (10.2) |
| Lower extremity amputations | 118 (7.6) | 63 (4.3)b | 20 (6.0) | 20 (12.5) | 25 (19.5)a |
| Open abdominal | 304 (19.5) | 370 (25.3)b | 88 (26.2) | 23 (14.4) | 21 (16.4) |
| Open carotid | 74 (4.8) | 31 (2.1)b | 3 (0.9)b | 11 (6.9) | 1 (0.8) |
| Endovascular peripheral | 111 (7.1) | 99 (6.8) | 24 (7.1) | 24 (15.0)b | 19 (14.8) |
| Open peripheral | 334 (21.5) | 194 (13.2)b | 43 (12.8)b | 25 (15.6) | 33 (25.8) |
| Open thoracic | 376 (24.1) | 508 (34.7)b | 98 (29.2) | 25 (15.6) | 16 (12.5)b |
| Admission hemoglobin level, g/dL, No. (%) | |||||
| Missing | 318 (20.4) | 215 (14.7) | 53 (15.8) | 27 (16.9) | 26 (20.3) |
| <10 | 249 (16.0) | 299 (20.4)b | 76 (22.6)b | 34 (21.3) | 35 (27.3)b |
| 10–12 | 460 (29.5) | 463 (31.6) | 113 (33.6) | 56 (35.0) | 35 (27.3) |
| ≥12 | 530 (34.0) | 488 (33.3) | 94 (28.0) | 43 (26.9) | 32 (25.0) |
| Admission medications, No. (%) | |||||
| Aspirin | 409 (26.3) | 318 (21.7)b | 75 (22.3) | 44 (27.5) | 31 (24.2) |
| Statin | 386 (24.8) | 307 (21.0) | 91 (27.1) | 50 (31.3) | 28 (21.9) |
| Angiotensin-converting enzyme inhibitor | 194 (12.5) | 205 (14.0) | 53 (15.8) | 25 (15.6) | 21 (16.4) |
| β-Blocker | 626 (40.2) | 572 (39.0) | 152 (45.2) | 76 (47.5) | 61 (47.7) |
| Chronic kidney disease, No. (%) | |||||
| Mild to moderatec | NA | NA | 248 (73.8) | 133 (83.1) | NA |
| Severed | NA | NA | 88 (26.2)e | 27 (16.9) | NA |
| AKI, No. (%) | |||||
| Mild to moderatef | NA | 1214 (82.9) | 135 (40.2) | NA | NA |
| Severeg | NA | 251 (17.1) | 201 (59.8)h | NA | NA |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; ESRD, end-stage renal disease; NA, not applicable.
SI conversion factor: To convert hemoglobin to grams per liter, multiply by 10.0.
Percentages have been rounded and may not total 100.
P < .05 for comparison with respect to the group with no known kidney disease using Bonferroni adjustment for 4 comparisons.
Indicates estimated glomerular filtration rate (eGFR) of 30 mL/min/1.73m2 or higher.
Indicates eGFR lower than 30 mL/min/1.73m2.
P < .05 for association between CKD severity (mild to moderate or severe) and 2 renal groups (AKI with CKD and CKD with no AKI).
Indicates stage 1 (a 50% change in serum creatinine level) or 2 (a doubling in serum creatinine level).
Indicates stage 3 (a tripling or increase in serum creatinine level to 4.0mg/dL or higher [to convert to micromoles per liter, multiply by 88.4] or the initiation of renal replacement therapy).
P < .05 for association between AKI severity (mild to moderate or severe) and 2 renal groups (AKI with no CKD and AKI with CKD).
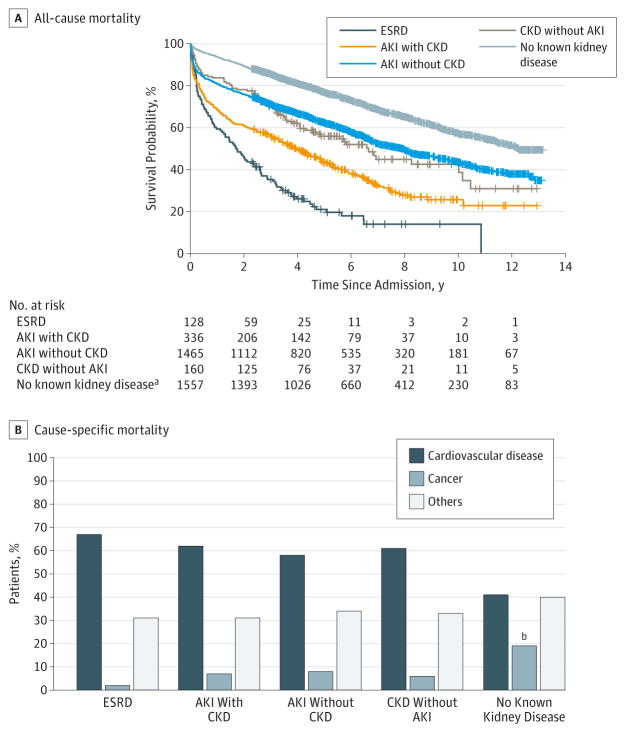
Long-term survival rates for patients with any form of acute or chronic kidney disease were significantly lower compared with patients with no known kidney disease after a median follow-up of 7 years (P < .001) (Figure 1 and Figure 2).At 10 years of follow-up, unadjusted cumulative survival probability considering all-cause mortality was 59% for patients with no known kidney disease, whereas the survival probability ranged from 13% to 44% for patients with any form of kidney disease. The 10-year cumulative survival for patients with AKI but no CKD was comparable to that for patients with CKD but no AKI. The top 2 causes of death in our cohort were cardiovascular disease (845 of 1577 [53.6%]) and cancer (173 of 1577 [11.0%]). A significantly greater proportion of patients died owing to cardiovascular disease in the group with AKI but no CKD (399 of 693 [57.6%]), AKI with underlying CKD (132 of 215 [61.4%]), CKD without AKI (47 of 76 [61.8%]), and ESRD (68 of 101 [67.3%]) compared with patients with no known kidney disease (199 of 494 [40.4%]). Conversely, the proportion of deaths due to cancer was less in patients with any kidney disease (78of 1085 [7.2%]) compared with patients with no kidney disease (95 of 492 [19.3%]).
Figure 1. Unadjusted Survival Probability.
Kaplan-Meier survival curves and cumulative survival probabilities are given for patients stratified by kidney disease for all-cause mortality and cause-specific mortality. AKI indicates acute kidney injury; CKD, chronic kidney disease; and ESRD, end-stage renal disease.
aLog-rank P < .001 for comparison of groups with respect to the group with no known kidney disease using Bonferroni adjustment.
bLog-rank P < .05 for comparison with respect to the group with no known kidney disease using Bonferroni adjustment.
Figure 2. Unadjusted Cumulative Incidence Curves for Cardiovascular-Specific Mortality by Kidney Disease Status.
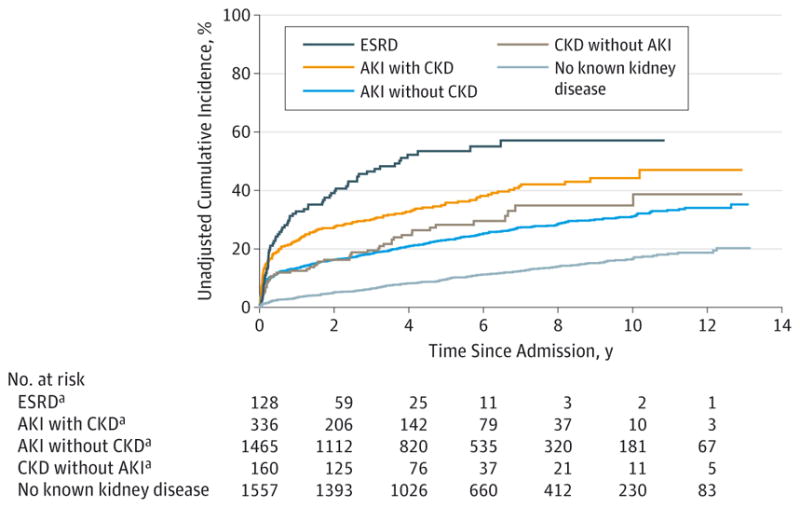
AKI indicates acute kidney injury; CKD, chronic kidney disease; and ESRD, end-stage renal disease.
aLog-rank P < .001 for comparison of groups with respect to the group with no known kidney disease using Bonferroni adjustment.
Competing Risk Models and Cardiovascular-Specific Mortality
Our final competing risk model for cardiovascular-specific mortality included the occurrence of kidney disease as the covariate of interest and was additionally adjusted for individual comorbidities, admission medications, surgery type and status, admission hemoglobin levels, and other demographic characteristics (Table 2 and Figure 3). Relative to the reference group with no known kidney disease, the multivariate proportional subdistribution hazards model (HR; 95%CI) showed that AKI with no CKD (2.07; 1.74–2.45), AKI with underlying CKD (2.99; 2.37–3.78),CKD without AKI (2.01; 1.46–2.78), and ESRD (4.90; 3.67–6.53) were independently associated with excess, long-term cardiovascular-specific mortality. Patients with AKI without CKD had an adjusted HR comparable to that of patients with CKD without AKI. Adjusted HRs were significantly elevated for age; the presence of previous myocardial infarction; comorbid congestive heart failure, chronic obstructive pulmonary disease, and diabetes mellitus; and emergent surgery status. With an admission hemoglobin level of 12 g/dL or higher as the reference group, hemoglobin levels lower than 10 and 10 to 12 g/dL were also significantly associated with cardiovascular-specific mortality. Peripheral vascular procedures and lower extremity amputations were additionally associated with excess long-term mortality when endovascular thoracic and abdominal procedures were treated as the reference group. As in the unadjusted results, the cancer-specific mortality was less in patients with any kidney disease compared with the cardiovascular-specific mortality.
Table 2.
Adjusted HRs for Cardiovascular-Specific Mortality Using Multivariable Subdistributional Hazards Models
| Variables | Adjusted HR (95% CI) | ||
|---|---|---|---|
| Model 1a | Model 2b | Model 3c | |
| Kidney disease | |||
| No known kidney disease | 1 [Reference] | NA | 1 [Reference] |
| AKI without CKD | 2.05 (1.73–2.43)d | NA | 2.07 (1.74–2.45)d |
| AKI with CKD | 2.73 (2.16–3.45)d | NA | 2.99 (2.37–3.78)d |
| CKD without AKI | 1.86 (1.35–2.58)d | NA | 2.01 (1.46–2.78)d |
| ESRD | 3.99 (2.95–5.40)d | NA | 4.90 (3.67–6.53)d |
| Kidney disease stratified by severity stagese | |||
| No known kidney disease | NA | 1 [Reference] | NA |
| ACI | |||
| Stage 1 | NA | 1.43 (1.17–1.75)d | NA |
| Stage 2 | NA | 2.08 (1.67–2.60)d | NA |
| Stage 3 | NA | 4.17 (3.40–5.12)d | NA |
| Mild CKD | NA | 1.33 (0.70–2.55) | NA |
| Moderate CKD | NA | 2.04 (1.35–3.06)d | NA |
| Severe CKD | NA | 2.49 (1.32–4.69)d | NA |
| ESRD | NA | 4.25 (3.14–5.76)d | NA |
| Age per 1-y increase | 1.03 (1.02–1.04)d | 1.03 (1.02–1.04)d | 1.03 (1.02–1.04)d |
| Male (vs female) | 1.01 (0.87–1.16) | 1.01 (0.87–1.16) | 1.03 (0.89–1.19) |
| African American vs other ethnicities | 1.08 (0.87–1.33) | 1.08 (0.87–1.34) | 1.06 (0.86–1.32) |
| Charlson-Deyo comorbidity index | |||
| 0 | 1 [Reference] | 1 [Reference] | NA |
| 1 | 0.99 (0.59–1.66) | 0.97 (0.57–1.65) | NA |
| 2 | 1.75 (1.05–2.94)d | 1.68 (0.996–2.85) | NA |
| ≥3 | 1.93 (1.15–3.22)d | 1.79 (1.06–3.03)a | NA |
| Comorbidities | |||
| Myocardial infarction (yes vs no) | NA | NA | 1.32 (1.11–1.58)d |
| Congestive heart failure (yes vs no) | NA | NA | 1.42 (1.18–1.71)d |
| Chronic obstructive pulmonary disease (yes vs no) | NA | NA | 1.28 (1.10–1.49)d |
| Diabetes mellitus (yes vs no) | NA | NA | 1.20 (1.02–1.41)d |
| Admission medications | |||
| Aspirin (yes vs no) | NA | NA | 1.01 (0.85–1.19) |
| Statin (yes vs no) | NA | NA | 0.83 (0.69–0.99)d |
| Angiotensin-converting enzyme inhibitor (yes vs no) | NA | NA | 0.86 (0.70–1.05) |
| β-Blocker (yes vs no) | NA | NA | 0.97 (0.83–1.12) |
| Surgery type | |||
| Endovascular thoracic and abdominal | NA | NA | 1 [Reference] |
| Peripheral vascularf | NA | NA | 1.40 (1.10–1.78)d |
| Open thoracic and abdominal | NA | NA | 1.24 (0.99–1.57) |
| Lower extremity amputations | NA | NA | 1.43 (1.02–1.99)d |
| Emergent surgery (vs elective) | 1.54 (1.34–1.78)d | 1.41 (1.22–1.63)d | 1.47 (1.27–1.71)d |
| Admission hemoglobin level, g/dL | |||
| ≥12 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| <10 | 1.36 (1.12–1.66)d | 1.32 (1.09–1.61)d | 1.39 (1.14–1.69)d |
| 10–12 | 1.20 (1.01–1.44)d | 1.20 (1.01–1.44)d | 1.24 (1.04–1.48)d |
| Missing | 1.10 (0.89–1.37) | 1.12 (0.91–1.39) | 1.10 (0.89–1.37) |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; ESRD, end-stage renal disease; HR, hazard ratio; NA, not applicable.
SI conversion factor: To convert hemoglobin to grams per liter, multiply by 10.0.
Adjusted for Charlson-Deyo comorbidity index.
Adjusted for Charlson-Deyo comorbidity index and kidney disease severity.
Adjusted for individual comorbidities, admission medications, and surgery type.
P < .05.
Stages of AKI and severity of CKD are described in Table 1.
Includes open carotid, open peripheral, and endovascular peripheral procedures.
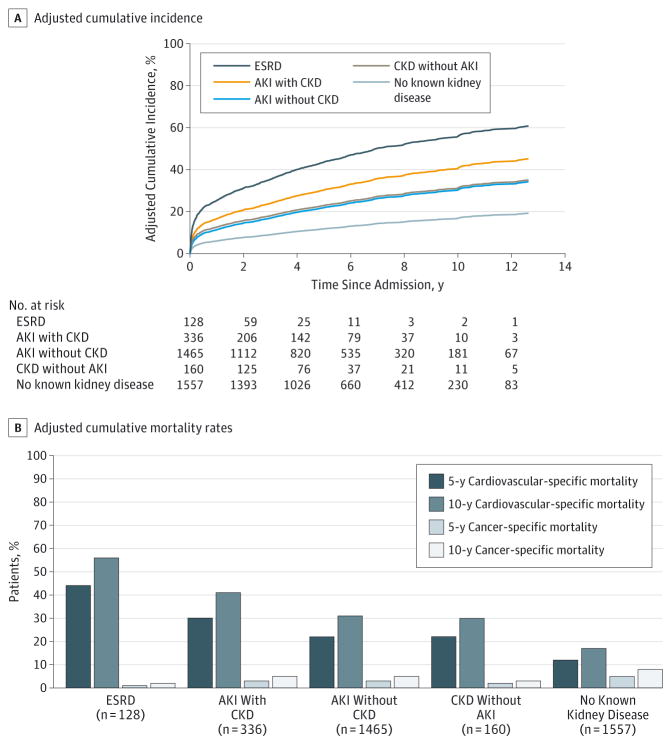
Figure 3. Model-Based Adjusted Cumulative Incidence and Mortality.
Cumulative incidence curves are given for cardiovascular-specific mortality by kidney disease status. Covariates are adjusted for age, sex, ethnicity, comorbidities, admission medications, emergent surgery status, surgery type, and admission hemoglobin level. All groups with acute kidney injury (AKI) or chronic kidney disease (CKD) have significantly higher hazards ratios compared with the group with no known kidney disease (P < .001). The bar graph shows adjusted 5- and 10-year cumulative cardiovascular and cancer-specific mortality rates for each group.
Compared with patients with no known disease, the risk for cardiovascular-specific mortality was proportional to the severity of the kidney disease. Among patients who developed AKI, the adjusted HRs(95%CIs) were 1.43 (1.17–1.75), 2.08 (1.67–2.60), and 4.17 (3.40–5.12) for patients with stages 1, 2, and 3 kidney injury, respectively. Patients with mild CKD before the index hospital admission had an insignificant increase in the HR (95% CI) for cardiovascular-specific mortality (1.33; 0.70–2.55), whereas those for cardiovascular specific mortality were significantly increased for patients with moderate (2.04; 1.35–3.06) or severe (2.49; 1.32–4.69)CKD before the index hospital admission.
For internal validation of the study, we compared the performance of models in the training and validation cohort from multiple iterations. The C index values for our final multivariable competing risk model from training cohorts were 0.76, 0.72, and 0.70 at 1, 5, and 10 years, respectively. This model performed well, with C index values (95% CI) of 0.75 (0.71–0.77),0.71 (0.69–0.73),and0.69(0.67–0.70) at 1, 5,and10 years, respectively, simultaneously in the validation data set. Similar performances were observed for the other models, and no significant differences were found in the C index values of competing risk models applied to training and validation cohorts (P > .05 for all).
The unadjusted and adjusted cumulative incidence of cardiovascular-specific mortality was lower for patients with no known kidney disease relative to patients with AKI or CKD (Figure 2 and Figure 3). Ten-year, adjusted, cardiovascular-specific mortality estimates were 17%,31%,30%,41%,and 56% for patients with no known kidney disease, AKI without CKD, CKD without AKI, AKI with CKD, and ESRD, respectively (P < .001) (Figure 3). Patients with AKI without CKD had a 10-year estimate of cardiovascular-specific mortality comparable to that of patients with CKD but no AKI. In contrast, 10-year adjusted cancer-specific mortality estimates were highest for patients with no known kidney disease at 8% and ranged from 2% for patients with ESRD to 5% for patients with AKI without CKD and AKI with CKD (Figure 3). Similar results were obtained in adjusted and unadjusted analyses when comparing patients grouped by sex, age, and type of procedure (eFigures 1–4 in the Supplement).
Discussion
In a cohort of patients undergoing major vascular surgery, AKI and CKD were associated with significant increases in long-term cardiovascular-specific mortality compared with patients with no kidney disease. This association was proportional to the severity of kidney disease independent of the patients’ age, sex, comorbidity burden at admission, or the type of operation in the cohort that included open and endovascular surgery. Postoperative AKI, with or without underlying CKD, was independently associated with cardiovascular-specific mortality. Even patients with stage 1 AKI, who in clinical practice are often not even considered to have true organ damage, had a 43%increase in the adjusted HR for cardiovascular-specific mortality compared with patients with no kidney disease. The incidence of AKI alone was more than 3 times that of CKD alone, and patients with AKI alone had a cardiovascular-specific mortality comparable to that seen in patients with CKD alone for all periods. Patients with AKI superimposed on underlying CKD constituted a smaller proportion of patients with AKI but had an even higher cardiovascular-specific mortality.
Declining eGFR and the development of albuminuria, the clinical manifestations of CKD, are both important determinants of longevity in the general population.32 Chronic kidney disease is a well-known risk factor for cardiovascular disease, and the absolute risk for death increases exponentially with decreasing renal function, even among patients without manifest cardiovascular disease.33,34 Individuals with lesser stages of CKD are more likely to die of cardiovascular disease than to develop kidney failure requiring dialysis, whereas those with ESRD have as much as 30 times the cardiovascular mortality of the general population.1–3,35 Recent studies suggest that AKI might also be a risk factor for cardiovascular disease through the progression to CKD or through independent mechanisms.36–40 Patients with AKI have an increased risk for coronary angiography, coronary artery bypass grafting surgery, myocardial infarction, congestive heart failure, and stroke regardless of any progression to CKD.41–43
Major open vascular surgery, with the risk for intraoperative hypotension and the frequent need to cross-clamp the aorta, has long had an association with renal failure.44 Modern vascular surgery relies heavily on preoperative and, especially with endovascular procedures, intraoperative contrast enhanced imaging and thus carries an increased risk for contrast-induced AKI. The use of nephrotoxic broad-spectrum antibiotics can increase the risk for AKI in the perioperative period for patients undergoing major vascular surgery.45 The novel finding in this study is that major vascular surgery has high cardiovascular-specific mortality associated with AKI and CKD. This finding has important implications for the preoperative and perioperative management of the patient undergoing major vascular surgery.
Efforts must then focus on AKI prevention in vascular surgery, mitigation of further injury when AKI has already occurred, and facilitation of renal recovery in patients with established AKI. More tools are available toward these goals than are commonly recognized. Goal-directed intraoperative management to reduce the risk for postoperative AKI through optimizing renal perfusion is feasible and underused.46 The emergence of new biomarkers and imaging techniques has provided new tools for early risk stratification and diagnosis in the peri-operative period.47,48 Standardized follow-up is important after an episode of AKI and can help to prevent the development of CKD.49 This area can be addressed quickly and relatively easily because less than50%ofpatientswith the most severe AKI will have a follow-up creatinine level measured within the first 3 months of hospitalization, and follow-up is even less likely to be obtained after less severe AKI.50
We acknowledge the limitations of all retrospective studies. With the use of multivariable adjustments and the evaluation of model discrimination on validation data sets, we have attempted to increase the internal validity of the competing risk models. Although our study used patients from a single center, which may limit our ability to generalize the findings, the study site is a large tertiary care center that receives a large number of referrals from all over the state and hence has a very heterogeneous patient profile with a wide range of procedures. To our knowledge, no prospective surgical cohort of this size and heterogeneity had concomitant data for kidney disease and cardiovascular mortality. We had only limited data concerning urine output or concerning AKI and preoperative albuminuria among patients with CKD; if available, those data could have strengthened our analysis. We used a combination of ICD-9-CM administrative codes and eGFR on admission to define CKD status. A recent systematic review demonstrated that, although sensitivity for coded CKD covariates was highly variable, specificity was high, with all studies reporting values of 0.90 or higher.51 We have not attempted to link progression to ESRD among patients with AKI as a main determinant of cardiovascular mortality, although the evidence from patients with mild CKD suggests that death from cardiovascular disease is more likely than progression to dialysis.1–3 Several traditional Framingham risk factors for cardiovascular disease were not recorded in our administrative database—including systolic blood pressure, total and high-density lipoprotein cholesterol levels, smoking history, and current use of antihypertensives—and thus could not be included as covariates.52 Patient cardiovascular comorbidity information, however, was available for previous myocardial infarctions, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes mellitus without complications, and diabetes mellitus with complications, and each patient’s Charlson-Deyo comorbidity index was calculated and included as a model covariate.
Conclusions
To the best of our knowledge, this study is the first to demonstrate that the deleterious effects of AKI and CKD on long-term survival after major vascular surgery are comparable and are primarily owing to the increase in cardiovascular-specific mortality. Both AKI and CKD were common in patients undergoing major vascular surgical procedures and were associated with as much as a 4-fold increase in long-term cardiovascular-specific mortality compared with patients with no kidney disease. This association was proportional to the severity of kidney disease and was unaffected by sex and markedly more pronounced in older patients. These findings reinforce the importance of preoperative CKD risk stratification through the application of consensus staging criteria for CKD using eGFR and albuminuria for all patients undergoing major vascular surgery. Preoperative and postoperative risk stratification for AKI using clinical scores and urinary biomarkers similarly can help to direct the implementation of simple and inexpensive preventive strategies in the perioperative period that could prevent or mitigate further decline in kidney function. The appropriate transition of patients undergoing surgery to follow-up in the outpatient setting with an emphasis on the prevention of kidney disease progression and mitigation of cardiovascular risk can be an important factor in improving the care of the patient undergoing vascular surgery who has AKI and/or CKD. Our findings present compelling evidence that such efforts are warranted and justifiable.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by Center for Sepsis and Critical Illness Award P50 GM-111152 from the National Institute of General Medical Sciences (Dr Bihorac); by research grants from the Society of Critical Care Medicine and Astute Medical, Inc (Dr Bihorac); by an award from the I Heermann Anesthesia Foundation, Inc, and Vision Grant for data acquisition (Dr Bihorac); by a medical student summer research fellowship from the University of Florida and T35 funds from the National Institutes of Health (NIH) (Mr Huber); and in part by Clinical and Translational Sciences award UL1 TR000064 from the National Center for Advancing Translational Sciences, NIH (University of Florida).
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: This paper was presented at the 39th Annual Meeting of the Association of Veterans Administration Surgeons; May 4, 2015; Miami Beach, Florida.
Supplemental content at jamasurgery.com
Author Contributions: Dr Hobson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ozrazgat-Baslanti, Bihorac.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Huber, Ozrazgat-Baslanti, Bihorac, Hobson.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ozrazgat-Baslanti, Thottakkara, Bihorac, Hobson.
Obtained funding: Bihorac.
Administrative, technical, or material support: Scali, Bihorac, Hobson.
Study supervision: Scali, Hobson.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 2.van der Velde M, Matsushita K, Coresh J, et al. Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality: a collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 3.Gaber AO, Moore LW, Aloia TA, et al. Cross-sectional and case-control analyses of the association of kidney function staging with adverse postoperative outcomes in general and vascular surgery. Ann Surg. 2013;258(1):169–177. doi: 10.1097/SLA.0b013e318288e18e. [DOI] [PubMed] [Google Scholar]
- 4.Mathew A, Devereaux PJ, O’Hare A, et al. Chronic kidney disease and postoperative mortality: a systematic review and meta-analysis. Kidney Int. 2008;73(9):1069–1081. doi: 10.1038/ki.2008.29. [DOI] [PubMed] [Google Scholar]
- 5.Patel VI, Mukhopadhyay S, Guest JM, et al. Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J Vasc Surg. 2014;59(2):368–375. doi: 10.1016/j.jvs.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Beck AW, Goodney PP, Nolan BW, Likosky DS, Eldrup-Jorgensen J, Cronenwett JL Vascular Study Group of Northern New England. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(4):838–843. doi: 10.1016/j.jvs.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 7.AbuRahma AF, Srivastava M, Stone PA, et al. The effect of chronic renal insufficiency by use of glomerular filtration rate versus serum creatinine level on late clinical outcome of carotid endarterectomy. J Vasc Surg. 2015;61(3):675–682. doi: 10.1016/j.jvs.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber M, Ozrazgat-Baslanti T, Thottakkara P, et al. Mortality and cost of acute and chronic kidney disease after vascular surgery [published online July 14, 2015] Ann Vasc Surg. doi: 10.1016/j.avsg.2015.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopolovic I, Simmonds K, Duggan S, Ewanchuk M, Stollery DE, Bagshaw SM. Risk factors and outcomes associated with acute kidney injury following ruptured abdominal aortic aneurysm. BMC Nephrol. 2013;14(1):99. doi: 10.1186/1471-2369-14-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora P, Davari-Farid S, Pourafkari L, Gupta A, Dosluoglu HH, Nader ND. The effect of acute kidney injury after revascularization on the development of chronic kidney disease and mortality in patients with chronic limb ischemia. J Vasc Surg. 2015;61(3):720–727. doi: 10.1016/j.jvs.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Kabbani LS, West CA, Viau D, et al. Survival after repair of pararenal and paravisceral abdominal aortic aneurysms. J Vasc Surg. 2014;59(6):1488–1494. doi: 10.1016/j.jvs.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Drews JD, Patel HJ, Williams DM, Dasika NL, Deeb GM. The impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repair. Ann Thorac Surg. 2014;97(6):2027–2033. doi: 10.1016/j.athoracsur.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 13.Adalbert S, Adelina M, Romulus T, et al. Acute kidney injury in peripheral arterial surgery patients: a cohort study. Ren Fail. 2013;35(9):1236–1239. doi: 10.3109/0886022X.2013.823830. [DOI] [PubMed] [Google Scholar]
- 14.Piffaretti G, Mariscalco G, Bonardelli S, et al. Predictors and outcomes of acute kidney injury after thoracic aortic endograft repair. J Vasc Surg. 2012;56(6):1527–1534. doi: 10.1016/j.jvs.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 15.Bihorac A, Brennan M, Ozrazgat-Baslanti T, et al. National surgical quality improvement program underestimates the risk associated with mild and moderate postoperative acute kidney injury. Crit Care Med. 2013;41(11):2570–2583. doi: 10.1097/CCM.0b013e31829860fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jim J, Owens PL, Sanchez LA, Rubin BG. Population-based analysis of inpatient vascular procedures and predicting future workload and implications for training. J Vasc Surg. 2012;55(5):1394–1399. doi: 10.1016/j.jvs.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 17.Murray CJ, Atkinson C, Bhalla K, et al. US Burden of Disease Collaborators. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61(6):1–51. [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712–719. doi: 10.2215/CJN.10821011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald R, Waikar SS, Liangos O, Pereira BJG, Chertow GM, Jaber BL. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg. 2006;43(3):460–466. doi: 10.1016/j.jvs.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–163. [Google Scholar]
- 23.Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–141. [Google Scholar]
- 24.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a US population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158(10):709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shavit L, Hitti S, Silberman S, et al. Preoperative hemoglobin and outcomes in patients with CKD undergoing cardiac surgery. Clin J Am Soc Nephrol. 2014;9(9):1536–1544. doi: 10.2215/CJN.00110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Wolbers M, Blanche P, Koller MT, Witteman JC, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014;15(3):526–539. doi: 10.1093/biostatistics/kxt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed March 14, 2015];cmprsk: Subdistribution Analysis of Competing Risks: R package version 2.2-2. computer program https://cran.r-project.org/web/packages/cmprsk/index.html.
- 32.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 34.Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarnak MJ, Levey AS, Schoolwerth AC, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 36.Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014;9(3):448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watabe H, Sato A, Hoshi T, et al. Association of contrast-induced acute kidney injury with long-term cardiovascular events in acute coronary syndrome patients with chronic kidney disease undergoing emergent percutaneous coronary intervention. Int J Cardiol. 2014;174(1):57–63. doi: 10.1016/j.ijcard.2014.03.146. [DOI] [PubMed] [Google Scholar]
- 39.Rydén L, Ahnve S, Bell M, et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. Int J Cardiol. 2014;172(1):190–195. doi: 10.1016/j.ijcard.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Holzmann MJ, Rydén L, Sartipy U. Acute kidney injury and long-term risk of stroke after coronary artery bypass surgery. Int J Cardiol. 2013;168(6):5405–5410. doi: 10.1016/j.ijcard.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 41.Wu VC, Wu CH, Huang TM, et al. NSARF Group. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu VC, Wu PC, Wu CH, et al. National Taiwan University Study Group on Acute Renal Failure (NSARF) Group. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc. 2014;3(4):e000933. doi: 10.1161/JAHA.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olsson D, Sartipy U, Braunschweig F, Holzmann MJ. Acute kidney injury following coronary artery bypass surgery and long-term risk of heart failure. Circ Heart Fail. 2013;6(1):83–90. doi: 10.1161/CIRCHEARTFAILURE.112.971705. [DOI] [PubMed] [Google Scholar]
- 44.Payne JH, Wood DL, Goethel JA. Oliguria and renal failure in abdominal aortic surgery: prophylaxis with mannitol. Am Surg. 1963;29:713–718. [PubMed] [Google Scholar]
- 45.Yamout H, Levin ML, Rosa RM, Myrie K, Westergaard S. Physician prevention of acute kidney injury. Am J Med. 2015;128(9):1001–1006. doi: 10.1016/j.amjmed.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K Optimisation Systematic Review Steering Group. Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev. 2012;11(11):CD004082. doi: 10.1002/14651858.CD004082.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Göcze I, Renner P, Graf BM, Schlitt HJ, Bein T, Pfister K. Simplified approach for the assessment of kidney perfusion and acute kidney injury at the bedside using contrast-enhanced ultrasound. Intensive Care Med. 2015;41(2):362–363. doi: 10.1007/s00134-014-3554-7. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez F, Vincent F. Biomarkers for acute kidney injury in critically ill patients. Minerva Anestesiol. 2012;78(12):1394–1403. [PubMed] [Google Scholar]
- 49.Meran S, Wonnacott A, Amphlett B, Phillips A. How good are we at managing acute kidney injury in hospital? Clin Kidney J. 2014;7(2):144–150. doi: 10.1093/ckj/sfu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Renal Data System. USRDS 2014 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2014. [Google Scholar]
- 51.Grams ME, Plantinga LC, Hedgeman E, et al. CDC CKD Surveillance Team. Validation of CKD and related conditions in existing data sets: a systematic review. Am J Kidney Dis. 2011;57(1):44–54. doi: 10.1053/j.ajkd.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.