Abstract
Preparation of small molecule based dual-modality probes remains a challenging task due to the complicated synthetic procedure. In this study, a novel concise and generic strategy for preparing dual-modality optical/PET imaging probes via photo-click chemistry was developed, in which the diazole photo-click linker functioned not only as a bridge between the targeting-ligand and the PET imaging moiety, but also as the fluorophore for optical imaging. A dual-modality AE105 peptidic probe was successfully generated via this strategy and subsequently applied in the fluorescent staining of U87MG cells and the 68Ga based PET imaging of mice bearing U87MG xenograft. In addition, dual-modality monoclonal antibody cetuximab has also been generated via this strategy and labeled with 64Cu for PET imaging studies, broadening the application of this strategy to include the preparation of macromolecule based imaging probes.
Graphical abstract
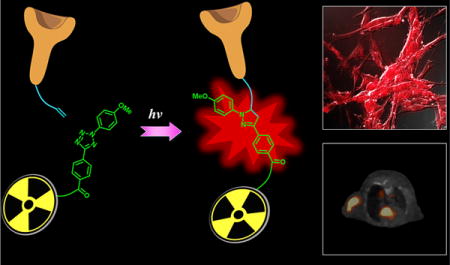
Dual-modality optical/PET imaging probes, which are featured by assembling optical imaging property and PET imaging property into one single probe to target a specific disease related biomarker, are considered as efficient tools for disease diagnosis and/or monitoring.1 In particular, PET imaging allows extremely sensitive in vivo imaging without penetration limitations, while optical imaging allows correlated fluorescent analysis (such as fluorescent staining, flow cytometry, IHC staining, etc.). Compared to using monomodality optical probes and PET probes, respectively, such dual-modality optical/PET probes intrinsically offer a better correlation between results of fluorescence experiments and those of PET imaging, especially when the biological effect of the structural difference between monomodality optical probes and the corresponding PET probes is not ignorable. Therefore, using in vitro fluorescent staining, the targeting capability of dual-modality probes on the biomarkers of interest could be assessed prior to performing in vivo PET imaging.
Depending on the type of carriers, which usually serve as transportation tools targeting disease associated abnormal cells or tissues, most dual-modality probes can be categorized into liposome based,2 quantum dots based,3 polymer based,4 protein based,5 and small molecule based6 probes, and each of them possesses its own advantages and disadvantages. In particular, small molecule based probes, rendered by their relatively faster body clearance (reduced radio dose exposure time)7 and better quality control (minimized batch to batch difference)8 compared with other larger size counterparts, play an important role in the area of molecular imaging. However, although small molecule based dual-modality probes possess desirable properties, their preparation usually involved abundant and complex chemistry work including the multiple-step synthesis as well as the frequent use of protection–deprotection groups9–11 (Figure 1A), which may limit their applications in research and/or in clinical studies.
Figure 1.
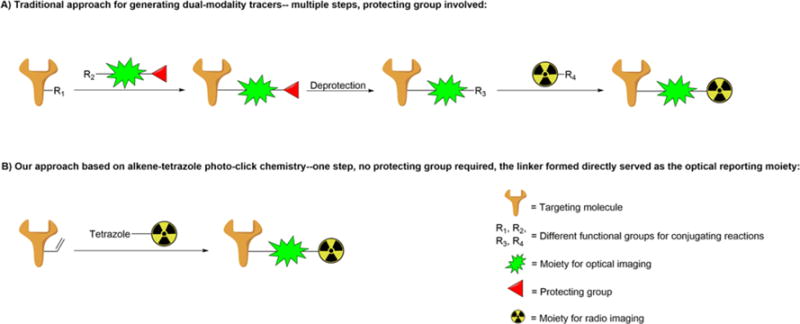
(A) Traditional approach for generating dual-modality probes. (B) Alkene tetrazole photo-click chemistry for generating dual-modality optical/PET probes.
Bioorthogonal click chemistry, represented by the strain-promoted alkyne–azide cycloaddition and inverse electron demand Diels–Alder cycloaddition,12 opens a new avenue for preparing imaging probes13 due to its biocompatible and one-pot straightforward properties. However, since it is difficult to introduce two or more different clickable functional groups into one targeting small molecule, most imaging probes prepared by click chemistry possessed just one modality,14–16 and only a few dual-modality probes generated by click chemistry were reported.17,18 Herein, we have this problem addressed by using an alkene tetrazole photo-click chemistry (Figure 1B), in which the linker formed after conjugation not only serves as a bridge between the targeting moiety and bifunctional chelator (BFC, for PET imaging), but also possesses fluorescent property (for optical imaging). The alkene tetrazole photo-click chemistry was developed by Dr. Lin and his co-workers, and it enables a rapid ligation between two bioorthogonal moieties upon light-irradiation.19 Because of its fast reaction rate (∼45 M−1 s−1,20 more than 100 times as fast as that of the widely used DBCO based click chemistry at ∼0.36 M−1 s−121) and capability of conducting both temporal and spatial controls, this promising bioorthogonal reaction has been utilized to generate a series of fluorescent photo-click products for the fluorescent imaging of living cells.22 Herein, we extend its application from preparing optical imaging probes for in vitro cell imaging to preparing optical/PET dual-modality probes for in vivo imaging. Compared to the traditional approach that usually needs at least three steps to get the desired probe (besides peptide and radioactive moiety modifications), our developed strategy takes only one step, thus significantly simplifying the preparation of dual-modality probes for preclinical and/or clinical applications, and will greatly benefit research groups without strong expertise on organic synthesis.
In a proof-of-principle study, peptidic targeting ligand AE105 (a reported antagonist of uPAR23,24) was functionalized with alkene and then photo-clicked with the tetrazole-attached BFC NOTA (Figure 2A). UPAR, the urokinase-type plasminogen activator receptor, has been demonstrated as an important biomarker involved in the development of cancer.25,26 The alkene and tetrazole functionalization compounds used in this project (Figure 2B) were synthesized via the previously reported procedures (Scheme S1).27 As illustrated in Scheme 1, the AE105 on resin was functionalized with an alkene group using compound 1 while Boc protected NOTA (compound 5) was functionalized with a tetrazole group using compound 2, and then the resulting two intermedates (compound 4 and compound 6) could be photo-clicked to generate the dual-modality optical/PET probe (compound 7) in aqueous solution quickly under mild conditions. Specifically, this photo-click reaction was carried out in phosphate buffer (pH = 7.4):acetonitrile = 1:1 solution with a concentration of alkene functionalized AE105 4 at 2 mM and a concentration of tetrazole functionalized NOTA 6 at 400 μM. Upon 2 min irradiation at 254 nm by a portable lab UV lamp, the reaction completed with high yield (>50%). The resulting dual-modality AE105 probe (compound 7) was then separated by the HPLC purification and characterized by LC-MS. In summary, both functionalization steps and the final photo-click step proceed smoothly, rendering it as an ideal approach for preparing dual-modality optical/PET probes. Besides the uPAR targeted peptidic ligand AE105, the EGFR (epidermal growth factor receptor) targeted cetuximab (monoclonal antibody) has also been successfully functionalized with acrylic-chloride in PBS buffer via the slightly modified procedures28 and subsequently photo-clicked with compound 6 to generate corresponding dual-modality optical/PET imaging probes targeting EGFR (Scheme S2).
Figure 2.
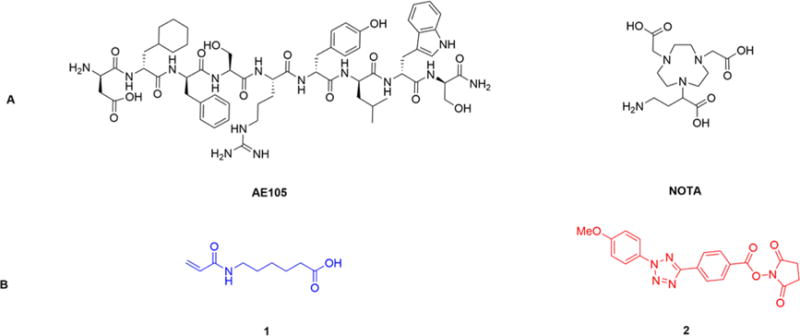
(A) AE105 and NOTA; (B) Compounds for modifying peptides and chelators with the alkene group (compound 1) and the tetrazole group (compound 2), respectively.
Scheme 1. Synthesis of the Dual-Modality AE105 Probe by the Alkene Tetrazole Photo-Click Chemistrya.
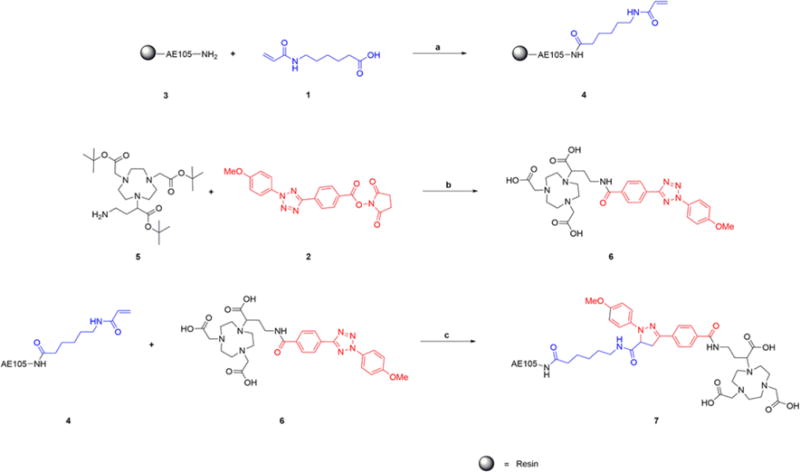
aReagents and conditions: (a) (1) EDCI, HOBT, Et3N, DMF, rt, overnight, (2) 95%TFA, rt, 1 h; (b) (1) DIPEA, DMF, rt, 2 h, (2). 95%TFA, rt, 1 h; (c) irradiated by UV254 nm 2 min, rt, 5 min.
The absorption and emission spectra of the prepared dual modality AE105 probe were then recorded using a Cary 100 Bio UV/vis spectrophotometer and a Cary Eclipse Fluorescence spectrophotometer, respectively. Corresponding curves were plotted subsequently (Figure 3). It was found that the fluorescent pyrazoline linker exhibited a maximum absorption at around 360 nm and a maximum emission at around 570 nm, both values were consistent with the previous reported results for compounds bearing the similar fluorescent core.29
Figure 3.
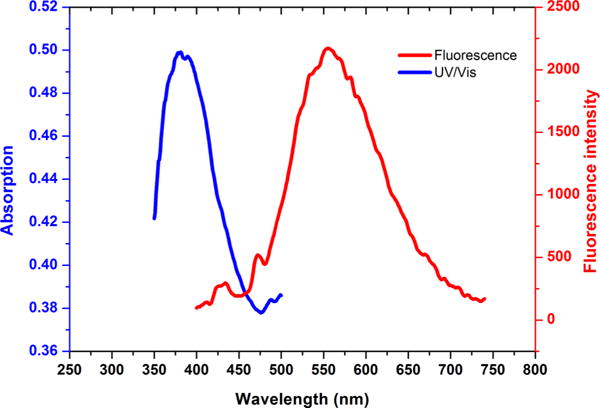
Absorption and emission curves of synthesized multimodal AE105 probe.
The fluorescent property confirmed by the absorption and emission curve prompted us to apply the synthesized dual-modality probe in the fluorescent staining of the uPAR overexpressed U87MG cell line. In particular, cells were incubated with the probe for 2 h at room temperature. Upon removal of the medium containing the unbounded probe, cells were washed, fixed, and imaged by the fluorescence microscopy using a specific filter (excitation at 365 nm, emission at 605 ± 35 nm). Red fluorescence was observed (Figure 4A), indicating the successful staining of U87MG with the synthesized probe. To investigate the specificity of this probe toward uPAR, another blocking group was set up as a negative control in which 500 times excess amount of AE105 was applied together with the probe (compound 7). It was found that for this blocking group, only very weak fluorescence was observed using the same setting of the fluorescence microscopy (Figure 4B). Taking into account fluorescent imaging results of both groups, it could be concluded that this probe can specifically bind to uPAR receptors on U87MG cells.
Figure 4.
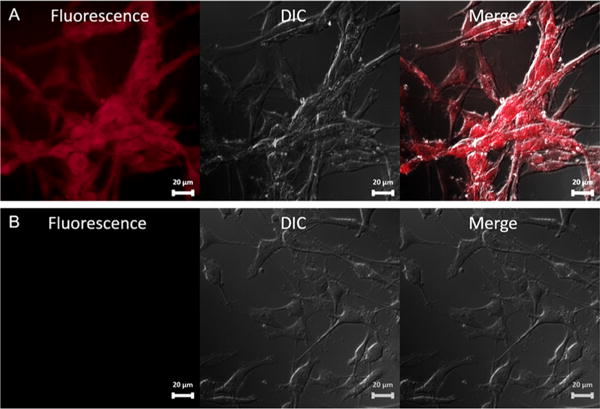
Fluorescence staining of U87MG cells with synthesized dual-modality AE105 probe: (A) Treated with synthesized dual-modality AE105 probe only. (B) Treated with synthesized dual-modality AE105 probe and blocking dose of AE105.
Encouraged by the promising result obtained in the cell fluorescent staining, we then investigated the application of this dual-modality AE105 probe in PET imaging. Equipped with the NOTA chelator, this probe could be radiolabeled with 68Ga in decent specific activity. Briefly, 1 nmol probe was incubated with 1 mCi 68Ga in 100 μL ammonium acetate buffer (0.1 M, pH = 4.0). 68Ga labeling was conducted at 70 °C, and over 95% incorporation yield could be obtained within 30 min, leading to a specific activity of 1.0 mCi/nmol. The resulting 68Ga labeled probe ((68Ga)7) was then injected (tail vein) into nude mice bearing U87MG xenografts at their right shoulders. PET/CT imaging was subsequently performed at 1 and 2 h after the tail vein injection. As shown in Figure 5, at different time points scanned, the tumor could be clearly visualized on PET images with a high signal to background ratio, rendering this probe as an ideal tool for the PET imaging of U87MG xenografts. Similar to the cell fluorescent staining study, a blocking group was step up to investigate the in vivo specificity of this imaging probe, in which abundant AE105 was coinjected with (68Ga)7. Results reflected that the intensity of the PET signal at the tumor site was significantly reduced in the blocking group, indicating the in vivo specific binding of this probe to uPAR. In addition to 68Ga, the AE105-photo-click-NOTA (compound 7) has also been successfully radiolabeled with other PET radioisotopes, such as 64Cu and Al18F. As mentioned before, besides small molecules, the antibody (cetxuimab) has also been functionalized with NOTA via the developed photo-click conjugation between alkene-cetuximab and compound 6. The cetuximab-based dual-modality PET/optical imaging probe could be radiolabeled with 64Cu in decent specific activity (∼10 mCi/mg) under mild conditions, and the resulting (64Cu)-cetuximab radiotracer was successfully used for the PET imaging of EGFR expression in mice bearing U87MG and 4T1 (mouse original tumor, served as a negative control tumor which would not be bound by cetuximab) xenografts (Figure 6).
Figure 5.
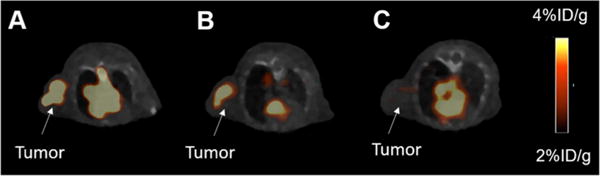
PET/CT imaging of mice bearing U87MG xenografts using 68Ga labeled dual-modality AE105 probe: (A) 1 h p.i; (B) 2 h p.i.; (C) with blockade, 1 h p.i.
Figure 6.
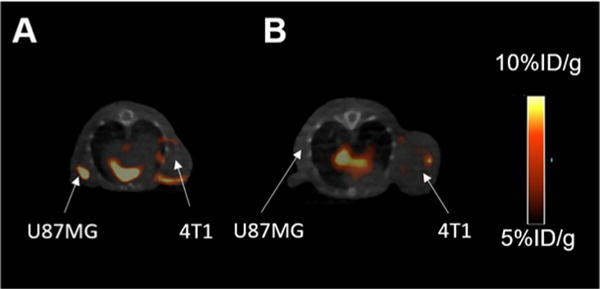
PET/CT imaging of mice bearing both U87MG and 4T1 xenografts at 48 h p.i of 64Cu labeled cetuximab probe: (A) the U87MG tumor was much brighter than the negative control 4T1 tumor; (B) coinjecting an excess amount of unlabeled cetuximab.
In summary, we have successfully developed a photo-click chemistry based strategy for the facile preparation of dual-modality optical/PET probes. The most important feature of this strategy is that the diazole photo-click linker between the targeting ligand and the chelator could also serve as the fluorescent core for the optical imaging, thus avoiding complex chemistry work to incorporate the optical imaging moiety. As demonstrated in the proof-of-principle study, the uPAR- and EGFR-targeted dual-modality probes prepared via this approach could be applied in cell fluorescence staining as well as in PET imaging. Moreover, by using the designed pair of functionalization compounds (Figure S1), different targeting ligands (peptides and antibodies) can be assembled with different types of chelators selected based on the specific radioisotopes suitable for that study, rendering this strategy a flexible approach for efficiently developing dual-modality optical/PET probes.
Supplementary Material
Acknowledgments
We thank Mr. Joseph Donald Latoche for his assistance in the fluorescence imaging study. This work was supported by the National Institute of Biomedical Imaging and Bioengineering grant R21-EB017317 and R21-EB020737. Preclinical PET/CT imaging is supported in part by P30CA047904 (UPCI CCSG).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bioconj-chem.6b00115.
Synthesis of functionalization compounds, preparation of AE105 and cetuximab based dual-modality probes, immunofluorescent staining, small animal PET/CT imaging, ex vivo biodistribution studies, and 18F labeling condition (PDF)
Notes
The authors declare no competing financial interest.
References
- 1.Louie A. Multimodality Imaging Probes: Design and Challenges. Chem Rev. 2010;110:3146–3195. doi: 10.1021/cr9003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng J, Liu J, Dunne M, Jaffray DA, Allen C. In vivo performance of a liposomal vascular contrast agent for CT and MR-based image guidance applications. Pharm Res. 2007;24:1193–201. doi: 10.1007/s11095-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862–70. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Lim EK, Lee HJ, Park J, Lee SC, Lee K, Yoon HG, Suh JS, Huh YM, Haam S. Fluorescent magnetic nanohybrids as multimodal imaging agents for human epithelial cancer detection. Biomaterials. 2008;29:2548–55. doi: 10.1016/j.biomaterials.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Glickson JD, Lund-Katz S, Zhou R, Choi H, Chen IW, Li H, Corbin I, Popov AV, Cao W, Song L, et al. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Molecular Imaging. 2008;7:101–10. [PubMed] [Google Scholar]
- 6.Mizukami S, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Dual-function probe to detect protease activity for fluorescence measurement and 19F MRI. Angew Chem, Int Ed. 2009;48:3641–3. doi: 10.1002/anie.200806328. [DOI] [PubMed] [Google Scholar]
- 7.Houghton JL, Zeglis BM, Abdel-Atti D, Sawada R, Scholz WW, Lewis JS. Pretargeted immunoPET of pancreatic cancer: overcoming circulating antigen and antibody internalization to reduce radiation doses. J Nucl Med. 2016;57:453. doi: 10.2967/jnumed.115.163824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal P, Bertozzi CR. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate Chem. 2015;26:176–92. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Wang W, Wu Q, Ke S, Houston J, Sevick-Muraca E, Dong L, Chow D, Charnsangavej C, Gelovani JG. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nucl Med Biol. 2006;33:349–58. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Baidoo K, Gunn AJ, Boswell CA, Milenic DE, Choyke PL, Brechbiel MW. Design, Synthesis, and Characterization of a Dual Modality Positron Emission Tomography and Fluorescence Imaging Agent for Monoclonal Antibody Tumor-Targeted Imaging. J Med Chem. 2007;50:4759–4765. doi: 10.1021/jm070657w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhushan KR, Misra P, Liu F, Mathur S, Lenkinski RE, Frangioni JV. Detection of Breast Cancer Microcalcifications Using a Dual-modality SPECT/NIR Fluorescent Probe. J Am Chem Soc. 2008;130:17648–17649. doi: 10.1021/ja807099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng D, Zeglis BM, Lewis JS, Anderson CJ. The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals. J Nucl Med. 2013;54:829–32. doi: 10.2967/jnumed.112.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Gai Y, Anderson CJ, Zeng D. Highly-efficient and versatile fluorous-tagged Cu(i)-catalyzed azide-alkyne cycloaddition ligand for preparing bioconjugates. Chem Commun. 2015;51:17072–17075. doi: 10.1039/c5cc06858d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sletten EM, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew Chem, Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beal DM, Jones LH. Molecular Scaffolds Using Multiple Orthogonal Conjugations: Applications in Chemical Biology and Drug Discovery. Angew Chem, Int Ed. 2012;51:6320–6326. doi: 10.1002/anie.201200002. [DOI] [PubMed] [Google Scholar]
- 16.Zeng D, Lee NS, Liu Y, Zhou D, Dence CS, Wooley KL, Katzenellenbogen JA, Welch MJ. 64Cu Core-labeled nanoparticles with high specific activity via metal-free click chemistry. ACS Nano. 2012;6:5209–19. doi: 10.1021/nn300974s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Ma X, Cheng K, Wu B, Duan J, Chen H, Bu L, Zhang R, Hu X, Deng Z, et al. Strained cyclooctyne as a molecular platform for construction of multimodal imaging probes. Angew Chem, Int Ed. 2015;54:5981–4. doi: 10.1002/anie.201500941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Radtke MA, Wong MQ, Lin K-S, Yapp DT, Perrin DM. Dual Mode Fluorescent 18F-PET Tracers: Efficient Modular Synthesis of Rhodamine-[cRGD]2-[18F]-Organotrifluoroborate, Rapid, and High Yielding One-Step 18F-Labeling at High Specific Activity, and Correlated in Vivo PET Imaging and ex Vivo Fluorescence. Bioconjugate Chem. 2014;25:1951–1962. doi: 10.1021/bc5003357. [DOI] [PubMed] [Google Scholar]
- 19.Lim RK, Lin Q. Photoinducible bioorthogonal chemistry: a spatiotemporally controllable tool to visualize and perturb proteins in live cells. Acc Chem Res. 2011;44:828–39. doi: 10.1021/ar200021p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Z, Pan Y, Wang Z, Wang J, Lin Q. Genetically Encoded Cyclopropene Directs Rapid, Photoclick-Chemistry-Mediated Protein Labeling in Mammalian Cells. Angew Chem. 2012;124:10752–10756. doi: 10.1002/anie.201205352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debets MF, van Berkel SS, Schoffelen S, Rutjes FP, van Hest JC, van Delft FL. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3 + 2) cycloaddition. Chem Commun (Cambridge, U K) 2010;46:97–9. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Ohulchanskyy TY, An P, Prasad PN, Lin Q. Fluorogenic, two-photon-triggered photoclick chemistry in live mammalian cells. J Am Chem Soc. 2013;135:16766–9. doi: 10.1021/ja407867a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li ZB, Niu G, Wang H, He L, Yang L, Ploug M, Chen X. Imaging of urokinase-type plasminogen activator receptor expression using a 64Cu-labeled linear peptide antagonist by microPET. Clin Cancer Res. 2008;14:4758–66. doi: 10.1158/1078-0432.CCR-07-4434. [DOI] [PubMed] [Google Scholar]
- 24.Gai Y, Xiang G, Ma X, Hui W, Ouyang Q, Sun L, Ding J, Sheng J, Zeng D. A Universal Molecular Scaffold for Facile Construction of Multivalent and Multimodal Imaging Probes. Bioconjugate Chem. 2016;27:515. doi: 10.1021/acs.bioconjchem.6b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 26.Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Rivera Vera CI, Lin Q. Convenient Synthesis of Highly Functionalized Pyrazolines via Mild, Photo-activated 1,3-Dipolar Cycloaddition. Org Lett. 2007;9:4155–4158. doi: 10.1021/ol7017328. [DOI] [PubMed] [Google Scholar]
- 28.Zeng D, Guo Y, White AG, Cai Z, Modi J, Ferdani R, Anderson CJ. Comparison of Conjugation Strategies of Cross-Bridged Macrocyclic Chelators with Cetuximab for Copper-64 Radiolabeling and PET Imaging of EGFR in Colorectal Tumor-Bearing Mice. Mol Pharmaceutics. 2014;11:3980–3987. doi: 10.1021/mp500004m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Zhang H, Sun Y, Pan Y, Zhou J, Wang J. Expanding the Genetic Code for Photoclick Chemistry in E. coli, Mammalian Cells, and A. thaliana. Angew Chem, Int Ed. 2013;52:9700–9704. doi: 10.1002/anie.201303477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


