SUMMARY
Some patients with cancer never develop metastasis, and their host response may provide cues for innovative treatment strategies. We previously reported an association between autoantibodies against complement factor H (CFH) and early stage lung cancer. CFH prevents complement-mediated cytotoxicity (CDC) by inhibiting formation of cell-lytic membrane attack complexes on self-surfaces. In an effort to translate these findings into a biologic therapy for cancer, we isolated and expressed DNA sequences encoding high affinity human CFH antibodies directly from single, sorted B cells obtained from patients with the antibody. The co-crystal structure of a CFH antibody-target complex shows a conformational change in the target relative to the native structure. This recombinant CFH antibody causes complement activation, release of anaphylatoxins, and promotes CDC of tumor cell lines, and inhibits tumor growth in vivo. The isolation of anti-tumor antibodies derived from single human B cells represents an alternative paradigm in antibody drug discovery.
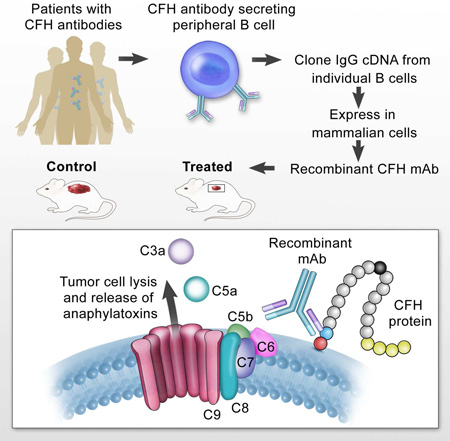
Graphical abstract
eTOC Blurb
Bushey et al. clone antibodies against complement factor H (CFH) from single human B cells. CFH protects tumor cells from complement dependent cytotoxicity (CDC). The authors demonstrate that a recombinant CFH antibody induces CDC of tumor cells, inhibits tumor growth in vivo, and stimulates infiltration of the tumor by lymphocytes.
INTRODUCTION
Metastatic disease is responsible for the majority of cancer deaths, and unfortunately many current drugs only offer modest benefits in progression-free survival. We have been studying the immune response in a distinct group of patients with early stage disease who do not develop metastasis as an approach to developing therapeutic strategies (Amornsiripanitch et al., 2010). Our goal was to identify tumor specific antibodies capable of initiating tumor cell death, while stimulating a durable, long term adaptive immune response. We previously reported an association of autoantibodies to a complement regulatory protein, complement factor H (CFH), with early stage non-small cell lung cancer (NSCLC), and found that patients with stage I NSCLC had a significantly higher incidence of anti-CFH antibody than those with late-stage NSCLC (P = 0.0051). This association led to the hypothesis that CFH antibodies that arise in lung cancer patients may promote anti-tumor cell activity, and that CFH antibody administration might provide a unique way to stimulate a long term immune response and treat cancer. We set out to isolate and characterize human CFH antibodies starting from the memory B cells of patients with the autoantibody in an effort to develop a therapy that would recapitulate the native immune response.
CFH is a regulatory protein that protects host cells from attack and destruction by the complement system by inhibiting the alternative pathway of complement-mediated lysis (Ferreira et al., 2010; Makou et al., 2013). CFH prevents the deposition of complement C3b on the cell surface by several mechanisms. Deposition of C3b initiates the formation of cell-lytic membrane attack complexes (MAC) leading to cell lysis. Thus CFH inhibition of the deposition of C3b on the cell surface protects against cell lysis. Tumor cells take advantage of the protection conferred by CFH in order to evade destruction by the complement system (Ajona et al., 2007; Junnikkala et al., 2000; Varsano et al., 1998; Wilczek et al., 2008). We hypothesize that by neutralizing this protective protein, patient antibodies to CFH allow complement activation and tumor cell lysis, resulting in the release of anaphylatoxins and modulation of the adaptive immune response, thus suppressing tumor growth while forestalling metastasis.
In order to develop a CFH antibody as a cancer therapeutic, targeting the same epitope that is recognized by the autoantibodies of cancer patients would be essential to prevent off-target effects, since CFH is a ubiquitous protein that binds to the surface of host cells. CFH is a multifunctional 150 kDa protein that is composed of 20 short consensus repeats (SCRs), each 60 amino acids long (Makou et al., 2013). The C-terminal SCR domains SCR19 and SCR20 bind to glycosaminoglycan and sialic acid polyanions, as well as to membrane-bound C3b and its proteolytic fragments, on the mammalian cell surface (Ferreira et al., 2010; Kajander et al., 2011; Makou et al., 2013; Morgan et al., 2011). CFH antibodies affinity purified from the sera of lung cancer patients bind an epitope within SCR19, PIDNGDIT (Campa et al., 2015). This epitope comprises residues that are predicted on mutational and structural grounds to be critical for the CFH-C3b interaction (Kajander et al., 2011; Morgan et al., 2011). In vitro, the CFH antibodies prevent CFH binding to tumor cells, increase C3b binding, and promote complement-mediated cytotoxicity (CDC). Although CFH is abundant in the blood, it is notable that patients who have these antibodies show no apparent off-target effects. This, and the fact that the autoantibodies bind preferentially to reduced over oxidized CFH in vitro, led us to propose that the autoantibodies bind to a conformationally distinct form of CFH that exists in tumors (Campa et al., 2015).
Here we report the isolation and characterization of high affinity, human CFH monoclonal antibodies (mAbs) from single B cells of patients with the autoantibody. Using our lead candidate mAb, we explore the mechanism of anti-tumor cell activity and demonstrate the ability of the antibody to kill tumor cells in vitro, and inhibit tumor growth in vivo.
RESULTS
Isolation of Human CFH Antibodies from Patients with the Autoantibody
We had previously mapped the binding region of the autoantibodies from NSCLC patient sera to a specific 8 amino acid segment (PIDNGDIT) of the SCR19 domain (Campa et al., 2015). In order to clone human mAbs that recognize this epitope, we pooled the peripheral blood mononuclear cells (PBMC) of 11 patients who were shown by immunoblot to express CFH autoantibody. Using a biotinylated CFH 15mer peptide containing the mapped 8 amino acid binding segment as bait, we sorted single memory B cells from the pooled PBMCs by flow cytometry for isolation of the immunoglobulin (Ig) variable region of heavy and light gene segments (VHDJH and VLJL) (Figure 1A). VHDJH and VLJL gene pairs were isolated by RT/PCR from each single CFH antigen-specific memory B cell. A total of 15 recombinant mAbs generated from the isolated VHDJH and VLJL gene pairs were identified that bound to the peptide containing the epitope (Figure 1B). Gene sequence analysis indicated that these 15 CFH-positive mAbs belong to 7 clonal lineages (Table 1). Although these lineages used different VH gene families, all used a kappa light chain with a CDR3 of 9 amino acids in length. The antibodies had VH gene mutation frequencies ranging from 2.7–10.9%, and VK chain mutation frequencies ranging from 3.0–6.3% except that both VHDJH and VLJL genes of Ab7966 had 0% mutation. Except for two IgM antibodies (Ab7962 and Ab7966), these antibodies were IgG3 subtype, as were all 22 autoantibodies previously subtyped in NSCLC patient sera (Campa et al., 2015).
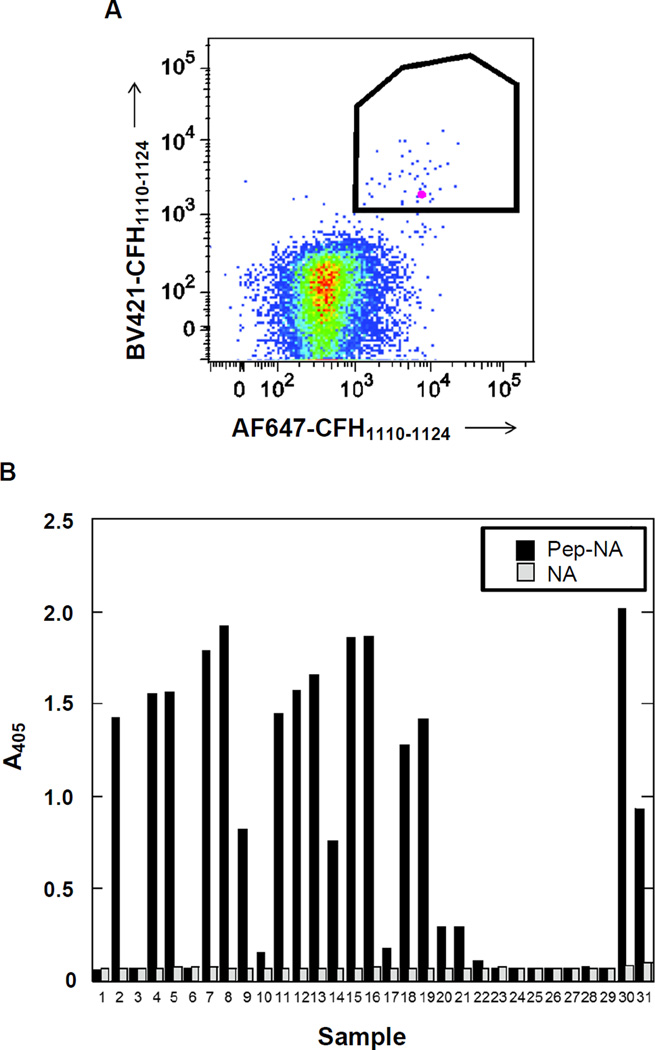
Figure 1. Cloning of Human CFH mAbs Specific to an Antigenic Peptide.
(A) Isolation of CFH-specific memory B cells that bind to the epitope of interest. PBMCs were obtained from patients positive for anti-CFH antibodies and used for sorting CFH antigen-binding memory B-cells via FACS. To minimize false-positives, streptavidin was labeled with Alexa Fluor 647 and Brilliant Violet 421. Labeling with each fluorophore was carried out on separate aliquots of streptavidin, which were then mixed together prior to interaction with biotinylated antigen peptide (CFH1110–1124). Cells showing elevated fluorescence for both fluorophores, indicated by the outlined region (0.32% of the total), were sorted into single wells for recombinant mAb synthesis. The B cell expressing mAb7968 is designated in pink.
(B) Identification of cloned mAbs that bind a CFH epitope-containing peptide by ELISA. Supernatants from HEK293 cells transiently expressing pairs of VH and VL chains were collected, adjusted to 1 µg/ml IgG, and equal volumes tested for binding to epitope peptide bound to neutravidin (Pep-NA) or to neutravidin alone (NA). Bound antibody was detected with anti-human IgGγ-HRP and ABTS/H2O2 substrate. Sample 31 is an affinity purified anti-CFH autoantibody serving as a positive control.
Table 1.
V(D)J Rearrangement of Immunoglobulin Genes of the Isolated CFH-Reactive mAbs
| Ab ID |
VH ID |
VH | DH | JH | VH Mutation Frequency % |
HCDR3 Length |
Isotype | VL ID |
VK | JK | VK Mutation Frequency % |
KCDR3 Length |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab7970 | H 7970 | 3~30 | 6~13 | 6 | 8.1 | 12 | G3 | K6004 | 4~1 | 1 | 4.6 | 9 |
| Ab7955 | H7955 | 3~30 | 6~13 | 1 | 10.7 | 12 | G3 | K5989 | 4~1 | 1 | 5.4 | 9 |
| Ab7957 | H7957 | 3~30 | 2~21 | 3 | 6.8 | 12 | G3 | K5991 | 4~1 | 1 | 6.3 | 9 |
| Ab7958 | H7958 | 3~30 | 2~21 | 3 | 6.8 | 12 | G3 | K5992 | 4~1 | 1 | 6.3 | 9 |
| Ab7963 | H7963 | 3~30 | 2~21 | 3 | 6.8 | 12 | G3 | K5998 | 4~1 | 1 | 6.3 | 9 |
| Ab7960 | H7960 | 3~30 | 6~25 | 4 | 7.1 | 12 | G3 | K5994 | 4~1 | 1 | 4.1 | 9 |
| Ab7967 | H7967 | 3~30 | 6~25 | 4 | 7.1 | 12 | G3 | K6002 | 4~1 | 1 | 4.1 | 9 |
| Ab7979 | H7979 | 3~30 | 6~25 | 4 | 6 | 12 | G3 | K6015 | 4~1 | 1 | 3.3 | 9 |
| Ab7961 | H7961 | 3~30 | 6~25 | 4 | 6.8 | 12 | G3 | K5995 | 4~1 | 1 | 3 | 9 |
| Ab7965 | H7965 | 3~30 | 6~25 | 4 | 6.8 | 12 | G3 | K6000 | 4~1 | 1 | 3 | 9 |
| Ab7964 | H7964 | 3~30 | 1~26 | 4 | 4.2 | 12 | G3 | K5999 | 4~1 | 1 | 3.5 | 9 |
| Ab7968 | H7968 | 3~30 | 6~13 | 6 | 2.9 | 12 | G3 | K6003 | 4~1 | 1 | 4.3 | 9 |
| Ab7971 | H7971 | 3~30 | 6~13 | 6 | 2.9 | 12 | G3 | K6005 | 4~1 | 1 | 4.3 | 9 |
| Ab7962 | H7962 | 3~48 | 6~6 | 6 | 0.3 | 14 | M | K5996 | 4~1 | 4 | 4.9 | 9 |
| Ab7966 | H7966 | 5~51 | 1~IR1 | 6 | 0 | 17 | M | K6001 | 1~5 | 1 | 0 | 9 |
Antibodies were regarded as clonally related if they were inferred to use the same V and J gene segments, identical CDR3 length and have 70% or greater nucleotide identity in CDR3 region while D-segment identification is subject to substantial uncertainty, so inferred differences in D-segment use were not sufficient to rule out clonal relatedness of two antibodies. Clonally related antibodies are grouped. Based on the analysis, 7 antibodies as highlighted in red color with unique VHDJH and VLJL sequences were selected to be produced as purified antibodies for further characterization.
Most of VHDJH and VLJL sequences in the individual clonal lineages had identical sequences except the 4th group in Table 1. Seven antibodies with unique VHDJH and VLJL sequences were selected and produced as purified recombinant antibodies for further characterization. These antibodies were demonstrated to bind CFH with specificity for the reduced form of both full length CFH and a fragment containing SCR19–20 (Figure S1). Thus, the isolated antibodies recapitulate the specificity of the autoantibodies identified in sera. By surface plasmon resonance analysis (SPR), six of the seven antibodies had affinities in the low nM range against the CFH epitope-peptide (Table S1). Taken together, these data confirm that single human B cells against this tumor antigen express high affinity antibodies that have undergone somatic hypermutation.
Alanine Scanning Mutagenesis and Crystal Structure of mAb7968 with an Epitope-Containing Peptide
To define the amino acid footprint of the recombinant CFH mAbs, we analyzed binding of seven recombinant mAbs to alanine-substituted peptides containing the originally described 8-amino acid binding domain (Figure S2). Mutation of Ile1120 to Ala completely ablated binding of all mAbs; mutation of Asn1117 resulted in 0–35% of wild type binding and Thr1121, 1–66% of wild type binding. None of the mAbs were affected by changes in the 4 residues upstream or 3 residues downstream of the epitope that were included in the peptide.
To investigate the interaction of the CFH antibody with its epitope, the crystal structure of Fab7968 in complex with a CFH1110–1122 peptide was solved by molecular replacement and refined to a resolution of 2.0 Å (Figure 2A; Table S2). Most of the CFH polypeptide backbone (residues 1112–1122) was visible in the scattering electron density, showing specific antibody-antigen interactions in detail. In particular, the CFH residues Asn1117 and Ile1120 had been shown by alanine scanning to be critical components of the mAb7968 epitope (Figure S2). The complex structure showed that Asn1117 made a through-water interaction with heavy chain residue Gly96. It is also possible that the substitution of Asn1117 with Ala disrupted an intra-peptide interaction between the Asn1117 and Thr1121 side chains that helped to stabilize the peptide’s alpha-helical conformation. In agreement with this, the Thr1121Ala mutation partially but not entirely abrogated binding (Figure S2). The complex structure also showed the Ile1120 side chain binding in a hydrophobic pocket formed by Val50 and Tyr58 on the heavy chain and Trp96 on the light chain. Mutating Ile1120 to Ala would allow solvent to penetrate this pocket, disrupting hydrophobic antibody-antigen contacts in the vicinity.
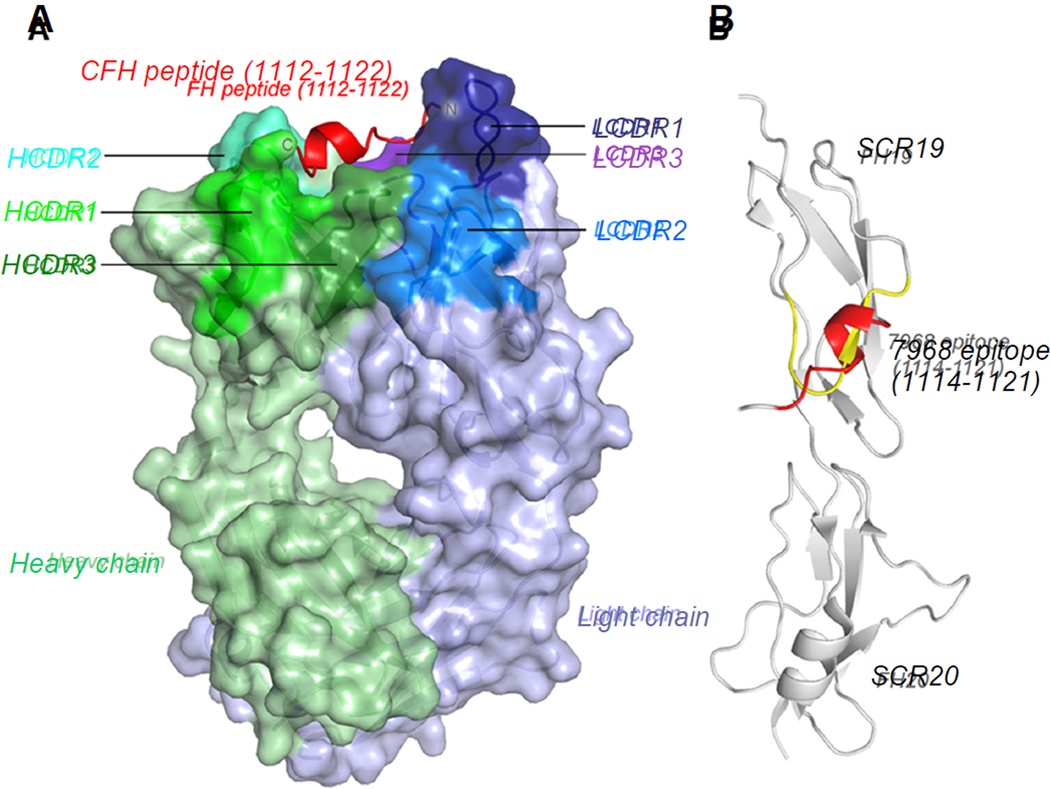
Figure 2. Structure of Fab7968 in Complex with CFH Peptide.
(A) Fab 7968 shown with the heavy chain in green and the light chain in blue. The paratope with bound peptide in red is oriented toward the top of the figure, and the CDRs are marked and labeled.
(B) CFH peptide in its antibody-bound conformation (red) contrasted in a superposition to the same region in the natively folded CFH protein (gray). Here, only the 7968 epitope is highlighted in red (antibody-bound conformation) and yellow (on the natively folded protein). See also Figure S2 and Table S2.
Asp1119 in the peptide made an H-bond with Thr56 on the heavy chain at a location on the surface of the complex. Disruption of this interaction by substitution with Ala resulted in varying impact on antibody binding (Figure S2). Thus with this structure, disrupting a through-water interaction (Asn1117) located on the interior of the antibody-antigen interface was more detrimental to binding than disrupting a superficial direct interaction (Asp1119).
Importantly, the peptide exhibited a conformation distinctly different from that observed in other structures of CFH protein constructs (Herbert et al., 2012; Kajander et al., 2011; Morgan et al., 2012; Morgan et al., 2011). In particular, residues 1117–1120 (sequence element NGDI) near the C-terminus of the peptide adopted an α-helical conformation in the antibody-bound complex, whereas the same region exhibited a β-strand conformation in natively folded structures of CFH (Figure 2A) (Herbert et al., 2012; Kajander et al., 2011; Morgan et al., 2012; Morgan et al., 2011). This was consistent with the observation that mAb7968 bound a conformationally distinct form of CFH (Figure S1). The presence of the helical element was also consistent with the Ala scanning results showing a discontinuous epitope for mAb7968 (Figure S2).
Complement Dependent Cytotoxicity of Cancer Cell Lines by Human CFH mAbs
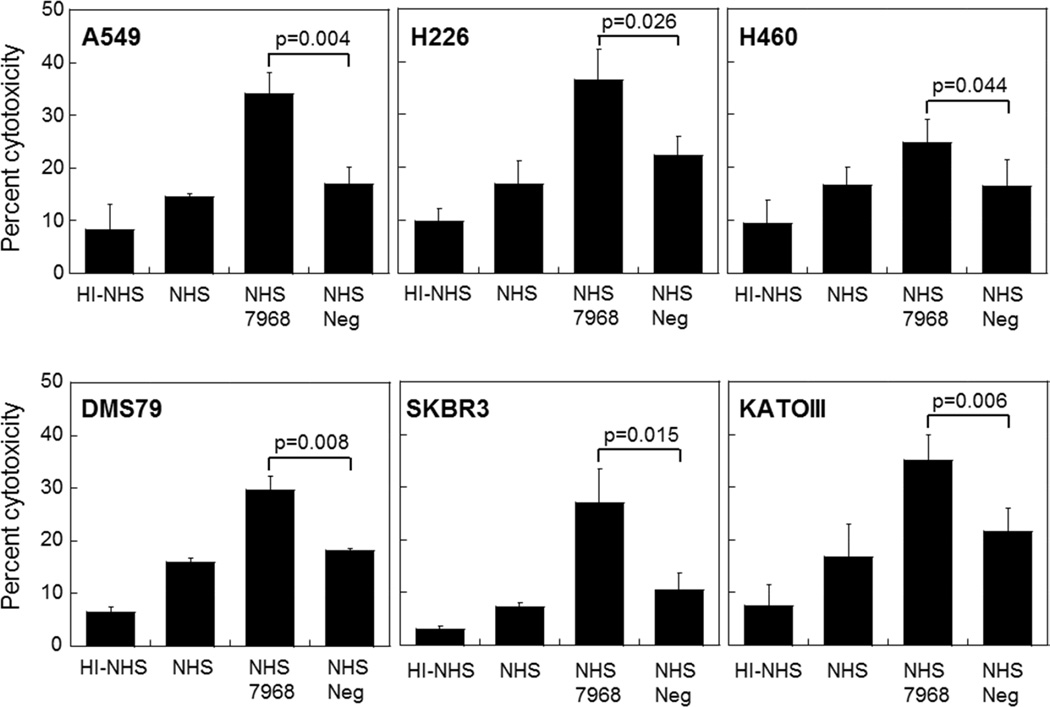
Because of the location of the epitope within the CFH protein and inferred mechanism of action, we tested five of the mAbs in a CDC assay using A549 lung cancer cells. Lung cancer cells were chosen because we originally discovered CFH autoantibodies in lung cancer patients (Amornsiripanitch et al., 2010). In the CDC assay, cells are mixed with antibodies and normal human serum (NHS) as a source of complement, incubated at 37 °C, and cytolysis is measured by lactate dehydrogenase (LDH) release. The five mAbs behaved similarly in the CDC assay; however, we chose mAb7968 for future development since, in addition to its low nanomolar affinity for the epitope-containing peptide, it was highly expressed in cell culture. When added to A549 cells, mAb7968 increased CDC over that seen with a negative control human antibody by 101% (Figure 3). Besides A549 (a NSCLC adenocarcinoma cell line), other cell lines that were killed by mAb7968 were the H226 squamous cell carcinoma and H460 large cell cancer cell lines (both NSCLC cell lines), the DMS79 small cell lung cancer cell line, the SKBR3 breast cancer cell line and the KATOIII gastric cancer cell line (Figure 3). Additional cell lines that showed increased cytotoxicity with mAb7968 were A431 (epidermoid carcinoma), RD-ES (Ewing’s sarcoma) and FaDu (pharyngeal squamous cell carcinoma) (data not shown). All cell lines were shown to express CFH and bind mAb7968 (data not shown).
Figure 3. Complement-Dependent Cytotoxicity of Six Tumor Cell Lines after Treatment with Recombinant Human CFH mAb7968 or Control mAb.
The A549 (NSCLC - adenocarcinoma), H226 (NSCLC - squamous cell carcinoma), H460 (NSCLC - large cell carcinoma), DMS79 (small cell lung cancer), SKBR3 (breast adenocarcinoma), and KATOIII (gastric carcinoma) cell lines were treated with CFH mAb7968, alongside a subtype-matched negative control mAb (Neg), in an LDH release assay for cytotoxicity.
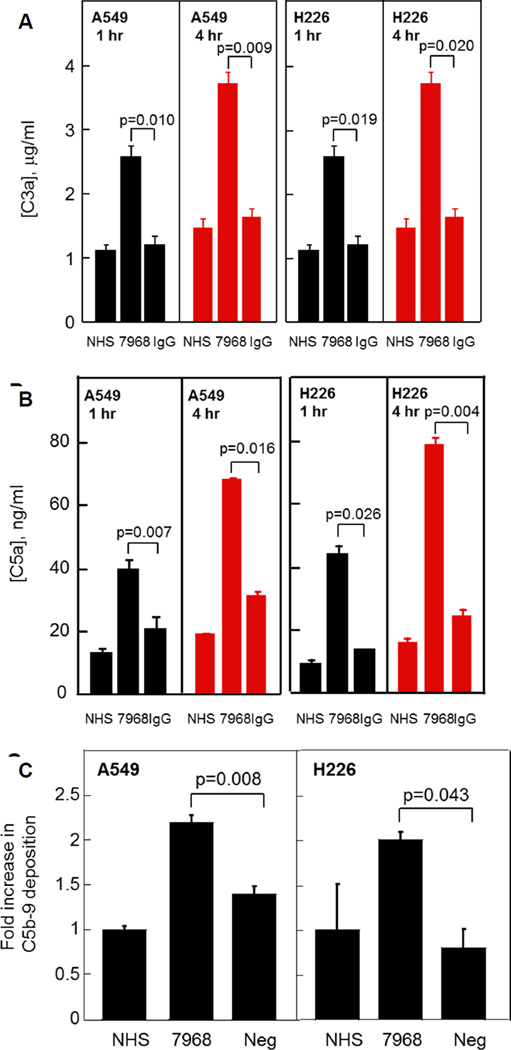
To confirm complement activation, we assayed products of the CDC reaction: C3a, C5a and C5b-9. The anaphylatoxins C3a and C5a are generated during complement activation, and as cytokines for immune cells, are links between the innate and adaptive immune systems (Klos et al., 2009). C5b-9 comprises the terminal MAC, a minimal number of which are required to assemble in order to lyse the cell (Koski et al., 1983). Addition of mAb7968 to A549 or H226 cells in the presence of NHS resulted in increased C3a release (Figure 4A), C5a release (Figure 4B) and C5b-9 deposition (Figure 4C).
Figure 4. Response to CFH mAb by Tumor Cells: C3a Release, C5a Release, and C5b-9 Deposition.
(A) Release of C3a from A549 or H226 lung cancer cells. Cells were incubated in the presence of either NHS alone, NHS plus mAb7968, or NHS plus human IgG. C3a was measured in cell supernatants at 1 hr and 4 hr by ELISA.
(B) Release of C5a from A549 or H226 cells. Using the same cell supernatants described in (A), C5a was measured by ELISA.
(C) C5b-9 deposition on A549 or H226 cells. After incubation with NHS, NHS plus mAb7968, or NHS plus a subtype-matched negative control mAb (Neg), C5b-9 deposition on cells was measured by flow cytometry.
In Vivo Tumor Growth Studies
For in vivo mouse studies, we developed a murine version of mAb7968 since initial experiments had shown that the human mAb triggered the formation of anti-human antibodies in nude mice. To test the effect of antibody on tumor growth, we initially used an adult patient-derived brain tumor xenograft, D-270MG, grown in nude mice (Bigner et al., 1990), performing intratumoral injections of either murine IgG1-mAb7968, a murine subtype-matched negative control antibody, or no antibody in each of 3 groups of mice. Injections were repeated biweekly for 3 weeks and tumors were measured. By the end of the 3 week study, there was significant tumor growth inhibition (Figure S3A, B) and prolonged survival (Figure S3C) in the group of animals that received murine mAb7968. The primary concern for side effects from inhibition of CFH by a CFH antibody is renal toxicity (Hofer et al., 2014). Stained sections from the kidneys of all animals were examined by hematoxylin and eosin (H&E) and were normal. There were no observed adverse reactions at necropsy in any of the animals treated with mAb7968. H&E-stained sections of tumor excised from mice receiving the negative control mAb show densely packed tumor cells whereas H&E-stained sections from the smallest palpable mass excised from a mAb7968-treated mouse show diffuse inflammatory cells without visible tumor cells (Figure S3D).
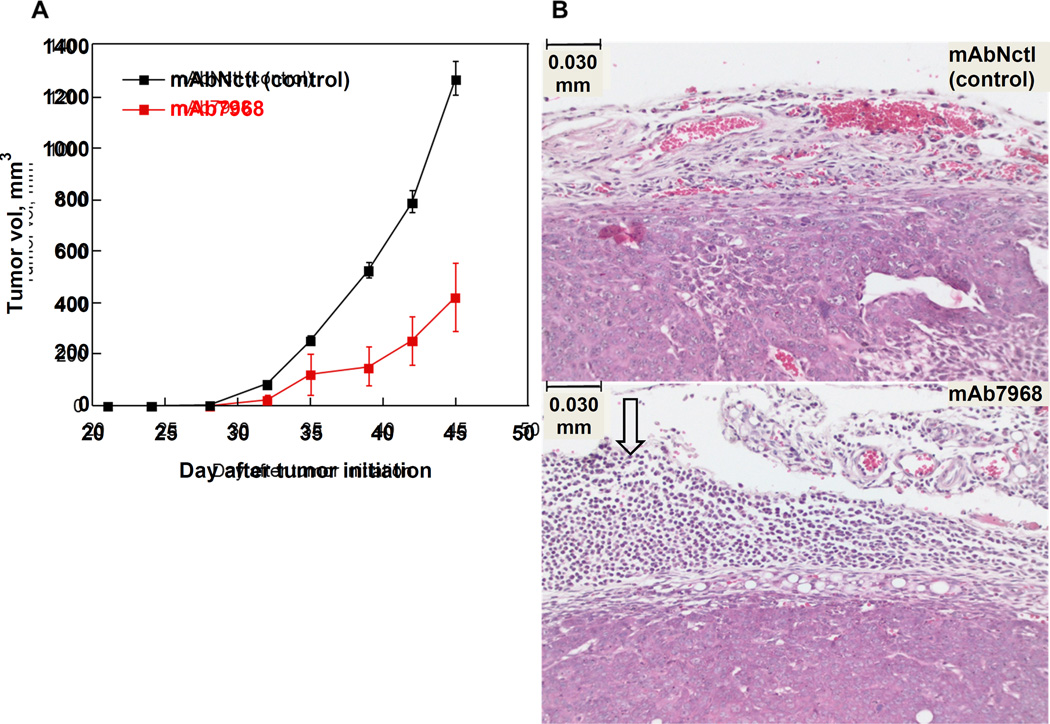
In order to test antibody efficacy in a mouse with a functional immune system, we used the KLN205 - DB/2 syngeneic lung cancer model (Kaneko and LePage, 1978). The murine KLN205 cell line expresses CFH and binds murine mAb7968 (data not shown). Tumor cells were injected s.c., then mAb7968 or negative control mAbNctl was injected i.p. on days 1, 4, 7, 10, and 13. Tumor volumes were measured periodically thereafter. Differences in mean tumor volume were observed in the two groups of mice, with systemically administered mAb7968 conferring growth delay and inhibition compared to negative control mAbNctl (Figure 5A). The magnitude of this difference reached statistical significance (P<0.05). H&E staining of a section from the residual tumor from a mAb7968-treated mouse showed an abundant lymphocytic infiltrate that was absent in the tumor section from a control mouse (Figure 5B).
Fig. 5. Tumor growth in the KLN205 - DBA/2 syngeneic lung cancer model with mAb treatment.
(A) Growth curves. KLN205 tumor cells were injected s.c. on day 0 and mAb7968 or mAbNctl was injected i.p. every 3 days between days 1–13, after which treatment was stopped. The mean tumor volumes +/− SEM for t=7 mice treated with each mAb are plotted. P-values for the difference in tumor volumes between mAb7968 and mAbNctl on days 39, 42, and 45 are 0.027, 0.030, and 0.077, respectively.
(B) Representative H&E images of the tumors. A lymphocytic component is evident in residual mAb7968-treated tumor (white arrow). The scale bar=0.030 mm and magnification is 100X.
DISCUSSION
In an effort to develop an immunotherapeutic strategy, we initially embarked on a search for autoantibodies associated with a distinct non-metastatic early stage phenotype that could cause cancer cell death, modulate the adaptive immune response, and ultimately produce a long-term cellular response against the tumor. The current study used a unique approach to develop a tumor specific antibody that would target cancer cells without creating off-target effects. Here we report the sequencing and expression of CFH antibodies starting from the B cells of patients who produced these antibodies. While this same technology has been used to isolate broadly neutralizing antibodies for HIV starting from B cells (Morris et al., 2011), this study isolates high-affinity antibodies with anti-tumor cell and anti-tumor growth activity directly from patients. The process of cloning and expressing antibody genes derived from selected B cells is significantly more efficient than production of mAbs in mice by immunization followed by “humanization”. This allowed us to generate an affinity matured antibody that recognizes a conformationally distinct epitope of CFH, that when originally targeted by the immune system, resulted in a desirable phenotype (i.e., limitation of early stage cancer and no apparent side effects).
The 15 isolated CFH-reactive antibodies can be classified into 7 clonal lineages because they share the same VH, JH, Vκ and Jκ gene families and had the same HCDR3 and KCDR3 lengths. Since the PBMCs that were used for sorting single B cells were pooled from 11 patients, it is unclear if antibody members from the individual clonal lineages were from one patient or from different patients.
The CFH mAbs have the same specificity for the conformationally distinct form of CFH and the SCR19–20 fragment as the serum autoantibodies previously described (Campa et al., 2015), which is important to avoid potential off-target effects. An altered conformation of the CFH epitope is seen in the peptide-antibody co-crystal structure, and recognition of this conformation in the tumor environment may be the basis for antibody tumor specificity. This finding also suggests that conformationally distinct epitopes may be relevant therapeutic targets.
We showed that CFH mAb7968 killed a variety of tumor cell lines in vitro including NSCLC (3 histological types), small cell lung cancer, gastric cancer and breast cancer. In vivo, growth of both a s.c. brain tumor in a nude mouse model and a s.c. lung tumor in a syngeneic model were substantially inhibited by this antibody without observable adverse reactions.
In addition to and possibly more important than the immediate effect of the CFH mAb on complement activation, is that the anaphylatoxins C3a and C5a produced, and subsequently other cytokines and chemokines produced when the cell is damaged, will modulate an adaptive immune response. In particular, the anaphylatoxins bind directly to dendritic cells, causing activation and maturation required for induction of T cell effector activity (Surace et al., 2015). It is this long term cellular response that could potentially have the most profound impact on cancer outcomes. Further in vivo studies addressing the immune program will be needed in order to understand the full potential of this approach.
EXPERIMENTAL PROCEDURES
Patient Samples
Samples of peripheral blood from patients diagnosed with NSCLC were collected under our IRB approved protocol with patient consent after the nature and possible consequences of the study were explained.
Experimental Animals and Cell Lines
DBA/2 mice were purchased from Charles River Laboratories (Wilmington, MA). All cell lines were obtained from the American Type Culture Collection (Manassas, VA) except for SKBR3 breast cancer cells that were a gift from Donald McDonnell.
Proteins and Peptides
CFH was purchased from Complement Technology, Inc. (Tyler, TX). SCR19–20 was purified as described (Campa et al., 2015). The CFH epitope-peptide, [GPPPPIDNGDITSFP(GGGK)-biotin], encompassing CFH1110–1124 that includes the 8-amino acid epitope (underlined), plus 3 glycine residues as a linker and a single lysine residue for biotinylation for protein capture and flow sorting was produced; alanine substitution variant peptides for epitope mapping were also produced (CPC Scientific). A shorter version of this peptide, CFH1110–1122 peptide (GPPPPIDNGDITS), was designed for crystal structure study and was commercially synthesized by CPC Scientific with an acetylated N-terminus and amidated C-terminus.
Isolation of VHDJH and VL Genes and Expression of VHDJH and VLJL genes as Full-Length IgG1 Recombinant mAbs
PBMCs were obtained from patients positive for anti-CFH antibodies and used for sorting CD19+/CD27+ CFH antigen-binding memory B-cells via FACS using the methods as described (Morris et al., 2011). To minimize false-positives, streptavidin was labeled with Alexa Fluor 647 and Brilliant Violet 421. Labeling with each fluorophore was carried out on separate aliquots of streptavidin, which were then mixed together prior to interaction with biotinylated antigen peptide (CFH1110–1124). Cells showing elevated fluorescence for both fluorophores were sorted into single wells of a 96-well tray. The VHDJH and VLJL gene segment pairs of the mAbs were amplified by RT/PCR from flow sorted human CFH antigen-specific memory B cells, sequenced and annotated using methods described (Liao et al., 2013). Genetic information for both VHDJH and VLJL gene segments of individual antibodies, including gene segment family usage, somatic mutation frequency, CDR3 length, and clonal lineage relationships, were inferred using the software program Cloanalyst (http://www.bu.edu/computationalimmunology/research/software/) as described previously (Kepler, 2013; Kepler et al., 2014). The isolated Ig VH and VL gene pairs were assembled by PCR into the linear full-length human Ig heavy- and light-chain gene expression cassettes for production of recombinant mAbs in 293T cells for initial screening of CFH reactive antibodies using methods as described (Liao et al., 2009). The VHDJH and VLJL genes of selected CFH binding antibodies were synthesized (GenScript, NJ) and cloned into modified pcDNA3.1 plasmid (Invitrogen, Grand Island, NY) for production of purified recombinant IgG1 and Fab antibodies as described (Liao et al., 2011).
Production of Purified Recombinant Antibodies
For production of purified recombinant mAbs for further characterization, the VHDJH and VLJL genes of selected CFH binding antibodies were synthesized (GenScript, NJ) and cloned into modified pcDNA3.1 plasmid containing the constant region of human IgG1 heavy chain (Liao et al., 2009). To study the effect of antibody isotype, the constant region of Ab7968 was substituted with the constant region of human IgG3 (Genbank accession number: M12958.1) (Huck et al., 1986). To study the in vivo effect of Ab7968 on tumor grown in mouse models and minimize the antigenicity of antibody in mice, both heavy- and kappa light-chain constant regions were substituted with mouse IgG1 (Genbank accession number: BC024405) (Strausberg et al., 2002) and kappa chain (Genbank accession number: P01837) (Svasti and Milstein, 1972), respectively. Recombinant Fab antibodies were produced for crystal structure study as described (Nicely et al., 2010). Recombinant antibodies were produced in 293F cells by transient transfection, purified from the supernatants of transfected 293F cells by using Protein A columns.
X-ray Crystallography
The Fab fragment of recombinant mAb 7968 was produced in 293F cells by transient transfection then purified using methods described previously (Nicely et al., 2010). For structural studies, after affinity capture using KappaSelect (GE Healthcare), the Fab was further purified via gel filtration chromatography using a HiLoad 26/60 Superdex 200pg 26/60 column at 2 ml/min with a buffer of 10 mM Hepes pH 7.2, 50 mM NaCl, 0.02% NaN3. Peak elution fractions were pooled and exchanged into ddH2O via five dilution/concentration cycles then brought to a final concentration of 18.8 mg/ml.
Unliganded Fab7968 crystallized in a drop composed of 0.2 µl protein plus 0.4 µl 25 mM citric acid pH 3.5, 8% PEG 3350 over a reservoir of 60 µl 24% PEG 3350 at 20 °C. CFH1110–1122 peptide was soaked into the crystal by diluting 0.1 µl of the 20 mg/ml peptide solution in ddH2O with 1 µl of the reservoir solution, then adding that solution to the crystal-containing drop and allowing it to incubate overnight. The crystal was cryoprotected in reservoir solution supplemented with ethylene glycol then flash frozen in liquid nitrogen. Data were collected at Southeast Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the Advanced Photon Source, Argonne National Laboratory using an incident beam of 1 Å in wavelength. The dataset was reduced in HKL-2000 (Otwinowski and Minor, 1997). The structure was phased by molecular replacement in PHENIX (Terwilliger et al., 2008) using as search models Fab and Fc domains composited from the source models 1NL0 (Huang et al., 2004) for the heavy chain and 3QOS (Malia et al., 2011) for the light chain as determined by BLAST search for sequence identity. Rebuilding and real-space refinements were done in Coot (Emsley et al., 2010) with reciprocal space refinements in PHENIX (Adams et al., 2010) and validations in MolProbity (Lovell et al., 2003).
C3a and C5a Release
A549 or H226 lung cancer cells (5 × 105 cells/well) were incubated in triplicate in 100 µl cell culture medium containing either 1:8 diluted NHS, 1:8 NHS and 250 µg/ml mAb7968, or 1:8 NHS and 250 µg/ml human IgG. Cell supernatants were assayed at 1 hr and 4 hr for C5a and C3a using commercial ELISA kits (R&D Systems and eBioscience, respectively).
C5b-9 Deposition
C5b-9 deposition was measured by flow cytometry. A549 and H226 cells (5 × 105 cells) in veronal buffer were incubated in triplicate for 30 min at 37 °C with an equal volume of DPBS + NHS that had been preincubated with CFH mAb or negative control antibody for 30 min at 4 °C. Final concentrations after mixing were 1:8 diluted NHS, 1:8 NHS and 300 µg/ml mAb7968, or 1:8 NHS and 300 µg/ml subtype-matched control mAb. After 30 min, cells were washed twice in 1% (w/v) BSA in DPBS (DPBS-BSA), and then incubated for 30 min at 4 °C with a mouse anti-human C5b-9 antibody (Abcam, Cambridge, MA) at a 1:100 dilution in PBS. Cells were washed three times in DPBS-BSA and then incubated for 30 min at 4 °C with an FITC-conjugated goat anti-mouse secondary antibody (Jackson Immunoresearch, West Grove, PA) at a 1:100 dilution in PBS. Cells were washed three times in DPBS-BSA and flow cytometry was carried out using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) at the Duke Cancer Center Core Facility. Mean fluorescence intensity on cells, corresponding to C5b-9 deposition on the cell surface, was determined using FlowJo software (Tree Star Inc., Ashland, OR).
CDC Assays
LDH release assays were performed using the CytoTox 96 Non-radioactive Cytotoxicity Assay (Promega, Madison, WI) to assess cytotoxicity. NHS, CFH and C1q depleted serum were purchased from Complement Tech, Inc. NHS was diluted 1:8, except in the B cell cytotoxicity experiments where it was diluted 1:16. The number of cells plated in each well (between 5 × 103 and 1 × 104 for solid tumor cell lines and 5 × 105 for B cell experiments) was experimentally determined based on spontaneous and maximum LDH release. After addition of anti-CFH or control antibodies (~100 µg/ml except where noted), cells were incubated overnight at 37 °C, except for the B cell experiments in which they were incubated for 2 hours. All reactions were performed in triplicate and LDH release was read in a plate reader at 490nm.
In Vivo Tumor Growth Study: Syngeneic Lung Cancer Model (KLN205-DBA/2)
Animal care was in accordance with Duke University IACUC guidelines. For in vivo mouse studies, we developed a mAb7968 chimeric antibody that contained the VH and VK region genes of Ab7968 and the constant region of mouse IgG2a (Strausberg et al., 2002) and mouse Ig kappa light chain (Svasti and Milstein, 1972), since initial experiments had shown that the human mAb triggered the formation of anti-human antibodies in nude mice. KLN205 tumor cells (4 × 105/0.1 ml/mouse) were injected s.c. on day 0. On days 1,4, 7, 10, and 13, mAb7968 or subtype-matched control mAbNctl (200 µg/0.1 ml/mouse; n=10 mice per group) was injected i.p. Tumor measurements were made by calipers and volumes calculated as (W2 × L)/2. Tumors did not form in three mice which received mAb7968 and one mouse that received mAbNctl; these mice were removed from the study analysis as to not bias the results. Two mice that received mAbNctl had to be euthanized due to ulcerated tumors and were also removed from the study analysis. All mice were euthanized at the end of the study. Tumors removed at necropsy were fixed in 10% formalin and embedded in paraffin. Five micron thick sections were stained with hematoxylin and eosin (H&E) for histologic evaluation.
Statistics
Statistical analysis was performed with Microsoft Excel using the Student’s t test for assessment of the significance of differences between treatment groups in the CDC assays, the ELISAs, and the tumor growth assays. P values < 0.05 were considered to be statistically significant.
Supplementary Material
Highlights.
Recombinant CFH mAbs were derived from B cells of patients with CFH autoantibodies.
Recombinant CFH mAbs promote tumor cell lysis and cause release of anaphylatoxins.
Recombinant murine CFH mAbs inhibit tumor growth in mice.
Acknowledgments
We thank Darell Bigner for the murine brain tumor model, Michael M. Frank for valuable discussions about complement, and J. Brice Weinberg for critical reading of the manuscript. This work was funded by grants to Edward F. Patz, Jr. from the LUNGevity Foundation, the Department of Defense (W81XWH-13-1-0189), the NIH (Transformative Grant UL1TR001117), and the Duke Translational Research Institute. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBERS
Coordinates and structure factors for the Fab7968 complex with a CFH1110–1122 peptide have been deposited in the Protein Data Bank (rcsb.org) with accession code 5EA0.
SUPPLEMENTAL INFORMATION
Supplemental information includes Supplemental Experimental Procedures, two tables and three figures.
AUTHOR CONTRIBUTIONS
RTB performed in vitro assays, MAM supervised and analyzed B cell sorting experiments resulting in the isolation of CFH-specific B cells, NN performed X-ray crystallography, SMA performed SPR experiments, STK performed in vivo experiments, RCB conducted pathology analysis of normal and tumor tissue, KRC provided statistical analysis, MJC performed in vitro assays and made intellectual contributions, EBG wrote the manuscript and made intellectual contributions, BFH provided invaluable advice, and HXL and EFP wrote the manuscript and directed the research. All authors read and approved the manuscript.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajona D, Hsu YF, Corrales L, Montuenga LM, Pio R. Down-regulation of human complement factor H sensitizes non-small cell lung cancer cells to complement attack and reduces in vivo tumor growth. J Immunol. 2007;178:5991–5998. doi: 10.4049/jimmunol.178.9.5991. [DOI] [PubMed] [Google Scholar]
- Amornsiripanitch N, Hong S, Campa MJ, Frank MM, Gottlin EB, Patz EF., Jr Complement factor H autoantibodies are associated with early stage NSCLC. Clinical Cancer Research. 2010;16:3226–3231. doi: 10.1158/1078-0432.CCR-10-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigner SH, Humphrey PA, Wong AJ, Vogelstein B, Mark J, Friedman HS, Bigner DD. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 1990;50:8017–8022. [PubMed] [Google Scholar]
- Campa MJ, Gottlin EB, Bushey RT, Patz EF., Jr Complement factor H antibodies from lung cancer patients induce complement dependent lysis of tumor cells, suggesting a novel immunotherapeutic strategy. Cancer Immunology Research. 2015;3:1325–1332. doi: 10.1158/2326-6066.CIR-15-0122. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira VP, Pangburn MK, Cortes C. Complement control protein factor H: the good, the bad, and the inadequate. Mol Immunol. 2010;47:2187–2197. doi: 10.1016/j.molimm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert AP, Kavanagh D, Johansson C, Morgan HP, Blaum BS, Hannan JP, Barlow PN, Uhrin D. Structural and functional characterization of the product of disease-related factor H gene conversion. Biochemistry. 2012;51:1874–1884. doi: 10.1021/bi201689j. [DOI] [PubMed] [Google Scholar]
- Hofer J, Giner T, Jozsi M. Complement factor H-antibody-associated hemolytic uremic syndrome: pathogenesis, clinical presentation, and treatment. Semin Thromb Hemost. 2014;40:431–443. doi: 10.1055/s-0034-1375297. [DOI] [PubMed] [Google Scholar]
- Huang M, Furie BC, Furie B. Crystal structure of the calcium-stabilized human factor IX Gla domain bound to a conformation-specific anti-factor IX antibody. J Biol Chem. 2004;279:14338–14346. doi: 10.1074/jbc.M314011200. [DOI] [PubMed] [Google Scholar]
- Huck S, Fort P, Crawford DH, Lefranc MP, Lefranc G. Sequence of a human immunoglobulin gamma 3 heavy chain constant region gene: comparison with the other human C gamma genes. Nucleic acids research. 1986;14:1779–1789. doi: 10.1093/nar/14.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junnikkala S, Jokiranta TS, Friese MA, Jarva H, Zipfel PF, Meri S. Exceptional resistance of human H2 glioblastoma cells to complement-mediated killing by expression and utilization of factor H and factor H-like protein 1. J Immunol. 2000;164:6075–6081. doi: 10.4049/jimmunol.164.11.6075. [DOI] [PubMed] [Google Scholar]
- Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci U S A. 2011;108:2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, LePage GA. Growth characteristics and drug responses of a murine lung carcinoma in vitro and in vivo. Cancer Res. 1978;38:2084–2090. [PubMed] [Google Scholar]
- Kepler TB. Reconstructing a B-cell clonal lineage. I. Statistical inference of unobserved ancestors. F1000Res. 2013;2:103. doi: 10.12688/f1000research.2-103.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepler TB, Munshaw S, Wiehe K, Zhang R, Yu JS, Woods CW, Denny TN, Tomaras GD, Alam SM, Moody MA, et al. Reconstructing a B-Cell Clonal Lineage. II. Mutation, Selection, and Affinity Maturation. Frontiers in immunology. 2014;5:170. doi: 10.3389/fimmu.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski CL, Ramm LE, Hammer CH, Mayer MM, Shin ML. Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A. 1983;80:3816–3820. doi: 10.1073/pnas.80.12.3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, et al. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. The Journal of experimental medicine. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, Parks R, Whitesides J, Marshall DJ, Hwang KK, et al. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods. 2009;158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature. 2013;496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell SC, Davis IW, Arendall WB, 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- Makou E, Herbert AP, Barlow PN. Functional anatomy of complement factor H. Biochemistry. 2013;52:3949–3962. doi: 10.1021/bi4003452. [DOI] [PubMed] [Google Scholar]
- Malia TJ, Obmolova G, Almagro JC, Gilliland GL, Teplyakov A. Crystal structure of human germline antibody 3-23/B3. Mol Immunol. 2011;48:1586–1588. doi: 10.1016/j.molimm.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Morgan HP, Mertens HD, Guariento M, Schmidt CQ, Soares DC, Svergun DI, Herbert AP, Barlow PN, Hannan JP. Structural analysis of the C-terminal region (modules 18–20) of complement regulator factor H (FH) PLoS One. 2012;7:e32187. doi: 10.1371/journal.pone.0032187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, Kavanagh D, Mertens HD, Svergun DI, Johansson CM, et al. Structural basis for engagement by complement factor H of C3b on a self surface. Nature structural & molecular biology. 2011;18:463–470. doi: 10.1038/nsmb.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L, Chen X, Alam M, Tomaras G, Zhang R, Marshall DJ, Chen B, Parks R, Foulger A, Jaeger F, et al. Isolation of a human anti-HIV gp41 membrane proximal region neutralizing antibody by antigen-specific single B cell sorting. PLoS One. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicely NI, Dennison SM, Spicer L, Scearce RM, Kelsoe G, Ueda Y, Chen H, Liao HX, Alam SM, Haynes BF. Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region. Nature structural & molecular biology. 2010;17:1492–1494. doi: 10.1038/nsmb.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation model. Methods in Enzymology: Macromolecular Crystallography Part A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace L, Lysenko V, Fontana AO, Cecconi V, Janssen H, Bicvic A, Okoniewski M, Pruschy M, Dummer R, Neefjes J, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity. 2015;42:767–777. doi: 10.1016/j.immuni.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Svasti J, Milstein C. The complete amino acid sequence of a mouse kappa light chain. Biochem J. 1972;128:427–444. doi: 10.1042/bj1280427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Grosse-Kunstleve RW, Afonine PV, Moriarty NW, Zwart PH, Hung LW, Read RJ, Adams PD. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr D Biol Crystallogr. 2008;64:61–69. doi: 10.1107/S090744490705024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsano S, Rashkovsky L, Shapiro H, Ophir D, Mark-Bentankur T. Human lung cancer cell lines express cell membrane complement inhibitory proteins and are extremely resistant to complement-mediated lysis; a comparison with normal human respiratory epithelium in vitro, and an insight into mechanism(s) of resistance. Clinical and experimental immunology. 1998;113:173–182. doi: 10.1046/j.1365-2249.1998.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Jr, Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A. 2012;109:E2083–E2090. doi: 10.1073/pnas.1208984109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehe K, Easterhoff D, Luo K, Nicely NI, Bradley T, Jaeger FH, Dennison SM, Zhang R, Lloyd KE, Stolarchuk C, et al. Antibody light-chain-restricted recognition of the site of immune pressure in the RV144 HIV-1 vaccine trial is phylogenetically conserved. Immunity. 2014;41:909–918. doi: 10.1016/j.immuni.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczek E, Rzepko R, Nowis D, Legat M, Golab J, Glab M, Gorlewicz A, Konopacki F, Mazurkiewicz M, Sladowski D, et al. The possible role of factor H in colon cancer resistance to complement attack. Int J Cancer. 2008;122:2030–2037. doi: 10.1002/ijc.23238. [DOI] [PubMed] [Google Scholar]
- Zhou T, Lynch RM, Chen L, Acharya P, Wu X, Doria-Rose NA, Joyce MG, Lingwood D, Soto C, Bailer RT, et al. Structural Repertoire of HIV-1-Neutralizing Antibodies Targeting the CD4 Supersite in 14 Donors. Cell. 2015;161:1280–1292. doi: 10.1016/j.cell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Zhu J, Wu X, Moquin S, Zhang B, Acharya P, Georgiev IS, Altae-Tran HR, Chuang GY, Joyce MG, et al. Multidonor analysis reveals structural elements, genetic determinants, and maturation pathway for HIV-1 neutralization by VRC01-class antibodies. Immunity. 2013;39:245–258. doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.