Abstract
Autophagy is an evolutionarily ancient process of intracellular catabolism necessary to preserve cellular homeostasis in response to a wide variety of stresses. In the case of post-mitotic cells, where cell replacement is not an option, finely tuned quality control of cytoplasmic constituents and organelles is especially critical. And due to the ubiquitous and critical role of autophagic flux in the maintenance of cell health, it comes as little surprise that perturbation of the autophagic process is observed in multiple disease processes. A large body of preclinical evidence suggests that autophagy is a double-edged sword in cardiovascular disease, acting in either beneficial or maladaptive ways, depending on the context. In light of this, the autophagic machinery in cardiomyocytes and other cardiovascular cell types has been proposed as a potential therapeutic target. Here, we summarize current knowledge regarding the dual functions of autophagy in cardiovascular disease. We go on to analyze recent evidence suggesting that titration of autophagic flux holds potential as a novel treatment strategy.
Keywords: cardiac hypertrophy, heart failure, remodeling
Introduction
Despite robust successes in recent decades to tame the acutely lethal manifestations of heart disease, it continues to grow as the number one cause of death worldwide. Identification of novel mechanisms of disease pathogenesis, exploitation of novel drug targets, advances in clinical care with new drugs, devices, and systems, together have culminated in a dramatic 75% decrease in age-adjusted mortality in the past 50 years [1]. Notably, these advances have not touched all patient groups [2]. Further, the scourge of heart disease continues to expand as it evolves in new directions. To cite one area of challenge, the worldwide pandemic of obesity, and consequent metabolic syndrome and diabetes, has emerged as one of the most vexing problems in cardiovascular medicine going forward.
One of the prominent features of the cardiovascular system is its ability to adapt to a wide range of environmental stresses. The myocardium itself manifests robust plasticity in the setting of both physiological and pathological stimuli [3]. A few of the intracellular signaling pathways active within the cardiomyocyte have been exploited already to accomplish therapeutic gains. Adrenergic signaling and the renin-angiotensin-aldosterone (RAAS) system are two prominent examples. Others are currently being explored, including the handling of intracellular Ca2+, nitric oxide- and protein kinase G (PKG)-dependent events, ion channels, and epigenetic control of gene expression. Further, adaptations within the cardiomyocyte typically involve multiple responses that encompass virtually all intracellular organelles [4].
Post-mitotic cells, such as cardiomyocytes and neurons, rely critically on the housekeeping mechanisms of proteostasis – regulation of protein synthesis, processing, and elimination – owing to the limited ability of these cells to divide. These cells must survive for many years, and they cannot simply be discarded when they become ill or dysfunctional. Among the critical mechanisms of cardiomyocyte proteostasis are recycling events governed by lysosomes. For several decades now, the role of these events in heart disease has been recognized and acknowledged [5]. Initial studies centered on lysosomal processing, but more recent efforts have focused on pathways that identify and target proteins and dysfunctional organelles, sequester them, and then deliver them as cargo to the lysosome. This process, termed autophagy, is ubiquitous and highly dynamic. Presently, our understanding of molecular events governing autophagic flux has emerged to the point that one could envision it as a relevant therapeutic target. Indeed, we and others, have suggested that the ubiquitous intracellular process of autophagy could be manipulated – titrated up or down – for therapeutic gain [6–8].
Autophagy basics
Autophagy is a highly conserved process of protein and organelle catabolism and recycling. Proteins and mitochondria targeted for elimination are sequestered and delivered to lysosomes for degradation, and the resulting molecular components – amino acids, lipids, nucleic acids, carbohydrates – are released into the cell to support metabolic demand. Under basal conditions, when nutrient supply is ample, autophagy is maintained at low levels. Critically, those low but finite levels of autophagic flux are required for cell survival; if autophagic activity is suppressed to zero, cellular demise ensues rapidly [9, 10].
Autophagic flux is rapidly activated by stress impinging on the cell. The exemplar of autophagy-activating cellular stress is that of nutrient deprivation. Starvation or growth factor deprivation triggers a robust activation of autophagic recycling of intracellular contents to sustain metabolic demand and support macromolecule biosynthesis.
With respect to the cardiomyocyte, many other forms of stress trigger changes in autophagic flux, including a number of cues relevant to heart disease. Pressure stress, such as that occurring in hypertension or aortic stenosis, triggers significant increases in cardiomyocyte autophagy, attaining a new, steady-state level of flux after the cell has responded with hypertrophic growth [11]. Ischemia triggers a transient increase in autophagic flux, as the cell responds to the “starved” state of ischemia. Some evidence suggests, however, that with time that increase in autophagic flux declines, falling below the normal steady state level of flux [12]. One possible explanation, which remains to be tested, is that autophagic flux, even when up-regulated by stress, is insufficient to sustain the ATP requirements of the continuously contracting myocyte all while providing ATP sufficient to support autophagic flux. As such, ATP levels decline and cardioprotective autophagic flux declines.
Three types of autophagy have been recognized in mammalian cells: chaperone-mediated autophagy, microautophagy and macroautophagy [13]. Chaperone-mediated autophagy is a selective degradation of cytosolic proteins harboring the amino acid motif KFERQ which is recognized by chaperones to facilitate protein transport into the lysosome through the lysosomal membrane protein LAMP2A. In microautophagy, substrate uptake occurs directly by means of invagination of the lysosomal membrane [14]. The specific roles in cardiovascular disease of these two forms of autophagy, chaperone-mediated autophagy and microautophagy, have not been elucidated to date, despite recent studies that reported a critical role for these types of autophagy in regulating the activity of certain metabolic enzymes [15–17].
Macroautophagy, by contrast, is the most prevalent and most extensively characterized form of autophagy. Macroautophagy (hereafter termed autophagy) involves a series of defined steps governed by multiple proteins encoded by autophagy-related genes (ATGs). Together, these proteins orchestrate the formation of a double-membrane vesicle, called an autophagosome, which fuses with a lysosome to deliver cytoplasmic cargo for acid hydrolase-dependent degradation. Finally, those degraded molecular elements are released into the cell as building blocks to support energy homeostasis and macromolecule biosynthesis.
Overview of the autophagic cascade
Our understanding of cellular and molecular mechanisms governing autophagy in mammals has expanded greatly over the past several years and will be presented here only in brief overview. Readers are referred to recent comprehensive reviews [18–20]. Autophagosome nucleation commences with the activation of several ATG proteins; class III phosphoinositide 3-kinase (PI3K) family members (vacuolar protein sorting 34 – Vps34) and Beclin 1 recruit membranes from intracellular sources (e.g. endoplasmic reticulum, mitochondria, Golgi apparatus, plasma membrane) or by de novo synthesis [21]. ATG8 and microtubule-associated protein 1 light-chain 3 (LC3; also known as MAP1LC3) protein play a crucial role during the ensuing autophagosome elongation step. In addition, they recruit adaptor proteins, such as ubiquitin-binding protein p62 (also called sequestosome 1) that facilitates cargo recognition and loading into the autophagosome. The last step, fusion of the autophagosome filled with cytoplasmic material with a lysosome, is regulated by soluble NSF attachment protein receptor (SNARE) proteins. The resulting autolysosome is the site where cytoplasmic material is degraded by lysosomal hydrolases [22].
A complex upstream signaling network regulates the activity of the autophagic process. Growth factors, nutrients, cellular energy status, and pathologic stresses impact autophagy and are integrated by the kinase activity of mechanistic target of rapamycin (mTOR). mTOR complex 1 (mTORC1) inhibition by nutrient deprivation or by pharmacological suppression (rapamycin, Torin 1) results in the induction of autophagy [13], activating the upstream signaling molecules involved in autophagosome formation (PI3K-III, Vps34, Beclin 1, ATG6 and ATG 14). The master role of mTOR in regulating autophagy has been confirmed in the cardiovascular system by genetic silencing of mTOR in cardiomyocytes [23, 24]. At a transcriptional level, autophagy can be positively regulated by the forkhead box O (FoxO) family of transcription factors [25], which are known to play an important role in various forms of cardiovascular disease [26].
Autophagy in cardiovascular biology: friend or foe?
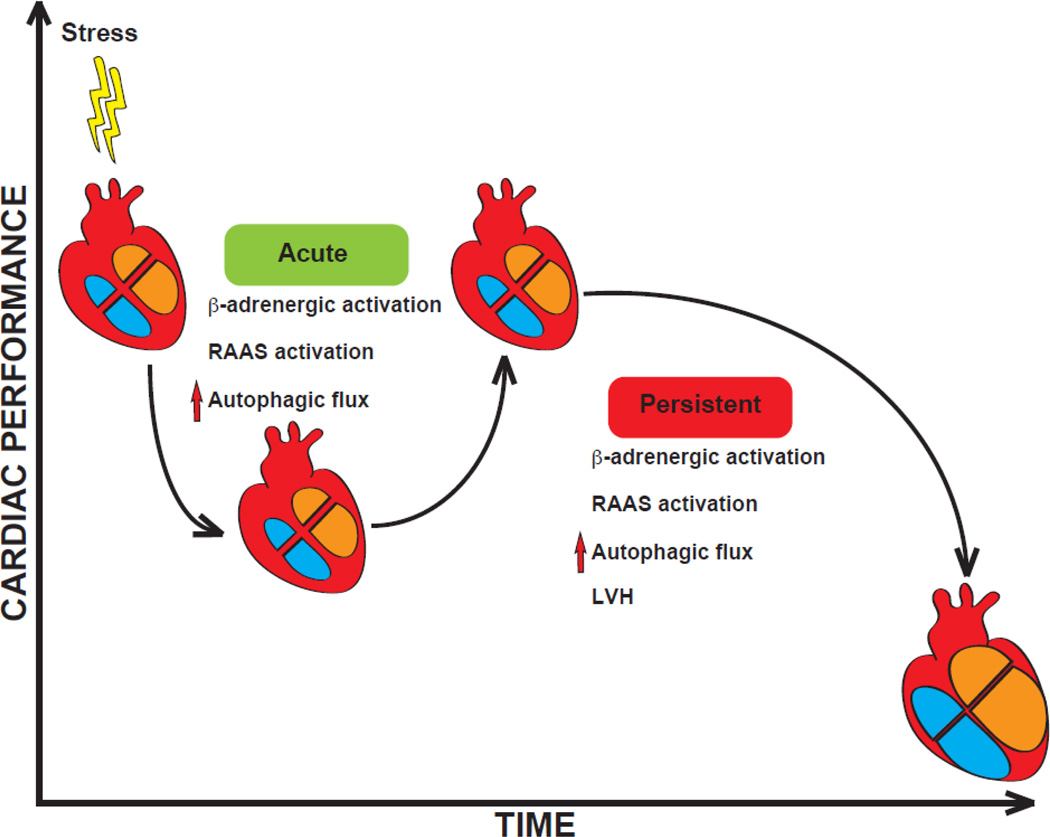
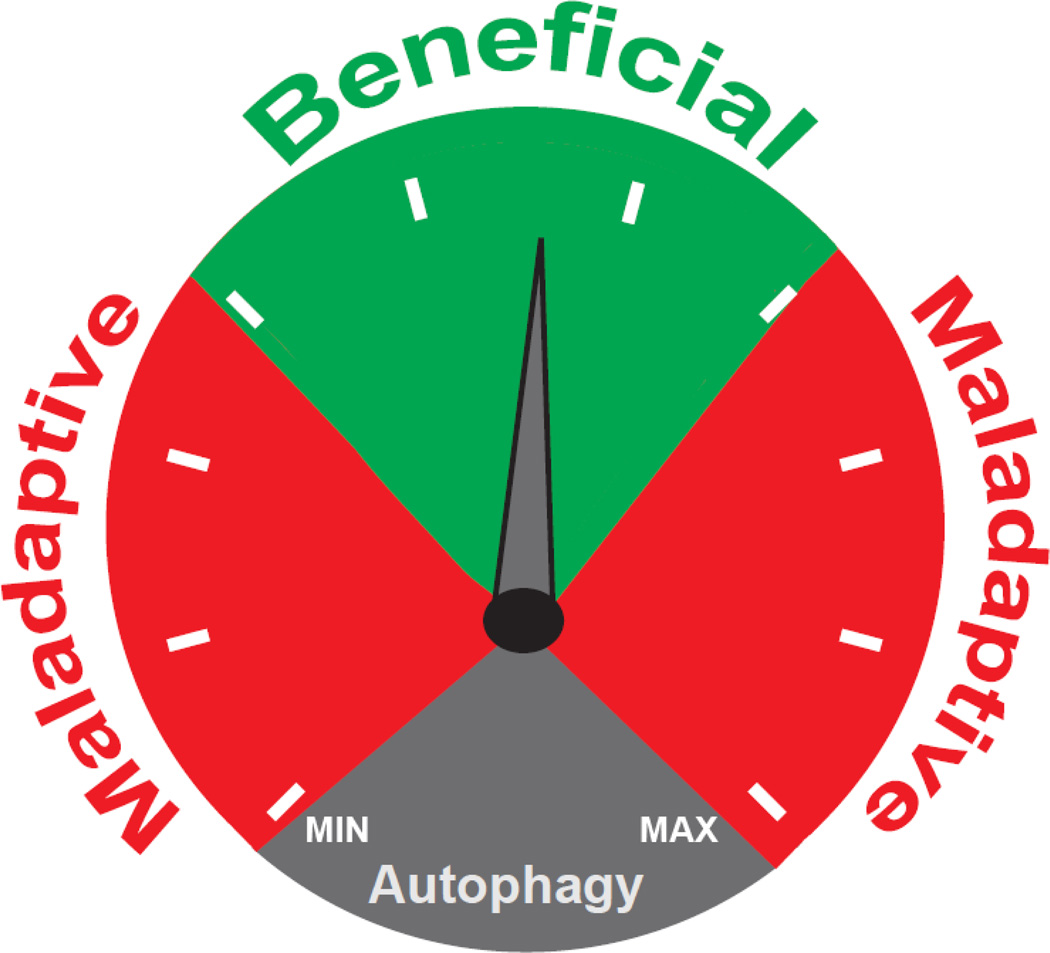
Existence of autophagic activity in the cardiovascular system has been recognized for some years [27–30]. More recently, insights have emerged regarding the role of this process in normal cellular homeostasis and disease. In some instances, disease-related activation of cardiomyocyte autophagy may reflect an evolutionarily conserved response to stress, analogous to activation of the adrenergic and RAAS systems or hypertrophic growth of the left ventricle [31] (Figure 1). According to this view, activation of autophagic flux in the heart may be beneficial or maladaptive, depending on the context [7]. Therefore, the spectrum of autophagic flux in cardiomyocytes ranges from low level activation required for cellular homeostasis to high level activation which is detrimental and disease promoting (Figure 2).
Figure 1. Autophagy as a homeostatic response within the cardiovascular system.
In response to disease-related cardiovascular stress, a number of systemic responses are triggered which support cardiovascular performance in the short term. Those same responses, when allowed to persist, are maladaptive. Indeed, antagonism of the β-adrenergic and renin-angiotensin-aldosterone (RAAS) pathways is a cornerstone of HF therapy. Emerging evidence suggests that both autophagy and LVH act similarly in their short-term and long-term actions. We speculate that each may emerge with time as a target of therapy. LVH: left ventricular hypertrophy.
Figure 2. The double-edged sword of autophagic flux.
Autophagic activity is beneficial when maintained within a narrow range. When flux is activated to high levels, or suppressed to low levels, the process is maladaptive.
It is now clear that perturbation of autophagic activity contributes to cardiovascular disease [7]. Depending on the timing and magnitude of the activation, and the cell type(s) involved, the autophagic process exhibits a dual role in several cardiovascular diseases. For example, it has been reported that reduced basal autophagy promotes heart failure (HF) [32, 33]. Whereas some studies have reported that enhanced autophagy in the heart can be cardioprotective, over-activation of the autophagic process is detrimental, leading to excessive degradation of intracellular components and in turn cardiomyocyte death [34]. In the end, the exact role of autophagic flux in heart disease remains a matter of debate.
Interplay between autophagy and apoptosis
Autophagy and apoptosis are both essential for cellular homeostasis, and perturbations of each cellular cascade have been implicated in cardiovascular disease. However, mechanisms governing the crosstalk between these two fundamental pathways remain unclear. In some contexts, autophagy and apoptosis occur independently [35]. In other scenarios, however, activation of autophagy can either inhibit or promote apoptosis [36]. Tight interplay between autophagy and apoptosis is also highlighted by the fact that fundamental regulators of apoptosis, such as components of the Bcl-2 family [37] also regulate autophagy; conversely, certain autophagic proteins (Atg5, Beclin 1) play a role in apoptosis.
Mitochondria are recognized as central mediators of programmed apoptotic cell death [38] and their elimination by mitochondria-specific autophagy (mitophagy) represents a link between these processes. During ischemia, reduced ATP production by mitochondria activates autophagy, serving as a cytoprotective mechanism; however, persistence of damaged mitochondria triggers their removal by mitophagy, thereby preventing apoptotic cell death.
At the molecular level, the dual role of autophagy can be explained in part by the degradation of negative and positive effectors of apoptosis, triggering, respectively, cell death or cell survival. To date, Atg5 and members of the Beclin 1 family have been recognized as molecular switches between autophagic and apoptotic pathways [39]. The roles of these two components of the autophagic machinery have been explored in several models of cardiovascular disease. For example, in cardiomyocyte-specific Atg5-deficient mice, insufficient autophagy promotes apoptosis and cell death [32], whereas apoptosis was prevented in Beclin 1 heterozygous mice [40].
Controversies persist regarding the reciprocal roles of autophagy in cellular homeostasis and with respect to crosstalk with apoptosis. Nevertheless, context-dependent titration of autophagic flux – sometimes up, sometimes down – holds promise as a novel therapeutic approach.
Autophagy in ischemic heart disease
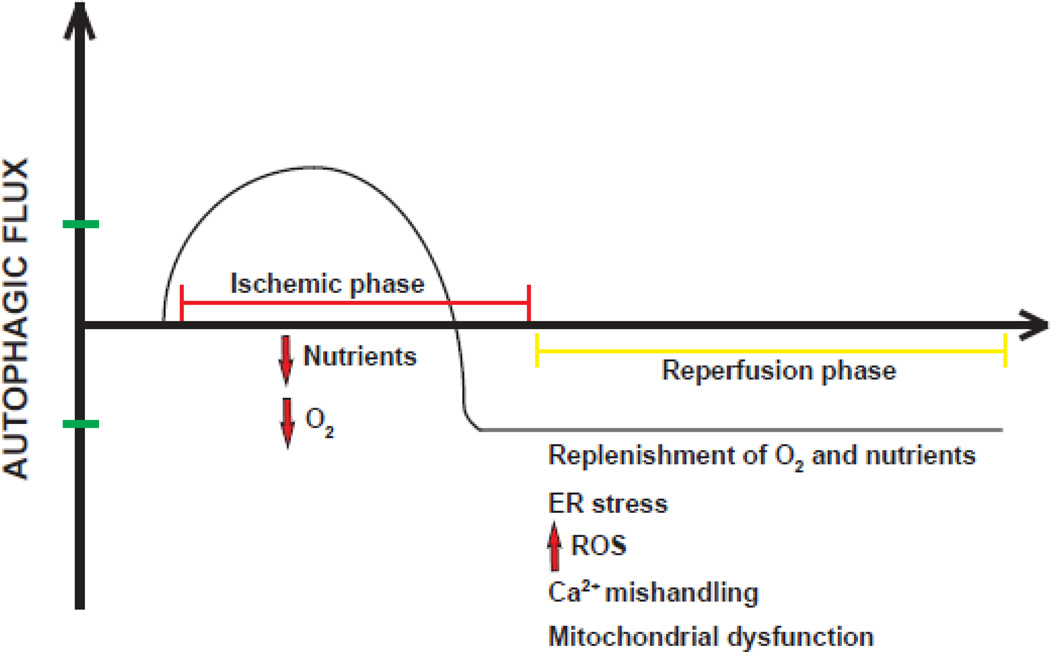
Ischemic heart disease is recognized as the most common cause of death globally [41]. The autophagic process in cardiomyocytes contributes to both major phases of myocardial injury, ischemia and reperfusion [40, 42, 43]. Although both events trigger cardiomyocyte stress, they are distinct in many ways, including abundance of nutrient supply, levels of reactive oxygen species (ROS) accumulation, and availability of oxygen (Figure 3). During ischemia, depleted energy supplies trigger cardiomyocyte autophagy to replenish metabolic substrates and to remove damaged organelles [44]. Nutrient depletion activates AMP-activated protein kinase (AMPK) which, in turn, inhibits mTOR, removing a major brake on the process [40]. In this context, autophagy is considered an adaptive response with cardioprotective effects. Consistent with this notion, inhibition of AMPK activity or other pharmacological strategies that inhibit autophagy result in increased cardiomyocyte death [45].
Figure 3. Autophagic flux kinetics during ischemia/reperfusion injury.
During ischemia, when a cell is “starved”, autophagy is activated. With time, however, autophagic flux during ischemia declines below baseline, possibly owing to inadequate ATP availability. During reperfusion, replenishment of O2 and nutrients is coupled with ER (endoplasmic reticulum) stress, accumulation of reactive oxygen species (ROS), Ca2+ mishandling and mitochondrial dysfunction. Autophagic flux remains low.
Then, during tissue reperfusion, when oxygen and nutrients are restored, the scenario is dominated by accumulation of ROS. Here, autophagy can be up-regulated but the ultimate effects of altered autophagic flux have been reported to be either beneficial or detrimental [46, 47]. One could speculate that contrasting evidence regarding the function of autophagy during ischemia/reperfusion (I/R) injury could be attributed to the dual actions of Beclin 1 during I/R injury. Activation of Beclin 1 is required for initiation of autophagy in the early phase of ischemia; however, its persistent activation during reperfusion may result in excessive catabolic activation and cell death [48]. Recently, our group confirmed the cardioprotective effects of autophagy induction in ischemic heart disease in both small and large animal models of I/R injury [49].
Autophagy in cardiac hypertrophy and failure
Hypertrophic growth of the myocardium results from increases in afterload (e.g. hypertension, aortic stenosis), as well as from several other disease-related stresses. Whereas some evidence suggests that cardiac hypertrophy is beneficial in the short-term, with time it leads inevitably to HF [31]. In recent years, studies have focused on the role of cardiomyocyte autophagy in the natural history of this progression.
In a mouse model of pressure overload induced by transverse aortic constriction (TAC) surgery, the increase in autophagic flux correlates with the degree of left ventricular hypertrophy; the flux response increases initially and then ultimately reverts to a new, higher steady-state level [34]. Reduced abundance of the autophagy repressing microRNA-30a accelerates progression of cardiac hypertrophy after TAC [50], and pharmacological inhibition of autophagy by 3-methyladenine (3-MA) reduces fibrosis in the same model [51]. Genetic models in which autophagic flux is increased in cardiomyocytes by over-expression of Beclin 1 or reduced by haploinsufficiency of the gene coding for this protein, result in amplified or attenuated pressure overload-induced HF, respectively. However, other evidence suggests that low-level autophagic flux is necessary for an adaptive response during cardiac hypertrophy [32]. More recently, findings have emerged pointing to the importance of preserved levels of protein acetylation in cells to inhibit the detrimental autophagy induced by TAC [52]. Together, these observations are consistent with a model in which autophagy is a maladaptive response to disease-related pressure overload [34, 53].
Overall, the blurred boundary separating adaptive and maladaptive autophagic responses in cardiac hypertrophy and HF derive, at least in part, from the bimodal nature of the autophagic process itself: low levels of intracellular catabolism are required for proteostasis, whereas increases in autophagic flux could ultimately confer harm to the cell. Accordingly, therapeutic interventions aimed at modulating autophagy in HF must take these realities into account in an effort to fine tune the autophagic flux response for benefit.
Autophagy in cardiac lysosomal storage disease
Defects in lysosome function cause a wide spectrum of metabolic disorders characterized by accumulation in several organs of undegraded lipids, glycoproteins and mucopolysaccharides [54]. Myocardial involvement is frequent in these conditions. Diminished catabolism due to dysfunctional or absent lysosomal enzymes results in accumulation of vacuoles within cardiomyocytes and, in turn, contractile dysfunction. In Pompe disease, a lysosomal storage disease characterized by massive accumulation of glycogen, suppression of autophagosome formation by genetic deficiency of ATG7 results in amelioration of the phenotype and facilitates enzyme replacement therapy [55]. However, more recently it was reported that fibroblasts from Pompe patients reprogrammed to a cardiomyocyte lineage exhibit normal contractile properties and preserved autophagic flux [56].
Impaired autophagy has been recognized in another class of lysosomal storage disease, mucopolysaccharidoses (MPS) [57, 58]. Perturbations in cardiac autophagy have been reported in a mouse model of MPS IIIB (Sanfilippo type B syndrome) that develops HF over time [59]. Defects in autophagosome-lysosome fusion have also been described in Danon syndrome [60], where mutations in the gene coding for LAMP2 disrupt intracellular catabolism and lead to accumulation of autophagic material in cardiomyocytes and skeletal muscle cells [61].
Autophagy in chemotherapy-induced cardiotoxicity
Cardiac dysfunction is a common side effect of antineoplastic drug therapy [62]. Clinical use of doxorubicin, an efficacious and commonly used chemotherapeutic agent, is limited due to dose-dependent cardiotoxicity [63, 64]. Numerous studies have probed underlying mechanisms [65], and a number of molecular elements have been implicated in the pathogenesis of doxorubicin cardiotoxicity, including DNA damage [66], mitochondrial damage [67], mitochondrial iron accumulation [68], and accumulation of ROS [69]. The possible role of autophagy in doxorubicin cardiomyopathy has been posited in recent years but has remained a question of debate. Anthracyclines are associated with increases in cardiomyocyte autophagy and down-regulation the transcription factor GATA4 [70] which regulates several autophagic genes. Enhanced autophagy was also reported in an in vivo model of anti-cancer-induced therapy cardiac toxicity [71]. We recently uncovered evidence that doxorubicin compromises lysosomal acidification and function, thereby inhibiting cardiomyocyte autophagic flux (manuscript in review).
Autophagy in cardiac arrhythmia
Cardiac arrhythmia, defined as a perturbation of normal sinus rhythm such that the heart beats abnormally fast, slow, or irregularly, is a large and important clinical problem in cardiovascular medicine. Involvement of autophagy in arrhythmia development and maintenance is not well established. Some evidence points to impairment in the autophagic process in atrial samples harvested from patients with post-operative atrial fibrillation [72]. However, given that ventricular myocytes manifest changes in autophagic flux in the setting of essentially all environmental perturbations, it is reasonable to speculate that the same may be true of atrial myocytes. In light of the fact that aging is a major risk factor for atrial fibrillation, one might speculate that age-related declines in autophagy may predispose atrial cells to fibrillation.
Autophagy and cardiovascular risk factors
A significant component of many cardiovascular diseases derives from modifiable risk factors, such as hypertension, obesity, diabetes and hyperlipidemia. In many of these disease triggers, cardiomyocyte autophagy seems to play a role. In diabetic cardiomyopathy, autophagic flux in cardiomyocytes is inhibited [73–75], and genetic manipulation of key autophagic elements has supported the notion of autophagy as an adaptive response in diabetes that serves to reduce cardiac dysfunction [73, 76–78]. A protective role of autophagy in cardiac dysfunction observed in metabolic disorders is also suggested by alteration in autophagosome maturation in high-fat diet animal models [79]. However, the specific role of autophagy in obesity and diabetes is cell-type specific and as yet poorly defined. For example, pancreatic β cells from patients with type 2 diabetes exhibit high levels of autophagy and increased cell death [80], suggesting that autophagic flux in this context is detrimental and serving to promote disease progression.
Evidence for perturbation of autophagy in human cardiovascular disease
Reports implicating changes in autophagic activity in human disease are limited. Whereas a large literature has emerged implicating perturbations in autophagy in preclinical models of heart disease, direct evidence of alterations of the autophagic process in human samples derive exclusively from myocardial samples obtained from patients with heart failure with reduced ejection fraction (HFrEF) [81]. One major reason underlying the absence of data in humans is the lack of noninvasive markers, either circulating or imaging, to track changes in cellular autophagy. Rather, sampling of diseased tissue is required, which is not routinely performed in heart disease.
Myocardial samples from patients with end-stage dilated cardiomyopathy exhibit ultrastructural changes which include accumulation of autophagic vacuoles and damaged organelles, each associated with morphological evidence of cardiomyocyte death. Interestingly, samples obtained from dilated hearts mechanically unloaded with a left ventricular assist device (LVAD) manifest reductions in autophagic markers [82], suggesting that mechanical unloading may restore autophagic flux in cardiomyocytes.
Whereas data in humans are limited, it is likely that the autophagic process is tightly regulated in human tissue, as well. As such, a fuller understating of details of autophagic regulatory control in human cardiovascular disease will be crucial to envision autophagy as a therapeutic target. Altogether, these data highlight the potential role of autophagic cell death as an important contributor to the progression of cardiomyocyte failure and suggest again that autophagy may be an adaptive process in the failing heart restorable by normalization of loading conditions.
Rationale for autophagy as a therapeutic target in cardiovascular disease
Studies discussed here point to dysregulation of the cardiomyocyte autophagic process in many cardiovascular diseases. This raises the tantalizing prospect of targeting this process for therapeutic gain. Due to the highly conserved nature of autophagic mechanisms across tissues and cell types, pharmacological approaches to manipulate autophagy are receiving growing attention in other human diseases, such as cancer, neurodegenerative disorders, and metabolic disease [21].
The dual role, adaptive versus maladaptive, of autophagy poses a special challenge in this regard. Indeed, the fact that highly dynamic catabolic processes across cell types participate in the cellular response to a wide range of stresses is another challenge. And as pointed out earlier, complete abolition of autophagic activity is poorly tolerated. Increased autophagic flux after pressure overload is beneficial in the initial phases of the process but can become detrimental when over-activated. Clearly, a nuanced approach is warranted.
The design and validation of effective drugs targeting autophagy in cardiovascular disease will require a comprehensive and sophisticated understanding of autophagy’s role across cells and during the course of disease natural history. To date, drugs that modulate autophagy in a cardiovascular setting were not designed specifically for this purpose and yet raise the alluring prospect that effects on autophagic activity contribute to their actions. For example, a recent study from our group has shown that beneficial effects of histone deacetylase inhibition in I/R injury are dependent on activation of cardiomyocyte autophagy [49]. Whereas these compounds were not designed specifically to target autophagy, some of their effects are autophagy dependent, highlighting again the importance of this pathway in cardiovascular disease.
Therapeutic modulation of autophagic flux
Several drugs have been reported to modulate autophagy by acting on various molecular elements within the flux cascade. As noted earlier, a cornerstone of autophagy control is the activity of the mTOR complex. Rapamycin inhibits mTORC1 and results in an increase in autophagic flux. Moreover, other mTOR-targeting molecules, such as Torin 1, which inhibits both mTORC1 and mTORC2, induce autophagy [83]. To date, the most important clinical cardiovascular applications of rapamycin and its analogues center on their effects as immunomodulators for preventing post-transplant rejection and in suppressing smooth muscle cell migration and proliferation, as capitalized upon with drug-eluting devices for coronary and peripheral angioplasty [84, 85]. Interestingly, a widely used class III antiarrhythmic drug, amiodarone, has been reported to inhibit mTORC1 [86].
Our group has sought to identify other means of titrating autophagic flux still centered on mTOR as a therapeutic target. To that end, reversible protein acetylation, controlled by enzymes which attach (histone acetyltransferases) or remove (histone deacetylases, HDACs) acetyl groups at lysine residues, is a major mechanism governing a wide range of cellular processes, both transcriptional and post-transcriptional [87]. In the context of cardiac hypertrophy, small molecule HDAC inhibitors attenuate pathological cardiac remodeling, including hypertrophic growth, fibrosis, and declines in ventricular function [88–90]. And as pharmacological suppression of HDACs has emerged in oncology, the prospect of clinical translation is promising. We have found that HDAC inhibitors suppress the activity of mTOR by up-regulating TSC2, a negative regulator of mTOR (unpublished observations).
Drugs with activity in mTOR-independent pathways that alter autophagic flux include molecules acting on phosphoinositol- and calcium-related pathways. Intracellular levels of inositol are inversely related to autophagic activity [20]; drugs such as lithium and carbamazepine induce autophagy by reducing cytoplasmic inositol levels [91, 92]. Notably inositol content is also regulated by intracellular Ca2+ and cyclic adenosine monophosphate (cAMP); therefore some antihypertensive drugs, such as clonidine and Ca2+ channel blockers, can induce autophagy by decreasing cAMP content. There are several other compounds or drugs capable of modulating autophagy but whose mechanisms of action remain unknown (Table).
Table.
Autophagy-inducing drugs in cardiovascular disease.
| Drug | Therapeutic application | Refs. (PMID) |
|---|---|---|
| Rapamycin and analogs | Preventing cardiac transplantation rejection and smooth muscle cell proliferation and migration (coated vascular stents and balloons) | 25812489, 25240980 |
| Amiodarone | Class III antiarrhythmic | 19771169 |
| Metformin | Antidiabetic agent | 16990266 |
| Verapamil, nicardipine, nimodipine (L-type Ca2+ channel blockers) | Antihypertensive | 18024584 |
| Clonidine | Antihypertensive | 18391949 |
| Minoxidil | Vasodilator | 18391949 |
| Statins | Cholesterol-lowering | 23901824 |
Translating preclinical insights into the clinic
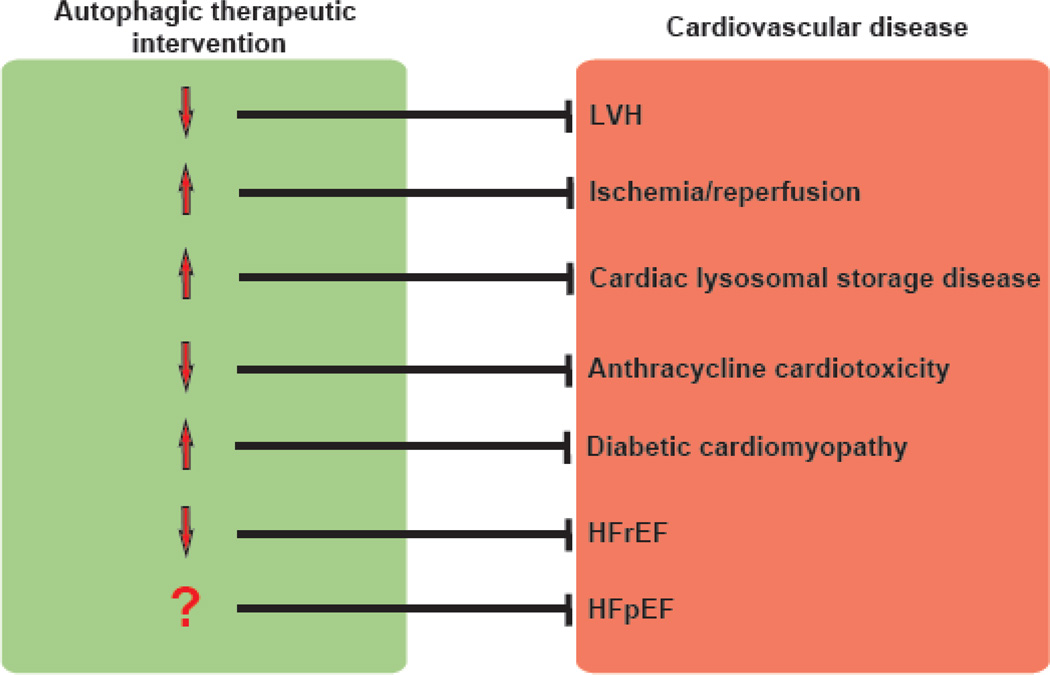
A number of significant hurdles challenge the translation of our understanding of autophagic mechanisms into clinically relevant interventions. Not least among them is the ubiquitous nature of autophagic activity, present in essentially all cells in the body. Titrating autophagy in a diseased organ might have untoward effects in other tissues. Also, the process must be titrated, not abrogated, as the latter is likely to be cytotoxic. In some instances, a therapeutic objective would be to titrate down the flux response; in other instances, one would seek to increase autophagic flux (Figure 4). Further, many cardiovascular diseases are chronic and progressive, so issues of timing, stage of pathogenesis, and duration of therapy are relevant.
Figure 4. Working model of potential therapeutic manipulation of autophagy in cardiovascular disease.
Whereas the field is still evolving, we propose the following as therapeutic objectives in titrating cardiomyocyte autophagy in heart disease. LVH: left ventricular hypertrophy; HFrEF: heart failure with reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction.
One of the most important limitations for implementing preclinical autophagic insights into the clinical domain is the lack of noninvasive biomarkers – imaging or circulating – able to report levels of autophagic flux. Absent that, it will be difficult to identify appropriate targets and optimize therapeutic intervention.
For all these reasons, chronic therapy targeting cardiac autophagy is not on the immediate horizon. However, there are instances where one could envision acute intervention. As one example, autophagic flux is perturbed in myocardial ischemia/reperfusion injury, and we have shown that a single dose of an HDAC inhibitor at the time of reperfusion biases autophagic flux up from abnormally low levels, conferring benefit [12]. Here, a single intervention during a period of severe disease-related stress could be acceptable, as effects in other tissues are likely to be short-lived.
Conclusions and future directions
The ultimate goal of cardiovascular translational research is to identify novel therapeutic targets and to design and test new drugs that confer clinical benefit. Notably, a number of compounds capable of modulating autophagic flux in preclinical models are still under investigation and may prove suitable for clinical use. Presently, lack of specificity of molecules capable of modulating the autophagic process limits the implementation of therapeutics with potential clinical efficacy. Both pharmacologic enhancers and inhibitors of autophagy are currently being tested in clinical trials outside cardiovascular disease [93]. However, none of these trials evaluates compounds that specifically target autophagic flux per se. As autophagic activity is ubiquitous, vigilance for off-target actions is a foremost consideration. We propose a model in which modulation of autophagy in heart can be beneficial in specific contexts depending on the timing and the amplitude of both the flux process and drug effect.
Highlights.
Autophagy is an intracellular process required to maintain cardiovascular homeostasis.
-
Perturbations in autophagy are involved in virtually all cardiovascular disease states.
Depending on the context, autophagic flux may be biased up or down.
Autophagy mediators hold promise as targets for cardiovascular disease therapy.
Acknowledgments
We gratefully acknowledge critical feedback from members of the Hill lab.
Sources of Funding
This work was supported by grants from the NIH (HL-120732; HL-100401), AHA (14SFRN20740000), CPRIT (RP110486P3), and the Leducq Foundation (11CVD04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
We declare no conflicts of interest.
References
- 1.Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. The New England journal of medicine. 2012;366:54–63. doi: 10.1056/NEJMra1112570. [DOI] [PubMed] [Google Scholar]
- 2.Wilmot KA, O'Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill JA, Olson EN. Cardiac plasticity. The New England journal of medicine. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 4.Galluzzi L, Bravo-San Pedro JM, Kroemer G. Organelle-specific initiation of cell death. Nature cell biology. 2014;16:728–736. doi: 10.1038/ncb3005. [DOI] [PubMed] [Google Scholar]
- 5.Decker RS, Poole AR, Griffin EE, Dingle JT, Wildenthal K. Altered distribution of lysosomal cathepsin D in ischemic myocardium. J Clin Invest. 1977;59:911–921. doi: 10.1172/JCI108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie M, Morales CR, Lavandero S, Hill JA. Tuning flux: autophagy as a target of heart disease therapy. Current opinion in cardiology. 2011;26:216–222. doi: 10.1097/HCO.0b013e328345980a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavandero S, Chiong M, Rothermel BA, Hill JA. Autophagy in cardiovascular biology. The Journal of clinical investigation. 2015;125:55–64. doi: 10.1172/JCI73943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ZV, Hill JA. Protein Quality Control and Metabolism: Bidirectional Control in the Heart. Cell Metab. 2015;21:215–226. doi: 10.1016/j.cmet.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159:1263–1276. doi: 10.1016/j.cell.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, et al. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7074–7079. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, et al. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 2014;129:1139–1151. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res. 116:456–467. doi: 10.1161/CIRCRESAHA.114.303788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 7:673–682. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 15.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuervo AM, Knecht E, Terlecky SR, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995;269:C1200–C1208. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- 17.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 2006;103:5805–5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 21.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreau K, Renna M, Rubinsztein DC. Connections between SNAREs and autophagy. Trends Biochem Sci. 38:57–63. doi: 10.1016/j.tibs.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Pires KM, Whitehead KJ, Olsen CD, Wayment B, Zhang YC, et al. Mechanistic target of rapamycin (Mtor) is essential for murine embryonic heart development and growth. PLoS One. 8:e54221. doi: 10.1371/journal.pone.0054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandri M. FOXOphagy path to inducing stress resistance and cell survival. Nat Cell Biol. 14:786–788. doi: 10.1038/ncb2550. [DOI] [PubMed] [Google Scholar]
- 26.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, et al. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 122:1109–1118. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol. 51:584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothermel BA, Hill JA. Autophagy in load-induced heart disease. Circulation research. 2008;103:1363–1369. doi: 10.1161/CIRCRESAHA.108.186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rifki OF, Hill JA. Cardiac autophagy: good with the bad. J Cardiovasc Pharmacol. 60:248–252. doi: 10.1097/FJC.0b013e3182646cb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiattarella GG, Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 131:1435–1447. doi: 10.1161/CIRCULATIONAHA.115.013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature medicine. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 33.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. The Journal of clinical investigation. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell death and differentiation. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 36.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature reviews Molecular cell biology. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 37.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 39.Nishida K, Yamaguchi O, Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circulation research. 2008;103:343–351. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- 40.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circulation research. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 41.Kehat I, Accornero F, Aronow BJ, Molkentin JD. Modulation of chromatin position and gene expression by HDAC4 interaction with nucleoporins. The Journal of cell biology. 2011;193:21–29. doi: 10.1083/jcb.201101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, et al. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:3549–3560. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, et al. Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res. 2009;105:1223–1231. doi: 10.1161/CIRCRESAHA.109.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. Journal of molecular and cellular cardiology. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 46.Gurusamy N, Lekli I, Gorbunov NV, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–387. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, Yitzhaki S, Perry CN, Liu W, Giricz Z, Mentzer RM, Jr, et al. Autophagy induced by ischemic preconditioning is essential for cardioprotection. J Cardiovasc Transl Res. 3:365–373. doi: 10.1007/s12265-010-9189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, et al. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation. 125:3170–3181. doi: 10.1161/CIRCULATIONAHA.111.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, et al. Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy. Circulation. 129:1139–1151. doi: 10.1161/CIRCULATIONAHA.113.002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin X, Peng C, Ning W, Li C, Ren Z, Zhang J, et al. miR-30a downregulation aggravates pressure overload-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 379:1–6. doi: 10.1007/s11010-012-1552-z. [DOI] [PubMed] [Google Scholar]
- 51.Weng LQ, Zhang WB, Ye Y, Yin PP, Yuan J, Wang XX, et al. Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta Pharmacol Sin. 35:1005–1014. doi: 10.1038/aps.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 53:710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, et al. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raben N, Shea L, Hill V, Plotz P. Monitoring autophagy in lysosomal storage disorders. Methods Enzymol. 2009;453:417–449. doi: 10.1016/S0076-6879(08)04021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raben N, Schreiner C, Baum R, Takikita S, Xu S, Xie T, et al. Suppression of autophagy permits successful enzyme replacement therapy in a lysosomal storage disorder--murine Pompe disease. Autophagy. 6:1078–1089. doi: 10.4161/auto.6.8.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raval KK, Tao R, White BE, De Lange WJ, Koonce CH, Yu J, et al. Pompe disease results in a Golgi-based glycosylation deficit in human induced pluripotent stem cell-derived cardiomyocytes. J Biol Chem. 290:3121–3136. doi: 10.1074/jbc.M114.628628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, et al. A block of autophagy in lysosomal storage disorders. Hum Mol Genet. 2008;17:119–129. doi: 10.1093/hmg/ddm289. [DOI] [PubMed] [Google Scholar]
- 58.Tessitore A, Pirozzi M, Auricchio A. Abnormal autophagy, ubiquitination, inflammation and apoptosis are dependent upon lysosomal storage and are useful biomarkers of mucopolysaccharidosis VI. Pathogenetics. 2009;2:4. doi: 10.1186/1755-8417-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiattarella GG, Cerulo G, De Pasquale V, Cocchiaro P, Paciello O, Avallone L, et al. The Murine Model of Mucopolysaccharidosis IIIB Develops Cardiopathies over Time Leading to Heart Failure. PloS one. 2015;10:e0131662. doi: 10.1371/journal.pone.0131662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D'Souza RS, Levandowski C, Slavov D, Graw SL, Allen LA, Adler E, et al. Danon disease: clinical features, evaluation, and management. Circ Heart Fail. 7:843–849. doi: 10.1161/CIRCHEARTFAILURE.114.001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 62.Perrino C, Schiattarella GG, Magliulo F, Ilardi F, Carotenuto G, Gargiulo G, et al. Cardiac side effects of chemotherapy: state of art and strategies for a correct management. Curr Vasc Pharmacol. 12:106–116. doi: 10.2174/157016111201140327163302. [DOI] [PubMed] [Google Scholar]
- 63.Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. Journal of molecular and cellular cardiology. 1987;19:817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 64.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. The New England journal of medicine. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 65.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. Journal of molecular and cellular cardiology. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature medicine. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 67.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. The Journal of clinical investigation. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. The Journal of clinical investigation. 2014;124:617–630. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. The New England journal of medicine. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. The Journal of biological chemistry. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol. 2009;134:82–90. doi: 10.1016/j.ijcard.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 72.Garcia L, Verdejo HE, Kuzmicic J, Zalaquett R, Gonzalez S, Lavandero S, et al. Impaired cardiac autophagy in patients developing postoperative atrial fibrillation. The Journal of thoracic and cardiovascular surgery. 2012;143:451–459. doi: 10.1016/j.jtcvs.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 73.Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, et al. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem. 288:18077–18092. doi: 10.1074/jbc.M113.474650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsh BJ, Soden C, Alarcon C, Wicksteed BL, Yaekura K, Costin AJ, et al. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Mol Endocrinol. 2007;21:2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 75.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation. 125:1134–1146. doi: 10.1161/CIRCULATIONAHA.111.078212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fierabracci A. The putative role of proteolytic pathways in the pathogenesis of Type 1 diabetes mellitus: the 'autophagy' hypothesis. Med Hypotheses. 82:553–557. doi: 10.1016/j.mehy.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 77.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Fujitani Y, Ebato C, Uchida T, Kawamori R, Watada H. beta-cell autophagy: A novel mechanism regulating beta-cell function and mass: Lessons from beta-cell-specific Atg7-deficient mice. Islets. 2009;1:151–153. doi: 10.4161/isl.1.2.9057. [DOI] [PubMed] [Google Scholar]
- 79.Xu X, Hua Y, Nair S, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 81.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 82.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, et al. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation. 2009;120:S191–S197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gogas BD, McDaniel M, Samady H, King SB, 3rd, et al. Novel drug-eluting stents for coronary revascularization. Trends Cardiovasc Med. 24:305–313. doi: 10.1016/j.tcm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Soderlund C, Radegran G. Immunosuppressive therapies after heart transplantation - The balance between under- and over-immunosuppression. Transplant Rev (Orlando) 29:181–189. doi: 10.1016/j.trre.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gillette TG, Hill JA. Readers, Writers, and Erasers: Chromatin as the Whiteboard of Heart Disease. Circulation research. 2015;116:1245–1253. doi: 10.1161/CIRCRESAHA.116.303630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Antos CL, McKinsey TA, Dreitz M, Hollingsworth LM, Zhang CL, Schreiber K, et al. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. The Journal of biological chemistry. 2003;278:28930–28937. doi: 10.1074/jbc.M303113200. [DOI] [PubMed] [Google Scholar]
- 89.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–2588. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, et al. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, et al. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24:69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]