SUMMARY
Memory can strongly influence how attention is deployed in future encounters. Though memory dependent on the medial temporal lobes has been shown to drive attention, how other memory systems could concurrently and comparably enhance attention is less clear. Here, we demonstrate that both reinforcement learning and context memory facilitate attention in a visual search task. Using functional magnetic resonance imaging, we dissociate the mechanisms by which these memories guide attention: trial by trial, the hippocampus (not the striatum) predicted attention benefits from context memory, while the striatum (not the hippocampus) predicted facilitation from rewarded stimulus-response associations. Responses in these regions were also distinctly correlated with individual differences in each type of memory-guided attention. This study provides novel evidence for the role of the striatum in guiding attention, dissociable from hippocampus-dependent context memory.
INTRODUCTION
Attention can be profoundly influenced by memory. Even something as simple as having previously viewed a picture or an array of shapes can inform where visual attention will be directed, enhancing perceptual sensitivity (Chun and Jiang, 1998; Patai et al., 2012; Summerfield et al., 2006). While the influence of memory on attention is a relatively recent topic (Hutchinson and Turk-Browne, 2012; Rosen et al., 2015), there is compelling evidence that hippocampal memory can guide attention. The hippocampal memory system rapidly encodes episodic memories, which are flexible and rich in contextual detail (Burgess et al., 2002). Long-term memory for complex scenes engages the hippocampus and facilitates attention and eye movements to targets, even in the absence of explicit recall (Hannula and Ranganath, 2009; Summerfield et al., 2006). The contextual cueing effect demonstrates that memory for a repeated spatial configuration guides attention and improves performance in visual search (Chun and Jiang, 1998). These memories depend on medial temporal lobe (MTL) structures and have frequently been shown to involve the hippocampus (Chun and Phelps, 1999; Giesbrecht et al., 2013; Greene et al., 2007; Preston and Gabrieli, 2008).
Although these studies have demonstrated the critical role of hippocampal memory in guiding attention, memory is not a unitary process (Squire, 1992). Different neural systems support encoding and retrieval of specific kinds of information (Henke, 2010). Thus, the ability of memory for diverse cues to guide attention may depend on distinct memory systems. Unlike hippocampal memory, the striatum slowly acquires rigid associations between stimuli and responses (Bayley et al., 2005; Graybiel, 1998; Yin and Knowlton, 2006). In healthy individuals, hippocampal and striatal systems can concurrently acquire information (Foerde and Shohamy, 2011), and lesion studies in rats (Packard and McGaugh, 1996) and human patients (Knowlton et al., 1996) have dissociated these systems. However, the influence of striatal memory on attention has not been studied.
We hypothesize that people can learn and use multiple informative cues to guide attention. Furthermore, we hypothesize that changing the type of cue can change the memory system that guides attention. We developed a way to directly compare how hippocampal and striatal memory influences attention. Contextual cueing demonstrates the impact of hippocampal memory on attention in visual search. During the search task, participants search for a target (a rotated “T”) and press a button once they find it, indicating the direction of the “T” (Figure 1). The influence of hippocampal memory is shown via repeated configurations of target and distractors; on these trials, memory for spatial context guides attention to the exact location of the target. This effect is implicit. Participants do not have explicit memory for the repeated context (Chun and Jiang, 1998, 2003; Chun and Phelps, 1999).
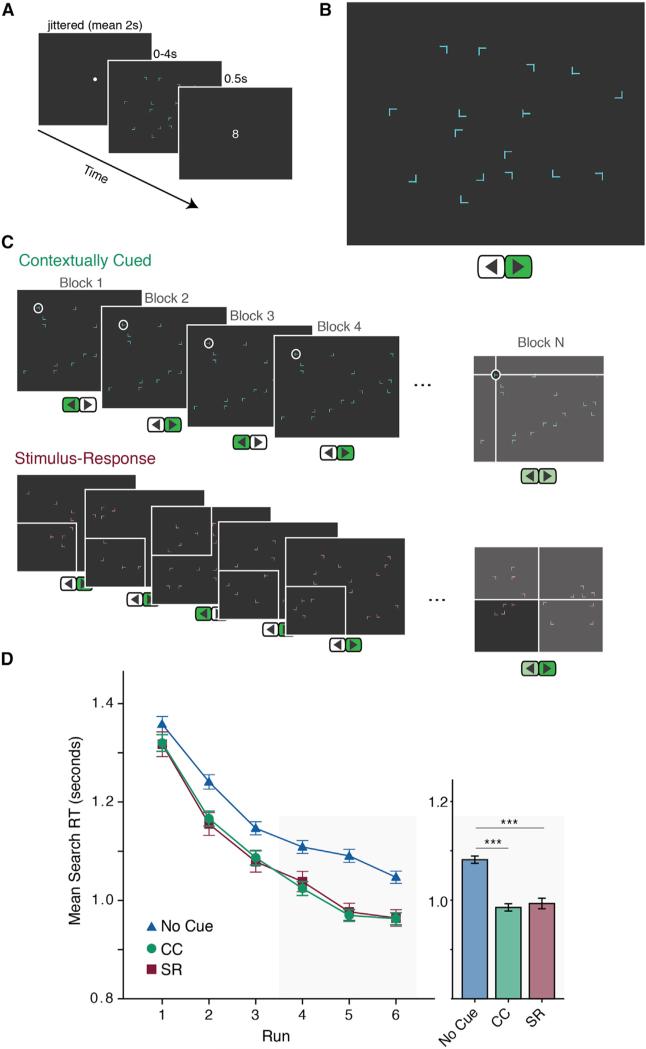
Figure 1. Experiment Design and Behavior.
(A) Trial sequence.
(B) Example search screen and correct response.
(C) Schematic of CC and SR associations.
(D) Behavioral results. Participants were significantly faster at finding the “T” in the presence of a CC or SR cue compared to no cue (replication, Figure S1). Left: learning throughout the experiment (one run = mean of four sequential blocks). Only responses for accurate, non-outlier trials are shown. Outliers were defined as RTs more than 3 SDs outside the mean for that trial type (1.5% of trials, no significant difference between trial types; Chun and Jiang, 2003). Right: performance during the second half of the experiment (gray background). Error bars, ±1 SE. ***p < 0.001.
While contextual cueing has been frequently replicated and provides an index of hippocampal memory guiding attention, other forms of predictive associations, potentially reliant on other memory systems, have been less studied. To address this, we modified the search task to include probabilistic stimulus-response (SR) associations known to rely on the striatum as mnemonic cues for attention. Specifically, on some trials, the target and distractors appeared in a different color. The predictive color probabilistically (80% validity) cued the target location (quadrant of the screen) and the button-press response (the direction of the “T”). Thus, the cue evoked a “chunked” response of orienting to the quadrant and preparing the button press. The striatum is critical to forming such SR associations, including the chunking of motor and cognitive actions following a cue (e.g., Graybiel, 1998). We randomly interleaved these cued trials, hereafter contextually cued (CC) and SR, with trials that had no mnemonic cue. The influence of both cues on attention was quantified as faster reaction time (RT) on cued compared to uncued trials.
RESULTS
Behavior
Participants improved their search performance (showed faster RT) for all trial types (F(5,170) = 108.5, p < 0.001; Figure 1D). Importantly, they showed superior performance on both CC and SR relative to no-cue trials (main effect of trial type, F(2,68) = 11.03, p < 0.001). This was replicated in a separate sample (Figure S1). There was no significant difference in RT between SR and CC trials, suggesting that both mnemonic cues comparably enhanced attention, although the learning rate differed (Figure S1).
Participants were accurate (97.5%). There were no speed-accuracy tradeoffs for memory-guided attention: accuracy on SR (97.7%) was not significantly different from that on no-cue trials (97.1%; t(34) = 1.35, p = 0.2), while CC (98%) were more accurate than no-cue trials (t(34) = 2.29, p = 0.03).
Critically, SR associations were specifically evoked by the SR cue: when the SR cue (color) was absent, participants were not faster at finding the “T” in the SR-cued quadrant (t(34) = 0.7, p = 0.5), and they were not faster in making the SR-cued response (t(34) = 1.36, p = 0.2), indi cating that the benefit on SR trials was specifically elicited by the SR cue. Furthermore, the cued response involved more than a simple button press after viewing the SR color (Figure S2).
To test whether memory for CC and SR associations was explicit, we probed memory for “T” location quadrant (CC and SR) and button-press response (SR). Participants did not differ from chance (25%) in recalling the “T” location on CC (mean accuracy = 23.9% [SEM = 1.8%]; t(34) = 0.59, p = 0.6) or SR trials (mean accuracy = 29.2% [SEM = 2.8%]; t(34) = 1.5, p = 0.14) and did not differ from chance (50%) in recalling the motor response on SR trials (mean accuracy = 45.7% [SEM = 4.2%]; t(34) = 1.0, p = 0.3).
These results demonstrate that memories for CC and SR associations implicitly guided attention. We hypothesized that changes in attention resulting from these memories depended on different neural systems. To test this, we examined blood oxygen level-dependent (BOLD) responses as participants performed the task, enabling us probe memory-guided attention as it unfolded (Karuza et al., 2014).
Subsequent Attention Effect: Trial-by-Trial BOLD Response Predicts Behavior
We investigated how neural systems supporting memory influence attention trial by trial. Earlier work on episodic memory has shown that activity at encoding predicts retrieval success (Wagner et al., 1998). Here, we examined whether trial-evoked BOLD responses (Figure S3) could predict memory-guided attention (RT) on the subsequent trial (Figure 2A). We anatomically defined a priori regions of interest (ROIs; hippocampus and striatum). In one set of linear regressions, signals from both hippocampus and striatum were predictors of subsequent RT. To understand the contributions of subregions, we also ran separate linear regressions in which one subregion at a time served as the predictor.
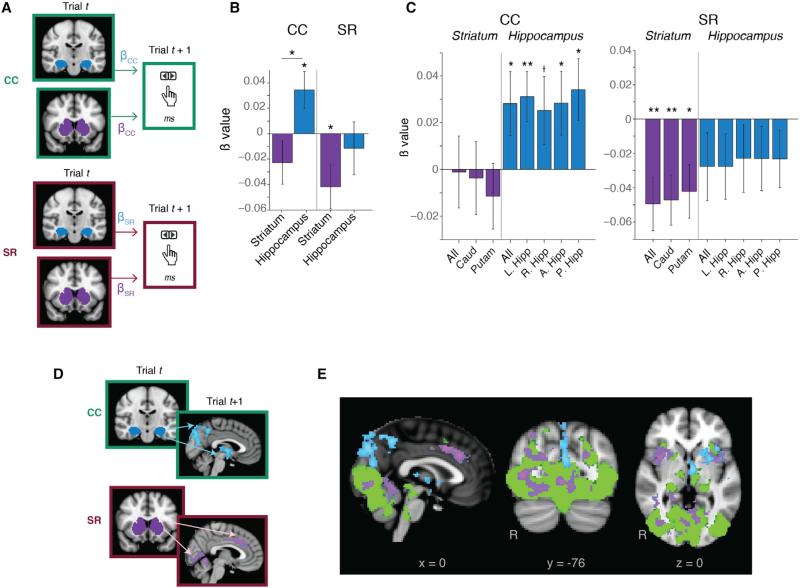
Figure 2. SAEs Are Predicted by Distinct Neural Regions.
(A) Analysis procedure, trial-by-trial prediction of behavior. For each trial, we extracted the trial-evoked response from each ROI and computed the area under the curve (Figure S3). We used these trial-evoked responses (trial t) to predict RT for the next trial with that memory cue (t + 1). For CC trials, this analysis was conducted for each repeated configuration separately and then averaged; for SR trials, this was run iteratively for all subsequent trials.
(B) Hippocampus and striatum dissociate SAEs. When trial-evoked responses from the hippocampus (blue) and striatum (purple) were both used as predictors of subsequent CC-guided attention, the hippocampus significantly predicted attention while the striatum did not. By contrast, trial-evoked responses in the striatum significantly predicted SR-guided attention while hippocampal responses did not. Positive β values, negative trial-evoked responses predict decreasing RT; negative β values, positive responses predict decreasing RT.
(C) Hippocampal regions predict CC-guided attention, while striatal regions predict SR-guided attention. Regressions were run separately for each region and graphed as described in (B). Left panel: CC trials. Right panel: SR trials. All, full ROI (striatum left, hippocampus right); Caud, caudate; Putam, putamen; L. Hipp, left hippocampus; R. Hipp, right hippocampus; A. Hipp, anterior hippocampus; P. Hipp, posterior hippocampus.
(D) Neural circuitry of SAE and analysis procedure. Trial-evoked responses from the hippocampus on CCt (as in A) modulated activity on CCt+1, and trial-evoked responses from the striatum on SRt modulated activity on SRt+1. Following (B), we examined regions negatively modulated by the hippocampus and positively modulated by the striatum.
(E) Distinct mechanisms of memory-guided attention. Blue, negatively modulated by hippocampus; purple, positively modulated by striatum; green, search regions, determined by conjunction analysis of significant activity on all accurate trials. Conjunction analyses are voxel-wise corrected p < 0.001, modulation analyses are cluster corrected (Z > 2.3) to a corrected threshold of p < 0.05. See the full list of significant clusters in Tables 1 and S1.
Error bars, ±1 SEM. *p < 0.05, **p < 0.01, †p = 0.09.
Our subsequent attention effect (SAE) analysis revealed that BOLD signal in the hippocampus significantly predicted subsequent attention on CC trials (mean β = 0.03, p = 0.03) while striatal signal did not (Figure 2B). The hippocampus was a stronger predictor of CC-based SAE than the striatum (β = –0.02, t(34) = 2.18, p = 0.04). In separate regressions, almost all hippocampal subregions significantly predicted CC-based SAE (full hippocampus: β = 0.028, p = 0.049; left: β = 0.03, p = 0.007; right: β = 0.025, p = 0.09, anterior: β = 0.028, p = 0.047; posterior: β = 0.034, p = 0.01; Figure 2C), but activity in striatal regions did not. Positive hippocampal β values indicate that lower hippocampal signal predicts greater SAE (lower RT) on CC trials.
We observed the opposite pattern on SR trials. BOLD signal in the striatum predicted SR-based SAE (β = –0.04, p = 0.02), while hippocampal signal did not (Figure 2B). When we ran separate regressions, signal in all striatal subregions predicted SR-based SAE (full striatum: β = –0.049, p = 0.003; caudate: β = –0.047, p = 0.003; putamen: β = –0.042, p = 0.01), but signal in hippocampal regions did not (Figure 2C). Negative β values indicate that a higher striatal signal predicts greater SAE on SR trials. These results dissociate hippocampal and striatal involvement in CC- and SR-guided attention.
SAE: Neural Circuits
To probe neural networks of SAEs, we ran an analogous analysis using trial-evoked responses in the hippocampus and striatum to predict subsequent BOLD responses in other regions (Figure 2D). We used trial-evoked responses from the hippocampus and striatum as parametric modulators for CC and SR trials, testing where hippocampal response on CC trial t predicted BOLD signal on CC trial t + 1 and where striatal response predicted subsequent BOLD on SR trials. As decreased hippocampal BOLD predicted CC-based SAE, we probed negative correlations with the hippocampus and, as increased striatal BOLD predicted SR-based SAE, we probed positive correlations with the striatum (Table 1). These analyses revealed distinct networks for striatum- and hippocampus-guided subsequent attention (Figure 2E).
Table 1.
Regions Tracking SAE: Activity on Trial t + 1 Predicted by Signal on Trial t
| CC-Based SAE: Hippocampal Signal | ||||
|---|---|---|---|---|
| X | Y | Z | Z Score | |
| Frontal | ||||
| L precentral gyrus | −48 | −4 | 34 | 3.47 |
| 14 | −24 | 46 | 4.24 | |
| L central opercular cortex/insula | −38 | −8 | 14 | 3.79 |
| L central opercular cortex | −46 | −8 | 18 | 3.59 |
| Parietal | ||||
| L central opercular cortex/postcentral gyrus | −54 | −18 | 20 | 3.62 |
| L central opercular cortex/parietal operculum cortex | −54 | −22 | 20 | 3.7 |
| R precuneus | 12 | −54 | 50 | 3.65 |
| L precuneus | 0 | −80 | 40 | 4.67 |
| 0 | −52 | 48 | 3.68 | |
| −2 | −64 | 54 | 3.51 | |
| Temporal | ||||
| L temporal pole | −40 | 12 -18 | 3.65 | |
| Occipital | ||||
| L occipital pole | −4 | −90 | 38 | 3.86 |
| Subcortical | ||||
| L putamen | −26 | 8 | −4 | 3.94 |
| L putamen/white matter | −20 | 2 | −12 | 3.5 |
| −24 | 8 | −12 | 3.48 | |
| L caudate | −16 | −14 | 4 | 3.73 |
| L thalamus | −4 | −8 | 2 | 4.11 |
| SR-Based SAE: Striatal Signal | ||||
|---|---|---|---|---|
| X | Y | Z | Z Score | |
| Frontal | ||||
| R frontal pole | 38 | 58 | 20 | 3.6 |
| 32 | 52 | 18 | 4.35 | |
| 38 | 48 | 6 | 3.27 | |
| 34 | 46 | 8 | 3.04 | |
| 34 | 40 | 22 | 4.55 | |
| R frontal pole/middle frontal gyrus | 36 | 40 | 32 | 3.5 |
| L frontal orbital cortex | −36 | 24 | −4 | 3.28 |
| R anterior cingulate | 2 | −4 | 38 | 3.99 |
| L anterior cingulate | 0 | 12 | 40 | 4.55 |
| R paracingulate | 2 | 28 | 32 | 4.12 |
| 4 | 20 | 40 | 3.71 | |
| R anterior insula | 38 | 8 | 0 | 3.81 |
| −42 | 8 | −6 | 3.21 | |
| L anterior insula | −38 | 4 2 | 3.58 | |
| Occipital | ||||
| L temporal-occipital-fusiform cortex | −28 | −54 | −14 | 4.04 |
| R lateral occipital cortex, superior | 28 | −80 | 22 | 4.49 |
| 16 | −86 | 20 | 3.98 | |
| Occipital | ||||
| R lateral occipital cortex, inferior | 42 | −66 | −6 | 4.17 |
| 38 | −74 | 4 | 4.35 | |
| R occipital pole | 16 | −90 | 18 | 4 |
| Subcortical | ||||
| L amygdala/white matter | −20 | 2 | −14 | 4.08 |
| L putamen | −26 | 4 | −12 | 3.71 |
Table includes peak and local maxima. Coordinates are in standard Montreal Neurological Institute atlas space. For CC-based SAE, signal on trial t + 1 is negatively predicted by hippocampal signal on trial t; for SR-based SAE, signal on trial t + 1 is positively predicted by striatal signal on trial t. Hippocampal and striatal signals on trial t are computed as in Figure 2 (for details, see Figure S3); clusters are shown in Figures 2D and 2E. R, right hemisphere; L, left hemisphere.
Common Activity in Memory- and Visually Guided Search
To better understand brain regions involved across memory- and visually guided search, we identified areas that were commonly active on accurate CC, SR, and no-cue trials (following Summerfield et al., 2006). Using a generalized linear model (GLM), we separately identified regions that were significantly active in three contrasts: (voxel-wise corrected p < 0.05): CC versus baseline, SR versus baseline, and no-cue versus baseline (accurate only). To test for significant activity in all contrasts, we performed a conjunction analysis, taking the maximum p value from these maps (Nichols et al., 2005). Peak and local maxima are shown in Table S1 and Figure 2E. These include the anterior insula, precentral sulcus, superior parietal lobule, and lateral occipital cortex.
BOLD Responses Correlate with Individual Differences in Memory-Guided Attention
Next, we probed whether BOLD signal in our ROIs correlated with individual differences in memory-guided attention. We quantified memory-guided attention as the difference in RT between no-cue and memory-cued trials during the second half of the experiment (Chun and Jiang, 1998). This revealed considerable variability across participants (Figure 3A). To understand how the hippocampus and striatum contribute to this variability, we used a GLM to derive BOLD estimates per trial type per ROI. We then correlated these responses with attention benefits from each mnemonic cue and compared the dependent correlations (Steiger, 1980, Equations 3, 10, and 14).
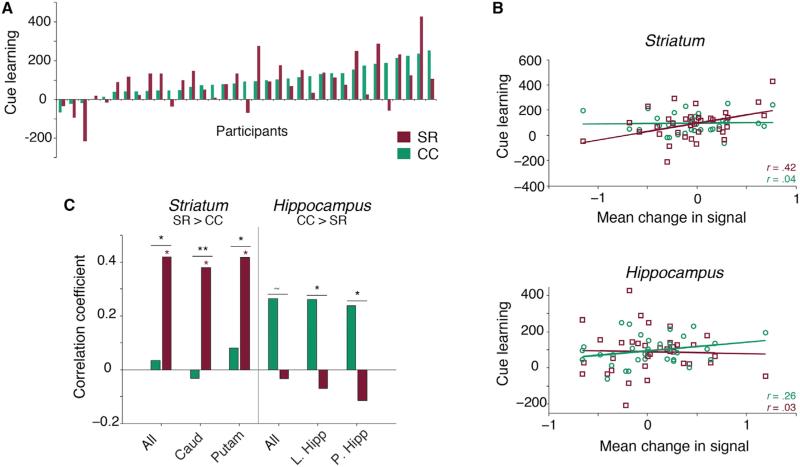
Figure 3. Distinct Neural Regions Correlate with Individual Differences in Memory-Guided Attention.
(A) Variability in memory-guided attention across participants. Cue learning (y axis) is the mean RT difference between no-cue and memory-cued (CC and SR) trials, second half of the experiment (as in Chun and Jiang, 1998). Positive values indicate memory-guided attention. Each participant is represented by two bars: red, SR-guided attention; green, CC-guided attention.
(B) Change in BOLD correlates with individual differences. Scatter plots show correlation between change in signal (first to second half) and memory-guided attention across participants. Negative x axis values indicate decrease in signal. Cue learning as in (A).
(C) Hippocampal and striatal BOLD dissociate individual differences. Change in striatal BOLD correlates with individual differences in SR-guided attention (red asterisks) and is more correlated with SR-guided than with CC-guided attention across participants (also B, top). Conversely, change in hippocampal BOLD is more correlated with individual differences in CC-guided than in SR-guided attention (B, bottom). x axis labels as described in Figure 2. **p < 0.01, *p < 0.05, ~p = 0.06.
Our metric of memory-guided attention uses performance in the second half of the experiment, raising the question of whether early or late BOLD signal would predict individual differences. Early hippocampal signal predicts later contextual cueing effects (Giesbrecht et al., 2013), but striatal learning tends to follow a slower time course (Henke, 2010). We examined whether BOLD responses during the first or second half of the experiment correlated with individual differences in later memory-guided attention. BOLD responses in the posterior hippocampus (CC > no cue) during the first half correlated with later CC-guided attention (r = 0.41, p = 0.01), such that participants with lower hippocampal responses on CC than on no-cue trials showed greater CC-guided attention. Striatal signal did not correlate with CC-guided attention. In contrast, for SR-guided attention, responses in the putamen (SR > no cue) during the second half trended toward correlating with SR-guided attention (r = 0.32, p = 0.06), such that those with higher putamen responses on SR than no-cue trials showed greater SR-guided attention. Hippocampal signal did not correlate with SR-guided attention.
To facilitate comparison of CC- and SR-guided attention, we contrasted BOLD responses on CC versus SR trials, further demonstrating divergent temporal trajectories. Striatal signal during the second half was significantly more correlated with individual differences in SR-guided attention than striatal signal during the first half (SR > CC—first half: r = –0.17, second half: r = 0.27; Z = 2.4, p = 0.02). Hippocampal BOLD during the first half trended toward correlating more with variability in CC-guided than in SR-guided attention (CC > SR—CC: r = 0.22, SR: r = 0.07; Z = 1.8, p = 0.07). This difference was significant in the left hippocampus (CC: r = –0.28, SR: r = 0.05; Z = 2.07, p = 0.04).
Given these different time courses, we examined whether change in signal from the first to the second half of the experiment correlated with individual differences in memory-guided attention (Figures 3B and 3C). Changes in striatal BOLD were significantly more correlated with variability in SR-guided attention (SR > CC—r = 0.42, p = 0.01) than in CC-guided attention (SR > CC—r = 0.04; Z = 2.5, p = 0.01; also caudate [SR: r = 0.38, p = 0.03; CC: r = –0.03; Z = 2.6, p = 0.008] and putamen [SR: r = 0.42, p = 0.01; CC: r = 0.08; Z = 2.2, p = 0.03]). Conversely, changes in hippocampal BOLD trended toward being more correlated with variability in CC-guided attention (CC > SR—r = 0.26) than in SR-guided attention (CC > SR—r = –0.03; Z = –1.88, p = 0.06). This dissociation was significant in the left hippocampus (CC: r = 0.26; SR: r = –0.07; Z = –2.08, p = 0.04) and posterior hippocampus (CC: r = 0.24; SR: r = –0.1; Z = –2.21, p = 0.03).
DISCUSSION
This study demonstrates that multiple mnemonic cues facilitate attention via distinct neural systems. In our visual search task, participants showed subsequent attention benefits (faster RT) after learning CC and SR associations. By changing the cue, this task changed the memory system that guided attention: the hippocampus for CC-guided attention and the striatum for SR-guided attention. Trial by trial, trial-evoked BOLD responses in each region predicted RT on the subsequent exposure to the corresponding mnemonic cue. BOLD signal in these regions also correlated with individual differences in memory-guided attention.
Our trial-by-trial subsequent attention analysis revealed a double dissociation of neural regions involved in CC and SR learning. Analogous to the subsequent memory effect (Wagner et al., 1998), imaging data were sorted based on later behavior, allowing us to probe BOLD activity that predicted memory-specific behavior changes. Trial-evoked responses in the hippocampus on a CC trial significantly predicted attention on the next CC trial, while striatal responses did not. The opposite pattern was observed on SR trials: striatal responses predicted subsequent attention, while hippocampal responses did not. This demonstrates the role of multiple memory systems in contributing to a dynamic, integrated priority map that guides attention (Awh et al., 2012).
To understand brain networks in memory-guided attention, we conducted an analogous analysis in which trial-evoked responses predicted BOLD activity in other regions. While both hippocampus and striatum predicted subsequent BOLD in the insula, occipital pole, and putamen, these networks also had distinct features, revealing differences in how these memory systems guide attention. Several regions have previously been related to memory-guided attention: for the hippocampus, the precuneus (Rosen et al., 2015; Sestieri et al., 2010) and left caudate (Summerfield et al., 2006) and, for the striatum, the anterior cingulate (Rosen et al., 2015; Summerfield et al., 2006). The anterior cingulate functions as part of a circuit with the striatum (Alexander et al., 1986). In general, regions identified by this analysis overlapped with areas identified by our conjunction analysis, which correspond to the dorsal frontoparietal network (Corbetta and Shulman, 2002).
Our individual difference analyses highlighted the ability of memory regions to explain variability in CC- and SR-guided attention. Hippocampal BOLD, particularly the left and posterior hippocampus (previously correlated with contextual cueing; Greene et al., 2007) correlated more with variability in CC-guided than in SR-guided attention. By contrast, striatal BOLD correlated more with variability in SR-guided than in CC-guided attention. We also observed distinct temporal trajectories: CC-guided attention correlated more with early hippocampal BOLD, while SR-guided attention correlated more with late striatal BOLD. Early hippocampal activity predicts later contextual cueing (Giesbrecht et al., 2013), suggesting the importance of initial encoding. By contrast, striatal projection neurons change firing patterns gradually, showing increased coherence later in learning (Graybiel, 1998). These trajectories have been shown previously, with the MTL active initially and the caudate active later in learning (Poldrack et al., 2001).
We observed distinct directionality in hippocampus- and striatum-guided attention, with lower hippocampal and higher striatal BOLD predicting facilitated attention. This striatal pattern is consistent with habit studies that show increased striatal activity over the course of training (Tricomi et al., 2009). However, the hippocampus does not show consistent directionality. Other studies have also found that lower hippocampal signal during repeated encoding (Vannini et al., 2013; Ward et al., 2013) and retrieval (Gonsalves et al., 2005; Suzuki et al., 2011) correlate with better memory. The role of the hippocampus in memory-guided attention is mixed, with decreased (Rosen et al., 2015) and increased (Stokes et al., 2012; Summerfield et al., 2006) activity related to facilitated attention. It is possible that the explicit nature of the memory plays a role. Repetition suppression in several MTL areas has been related to (implicit) repetition priming but not explicit memory (Ward et al., 2013), although increased hippocampal activity has been shown before retrieval of implicit memory (Hannula and Ranganath, 2009). Thus, while the hippocampus is commonly recruited in memory-guided attention, the directionality of hippocampal BOLD activity in relation to implicit and explicit memory deserves further study.
Using a stimulus that probabilistically (80% validity) predicted a response, we demonstrated that striatal memory could guide attention. Previous research on top-down control of attention has shown that participants can successfully orient attention based on probabilistic cues. However, in prior studies, the association between cue and response was instructed and explicit, leading to specific preparatory control signals (Corbetta and Shulman, 2002) and enhanced efficiency. Such explicit associations between stimuli and responses are unlikely to rely on the striatal memory system (Graybiel, 1998; Yin and Knowlton, 2006). By contrast, in our study, the relationship was implicit. Striatal memory is recruited in situations for which the appropriate response to the cue is unclear (Foerde and Shohamy, 2011; Knowlton et al., 1996), requiring gradual trial-and-error learning.
Although the goal of the current study was to dissociate the contributions of hippocampal and striatal memory to attention, these memory systems do not typically function in isolation. In real-world search, multiple types of information (which may be learned by distinct systems) can facilitate target detection (Chun and Turk-Browne, 2007). In some situations, both systems are required for optimal performance (Shohamy et al., 2009), while in others, factors such as distraction (Foerde et al., 2006), duration of training (Poldrack et al., 2001), or stress (Schwabe and Wolf, 2012) influence which memory system will dominate performance. Future research is necessary to determine how hippocampal and striatal memory interact to influence attention in such scenarios.
EXPERIMENTAL PROCEDURES
This research was approved by New York University's University Committee on Activities Involving Human Subjects. For monetary compensation, 35 right-handed participants (51% female, mean age = 21.7 years) completed the study. Based on power analyses from prior behavioral data (Figure S1), 35 participants were needed for a power level of 0.08 (α = 0.05).
After 24 practice trials, participants completed 576 trials of visual search for a target among distractors (Figure 1). Following fixation, participants had up to 4 s to locate the “T” and make a response. As soon as the participant responded, this screen disappeared. Next, a feedback screen showed points earned (based on speed and accuracy). Participants completed 576 trials over six scanning runs. Across trials, we controlled for factors (such as “T” location) that could bias visual search (details in the Supplemental Experimental Procedures). Search trials had no mnemonic cue, CC, or probabilistic SR cues. On CC trials, repeated spatial configurations cued the exact “T” location but did not cue the response (50% probability). On SR trials, the color of the shapes cued (80% probability) the location of the “T” and the response (see Figure S2 for design of SR trials). These mnemonic cues were never presented together—that is, the CC trials were never presented in the SR-predictive color, and the spatial configuration of SR trials was randomly generated. Immediately following search, participants were tested for explicit memory for these mnemonic associations.
Scanning was performed on a 3T Siemens Allegra head-only scanner with a Siemens standard head coil at the New York University Center for Brain Imaging. SAE analyses are described in Figure 2. For detailed experimental procedures, please see Supplemental Information.
Supplementary Material
Highlights.
Multiple memory systems concurrently and implicitly facilitate attention
The striatum predicts attention benefits from reinforcement learning
The hippocampus predicts attention benefits from implicit context memory
The hippocampus quickly guides attention, while the striatum is slower
ACKNOWLEDGMENTS
This work was supported by NIH grant 1R01MH097085 (E.A.P.) and a National Science Foundation Graduate Research Fellowship (E.V.G.). We thank members of the E.A.P. and Davachi labs, especially Vishnu Murty, Sarah DuBrow, Alexa Tompary, and Peter Sokol-Hessner, for helpful discussions, and we thank Jackie Reitzes for assistance with data collection.
Footnotes
AUTHOR CONTRIBUTIONS
Conceptualization & Methodology, E.V.G., M.M.C., E.A.P.; Investigation, E.V.G.; Writing – Original Draft, E.V.G.; Writing – Review & Editing, M.M.C. and E.A.P.; Funding Acquisition, E.A.P.; Resources, E.V.G. and M.M.C.; Supervision, E.A.P.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, three figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.neuron.2015.12.014.
REFERENCES
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn. Sci. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: implicit learning and memory of visual context guides spatial attention. Cognit. Psychol. 1998;36:28–71. doi: 10.1006/cogp.1998.0681. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat. Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Implicit, long-term spatial contextual memory. J. Exp. Psychol. Learn. Mem. Cogn. 2003;29:224–234. doi: 10.1037/0278-7393.29.2.224. [DOI] [PubMed] [Google Scholar]
- Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr. Opin. Neurobiol. 2007;17:177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Foerde K, Shohamy D. Feedback timing modulates brain systems for learning in humans. J. Neurosci. 2011;31:13157–13167. doi: 10.1523/JNEUROSCI.2701-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc. Natl. Acad. Sci. USA. 2006;103:11778–11783. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht B, Sy JL, Guerin SA. Both memory and attention systems contribute to visual search for targets cued by implicitly learned context. Vision Res. 2013;85:80–89. doi: 10.1016/j.visres.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol. Learn. Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Gross WL, Elsinger CL, Rao SM. Hippocampal differentiation without recognition: an fMRI analysis of the contextual cueing task. Learn. Mem. 2007;14:548–553. doi: 10.1101/lm.609807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nat. Rev. Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Turk-Browne NB. Memory-guided attention: control from multiple memory systems. Trends Cogn. Sci. 2012;16:576–579. doi: 10.1016/j.tics.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karuza EA, Emberson LL, Aslin RN. Combining fMRI and behavioral measures to examine the process of human learning. Neurobiol. Learn. Mem. 2014;109:193–206. doi: 10.1016/j.nlm.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Patai EZ, Doallo S, Nobre AC. Long-term memories bias sensitivity and target selection in complex scenes. J. Cogn. Neurosci. 2012;24:2281–2291. doi: 10.1162/jocn_a_00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Paré-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Preston AR, Gabrieli JD. Dissociation between explicit memory and configural memory in the human medial temporal lobe. Cereb. Cortex. 2008;18:2192–2207. doi: 10.1093/cercor/bhm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Stern CE, Michalka SW, Devaney KJ, Somers DC. Cognitive control network contributions to memory-guided visual attention. Cereb. Cortex. 2015 doi: 10.1093/cercor/bhv028. Published online March 5, 2015. http://dx.doi.org/10.1093/cercor/bhv028. [DOI] [PMC free article] [PubMed]
- Schwabe L, Wolf OT. Stress modulates the engagement of multiple memory systems in classification learning. J. Neurosci. 2012;32:11042–11049. doi: 10.1523/JNEUROSCI.1484-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J. Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Hopkins RO, Sage J, Gluck MA. Distinct hippocampal and basal ganglia contributions to probabilistic learning and reversal. J. Cogn. Neurosci. 2009;21:1821–1833. doi: 10.1162/jocn.2009.21138. [DOI] [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J. Cogn. Neurosci. 1992;4:232–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980;87:245–251. [Google Scholar]
- Stokes MG, Atherton K, Patai EZ, Nobre AC. Long-term memory prepares neural activity for perception. Proc. Natl. Acad. Sci. USA. 2012;109:E360–E367. doi: 10.1073/pnas.1108555108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Lepsien J, Gitelman DR, Mesulam MM, Nobre AC. Orienting attention based on long-term memory experience. Neuron. 2006;49:905–916. doi: 10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD. Decrements in hippocampal activity with item repetition during continuous recognition: an fMRI study. J. Cogn. Neurosci. 2011;23:1522–1532. doi: 10.1162/jocn.2010.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur. J. Neurosci. 2009;29:2225–2232. doi: 10.1111/j.1460-9568.2009.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Sullivan C, Sperling RA. Differential functional response in the posteromedial cortices and hippocampus to stimulus repetition during successful memory encoding. Hum. Brain Mapp. 2013;34:1568–1578. doi: 10.1002/hbm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Chun MM, Kuhl BA. Repetition suppression and multi-voxel pattern similarity differentially track implicit and explicit visual memory. J. Neurosci. 2013;33:14749–14757. doi: 10.1523/JNEUROSCI.4889-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.