Abstract
Brain has exceptional high requirement for energy metabolism with glucose as the exclusive energy source. Decrease of brain energy metabolism and glucose uptake has been found in patients of Alzheimer’s, Parkinson’s and other neurodegenerative diseases, providing a clear link between neurodegenerative disorders and energy metabolism. On the other hand, cancers, including glioblastoma, have increased glucose uptake and rely on aerobic glycolysis for energy metabolism. The switch of high efficient oxidative phosphorylation to low efficient aerobic glycolysis pathway (Warburg effect) provides macromolecule for biosynthesis and proliferation. Current research indicate that methylene blue, a century old drug, can receive electron from NADH in the presence of complex I and donates it to cytochrome C, providing an alternative electron transfer pathway. Methylene blue increases oxygen consumption, decrease glycolysis, and increases glucose uptake in vitro. Methylene blue enhances glucose uptake and regional cerebral blood flow in rats upon acute treatment. In addition, methylene blue provides protective effect in neuron and astrocyte against various insults in vitro and in rodent models of Alzheimer’s, Parkinson’s, and Huntington’s disease. In glioblastoma cells, methylene blue reverses Warburg effect by enhancing mitochondrial oxidative phosphorylation, arrests glioma cell cycle at s-phase, and inhibits glioma cell proliferation. Accordingly, methylene blue activates AMP-activated protein kinase, inhibits downstream acetyl-coA carboxylase and cyclin-dependent kinases. In summary, there is accumulating evidence providing a proof of concept that enhancement of mitochondrial oxidative phosphorylation via alternative mitochondrial electron transfer may offer protective action against neurodegenerative diseases and inhibit cancers proliferation.
Introduction
Cancer and neurodegeneration are often believed to be two distinct pathological disorders of opposite etiology and therapeutic intervention. While cancers are characterized by the enhanced resistance to cell death, neurodegenerative diseases are featured by the progressive premature neuron death (Plun-Favreau et al., 2010). Indeed, neurodegenerative diseases such as Alzheimer’s disease (AD) have been found to be inversely associated with cancers (Bajaj et al., 2010; Driver et al., 2012). On the other hand, there is increasing evidence that cancers and neurodegenerative diseases might share common etiologic mechanisms and therapeutic targets. Age is the single most important risk factor for both neurodegeneration and cancers (Niccoli and Partridge, 2012). Dietary restriction has been found to be one of the most effective interventions to extend lifespan and retard age-related diseases including cancers and neurodegenerative diseases (Hursting et al., 2013)(Graff et al., 2013; Hursting et al., 2010; Prolla and Mattson, 2001). There are many drugs that may be effective for the treatment of both cancers and neurodegenerative diseases. For example, Bexarotene, a retinoid X receptor agonist for the treatment of T cell lymphoma, has been shown to reduce Aβ plaques and attenuates cognitive deficits in murine models of AD (Aicardi, 2013; Cramer et al., 2012; Tai et al., 2014).
From the discoveries of Krebs cycle (Krebs and Johnson, 1937) to mitochondrial oxidative phosphorylation (Boyer et al., 1973; Gresser et al., 1982), biology has experienced great advance of our knowledge in cellular metabolism from 1920s to 1980s. For the past 3 decades, molecular biology approaches have been dominating biological research without paying much attention to the metabolic state of cells. Despite the dramatic advantage of molecular biology and modern medicine, neurodegenerative disorders and cancers remain the most devastating diseases and effective therapeutic interventions are desperately needed. Recently, it is beginning to be accepted that transcription participates in dictating cellular metabolism (McKnight, 2010). The integrative molecular, cellular, and metabolic approaches might provide a better understanding of neurodegenerative disorders and cancers, thus, leads to discover novel therapeutics for those incurables.
Abnormal metabolism is the key feature of many disorders. Neurodegenerative diseases are a heterogeneous group of disorders which presume to primarily affect different subset of neurons at the CNS, mainly including AD, Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). Despite the heterogeneity, both experimental and clinical studies have indicated that many neurodegenerative disorders often coexist metabolic dysfunction (Cai et al., 2012). Similarly, cancer cells are genetically and phenotypically heterogeneous given the different origination and the inter-tumor genomic instability (Burrell et al., 2013). Nonetheless, cancer cells have long been known to have characteristic alterations in their metabolism since 1920s (Warburg, 1956a, b). Reprogramming of energy metabolism has recently been proposed as an emerging cancer hallmark (Hanahan and Weinberg, 2011). Accordingly, novel therapeutic targets on metabolic pathway have been exploring for the treatment of cancers and neurodegeneration. In this review, we recapitulate the findings that highlight the metabolic alteration in neurodegenerative diseases and cancers, summarize the novel function of methylene blue, a century old drug, as an alternative mitochondrial electron transfer carrier, and propose that alternative mitochondrial electron transfer as a common therapeutic mechanism for the treatment of both neurodegenerative diseases and cancers.
Brain Energy Metabolism
Brain bioenergetics
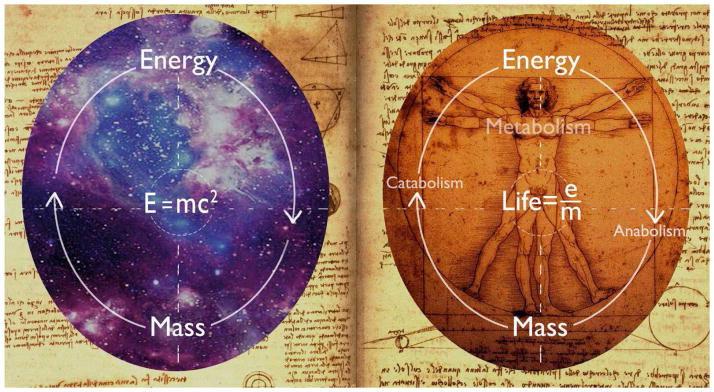
Einstein’s famous equation, E=MC2, simplifies the relationship of two fundamental physics entities, energy and mass, and linked them with the speed of light. Thus, mass and energy, used to be thought as separate entities, are known to be interchangeable. The significance of Einstein’s formula is even beyond physics. In biology, energy is an attribute of all living organisms from bacteria to human being. The conversion between mass and energy are fundamental to the biological processes defined as metabolism by which living organisms cycle energy through different mechanisms to produce the necessary molecules and perform the essential functions of life. Through anabolism, complex compounds are biosynthesized from simpler molecules with the energy expense provided by ATP hydrolysis. Through catabolism, complex nutrients are broken down to simpler oxidized molecules with an energy releasing process coupled to ATP production. Life is the interplay between energy and structure (Wallace, 2005). As the metabolism goes on, the life goes on (Figure 1).
Figure 1.
Energy and mass: from Einstein equation to energy metabolism. Einstein’s famous equation reveals the interchangeable relationship of mass and energy. The significance of Einstein’s formula is even beyond physics. In biology, conversion between mass and energy are fundamental processes defined as metabolism. Through anabolism, complex compounds are biosynthesized from simpler molecules with the energy expense provided by ATP hydrolysis. Through catabolism, complex nutrients are broken down to simpler oxidized compounds with an energy releasing process coupled to ATP production. Life is the interplay between energy and structure. As the metabolism goes on, the life goes on. E: energy. M: mass. C: speed of light.
Mammalian brain is characterized by high metabolic activity with fine regulatory mechanisms to ensure adequate energy substrates supply in register with neuronal activity. The human brain constitutes only 2% of the body weight, but receives 15% of cardiac output, accounts for almost 20% of the total oxygen consumption, and consumes approximately 25% of total body glucose utilization. The human brain is by far the most expensive organ in term of energy expenditure in the whole body. Maintenance and restoration of transmembrane resting potential dissipated by postsynaptic and action potential and neurotransmitters recycling represent the main energetic cost at the brain (Alle et al., 2009; Attwell and Laughlin, 2001; Howarth et al., 2012). An updated computation study has indicated that postsynaptic glutamate receptors, action potentials, resting potentials, presynaptic transmitter release, and transmitter recycling consume 50, 21, 20, 5, and 4% of energy budget at cerebral cortex, respectively (Howarth et al., 2012).
In addition to the extreme high energy consumption, brain energy supply and expenditure are tightly coupled by neurovascular and neurometabolic mechanisms. As the brain has very limited energy storage, local brain activity has to be complied with a coincidental increase of cerebral blood flow (CBF), referred as neurovascular coupling. Brain vasculature has many unique structural and functional features that are distinct from the peripheral characterized by the intimate relationships between endothelium, pericyte, astrocyte, and neuron, termed together as the neurovascular unit (Del Zoppo, 2013; Iadecola, 2004). Anatomically, the neurovascular coupling has to be orchestrated by the synergistic action of neurovascular unit. Therefore, brain activity evoked increase of CBF, functional hyperemia, is likely mediated through the concerted action of numerous vasoactive agents derived from the neurovascular unit components (Girouard and Iadecola, 2006; Petzold and Murthy, 2011).
Under normal physiological condition, adult brain almost exclusively uses glucose as substrate for energy metabolism in which glucose is delivered from the circulation to the brain through facilitated diffusion predominantly mediated by glucose transporter 1 (GLUT1) and GLUT3. GLUT1 was the first GLUT to be cloned with two isoforms been detected in the brain (Gerhart et al., 1989; McCall et al., 1996). The 45 kDa isoform has been found to be localized in glial cells and the higher molecular weight (55 kDa) isoform, the glycosylated GLUT1, present in both the luminal and abluminal membranes of endothelium (Birnbaum et al., 1986). In addition, the 55 kDa GLUT1 reside in endothelial cytoplasm as an intracellular pool (Duelli and Kuschinsky, 2001). On the other hand, GLUT3 has been found to be almost exclusively expressed in neurons (Simpson et al., 2007). Brain is considered to be an insulin insensitive organ as GLUT1 and GLUT3 are less insulin sensitive as comparing to GLUT4 (Heidenrich et al., 1989; Mueckler et al., 1985). The heterogeneous metabolism rate at the brain is companied with a heterogeneous distribution of GLUTs in the brain. The correlations between the metabolic rate and GLUTs distribution suggest that the densities of GLUT1 and GLUT3 might contribute to the local metabolic demand of the different brain structures (Duelli and Kuschinsky, 2001). Upon transported into the brain, as in other organs, glucose is phosphorylated by hexokinase to produce glucose-6-phosphate which can be further processed via different metabolic pathways: glycolysis, pentose phosphate pathway, and glycogenesis. Under normal condition, glucose is almost entirely metabolized to CO2 and water through glycolysis, tricarboxylic acid (TCA) cycle, and mitochondrial oxidative phosphorylation (Belanger et al., 2011).
Glycolysis is an evolutionary preserved and nearly universal energy production pathway whereby glucose is anaerobic fermented to lactic acid with the production of 3 ATP. In the presence of oxygen, glycolysis is tightly coupled with TCA cycle and mitochondrial oxidative phosphorylation and serves as a molecular interconversion system. Lactate, the product of glycolysis, is shuttled into mitochondria via the mitochondrial monocarboxylate transporter (MCT) where it is oxidized to pyruvate. Then, pyruvate is converted into a series of organic acid through TCA cycle with the liberation of carbon dioxide (Schurr, 2014). In addition, the TCA cycle reduces NAD+ to NADH, which is fed into the mitochondrial oxidative phosphorylation to generate ultimate biochemical energy, ATP.
Mitochondrial oxidative phosphorylation encompasses electron transfer through the mitochondrial respiratory chain, trans-inner mitochondrial membrane proton pumping, generating of mitochondrial membrane potential, and the ultimate ATP synthesis. The mitochondria produce energy in the form of ATP by oxidizing carbohydrates and fats-derived hydrogen. Electrons derived from NADH or succinate are passed sequentially through electron transfer chain (ETC) complexes and the released energy is used to pump protons into the intermembrane space through complex I, III, and IV to create mitochondrial membrane potential which is coupled to ATP synthesis. As protons flow cross mitochondrial inner membrane back into the mitochondrial matrix through complex V, Pi is bound to ADP to produce ATP (Wallace, 2005). As the by-product of mitochondrial oxidative phosphorylation, electrons leaked from the ETC complexes, mainly at complex I and III, can be directly donated to O2 to produce superoxide anion and other reactive oxygen species (ROS) (Birch-Machin, 2006; Murphy, 2009).
Although mitochondrial structure and function are highly conserved, there are many qualitative and quantitative differences in mitochondria between different cells and tissues. In addition, mitochondria dynamically undergo shape and number changes through regulated processes of fusion and fission. There are studies indicated that brain mitochondria were complicated by the cellular and regional heterogeneity and aging and that mitochondria in the brain can generate ATP at a faster pace than peripheral (Battino et al., 1991; Kwong and Sohal, 2000; Tesco et al., 2007). Perturbation of mitochondrial dynamics has been indicated to be involved in a wide spectrum of human disorders, including neurodegenerative diseases and cancers (Chen and Chan, 2009; Grandemange et al., 2009).
Beyond Bioenergetics
Glucose derived from carbohydrates provides not only the major fuel for ATP production through mitochondrial oxidative phosphorylation but also the essential substrate for de novo synthesis of proteins, lipids, and nuclei acids (Bauer et al., 2005). Similarly, in addition to its role as cellular bioenergetics center, mitochondria function as biosynthetic hub and play critical roles in synthesis of macromolecules to meet the biosynthetic demand for cell proliferation (Jones and Thompson, 2009). For bioenergetic pathway, pyruvate, generated from glycolysis, is converted to acetyl-CoA which feeds into the TCA cycle to be oxidized for energy production. For biosynthetic pathway, carbon atoms derived from glucose enter mitochondria and are then extruded from the TCA cycle at various steps to provide precursors for biosynthesis of lipids, nucleotides, and amino acids (Jones and Thompson, 2009).
In TCA cycle, 2-carbon acetyl group derived from acetyl-CoA is transferred to 4-carbon oxaloacetate to form 6-carbon citrate which is further converted to isocitrate. As an alternative, citrate is exported to the cytosol through pyruvate-citrate shuttle for lipid biosynthesis and protein acetylation. In the cytosol, citrate is cleaved back to form oxaloacetate and acetyl-CoA. The citrate is further converted into malonyl-CoA by acetyl-CoA carboxylase and feed into fatty acid synthesis pathway (Ahn and Metallo, 2015). Thus, in additional to its critical role in bioenergetics, metabolic intermediate acetyl-CoA functions as a central biosynthetic precursor for fatty acid synthesis. Consistently, eukaryote mitochondria also harbor a highly conserved fatty acid synthetic machinery independent of the cytosolic fatty acid synthesis apparatus although its end products and functions are largely unclear (Hiltunen et al., 2009).
Glucose metabolites also provide substrates for de novo biosynthesis of amino acids. Pyruvate, α-ketoglutarate, and oxaloacetate can be converted into amino acids through the addition of an amino group. α-ketoglutarate can be converted into glutamate by reductive amination. Then the amino group from glutamate can be transferred to pyruvate and oxaloacetate to produce alanine and aspartate, respectively. Glutamate is also the precursor of proline and arginine. Glucose metabolism through the pentose phosphate pathway produces a key intermediate in nucleotide biosynthesis, ribose-5-phosphate (Munoz-Pinedo et al., 2012). In addition, pentose phosphate pathway produces NADPH, which supplies reducing equivalents for the biosynthesis of fatty acid and nucleotide (Jones and Thompson, 2009).
Mitochondrial oxidative phosphorylation is the dominant metabolic pathway in the brain. The central dogma of brain energy metabolism assumed that brain glucose utilization fuels neuronal activity through mitochondrial oxidative phosphorylation in both basal and activated state (Simpson et al., 2007; Sokoloff et al., 1977). However, there is increasing evidence suggesting an uncoupling between the brain glucose and oxygen utilization. The measurement of glucose utilization has been found to be up to 31 mmol/100g weight/min calculated from the oxygen consumption with a respiratory quotient of 1 (Kety and Schmidt, 1948) (Pellerin and Magistretti, 2012). Therefore, in addition to bioenergetic production of ATP through mitochondrial oxidative phosphorylation, biosynthetic pathways involved in both glycogenesis and lipogenesis may contribute to the glucose metabolism by the brain in excess of oxygen consumption. Indeed, aerobic glycolysis has been found to account for ~10% of the glucose consumed by the adult human brain (Goyal et al., 2014).
Although brain is believed to be have very limited energy storage, glycogen, the single largest glucose reserve, has been found in the brain which predominately localize in astrocytic end-feet, bodies, and processes (DiNuzzo et al., 2011; Oz et al., 2007). Theoretically, given their intimate relationship with neurons both anatomically and functionally, astrocytes can mobilize glycogen for neuronal metabolism during normal circumstance and in case of increased energy demand or failure of neurons. Given the relatively low content of glycogen in the brain as comparing to liver and skeletal muscle, the astrocytic glycogen is unlikely accounted for as an alternative energy supply in the brain during hypoglycemia. Instead, astrocytic glycogen more likely serve as an integral factor for brain metabolism under normal condition (DiNuzzo et al., 2011; Obel et al., 2012). The glycogenesis and glycogen degradation is fine-tuned by glycogen synthase and glycogen phosphorylase (Obel et al., 2012). There is much controversy regarding the function of astrocytic glycogen in term of neuron-glial metabolic coupling which is beyond the scope of this review. Importantly, astrocytic glycogen seems play a role in learning process (Hertz and Gibbs, 2009). Furthermore, disturbance of glycogen homeostasis between astrocyte and neuron has been found to result neurodegeneration. Recent studies have indicated that neurons have the enzymatic machinery for glycogenesis and that Lafora disease (progressive myoclonus epilepsy) related gene mutations activated glycogen synthesis in neurons and caused apoptosis (Duran et al., 2012; Magistretti and Allaman, 2007; Vilchez et al., 2007).
Lipid metabolism is of particular interest due to its high concentration in the brains and that mutation in lipid metabolizing enzymes result in debilitating neurological diseases. Lipids could potentially serve two principle functions as substrate for energy production through β oxidation and as structural components of cell membranes. It was conjectured that the selection of glucose, the predominant substrate for brain energy metabolism, over fatty acids for energy metabolism in the brain may be evolutionarily beneficial to avoid the disadvantages of β-oxidation (Schonfeld and Reiser, 2013). While fatty acids are used by the brain as well (Ebert et al., 2003; Kuge et al., 1995; Panov et al., 2014), neurons have low level rate-setting fatty acid catabolism enzymes and are generally not thought to rely on fatty acid β-oxidation for bioenergetics (Cahoy et al., 2008; Lee and Wolfgang, 2012) (Schonfeld and Reiser, 2013).
Beside potential energy substrate, lipids are structural components of cell membranes and have been found to be involved in intracellular architecture, membrane trafficking, and regulation of proteins and sub-compartments in the membranes (Adibhatla and Hatcher, 2007; Liu and Zhang, 2014). Cholesterol is an indispensable biological membrane component. Brain is a highly cholesterol-enriched organ resided with ~25% of total body cholesterol (Dietschy and Turley, 2001). The majority of brain cholesterol resides in the myelin sheaths and plasma membranes required for brain development, synapse and dendrite formation, and axonal guidance (Orth and Bellosta, 2012). Cholesterol synthesis is at the highest in oligodendrocytes during myelination at the developmental stage (Dietschy and Turley, 2004). In the adult brain, BBB prevents the cholesterol uptake from the blood, thus, de novo cholesterol synthesis continues in astrocytes at a low rate (Nieweg et al., 2009) (Vance, 2012). Consistently, lipid synthetic pathways are highly enriched in astrocytes (Cahoy et al., 2008).
In addition as the pivotal structural component, brain lipids have critical cell signaling function mainly through three mechanisms. First, bioactive lipids as lysophospholipids, eicosanoids, and endocannabinoids are known to interact with G-protein coupled membrane receptors that activate the downstream signaling (Bieberich, 2012). Secondly, brain bioactive lipids as phosphatidylinositols and ceramide bind to protein kinases, phosphatases, and other signaling proteins that affect intracellular cascades (Bieberich, 2012). In addition, brain bioactive lipids regulate cell signaling pathways through cholesterol- and sphingolipid-enriched lipid rafts (Lingwood and Simons, 2010). Lipid rafts have been shown to be involved in many brain functions both physiologically and pathologically. For example, lipid rafts have been shown to influence the potency and efficacy of neurotransmitter receptors and transporters (Allen et al., 2007). lipid L-carnitine, its primary role appears to facilitate the transport of long chain fatty acids into mitochondria for β-oxidation cycle, has been indicated to be neuroprotective (Rau et al., 2012; Wang et al., 2007). Lipogenesis has been shown to regulate adult neural stem proliferation (Folmes et al., 2013; Knobloch et al., 2013).
Astrocyte-Neuron Metabolic Coupling and Astrocyte Neuron Lactate Shuttling
In the last decade, our knowledge in neuroenergetics has been rapidly evolving from the “neurocentric” view to an integrated picture of neuron-astrocyte coupling (Allaman et al., 2011; Belanger et al., 2011). Anatomically, neurons are separated from circulation by BBB and do not have direct access to blood-born fuel. Glucose from the circulation has to cross 4 plasma membrane to reach neurons, 2 endothelial and 2 astrocytic. Thus, neurons might not be able to directly control their own fuel supply. On the other hand, astrocytes, with their end feet cover 99.7% of the brain capillary, have an anatomical advantage to control over glucose flux into the brain (Mathiisen et al., 2010). Therefore, astrocytes may server as an intermediary between neuron and vasculature for neurovascular coupling.
Consistent to the anatomical evidence, the knockout mice studies have indicated that glia/endothelial GLUT1 play a major role in glucose transport at the mammalian brain as comparing with neuronal GLUT3. Heterogeneous GLUT1 knockout mice have decreased brain GLUT1 expression and display many features similar to the human GLUT1 deficiency syndrome with the decreased brain glucose uptake, seizures, impaired motor activity, hypoglycorrhachia, and disturbances of microencephaly incoordination and learning (Wang et al., 2006). On the other hand, GLUT3 haploinsufficiency is not associated with any brain dysfunction and impairment of brain glucose uptake (Stuart et al., 2011). Thus, astrocytes may play an even more important role in glucose metabolism compared with neurons in the brain. Indeed, at the hippocampus and cerebellum, glucose transport and metabolism has been found to be faster in the glial compartment than in the neuronal compartment (Jakoby et al., 2014). In vivo imaging of brain slices have indicated that stimulation of astrocytes triggers an intra-astrocytic calcium surge which subsequent dilate or constrict adjacent arterioles (Girouard et al., 2010).
Besides glucose, a wide range of metabolic intermediates can be oxidized for energy production in the brain, including lactate, pyruvate, acetate, glutamate, and glutamine (Belanger et al., 2011; Zielke et al., 2009). Importantly, neurons and astrocytes present different metabolic profiles in physiological condition. Beside GLUT1 and GLUT3, glycolytic products are transported into and out of neurons by monocarboxylate transporters (MCT) with MCT1 been found in the BBB and astrocytes and MCT2 in neurons (Simpson et al., 2007). In addition, astrocyte expresses MCT4 (Pierre and Pellerin, 2005). MCT1-4 have been shown to transport endogenously generated monocarboxylate such as pyruvate, lactate, and ketone bodies (Pierre and Pellerin, 2005). Glycolytic product, lactate, has been shown to have a role in long-term memory formation. Antisense oligonucleotides targeting either astrocytic MCT4 or neuronal MCT2 cause memory defects. Interestingly, addition of exogenous lactate only rescued the memory consolidation defects caused by MCT4 knockout, but not MCT2 knockout, suggesting lactate shuttling from astrocytes into neurons (Bezzi and Volterra, 2011; Suzuki et al., 2011). Although there are substantial evidence that pyruvate derived from neuronal glucose is the major oxidative fuel for neurons (Patel et al., 2014), there is increasing evidence suggested that astrocytes play critical roles in both bioenergetic and biosynthetic metabolism at the brain. Furthermore, dysfunction of astrocyte metabolism has been found in many neurodegenerative diseases (Stobart and Anderson, 2013).
AMPK, a Master Sensor and Regulator of Energy Metabolism
Matching energy supply with expenditure is essential for brain function. ATP, produced from ADP and inorganic phosphate, is the universal energy currency in the cells. Reversely, energy-required cellular processes are powered by the hydrolysis of ATP to ADP and phosphate. Thus ADP/ATP ratio provides an important parameter for both cellular ATP consumption and synthesis. In addition, the ubiquitously expressed adenylate kinase catalyze the inter conversion of adenine nucleotides which make AMP/ATP ratio another important indicator for energy status (Hardie, 2011). At the cellular level, maintenance of the energy homeostasis as reflected in relative level of ATP, ADP, and AMP is of critical importance for energy metabolism. Recent studies demonstrated that the relative level of ATP, ADP, and AMP, in term of ADP/ATP and AMP/ATP ratios, play a critical role in the regulation of energy metabolism through AMP-activated protein kinase (AMPK) signaling (Gowans et al., 2013; Oakhill et al., 2011).
AMPK, a Ser/Thr kinase, is an evolutionarily conserved energy sensor and regulator for energy metabolism (Kahn et al., 2005). AMPK is a heterotrimeric protein comprised of catalytic α subunits (α1, α2) and regulatory β and γ subunits (β1, β2, γ1, γ2, γ3) (Hardie, 2007). It is activated by events that either increase ATP consumption or compromise cellular ATP production (Hardie, 2007; Long and Zierath, 2006). The glycogen-binding domain on the β subunit also allows AMPK to function as a glycogen sensor (McBride et al., 2009; McBride and Hardie, 2009). AMPK elicits diverse effects on glucose and lipid metabolism, cell cycle, and protein synthesis. Activated AMPK stimulates catabolic pathways that generate ATP through increasing glucose and fatty acid uptake, enhancing glycolysis and fatty acid oxidation, and stimulating mitochondrial biogenesis. Concomitantly, AMPK activation inhibits the rate of anabolic pathways that consume ATP, thus, restores the correct adenylate energy charge (Hardie, 2011). AMPK activation inhibits glycogen and fatty acid synthesis by phosphorylates and inactivates glycogen synthase and Acetyl-CoA carboxylase (ACC), respectively (Hardie, 2007). In addition, AMPK inhibits protein synthesis and cell proliferation (Horman et al., 2002; Motoshima et al., 2006). When nutrients are limited, AMPK inhibits cell growth via the suppression of mammalian target of rapamycin complex I (mTORC1) (Gowans et al., 2013). Furthermore, AMPK activation promotes autophagy to maintain essential cellular activity in response to nutrient scarcity (Kim et al., 2011).
In the mammalian adult brain, AMPK predominately expresses in neurons characterized by high catabolic activity (Culmsee et al., 2001; Turnley et al., 1999; Vingtdeux et al., 2011). Different AMPK expression levels have been identified in different neuronal subtypes of the adult brain which higher glucose metabolism have higher AMPK expression (Spasic et al., 2009). At the hypothalamus, AMPK is expressed in energy sensing neurons and functions as a master regulator of organismal energy balance (Ronnett et al., 2009). Energy deficit signals such as hypoglycemia (McCrimmon et al., 2004), thyroid hormones (Ishii et al., 2008), glucocorticoids (Shimizu et al., 2008), cannabinoids (Kola et al., 2005), and adiponectin (Kubota et al., 2007) enhance hypothalamic AMPK activity to increase feeding. On the other hand, energy surplus signals induced by high glucose, leptin, insulin, and resistin inhibit feeding behavior via decreasing hypothalamic AMPK activity (Minokoshi et al., 2004). AMPK activation is mediated through phosphorylation by tumor suppressor LKB-1 complex and calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ). LKB-1 has been found to be ubiquitously expressed which enable basal constitutive AMPK activation, while, CaMKKβ is expressed predominantly in neurons (Spasic et al., 2009). On the other hand, consistent with its pro-anabolic phenotype, astrocytes only express minimal AMPK under physiological condition (McCullough et al., 2005).
Energy Metabolism in Neurodegenerative Diseases
Neurodegenerative diseases are heterogeneous in term of clinical symptom and pathophysiology due to the progressive loss of different subsets of neurons at the CNS (Przedborski et al., 2003). Most of neurodegenerative diseases are aging-related and present progressive motor and/or cognitive dysfunction that causes reduced life quality, increase medical costs, and eventual death. As the aging population increases worldwide, neurodegenerative diseases are becoming an enormous social and economic burden in the world. Unfortunately, efforts towards discovering effective treatment for neurodegeneration have been fruitless and no cure has been developed so far for any of the neurodegenerative disorders.
For decades, research in neurodegenerative diseases has been dominated by the neurocentric view holds that neurodegeneration is primarily caused by neuronal defects. However, there is increasing evidence indicates that vasculature and astroglia might also contribute the onset and progression of many neurodegenerative disorders (Maragakis and Rothstein, 2006; Zacchigna et al., 2008). The heterogeneity of the neurodegenerative diseases does not forestall common biochemical features. An integrative view of the neurovascular unit and their common biochemical feature in neurodegenerative diseases might shed light on our future research in neurodegeneration that ultimately lead to the discovery of effective therapies. There is accumulating evidence indicating a causative role of mitochondrial dysfunction in both aging and age-related neurodegenerative disorders (Bratic and Larsson, 2013; Chen and Chan, 2009). In fact, the causative factor is likely beyond the mitochondria dysfunction and involved in energy metabolism.
Metabolic Alteration in AD
AD is the most common neurodegenerative disease hallmarked pathologically by the amyloid-β plague in neuropil and cerebral vessels, and by the hyperphosphorylated neurofibrillary tangles in neurons (Girouard and Iadecola, 2006). Accordingly, AD research has been dominated by the “amyloid cascade hypothesis” and “tau and tangle hypothesis” (Korczyn, 2008; Mudher and Lovestone, 2002). However, decades of research focusing on Aβ and tau has not lead to the identification of any effective treatment for AD. All therapeutics purported to decrease amyloid-β plague have failed to demonstrate any efficacy in clinical trials (Karran and Hardy, 2014; Karran et al., 2011). The fact that amyloid deposition is not strongly correlated with cognition argues against amyloid hypothesis. Furthermore, it has been clear that amyloid cascade and tau hyperphosphorylation can be induced by a plethora of brain damages. In experimental models, ischemic stroke induces overexpression of β-amyloid precursor protein (Nihashi et al., 2001; Shi et al., 2000), increases beta-secretase expression and activity (Tesco et al., 2007; Wen et al., 2004a), induces site-specific hyperhposphorylation (Wen et al., 2004c) and AD like tauopathy (Wen et al., 2004b). Consistently, accumulation of Aβ1-40 and Aβ1-42 were found in human hippocampus after ischemic stroke (Qi et al., 2007). Amyloid-β and tau pathology have also been observed after traumatic brain injury in human (Chen et al., 2009; Johnson et al., 2012b). These evidence suggests that neither amyloid nor tau cascade may be the cause of neurodegeneration in AD. Thus, alternative hypotheses are needed to be explored beyond the amyloid-β and tau cascade.
Metabolic syndrome has been found to contribute to cognitive impairment in elderly persons and be associated with AD (Razay et al., 2007; Yaffe et al., 2004). Hypometabolism has emerged as a robust biomarker of neurodegeneration (Johnson et al., 2012a). Reduction of glucose metabolism has long been implicated in AD brain (Iadecola, 2015). Impairments in brain energy metabolism and glucose utilization have been found to be among the very early abnormalities that accompany or even precede the early stage of cognitive dysfunction (Hoyer, 2004; Nordberg et al., 2010; Sims et al., 1980). The reductions in brain glucose transport and utilization were found in cognitively normal people at genetic risk for AD and at the early state in AD patients (Iadecola, 2015; Nordberg et al., 2010). There are many underlying mechanisms that may contribute to the impairment of glucose metabolism in AD brain. Endothelial and astrocyte localized GLUT1 plays a major role in glucose transport at the mammalian brain. Reduction of GLUT1 has been found in AD brain (Kalaria and Harik, 1989; Mooradian et al., 1997). A very recent study indicated that GLUT1 deficiency exacerbated the pathophysiology in the brains of AD mice and may act synergistically with AD pathology to promote the progression of dementia (Winkler et al., 2015). Reciprocally, restoration of GLUT1 reduced Aβ levels in AD mice brains (Winkler et al., 2015). In addition, alternative energy substrates such as ketogenetic diet and enhancement glucose uptake by intranasal insulin have demonstrated beneficial effects (Mamelak, 2012; Stafstrom and Rho, 2012; Yarchoan and Arnold, 2014).
Vascular mechanisms might also contribute to the metabolic alteration in AD. Cerebral amyloid angiopathy characterized by amyloid deposition within brain vasculature is common in aged individuals and even more common in AD patients (Castellani et al., 2004). Thus, it is no surprise that cerebrovascular function is profoundly altered in AD. Ironically, the presence of cerebrovascular disease is considered an exclusive criterion for AD diagnosis and the contribution of vascular factor to the onset and progression of AD has been long debated (Iadecola, 2004; McKhann et al., 2011). There is increasing evidence from both autopsy and long-term observational studies indicated that AD are highly pathologically heterogeneous with many exhibiting mixed pathologies, including infarcts (Schneider et al., 2009). Indeed, the coexistence of AD and vascular dementia, termed mixed dementia, is the most common dementia (Langa et al., 2004). In the brain, neurons, astrocyte, and vasculature constitute the neurovascular unit to maintain the brain homeostasis. Alteration of the neurovascular coupling has been found not only in cerebrovascular disease but also neurodegenerative diseases including AD. Cerebral amyloid angiopathy induces progressive neurovascular unit dysfunction featured by the failure of vascular reactivity, smooth muscle cell loss, and breakdown of vessel integrity (Zipfel et al., 2009). Amyloid-β, the main component of amyloid plaques in AD brain, is vasoactive both in vitro and in vivo (Crawford et al., 1998; Niwa et al., 2000). Functional hyperemia has been found to be perturbed in both animal models and AD patients (Iadecola, 2004; Petzold and Murthy, 2011). Thus, the impairment of neurovascular coupling, together with other cellular mechanisms, might trigger and promote neurodegeneration in AD.
Besides the amyloid β plaque and neurofibrillary tangles, AD brain also display high occurrence of adipose inclusions (Foley, 2010). The risk of AD is affected by inheritance of different isoforms of Apolipoprotein E (APOE), a pivotal regulator for cholesterol metabolism, with the ε4 allele as the strongest genetic risk factor for AD (Bertram and Tanzi, 2008; Corder et al., 1993), providing strong evidence that lipid metabolism dysfunction may be involved in AD pathophysiology (Smith, 2000). It is well established that many lipids are implicated in AD pathogenesis (Di Paolo and Kim, 2011). Studies have indicated that change of brain cholesterol level influence APP processing and Aβ production (Lim et al., 2014). There is also evidence that lipid rafts might function as platform for the production of neurotoxic proteins, thus, involve in the AD pathogenesis (Allen et al., 2007; Ehehalt et al., 2003).
Mitochondria play pivotal roles in cellular functions including energy production, cell proliferation, and apoptosis (Birch-Machin, 2006; Wallace, 2005). At the brain, mitochondria regulate synaptic transmission, brain function, and cognition (Picard and McEwen, 2014). Giving the identified metabolic dysfunction in AD, it might not be surprised that mitochondria deficit is involved in the onset and progression of AD. Abnormalities of mitochondrial morphology, biochemistry, and genetics have been found in AD brains although its causative action in AD is still debatable (Mancuso et al., 2008; Swerdlow et al., 2014; Swerdlow and Khan, 2004). AD related oxidative stress has been associated with alteration of mitochondrial ETC complexes (Morais and De Strooper, 2010). Aβ has been shown to alter the assembly and enzyme activities of ETC complexes (Moran et al., 2012). Accordingly, studies have indicated that Aβ could gain access into mitochondria in both AD patients and transgenic AD mice (Chen and Yan, 2006). In addition, neurofibrillary tangles detected in AD brains have been shown to inhibit ETC complexes activity, increase ROS production, and decrease mitochondrial membrane potential (Moran et al., 2012). Reciprocally, AD transmitochondrial cybrids, cell depleted endogenous mtDNA repopulated with mtDNA from AD patients, showed overproduction of Aβ although no causative mtDNA nutation has been discovered in AD patients (Mancuso et al., 2009). Therefore, Aβ, neurofibrillary tangles, and mitochondrial deficit may synergistically initiate the AD pathophysiology and promote AD progression in a vicious cycle.
Considering AMPK as a master energy sensor and regulator, functional defects in AMPK signaling might be potentially involved in the deficiencies of energy metabolism in the AD brains. Consistently, increased AMPK phosphorylation has been found in the brains of AD patients and AD mouse models (Ma et al., 2014; Mairet-Coello et al., 2013; Vingtdeux et al., 2011). There is evidence that AMPK regulates tau phosphorylation at numerous sites (Thornton et al., 2011). In the normal brains, phosphorylated AMPK is predominately localized in the nuclei of neurons. On the other hand, cytoplasmic accumulation of activated AMPK have been found in neurons with hyperphosphorlation tau in AD brains, indicating that the cytoplasmic translocation and activation of AMPK may precede the tau hyperphosphorylation and tangle formation (Vingtdeux et al., 2011). Paradoxically, 5-aminoimidazole-4-carboxamide-1-d-ribofuranoside (AICAR), an AMPK activator, has been shown to inhibit tau phosphorylation. In addition, it has been suggested that leptin decreased tau phosphorylation through AMPK activation in primary neurons (Greco et al., 2009a; Greco et al., 2009b). Similarly, the paradoxical action of AMPK in amyloidogenesis has also been indicated. Aβ42 oligomers have been shown to activate AMPK (Mairet-Coello et al., 2013). On the other hand, studies have demonstrated that AMPK can inhibit amyloidogenesis (Salminen et al., 2011). Genetic and pharmacological activation of AMPK decreased Aβ accumulation both in vitro and in vivo (Vingtdeux et al., 2010; Won et al., 2010). Taken together, the effect of AMPK on tauopathy and amyloidogenesis may be context-dependent. While amyloid β can activate AMPK which further directly phosphorylate tau protein under certain conditions, activation of AMPK can inhibit amyloidogenesis and tau aggregation in other instances (Salminen et al., 2011).
Metabolic Alteration in PD
PD is the second most common neurodegenerative disorder featured pathologically by the progressive death of dopaminergic neurons in the substantia nigra. The typical symptoms of PD include slowness of movements (bradykinesia), muscle stiffness (rigidity), tremor, and balance disturbance. Etiopathologically, PD is due to the significant loss of dopaminergic neurons in the substantia nigra and the subsequent dopamine depletion at the striatum. To date, there are only symptomatic treatments available for PD patients, particularly at the early stages. No therapy has been discovered that can cure or halt the progression of PD.
Dysregulation of glucose metabolism has been found as an early event in sporadic PD patients. FDG-PET studies have indicated significant reduction in glucose metabolism in PD brains (Borghammer, 2012; Borghammer et al., 2010; Borghammer et al., 2009; Dunn et al., 2014). Interestingly, glucose hypometabolism was found extensively in cerebral cortex in PD patients with and without dementia (Edison et al., 2013).
The pathophysiology underlying the degeneration of dopaminergic neurons at the substantia nigra is still unclear. Mitochondrial dysfunction and oxidative stress have been consistently observed in PD brains. There is increasing pharmacological and genetic evidence sustain a link between PD and mitochondrial respiratory chain dysfunction, particular a deficit in mitochondrial ETC complex I (Franco-Iborra et al., 2015). Accidental exposure to 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine (MPTP), a mitochondrial complex I inhibitor, has been known to result in acute and irreversible PD syndrome (Calne and Langston, 1983; Langston and Ballard, 1983). Later on, mitochondrial complex I inhibition has been identified in the brains of sporadic PD patients (Schapira et al., 1990). In addition, chronic systemic inhibition of mitochondrial ETC complex I by pesticide rotenone has been found to link to sporadic PD (Betarbet et al., 2000). Interestingly, complex I deficiency has been found not only in the postmortem substantia nigra but also in cerebral cortex (Schapira et al., 1990), which is consistent to the cortical glucose hypometabolism observed in PD patients. Indeed, the pathology of PD has been found to involve several brain regions other than the SNc and many neurotransmitters other than dopamine (Lang and Obeso, 2004a, b). PD models using MPTP and rotenone have now been used extensively in PD research (Beal, 2010).
Although majority of PD cases are sporadic, mutations of many genes have been found to be related directly or indirectly to mitochondrial dysfunction identified in familial PD. Parkin and PINK1 mutations have been identified in autosomal recessive Parkinsonism (Pickrell and Youle, 2015). The functions of PINK1 and Parkin have been indicated in the maintenance of healthy mitochondria through regulating mitochondrial dynamics and autophagy that eliminate dysfunctional mitochondria (Moran et al., 2012). Recently, the DJ-1 protein, an antioxidant and transcriptional modulator, has been implicated to work with PINKs and Parkin to regulate mitochondrial function (Irrcher et al., 2010).
The deficiencies of energy metabolism in PD pathophysiology might be associated with potential functional defects in AMPK signaling. Activation of AMPK signaling has been demonstrated in PD models using MPTP, MPP+, and 6-OHDA, both in vitro and in vivo (Choi et al., 2010; Kim et al., 2013). In addition, inhibition of AMPK by compound C resulted in MPP+ induced cell death (Choi et al., 2010). On the other hand, both pharmacological and genetic activation of AMPK have been shown to provide protective action in PD models (Choi et al., 2010; Ng et al., 2012).
Metabolic Alteration in HD and FRDA
Beside AD and PD, dysfunctions of mitochondria and key energy metabolism signaling have been observed in other neurodegenerative diseases. HD is known as the result of mutant Huntingin gene and featured by the preferential loss of medium spiny GABAergic neurons in the striatum (Damiano et al., 2010). There is increasing evidence indicated that mutant huntingtin binds directly to mitochondria and alter mitochondrial functions (Choo et al., 2004; Orr et al., 2008). Reduction of mitochondrial ETC complex II, III, and IV were found at the caudate putamen in HD patients (Damiano et al., 2010). In addition, hyper-activation of AMPK has been found in the brains of HD patients and mice HD models (Ju et al., 2011). Consistently, AICAR, an AMPK activator, induced neuronal death and decreased the lifespan of HD mice (Ju et al., 2011).
Friedreich’s ataxia (FRDA) is the most common early-onset inherited ataxia due to the frataxin deficiency. The GAA trinucleotide expansion of the frataxin gene (FXN) results in defective frataxin transcription and reduces the amount of frataxin expression (Gonzalez-Cabo and Palau, 2013; Kaplan, 1999). Frataxin is known to be associated with iron metabolism and play an important role in heme biogenesis and the formation of iron-sulfur clusters (Gonzalez-Cabo et al., 2005; Karthikeyan et al., 2003). Deficiency of the iron-sulfur cluster-containing subunits of mitochondrial ETC complexes I, II, and III as well as aconitase have been identified in FRDA patients. In addition, sever deficit of mitochondrial oxidative phosphorylation and elevated intramitochondrial iron have been observed in FRDA patients. Consistently, reduction of maximum rate of mitochondrial ATP production was found in muscle of FRDA patients (Lodi et al., 1999). Accordingly, mitochondrial respiration has been the main target for discovery novel therapy for FRDA.
Energy Metabolism and Cancers
Revisit Warburg’s Effect
A century ago, in 1924, Otto Warburg postulated that cancers may be caused by the increased glycolysis and impaired respiration based on his observations that tumor tissue actively metabolizes glucose in oxygen-rich environment and produces excessive lactic acid while exhibiting a comparably low respiratory rate (Koppenol et al., 2011; Warburg, 1956b). This unique caner metabolism was later known as Warburg effect which was subsequently substantiated by many cancer researchers. The Warburg effect presented a paradox phenomenon that highly proliferating cancer cells in a great need for ATP use a ~18-fold lower efficient ATP production pathway. Without knowing the underlying mechanisms and significance of the Warburg effect, Warburg’s hypothesis on the origin of cancers had limited impact in cancer research and the metabolic signature had not been recognized as a cancer hallmark even toward the end of last millennium (Hanahan and Weinberg, 2000).
In the beginning of the new millennium the Warburg effect re-catches the attention of cancer researchers. The development of biology and the accumulating knowledge in energy metabolism and genetics have enabled us to further elaborate the cellular and molecular mechanisms underlying metabolic switch of cancer cells. We now know that the lower efficiency of ATP production through aerobic glycolysis in cancer cells is compensated by up-regulating glucose transporters. In addition, highly proliferative cancer cells do not necessary have defects in mitochondrial oxidative phosphorylation (DeBerardinis et al., 2008a; Moreno-Sanchez et al., 2007). Given that energy metabolism pathway can serve both bioenergetic and biosynthetic function, the gaining function of aerobic glycolysis may mainly support the unusually high proliferation and growth rate of cancer cells (DeBerardinis, 2008; DeBerardinis et al., 2008a; Jones and Thompson, 2009; Vander Heiden et al., 2009).
The insensitivity to antigrowth signals and self-sufficiency in growth signals, two of the cancer hallmarks (Hanahan and Weinberg, 2000), render the cancer cells not only high proliferation rate but also huge demands of protein, lipids, and nucleic acids for self-replication. To meet the challenge, cancer cells have to gain ability to capture sufficient nutrients and establish appropriate metabolism to produce macromolecules to build up the cancer biomass. Lipid and nucleotide biosynthesis use glucose as a carbon source, consume TCA cycle intermediates, and requires NADPH as reductive power (Deberardinis et al., 2008b). Cancer cells redirect glucose metabolism away from TCA cycle and mitochondrial oxidative phosphorylation to generate acetyl CoA through pyruvate-citrate shuttle for fatty acid synthesis and protein acetylation (Kamphorst et al., 2013; Metallo et al., 2012). In addition, cancer cells divert carbon from glycolysis into pentose phosphate pathway to generate ribose-5-phosphae for nucleotide biosynthesis (Deberardinis et al., 2008b). Thus, the tradeoff between the efficient energy production through mitochondrial oxidative phosphorylation and aerobic glycolysis ultimately benefit the biomass increase of cancers (Deberardinis et al., 2008b; Jones and Thompson, 2009). Now, switch of energy metabolism has been proving to be widespread in cancers and reprogramming of energy metabolism has been recognized as an emerging cancer hallmark (Hanahan and Weinberg, 2011).
Oncogenesis and Aerobic Glycolysis of Cancer Cells
No long after the Warburg proposed his theory of cancer origin, his contention of mitochondrial defects in cancers was negated by others (Chance and Castor, 1952; Weinhouse, 1956). Indeed, mitochondria oxidative phosphorylation capacity is not defective in most cancers (DeBerardinis et al., 2008a; Moreno-Sanchez et al., 2007; Ward and Thompson, 2012a). Consistently, we observed a dramatic increase in glycolytic flux which was accompanied with a less increase of oxidative phosphorylation in glioblastoma cells (Poteet et al., 2013). Thus, cancer cells still produce significant fraction of ATP through mitochondrial oxidative phosphorylation and the Warburg effect in cancer is not due to mitochondrial damage. The technological improvements in genetics and molecular biology have enabled us to reconcile the mechanisms underlying the metabolic switch of cancer cells and re-evaluate the connection of Warburg effect and tumorigenesis.
Through the advance of molecular biology in the last 30 more years, we now understand that tumor suppressor genes and oncogenes encode many cellular signal pathways that not only sustain cancer proliferation but also reprogram cancer energy metabolism to meet the biosynthetic challenge associated with the cancer cells growth and proliferation (Hanahan and Weinberg, 2011; Vander Heiden et al., 2009; Ward and Thompson, 2012a). Many signaling pathways including PI3K/Akt/mTORC1 and HIF are involved in cancer metabolic reprograming for cellular biosynthesis (Jones and Thompson, 2009; Ward and Thompson, 2012a, b). Furthermore, oncogenes and tumor suppressors have been found to be critical components of these metabolic signaling networks. PI3K/Akt activation is tightly controlled by PTEN, a key tumor suppressor. PTEN loss and/or PI3K mutations compose the most common genotype in human cancers that lead to biosynthetic reprograming (Jones and Thompson, 2009). Many oncogenes such as H-Ras, c-Myc, and src are known to enhance anabolic metabolism in cancer cells (Dang, 2011; Jones and Thompson, 2009). Taken together, mutations of oncogenes, loss of tumor suppressor genes, and many aberrant signaling are involved in the metabolic reprograming for cancers to arise and proliferate. In addition, the relationship between cellular signaling and metabolism could be bidirectional (Metallo and Vander Heiden, 2010; Ward and Thompson, 2012b). The feedback control of cellular metabolites on biosynthetic activities has been found at the level of both posttranslational modification and gene expression (Metallo and Vander Heiden, 2010; Ward and Thompson, 2012b).
MB, New Application for a Century Old Drug
A Century Old Drug with Broad Medical Application Against an Ever-expanding Spectrum of Diseases
In the late 1800s, the prospering textile industry dramatically increased the dye demands and brought rapid development of the synthetic dye research (Oz et al., 2011). In 1876, methylene blue (MB), an aniline-based dye, was synthesized for cotton staining by Heinrich Caro (Schirmer et al., 2011). Although MB failed to meet the standards of the textile industry, its biological application was soon discovered. Given the ever-expanding drugs derived from MB structure for over 100 years and its broad medical applications for the treatment of an ever-expanding spectrum of diseases, MB is probably by far the most important pharmaceutical lead structure (Ohlow and Moosmann, 2011).
MB is the first lead chemical structure of phenothiazine and its derivatives. In 1883, German chemist Heinrich August Bernthsen synthesized phenothiazine. In 1891, the staining of MB for plasmodia and its effect on malaria were developed by Paul Guttmann and Paul Ehrlich which provided the foundation of modern chemotherapy (Kaufmann, 2008; Parascandola, 1981). In 1890s, MB was administered in psychiatric patients to monitor their compliance, which led to the discovery its antipsychotic effects and the later discovery of chlorpromazine, the first synthetic antipsychotic drug, in 1951 (Ohlow and Moosmann, 2011; Schirmer et al., 2011). In the early 20th century, the antifungal, insecticidal, and anthelmintic activities of phenothiazine, a methylene blue derivative, were discovered (Ohlow and Moosmann, 2011). Since 1930s, intravenous MB has been used as the first-line antidotal agent for methemoglobinemia (Wendel, 1939) and cyanide poisoning (Alston, 2014). Since 1990s, MB has been introduced for the treatment of ifosfamide-induced encephalopathy (Kupfer et al., 1994; Pelgrims et al., 2000; Zulian et al., 1995). In addition, MB has also been used as a tracer for cancer diagnosis and as a photosensitizer for cancer treatment (Chen et al., 2007).
Since 1940s, phenothiazine has been used as an antioxidant for preventing oxidative changes in polyethylene oils (Murphy et al., 1950). The neuroprotective effect of phenothiazine and some of its derivatives have been explored since 1990s (Yu et al., 1992a; Yu et al., 1992b). The protective action of phenothiazine derivatives have been demonstrated in models of ischemic stroke (Yu et al., 1992a) and PD (Mocko et al., 2010). On the other hand, the protective action of MB was discovered more recently.
MB has been known as an electron carrier for more than half century, manifested by its action to accelerate cytochrome c reduction in isolated mitochondria (Weinstein et al., 1964). There is increasing evidence indicated that MB may exist neuroprotective action through mechanisms different from other phenothiazine derivatives (Poteet et al., 2012). Our recent structure-activity relationship study indicated that the cytoprotective effects of MB are distinctive to other phenothiazine derivatives in its action as an alternative mitochondrial electron carrier and as a re-generable anti-oxidant in the mitochondria (Poteet et al., 2012).
5.2. MB as an Alternative Mitochondrial Electron Transfer Carrier
MB has very unique redox property that exists in equilibrium between oxidized state in dark blue (MB) and colorless reduced state (leucomethylene blue), making it both prooxidant and antioxidant under different conditions. The unique redox property of MB might directly contribute to its therapeutic effect on several disorders such as ifosfamide-induced encephalopathy. Although the exact pathophysiological mechanisms underlying the ifosfamide-induced encephalopathy are not known, there is evidence indicated that it might due to the inhibition of mitochondrial respiratory chain by ifosfamide metabolite, chloroethylamine (Kupfer et al., 1996; Pelgrims et al., 2000). For the treatment of methemoglobinemia, MB is reduced to leukomethylene blue by erythrocyte’s methemoglobin reductase, thus, reducing methemoglobin to hemoglobin (Clifton and Leikin, 2003).
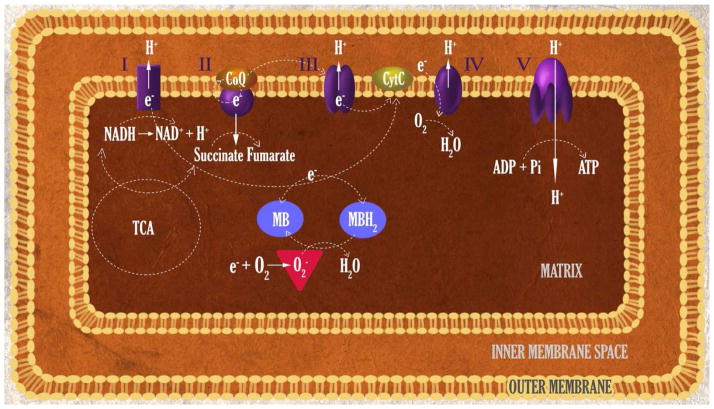
The capability of MB as an electron carrier has long been recognized. MB directly accepts electrons from NADH, NADPH, and FADH2 (Atamna et al., 2008; Buchholz et al., 2008; Dixon, 1971; May et al., 2004; Wen et al., 2011b). MB is able to mediate the electron flow from certain enzymes to cytochrome c under both anaerobic and aerobic conditions (McCord and Fridovich, 1970). Thus, MB might be able to act as an alternative electron transfer carrier that replaces the damaged mitochondrial respiratory complexes. This notion is supported by the fact that MB attenuates rotenone-induced neuropathy in the retina and striatum (Rojas et al., 2009a; Rojas et al., 2009b; Zhang et al., 2006). Consistently, using isolated mitochondria, MB has been demonstrated to enhance mitochondrial ETC complex I, I–III activities, but not complex II–III activities (Atamna et al., 2008; Poteet et al., 2012; Wen et al., 2011a). More importantly, the MB-induced increase of mitochondrial complex I and I–III activities is insensitive to complex I and III inhibition (Atamna et al., 2008; Poteet et al., 2012; Wen et al., 2011b). Furthermore, reduced form MB (MBH2) has been found to be able to deliver the electrons to cytochrome c in the presence of oxygen in mitochondria (Atamna et al., 2008; Wen et al., 2011b). Taken together, there is growing consensus that MB could function as a mitochondrial alternative electron carrier. Upon its redox cycle (MB- MBH2-MB), electrons from NADH are delivered to cytochrome c in an alternate route despite the inhibition of complex I and III (Atamna et al., 2008; Bruchey and Gonzalez-Lima, 2008; Wen et al., 2011b) (Poteet et al., 2012; Rojas et al., 2012) (Figure 2).
Figure 2.
Function of methylene blue (MB) as an alternative mitochondrial electron transfer carrier and regenerable antioxidant. MB accepts electron from NADH in the presence of complex I. Upon the redox cycle (MB- MBH2-MB), electrons are delivered to cytochrome c in an alternate route despite the inhibition of complex I and III. The distinct redox property enables MB as a regenerable anti-oxidant in mitochondria that distinct from the traditional free radical scavenges.
Cytochrome c oxidase, the mitochondrial ETC complex IV, is the terminal enzyme of mitochondrial respiration that reduces oxygen to water coupled pumping proton across the inner mitochondrial membrane (Wallace, 2005). MB has been demonstrated to enhance cytochrome c oxidase activity both in vitro and in vivo (Atamna et al., 2008; Callaway et al., 2004; Gonzalez-Lima and Bruchey, 2004; Poteet et al., 2012; Rojas et al., 2012). It was proposed that a direct interaction between MB and cytochrome c oxidase may underlie the memory-enhancing action of MB (Rojas et al., 2012). On the other hand, the MB-induced increase of cytochrome c oxidase activity might be secondary, at least in part, to the action of MB as an alternative electron carrier that enhances electron transport and increase cytochrome c reduction (Atamna et al., 2008; Poteet et al., 2012). Our recent study demonstrated that 2-chlorophenothiazine, a phenothiazine derivative, has similar neuroprotective action against glutamate-induced oxidative stress in HT-22 cells without increase of oxygen consumption rate. Interestingly, 2-chlorophenothiazine has no effect on cytochrome c oxidase expression and activity suggesting that the action of MB as an alternative mitochondrial electron transport might directly contribute to its effect on cytochrome c oxidase (Poteet et al., 2012).
The action of MB on mitochondrial electron transport is consistent to its action on mitochondrial oxidative phosphorylation. The effect of MB on the oxygen consumption was demonstrated back to 1930s in which MB was found to increase oxygen consumption of normal tissues having aerobic glycolysis and of tumors (Barron, 1930). Using fiber optic oxygen sensor, Riha et al. have found that leucomethylene blue decreased oxygen concentration in rat brain homogenates in vitro (Riha et al., 2005). In addition, oxygen concentration was found to be significantly lower in brain homogenates obtained from rats at 24 hours after MB treatment in vivo (Riha et al., 2005). Using Clark oxygen electrode, Atamna et al. have discovered that MB increased oxygen consumption in fibroblast cells (Atamna et al., 2008). Using Seahorse extracellular flux analyzer and ruthenium fluorescence-lifetime imaging microscopy, we have discovered that MB increased oxygen consumption rate and decreased extracellular acidification rate in both neuronal cells and astrocytes (Poteet et al., 2012; Roy Choudhury et al., 2015). Using multimetric neuroimaging and vascular blood oxygenation measurement, we further observed that acute treatment of MB increased cerebral metabolic rate of oxygen (CMRO2) in rat brains (Lin et al., 2012). The stimulating effect of MB on mitochondrial electron transport is in line with its action on glucose metabolism. In fibroblast cells, MB has been shown to stimulate 2-deoxyglucose uptake (Louters et al., 2006; Roelofs et al., 2006). Using MRI and PET, we demonstrated that acute treatment of MB significantly enhance glucose uptake and increase regional CBF in rats (Lin et al., 2012).
Mitochondrial oxidative phosphorylation is the dominant metabolic pathway in the brain with tight coupling between brain energy supply and expenditure. Thus, MB, as a brain metabolic and hemodynamic enhancer, may improve brain function in physiological condition. The effect of MB on nerve system was explored in late 1880s. Paul Ehrlich found that MB had a selectively affinity to living nerve tissue and was expected to be neurotropic, in Ehrlich’s word (Parascandola, 1981). Indeed, pharmacokinetic study in rat has shown that MB can cross BBB and reach brain at concentrations 10 times higher than that in the circulation (Peter et al., 2000). Since 1970s, MB has been found to improve various experimental memory tasks in rodents (see review (Rojas et al., 2012)). In addition, the potential causative role of brain metabolic dysfunction in both aging and aging-related neurodegenerative disorders indicated that MB might be an ideal candidate for future investigations for the treatment of neurodegenerative diseases.
MB is different from the traditional antioxidants which have failed in all clinical trials for the treatment of neurological disorders. First, the action of MB provides an alternative mitochondrial electron transport route bypass complex I and III inhibition not only preserves mitochondrial oxidative phosphorylation but also attenuates the production of superoxide and reactive oxygen species (Atamna et al., 2008; Poteet et al., 2012; Wen et al., 2011b). Secondly, the distinct redox property enables MB as a regenerable anti-oxidant in mitochondria that distinct from the traditional free radical scavenges (Poteet et al., 2012). In addition, the alternative mitochondrial electron transfer action might not only enable MB to enhance brain metabolism but also to switch the Warburg’s effect, hence, inhibit cancer proliferation.
MB for the Treatment of Neurodegenerative Diseases
MB and AD
Despite decades of drug development for AD, only memantine and 4 cholinesterase inhibitors have been approved for clinical application on AD treatment. However, these approved drugs have shown very limited effect and there is controversy whether they are therapeutically useful (Schneider et al., 2014). MB has recently attracted increasing scientific attention after more than one century of its synthesis. At the 2008 annual meeting of the Alzheimer’s Association, promising results were emerged from a clinical Phase II trial testing MB treatment on the cognitive dysfunction in 332 probable AD patients. Over the course of a year, MB treatment significantly improved cognitive function with an 81% reduction in the rate of cognitive decline as compared with those receiving placebo (Gura, 2008; Oz et al., 2009). Concerns have been raised regarding the drug formulation, the potential underlying tau-dissolving mechanism, the problem with blinding due to that MB turns the urine in blue, and lack of peer-review publications derived from the clinical trial (Gravitz, 2011; Oz et al., 2009). Nonetheless, the MB clinical trial triggered global interests in exploring the effect of MB on AD in both experimental models as well as clinical studies. MB is currently in on global phase III trials for AD and frontotemporal dementia.
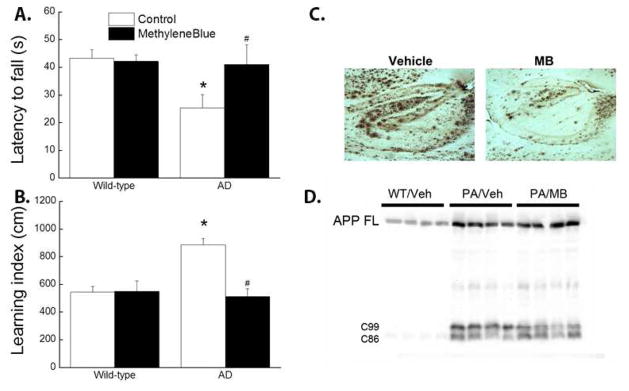
The Phase II clinical trial of MB treatment in AD was based on the early identified tau-dissolving properties of MB in vitro without any publication testing of MB in preclinical animal models. MB, at a higher concentration that may not be able to achieve clinically, blocks tau-tau binding interaction through the repeat domain (Wischik et al., 1996). Similarly, MB, at the concentration of 50 μM, has been demonstrated to reduce tau phosphorylation in organotypic slices derived from transgenic AD mice (Jinwal et al., 2009). Late on, chronic MB administration has been shown to decrease amyloid deposits and neurofibrillary tangles in vivo (Hochgrafe et al., 2015; Hosokawa et al., 2012; Melis et al., 2015). Consistently, we found that 8 months feeding of MB containing diet significantly reduced Aβ plaque and improved water maze and bridge walking performance in 5 X FAD mice (Figure 3).
Figure 3.
Methylene blue feeding improved water-maze and bridge walking performance in 5 X FAD mice. Wild type and 5 X FAD female mice were switched to diets containing MB or vehicle at the age of 8 weeks and continually fed for 8 months. At the age of 10 months, the mice were subjected to batteries of behavioral testing of water-maze and bridge walking as described previously (Shetty et al., 2014). MB diet group had significantly reduced average latency to fall in the bridge walking test as compared to control diet group (A). In water-maze test, MB feeding significantly improved learning index (B). All mice were sacrificed and the effect of MB on brain Aβ plaques were determined. MB feeding decreases Aβ plaque evidenced by 6E10 immunohistochemistry (C) and Western blot analysis (D). # p<0.05 vs Control AD. * p<0.05 vs Control wild-type.
There is increasing evidence that the beneficial effects of MB on cognition decline in AD might be independent of the action on tauopathy. In fact, MB enhances memory function in normal rodents potentially through neurometabolic mechanisms (Rojas et al., 2012). The anti-tau aggregation action of MB requires higher concentrations that may not be able to reach in vivo. MB failed to reduces tau phosphorylation and aggregation in zebrafish expressing mutant human tau (van Bebber et al., 2010). Chronic MB treatment decreases brain Aβ levels and improves learning and memory in an AD mouse model (3 x Tg-AD) without affecting tau pathology (Medina et al., 2011). These studies argue for additional mechanisms underlying the beneficial effect of MB beyond its inhibitory action on the tau aggregation and Aβ plaques. Interestingly, the reduction of tauopathy and amyloid plaque upon chronic MB treatment in AD mice were found to be accompanied by an upregulation of autophagy (Hochgrafe et al., 2015). It has been indicated that elevated autophagic activity prevented protein aggregates in neurons, including tauopathy (Lee et al., 2013). Indeed, MB has been demonstrated to induce autophagy and attenuate tauopathy through inhibition of mTOR signaling both in vitro and in vivo (Congdon et al., 2012). AMPK, a master regulator of cellular energy homeostasis, plays import role in autophagy (Hardie, 2011). MB increases mitochondrial respiration and enhances AMPK signaling which may further activate autophagy (Xie et al., 2013). Taken together, all these studies demonstrated that MB, as an alternative electron carrier, maybe effective for AD therapy.
MB and PD
Levodopa, a dopamine precursor to restore intracerebral dopamine, remains the single most effective treatment for PD. However, levodopa does not affect the disease progression and dyskinesia complicates its treatment in most patients within 5 to 10 years after treatment initiation (Lang and Obeso, 2004a). No treatment so far has been developed that change the progressive course of PD.
There are substantial evidence indicates the involvement of the dysfunction of mitochondrial ETC complexes and oxidative stress in the etiopathogenesis of PD (Abou-Sleiman et al., 2006; Sayre et al., 2008). Consistently, as discussed above, mitochondrial complex I inhibitors such as rotenone and MPTP have been indicated to be involved in the etiology of sporadic PD. The unique action of MB as an alternative mitochondrial electron carrier bypasses the ETC complex I and III, hence, renders it protection against rotenone and MPTP toxicity. In isolated mitochondria, MB preserves respiration despite mitochondrial complex I and III inhibition by rotenone and antimycin, respectively (Poteet et al., 2012; Wen et al., 2011b). In cell cultures, MB protects neuronal HT-22 cells and primary rat retinal ganglion cells against rotenone insult (Daudt et al., 2012; Poteet et al., 2012; Wen et al., 2011b). In rodents, MB has been shown to prevent neurodegeneration induced by intravitreal or intracerebral administration of rotenone in retina and striatum, respectively (Rojas et al., 2009a; Rojas et al., 2009b; Zhang et al., 2006). Experimental animal models of PD using MPTP and rotenone are valuable tools for the development of novel therapeutics. Systemic administration of MPTP or rotenone induces dopaminergic neurodegeneration in Caenorhabditis elegans (Braungart et al., 2004), mouse, rat, and primates (Beal, 2001). MB has been shown to be protective in caenorhabditis elegans models of PD (Mocko et al., 2010). Importantly, our study demonstrated that MB attenuated rotenone-induced loss of nigral dopaminergic neurons and resulted in a better locomotor function in a rat PD model (Wen et al., 2011b). On the other hand, dopamine agonists only rescue locomotor deficit in PD. Therefore, MB might be a superior therapy compared to levodopa that not only ameliorate symptoms but also prevent the dopaminergic neurons degeneration, thus, slow down the disease progression.
MB and HD
HD is a devastating neurodegenerative disease manifested with a typical sporadic, rapid, involuntary limb movement, limbs’ stiffness, psychiatric disturbance, and cognitive impairment (Kumar et al., 2015). The current therapeutics in HD is mainly designed to improve the life quality of the patients by symptom controlling and no disease-modifying treatment is available. It has been indicated that mutant huntingtin impairs mitochondrial respiration, leading to reduction of ATP production, thus, promoting oxidative stress, excitotoxicity, and apoptosis (Kumar et al., 2015). Consequently, mitochondrial respiration represents a potential therapeutic target for HD. Co-enzyme Q10, a mitochondrial ETC complex component, has been shown to enhance mitochondrial activity, delay motor deficits development, decrease weight loss, prevent cerebral atrophy, and extend survival in HD mouse model (Ferrante et al., 2002). Now Co-enzyme Q10 is in phase II clinical trial for HD (Kumar et al., 2015).
The unique function of MB as an alternative mitochondrial electron carrier that enhances mitochondrial respiration may render its therapeutic effect in HD. MB treatment has been found to reduce behavioral phenotypes and delay disease progression in both Drosophila and R6/2 mouse models of HD (Sontag et al., 2012). The aggregation of misfolded Huntingtin has been considered as a main cause of HD pathogenesis (Kim and Kim, 2014). And the protective action of MB on HD was proposed to be secondary to its action on the aggregation of huntingtin variant (Sontag et al., 2012). MB indeed prevented aggregation and deposition of poly-Q-expanded huntingtin in zebrafish at a dose of 10 and 100 μM. However, no protective effect of MB was observed even it almost completely reduced huntingtin aggregates at these concentrations (van Bebber et al., 2010). Similarly, MB blocked aggregation of mutant huntingtin in primary neuron culture at the concentrations of 1, 10, and 100 μM upon acute treatment (Sontag et al., 2012). On the other hand, it took up to 10 day for low dose of MB (100 nM) treatment to decrease oligomer formation of mutant huntingtin. Interestingly, 100 nM MB was found to increase the survival of mutant huntingtin expressing primary neurons by 25% over a time period of 3 days (van Bebber et al., 2010). Therefore, the anti-aggregation effect of MB is likely secondary to the protective action, potentially through its function as an alternative mitochondrial electron carrier.
6.4 MB and FRAD
FRAD is debilitating progressive neurodegenerative disease featured by the progressive ataxia and dysarthria due to the degeneration of the sensory neurons at the dorsal root ganglia, the corticospinal and spinocerebellar tracts of the spinal cord, and the dentate nucleus. FRAD also is associated other non-neurological features as muscle weakness, diabetes, cardiomyopathy and ultimate cardiac failure, which is the most common cause of death in FRAD. The current therapy is limited to the standard treatment for heart failure, arrhythmias, and diabetes mellitus (Pandolfo, 2009). Over the last decade, co-enzyme Q10 and its analogue, idebenone, have been extensively studied for FRAD treatment. So far, the efficacy of co-enzyme Q10 and idebenone on FRAD remains elusive (Parkinson et al., 2013). Co-enzyme Q10 is an essential cofactor of the mitochondrial ETC and its deficiency has been implicated in several neurodegenerative disorders including FRAD (Molyneux et al., 2008). Co-enzyme Q10 transfers electrons from complex I or II to complex III. In addition, the reduced form of co-enzyme Q10 is an antioxidant (Sohal, 2004). As an endogenous mitochondrial ETC component, the effect of co-enzyme Q10 on mitochondrial respiration is greatly limited by the activities of complex II and III. Indeed, co-enzyme Q10 had no effect on basal oxygen consumption rate in isolated mitochondria and neuroblastoma cells (Fink et al., 2009; Sadli et al., 2013).
MB differs from co-enzyme Q10 in its unique action that bypassing mitochondrial ETC complex I to III inhibition and increasing mitochondrial respiration. MB has been shown to be protective against L-buthionine (S,R)-sulfoximine (BSO)-induced oxidative stress in FRAD fibroblast cultures (Richardson et al., 2013). A very recent study demonstrated that MB rescues heart defect in a frataxin-depleted drosophila model of FRDA. Further analysis of MB derivatives indicated that only compounds with electron carrier properties, but not idebenone, were able to prevent the heart dysfunction (Tricoire et al., 2014). Therefore, the alternative mitochondrial electron transferring mechanism may render MB a better therapeutic strategy for the treatment of FRAD.
MB for Cancer Treatment
Targeting Metabolic Signaling and Enzymes for Cancer Therapy
The renewed interest in Warburg effect makes metabolism an emerging hot target for anti-cancer drug discovery (Vander Heiden et al., 2009). Oncogenes and tumor suppressor genes orchestrate many cellular signal pathways that are involved in the metabolic reprogramming of cancer cells. Therapeutic targets on the identified metabolic signaling pathways for anticancer therapies have been exploring. The PI3K/Akt/mTOR signaling has long been recognized as a key pathway downstream of growth factor receptor tyrosine kinase that is involved in cancer cells survival, growth, and metabolism (Cantley, 2002; Shaw and Cantley, 2006). Correspondingly, inhibitors of PI3K/Akt/mTOR signaling pathway have been extensively exploited for cancer therapies (Engelman, 2009). There are concerns that cancer cells may take advantage of signaling redundancy and cross-talk to tackle the anabolic challenge (Logue and Morrison, 2012). Indeed, despite the advance of our understanding of cancer metabolism, our knowledge in the metabolic signaling regulation is incomplete. Further studies are warranted for determine the high plasticity of the metabolic network in cancer. Nonetheless, the complexity nature of the signaling network argues against targeting a single signal component for cancer therapy.
The signature catabolic metabolism and metabolic dependencies of cancer cells have raised interest in targeting metabolic enzymes for cancer treatments (Vander Heiden, 2011). Many inhibitors against key metabolic enzymes have been developed as potential anticancer therapies. The targeted enzymes are distributed cross the wide spectrum of glucose uptake, pentose pathway, glycolysis, TCA cycle, and lipid synthesis including GLUTs, hexokinase, phosphofructose kinase 2, pyruvate kinase M2 (PKM2), pyruvate dehydrogenase, pyruvate dehydrogenase kinase, lactate dehydrogenase, MCT, glutaminase, ATP citrate lyase, monoacylglycerol lipase, and fatty acid synthase (Jones and Schulze, 2012; Vander Heiden, 2011). It is uncertain whether targeting these enzymes provides a sufficient therapeutic window for cancer treatment (Vander Heiden, 2011). And the clinical efficacy of these inhibitors are to be further determined. There are two major obstacles that challenge the anticancer drug development through targeting metabolic enzymes. First, the redundancy of synthetic pathway may impair the efficacy of a drug inhibiting a specific metabolic enzyme for cancer treatment. Luckily, there is increasing evidence that genetic mutations render cancer cells specific metabolic pathways with less redundancy (Vander Heiden, 2011). Nonetheless, inter-tumor genomic instability leads to an increased mutation rate of cancer cells that may further develop drug resistance (Burrell et al., 2013). Second, metabolism, including both catabolic and anabolic pathways, is a key function of normal cells. Identification of specific metabolic enzymes in cancer cells is crucial for drug development that has minimal side effects on normal tissue. Thus, the unacceptable side effect of inhibiting metabolic enzymes in normal proliferating cells will likely be another major challenge for the cancer drug development (Vander Heiden, 2011).
MB Functions as an Alternative Mitochondrial Electron Transfer Carrier for Cancer Therapy
The effect of MB on cell metabolism was studies as early as 1920s. MB was found to increase sugar degradation and diminish lactic acid formation in blood cells, indicating that MB treatment shifts cellular carbohydrate metabolism from anaerobic toward oxidative path (Harrop and Barron, 1928). MB was further found to increase oxygen consumption rate of 19.2 to 116% in human and animal tumors (Barron, 1930). However, without understanding the mechanisms and significance of the Warburg effect, the dramatic effect of MB on cancer metabolism has not caught much attention of cancer researchers.
MB has been found to be selectively accumulated in certain tumors in vivo (Fukui et al., 1983; Gill et al., 1984; Gill and Strauss, 1984). Interestingly, MB also has high affinity to brain and nerve tissues when administered systemically or supravitally (Kristiansen, 1989; Oz et al., 2011; Parascandola, 1981; Peter et al., 2000). Although the mechanism underlying the selectivity of MB to neuronal and cancer tissues is not clear, the high metabolic rate of both tissues indicates that cellular metabolism may play a role in the accumulation of MB in these tissues. Consistently, MB has been found to actively bind to mitochondria and enters the mitochondrial matrix in a manner enhanced by the mitochondrial membrane potential (Gabrielli et al., 2004). The high affinity of MB towards nerve and cancer tissues lead to its application for staining neuronal structures and cancer tissues in both clinical and histochemistry studies (Oz et al., 2011). MB-assisted lymph node dissection has also been introduced in breast cancer and rectal cancer patients (Chen et al., 2007; Markl et al., 2007; Resch and Langner, 2013; Thevarajah et al., 2005).
The electron transfer reaction has been indicated to involve in photosensitizer to initiate radical-induced damage in biomolecules (Cappugi et al., 2001). The unique redox action of MB for reversible electron transfer may confer its action as a photosensitizer. Indeed, MB has been used as a photosensitizer for various cancers treatment, including basal cell carcinoma, melanoma, and Kaposi’s sarcoma (Tardivo et al., 2005).
Mitochondrial ETC sits at the very downstream of anabolic pathway which couples electron transferring with proton pumping cross inner mitochondrial membrane and provides driving force for ATP production. Targeting ETC complexes has been proposed as a possible strategy for cancer therapy (Rohlena et al., 2011). Indeed, some anti-cancer drugs do inhibit components of ETC complexes. For example, tamoxifen, a first-line anti-breast cancer drug, has inhibitory action on mitochondrial complex I (He et al., 2015). The role of mitochondrial ETC complex I inhibition in the anti-cancer effect of tamoxifen is till debatable. Nonetheless, the strategy of inhibiting mitochondrial ETC for cancer therapy may bear unacceptable side effect, given the indispensable role of mitochondrial ETC in ATP production in normal cells. On the other hand, our recent study indicated that enhancement of mitochondrial respiration through alternative mitochondrial electron transfer approach may reverse Warburg effect and, thus, inhibit cancer proliferation (Poteet et al., 2013).
The Warburg effect characterizes the metabolic signature of cancer that cancer cells exhibit a predominate anabolism with a comparably low mitochondrial respiration rate. Interestingly, increase in oxidative phosphorylation has been shown to suppress cancer growth in vitro and in vivo (Schulz et al., 2006). Our recent study provided the proof-of-concept that MB, as an alternative mitochondrial electron carrier, reverses the Warburg effect, attenuates anabolism, and inhibits glioblastoma cells proliferation (Poteet et al., 2013). In glioblastoma cells, MB increase oxygen consumption rate, decrease lactic acid production and extracellular acidification rate, reduce NADPH, and inhibit proliferation (Poteet et al., 2013). These results indicated that MB, as an alternative mitochondrial electron carrier, enhances mitochondrial electron transfer, increases oxidative phosphorylation, decreases glycolytic flux and metabolic intermediates, hence, exhausts the building brick for cancer cell proliferation. Consistently, MB arrests cancer cells at S phase corresponding to a reduction of cyclin A2, B1, and D1 expression (Poteet et al., 2013). We further demonstrated that MB is capable of activating AMPK signal pathway, likely secondary to its bioenergetic action (Poteet et al., 2013). Thus, the direct targeting mitochondria electron transfer may provide further advantage given the potential feedback of cellular metabolites on biosynthetic activities (Metallo and Vander Heiden, 2010; Ward and Thompson, 2012b).
The alternative mitochondrial electron transfer provides a novel mechanism for anti-cancer drug development with many advantage compared with other metabolism targeting strategies. First, targeting metabolic adaptations could be applied to many cancers given that catabolic metabolism as a signature of all cancers. Second, inhibiting cellular signaling and/or metabolic enzymes to attenuate cancer anabolism might not be effective due to the redundancy of metabolic signaling and anabolic pathways. On the other hand, mitochondrial ETC is indispensable for mitochondrial oxidative phosphorylation. Thus, the alternative mitochondrial electron transfer approach enhances mitochondrial respiration may provide a more effective way for cancer therapy. Third, the redundancy of metabolic signaling and anabolic pathways make request targeting multiple metabolic signaling pathways and/or enzymes to increase cancer treatment efficacy, which will likely induce severe side effects as no metabolic signaling and enzyme are unique to cancer cells. The alternative mitochondrial electron transfer strategy differs from the traditional chemotherapeutic agents in that it is less cytotoxic. The increase of mitochondrial electron transfer may reciprocally decrease glycolysis and other anabolic pathways, switch the role of mitochondria from biosynthetic hub to bioenergetic hub, thus, inhibit cancer growth and proliferation. Thus, alternative mitochondrial electron transfer strategy might be a safer anti-cancer therapy approach with less serious side effects.
Conclusion
Neurodegenerative diseases and cancers are among the most leading causes of death and pose the worst threat to public health worldwide (Fitzmaurice et al., 2015; Jin et al., 2015). Due to the declining fertility and increasing life expectancies, the speed of global population aging will accelerate over the coming decades (Lutz et al., 2008). Without a doubt the global burden of neurodegenerative disorders and cancers, both as major aging-related diseases, will continue to increase significantly over the next century. As reiterated in this review, neurodegenerative diseases and cancers may share common mechanistic foundations such as metabolic reprogramming. Accordingly, targeting energy metabolism may provide a one stone two birds strategy for the treatment of both neurodegenerative diseases and cancers. While enforcing brain bioenergetics may enhance brain function hence slow down or prevent the progression of neurodegenerative diseases, switching cancer metabolic phenotype from biosynthetic back to bioenergetic might exhaust building bricks for cancer biomass, thus, inhibit cancers proliferation (Figure 4). As recapitulated in the review, MB functions as an alternative mitochondrial electron transfer carrier and is effective for the treatment of both neurodegenerative diseases and glioblastoma in various experimental models, providing a proof-of-concept that alternative mitochondrial electron transfer might be a common novel therapeutic mechanism for cancers and many neurodegenerative disorders.
Figure 4.
Reprograming energetic metabolism for the treatment of neurodegenerative diseases and brain tumor. Metabolic reprograming may serve as a common mechanistic foundation for neurodegenerative diseases and cancers. Accordingly, targeting energy metabolism may provide a one stone two birds strategy for the treatment both neurodegenerative diseases and cancers. While enforcing brain bioenergetics may enhance brain function hence slow down or prevent the progression of neurodegenerative diseases, switching cancer metabolic phenotype from biosynthetic back to bioenergetic might exhaust building brick for cancer biomass, thus, inhibit cancers proliferation.
As a more than one century old drug, MB has many desirable properties for the treatment of neurodegenerative disorders and cancers, including its well-known pharmacokinetics, the ability to cross the BBB and accumulate in the brain with low toxicity, and the high affinity to both neuronal and cancer tissues (Kupfer et al., 1994; Peter et al., 2000). The effect of MB on neurodegenerative diseases and cancers require further investigations. However, MB is unlikely a magic bullet for all neurodegenerative diseases given the heterogeneous etiopathology of the neurodegenerative disorders. Indeed, MB treatment failed to confer neuroprotection in both SOD1 G93A and TDP-43 mouse models of amyotrophic lateral sclerosis (ALS) (Audet et al., 2012; Lougheed and Turnbull, 2011). Similarly, alternative mitochondrial electron transfer strategy may also have its limitation for cancer treatment. AMPK can exert both anti- and pro-tumorigenic roles in cancer depending on context. Thus, AMPK activation, induced by MB, may provide a survival advantage to cancer cells during chemotherapy by providing them the flexibility to cope the metabolic stress (Faubert et al., 2015).
Highlights.
Reprogramming energetic metabolism is a common feature for neurodegenerative diseases and cancers.
Methylene blue functions as an alternative mitochondrial electron transfer carrier, enhancing bioenergetics and inhibiting biosynthetics.
Methylene blue provides protective effect in rodent models of Parkinson disease, Alzheimer’s disease, Huntington’s disease, and Friedreich’s ataxia.
Methylene blue reverses Warburg’s effect and inhibits cancers proliferation.
Alternative mitochondrial electron transfer may provide a common novel therapeutic mechanism for cancers and neurodegenerative diseases.
Acknowledgments
This work was partly supported by National Institutes of Health grants R01NS054651 (SY), R01NS088596 (SY), American Heart Association grant SDG16960084 (RL).
Abbreviation list
- MB
methylene blue
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- ALS
amyotrophic lateral sclerosis
- HD
Huntington’s disease
- CBF
cerebral blood flow
- GLUT
glucose transporter
- TCA
tricarboxylic acid
- ETC
electron transfer chain
- NADH
dihydronicotinamide adenine dinucleotide
- NADPH
Nicotinamide Adenine Dinucleotide Phosphate Hydrogen
- MCT
monocarboxylate transporter
- AMPK
AMP-activated protein kinase
- ACC
Acetyl-CoA carboxylase
- CaMKKβ
calcium/calmodulin-dependent protein kinase kinase β
- APOE
Apolipoprotein E
- MPTP
1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine
- FRDA
Friedreich’s ataxia
- FXN
frataxin
- mTORC1
mammalian target of rapamycin complex I
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Role of Lipids in Brain Injury and Diseases. Future Lipidol. 2007;2:403–422. doi: 10.2217/17460875.2.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3:1. doi: 10.1186/s40170-015-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicardi G. New hope from an old drug: fighting Alzheimer’s disease with the cancer drug bexarotene (targretin)? Rejuvenation Res. 2013;16:524–528. doi: 10.1089/rej.2013.1497. [DOI] [PubMed] [Google Scholar]
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Alston TA. Why does methylene blue reduce methemoglobin in benzocaine poisoning but beneficially oxidize hemoglobin in cyanide poisoning? J Clin Anesth. 2014;26:702–703. doi: 10.1016/j.jclinane.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, Ames BN. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–712. doi: 10.1096/fj.07-9610com. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Audet JN, Soucy G, Julien JP. Methylene blue administration fails to confer neuroprotection in two amyotrophic lateral sclerosis mouse models. Neuroscience. 2012;209:136–143. doi: 10.1016/j.neuroscience.2011.12.047. [DOI] [PubMed] [Google Scholar]
- Bajaj A, Driver JA, Schernhammer ES. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- Barron ES. The Catalytic Effect of Methylene Blue on the Oxygen Consumption of Tumors and Normal Tissues. J Exp Med. 1930;52:447–456. doi: 10.1084/jem.52.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battino M, Bertoli E, Formiggini G, Sassi S, Gorini A, Villa RF, Lenaz G. Structural and functional aspects of the respiratory chain of synaptic and nonsynaptic mitochondria derived from selected brain regions. J Bioenerg Biomembr. 1991;23:345–363. doi: 10.1007/BF00762227. [DOI] [PubMed] [Google Scholar]
- Bauer DE, Hatzivassiliou G, Zhao F, Andreadis C, Thompson CB. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- Beal MF. Experimental models of Parkinson’s disease. Nat Rev Neurosci. 2001;2:325–334. doi: 10.1038/35072550. [DOI] [PubMed] [Google Scholar]
- Beal MF. Parkinson’s disease: a model dilemma. Nature. 2010;466:S8–10. doi: 10.1038/466S8a. [DOI] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Volterra A. Astrocytes: powering memory. Cell. 2011;144:644–645. doi: 10.1016/j.cell.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Bieberich E. It’s a lipid’s world: bioactive lipid metabolism and signaling in neural stem cell differentiation. Neurochem Res. 2012;37:1208–1229. doi: 10.1007/s11064-011-0698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin MA. The role of mitochondria in ageing and carcinogenesis. Clin Exp Dermatol. 2006;31:548–552. doi: 10.1111/j.1365-2230.2006.02161.x. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, Haspel HC, Rosen OM. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986;83:5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghammer P. Perfusion and metabolism imaging studies in Parkinson’s disease. Dan Med J. 2012;59:B4466. [PubMed] [Google Scholar]
- Borghammer P, Chakravarty M, Jonsdottir KY, Sato N, Matsuda H, Ito K, Arahata Y, Kato T, Gjedde A. Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010;214:303–317. doi: 10.1007/s00429-010-0246-0. [DOI] [PubMed] [Google Scholar]
- Borghammer P, Cumming P, Aanerud J, Gjedde A. Artefactual subcortical hyperperfusion in PET studies normalized to global mean: lessons from Parkinson’s disease. Neuroimage. 2009;45:249–257. doi: 10.1016/j.neuroimage.2008.07.042. [DOI] [PubMed] [Google Scholar]
- Boyer PD, Cross RL, Momsen W. A new concept for energy coupling in oxidative phosphorylation based on a molecular explanation of the oxygen exchange reactions. Proc Natl Acad Sci U S A. 1973;70:2837–2839. doi: 10.1073/pnas.70.10.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braungart E, Gerlach M, Riederer P, Baumeister R, Hoener MC. Caenorhabditis elegans MPP+ model of Parkinson’s disease for high-throughput drug screenings. Neurodegener Dis. 2004;1:175–183. doi: 10.1159/000080983. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Behavioral, Physiological and Biochemical Hormetic Responses to the Autoxidizable Dye Methylene Blue. Am J Pharmacol Toxicol. 2008;3:72–79. doi: 10.3844/ajptsp.2008.72.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz K, Schirmer RH, Eubel JK, Akoachere MB, Dandekar T, Becker K, Gromer S. Interactions of methylene blue with human disulfide reductases and their orthologues from Plasmodium falciparum. Antimicrob Agents Chemother. 2008;52:183–191. doi: 10.1128/AAC.00773-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345. doi: 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Cong WN, Ji S, Rothman S, Maudsley S, Martin B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012;9:5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway NL, Riha PD, Bruchey AK, Munshi Z, Gonzalez-Lima F. Methylene blue improves brain oxidative metabolism and memory retention in rats. Pharmacol Biochem Behav. 2004;77:175–181. doi: 10.1016/j.pbb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Calne DB, Langston JW. Aetiology of Parkinson’s disease. Lancet. 1983;2:1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cappugi P, Campolmi P, Mavilia L, Prignano F, Rossi R. Topical 5-aminolevulinic acid and photodynamic therapy in dermatology: a minireview. J Chemother. 2001;13:494–502. doi: 10.1179/joc.2001.13.5.494. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Smith MA, Perry G, Friedland RP. Cerebral amyloid angiopathy: major contributor or decorative response to Alzheimer’s disease pathogenesis. Neurobiol Aging. 2004;25:599–602. doi: 10.1016/j.neurobiolaging.2003.12.019. discussion 603–594. [DOI] [PubMed] [Google Scholar]
- Chance B, Castor LN. Some Patterns of the Respiratory Pigments of Ascites Tumors of Mice. Science. 1952;116:200–202. doi: 10.1126/science.116.3008.200. [DOI] [PubMed] [Google Scholar]
- Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yan SD. Mitochondrial Abeta: a potential cause of metabolic dysfunction in Alzheimer’s disease. IUBMB Life. 2006;58:686–694. doi: 10.1080/15216540601047767. [DOI] [PubMed] [Google Scholar]
- Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Lin JS, Fong JH, Wang IK, Chou SJ, Wu CH, Lui MT, Chang CS, Kao SY. Use of methylene blue as a diagnostic aid in early detection of oral cancer and precancerous lesions. Br J Oral Maxillofac Surg. 2007;45:590–591. doi: 10.1016/j.bjoms.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Choi JS, Park C, Jeong JW. AMP-activated protein kinase is activated in Parkinson’s disease models mediated by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Biochem Biophys Res Commun. 2010;391:147–151. doi: 10.1016/j.bbrc.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- Clifton J, 2nd, Leikin JB. Methylene blue. American journal of therapeutics. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- Congdon EE, Wu JW, Myeku N, Figueroa YH, Herman M, Marinec PS, Gestwicki JE, Dickey CA, Yu WH, Duff KE. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8:609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F, Suo Z, Fang C, Mullan M. Characteristics of the in vitro vasoactivity of beta-amyloid peptides. Exp Neurol. 1998;150:159–168. doi: 10.1006/exnr.1997.6743. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Damiano M, Galvan L, Deglon N, Brouillet E. Mitochondria in Huntington’s disease. Biochim Biophys Acta. 2010;1802:52–61. doi: 10.1016/j.bbadis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Dang CV. Therapeutic targeting of Myc-reprogrammed cancer cell metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:369–374. doi: 10.1101/sqb.2011.76.011296. [DOI] [PubMed] [Google Scholar]
- Daudt DR, 3rd, Mueller B, Park YH, Wen Y, Yorio T. Methylene blue protects primary rat retinal ganglion cells from cellular senescence. Invest Ophthalmol Vis Sci. 2012;53:4657–4667. doi: 10.1167/iovs.12-9734. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10:767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008a;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008b;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Zoppo GJ. Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture. Stroke. 2013;44:263–269. doi: 10.1161/STROKEAHA.112.653618. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12:284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Maraviglia B, Giove F. Why does the brain (not) have glycogen? Bioessays. 2011;33:319–326. doi: 10.1002/bies.201000151. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. II. Free flavins. Biochim Biophys Acta. 1971;226:259–268. doi: 10.1016/0005-2728(71)90093-4. [DOI] [PubMed] [Google Scholar]
- Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP, Lu KP, Seshadri S, Wolf PA. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci. 2001;16:71–76. doi: 10.1152/physiologyonline.2001.16.2.71. [DOI] [PubMed] [Google Scholar]
- Dunn L, Allen GF, Mamais A, Ling H, Li A, Duberley KE, Hargreaves IP, Pope S, Holton JL, Lees A, Heales SJ, Bandopadhyay R. Dysregulation of glucose metabolism is an early event in sporadic Parkinson’s disease. Neurobiol Aging. 2014;35:1111–1115. doi: 10.1016/j.neurobiolaging.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran J, Tevy MF, Garcia-Rocha M, Calbo J, Milan M, Guinovart JJ. Deleterious effects of neuronal accumulation of glycogen in flies and mice. EMBO Mol Med. 2012;4:719–729. doi: 10.1002/emmm.201200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2003;23:5928–5935. doi: 10.1523/JNEUROSCI.23-13-05928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, Walker Z, Turkheimer FE, Brooks DJ. Microglia, amyloid, and glucose metabolism in Parkinson’s disease with and without dementia. Neuropsychopharmacology. 2013;38:938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Lett. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BD, O’Malley Y, Dake BL, Ross NC, Prisinzano TE, Sivitz WI. Mitochondrial targeted coenzyme Q, superoxide, and fuel selectivity in endothelial cells. PLoS One. 2009;4:e4250. doi: 10.1371/journal.pone.0004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catala-Lopez F, deVeber G, Gotay C, Khan G, Hosgood HD, 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas J, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castaneda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillman T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley P. Lipids in Alzheimer’s disease: A century-old story. Biochim Biophys Acta. 2010;1801:750–753. doi: 10.1016/j.bbalip.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Folmes CD, Park S, Terzic A. Lipid metabolism greases the stem cell engine. Cell Metab. 2013;17:153–155. doi: 10.1016/j.cmet.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Franco-Iborra S, Vila M, Perier C. The Parkinson Disease Mitochondrial Hypothesis: Where Are We at? Neuroscientist. 2015 doi: 10.1177/1073858415574600. [DOI] [PubMed] [Google Scholar]
- Fukui I, Yokokawa M, Mitani G, Ohwada F, Wakui M, Washizuka M, Tohma T, Igarashi K, Yamada T. In vivo staining test with methylene blue for bladder cancer. J Urol. 1983;130:252–255. doi: 10.1016/s0022-5347(17)51088-5. [DOI] [PubMed] [Google Scholar]
- Gabrielli D, Belisle E, Severino D, Kowaltowski AJ, Baptista MS. Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem Photobiol. 2004;79:227–232. doi: 10.1562/be-03-27.1. [DOI] [PubMed] [Google Scholar]
- Gerhart DZ, LeVasseur RJ, Broderius MA, Drewes LR. Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res. 1989;22:464–472. doi: 10.1002/jnr.490220413. [DOI] [PubMed] [Google Scholar]
- Gill WB, Huffman JL, Lyon ES, Bagley DH, Schoenberg HW, Straus FH., 2nd Selective surface staining of bladder tumors by intravesical methylene blue with enhanced endoscopic identification. Cancer. 1984;53:2724–2727. doi: 10.1002/1097-0142(19840615)53:12<2724::aid-cncr2820531230>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gill WB, Strauss FH. In vivo mapping of bladder cancer (chromocystoscopy for in vivo detection of neoplastic urothelial surfaces) Urology. 1984;23:63–66. doi: 10.1016/s0090-4295(84)80071-0. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabo P, Palau F. Mitochondrial pathophysiology in Friedreich’s ataxia. J Neurochem. 2013;126(Suppl 1):53–64. doi: 10.1111/jnc.12303. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Cabo P, Vazquez-Manrique RP, Garcia-Gimeno MA, Sanz P, Palau F. Frataxin interacts functionally with mitochondrial electron transport chain proteins. Hum Mol Genet. 2005;14:2091–2098. doi: 10.1093/hmg/ddi214. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Bruchey AK. Extinction memory improvement by the metabolic enhancer methylene blue. Learn Mem. 2004;11:633–640. doi: 10.1101/lm.82404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, Tsai LH. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J Neurosci. 2013;33:8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandemange S, Herzig S, Martinou JC. Mitochondrial dynamics and cancer. Semin Cancer Biol. 2009;19:50–56. doi: 10.1016/j.semcancer.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Gravitz L. Drugs: a tangled web of targets. Nature. 2011;475:S9–11. doi: 10.1038/475S9a. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Casadesus G, Zhu X, Smith MA, Ashford JW, Johnston JM, Tezapsidis N. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009a;455:191–194. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco SJ, Sarkar S, Johnston JM, Tezapsidis N. Leptin regulates tau phosphorylation and amyloid through AMPK in neuronal cells. Biochem Biophys Res Commun. 2009b;380:98–104. doi: 10.1016/j.bbrc.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser MJ, Myers JA, Boyer PD. Catalytic site cooperativity of beef heart mitochondrial F1 adenosine triphosphatase. Correlations of initial velocity, bound intermediate, and oxygen exchange measurements with an alternating three-site model. J Biol Chem. 1982;257:12030–12038. [PubMed] [Google Scholar]
- Gura T. Hope in Alzheimer’s fight emerges from unexpected places. Nat Med. 2008;14:894. doi: 10.1038/nm0908-894. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrop GA, Barron ES. Studies on Blood Cell Metabolism: I. The Effect of Methylene Blue and Other Dyes Upon the Oxygen Consumption of Mammalian and Avian Erythrocytes. J Exp Med. 1928;48:207–223. doi: 10.1084/jem.48.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Liu R, Yang SH, Yuan F. Chemotherapeutic effect of tamoxifen on temozolomide-resistant gliomas. Anticancer Drugs. 2015;26:293–300. doi: 10.1097/CAD.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenrich KA, Gilmore PR, Garvey WT. Glucose transport in primary cultured neurons. J Neurosci Res. 1989;22:397–407. doi: 10.1002/jnr.490220405. [DOI] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009;109(Suppl 1):10–16. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- Hiltunen JK, Schonauer MS, Autio KJ, Mittelmeier TM, Kastaniotis AJ, Dieckmann CL. Mitochondrial fatty acid synthesis type II: more than just fatty acids. J Biol Chem. 2009;284:9011–9015. doi: 10.1074/jbc.R800068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgrafe K, Sydow A, Matenia D, Cadinu D, Konen S, Petrova O, Pickhardt M, Goll P, Morellini F, Mandelkow E, Mandelkow EM. Preventive methylene blue treatment preserves cognition in mice expressing full-length pro-aggregant human Tau. Acta Neuropathol Commun. 2015;3:25. doi: 10.1186/s40478-015-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Arai T, Masuda-Suzukake M, Nonaka T, Yamashita M, Akiyama H, Hasegawa M. Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS One. 2012;7:e52389. doi: 10.1371/journal.pone.0052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Dunlap SM, Ford NA, Hursting MJ, Lashinger LM. Calorie restriction and cancer prevention: a mechanistic perspective. Cancer Metab. 2013;1:10. doi: 10.1186/2049-3002-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Sugar and Alzheimer’s disease: a bittersweet truth. Nat Neurosci. 2015;18:477–478. doi: 10.1038/nn.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Triiodothyronine (T3) stimulates food intake via enhanced hypothalamic AMP-activated kinase activity. Regul Pept. 2008;151:164–169. doi: 10.1016/j.regpep.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Jakoby P, Schmidt E, Ruminot I, Gutierrez R, Barros LF, Deitmer JW. Higher transport and metabolism of glucose in astrocytes compared with neurons: a multiphoton study of hippocampal and cerebellar tissue slices. Cereb Cortex. 2014;24:222–231. doi: 10.1093/cercor/bhs309. [DOI] [PubMed] [Google Scholar]
- Jin K, Simpkins JW, Ji X, Leis M, Stambler I. The Critical Need to Promote Research of Aging and Aging-related Diseases to Improve Health and Longevity of the Elderly Population. Aging Dis. 2015;6:1–5. doi: 10.14336/AD.2014.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, O’Leary J, Morgan D, Lee DC, Shults CL, Rousaki A, Weeber EJ, Zuiderweg ER, Gestwicki JE, Dickey CA. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012a;2:a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012b;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NP, Schulze A. Targeting cancer metabolism--aiming at a tumour’s sweet-spot. Drug Discov Today. 2012;17:232–241. doi: 10.1016/j.drudis.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, Sun CP, Tao MH, Tu PH, Chang C, Dickson DW, Chern Y. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. J Cell Biol. 2011;194:209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harik SI. Reduced glucose transporter at the blood-brain barrier and in cerebral cortex in Alzheimer disease. J Neurochem. 1989;53:1083–1088. doi: 10.1111/j.1471-4159.1989.tb07399.x. [DOI] [PubMed] [Google Scholar]
- Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A. 2013;110:8882–8887. doi: 10.1073/pnas.1307237110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. Friedreich’s ataxia is a mitochondrial disorder. Proc Natl Acad Sci U S A. 1999;96:10948–10949. doi: 10.1073/pnas.96.20.10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol. 2014;76:185–205. doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- Karthikeyan G, Santos JH, Graziewicz MA, Copeland WC, Isaya G, Van Houten B, Resnick MA. Reduction in frataxin causes progressive accumulation of mitochondrial damage. Hum Mol Genet. 2003;12:3331–3342. doi: 10.1093/hmg/ddg349. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Paul Ehrlich: founder of chemotherapy. Nat Rev Drug Discov. 2008;7:373. doi: 10.1038/nrd2582. [DOI] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF. The Nitrous Oxide Method for the Quantitative Determination of Cerebral Blood Flow in Man: Theory, Procedure and Normal Values. J Clin Invest. 1948;27:476–483. doi: 10.1172/JCI101994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim KT. Therapeutic Approaches for Inhibition of Protein Aggregation in Huntington’s Disease. Exp Neurobiol. 2014;23:36–44. doi: 10.5607/en.2014.23.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Cho HM, Choi SY, Suguira Y, Hayasaka T, Setou M, Koh HC, Hwang EM, Park JY, Kang SJ, Kim HS, Kim H, Sun W. (ADP-ribose) polymerase 1 and AMP-activated protein kinase mediate progressive dopaminergic neuronal degeneration in a mouse model of Parkinson’s disease. Cell Death Dis. 2013;4:e919. doi: 10.1038/cddis.2013.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Braun SM, Zurkirchen L, von Schoultz C, Zamboni N, Arauzo-Bravo MJ, Kovacs WJ, Karalay O, Suter U, Machado RA, Roccio M, Lutolf MP, Semenkovich CF, Jessberger S. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature. 2013;493:226–230. doi: 10.1038/nature11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Korczyn AD. The amyloid cascade hypothesis. Alzheimers Dement. 2008;4:176–178. doi: 10.1016/j.jalz.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Johnson WA. Metabolism of ketonic acids in animal tissues. Biochem J. 1937;31:645–660. doi: 10.1042/bj0310645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen JE. Dyes, antipsychotic drugs, and antimicrobial activity. Fragments of a development, with special reference to the influence of Paul Ehrlich. Dan Med Bull. 1989;36:178–185. [PubMed] [Google Scholar]
- Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kuge Y, Yajima K, Kawashima H, Yamazaki H, Hashimoto N, Miyake Y. Brain uptake and metabolism of [1-11C]octanoate in rats: pharmacokinetic basis for its application as a radiopharmaceutical for studying brain fatty acid metabolism. Ann Nucl Med. 1995;9:137–142. doi: 10.1007/BF03165040. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kumar Singh S, Kumar V, Kumar D, Agarwal S, Rana MK. Huntington’s disease: an update of therapeutic strategies. Gene. 2015;556:91–97. doi: 10.1016/j.gene.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Aeschlimann C, Cerny T. Methylene blue and the neurotoxic mechanisms of ifosfamide encephalopathy. European journal of clinical pharmacology. 1996;50:249–252. doi: 10.1007/s002280050102. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Aeschlimann C, Wermuth B, Cerny T. Prophylaxis and reversal of ifosfamide encephalopathy with methylene-blue. Lancet. 1994;343:763–764. doi: 10.1016/s0140-6736(94)91839-2. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- Lang AE, Obeso JA. Challenges in Parkinson’s disease: restoration of the nigrostriatal dopamine system is not enough. Lancet Neurol. 2004a;3:309–316. doi: 10.1016/S1474-4422(04)00740-9. [DOI] [PubMed] [Google Scholar]
- Lang AE, Obeso JA. Time to move beyond nigrostriatal dopamine deficiency in Parkinson’s disease. Ann Neurol. 2004b;55:761–765. doi: 10.1002/ana.20102. [DOI] [PubMed] [Google Scholar]
- Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard PA., Jr Parkinson’s disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med. 1983;309:310. doi: 10.1056/nejm198308043090511. [DOI] [PubMed] [Google Scholar]
- Lee J, Wolfgang MJ. Metabolomic profiling reveals a role for CPT1c in neuronal oxidative metabolism. BMC Biochem. 2012;13:23. doi: 10.1186/1471-2091-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Lee JH, Rubinsztein DC. Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system. Prog Neurobiol. 2013;105:49–59. doi: 10.1016/j.pneurobio.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Lim WL, Martins IJ, Martins RN. The involvement of lipids in Alzheimer’s disease. J Genet Genomics. 2014;41:261–274. doi: 10.1016/j.jgg.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Lin AL, Poteet E, Du F, Gourav RC, Liu R, Wen Y, Bresnen A, Huang S, Fox PT, Yang SH, Duong TQ. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One. 2012;7:e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang J. Lipid metabolism in Alzheimer’s disease. Neurosci Bull. 2014;30:331–345. doi: 10.1007/s12264-013-1410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi R, Cooper JM, Bradley JL, Manners D, Styles P, Taylor DJ, Schapira AH. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc Natl Acad Sci U S A. 1999;96:11492–11495. doi: 10.1073/pnas.96.20.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue JS, Morrison DK. Complexity in the signaling network: insights from the use of targeted inhibitors in cancer therapy. Genes Dev. 2012;26:641–650. doi: 10.1101/gad.186965.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lougheed R, Turnbull J. Lack of effect of methylene blue in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. PLoS One. 2011;6:e23141. doi: 10.1371/journal.pone.0023141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO, Walters L, Whalen T. Methylene blue stimulates 2-deoxyglucose uptake in L929 fibroblast cells. Life Sci. 2006;78:586–591. doi: 10.1016/j.lfs.2005.05.082. [DOI] [PubMed] [Google Scholar]
- Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E. Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid beta. J Neurosci. 2014;34:12230–12238. doi: 10.1523/JNEUROSCI.1694-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. Glycogen: a Trojan horse for neurons. Nat Neurosci. 2007;10:1341–1342. doi: 10.1038/nn1107-1341. [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78:94–108. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelak M. Sporadic Alzheimer’s disease: the starving brain. J Alzheimers Dis. 2012;31:459–474. doi: 10.3233/JAD-2012-120370. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Calsolaro V, Orsucci D, Siciliano G, Murri L. Is there a primary role of the mitochondrial genome in Alzheimer’s disease? J Bioenerg Biomembr. 2009;41:411–416. doi: 10.1007/s10863-009-9239-1. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Orsucci D, Siciliano G, Murri L. Mitochondria, mitochondrial DNA and Alzheimer’s disease. What comes first? Curr Alzheimer Res. 2008;5:457–468. doi: 10.2174/156720508785908946. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Markl B, Kerwel TG, Wagner T, Anthuber M, Arnholdt HM. Methylene blue injection into the rectal artery as a simple method to improve lymph node harvest in rectal cancer. Mod Pathol. 2007;20:797–801. doi: 10.1038/modpathol.3800824. [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Cobb CE. Reduction and uptake of methylene blue by human erythrocytes. Am J Physiol Cell Physiol. 2004;286:C1390–1398. doi: 10.1152/ajpcell.00512.2003. [DOI] [PubMed] [Google Scholar]
- McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34. doi: 10.1016/j.cmet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A, Hardie DG. AMP-activated protein kinase--a sensor of glycogen as well as AMP and ATP? Acta Physiol (Oxf) 2009;196:99–113. doi: 10.1111/j.1748-1716.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- McCall AL, Van Bueren AM, Nipper V, Moholt-Siebert M, Downes H, Lessov N. Forebrain ischemia increases GLUT1 protein in brain microvessels and parenchyma. J Cereb Blood Flow Metab. 1996;16:69–76. doi: 10.1097/00004647-199601000-00008. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970;245:1374–1377. [PubMed] [Google Scholar]
- McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53:1953–1958. doi: 10.2337/diabetes.53.8.1953. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight SL. On getting there from here. Science. 2010;330:1338–1339. doi: 10.1126/science.1199908. [DOI] [PubMed] [Google Scholar]
- Medina DX, Caccamo A, Oddo S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011;21:140–149. doi: 10.1111/j.1750-3639.2010.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis V, Magbagbeolu M, Rickard JE, Horsley D, Davidson K, Harrington KA, Goatman K, Goatman EA, Deiana S, Close SP, Zabke C, Stamer K, Dietze S, Schwab K, Storey JM, Harrington CR, Wischik CM, Theuring F, Riedel G. Effects of oxidized and reduced forms of methylthioninium in two transgenic mouse tauopathy models. Behav Pharmacol. 2015;26:353–368. doi: 10.1097/FBP.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Vander Heiden MG. Metabolism strikes back: metabolic flux regulates cell signaling. Genes Dev. 2010;24:2717–2722. doi: 10.1101/gad.2010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mocko JB, Kern A, Moosmann B, Behl C, Hajieva P. Phenothiazines interfere with dopaminergic neurodegeneration in Caenorhabditis elegans models of Parkinson’s disease. Neurobiol Dis. 2010;40:120–129. doi: 10.1016/j.nbd.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Molyneux SL, Young JM, Florkowski CM, Lever M, George PM. Coenzyme Q10: is there a clinical role and a case for measurement? Clin Biochem Rev. 2008;29:71–82. [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Morais VA, De Strooper B. Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis. 2010;20(Suppl 2):S255–263. doi: 10.3233/JAD-2010-100345. [DOI] [PubMed] [Google Scholar]
- Moran M, Moreno-Lastres D, Marin-Buera L, Arenas J, Martin MA, Ugalde C. Mitochondrial respiratory chain dysfunction: implications in neurodegeneration. Free Radic Biol Med. 2012;53:595–609. doi: 10.1016/j.freeradbiomed.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63–71. doi: 10.1113/jphysiol.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A, Lovestone S. Alzheimer’s disease-do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Munoz-Pinedo C, El Mjiyad N, Ricci JE. Cancer metabolism: current perspectives and future directions. Cell Death Dis. 2012;3:e248. doi: 10.1038/cddis.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, Ravner H, Smith NL. Mode of Action of Phenothiazine-Type Antioxidants. Industrial & Engineering Chemistry. 1950;42:2479–2489. [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CH, Guan MS, Koh C, Ouyang X, Yu F, Tan EK, O’Neill SP, Zhang X, Chung J, Lim KL. AMP kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in Drosophila models of Parkinson’s disease. J Neurosci. 2012;32:14311–14317. doi: 10.1523/JNEUROSCI.0499-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol. 2012;22:R741–752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Nieweg K, Schaller H, Pfrieger FW. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J Neurochem. 2009;109:125–134. doi: 10.1111/j.1471-4159.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- Nihashi T, Inao S, Kajita Y, Kawai T, Sugimoto T, Niwa M, Kabeya R, Hata N, Hayashi S, Yoshida J. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir (Wien) 2001;143:287–295. doi: 10.1007/s007010170109. [DOI] [PubMed] [Google Scholar]
- Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1-40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A, Rinne JO, Kadir A, Langstrom B. The use of PET in Alzheimer disease. Nat Rev Neurol. 2010;6:78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, Tam S, Kemp BE. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- Obel LF, Muller MS, Walls AB, Sickmann HM, Bak LK, Waagepetersen HS, Schousboe A. Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front Neuroenergetics. 2012;4:3. doi: 10.3389/fnene.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlow MJ, Moosmann B. Phenothiazine: the seven lives of pharmacology’s first lead structure. Drug Discov Today. 2011;16:119–131. doi: 10.1016/j.drudis.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol. 2012;2012:292598. doi: 10.1155/2012/292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van De Moortele PF, Gruetter R. Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab. 2007;292:E946–951. doi: 10.1152/ajpendo.00424.2006. [DOI] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Hasan M, Petroianu GA. Cellular and molecular actions of Methylene Blue in the nervous system. Med Res Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009;78:927–932. doi: 10.1016/j.bcp.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol. 2009;256(Suppl 1):3–8. doi: 10.1007/s00415-009-1002-3. [DOI] [PubMed] [Google Scholar]
- Panov A, Orynbayeva Z, Vavilin V, Lyakhovich V. Fatty acids in energy metabolism of the central nervous system. Biomed Res Int. 2014;2014:472459. doi: 10.1155/2014/472459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parascandola J. The theoretical basis of Paul Ehrlich’s chemotherapy. J Hist Med Allied Sci. 1981;36:19–43. doi: 10.1093/jhmas/xxxvi.1.19. [DOI] [PubMed] [Google Scholar]
- Parkinson MH, Schulz JB, Giunti P. Co-enzyme Q10 and idebenone use in Friedreich’s ataxia. J Neurochem. 2013;126(Suppl 1):125–141. doi: 10.1111/jnc.12322. [DOI] [PubMed] [Google Scholar]
- Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proc Natl Acad Sci U S A. 2014;111:5385–5390. doi: 10.1073/pnas.1403576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prove A, Vermorken JB. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer. 2000;82:291–294. doi: 10.1054/bjoc.1999.0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter C, Hongwan D, Kupfer A, Lauterburg BH. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. European journal of clinical pharmacology. 2000;56:247–250. doi: 10.1007/s002280000124. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Picard M, McEwen BS. Mitochondria impact brain function and cognition. Proc Natl Acad Sci U S A. 2014;111:7–8. doi: 10.1073/pnas.1321881111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- Plun-Favreau H, Lewis PA, Hardy J, Martins LM, Wood NW. Cancer and neurodegeneration: between the devil and the deep blue sea. PLoS Genet. 2010;6:e1001257. doi: 10.1371/journal.pgen.1001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteet E, Choudhury GR, Winters A, Li W, Ryou MG, Liu R, Tang L, Ghorpade A, Wen Y, Yuan F, Keir ST, Yan H, Bigner DD, Simpkins JW, Yang SH. Reversing the Warburg effect as a treatment for glioblastoma. J Biol Chem. 2013;288:9153–9164. doi: 10.1074/jbc.M112.440354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, Wen Y, Yang SH. Neuroprotective actions of methylene blue and its derivatives. PLoS One. 2012;7:e48279. doi: 10.1371/journal.pone.0048279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prolla TA, Mattson MP. Molecular mechanisms of brain aging and neurodegenerative disorders: lessons from dietary restriction. Trends Neurosci. 2001;24:S21–31. doi: 10.1016/s0166-2236(00)01957-3. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111:3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JP, Wu H, Yang Y, Wang DD, Chen YX, Gu YH, Liu T. Cerebral ischemia and Alzheimer’s disease: the expression of amyloid-beta and apolipoprotein E in human hippocampus. J Alzheimers Dis. 2007;12:335–341. doi: 10.3233/jad-2007-12406. [DOI] [PubMed] [Google Scholar]
- Rau TF, Lu Q, Sharma S, Sun X, Leary G, Beckman ML, Hou Y, Wainwright MS, Kavanaugh M, Poulsen DJ, Black SM. Oxygen glucose deprivation in rat hippocampal slice cultures results in alterations in carnitine homeostasis and mitochondrial dysfunction. PLoS One. 2012;7:e40881. doi: 10.1371/journal.pone.0040881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64:93–96. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- Resch A, Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J Gastroenterol. 2013;19:8515–8526. doi: 10.3748/wjg.v19.i46.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson TE, Kelly HN, Yu AE, Simpkins JW. Therapeutic strategies in Friedreich’s ataxia. Brain Res. 2013;1514:91–97. doi: 10.1016/j.brainres.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha PD, Bruchey AK, Echevarria DJ, Gonzalez-Lima F. Memory facilitation by methylene blue: dose-dependent effect on behavior and brain oxygen consumption. Eur J Pharmacol. 2005;511:151–158. doi: 10.1016/j.ejphar.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Roelofs B, Tidball A, Lindborg AE, TenHarmsel A, Vander Kooy TO, Louters LL. Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells. Biochimie. 2006;88:1941–1946. doi: 10.1016/j.biochi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Rohlena J, Dong LF, Ralph SJ, Neuzil J. Anticancer drugs targeting the mitochondrial electron transport chain. Antioxid Redox Signal. 2011;15:2951–2974. doi: 10.1089/ars.2011.3990. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012;96:32–45. doi: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas JC, John JM, Lee J, Gonzalez-Lima F. Methylene blue provides behavioral and metabolic neuroprotection against optic neuropathy. Neurotox Res. 2009a;15:260–273. doi: 10.1007/s12640-009-9027-z. [DOI] [PubMed] [Google Scholar]
- Rojas JC, Simola N, Kermath BA, Kane JR, Schallert T, Gonzalez-Lima F. Striatal neuroprotection with methylene blue. Neuroscience. 2009b;163:877–889. doi: 10.1016/j.neuroscience.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109(Suppl 1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Choudhury G, Winters A, Rich RM, Ryou MG, Gryczynski Z, Yuan F, Yang SH, Liu R. Methylene Blue Protects Astrocytes against Glucose Oxygen Deprivation by Improving Cellular Respiration. PLoS One. 2015;10:e0123096. doi: 10.1371/journal.pone.0123096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadli N, Barrow CJ, McGee S, Suphioglu C. Effect of DHA and coenzymeQ10 against Abeta- and zinc-induced mitochondrial dysfunction in human neuronal cells. Cell Physiol Biochem. 2013;32:243–252. doi: 10.1159/000354433. [DOI] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Haapasalo A, Soininen H, Hiltunen M. AMP-activated protein kinase: a potential player in Alzheimer’s disease. J Neurochem. 2011;118:460–474. doi: 10.1111/j.1471-4159.2011.07331.x. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem Res Toxicol. 2008;21:172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you--methylene blue…. Neurobiol Aging. 2011;32:2325e2327–2316. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33:1493–1499. doi: 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Thierbach R, Voigt A, Drewes G, Mietzner B, Steinberg P, Pfeiffer AF, Ristow M. Induction of oxidative metabolism by mitochondrial frataxin inhibits cancer growth: Otto Warburg revisited. J Biol Chem. 2006;281:977–981. doi: 10.1074/jbc.M511064200. [DOI] [PubMed] [Google Scholar]
- Schurr A. Cerebral glycolysis: a century of persistent misunderstanding and misconception. Front Neurosci. 2014;8:360. doi: 10.3389/fnins.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shetty RA, Ikonne US, Forster MJ, Sumien N. Coenzyme Q10 and alpha-tocopherol reversed age-associated functional impairments in mice. Exp Gerontol. 2014;58:208–218. doi: 10.1016/j.exger.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Yang SH, Stubley L, Day AL, Simpkins JW. Hypoperfusion induces overexpression of beta-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. 2000;853:1–4. doi: 10.1016/s0006-8993(99)02113-7. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Arima H, Watanabe M, Goto M, Banno R, Sato I, Ozaki N, Nagasaki H, Oiso Y. Glucocorticoids increase neuropeptide Y and agouti-related peptide gene expression via adenosine monophosphate-activated protein kinase signaling in the arcuate nucleus of rats. Endocrinology. 2008;149:4544–4553. doi: 10.1210/en.2008-0229. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NR, Bowen DM, Smith CC, Flack RH, Davison AN, Snowden JS, Neary D. Glucose metabolism and acetylcholine synthesis in relation to neuronal activity in Alzheimer’s disease. Lancet. 1980;1:333–336. doi: 10.1016/s0140-6736(80)90884-3. [DOI] [PubMed] [Google Scholar]
- Smith JD. Apolipoprotein E4: an allele associated with many diseases. Ann Med. 2000;32:118–127. doi: 10.3109/07853890009011761. [DOI] [PubMed] [Google Scholar]
- Sohal RS. Coenzyme Q and vitamin E interactions. Methods Enzymol. 2004;378:146–151. doi: 10.1016/S0076-6879(04)78010-6. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sontag EM, Lotz GP, Agrawal N, Tran A, Aron R, Yang G, Necula M, Lau A, Finkbeiner S, Glabe C, Marsh JL, Muchowski PJ, Thompson LM. Methylene blue modulates huntingtin aggregation intermediates and is protective in Huntington’s disease models. J Neurosci. 2012;32:11109–11119. doi: 10.1523/JNEUROSCI.0895-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasic MR, Callaerts P, Norga KK. AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist. 2009;15:309–316. doi: 10.1177/1073858408327805. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart CA, Ross IR, Howell ME, McCurry MP, Wood TG, Ceci JD, Kennel SJ, Wall J. Brain glucose transporter (Glut3) haploinsufficiency does not impair mouse brain glucose uptake. Brain Res. 2011;1384:15–22. doi: 10.1016/j.brainres.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Tai LM, Koster KP, Luo J, Lee SH, Wang YT, Collins NC, Ben Aissa M, Thatcher GR, LaDu MJ. Amyloid-beta pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo. J Biol Chem. 2014;289:30538–30555. doi: 10.1074/jbc.M114.600833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardivo JP, Del Giglio A, de Oliveira CS, Gabrielli DS, Junqueira HC, Tada DB, Severino D, de Fatima Turchiello R, Baptista MS. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn Ther. 2005;2:175–191. doi: 10.1016/S1572-1000(05)00097-9. [DOI] [PubMed] [Google Scholar]
- Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, Hiltunen M, Yang SH, Zhong Z, Shen Y, Simpkins JW, Tanzi RE. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54:721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajah S, Huston TL, Simmons RM. A comparison of the adverse reactions associated with isosulfan blue versus methylene blue dye in sentinel lymph node biopsy for breast cancer. Am J Surg. 2005;189:236–239. doi: 10.1016/j.amjsurg.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J. 2011;434:503–512. doi: 10.1042/BJ20101485. [DOI] [PubMed] [Google Scholar]
- Tricoire H, Palandri A, Bourdais A, Camadro JM, Monnier V. Methylene blue rescues heart defects in a Drosophila model of Friedreich’s ataxia. Hum Mol Genet. 2014;23:968–979. doi: 10.1093/hmg/ddt493. [DOI] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem. 1999;72:1707–1716. doi: 10.1046/j.1471-4159.1999.721707.x. [DOI] [PubMed] [Google Scholar]
- van Bebber F, Paquet D, Hruscha A, Schmid B, Haass C. Methylene blue fails to inhibit Tau and polyglutamine protein dependent toxicity in zebrafish. Neurobiol Dis. 2010;39:265–271. doi: 10.1016/j.nbd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Vance JE. Dysregulation of cholesterol balance in the brain: contribution to neurodegenerative diseases. Dis Model Mech. 2012;5:746–755. doi: 10.1242/dmm.010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, Garcia-Rocha M, Soriano E, Rodriguez de Cordoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol. 2011;121:337–349. doi: 10.1007/s00401-010-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Sadovova N, Ali HK, Duhart HM, Fu X, Zou X, Patterson TA, Binienda ZK, Virmani A, Paule MG, Slikker W, Jr, Ali SF. L-carnitine protects neurons from 1-methyl-4-phenylpyridinium-induced neuronal apoptosis in rat forebrain culture. Neuroscience. 2007;144:46–55. doi: 10.1016/j.neuroscience.2006.08.083. [DOI] [PubMed] [Google Scholar]
- Wang D, Pascual JM, Yang H, Engelstad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956a;124:269–270. [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956b;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012a;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PS, Thompson CB. Signaling in control of cell growth and metabolism. Cold Spring Harb Perspect Biol. 2012b;4:a006783. doi: 10.1101/cshperspect.a006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S. On respiratory impairment in cancer cells. Science. 1956;124:267–269. doi: 10.1126/science.124.3215.267. [DOI] [PubMed] [Google Scholar]
- Weinstein J, Scott A, Hunter FE., Jr The Action of Gramicidin D on Isolated Liver Mitochondria. J Biol Chem. 1964;239:3031–3037. [PubMed] [Google Scholar]
- Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. Journal of Biological Chemistry. 2011a;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH. Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem. 2011b;286:16504–16515. doi: 10.1074/jbc.M110.208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004a;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer’s disease-like tauopathy in female rats. J Biol Chem. 2004b;279:22684–22692. doi: 10.1074/jbc.M311768200. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Simpkins JW. Transient cerebral ischemia induces site-specific hyperphosphorylation of tau protein. Brain Res. 2004c;1022:30–38. doi: 10.1016/j.brainres.2004.05.106. [DOI] [PubMed] [Google Scholar]
- Wendel WB. The Control of Methemoglobinemia with Methylene Blue. J Clin Invest. 1939;18:179–185. doi: 10.1172/JCI101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Nishida Y, Sagare AP, Rege SV, Bell RD, Perlmutter D, Sengillo JD, Hillman S, Kong P, Nelson AR, Sullivan JS, Zhao Z, Meiselman HJ, Wenby RB, Soto J, Abel ED, Makshanoff J, Zuniga E, De Vivo DC, Zlokovic BV. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. 2015;18:521–530. doi: 10.1038/nn.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won JS, Im YB, Kim J, Singh AK, Singh I. Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis. Biochem Biophys Res Commun. 2010;399:487–491. doi: 10.1016/j.bbrc.2010.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Li W, Winters A, Yuan F, Jin K, Yang S. Methylene blue induces macroautophagy through 5′ adenosine monophosphate-activated protein kinase pathway to protect neurons from serum deprivation. Front Cell Neurosci. 2013;7:56. doi: 10.3389/fncel.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253–2261. doi: 10.2337/db14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MJ, McCowan JR, Smalstig EB, Bennett DR, Roush ME, Clemens JA. A phenothiazine derivative reduces rat brain damage after global or focal ischemia. Stroke. 1992a;23:1287–1291. doi: 10.1161/01.str.23.9.1287. [DOI] [PubMed] [Google Scholar]
- Yu MJ, McCowan JR, Thrasher KJ, Keith PT, Luttman CA, Ho PP, Towner RD, Bertsch B, Horng JS, Um SL, et al. Phenothiazines as lipid peroxidation inhibitors and cytoprotective agents. J Med Chem. 1992b;35:716–724. doi: 10.1021/jm00082a012. [DOI] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rojas JC, Gonzalez-Lima F. Methylene blue prevents neurodegeneration caused by rotenone in the retina. Neurotox Res. 2006;9:47–57. doi: 10.1007/BF03033307. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. J Neurochem. 2009;109(Suppl 1):24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel GJ, Han H, Ford AL, Lee JM. Cerebral amyloid angiopathy: progressive disruption of the neurovascular unit. Stroke. 2009;40:S16–19. doi: 10.1161/STROKEAHA.108.533174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulian GB, Tullen E, Maton B. Methylene blue for ifosfamide-associated encephalopathy. N Engl J Med. 1995;332:1239–1240. doi: 10.1056/NEJM199505043321817. [DOI] [PubMed] [Google Scholar]