Abstract
What is known and objectives
Some studies, howbeit with conflicting reports have suggested that consumption of honey has a potential to modulate drug metabolising enzymes which may result in a honey - drug interaction. Numerous studies have established that honey varies in composition, influenced by the dominant floral, processing and environmental factors. Thus, variation in honey composition may be a contributing factor to the controversial results obtained. No previous drug interaction study has been done with any honey from Africa. CYP 3A4 is an important enzyme in drug metabolism studies as it is involved in the metabolism of over 50 % of drugs in clinical use and quinine remains very relevant in malaria treatment in the tropics, we therefore determined whether there is potential drug interaction between a Nigeria honey and quinine, a drug whose metabolism to 3 –hydroxyquinine is mediated majorly by CYP3A4.
Methods
In a three phase randomized cross-over study with a wash out period of two weeks between each treatment phase, ten (10) healthy volunteers received quinine sulphate tablet (600 mg single dose) alone (phase 1) or after administration of 10 ml of honey (Phase 2) and 20 ml of honey (Phase 3) twice daily for seven (7) days. Blood samples were collected at the 16th hour post quinine administration in each phase and quinine and its major metabolite, 3-hydroxyquinine were analyzed using a validated HPLC method.
Results
After scheduled doses of honey, the mean metabolic ratios of quinine (3-hydroxyquinine/quinine) increased by 24.4 % (with 10 ml of honey) and reduced by 23.9 % (with 20 ml of honey) when compared to baseline. These magnitudes of alteration in the mean metabolic ratios were not significant (p > 0.05; Friedman-test). The geometric mean (95 % CI) for the metabolic ratio of quinine before and after honey intake at the two dose levels studied were 0.82 (0.54, 1.23) and 1.29 (0.96, 1.72) respectively and were also not significant (P = 0.296 and 0.081 respectively; student t-test).
What is new and conclusion
This is a pioneer study on the effect of Nigeria/Africa honey on quinine metabolism. The findings indicated that low and high doses of honey did not significantly affect metabolism of quinine to 3-hydroxyquinine. This suggest that CYP3A4 activity is not significantly altered following low or high dose of honey, since CYP3A4 has been reported to be responsible for the conversion of quinine to 3-hydroxyquinine. In conclusion, the outcome of this study suggests that there may be no potential significant metabolic interaction between Nigeria honey and quinine administration.
Keywords: Quinine, Nigeria-Honey, CYP3A4, Herb-drug interaction
INTRODUCTION
Several lines of evidence indicate that an increase in concurrent consumption of medicinal herbs or dietary supplements with conventional therapeutic agents increases the possibility of drug-food/herb interaction with consequent therapeutic outcomes1, 2. Since herbal dietary supplements or products are not regulated like drugs, their compositions, doses and frequencies of use are more variable and may deliver compounds with the potential to modulate drug metabolising enzymes.3 Honey is a common household nutritional supplement of plant-origin with variable bioactive components from the nectars of various plants4, with no restriction on the amount taken. Climate and environmental conditions have been shown to alter the composition of plant. For instance, the fruit of grape fruit tree exposed to freezing temperature has been reported to produce more naringin in response to environmental stress5. Similarly, numerous studies have reported that floral sources, seasonal and environmental factors in different geographical regions affect the type and amount of components, especially the phenolic constituents present in honey 4, 6–11 leading to a variation in honey constituents. Honey components include sugar, and flavonoids (quercetin, kaemferol, and luteolin) which have been reported to alter activities of drug metabolising enzymes7–9. CYP3A is an important enzyme in drug metabolism because it is involved in the metabolism of over 50 % of drugs in clinical use15, 16. Existing studies that evaluated the effect of honey from various geographical regions on the activities of drug metabolizing enzymes in rats and humans revealed conflicting results. Specifically, results from such previous studies showed that honey from India significantly induced CYP3A in animal models17–19 and man20. On the contrary, studies by Fetzner et al21, suggested that honey from Germany did not significantly modulate CYP3A activity in human.
Following an exhaustive literature search, no study was found to have assessed the effect of any honey from Nigeria or any African region on drug metabolising enzyme. A survey (unpublished) we conducted prior to the commencement of this study revealed that honey is largely and commonly consumed both as sweetener and for its medicinal benefit. Since geographical location has been reported to influence the composition of honey, and there are no examples of drug interaction studies between Nigeria honey from tropical climate and any drug, we therefore evaluated the effect of honey from Nigeria on CYP3A4 mediated metabolism of quinine. Several lines of evidence indicate that the metabolism of quinine to 3-hydroxyquinine is mediated by CYP3A22–26. Although the use of quinine as a drug to probe the activity of CPY3A4 has not been fully resolved, recent study 29, demonstrated that quinine metabolic ratio has been found to be comparable to midazolam clearance in plasma as a measure of CYP3A-activity. Midazolam has been established as a validated and recommended probe drug for CYP3A activity30. Quinine used as a substrate marker for CYP3A4 in this study has continued to find use in the treatment of severe and complicated malaria in Sub-Sahara Africa where malaria is endemic31 coupled with honey being a highly popular food supplement in Africa. Hence assessment of the effect of honey on CYP3A4 mediated quinine metabolism may suggest the effect of honey-quinine interaction on the outcome of quinine therapy. The metabolic ratio of 16th hour plasma sample of quinine22, 23, 27, 28 was used to assess the modulating effect of multiple dose honey intake on CYP3A mediated metabolism of quinine to 3-hydroxyquinine in healthy volunteers.
METHODS
Subjects
Ethical approval was obtained from Obafemi Awolowo University Teaching Hospital Complex Research Ethics Board and Safety Committee. Ten healthy subjects (range; mean ± SD: age, 20–28 years; 23.5 ± 3.0 and weight, 51–77 kg; 64.4 ± 7.9 kg) who gave written informed consent were recruited for this study. All subjects were declared healthy and fit for the study following assessment by a medical doctor, laboratory and clinical investigations. Volunteers were excluded from the study if on alcohol, on tobacco, pregnant, breast feeding, suffering from chronic disease, on quinine therapy or with known hypersensitivity reaction to quinine or similar agent. Subjects were told not to take any herbal dietary supplements, fruits juices, honey and quinine one week prior to and during the study.
Honey sample
Honey sample used in this study was purchased from a bee keeper in Ewu, a town located in the Western region of Nigeria. The floral (presumably what the bees fed on) within about 5 km distance from the beehives were Chromolaena odorata (Siam weed), Mangifera indica (Mango), Tectoa grandis (Teak), Elaeis guineensis (Palm) and Morinda lucida (Moringa) tree. Prior to the commencement of this study, a survey (Unpublished) was conducted and the result showed that people who used honey regularly took between 20 – 40 ml of honey per time. This was the rationale for the amount of honey used in this study
Study Design
The study was a randomized open label, three-phase crossover pharmacokinetic design, with each subject being his own control in order to minimize inter-individual variation in the ten healthy subjects who participated in the study. A wash-out period of two weeks was allowed between each study phase. In phase 1, each of the ten healthy volunteers after an overnight fast, received a single oral dose of 600 mg of quinine sulphate tablet (Maderich Ltd, Surrey, England). Blood samples (5 ml) were withdrawn by venepuncture from the forearm before and at the 16th hour post drug administration into EDTA tubes, centrifuged (3000 g for 10 mins) immediately and the resulting plasma was stored at −20º C until analysis. In subsequent phases, each subject ingested honey (10 ml in phase 2 and 20 ml in phase 3) twice daily for seven days and thereafter received quinine as given in phase 1. Blood samples were again collected and analyzed for quinine and its metabolite, 3-hydroxyquinine.
Analytical methods
The concentrations of quinine and its metabolite, 3-hydroxyquinne in plasma were determined using a high performance liquid chromatographic method described by Babalola et al32, but with slight modification. Before extraction of drug and metabolite, 3.0 μg/ml of primaquine (Internal standard) was spiked into 1.0 ml of plasma samples. Sample extraction involved protein precipitation with 0.64 ml perchloric acid (70 % w/w, density 1.664 g/ml), followed by basification with 1ml of 5 M NaOH and subsequent extraction using 4ml of diethylether and back extraction into 100 μl of 0.1 M HCl. 50 μl of the aqueous layer was loaded onto the HPLC 20 μl injector with the excess flowing out of the injector outlet tube. The high performance liquid chromatographic system used consisted of an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, Califonia, USA) fitted with an isocratic pump (model G1341A), a UV detector (model G1341B) and manual injector valve with a 20 μl sample loop. The chromatographic separation was achieved with an Eclipse XDB-C18 reverse phase HPLC column (150 x 4.6 mm internal diameter) with a 5-μm particle size (Agilent USA). The mobile phase (pH = 2.6) consisting of methanol, acetonitrile and 0.02 M KH2PO4 buffer (15:15: 70) containing 0.64 ml of perchloric acid (70% w/w, density 1.664 g/ml) was pumped at a flow rate of 1.6 ml/min. The column eluent was monitored at a wavelength of 254 nm. LC3D Chemstation software was used for data acquisition. Calibration procedure used has been previously reported32. The elution time for metabolite, drug and internal standard was less than 10 min. The Standard curve was linear over the concentrations range (0.25 – 4.0 μg/ml) for both quinine and metabolite. The coefficient of determination was 0.999 and 0.9995; limit of quantitation was 0.37μg/ml and 0.5μg/ml for quinine and 3- hydroxyquinine respectively. At concentrations of 0.25 and 4.0 μg/ml, the intra-day and inter-day coefficient of variation was less than 4 %, recovery was greater than 93.9 % for quinine and 73.4 % for 3-hydroxyquinine. The accuracy ranged between 93.1 % and 105.9 % for both drug and metabolite.
Data and Statistical Analysis
The Metabolic ratio (MR) of quinine calculated as the molar concentration ratio of 3-hydroxyquinine (metabolite) and quinine (drug) in the 16th hour plasma collection17, 22, was used to estimate the effect of honey on the metabolism of quinine.
Statistical analysis
Data was expressed as mean ± SD and p < 0.05 was considered statistically significant for all procedure. Friedman test was used to determine significant difference across the mean metabolic ratio of 3-hydroxyquinine/quinine of the ten healthy volunteers obtained upon administration of quinine alone (baseline: phase 1); quinine plus 10 ml of honey twice daily for seven days (Phase 2); and quinine plus honey 20 ml of honey twice daily for seven days (Phase 3). Additionally, the individual raw data was log transformed and the mean difference between the metabolic ratio obtained with quinine alone and quinine in the presence of honey at the respective honey dose level studied was calculated (log MR quinine alone _ log MR quinine and honey) and presented with the antilog. The corresponding 95% confidence intervals for the MR were calculated from the antilog of the mean. Student t- test was used to determine any statistically significant differences in the mean MR of quinine at baseline versus the respective doses of honey studied.
RESULTS
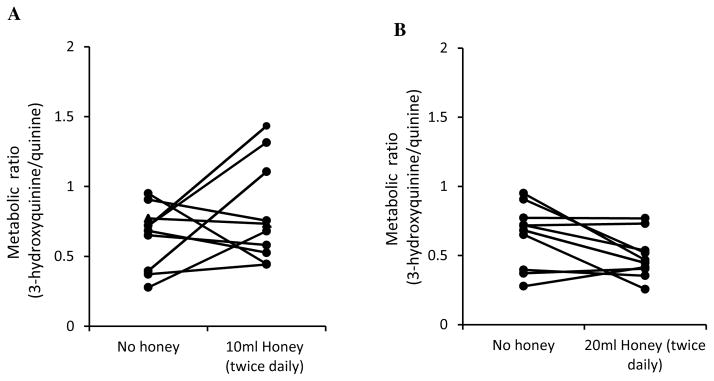
Overall quinine given alone or with honey was well tolerated as only three out of the ten volunteers complained of side effects as nausea, headache and dizziness. These were mild and transient and all enrolled subjects completed the study. Figure 1 presents the mean metabolic ratio of 3-hydroxyquinine to quinine for the ten volunteers who received quinine (600 mg) alone and with multiple adminstration of 10 ml or 20 ml honey twice daily for seven days. Table 1 presents the summary of the metabolic ratio (mean ± SD) for quinine at baseline and with ingestion of honey. As shown in the table, the mean metabolic ratio of quinine at baseline (0.64 ± 0.23), or with 10 ml twice daily (0.80 ± 0.36) or 20 ml twice daily of honey (0.49 ± 0.16) was not significantly different (p > 0.05). The magnitude of alteration in the mean metabolic ratio of quinine in the presence of 10 ml of honey taken twice daily increased by 24.4% but reduced by 23.8 % with 20 ml twice daily dose of honey (Table 1). The geometric mean [95 % Confidence Interval (CI)] for the metabolic ratio of quinine before and after honey intake at the two dose levels studied were 0.82(0.54, 1.23) with 10ml of honey; and 1.29 (0.96, 1.72) for 20 ml of honey (Table 2). Statistical analysis of the geometric means gave P = 0.296 and P = 0.081 respectively (Table 2), showing that the metabolic ratio of quinine in the presence of the lower dose or higher dose of honey compared to baseline were comparable (Student t-test; p > 0.05).
Fig. 1.
Metabolic ratio of 3-hydroxyquinine and quinine following oral administration of 600 mg quinine sulphate tablet to ten healthy volunteers before and after each volunteer received 10mls (A) or 20mls (B) of honey twice daily for 7 days (n = 10)
Table 1.
Summary of 16th hour mean metabolic ratio of quinine (3-hydroxyquinine/quinine) following oral administration of 600 mg of quinine sulphate tablets alone or after honey intake for seven days. (n=10)
| Treatment | Mean Metabolic Ratio (3-hydroxyquinine/quinine) | % change in Mean Metabolic ratio | P-value |
|---|---|---|---|
| Quinine alone (baseline) | 0.64 ± 0.23 | ||
| Quinine + Honey (10ml twice daily) | 0.80 ± 0.36 | ↑24.4 % | 0.15 |
| Quinine + Honey (20ml twice daily) | 0.49 ± 0.16 | ↓23.9 % |
(Friedman- test; Statistically significant P<0.05)
Table 2.
Log-transformed mean metabolic ratio of quinine (3-hydroxyquinine/quinine) following oral administration of 600 mg of quinine sulphate tablets alone or after honey intake.
| Treatment | Mean Metabolic Ratio (3-hydroxyquininelog – quininelog) | 95% CI | P value |
|---|---|---|---|
| Quinine alone and Quinine + Honey (10ml twice daily) | 0.82 | 0.54, 1.23 | 0.296 |
| Quinine alone and Quinine + Honey (20ml twice daily) | 1.29 | 0.96, 1.72 | 0.081 |
CI, Confidence interval.
Statistically significant P <0.05; Paired t- test calculated for log-transformed MR of quinine (with and without honey).
DISCUSSION
We investigated the potential effect of a Nigerian honey on the metabolism of quinine, a CYP3A substrate. To the best of our knowledge, this is the first study that evaluates the effect of any honey from Nigeria or Africa on the disposition of any drug. Our results (Tables 1 and 2) revealed that multiple doses of honey did not significantly alter the 16th hour mean metabolic ratio of quinine (3-hydroxyquinine/quinine) when compared to baseline. Previous studies have established that the metabolism of quinine to 3-hydroxyquinine is mediated by CYP3A and also, the metabolic ratio in a single plasma or urine sample collected 16 hours post quinine administration can serve as a stable measure of the hepatic activity of CYP3A mediated formation of 3-hydroxyquinine in humans22–27.. Even though the use of quinine has not yet been recommended or recognized as a CYP3A4 probe33, a recent study evaluated a single metabolic ratio of quinine and validated CYP3A4 probes, midazolam and 4β-hydroxycholesterol and found it to be comparable for determining CYP3A-induction30. In a previous study, Wanwimolruk et al23 suggested that quinine may serve as an in vivo probe to assess within-subject inhibition of liver CYP3A4 activity. However, just as for other recommended CYP3A probe, further studies may be needed to further investigate quinine as a potential and validated CYP3A4 probe during various conditions. For this reason, we designed a within subject study where the metabolic ratio of 16th hour plasma sample of quinine was used to assess the modulating effect of honey on CYP3A mediated metabolism of quinine to 3-hydroxyquinine in healthy volunteers.
Even though the results of our study suggest that honey did not significantly modulate CYP3A-mediated metabolism in healthy human volunteers as evidenced from the metabolic ratio of 3-hydroxyquinine/quinine observed, the mean metabolic ratio of quinine compared to baseline increased by 24.4 % with lower dose of honey but reduced by 23.9 % when the amount of honey taken by the volunteers was doubled. This result indicates that honey produced a dose dependent biphasic effect on the pattern of quinine metabolism with a lower dose of honey suggestive of stimulation (Fig. 1), and higher dose indicative of inhibition (Fig. 2) of CYP3A4 activity. This observation is consistent with the findings of Kang et al34 where quercetin (one of the flavonoids found in honey) had a concentration dependent effect, where lower doses stimulated and higher doses inhibited CYP1A2 activity in a system expressing human CYP1A2. A fuller dose-response evaluation between quinine and honey using a larger population and an extensive sampling procedure seems necessary to confirm this possibility of a dose dependent effect of honey on quinine or as the case may be, other CYP substrates.
It is noteworthy that the few drug-honey interaction studies reported in literature observed different effects of honey on CYP3A. For example, in a study were 10 ml of an Indian- honey was used twice daily for seven days in healthy volunteers, the excretion of endogenous 6-betahydroxycortisol, a CYP3A marker was significantly increased20, while in a more recent study with midazolam as the marker; authors reported that German-honey at a dose of 14.3 ml for 10 days did not significantly alter the activities of CYP3A21. Although, we used a different CYP3A substrate and a much higher amount of Nigeria-honey, the result of our finding is consistent with the more recent study21. This present study was premised on the knowledge that since honey is not a standardized substance and its composition varies, one might expect to observe differences in the result of drug interaction studies conducted with honey from different climatic region. This study had some limitations in that we did not analyse the flavonoids composition of the honey used in this present study. Previous honey drug interaction studies also had this limitation except the recent study by Fetzner et al21.
In conclusion, the results of this study indicate that multiple administration of honey in the amount used in this study showed a dose dependent biphasic effects on quinine metabolism though honey did not significantly alter quinine hepatic CYP3A biotransformation of quinine to its major metabolite, 3-hydroxyquinine. This indicates that ingestion of honey with quinine, may not elicit any serious drug-nutrient metabolic interaction.
Acknowledgments
We gratefully acknowledge the contribution of: Prof Chinedum. O. Babalola for the kind donation of primaquine; Dr Olukemi Taiwo and Mr Dimeji Salau for helping with sample collection and Mr Ayorinde Adehin for assisting with statistical analysis of data.
Source of funding
The work was partly supported financially by “Carnegie Corporation of New York Sponsored Fellowships” for Obafemi Awolowo University Female Staff under the auspices of Centre for Gender and Social Policy Studies, Obafemi Awolowo University, Osun State, Nigeria.
Footnotes
No conflicts of interest are declared by the authors
References
- 1.Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of Herbs with Cytochrome P450. Drug Metab Rev. 2003;35:35–98. doi: 10.1081/dmr-120018248. [DOI] [PubMed] [Google Scholar]
- 2.Morris ME, Zhang S. Flavonoid–drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006;78:2116–2130. doi: 10.1016/j.lfs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Harris RZ, Jang GR, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42:1071–1088. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrova B, Gevrenova R, Anklam E. Analysis of phenolic acids in honeys of different floral origin by solid-phase extraction and high-performance liquid chromatography. Phytochem Anal. 2007;18:24–32. doi: 10.1002/pca.948. [DOI] [PubMed] [Google Scholar]
- 5.Mansell RL, McIntosh CA, Vest SE. An analysis of the limonin and naringin content of grapefruit juice samples collected from Florida state test houses. J Agric Food Chem. 1983;31:156–162. [Google Scholar]
- 6.Gheldof N, Engeseth NJ. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J Agric Food Chem. 2002;50:3050–3055. doi: 10.1021/jf0114637. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutri Res. 2002;22:104–1047. [Google Scholar]
- 8.Chen L, Mechta A, Berebaum M, Zangerl AR, Egeseth NJ. Honeys from different floral sources as inhibitors of enzymatic browning in fruit and vegetable homogenates. J Agric Food Chem. 2000;48:4997–5000. doi: 10.1021/jf000373j. [DOI] [PubMed] [Google Scholar]
- 9.Frankel S, Robinson GE, Berenbaum MR. Antioxidant capacity and correlated characteristics of 14 unifloral honeys. J Apicul Res. 1998;37:27–31. [Google Scholar]
- 10.Yao L, Datta N, Tomas-Barberan FA, Ferreres F, Martos I, Singanusong R. Flavonoids, phenolic acids and abscissic acidin Australian and New Zealand Leptospermum honeys. Food Chem. 2003;81:159–168. [Google Scholar]
- 11.Martos I, Ferreres F, Tomas-Barberan FA. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J Agric Food Chem. 2000;48:1498–1502. doi: 10.1021/jf991166q. [DOI] [PubMed] [Google Scholar]
- 12.Raucy JL. Regulation of CYP3A expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab Dispos. 2003;31:533–539. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 13.Pal D, Mitra AK. MDR- and CYP3A-mediated drug-herbal interactions. Life Sci. 2006;78:2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Liu DY, Yang M, Zhu HJ, Zheng YF, Zhu XQ. Human pregnane X receptor-mediated transcriptional regulation of cytochrome P450 3A4 by some phytochemicals. Med Sci. 2006;35:8–13. doi: 10.3785/j.issn.1008-9292.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Pankaj B, Desai PB, Nallani SC, Sane RS, Moore LB, Goodwin BJ, Buckley DJ, Buckley AR. Induction of cytochrome p450 3A4 in primary human hepatocytes and activation of the human pregnane x receptor by tamoxifen and 4-hydroxytamoxifen. Drug Metab Dispos. 2002;30:608–612. doi: 10.1124/dmd.30.5.608. [DOI] [PubMed] [Google Scholar]
- 16.Fujita K. Food-drug interactions via human cytochrome P450 3A (CYP3A) Drug Metab Drug Interact. 2004;20:195–217. doi: 10.1515/dmdi.2004.20.4.195. [DOI] [PubMed] [Google Scholar]
- 17.Koumaravelou K, Adithan C, Shashindran CH, Asad M, Abraham BK. Effect of honey on carbamazepine kinetics in rabbits. Indian J Exp Biol. 2002a;40:560–563. [PubMed] [Google Scholar]
- 18.Koumaravelou K, Adithan C, Shashindran CH, Asad M, Abraham BK. Influence of honey on orally and intravenously administered diltiazem kinetics in rabbits. Indian J Exp Biol. 2002b;40:1164–1168. [PubMed] [Google Scholar]
- 19.Sukriti J, Garg SK. Influence of honey on the pharmacokinetics of phenytoin in healthy rabbits. Methods Find Exp Clin Pharmacol. 2003;25:367–370. doi: 10.1358/mf.2003.25.5.769658. [DOI] [PubMed] [Google Scholar]
- 20.Tushar T, Vinod T, Rajan S, Shashindran C, Adithan C. Effect of honey on CYP3A, CYP2D6 and CYP2C19 enzyme activity in healthy human volunteers. Basic Clin Pharmacol Toxicol. 2007;100:269–272. doi: 10.1111/j.1742-7843.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- 21.Fetzner L, Burhenne J, Weiss J, Völker M, Unger M, Mikus G, Haefeli WE. Daily Honey Consumption Does Not Change CYP3A Activity in Humans. J Clin Pharmacol. 2011;51:1223–1232. doi: 10.1177/0091270010382022. [DOI] [PubMed] [Google Scholar]
- 22.Mirghani RA, Ericsson O, Tybring G, Gustafsson LL, Bertilsson L. Quinine 3-hydroxylation as a biomarker reaction for the activity of CYP3A in man. Eur J Clin Pharmacol. 2003;59:23–28. doi: 10.1007/s00228-003-0575-5. [DOI] [PubMed] [Google Scholar]
- 23.Wanwimolruk S, Paine MF, Pusek SN, Watkins PB. Is quinine a suitable probe to assess the hepatic drug metabolizing enzyme CYP3A. Br J Clin Pharmacol. 2002;54:643–651. doi: 10.1046/j.1365-2125.2002.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirghani RA, Hellgren U, Westerberg PA, Ericsson O, Bertilsson L, Gustafsson LL. The roles of cytochrome P450 3A4 and 1A2 in the 3-hydroxylation of quinine in vivo. Clin Pharmacol Ther. 1999;66:454–460. doi: 10.1016/S0009-9236(99)70008-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhao XJ, Yokoyama H, Chiba K, Wanwimolruk S, Ishizaki T. Identification of human cytochrome P450 isoforms involved in the 3-hydroxylation of quinine by human live microsomes and nine recombinant human cytochrome P450. J Pharmacol Exp Ther. 1996;279:1327–1334. [PubMed] [Google Scholar]
- 26.Zhang H, Coville PF, Walker RJ, Miners JO, Birkett DJ, Wanwimolruk S. Evidence for involvement of human CYP3A in the 3-hydroxylation of quinine. Br J Clin Pharmacol. 1997;43:245–252. doi: 10.1046/j.1365-2125.1997.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen M, Andersson K, Dalén P, Mirghani RA, Muirhead GJ, Nordmark A, Tybring G, Wahlberg A, Yaşar U, Bertilsson L. Pharmacokinetics and drug disposition the karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther. 2003;73:517–528. doi: 10.1016/S0009-9236(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 28.Ho PC, Chalcroft SC, Coville PF, Wanwimolruk S. Grapefruit juice has no effect on quinine pharmacokinetics. Eur J Clin Pharmacol. 1999;55:393–398. doi: 10.1007/s002280050646. [DOI] [PubMed] [Google Scholar]
- 29.Björkhem-Bergman L, Bäckström T, Nylén H, Rönquist-Nii Y, Bredberg E, Andersson TB, Bertilsson L, Diczfalusy U. Quinine compared to 4β-hydroxycholesterol and midazolam as markers for CYP3A induction by rifampicin. Drug Metab Pharmacokinet. 2014;29(4):352–355. doi: 10.2133/dmpk.dmpk-13-sh-138. [DOI] [PubMed] [Google Scholar]
- 30.Zhu B, Ou-Yang DS, Cheng ZN, Huang SL, Zhou HH. Single plasma sampling to predict oral clearance of CYP3A probe midazolam. Acta Pharmacol Sin. 2001;22:634–638. [PubMed] [Google Scholar]
- 31.Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, Alessandro U. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. on-line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babalola CP, Bolaji OO, Dixon PAF, Ogunbonna FA. Column liquid chromatographic analysis of quinine in human plasma, saliva and urine. J chromatogr. 1993;616:151–154. doi: 10.1016/0378-4347(93)80482-j. [DOI] [PubMed] [Google Scholar]
- 33.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, Kao J, King SP, Miwa G, Ni L, Kumar G, McLeod J, Obach SR, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug-drug interaction studies: a PhRMA perspective. J Clin Pharmacol. 2003;43:443–469. [PubMed] [Google Scholar]
- 34.Kang IH, Kim HJ, Oh H, Park YI, Dong MS. Biphasic effects of the flavonoids quercetin and naringenin on the metabolic activation of 2-amino-3,5 dimethylimidazo[4,5-f]quinoline by Salmonella typhimurium TA1538 co-expressing human cytochrome P450 1A2, NADPH-cytochrome P450 reductase, and cytochrome b5. Mut Res. 2004;545:37–47. doi: 10.1016/j.mrfmmm.2003.08.002. [DOI] [PubMed] [Google Scholar]