SUMMARY
A 25kDa SloR metalloregulatory protein in Streptococcus mutans modulates the expression of multiple genes, including the sloABC operon that encodes essential Mn2+ transport and genes that promote cariogenesis. In this study, we report on SloC- and SloR-deficient strains of S. mutans (GMS284 and GMS584, respectively) that demonstrate compromised survivorship compared to their UA159 wildtype progenitor and their complemented strains (GMS285 and GMS585, respectively), when challenged with streptonigrin and/or in growth competition experiments. The results of streptonigrin assays revealed significantly larger zones of inhibition for GMS584 than for either UA159 or GMS585, indicating weakened S. mutans survivorship in the absence of SloR. Competition assays revealed a compromised ability for GMS284 and GMS584 to survive peroxide challenge compared with their SloC- and SloR-proficient counterparts. These findings are consistent with a role for SloC and SloR in S. mutans aerotolerance. We also predicted differential expression of oxidative stress tolerance genes in GMS584 versus UA159 and GMS585 when grown aerobically. The results of qRT-PCR experiments revealed S. mutans sod, tpx, and sloC expression that was up-regulated in GMS584 compared to UA159 and GMS585, indicating that the impact of oxidative stress on S. mutans is more severe in the absence of SloR than in its presence. The results of electrophoretic mobility shift assays indicate that SloR does not bind to the sod or tpx promoter regions directly, implicating intermediaries that may arbitrate the SloR response to oxidative stress.
Keywords: streptococci, dental caries, oxidative stress, SloR
INTRODUCTION
Dental caries disproportionately affect populations in lower socioeconomic groups, marking this infectious disease as an escalating public health disparity concern. To date, over 700 different microbial species have been detected in the human oral cavity (Aas et al., 2005). Among the colonizing streptococci, Streptococcus mutans is considered to be the most cariogenic, and it is the most prevalent species in active carious lesions that localize to the supra- and subgingival margins (Ajdić et al., 2002; Becker et al., 2002). It possesses unique adherence and metabolic attributes, which allow it to compete against other oral streptococci that occupy the same ecological niche in the plaque environment. S. mutans gets its energy by fermenting carbohydrates, many of which are consumed in the diet, to produce lactic acid as a metabolic byproduct (Ajdić et al., 2002; Hamada and Slade, 1980; Smart and Thomas, 1987). It is especially capable of withstanding and proliferating in low pH environments (Dunning et al., 2008; Takahashi-Abbe et al., 2003). A sustained pH in the 4.5 – 5.5 range, owing in part to S. mutans lactic acid production, leads to significant enamel demineralization and subsequent cariogenesis (Banas, 2004; Becker et al., 2002, Featherstone, 2008).
The survival and persistence of S. mutans in the oral cavity also depends on its ability to withstand the toxic effects of reactive oxygen species (ROS), some deriving from Fenton chemistry as part of the bacterial oxidative stress pathway. Damaging ROS include superoxide anion radical (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (HO·) (Cabiscol et al., 2000). ROS can react more readily with biological macromolecules, especially DNA, due to their thermodynamic properties and reduction potentials (Imlay, 2003). In addition to oxygen that is present in the highly variable environment of the oral cavity, there are many streptococcal species that vie to occupy the same ecological niche as S. mutans that produce toxic levels of H2O2 as a competitive strategy. Specifically, the early colonizers S. sanguinis and S. gordonii produce H2O2 during aerobic metabolism at levels ranging from 0.12 mM to 0.20 mM when grown in single-species planktonic cultures (Kreth et al., 2008; Xu et al., 2014). Interestingly, Kreth et al. (2005) noted that S. mutans and S. sanguinis compete for the same niche in solid-state competition cultures, and that their prevalence is mutually exclusive when the former is the primary colonizer, owing to the production of mutacins that can inhibit the growth of peroxigenic species. In sum, S. mutans’ ability to tolerate oxidative stress is essential to its prevalence in the dental plaque biofilm and ultimately to its success as an oral pathogen.
Although S. mutans does not perform oxidative phosphorylation, it can metabolize oxygen via various oxidative stress tolerance pathways. While many bacterial species employ glutathione, cytochromes, heme-containing proteins, and/or catalase to sequester or neutralize toxic ROS, S. mutans lacks the genes and protein machinery necessary to synthesize these protective molecules. Rather, a few of the most studied protein pathways associated with ROS tolerance in S. mutans involve superoxide dismutase (SOD), thiol peroxidase (Tpx), alkyl-hydroperoxide reductase (AhpCF), NADH oxidase (Nox), and a Dps-like peroxide resistance protein (Dpr). Specifically, MnSOD is a manganese-containing metalloenzyme that catalyzes the dismutation of superoxide radical into hydrogen peroxide and oxygen, a function that is important in defending S. mutans against oxygen toxicity. Also contributing to S. mutans aerotolerance is Tpx, an antioxidant enzyme that reduces peroxides to H2O, and Dpr, an iron-binding protein that inhibits Fenton chemistry by scavenging free Fe ions. The Spx transcriptional regulator has also been implicated in the S. mutans oxidative stress response (Galvão et al., 2015), as has the VicRK two-component signal transduction system (Downey et al., 2014). Genes encoding these proteins appear to be ubiquitously present in the genomes of S. mutans UA159, UA130, GS5, LJ23, and NN2025 (Ajdić et al., 2002), but the details of their involvement in the oxidative stress response and how they might be regulated in S. mutans remain to be comprehensively examined.
Intracellular metal ion transport and its regulation are imperative for S. mutans survival, especially in the context of managing ROS. Unlike iron, manganese does not undergo Fenton chemistry but rather plays a protective role against ROS, notwithstanding its role as a cofactor for MnSOD. Divalent manganese and iron share similar sizes and coordination geometries, and when present at high intracellular concentrations, manganese can outcompete unincorporated iron for binding to selective or adventitious cationic binding sites on proteins and/or nucleic acids (Imlay, 2008). This, in turn, could prevent site-specific Fenton reactions that might otherwise cause damage to these essential biomolecules. The S. mutans sloABC gene products comprise a high-affinity Mn-transport system, which also has the ability to transport Fe. Specifically, the sloABC operon encodes the three primary components of a metal-ion ATP-binding cassette (ABC) transport system: an ATP-binding protein (SloA), an integral membrane protein (SloB), and a lipoprotein receptor antigen (LraI) protein (SloC). Immediately downstream is the operon’s associated metalloregulatory protein (SloR) that is transcribed from its own promoter as well as via transcriptional readthrough from the sloABC promoter (Claverys, 2001; Fenno et al., 1995; Higgins et al., 1990; Kitten et al., 2000; Paik et al., 2003; Spatafora et al., 2001). In the present study, we hypothesize that both SloC and SloR are important contributors to S. mutans oxidative stress tolerance given their role in regulating Mn2+ transport across the bacterial cell envelope, and the protective role that Mn2+ plays in S. mutans aerotolerance.
SloR is a 25-kDa transcriptional regulator that shares 35% amino acid sequence identity, and presumably evolutionary homology, with the DtxR metal ion-dependent regulator in Corynebacterium diphtheriae (Kitten et al., 2000; Lee et al., 1997; O’Rourke et al., 2010; Schmitt et al., 1995). SloR represses transcription of the sloABC ABC-transport cassette as well as that of other S. mutans virulence genes, when metal ion concentrations are plentiful (Kitten et al., 2000). The results of DNA microarray and qRT-PCR experiments reveal SloR as a pleiotrophic transcriptional regulator, with Mn2+ as a co-repressor of S. mutans genes that are involved in both metal ion homeostasis and virulence (O’Rourke et al., 2010). We propose that SloR binds to an interrupted palindromic SloR Recognition Element (SRE) comprised of a 42bp consensus sequence that was originally identified through computational analyses (Spatafora et al. 2015). While the SRE is located promoter-proximal to the sloABC operon, our in silico analysis of the S. mutans genome revealed many additional putative SRE sequences that localize either promoter-proximal or -distal to the virulence genes they regulate (O’Rourke et al., 2010). The results of expression profiling experiments also revealed differential transcription of multiple oxidative stress genes in the SloR knockout mutant GMS584 versus the UA159 wild-type progenitor strain, and among them are the S. mutans tpx and sod genes.
The present study describes a regulatory role for SloR in the S. mutans oxidative stress response at the level of phenotype via streptonigrin disk assays and competition experiments with the peroxigenic S. gordonii and S. sanguinis species, and at the level of gene transcription in qRT-PCR studies. We also performed competition assays that support SloC as a contributor to the S. mutans oxidative stress response. Electrophoretic mobility shift assays (EMSA) were conducted to determine whether the impact of SloR on oxidative stress gene transcription is direct. Taken together, these experiments were performed in the interest of promoting dental health, and with a focus on SloR and/or SloC as a candidate target for rational drug design aimed at alleviating and/or preventing S. mutans-induced cariogenesis.
METHODS
Bacterial strains, plasmids and primers
The bacterial strains and plasmids used in this study are presented in Table 1. Oligonucleotide primers are presented in Table 2. Primers designed using MacVector 7.0 software were purchased from Sigma-Aldrich (St. Louis, MO) and are based on the sequence of the S. mutans UA159 genome.
Table 1.
Bacterial strains and plasmids used in this study.
| Strain/Plasmid | Genotype or phenotype | Source or reference |
|---|---|---|
| pER7 | pDL277-derived, harbors wildtype sloC coding sequence and promoter region, Spr |
This study |
| Escherichia coli | F- supE44 ΔlacU169 | Hanahan (1983) |
| DH5α | Φ80dlacZΔM15 hsdR17 recA1 endA1 gyrA96 thi-1 relA |
|
| Streptococcus mutans | ||
| UA159 | Wild-type, serotype c | ATCC 700610 |
| GMS284 | UA159-derived, sloC-deficient, Emr |
This study |
| GMS285 | GMS284 containing wildtype sloC gene in trans on plasmid pER7, Emr, Spr |
This study |
| GMS584 | UA159-derived, sloR-deficient, Emr |
Rolerson et al. (2006) |
| GMS585 | GMS584 containing wildtype sloR gene in trans on plasmid pER4, Emr, Spr |
Rolerson et al (2006) |
| SMCitM | UA159-derived, citM-deficient, Emr | Korithoski et al (2005) |
|
Streptococcus gordonii DL1 |
Wild-type H2O2 -producing primary colonizer |
Kreth et al. (2008) |
|
Streptococcus sanguinis SK36 |
Wild-type H2O2 -producing primary colonizer |
Kreth et al. (2008) |
Table 2.
List of oligonucleotide primers used in this study.
| Assay and primer name |
Nucleotide sequence (5’ to 3’) |
Annealing temp (°C) |
Amplicon size (bp) |
|---|---|---|---|
| cloning | |||
| fimA.LR.BamH1.F fimA.LR.BamH1.R |
CGGGATCCCGCAGGATATGGATAA GTGGATTACTGTG CGGGATCCCGGATCTTCTCATCAC GTTCACTGAGTTC |
52.5 | 1664 |
| PCR lig. mut. | |||
| fimA-SB-pim-P1 | CGCTGGGTGTCATTTTGATTG | 53 | 867 |
| fimA-SB-pim-P2 | GGCCGGCCCGGTAAACCAAGCATT GCCTCC |
||
| fimA-SB-pim-P3 | GGCGCGCCAAAAGTCGGGTGTCTG CG |
53 | 805 |
| fimA-SB-pim-P4 | GCCCTTAGTAGAGAGGTATCGT | ||
| erm.AscI.F |
GGCGCGCCCCGGGCCCAAAATTTG TTTGAT |
52.3 | 860 |
| erm.FseI.R | ATTCTATGAGTCGCTGCCGACTGGC CGGCC |
||
| qRT-PCR | |||
| SC_sod_qRT_F | ATTGATGCTGAAACGATGACCC | 60* | 209 |
| SC_sod_qRT_R | AAGAGTTCCCAGAAAAGAGCGTG | ||
| SC_tpx_qRT_F | AAATACGGTGACACTTGCTGGTAAG | 144 | |
| SC_tpx_qRT_R | TCAATAGATGGCACAACGGTAATC | ||
| SC_dpr_qRT_F | TGGTTCAGGCTTCCTTTATCTGC | 266 | |
| SC_dpr_qRT_R | CTTCCTCATCTGTCACATCAAGACC | ||
| SC_sloC_qRT_F | CGAAGAAGAGGGAACACCAAATC | 186 | |
| SC_sloC_qRT_R | CCAGCCTGTCCTTTTTTAGCAAC | ||
| Hk11.qPCR.F | GCTGGCTAATAATGTCATCAAGC | 88 | |
| Hk11.qPCR.F | CTCAACAGTTACTTCAATCTCCTCC | ||
| EMSA | |||
| MP_sloA_F | ATCGGTGAATCGCACTGTCG | 65 | 364 |
| MP_sloA_R2 | GCCATCAATAAAACTTGTCCCTTC | ||
| recA.Gel.LN.F | CGGTTATCCAAAAGGGCGTATC | 67 | 212 |
| recA.Gel.LN.R | CCTGTTCTCCTGAATCTGGTTGTG | ||
| SC_sod_EMSA_F | GGGGATGATTTCTGTCAAAGCAAG | 67 | 291 |
| SC_sod_EMSA_R | TGTTTTTCAAGAGCCGCATTAGC | ||
| SC_dpr_EMSA_F | CGCAATGGAATAGGGTAACCG | 66 | 434 |
| SC_dpr_EMSA_R | TTTGATAAATCCGCTACAGCCTG | ||
| SC_tpx_EMSA_F | CGTCTGTCAACTATTCGCAATGC | 68 | 388 |
| SC_tpx_EMSA_R | TTTCTTACCAGCAAGTGTCACCG | ||
| JPX_spxA_F | GGGGATGATTTCTGTCAAAGCAAG | 63 | 274 |
| JPC_spxA_R | TGTTTTTCAAGAGCCGCATTAGC |
The annealing temperature for qRT-PCR primers used in the CFX96 system (Bio-Rad) is 60°C. Restriction enzyme sites appear in boldface type.
Bacteriological media and reagents
Streptococcus mutans UA159, S. gordonii DL1, and S. sanguinis SK36 cultures for streptonigrin disk and hydrogen peroxide competition assays were grown as standing cultures at 37°C and 5% CO2 in Todd-Hewitt broth supplemented with 0.3% yeast extract (THYE). THYE broth was supplemented with erythromycin (10 µg/ml) when growing S. mutans GMS284 and GMS584 cultures, and with spectinomycin (1,200 µg/ml) when growing GMS285 and GMS585 cultures. E. coli DH5α transformants were grown on Luria agar plates supplemented with 100 µg/ml spectinomycin. For RNA isolation, S. mutans cultures were grown with aeration at 37°C in 50-ml Falcon tubes (Fisher Scientific, Pittsburgh, PA) on a Thermo Scientific Vari-Mix continuously rocking platform (Fisher Scientific, Pittsburgh, PA) or as standing cultures at 37°C and 5% CO2 in 15-ml Falcon tubes (Fisher Scientific, Pittsburgh, PA).
Construction of a S. mutans sloC-deficient mutant
An established PCR ligation mutagenesis approach was used, as described previously by Rolerson et al (2006), to disrupt the S. mutans sloC coding sequence on the UA159 chromosome. Specifically, primers fimA-SB-pim-P1-P4 (Table 2) were used to amplify the 5’ and 3’ ends of the sloC gene from the S. mutans UA159 chromosome, generating a 575bp gap in the coding sequence. An erm.AscI.F and erm.FseI.R primer pair was used in parallel to amplify an 860bp erythromycin resistance cassette from the genome of SMCitM, a citM deficient strain of UA159 (Table 1). The resulting amplicons were digested with AscI and FseI and ligated so as to position the antibiotic cassette 215bp downstream of the sloC ATG start codon. The ligation mixture was then used to transform S. mutans UA159 in the presence of 150µg of competence-stimulating peptide (CSP). Chromosomal DNA, isolated from selected transformants grown on THYE-erm plates, was used as a template for PCR and subsequent nucleotide sequencing with primers fimA-SB-pim-P1 and fimA-SB-pim-P4 to confirm sloC disruption by allelic exchange. The resulting sloC insertion-deletion mutant was named GMS284.
Complementation of the sloC mutation in S. mutans GMS284
Plasmid pER7 (Table 1) was constructed by cloning a 1.8kb amplicon containing the sloC coding sequence and its upstream regulatory region into the BamH1 site of plasmid pDL277 using primers fimA.LR.BamH1.F and fimA.LR.BamH1.R (Table 2). The resulting recombinant was transformed into E. coli DH5α and transformants were selected on Luria agar plates supplemented with spectinomycin. Plasmid DNA isolated with a mini-prep spin kit (Qiagen) was then used to complement the sloC mutation in S. mutans GMS284 in trans. Transformation occurred in the presence of CSP as described above, and transformants resistant to spectinomycin were selected on THYE-spec agar plates. The complemented strain was named GMS285.
Streptonigrin disk assays
Streptonigrin is a quinone antibiotic that promotes the formation of toxic oxygen radicals that can cause damage to nucleic acid, thereby accounting for its bactericidal action. Disk assays with this antibiotic were adapted from the Kirby-Bauer disk-diffusion method (Bauer et al., 1966). Briefly, sterilized filter paper disks were impregnated with 5 µg streptonigrin from Streptomyces flocculus (Sigma-Aldrich, St. Louis, MO). S. mutans cultures were incubated overnight as described previously and diluted to OD600 = 0.40 ± 0.05 before swabbing for confluency on THYE (UA159), THYE-erm (GMS584), or THYE-spec (GMS585) plates. Two streptonigrin disks were then positioned on each plate prior to incubation for 22 hours at 5% CO2 and 37°C. Zones of inhibition (ZOI) were quantified by measuring the diameter of the clear zone surrounding each streptonigrin disk. ANOVA analyses comparing average ZOI values were performed using the statistical software package SPSS.
Hydrogen peroxide competition assays on agar plates
All S. mutans, S. gordonii, and S. sanguinis overnight cultures were standardized to an OD600 = 0.40 ± 0.05. The peroxigenic primary colonizers S. gordonii and S. sanguinis were then serially diluted and spot-inoculated (8 µl) onto THYE agar plates without antibiotic selection. Control plates were prepared in parallel to confirm that growth inhibition was indeed due to H2O2 production, by spotting 8µl of catalase (0.75µg/µl) directly on top of the primary colonizer. After aerobic incubation at 37°C and 5% CO2 for 16 hours, (conditions that favor H2O2 production), 8 µl of a standardized S. mutans UA159, GMS284, GMS285, GMS584 or GMS585 overnight culture was spot inoculated adjacent to the primary colonizer such that the spot cultures nearly touched. These “sequential competition cultures” were incubated aerobically at 37°C and 5% CO2 for 22 hours. Growth inhibition was assessed by the presence of a proximal zone of inhibition between the primary and secondary colonizers. Zones of inhibition (ZOIs) between the pioneer culture (ie. Streptococcus gordonii or Streptococcus sanguinis) and Streptococcus mutans were measured and expressed as a ratio of the ZOI (in mm) divided by the distance between the spotted inocula. These values were then normalized against the ZOI ratio for UA159 to facilitate comparisons across experimental conditions.
Co-culture competition plates were prepared on THYE agar plates such that the serially diluted peroxigenic species (S. gordonii or S. sanguinis) and the aforementioned S. mutans cultures were spot inoculated (8 µl) simultaneously. All co-culture plates were incubated aerobically at 37°C and 5% CO2 for 22 hours.
RNA isolation and cDNA synthesis
Total RNA was isolated from S. mutans according to a modified protocol from Chen et al. (1998). Aerated and control S. mutans UA159 and GMS584 cultures were grown to mid-logarithmic-phase (OD600 = 0.40-0.80) as described previously, then centrifuged at 11,000 × g for 5 min prior to resuspending the cell pellet in RNAprotect bacterial reagent (Qiagen) according to the recommendations of the supplier. Cultures were centrifuged again as described above, and the cell pellet was resuspended in 250 µl of 50 mM Tris-10 mM EDTA buffer. The cell suspensions were transferred to separate lysing matrix B tubes (MP Biomedicals) containing zirconium beads, 10 µl of 10% SDS and 300 µl of acid phenol. Cell disruption proceeded by placing the tubes in a FastPrep-24 mechanical disrupter (MP Biomedicals) for two rounds of 30 s disruptions with intermittent chilling on ice. The cell slurry was centrifuged at 17,000 × g and 4°C in a Sorvall Legend Micro 21R centrifuge (Thermo-Scientific) for 10 min. 200 µl of the RNA-containing supernatant was DNase I treated and purified on an RNeasy column (Qiagen) according to the instructions provided by the manufacturer. Total RNA was examined for integrity on a 1% agarose gel and adjusted to a final concentration of 100 ng/µl using a NanoDrop Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The total intact RNAs were then reverse transcribed using a Revert-Aid First-Strand cDNA Synthesis Kit (Thermo-Scientific) according to the manufacturer’s instructions. Reaction mixtures without RNA served as no-template controls (NTC), and those without reverse transcriptase were included as negative controls (RT−).
Quantitative reverse transcription PCR (qRT-PCR)
Real-time quantitative reverse transcription PCR (qRT-PCR) was performed with the cDNA products described above (diluted 1:39) as the templates. Amplification was performed in a CFX96 real-time system with EvaGreen as the intercalating dye (Bio-Rad, Hercules, California) and 500 nM sod, dpr, tpx, sloC, or hk11 primers (see Table 2). RT− and NTC controls were run simultaneously with the same primers for each cDNA template to verify the absence of contaminating genomic DNA. The PCR thermal cycling program was set as follows: 95°C for 3 min followed by 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 15 s. Primer efficiency was calculated using a standard curve with three 5X serial dilutions of UA159 control cDNA, and each amplification was performed in triplicate for N = 3 independent experiments. Expression of the sod, tpx, dpr, and sloC genes was normalized against that of hk11, an endogenous control gene with steady state expression under the experimental test conditions. Expression under aerated conditions was further normalized relative to the expression in control cultures to allow for direct comparison between the S. mutans UA159 wild-type and GMS584 SloR-deficient strains.
EMSA
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (Haswell et al., 2013). Primers were designed to amplify the promoter regions of the S. mutans UA159 sod, tpx, and spxA1 genes and to include their predicted promoter-proximal SREs (O’Rourke et al., 2010). Specifically, the 291bp sod probe included 91 nucleotides upstream and 171 nucleotides downstream of the promoter, whereas the 388bp tpx probe included 283 nucleotides upstream and 75 nucleotides downstream of the promoter. A 274bp probe was used to reveal SloR binding to the spxA locus. This probe included 136 nucleotides upstream and 102bp downstream of the predicted promoter. The sloABC promoter region was also included in these experiments as a positive control and an internal recA sequence devoid of palindromes was used as a negative control (Table 2). Q5 High-Fidelity DNA Polymerase (New England BioLabs) and the accompanying supplier’s protocol, including the online Tm calculator (“NEBtools: Tm Calculator, v.1.7.2,” n.d.), were used to PCR amplify the SloR target sequences for gel shift. PCR amplicons were purified and concentrated using the QIAquick PCR Purification Kit (Qiagen) according to the supplier’s recommendations, then examined for integrity and amplicon size on a 1% agarose gel. The resultant PCR products were end-labeled with 10 µCi [γ-32P]ATP using 10 U T4 polynucleotide kinase (New England BioLabs) according to established protocols. The reaction mixture was incubated at 37°C for 30 min and then at 70°C for 20 min. The final volume of the samples was brought to 50 µl with sterile nuclease-free H2O before passing through a TE Select-D G-25 spin column (Roche Applied Science) to remove unincorporated radiolabel. Binding reactions were performed in a 16-µl total reaction volume and incubated at 25°C for 20 min. The reactions contained: 1 µl of end-labeled DNA of interest (approximately 2 picomol), purified SloR protein prepared previously (Haswell et al., 2013) at varying concentrations (10 nM, 60nM, or 100nM) , and 3.2 µl of 5X binding buffer (42 mM NaH2PO4, 58 mM Na2HPO4, 250 mM NaCl, 25 mM MgCl2, 50 µg/ml bovine serum albumin, 1 mg sonicated salmon sperm DNA, 50% [vol/vol] glycerol, and 37.5 µM MnCl2). Specific reaction conditions are described in the figure legends. EDTA was added at a final concentration of 15 mM to select reaction mixtures to confirm that SloR binding was metal ion- dependent. The binding reaction mixtures were resolved on 6% nondenaturing polyacrylamide gels (3 ml 20X bis-Tris borate [pH 7.70 ± 0.05], 74 µl 100 mM MnCl2, 1.5 ml 100% glycerol, 43 ml Millipore H2O, 12 ml 30% acrylamide [37.5:1 acrylamide-bis], 300 µl 15% freshly prepared ammonium persulfate [APS], and 90 µl TEMED [N,N,N’,N’-tetramethylethylenediamine]) for 80 min at 300 V. All gels were live-loaded under 40 V of electric current. Carestream BIOMAX XAR Film was used for autoradiography for up to 22 h at −80°C in the presence of an intensifying screen.
RESULTS
Differential susceptibility of S. mutans UA159, GMS584, and GMS585 to streptonigrin
To investigate a role for SloR in S. mutans oxidative stress tolerance, we performed disk assays with streptonigrin as the oxidative stressor (N = 5 independent experiments, each plated in triplicate). The average ZOI for UA159, GMS584, and GMS585 was significantly different according to one-way analysis of variance (ANOVA) (df = 58, P < 0.01) and the post-hoc Bonferroni correction method (P < 0.01), with greatest susceptibility demonstrated by the SloR-deficient GMS584 strain. Notably, GMS584 demonstrated a significantly larger average ZOI (4.03 ± 0.019 cm) compared to UA159 (3.85 ± 0.014 cm) and GMS585 (3.72 ± 0.015 cm).
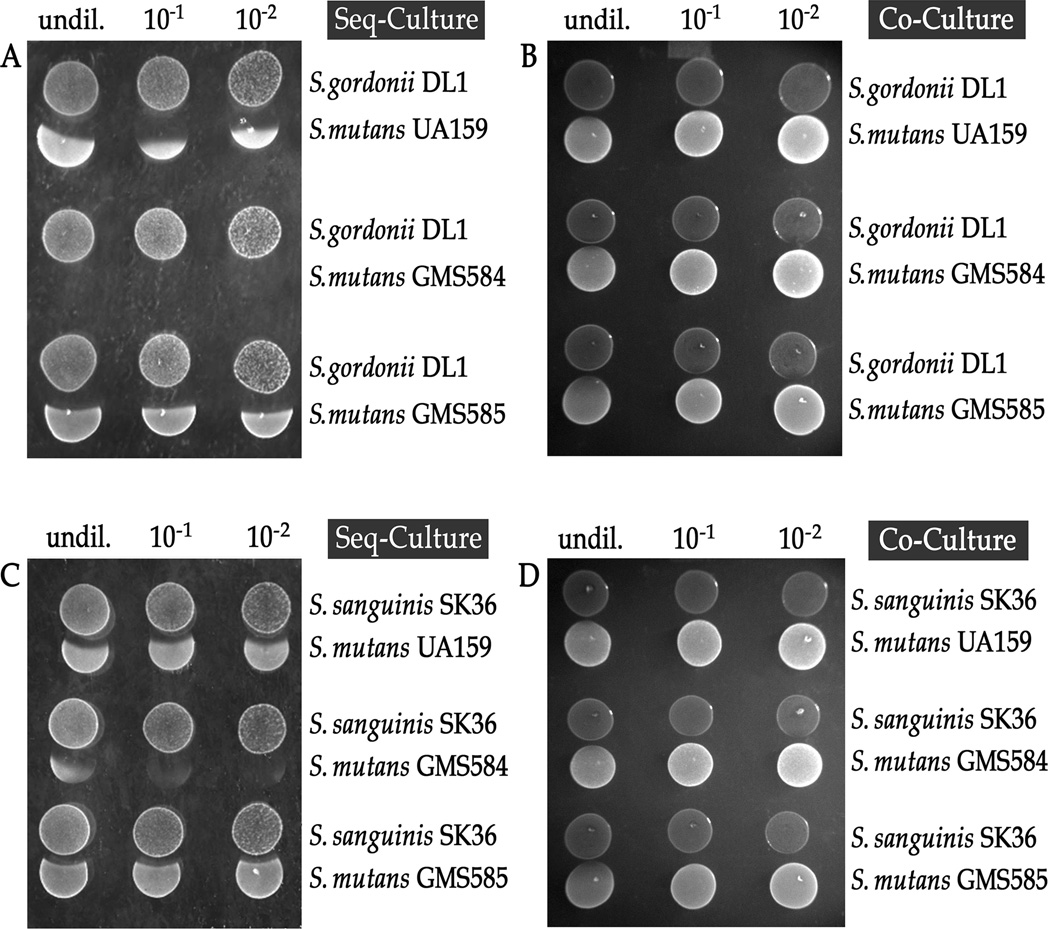
Increased susceptibility of S. mutans GMS584 (SloR-) and GMS284 (SloC-) to physiological H2O2 generated by S. gordonii and/or S. sanguinis
To further explore a role for SloR in S. mutans oxidative stress tolerance, we spot inoculated S. mutans UA159, GMS584, and GMS585 next to the peroxigenic niche competitors S. gordonii and S. sanguinis. The SloR-deficient, GMS584 strain demonstrated compromised survivorship compared to UA159 and GMS585 when challenged with the H2O2 producing competitor strains (Figs. 1A and 1C). In contrast, control studies performed in the presence of catalase revealed no zones of inhibition, indicating that H2O2 was the sole stressor in these experiments, and that S. gordonii and S. sanguinis peroxigenesis was responsible for S. mutans growth inhibition (data not shown). The results of co-culture experiments in which the peroxigenic and S. mutans strains were inoculated at the same time similarly confirmed the absence of a H2O2 impact on S. mutans growth, concurrent with the serial dilution of S. gordonii and S. sanguinis (Fig. 1B and 1D).
Fig. 1.
The SloR-deficient S. mutans GMS584 strain demonstrates compromised survivorship compared to the UA159 wild-type and GMS585 SloR-proficient strains (normalized ZOI ratios = 1.3, 1.0, and 0.84, respectively) when spot inoculated adjacent to streptococcal H2O2 producers., S. mutans was grown overnight to stationary phase and the bacterial concentration was standardized to OD600 = 0.400 ± 0.05 for subsequent spot inoculation., Streptococcus gordonii or S. sanguinis stationary phase cultures grown to OD ∼0.400 ± 0.05 were serially diluted (in triplicate) by a factor of 10 (N = 5 independent experiments). Shown are representative culture competition assays in which either S. gordonii or S. sanguinis was spot inoculated and grown for 16 hours before inoculating the adjacent S. mutans strains (A and C) or all strains were spot inoculated simultaneously (B and D). The S. mutans GMS584 SloR-deficient strain demonstrated the greatest susceptibility to H2O2 challenge.
To reveal the effect of Mn2+ on oxidative stress tolerance and identify the SloC metal ion permease as another important contributor to S. mutans aerotolerance, we repeated the competition assays described above with S. mutans UA159 and its isogenic GMS284 sloC-deficient mutant, grown in the presence of S. gordonii. Under these test conditions, the permease-deficient mutant was significantly more sensitive to the effects of H2O2 than either the wildtype strain or the GMS285 complement (Fig. S1), indicating that the SloC Mn2+ transporter is important for S. mutans growth under conditions of oxidative stress, and that Mn2+ serves as an important protectant against the deleterious effects of intracellular ROS.
Transcription of sod, tpx and sloC is increased in the absence of SloR and under conditions of oxidative stress
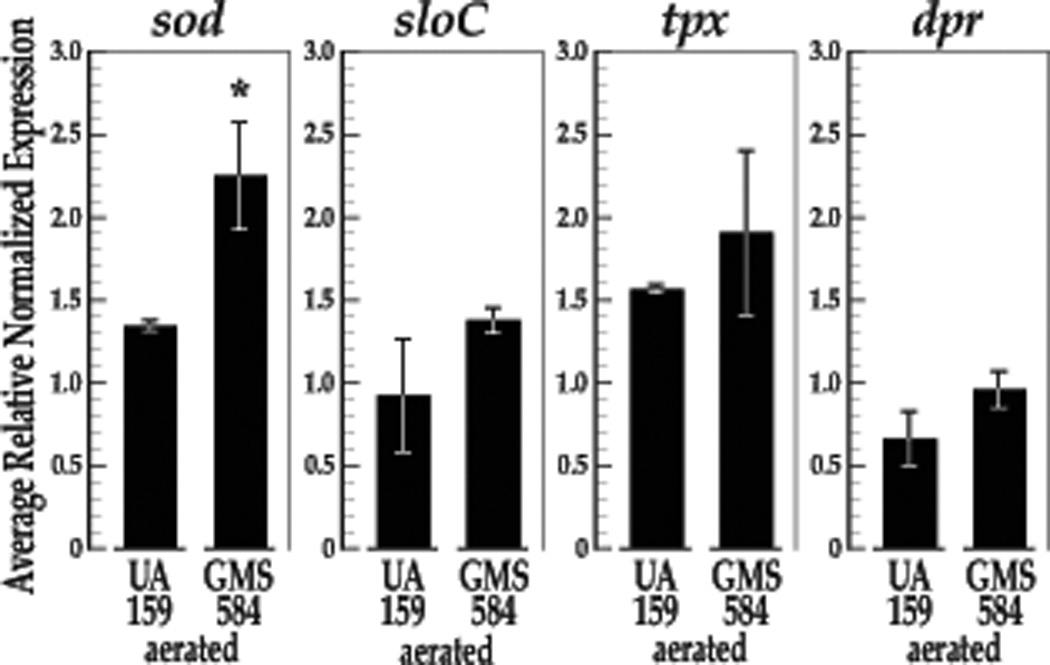
To reveal the impact of SloR on oxidative stress gene transcription and to determine whether sloC is co-regulated with genes known to contribute to S. mutans oxidative stress tolerance, we performed qRT-PCR experiments to monitor the expression of the sod, tpx, dpr and sloC genes in continuously aerated S. mutans cultures. Importantly, S. mutans UA159 and GMS584 demonstrate comparable doubling times (56.35 minutes for UA159 and 57.5 minutes for GMS584) when grown with aeration. Not surprisingly, we observed a significant increase in sod gene transcription under these test conditions (P = 0.050, Fig. 2) and similar expression trends for dpr, tpx, and sloC transcription. While the latter trends were not significant in independent t-test comparisons, the results of a Cohen’s d test revealed a large effect for SloR on sloC expression (Cohen’s d = 1.22) and a moderate effect for SloR on tpx expression (Cohen’s d = 0.55) (Fig. 3). The effect for SloR on dpr expression was unremarkable.
Fig. 2.
The results of qRT-PCR experiments reveal increased transcription of the S. mutans sod, tpx, and sloC genes in the SloR-deficient GMS584 strain compared to the UA159 wild-type SloR-proficient progenitor strain UA159 under conditions of oxidative stress. Gene expression was normalized against that of an endogenous hk11 control gene. Expression deriving from continuously aerated S. mutans cultures was normalized against that of control cultures of the same strain to allow for expression comparisons between UA159 and GMS584 (N = 3 independent experiments, with each expression level measured in triplicate). An independent samples t-test revealed significant up-regulation of sod gene expression in GMS584 compared to UA159 under conditions of continuous aeration compared to controls (p = 0.050, df = 4). Expression trends for tpx and sloC were similar and remarkable in a Cohen’s d test, demonstrating increased transcription in the SloR-deficient mutant compared to the SloR-proficient wild-type progenitor. Error bars represent the standard error of the mean (SEM) for normalized gene expression.
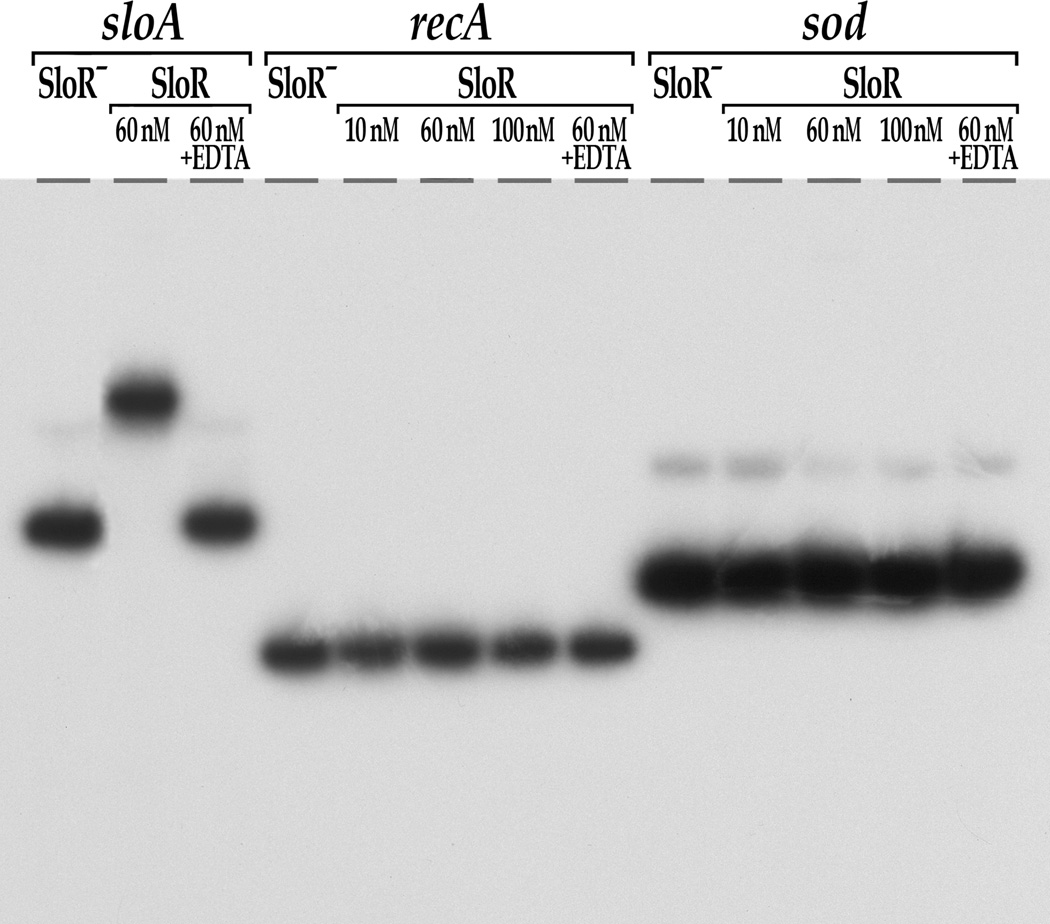
Fig. 3.
SloR shifts an SRE-containing promoter fragment that precedes the sloABC operon (positive control) but not the sod promoter region or a recA negative control. Shown are the results of EMSA performed on a 6% non-denaturing polyacrylamide gel with [γ-32P] end-labeled sloA-, recA- or sod-specific target sequences and varying concentrations of SloR. Protein-DNA complexes were resolved for a total of 350 volt-hours. Taken together, these findings indicate that the impact of SloR on the sod oxidative stress gene is indirect. The addition of EDTA abrogated the sloABC band shift, consistent with metal ion-dependent SloR binding.
SloR does not bind directly to the sod or tpx promoter regions
In previous reports we describe significant de-repression of the sloABC genes in the S. mutans SloR-deficient GMS584 strain, and direct SloR binding to the sloABC promoter region which harbors a 42bp promoter proximal SRE (O’Rourke et al., 2010; Rolerson et al., 2006; Spatafora et al., 2002). To determine whether the impact of SloR on sod and tpx transcription is the result of direct SloR binding to the predicted SREs in the promoter regions of these genes, we performed EMSA experiments with sod, and tpx promoter fragments with sufficient coverage to include their predicted SREs. In parallel, we used a 364bp sloABC promoter fragment with its resident 42bp SRE as a positive control for SloR binding, and an internal 212bp recA sequence that is devoid of any palindromes as a negative control (Table 2). At concentrations as low as 60nM, SloR shifted the SRE that localizes to the sloABC promoter but did not shift the recA control sequence or the promoter regions of the sod and tpx genes (Fig. 3 and Fig. 4). In fact, even when provided in 40-fold molar excess, SloR failed to shift the sod and tpx promoter regions, as well as the recA control (data not shown). Addition of the chelating agent EDTA to the sloABC containing reaction mixture abrogated the band shift, indicating that SloR binding to this promoter is metal ion-dependent.
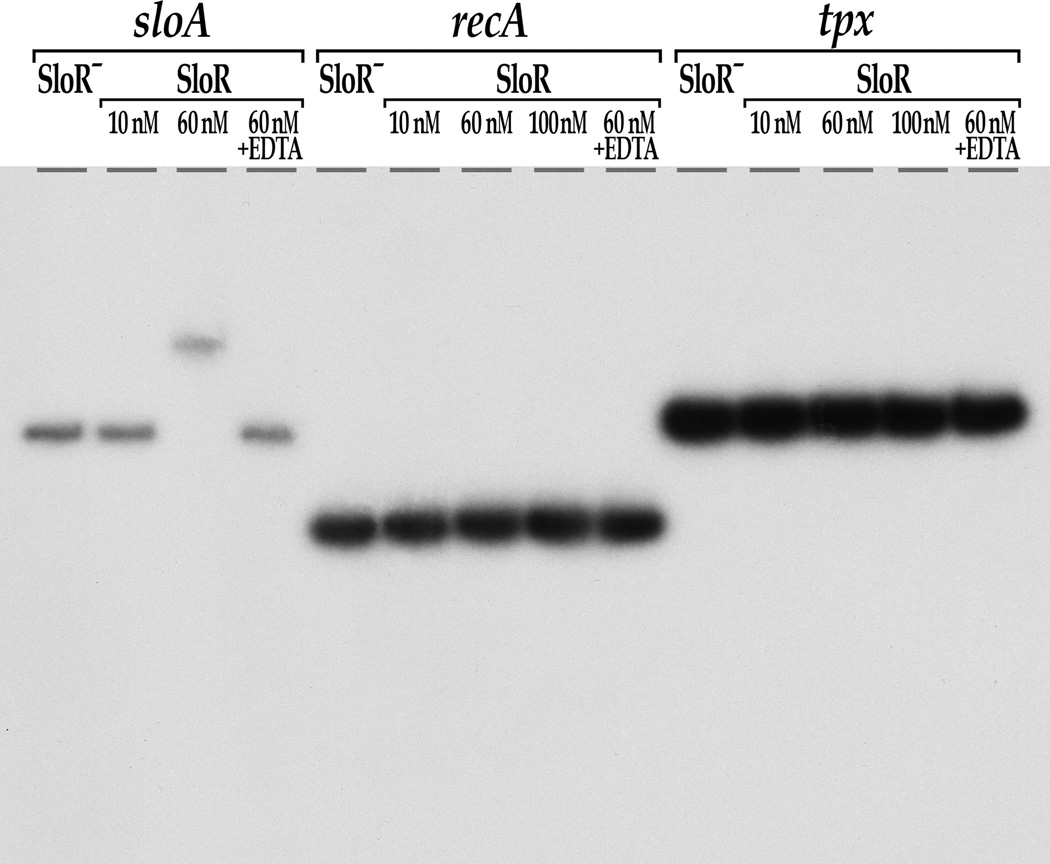
Fig. 4.
SloR shifts an SRE-containing promoter fragment that precedes the sloABC operon (positive control) but not the tpx promoter region or a recA negative control. Shown are the results of EMSA performed on a 6% non-denaturing polyacrylamide gel with a [γ-32P] end-labeled tpx-specific target sequence and varying concentrations of SloR. Protein-DNA complexes were resolved for a total of 350 volt-hours. Similar results were generated when SloR was provided in 40 molar excess (data not shown). These findings indicate that the impact of SloR on the tpx oxidative stress gene is indirect. The addition of EDTA abrogated the sloABC band shift, consistent with metal ion-dependent SloR binding at this locus.
SloR binds to the spxA promoter region
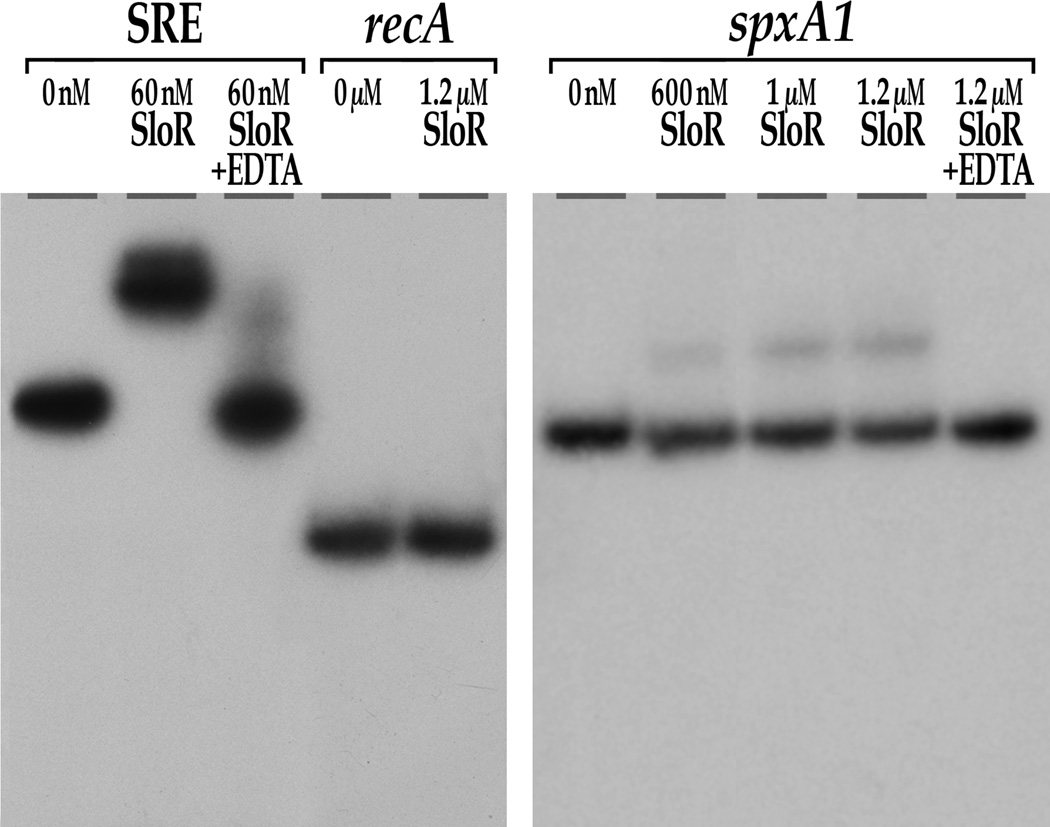
To determine if the impact of SloR on S. mutans oxidative stress gene transcription involves the SpxA1 transcriptional regulator as a putative intermediary, we performed EMSA experiments to reveal whether SloR binds directly to a 274bp spxA1 promoter region that harbors a putative SRE. The results of these studies support direct SloR binding to the spxA1 promoter at concentrations equal to or greater than 600nM (Fig. 5). SloR shifted only 24% of the total spxA1 target sequence available for binding however, suggesting that SloR’s affinity for this promoter is considerably lower than its affinity for the sloABC promoter. Addition of the EDTA metal ion chelator abrogated the SloR-spxA1 band shift, consistent with DNA binding that is metal ion-dependent. Importantly, no band shift was ever observed for the recA negative control sequence, even at SloR concentrations as high as 3µM.
Fig. 5.
SloR shifts a promoter fragment that precedes the S. mutans spxA1 gene. Shown are the results of EMSA performed on a 6% non-denaturing polyacrylamide gel with a 274bp [γ-32P] end-labeled spxA1-specific target sequence and varying concentrations of SloR. SloR-spxA1 complexes were resolved for a total of 350 volt-hours. SloR binds directly to the spxA1-containing DNA fragment when provided at concentrations of 600nM or greater. This binding was abrogated upon addition of the metal ion-chelating agent, EDTA.
DISCUSSION
Conditions in the human oral cavity are highly variable, especially with respect to pH, osmolarity, and oxygen content that fluctuate throughout the dental plaque biofilm. The role of metalloregulatory proteins in streptococcal ROS sensing and adaptation to oxidative stress remains relatively unexplored in the literature, with only a few exceptions. The Fur-like PerR response regulator in Streptococcus pyogenes is among the most extensively studied metalloregulators in the streptococci, and its impact on dpr transcription, iron homeostasis, and the oxidative stress response in the GAS is well established (Grifantini et al., 2011; Ricci et al., 2002). A PerR homolog has also been described in the economically important Streptococcus suis pathogen where it plays a crucial role in H2O2-resistance (Zhang et al., 2012). A perR deletion in S. suis gave rise to a mutant that was highly resistant to peroxide challenge, the likely result of derepressed dpr transcription according to the investigators. A PerR homolog is also present in S. mutans enabling it to respond quickly and effectively to ROS. As in S. suis, the S. mutans PerR homolog has been associated with resistance to H2O2 stress via its impact on dpr expression (Fujishima et al., 2013). According to this same report, PerR had relatively little impact on S. mutans sod expression however, and its effect on tpx transcription was not investigated.
In the present study, we proposed a role for the DtxR-like SloR protein and the SloC Mn2+ transporter that is subject to its control in S. mutans oxidative stress tolerance, and hypothesized that these proteins will have a significant impact on sod and tpx transcription, and perhaps a moderate impact on PerR-regulated dpr expression. In fact, our experimental findings supported a role for both SloR and SloC in S. mutans oxidative stress tolerance. Specifically, the SloR-deficient S. mutans GMS584 strain demonstrated significantly compromised survivorship compared to its wild-type UA159 progenitor and the SloR-complemented mutant GMS585, when challenged with streptonigrin as the oxidative stressor, or with endogenous H2O2 produced by its niche competitors S. gordonii or S. sanguinis. Growth of the GMS284 sloC mutant was similarly compromised in competition growth assays. Moreover, the impact of SloR on sod, tpx, and sloC transcription in S. mutans cultures grown under conditions of continuous aeration lend further support to a regulatory role for SloR in oxidative stress tolerance, given that the effect of oxygen stress on expression of these genes was more severe in the absence of SloR than in its presence. We recognize that de-repression of tpx and sloC transcription approached but did not reach statistical significance in an independent t-test, but note that the absence of SloR did indeed have a large to moderate impact on tpx and sloC expression (and no impact on dpr expression) according to a Cohen’s d analysis. We believe the redundancy of oxidative stress tolerance pathways in S. mutans involving the gene products of nox, and ahpCF for instance (both of which are subject to PerR control), could compensate for and hence mask true differences in tpx and sloC gene transcription in the GMS584 SloR-deficient strain when oxygen stressed (Ajdić et al., 2002). Alternatively, S. mutans growth with aeration will up-regulate the expression of oxidative stress genes in the UA159 wild-type strain, and hence this could mask significant differences in tpx, dpr and sloC gene transcription when compared with the mutant. That dpr is primarily controlled by the PerR response regulator might explain why we observed little if any impact on its transcription in aerated GMS584 cultures.
A central player in S. mutans oxidative stress tolerance is MnSOD, an enzyme that provides protection against toxic superoxide anions (Jakuobovics et al. 2002). An increase in S. mutans MnSOD activity in the wake of aerobic stress would be supported by the increase in sod expression that we describe herein. Since S. mutans does not produce a catalase however, protection against the H2O2 byproduct of superoxide anion detoxification would likely derive from an inherent thiol peroxidase. In fact, we observed heightened tpx transcription in the SloR-deficient GMS584 strain and propose that the S. mutans tpx gene product likely coordinates its protective response to oxidative stress with SOD under conditions of aerobiosis. In previous work we described similar upregulation of a tpx homolog in Streptococcus parasanguis cultures when challenged with H2O2 as the environmental stressor (Fenno et al., 1995; Spatafora et al., 2002).
The results of our EMSA experiments support an indirect mechanism for SloR regulation of sod and tpx transcription. Specifically, SloR did not shift these promoter regions that presumably harbor their associated SREs in these EMSA studies, but it did shift the SRE-containing sloABC promoter fragment at concentrations as low as 60nM, as well as the spxA1 promoter region when SloR was provided in 10-fold molar excess. Quantification of the band shifts by pixel counting revealed that 60nM SloR was capable of shifting 100% of the total sloABC target sequence that was available for binding (Figs. 3 and 4) whereas 600nM SloR was needed to shift up to 24% of the total available spxA1 target sequence (Fig. 5). These findings support SloR binding with lower affinity to the spxA1 promoter when compared with SloR’s binding affinity for the sloABC promoter. Importantly however, band shifts for the sloABC and spxA1 containing fragments were abolished upon addition of the EDTA metal ion chelator, consistent with SloR-DNA binding that is metal ion dependent. Whether the 600nM concentration of SloR required to shift the spxA1 promoter reflects physiological conditions for SloR-DNA binding in vivo is not known, although previous reports in the literature describe binding of the DtxR metalloregulator to so-called “DtxR boxes” in the promoter regions of the Corynebacterium diphtheria tox, sidA and htaA genes at protein concentrations in the 250nM range (Kunkle and Schmitt, 2003).
In light of these findings, it is tempting to suggest that control of the S. mutans sod and tpx genes by SloR may involve SpxA1 as an intermediary. SpxA has been the focus of recent research that revealed the oxidation of SpxA as having a positive effect on S. mutans sod, tpx, dpr, nox, gor, trxA (thioredoxin), trxB (thioredoxin reductase), and ahpCF transcription (Baker et al., 2014; Kajfasz et al., 2010). This increase in transcription is further supported by an increased susceptibility of SpxA-deficient mutants to oxidative stress (Kajfasz et al., 2010). Accordingly, it has been proposed that SpxA may function as a positive regulator of oxidative stress by interacting with the RNA polymerase α-subunit, since it lacks a DNA-binding domain (Galvão et al., 2015). SpxA also contains a highly conserved Cys-X-X-Cys motif in its N-terminal domain, which is likely the redox-sensing control region of the protein (Nakano et al., 2005). Future investigations merit more powerful computational analysis of the spxA1 promoter region for potential consensus SloR binding sites since they represent A-T rich palindromic sequences in a genome with less than 40% G-C content. Expression profiling experiments are also warranted to determine whether SpxA might have an impact on S. mutans sloR transcription.
Additional reports implicate the VicRK two-component signal transduction system in S. mutans oxidative stress tolerance (Downey et al., 2014). For instance, when paraquat was used as the oxidative stressor, expression of the S. mutans vicRK genes was significantly up-regulated (Senadheera et al., 2007), possibly owing to cross-regulatory networks between SloR and VicRK (Downey et al. 2014). Manganese is the common denominator that is shared by the VicK and SloR regulatory networks, and ROS are known to induce transcription of multiple S. mutans virulence and oxidative stress tolerance genes. Hence, cross-communication between the Mn- and ROS-sensing VicRK/SloR systems could also account for the indirect impact that SloR has on sod and tpx transcription in the present study. Future work should explore this cross talk to elucidate whether VicRK and possibly other intermediaries play a role in regulating S. mutans oxidative stress genes that are subject to SloR control.
SloR functions as a situational metalloregulator of S. mutans virulence genes
The large number of cellular processes involved in S. mutans oxidative stress tolerance speaks not only to the vital importance of this response for cell survival, but also to the many contributions that each system brings to this survival mechanism. Using a microarray platform, the results of which have been corroborated by qRT-PCR, Ahn et al. (2007) revealed that about 5% of genes in the S. mutans genome displayed differential expression in response to aeration. Notably, substantially more genes were upregulated, including sod and tpx, than were down-regulated. Expression profiling studies conducted previously in our laboratory support the regulation of multiple S. mutans virulence genes by SloR, including but not limited to those that contribute to amino acid biosynthesis, energy metabolism, signal transduction and gene regulation (O’Rourke et al., 2010). Combined with the present study, we propose SloR as a situational regulator that can respond to a variety of different conditions in the plaque environment. That is, SloR may modulate the expression of ROS tolerance genes early during S. mutans colonization of the tooth surface when S. mutans is challenged with H2O2 deriving from its peroxigenic S. gordonii or S. sanguinis niche competitors. Later on during the cariogenic process when S. mutans is growing exponentially and assuming a biofilm lifestyle, SloR might switch its regulatory target genes to include those that are specific to energy metabolism and sucrose-dependent adherence. From sod and tpx whose gene products directly address intracellular ROS, to the SpxA transcription factor and its connection to SloR as a metalloregulator, the varied and coordinated response to oxidative stress in this oral pathogen is clearly complex and has yet to be fully elucidated. Nevertheless, an improved understanding of the mechanisms by which SloR regulates the S. mutans oxidative stress response can bolster our ability to exploit interspecies interactions in the plaque biofilm to reduce S. mutans-induced cariogenesis.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health (NIH), grant R01 DE014711 to G.A.S., by the Ostro Family Molecular Biology and Biochemistry Endowment for Undergraduate Summer Research Fund, and by the Middlebury College Department of Biology.
We thank Gary Nelson for figure preparation, Patrick Monette and Shelby Redfield for their valuable contributions to scientific discussion, and Frank Spatafora and Timothy Allen for technical assistance.
REFERENCES
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S-J, Wen ZT, Burne RA. Effects of Oxygen on Virulence Traits of Streptococcus mutans. J. Bacteriol. 2007;189:8519–8527. doi: 10.1128/JB.01180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdić D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JL, Derr AM, Karuppaiah K, et al. Streptococcus mutans NADH Oxidase Lies at the Intersection of Overlapping Regulons Controlled by Oxygen and NAD+ Levels. J. Bacteriol. 2014;196:2166–2177. doi: 10.1128/JB.01542-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas JA. Virulence properties of Streptococcus mutans. Front. Biosci. J. Virtual Libr. 2004;9:1267–1277. doi: 10.2741/1305. [DOI] [PubMed] [Google Scholar]
- Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Becker MR, Paster BJ, Leys EJ, et al. Molecular Analysis of Bacterial Species Associated with Childhood Caries. J. Clin. Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2000;3:3–8. [PubMed] [Google Scholar]
- Chen YY, Weaver CA, Mendelsohn DR, Burne RA. Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J. Bacteriol. 1998;180:5769–5775. doi: 10.1128/jb.180.21.5769-5775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys J-P. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 2001;152:231–243. doi: 10.1016/s0923-2508(01)01195-0. [DOI] [PubMed] [Google Scholar]
- Downey JS, Mashburn-Warren L, Ayala EA, et al. In vitro Manganese-Dependent Cross-Talk between Streptococcus mutans VicK and GcrR: Implications for Overlapping Stress Response Pathways. PLoS One. 2014;9:e115975. doi: 10.1371/journal.pone.0115975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning DW, McCall LW, Powell WF, et al. SloR modulation of the Streptococcus mutans acid tolerance response involves the GcrR response regulator as an essential intermediary. Microbiology. 2008;154:1132–1143. doi: 10.1099/mic.0.2007/012492-0. [DOI] [PubMed] [Google Scholar]
- Featherstone JD. Dental caries: a dynamic disease process. Aust. Dent. J. 2008;53:286–291. doi: 10.1111/j.1834-7819.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- Fenno JC, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol. Microbiol. 1995;15:849–863. doi: 10.1111/j.1365-2958.1995.tb02355.x. [DOI] [PubMed] [Google Scholar]
- Fujishima K, Kawada-Matsuo M, Oogai Y, Tokuda M, Torii M, Komatsuzawa H. dpr and sod in Streptococcus mutans Are Involved in Coexistence with S. sanguinis, and PerR Is Associated with Resistance to H2O2. Appl. Environ. Microbiol. 2013;79:1436–1443. doi: 10.1128/AEM.03306-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão LCC, Miller JH, Kajfasz JK, et al. Transcriptional and Phenotypic Characterization of Novel Spx-Regulated Genes in Streptococcus mutans. PLoS One. 2015;10:e0124969. doi: 10.1371/journal.pone.0124969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifantini R, Toukoki C, Colaprico A, Gryllos I. Peroxide stimulon and role of PerR in group A Streptococcus. J. Bacteriol. 2011;193:6539–6551. doi: 10.1128/JB.05924-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980;44:331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Haswell JR, Pruitt BW, Cornacchione LP, Coe CL, Smith EG, Spatafora GA. Characterization of the Functional Domains of the SloR Metalloregulatory Protein in Streptococcus mutans. J. Bacteriol. 2013;195:126–134. doi: 10.1128/JB.01648-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins CF, Hyde SC, Mimmack MM, Gileadi U, Gill DR, Gallagher MP. Binding protein-dependent transport systems. J. Bioenerg. Biomembr. 1990;22:571–592. doi: 10.1007/BF00762962. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajfasz JK, Rivera-Ramos I, Abranches J, et al. Two Spx Proteins Modulate Stress Tolerance, Survival, and Virulence in Streptococcus mutans. J. Bacteriol. 2010;192:2546–2556. doi: 10.1128/JB.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitten T, Munro CL, Michalek SM, Macrina FL. Genetic Characterization of a Streptococcus mutans LraI Family Operon and Role in Virulence. Infect. Immun. 2000;68:4441–4451. doi: 10.1128/iai.68.8.4441-4451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korithoski B, Krastel K, Cvitkovitch D. Transport and metabolism of citrate by Streptococcus mutans. J. Bacteriol. 2005;187:4451–4456. doi: 10.1128/JB.187.13.4451-4456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Competition and Coexistence between Streptococcus mutans and Streptococcus sanguinis in the Dental Biofilm. J. Bacteriol. 2005;187:7193–7203. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, Herzberg MC. Streptococcal Antagonism in Oral Biofilms: Streptococcus sanguinis and Streptococcus gordonii Interference with Streptococcus mutans. J. Bacteriol. 2008;190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Wang T, Ault K, Liu J, Schmitt MP, Holmes RK. Identification and characterization of three new promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. Infect. Immun. 1997;65:4273–4280. doi: 10.1128/iai.65.10.4273-4280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Erwin KN, Ralle M, Zuber P. Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol. Microbiol. 2005;55:498–510. doi: 10.1111/j.1365-2958.2004.04395.x. [DOI] [PubMed] [Google Scholar]
- NEBtools: Tm Calculator, v.1.7.2 [WWW Document], n.d. . N. Engl. Biolabs. [(accessed 1.10.15)]; URL http://tmcalculator.neb.com/#!/
- O’Rourke KP, Shaw JD, Pesesky MW, et al. Genome-Wide Characterization of the SloR Metalloregulome in Streptococcus mutans. J. Bacteriol. 2010;192:1433–1443. doi: 10.1128/JB.01161-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S, Brown A, Munro CL, Cornelissen CN, Kitten T. The sloABCR Operon of Streptococcus mutans Encodes an Mn and Fe Transport System Required for Endocarditis Virulence and Its Mn-Dependent Repressor. J. Bacteriol. 2003;185:5967–5975. doi: 10.1128/JB.185.20.5967-5975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci S, Janulczyk R, Björck L. The regulator PerR is involved in oxidative stress response and iron homeostasis and is necessary for full virulence of Streptococcus pyogenes. Infect. Immun. 2002;70:4968–4976. doi: 10.1128/IAI.70.9.4968-4976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolerson E, Swick A, Newlon L, et al. The SloR/Dlg Metalloregulator Modulates Streptococcus mutans Virulence Gene Expression. J. Bacteriol. 2006;188:5033–5044. doi: 10.1128/JB.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MP, Predich M, Doukhan L, Smith I, Holmes RK. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect. Immun. 1995;63:4284–4289. doi: 10.1128/iai.63.11.4284-4289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera MD, Lee AWC, Hung DCI, Spatafora GA, Goodman SD, Cvitkovitch DG. The Streptococcus mutans vicX gene product modulates gtfB/C expression, biofilm formation, genetic competence, and oxidative stress tolerance. J. Bacteriol. 2007;189:1451–1458. doi: 10.1128/JB.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart JB, Thomas TD. Effect of Oxygen on Lactose Metabolism in Lactic Streptococci. Appl. Environ. Microbiol. 1987;53:533–541. doi: 10.1128/aem.53.3.533-541.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatafora G, Moore M, Landgren S, Stonehouse E, Michalek S. Expression of Streptococcus mutans fimA is iron-responsive and regulated by a DtxR homologue. Microbiology. 2001;147:1599–1610. doi: 10.1099/00221287-147-6-1599. [DOI] [PubMed] [Google Scholar]
- Spatafora G, Van Hoeven N, Wagner K, Fives-Taylor P. Evidence that ORF3 at the Streptococcus parasanguis fimA locus encodes a thiol-specific antioxidant. Microbiol. Read. Engl. 2002;148:755–762. doi: 10.1099/00221287-148-3-755. [DOI] [PubMed] [Google Scholar]
- Spatafora G, Corbett J, Cornacchione L, et al. Interactions of the metalloregulatory protein SloR from Streptococcus mutans with its metal ion effectors and DNA binding site. J. Bacteriol. 2015;197:3601–3615. doi: 10.1128/JB.00612-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Abbe S, Abe K, Takahashi N. Biochemical and functional properties of a pyruvate formate-lyase (PFL)-activating system in Streptococcus mutans. Oral Microbiol. Immunol. 2003;18:293–297. doi: 10.1034/j.1399-302x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Itzek A, Kreth J. Comparison of genes required for H2O2 resistance in Streptococcus gordonii and Streptococcus sanguinis. Microbiology. 2014;160:2627–2638. doi: 10.1099/mic.0.082156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Ding Y, Li T, et al. A Fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis. BMC Microbiol. 2012;12:85. doi: 10.1186/1471-2180-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.