Abstract
Background
Postoperative chemotherapy is standard following preoperative chemoradiation therapy (CRT) and curative resection for clinically staged II/III rectal cancer. Recent trials have questioned whether postoperative chemotherapy improves overall survival. We evaluated the comparative effectiveness of postoperative chemotherapy following CRT or radiation therapy (RT) with specific attention on the impact of age on postoperative chemotherapy effectiveness.
Methods
Patients treated with CRT or RT then resection of pathologically staged 0-III rectal cancer diagnosed from 2004-2009 were identified from the Surveillance, Epidemiology and End Results program-Medicare database. Propensity score weighted Cox proportional hazards models and Kaplan Meier methods were used to compare the effectiveness of 1) postoperative 5-fluorouracil (5-FU) or capecitabine to no treatment and 2) postoperative oxaliplatin+5-FU/capecitabine to 5-FU/capecitabine alone on mortality. Results were stratified by age.
Results
We identified 1,316 patients; 49% received postoperative chemotherapy, 341 (52%) included oxaliplatin. After weighting, postoperative 5-FU/capecitabine alone was associated with decreased mortality in patients aged 66-74 (adjusted hazard ratio (aHR)=0.46, 95%CI: 0.30,0.72), corresponding to a 5-year risk difference of -0.23, (95%CI: -0.33,-0.12). No further mortality reduction from adding oxaliplatin to 5-FU/capecitabine was seen in patients aged 66-74 (aHR=1.57, 95%CI: 0.93,2.65). No mortality reduction for 5-FU/capecitabine alone was observed among patients aged 75+ (aHR=1.11, 95%CI: 0.76,1.63).
Conclusions
Among patients<75 years, postoperative 5-FU/capecitabine was associated with reduced mortality after preoperative CRT/RT and surgical resection; however, addition of oxaliplatin was not associated with further mortality reduction. Decisions regarding postoperative chemotherapy after age 75 warrants consideration of individual patient risks and preferences, as benefits may be limited.
Keywords: rectal cancer, chemotherapy, aging, comparative effectiveness research
Introduction
For over 30 years the standard curative approach for stage II and III rectal cancer has included surgical resection, chemoradiotherapy (CRT), and 5-fluoruracil (5-FU) based postoperative chemotherapy. This approach is based on results of randomized controlled trials (RCTs) conducted in the 1980s-90s where patients treated with all three modalities had the lowest rates of local and distant recurrence and the longest survival.[1, 2] Rectal cancer treatment has since evolved. Total mesorectal excision is now standard with resultant lower rates of local recurrence.[3-6] CRT is administered preoperatively, providing better functional outcomes and possibly better local control and DFS.[7, 8] In 2004, the addition of oxaliplatin to 5-FU or capecitabine was shown to offer incremental survival benefit for patients with stage III colon cancer,[9-12] and its use was rapidly incorporated into treatment guidelines for rectal cancer and disseminated into routine clinical practice.[13]
While the best outcomes have been observed in patients treated with all modalities, early trials were not designed to test the individual contribution of each component of the preoperative CRT and postoperative chemotherapy platform on rectal cancer outcomes. The Cochrane Collaboration conducted a meta-analysis of 21 RCTs to examine whether the postoperative chemotherapy component in isolation reduces mortality over observation.[14] The meta-analysis reported a 17% reduction in the relative risk of all-cause mortality associated with postoperative chemotherapy; however, only a single trial, EORTC 22921, specifically evaluated chemotherapy following modern preoperative CRT. Now after 10 years of follow-up, 5-FU only marginally reduced mortality compared with observation (hazard ratio (HR)=0.91, 95%CI: 0.77,1.09); however, fewer than half of patients in EORTC 22921 were able to complete postoperative therapy at the planned dose and schedule.[15, 16] A second multi-trial analysis in which individual patient data from EORTC 22921 were combined with that of three additional trials of chemotherapy following preoperative treatment showed no benefit of postoperative chemotherapy (HR=0.97, 95%CI: 0.81,1.17).[17]
The three recently completed randomized trials comparing postoperative 5-FU/capecitabine with and without oxaliplatin have done little to elucidate the role of postoperative oxaliplatin, as in two trials the addition of oxaliplatin resulted in an improvement in disease free survival (DFS) of a similar magnitude as is seen in stage III colon cancer, but the third reported no DFS benefit.[18-20] Though long-term follow-up is immature, it seems unlikely that these results will markedly change given the close relationship between DFS and overall survival in colorectal cancer.[21]
While expert guidelines from the National Comprehensive Cancer Network,[22] European Society of Medical Oncology,[23] and the National Institute for Health and Care Excellence [24] continue to include the recommendation to at least consider postoperative chemotherapy, there is insufficient data about the effectiveness of postoperative chemotherapy (with our without oxaliplatin) to reduce recurrence or cancer mortality in preoperatively-treated patients.[25, 26] Because older adults tend to be underrepresented in trials,[27] there are even less data regarding the effectiveness of postoperative chemotherapy approaches in this population.
We evaluated the effectiveness of postoperative chemotherapy with 5-FU or capecitabine alone - and the incremental effectiveness of oxaliplatin - following preoperative CRT and surgery for rectal cancer in routine care settings in the US. With over half of all patients with rectal cancer diagnosed over the age of 65, [28] we also specifically evaluated the impact of age on the comparative effectiveness of postoperative chemotherapy approaches.
Methods
Data sources
Cancer cases were obtained from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program which collects demographic and tumor data for individuals diagnosed with cancer residing within one of the 18 SEER regions.[29] The linkage of persons in SEER with their Medicare enrollment and claims data allows for the identification of cancer treatments [30, 31] and extended mortality follow-up.
Study population
We included patients diagnosed with pathologically confirmed, first primary cancer of the rectum/rectosigmoid junction from 2004-2009, with continuous Medicare Parts A and B fee-for-service and no managed care enrollment for the 12-months before and 6-months following the cancer diagnosis date, ensuring complete claims capture and to define covariates and treatments. Patients with American Joint Commission on Cancer (AJCC) 6th Edition stage 0-III were included to ensure inclusion of patients with clinical stage II and III rectal cancer (for which CRT is standard) who were down staged by preoperative therapy because pathologic stage (e.g. ypT and ypN stage) supersedes clinical stage in SEER. Patients who underwent complete surgical resection within 180 days from diagnosis and received preoperative CRT or radiation therapy alone (RT) between the date of diagnosis and surgery date were included in the study.
Ascertainment of treatment
Administrative codes identified cancer-directed treatments (Appendix A) and have high validity for identifying specific chemotherapy agents.[30-32] Postoperative chemotherapy was assessed from the date of surgery through the following 122 days (4 months). Specific chemotherapeutic agents administered within the 60 days of the first chemotherapy administration were used to define the initial postoperative chemotherapy group (i.e., 5-FU/capecitabine alone or in combination with oxaliplatin).[30, 33-35]
Ascertainment of all-cause and cancer-specific mortality
The primary outcome of interest was all-cause mortality ascertained through December 2011 from the Medicare enrollment database.[36] Follow-up began 122 days from the surgery date (i.e., the landmark). The secondary outcome of cancer-specific mortality was defined from the death certificate cause of death reported to SEER; however, ascertainment was limited through December 2009.
Ascertainment of covariates
The diagnosis year, sex, age at diagnosis, race/ethnicity, marital status, region of residence, county and census tract level socioeconomic data were obtained from SEER.[37] Comorbidity was measured using conditions from Klabunde's adaptation of the Charlson Comorbidity Index (CCI).[38] Hospitalization within 30 days following surgical resection was a proxy for surgical complications, shown to be associated with both chemotherapy receipt and all-cause mortality.[39] For each patient, we estimated the predicted probability of activities of daily living dependence (ADL-D) as a proxy for frailty using a previously validated model.[40]
Statistical Analysis
We evaluated the association between postoperative chemotherapy with 5-FU/capecitabine alone versus observation and all-cause and cancer-specific mortality. We constructed a rich propensity score (PS) model that estimated the probability that each patient received postoperative 5-FU/capecitabine alone versus observation to control for measured confounding.[41] This model drew upon prior work,[13] including age, sex, race/ethnicity, marital status, metropolitan area, the proportion of individuals living below the poverty line within the same census tract, pathologic stage, cancer site, receipt of preoperative CRT or RT, specific comorbidities, hospitalization within 30 days from surgery, and the predicted probability of ADL-D. To control for measured confounders, we balanced the covariates across treatment groups using standardized mortality ratio (SMR)-weighting, assigning a weight of 1 for patients treated with postoperative 5-FU/capecitabine alone and a weight of the propensity odds [(PS)/(1-PS)] for patients who did not receive postoperative chemotherapy, creating a “pseudo-population” of patients who did not receive postoperative chemotherapy with the same covariate distribution as that observed in patients who received postoperative chemotherapy with 5-FU/capecitabine alone.[42, 43] Covariate balance was evaluated using standardized absolute mean difference (SAMD); adequate balance was considered as SAMD<0.1.[44]
The same analytic approach was implemented among all patients receiving postoperative chemotherapy in order to evaluate the association between the addition of oxaliplatin to postoperative 5-FU/capecitabine alone and all-cause and cancer-specific mortality. However, this analysis was restricted to patients aged 66-74 years in an effort to further reduce the potential for residual confounding by frailty among the oldest patient subgroups (where oxaliplatin treatment is least likely). We constructed a separate PS model to estimate the probability that each patient was treated with postoperative oxaliplatin (compared with 5-FU or capecitabine alone).
Cox proportional hazards models with SMR weights and robust standard errors (to account for the lack of independence due to weighting) were used to estimate adjusted hazard ratios (aHRs) and their 95% confidence intervals (CIs) for all-cause and cancer-specific mortality. Analyses of cancer-specific mortality accounted for non-cancer death as a competing risk.[45] The weighted cumulative incidence of all-cause and cancer-specific mortality was plotted for each postoperative treatment comparison and used to calculate adjusted 3- and 5-year risk ratios (aRRs) and risk differences (aRDs).[46] Ninety-five percent confidence intervals (CIs) were generated using the standard deviation of 200 bootstraps. Analyses for the comparison of postoperative chemotherapy versus observation were further stratified by age group (<75 vs.75+ years, based on clinical input), pathologic stage (0-I, II, III) and preoperative treatment (CRT vs. RT only). Exploratory analyses evaluated treatment effect heterogeneity by age on all-cause mortality in greater detail using a 5-year age-stratified moving window approach. All data were analyzed in SAS, version 9.3 (SAS Institute, Cary, NC). The institutional review board at the University of North Carolina at Chapel Hill determined that this research was exempt from review.
Results
Patterns of postoperative chemotherapy
In total, 1,316 patients with non-metastatic rectal cancer 66 years and older were included (Figure 1). Approximately half of eligible patients received postoperative chemotherapy following preoperative CRT and resection (n=650, 49%) and 341 (52%) of these chemotherapy treated patients received oxaliplatin (Table 1). Older age, lower stage, metropolitan residence and 30-day post-surgical hospitalization were associated with lower use of postoperative chemotherapy with 5-FU/capecitabine alone (versus observation), while comorbid peripheral vascular disease and preoperative CRT receipt were associated with higher use (Supplemental Table 1a). Among patients age 66-74 years who received postoperative chemotherapy, older age, higher census tract poverty level, lower stage and 30-day post-surgical hospitalization were associated with lower oxaliplatin receipt (Supplemental Table 1b). After PS weighting, measured characteristics were well balanced across treatment groups (SAMD <0.1, Supplemental Tables 2a-b).
Figure 1. Study population flowchart.
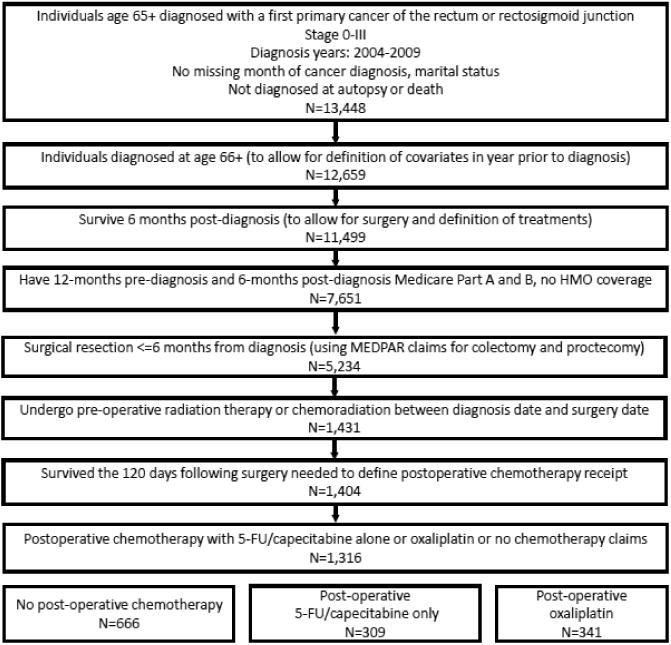
Cohort inclusion and exclusion criteria for older individuals diagnosed with pathologically staged, non-metastatic rectal cancer at age 66 years or older using the Surveillance, Epidemiology and End Results program (SEER)-Medicare linked data from 2003-2009.
Table 1. Characteristics of 1,316 older patients with non-metastatic rectal cancer receiving preoperative treatment and surgical resection by postoperative treatment type, 2004-2009.
| Characteristics | No postoperative chemotherapy | Postoperative 5-FU/ capecitabine alone | Postoperative oxaliplatin | |||
|---|---|---|---|---|---|---|
| n=666 | % | n=309 | % | n=341 | % | |
| Age at diagnosis | ||||||
| 66-69 years | 129 | 19 | 85 | 28 | 141 | 41 |
| 70-74 years | 213 | 32 | 98 | 32 | 113 | 33 |
| 75-79 years | 177 | 27 | 73 | 24 | 74 | 22 |
| 80+ years | 147 | 22 | 53 | 17 | 13 | 4 |
| Male | 381 | 57 | 178 | 58 | 214 | 63 |
| Race/ethnicity | ||||||
| White, Non-Hispanic | 526 | 79 | 244 | 79 | 287 | 84 |
| Black, Non-Hispanic | 43 | 6 | 16 | 5 | 14 | 4 |
| Other, Non-Hispanic | 46 | 7 | 23 | 7 | 23 | 7 |
| Hispanic | 51 | 8 | 26 | 8 | 17 | 5 |
| Marital status | ||||||
| Married | 385 | 58 | 196 | 63 | 220 | 65 |
| Widowed | 173 | 26 | 62 | 20 | 61 | 18 |
| Single | 58 | 9 | 23 | 7 | 30 | 9 |
| Separated or Divorced | 50 | 8 | 28 | 9 | 30 | 9 |
| Metropolitan residence | 598 | 90 | 257 | 83 | 309 | 91 |
| % living below poverty line (census tract) | ||||||
| Quartile 1 (low) | 151 | 23 | 65 | 21 | 107 | 31 |
| Quartile 2 | 168 | 25 | 71 | 23 | 90 | 26 |
| Quartile 3 | 173 | 26 | 82 | 27 | 76 | 22 |
| Quartile 4 (high) | 174 | 26 | 91 | 29 | 68 | 20 |
| AJCC pathologic stage | ||||||
| 0 and I | 204 | 31 | 75 | 24 | 54 | 16 |
| II | 298 | 45 | 121 | 39 | 120 | 35 |
| III | 164 | 25 | 113 | 37 | 167 | 49 |
| Diagnosis year | ||||||
| 2004 | 112 | 17 | 62 | 20 | 26 | 8 |
| 2005 | 125 | 19 | 61 | 20 | 62 | 18 |
| 2006 | 110 | 17 | 46 | 15 | 60 | 18 |
| 2007 | 118 | 18 | 46 | 15 | 64 | 19 |
| 2008 | 91 | 14 | 43 | 14 | 77 | 23 |
| 2009 | 110 | 17 | 51 | 17 | 52 | 15 |
| Rectum vs. Rectosigmoid | 597 | 90 | 278 | 90 | 309 | 91 |
| Preoperative chemoradiation vs. radiation therapy only | 527 | 79 | 290 | 94 | 312 | 91 |
| Selected comorbidities | ||||||
| Congestive heart failure | 43 | 6 | 14 | 5 | NR | NR |
| COPD | 65 | 10 | 23 | 7 | 26 | 8 |
| Diabetes without sequelae | 141 | 21 | 72 | 23 | 60 | 18 |
| Diabetes with sequelae | 19 | 3 | 11 | 4 | 13 | 4 |
| 30-day post-operative hospitalization | 245 | 37 | 69 | 22 | 58 | 17 |
| Predicted probability of ADL-D (median, IQR) | 0.034 (0.023, 0.054) | 0.031 (0.021, 0.046) | 0.026 (0.020, 0.038) | |||
Abbreviations: 5-FU=5-fluorouracil; NR=not reportable due to cell size less than 11, AJCC=American Joint Commission on Cancer, COPD=chronic obstructive pulmonary disease, ADL-D=activities of daily living dependence.
Effectiveness of postoperative 5-FU or capecitabine alone
Over a mean follow-up of 3.8 and 3.5 years for postoperative 5-FU/capecitabine groups and observation, respectively, all-cause mortality was significantly lower for those patients treated with postoperative 5-FU/capecitabine alone compared to observation (HR=0.68, 95%CI: 0.52, 0.88, Supplemental Table 3a). This survival benefit was not substantially altered by control for measured confounding (aHR=0.72, 95%CI 0.55, 0.96).
The benefit of postoperative 5-FU/capecitabine over observation was restricted to patients aged 66-74 years (aHR=0.46, 95%CI: 0.30, 0.72, Figure 2, Supplemental Table 3a). This translates to an absolute risk reduction in all-cause mortality of 14% at 3 years (aRD=-0.14, 95%CI: -0.23, -0.04) and 23 % at 5 years (aRD=-0.23, 95%CI: -0.33, -0.12), leading to a 3- and 5-year number needed to treat (NNT) of 7 and 4 (Table 2), assuming a causal effect. In contrast, patients aged 75+ years did not appear to benefit from postoperative 5-FU/capecitabine alone compared with observation (aHR=1.11, 95%CI: 0.76, 1.63, Figure 2, Supplemental Table 3a).
Figure 2. Figure 2a-f. Propensity score-weighted cumulative all-cause and cancer-specific mortality among patients with non-metastatic rectal cancer who received postoperative 5-FU or capecitabine alone vs. no postoperative chemotherapy by age group (panels a-d) and postoperative oxaliplatin vs. postoperative 5-FU/capecitabine alone among patients age 66-74 years (panels e-f).
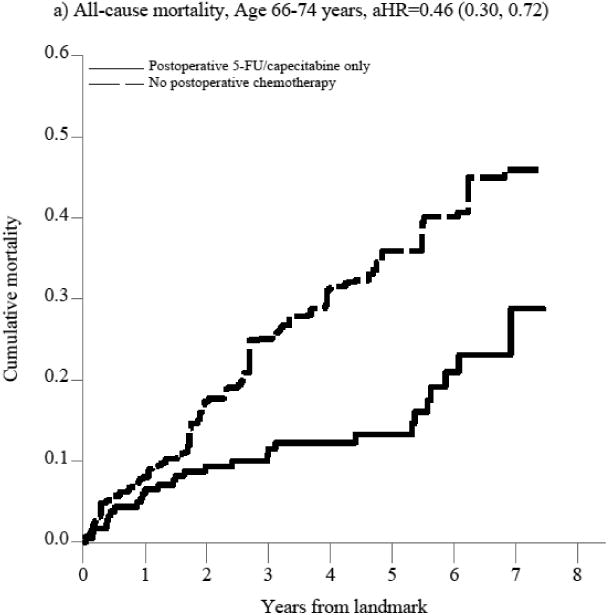
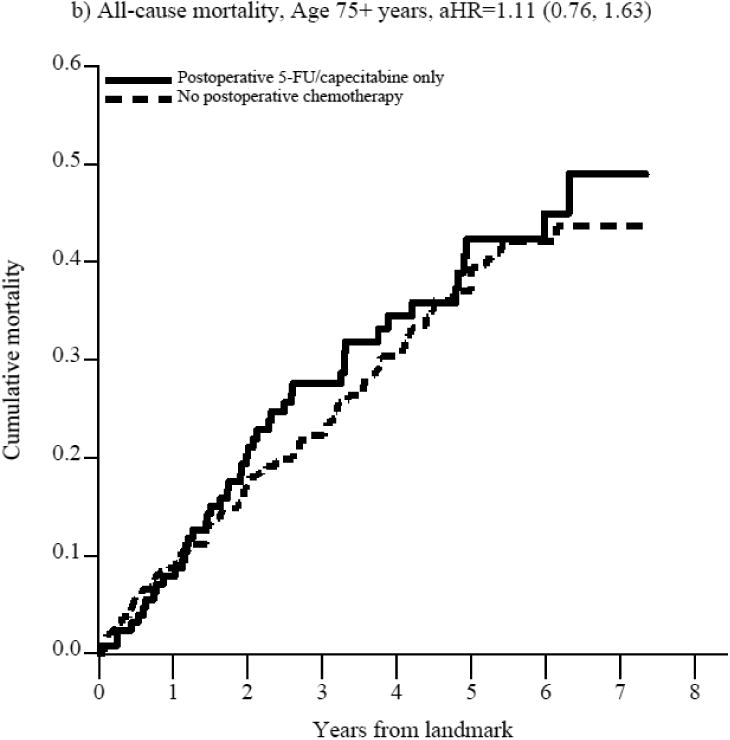
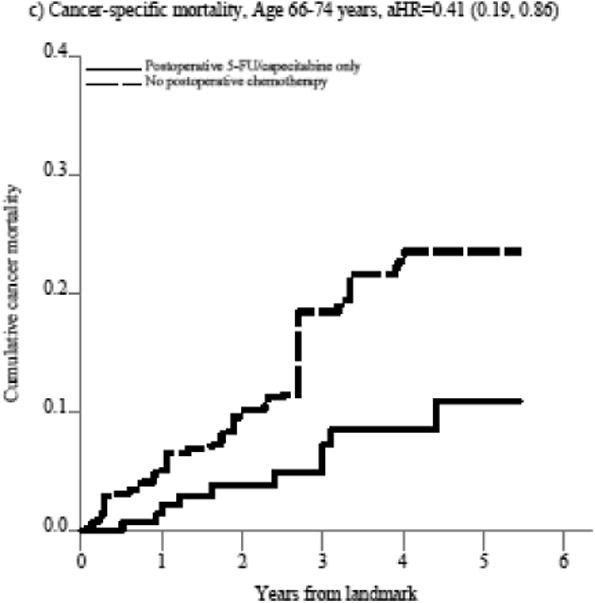
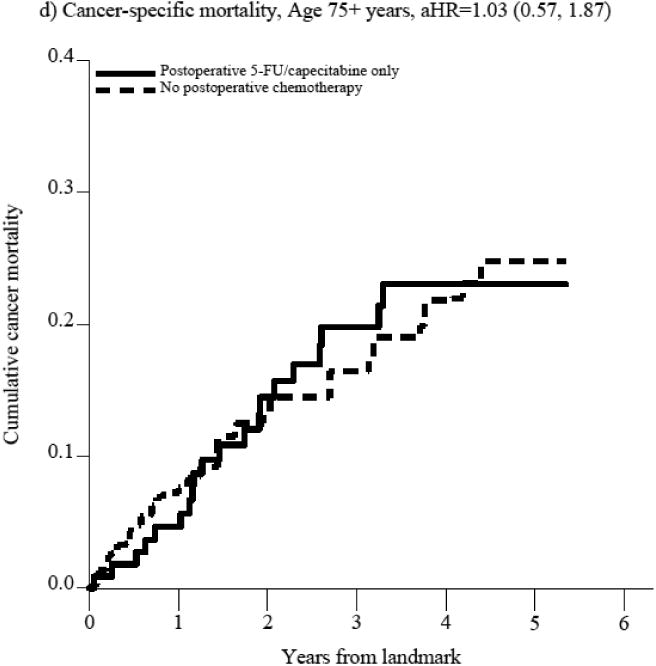
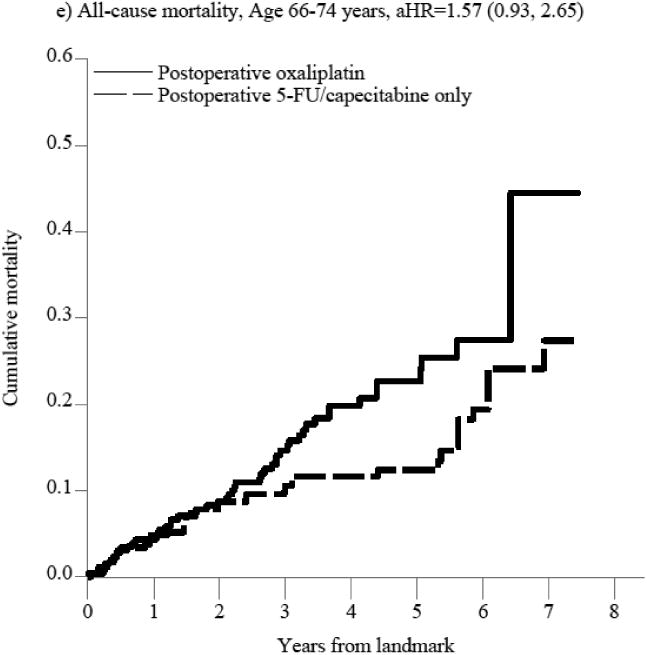
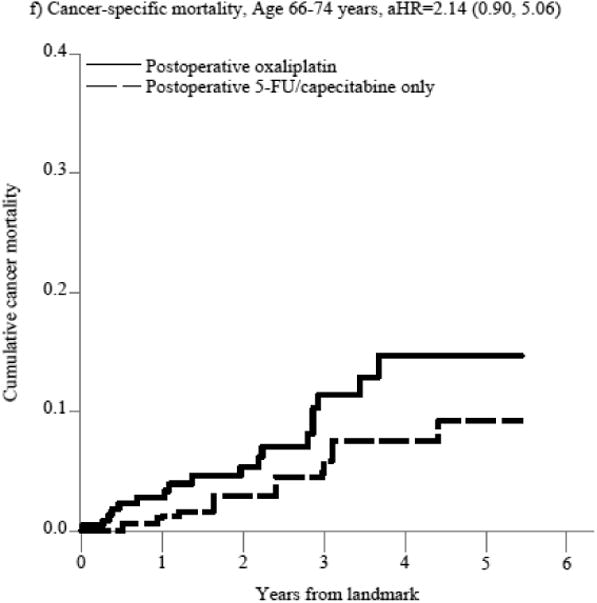
In panels a-d, the blue (solid) line represents the cumulative incidence of the specific outcome (i.e., all-cause or cancer specific mortality) for individuals who received postoperative 5-FU/capecitabine alone while the red (dashed) line represents the cumulative incidence of the outcome for individuals who did not receive postoperative chemotherapy. In panels e-f, the blue (solid) line represents the cumulative incidence of the outcome for individuals who received postoperative oxaliplatin and the red (dashed) line represents the cumulative incidence of the outcome for individuals who received postoperative 5-FU/capecitabine alone.
Table 2. Crude and weighted 3- and 5-year risk differences and risk ratios comparing postoperative 5-FU/capecitabine alo vs. no postoperative chemotherapy and all-cause and cancer-specific mortality.
| Outcome risk | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Outcome | Subgroup | Crude/Weighted and time period | No postoperative chemotherapy | Postoperative 5-FU/capecitabine alone | Risk difference | 95% CI | Risk ratio | 95% CI |
| All-cause mortality | Age 66-74 years | Crude | ||||||
| 3-year | 0.23 | 0.12 | -0.11 | (-0.18, -0.05) | 0.51 | (0.32, 0.80) | ||
| 5-year | 0.34 | 0.13 | -0.21 | (-0.29, -0.13) | 0.39 | (0.25, 0.61) | ||
| Age 75+ years | Crude | |||||||
| 3-year | 0.25 | 0.28 | 0.03 | (-0.06, 0.12) | 1.12 | (0.79, 1.58) | ||
| 5-year | 0.41 | 0.42 | 0.01 | (-0.10, 0.12) | 1.02 | (0.78, 1.34) | ||
| Age 66-74 years | Weightedb | |||||||
| 3-year | 0.25 | 0.12 | -0.14 | (-0.23, -0.04) | 0.46 | (0.28, 0.76) | ||
| 5-year | 0.36 | 0.13 | -0.23 | (-0.33, -0.12) | 0.37 | (0.23, 0.60) | ||
| Age 75+ years | Weightedb | |||||||
| 3-year | 0.22 | 0.28 | 0.05 | (-0.05, 0.16) | 1.24 | (0.81, 1.91) | ||
| 5-year | 0.39 | 0.42 | 0.03 | (-0.10, 0.16) | 1.07 | (0.77, 1.49) | ||
| Cancer-specific mortality | Age 66-74 years | Crude | ||||||
| 3-year | 0.12 | 0.07 | -0.05 | (-0.11, 0.01) | 0.59 | (0.30, 1.16) | ||
| 5-year | 0.19 | 0.11 | -0.08 | (-0.17, 0.01) | 0.58 | (0.25, 1.33) | ||
| Age 75+ years | Crude | |||||||
| 3-year | 0.15 | 0.20 | 0.05 | (-0.05, 0.15) | 1.34 | (0.75, 2.39) | ||
| 5-year | 0.22 | 0.23 | 0.01 | (-0.12, 0.14) | 1.05 | (0.56, 1.97) | ||
| Age 66-74 years | Weightedb | |||||||
| 3-year | 0.18 | 0.07 | -0.11 | (-0.29, 0.06) | 0.39 | (0.13, 1.16) | ||
| 5-year | 0.24 | 0.11 | -0.13 | (-0.29, 0.04) | 0.46 | (0.17, 1.28) | ||
| Age 75+ years | Weightedb | |||||||
| 3-year | 0.16 | 0.20 | 0.03 | (-0.09, 0.15) | 1.20 | (0.59, 2.43) | ||
| 5-year | 0.25 | 0.23 | -0.02 | (-0.17, 0.14) | 0.93 | (0.46, 1.88) | ||
Abbreviations: CI=confidence interval
Standard error based on standard deviation of 200 nonparametric bootstrap resamples.
Weighted for age, sex, race/ethnicity, AJCC pathologic stage, cancer site, marital status, metropolitan residence, census tract poverty level (%), congestive heart failure, peripheral vascular disease, cerebrovascular disease, COPD, diabetes without sequelae, diabetes with sequelae, renal disease, 30-days post-operative hospitalization, preoperative therapy (chemoradiation or radiation therapy alone), predicted probability of ADL-D.
The substantial mortality reduction associated with postoperative 5-FU/capecitabine alone does not seem largely related to unmeasured confounding based on treatment selection as cancer-specific mortality was similarly better in the postoperative 5-FU/capecitabine treated patients compared to untreated patients in the entire cohort (aHR=0.81, 95%CI 0.50-1.31, Supplemental Table 3a) and in patients aged 66-74 (aHR=0.41, 95%CI 0.19, 0.86, Figure 2, Supplemental Table 3a) though not in patients over 75 (aHR=1.03, 95%CI 0.57, 1.87, Figure 2, Supplemental Table 3a). Stratified results did not indicate effect measure modification by pathologic stage, although associations by preoperative treatment receipt were imprecise (Supplemental Figure 1).
The exploratory analysis of treatment effect heterogeneity revealed a pattern of attenuating aHRs with increasing age (Figure 3), with the aHR reaching 1.0 at the age midpoint of 74 years (aHR=1.00, 95%CI: 0.59, 1.69). This analysis confirms the approximate clinical selection of age 75 years as a cutpoint for stratified analyses.
Figure 3. Exploratory analysis of treatment effect heterogeneity by age at diagnosis comparing postoperative 5-FU/capecitabine alone versus no postoperative chemotherapy and all-cause mortality.
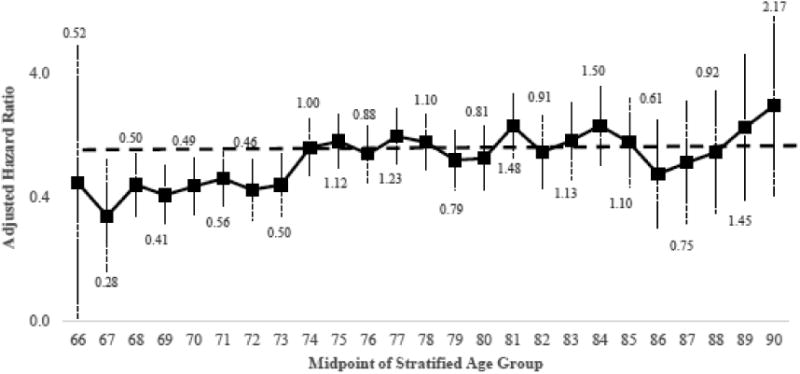
The solid black line denotes the adjusted hazard ratio estimated using age-stratified subgroups and a one-year moving window, resulting in 25 estimates. All estimates used 5-year age groupings (e.g., 71-75 years, age midpoint=73 years), when possible. The estimates for age 66 and 67 used one and three-year age groups, respectively. Estimates for ages beyond 90 years were not calculated, as data was sparse. The adjusted hazard ratios are plotted at the midpoint of each age group along with robust 95% confidence intervals (plotted on the log scale).
Comparative effectiveness of postoperative oxaliplatin versus 5-FU/capecitabine alone
Because of the observed lack of benefit of postoperative chemotherapy and the small number of patients over the age of 75 treated with oxaliplatin, we only evaluated the comparative effectiveness of adding oxaliplatin to 5-FU/capecitabine alone on all-cause and cancer-specific mortality in patients aged 66-74 years. Overall, there were 79 deaths; 29 in the 5-FU or capecitabine alone group and 50 in the postoperative oxaliplatin group with a median follow-up of 4.1 and 3.6 years, respectively. Oxaliplatin was not associated with an improvement in all-cause mortality compared with 5-FU/capecitabine alone in patients aged 66-74 years (aHR=1.57, 95%CI: 0.93, 2.65, Figure 2, Supplemental Table 3b). This translated into a non-significant increase in absolute risk at 3 years (aRD=0.04, 95%CI: -0.03, 0.11, Table 3), and significant increase in the risk of death at 5 years (aRD=0.10, 95%CI: 0.03, 0.18), leading to a 5-year number needed to treat to harm (NNTH)[47] of 10. The lack of observed mortality reduction and potential harm of oxaliplatin among patients aged 66-74 was also seen in cancer-specific mortality analyses, although these results were imprecise.
Table 3. Crude and weighted 3- and 5-year risk differences and risk ratios comparing postoperative oxaliplatin vs. postoperative 5-FU/capecitabine alone, patients age 66-74 years old.
| Outcome risk | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Outcome | Crude/Adjusted and time period | Postoperative 5-FU/capecitabine alone | Postoperative oxaliplatin | Risk difference | 95% CIa | Risk ratio | 95% CIa |
| All-cause mortality | Crude | ||||||
| 3-year | 0.12 | 0.15 | 0.03 | (-0.03, 0.10) | 1.28 | (0.76, 2.13) | |
| 5-year | 0.13 | 0.23 | 0.09 | (0.02, 0.17) | 1.71 | (1.03, 2.82) | |
| Weightedb | |||||||
| 3-year | 0.11 | 0.15 | 0.04 | (-0.03, 0.11) | 1.38 | (0.81, 2.36) | |
| 5-year | 0.12 | 0.23 | 0.10 | (0.03, 0.18) | 1.83 | (1.11, 3.03) | |
| Cancer-specific mortality | Crude | ||||||
| 3-year | 0.07 | 0.11 | 0.04 | (-0.03, 0.11) | 1.58 | (0.67, 3.69) | |
| 5-year | 0.11 | 0.15 | 0.04 | (-0.06, 0.13) | 1.34 | (0.57, 3.15) | |
| Weightedb | |||||||
| 3-year | 0.06 | 0.11 | 0.06 | (-0.02, 0.13) | 1.95 | (0.74, 5.15) | |
| 5-year | 0.09 | 0.15 | 0.05 | (-0.04, 0.15) | 1.59 | (0.62, 4.09) | |
Abbreviations: CI=confidence interval
Standard error based on standard deviation of 200 nonparametric bootstrap resamples.
Weighted for age (66-69, 70-74 years), sex, race/ethnicity, AJCC pathologic stage, married, metropolitan residence, census tract poverty level (%), COPD, diabetes, 30-day post-operative hospitalization, pre-operative therapy (chemoradiation or radiation therapy alone), predicted probability of ADL-D.
Discussion
Postoperative 5-FU/capecitabine alone following preoperative CRT and surgical resection was associated with lower all-cause and cancer-specific mortality compared with observation in this large cohort of Medicare beneficiaries. However, the benefits of postoperative chemotherapy on mortality reduction appear to be attenuated with advancing age, with no clear benefit observed in patients 75 years or older. We found no evidence that oxaliplatin incrementally reduced mortality compared with 5-FU/capecitabine alone among patients aged 66-74 years old.
Multiple European trials have specifically addressed the role of postoperative chemotherapy versus observation following CRT for rectal cancer.[17] In the analysis of patient data from four of these trials, three of which used 5-FU and leucovorin and one which used oxaliplatin with capecitabine, there was no suggestion of benefit from postoperative chemotherapy on survival (HR 0.97, 95%CI 0.81-1.17) or DFS (HR 0.91, 95%CI 0.77-1.07).[17] Low adherence to the protocol prescribed postoperative chemotherapy (where most patients were randomized at diagnosis and not after surgery) is a key limitation that has prevented these results from changing the long accepted belief that postoperative chemotherapy improves outcomes in resected rectal cancer. While seemingly contradictory, our study was designed to specifically address the role of postoperative chemotherapy among patients treated with timely preoperative CRT and surgery, rather than the entire CRT and chemotherapy program and therefore may better represent the isolated impact of postoperative chemotherapy among patients able to initiate treatment following surgical resection.
Although we used SMR-weighting methods with validated, novel measures of frailty to address measured confounding, it is likely that our estimate of an approximately 50% reduction in the risk of death following postoperative 5-FU/capecitabine alone versus observation among patients aged 66-74 is an overestimate of the actual treatment effect. In observational studies estimating treatment effectiveness, a key limitation is the inability to directly measure a number of key prognostic factors, notably frailty, performance status, and pretreatment clinical staging. This is particularly problematic in the comparison of postoperative 5-FU/capecitabine alone to observation, as the reasons that underlie the decision to forgo chemotherapy (e.g. frailty) are likely also related to increased mortality, thus failure to adequately control for these factors would lead us to overestimate the benefit of postoperative chemotherapy. Our study design, however, inherently decreases the potential for residual confounding by frailty, as all patients had to undergo active preoperative CRT or RT followed by curative surgical resection (e.g. not just local excision), thereby excluding patients deemed ineligible for aggressive treatment based on frailty or comorbidity. This type of restriction strategy has been found to be effective in reducing residual confounding by frailty.[48, 49] Bias related to treatment selection would be likely to have the greatest effect on all-cause mortality, yet we also observed a substantial reduction in cancer-related mortality associated with 5-FU/capecitabine alone. Thus, while it is unlikely that we could estimate the exact magnitude of the effect of 5-FU/capecitabine alone, we believe the design and findings of this study support the conclusion that postoperative 5-FU/capecitabine alone compared with observation likely reduces mortality following preoperative CRT/RT and surgery in patients with rectal cancer under 75 years.
While postoperative 5-FU/capecitabine alone reduced mortality among the entire cohort, there was no reduction observed among patients over age 75 at diagnosis. This decreasing benefit of postoperative therapy with increasing age among the elderly has been reported in subgroup analyses of patients with colon cancer treated in clinical trials. The landmark pooled analysis of patient data from randomized adjuvant 5-FU trials by Sargent in 2001 confirmed a similar benefit of 5-FU on freedom from recurrence in patients 70 years and over; however while there was no statistical evidence of interaction of age and survival, the survival difference between treated and untreated patients diminished with increasing follow up, presumably due to the higher rates of death from other causes.[50] Limitations of our data preclude us from evaluating the effect on recurrence specifically, and the follow-up time for cancer-specific survival is too short to make definitive conclusions based on those results.
The balance of evidence from the three recently reported trials supports the benefit of postoperative oxaliplatin versus 5-FU/capecitabine alone on DFS.[18, 20] In the one negative trial, patients were randomized at diagnosis and the resultant low completion rates make it difficult to interpret the postoperative chemotherapy effect (only 52% of patients randomized to oxaliplatin and 66% of patients randomized to capecitabine completed planned postoperative therapy).[19] Limitations of our data did not allow us to incorporate therapy completion into our analyses, which could mask the potential benefits of postoperative oxaliplatin. However, our analysis was designed to estimate the association of starting oxaliplatin versus 5-FU/capecitabine alone as an initial strategy, which answers a clinically relevant, but different question.
If oxaliplatin likely improves DFS in patients following resection of preoperatively treated rectal cancer, the pattern of benefit in rectal cancer may be akin to what is seen in colon cancer where oxaliplatin unequivocally works in younger patients but not in older patients. Just as we found no mortality reduction attributable to postoperative oxaliplatin in patients 66-74 years old, a pooled analysis of oxaliplatin in patients 70 and older with stage III colon cancer found oxaliplatin was associated with a slight improvement in time to recurrence over 5-FU alone, but there was no improvement in DFS or overall survival.[51] In the pooled analysis in colon cancer and our evaluation of patients aged 66-74 in SEER-Medicare, there was a slight suggestion that the addition of oxaliplatin may actually lead to harm. Because of the short follow-up for cancer-specific mortality and lack of information on recurrence, it is premature to conclude that oxaliplatin increases mortality. The possibility for harm should, however,be considered when counseling older patients about the addition of oxaliplatin to 5-FU/capecitabine alone following preoperative CRT and surgical resection of rectal cancer.
In summary, postoperative 5-FU/capecitabine alone was associated with a considerable reduction in mortality following preoperative CRT and surgery for non-metastatic rectal cancer in patients younger than 75 years compared with observation. Patients over the age of 75 should be counseled that any potential benefit of postoperative therapy after resection is likely to be small and treatment decisions should consider individual risks and preferences. Similar to the colon cancer setting, our data do not support the addition of oxaliplatin to postoperative chemotherapy in older patients with resected rectal cancer.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (R01 AG023178 and R01 AG042845 to T.S., K07 CA160722 to H.K.S., and K12 CA120780 to J.L.L.).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The authors would also like to acknowledge Dr. Stephen R. Cole and Dr. Jessie K. Edwards for providing SAS code used in the analyses of standardized risk differences and risk ratios in the presence of competing risks.
Footnotes
Disclosures and Conflict of Interest Statements: H Sanoff is a consultant for Amgen and has received research funding from PhRMA Foundation, Bayer, and Novartis. T Sturmer has received research funding from Amgen and Asta Zeneca and owns stock in Novartis, Roche, Astra Zeneca, and Johnson & Johnson. The authors have no other disclosure to report.
Author Contributions: Study Concepts: J Lund, H Sanoff, Study Design: J Lund, H Sanoff, T Sturmer, Data Acquisition: J Lund, H Sanoff, Quality Control of Data and Algorithms: J Lund, T Sturmer, Data Analysis and Interpretation: J Lund, H Sanoff, T Sturmer, Statistical Analysis: J Lund, H Sanoff, T Sturmer, Manuscript Preparation: J Lund, H Sanoff, Manuscript Editing: J Lund, H Sanoff, T Sturmer, Manuscript Review: J Lund, H Sanoff, T Sturmer
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gunderson LL, Sargent DJ, Tepper JE, Wolmark N, O'Connell MJ, Begovic M, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22(10):1785–96. doi: 10.1200/JCO.2004.08.173. Epub 2004/04/07. [DOI] [PubMed] [Google Scholar]
- 2.Douglass HO, Jr, Moertel CG, Mayer RJ, Thomas PR, Lindblad AS, Mittleman A, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315(20):1294–5. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 3.Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373(9666):821–8. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–60. doi: 10.1016/0140-6736(93)90207-w. Epub 1993/02/20. [DOI] [PubMed] [Google Scholar]
- 5.Kariv Y, Kariv R, Hammel JP, Lavery IC. Postoperative radiotherapy for stage IIIA rectal cancer: is it justified? Diseases of the colon and rectum. 2008;51(10):1459–66. doi: 10.1007/s10350-008-9346-9. [DOI] [PubMed] [Google Scholar]
- 6.Merchant NB, Guillem JG, Paty PB, Enker WE, Minsky BD, Quan SH, et al. T3N0 rectal cancer: results following sharp mesorectal excision and no adjuvant therapy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 1999;3(6):642–7. doi: 10.1016/s1091-255x(99)80087-0. Epub 1999/12/20. [DOI] [PubMed] [Google Scholar]
- 7.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 8.Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27(31):5124–30. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51. doi: 10.1056/NEJMoa032709. Epub 2004/06/04. [DOI] [PubMed] [Google Scholar]
- 10.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(19):3109–16. doi: 10.1200/JCO.2008.20.6771. Epub 2009/05/20. [DOI] [PubMed] [Google Scholar]
- 11.Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(11):1465–71. doi: 10.1200/JCO.2010.33.6297. Epub 2011/03/09. [DOI] [PubMed] [Google Scholar]
- 12.Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(16):2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 13.Lund JL, Sturmer T, Sanoff HK, Brookhart A, Sandler RS, Warren JL. Determinants of adjuvant oxaliplatin receipt among older stage II and III colorectal cancer patients. Cancer. 2013;119(11):2038–47. doi: 10.1002/cncr.27991. Epub 2013/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen SH, Harling H, Kirkeby LT, Wille-Jorgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. The Cochrane database of systematic reviews. 2012;3:CD004078. doi: 10.1002/14651858.CD004078.pub2. Epub 2012/03/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. The lancet oncology. 2014 doi: 10.1016/S1470-2045(13)70599-0. Epub 2014/01/21. [DOI] [PubMed] [Google Scholar]
- 16.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. doi: 10.1056/NEJMoa060829. Epub 2006/09/15. [DOI] [PubMed] [Google Scholar]
- 17.Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer a systematic review and meta-analysis of individual patient data. The lancet oncology. 2015;16(2):200–7. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 18.Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. The lancet oncology. 2014;15(11):1245–53. doi: 10.1016/S1470-2045(14)70377-8. Epub 2014/09/10. [DOI] [PubMed] [Google Scholar]
- 19.Schmoll HJ, Haustermans K, Price TJ, Nordlinger B, Hofheinz R, Daisne JF, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: Disease-free survival results at interim analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(15S) Abstr 3501. [Google Scholar]
- 20.Rodel C, Liersch T, Fietkau R, Hohenberger W, Graeven U, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with 5-fluorouracil and oxaliplatin versus 5-fluorouracil alone in locally advanced rectal cancer: Results of the German CAO/ARO/AIO-04 randomized phase III trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(15S) Abstr 3500. [Google Scholar]
- 21.Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(34):8664–70. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 22.Rectal Cancer. Version 2.2015. NCCN Clinical Practice Guidelines in Oncology [Internet] 2015 Feb; Available from: www.nccn.org.
- 23.Glimelius B, Tiret E, Cervantes A, Arnold D, Group EGW. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24(6):vi81–8. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 24.NICE clinical guideline 131. Colorectal cancer: The diagnosis and management of colorectal cancer. 2015 Feb; Available from: guidance.nice.org.uk/cg131.
- 25.Glimelius B, Pahlman L, Cervantes A. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(5):v82–6. doi: 10.1093/annonc/mdq170. Epub 2010/06/29. [DOI] [PubMed] [Google Scholar]
- 26.Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(10):2479–516. doi: 10.1093/annonc/mds236. Epub 2012/09/27. [DOI] [PubMed] [Google Scholar]
- 27.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA : the Journal of the American Medical Association. 2004;291(22):2720–6. doi: 10.1001/jama.291.22.2720. Epub 2004/06/10. [DOI] [PubMed] [Google Scholar]
- 28.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. Epub 2011/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365(9458):475–81. doi: 10.1016/S0140-6736(05)17864-7. Epub 2005/02/12. [DOI] [PubMed] [Google Scholar]
- 30.Lund JL, Sturmer T, Harlan LC, Sanoff HK, Sandler RS, Brookhart MA, et al. Identifying specific chemotherapeutic agents in Medicare data: a validation study. Medical care. 2013;51(5):e27–34. doi: 10.1097/MLR.0b013e31823ab60f. Epub 2011/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Medical care. 2002;40(8 Suppl):IV-55–61. doi: 10.1097/01.MLR.0000020944.17670.D7. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 32.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 33.Sanoff HK, Carpenter WR, Freburger J, Li L, Chen K, Zullig LL, et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: A Population-based analysis. Cancer. 2012 doi: 10.1002/cncr.27422. Epub 2012/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanoff HK, Carpenter WR, Martin CF, Sargent DJ, Meyerhardt JA, Sturmer T, et al. Comparative Effectiveness of Oxaliplatin vs Non-Oxaliplatin-containing Adjuvant Chemotherapy for Stage III Colon Cancer. J Natl Cancer Inst. 2012;104(3):211–27. doi: 10.1093/jnci/djr524. Epub 2012/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanoff HK, Carpenter WR, Sturmer T, Goldberg RM, Martin CF, Fine JP, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol. 2012;30(21):2624–34. doi: 10.1200/JCO.2011.41.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Medical care. 2002;40(8 Suppl):IV-19–25. doi: 10.1097/00005650-200208001-00003. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 37.Mandelblatt JS, Kerner JF, Hadley J, Hwang YT, Eggert L, Johnson LE, et al. Variations in breast carcinoma treatment in older medicare beneficiaries: is it black or white. Cancer. 2002;95(7):1401–14. doi: 10.1002/cncr.10825. Epub 2002/09/19. [DOI] [PubMed] [Google Scholar]
- 38.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. Epub 2001/01/09. [DOI] [PubMed] [Google Scholar]
- 39.Haynes AB, You YN, Hu CY, Eng C, Kopetz ES, Rodriguez-Bigas MA, et al. Postoperative chemotherapy use after neoadjuvant chemoradiotherapy for rectal cancer: Analysis of Surveillance, Epidemiology, and End Results-Medicare data, 1998-2007. Cancer. 2014;120(8):1162–70. doi: 10.1002/cncr.28545. Epub 2014/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiology and drug safety. 2015;24(1):59–66. doi: 10.1002/pds.3719. Epub 2014/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturmer T, Jonsson Funk M, Poole C, Brookhart MA. Nonexperimental comparative effectiveness research using linked healthcare databases. Epidemiology. 2011;22(3):298–301. doi: 10.1097/EDE.0b013e318212640c. Epub 2011/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–6. doi: 10.1097/01.EDE.0000081989.82616.7d. Epub 2003/10/22. [DOI] [PubMed] [Google Scholar]
- 43.Sturmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiology and drug safety. 2006;15(10):698–709. doi: 10.1002/pds.1231. Epub 2006/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate behavioral research. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. Epub 2011/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 46.Cole SR, Lau B, Eron JJ, Brookhart MA, Kitahata MM, Martin JN, et al. Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. American journal of epidemiology. 2015;181(4):238–45. doi: 10.1093/aje/kwu122. Epub 2014/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stang A, Poole C, Bender R. Common problems related to the use of number needed to treat. J Clin Epidemiol. 2010;63(8):820–5. doi: 10.1016/j.jclinepi.2009.08.006. Epub 2009/11/03. [DOI] [PubMed] [Google Scholar]
- 48.McGrath LJ, Ellis AR, Brookhart MA. Controlling Time-Dependent Confounding by Health Status and Frailty: Restriction Versus Statistical Adjustment. American journal of epidemiology. 2015;182(1):17–25. doi: 10.1093/aje/kwu485. Epub 2015/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneeweiss S, Patrick AR, Sturmer T, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Medical care. 2007;45(10 Supl 2):S131–42. doi: 10.1097/MLR.0b013e318070c08e. Epub 2007/10/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. Epub 2001/10/13. [DOI] [PubMed] [Google Scholar]
- 51.McCleary NJ, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, et al. Impact of Age on the Efficacy of Newer Adjuvant Therapies in Patients With Stage II/III Colon Cancer: Findings From the ACCENT Database. J Clin Oncol. 2013;31(20):2600–6. doi: 10.1200/JCO.2013.49.6638. Epub 2013/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


