Abstract
Millions of people die of infectious diseases each year, mostly in developing countries, which could largely be prevented by the use of vaccines. While immunization rates have risen since the introduction of the Expanded Program on Immunization (EPI), there remain major challenges to more effective vaccination in developing countries. As a possible solution, microneedle patches containing an array of micron-sized needles on an adhesive backing have been developed to be used for vaccine delivery to the skin. These microneedle patches can be easily and painlessly applied by pressing against the skin and, in some designs, do not leave behind sharps waste. The patches are single-dose, do not require reconstitution, are easy to administer, have reduced size to simplify storage, transportation and waste disposal, and offer the possibility of improved vaccine immunogenicity, dose sparing and thermostability. This review summarizes vaccination challenges in developing countries and discusses advantages that microneedle patches offer for vaccination to address these challenges. We conclude that microneedle patches offer a powerful new technology that can enable more effective vaccination in developing countries.
Graphical abstract
1. Barriers to vaccination in developing countries
According to 2014 WHO estimates, 1.5 million children die each year from vaccine-preventable diseases for which there are vaccines recommended by the WHO and 29% of deaths among children 1–59 months old are vaccine preventable [1]. For example, measles vaccine is 97% effective after two doses [2], yet, as of 2010, more than 100,000 children under the age of five died each year from measles, most of whom were unvaccinated children [3].
Vaccines are currently administered in developing countries primarily in two scenarios: routine vaccination and mass vaccination campaigns. Routine vaccination is used to achieve high immunization coverage on an on-going basis, but can fall short by itself due to infrastructural challenges in developing countries. Instead, or in addition, mass vaccination campaigns are employed to target large populations in specific regions more effectively [4, 5]. Mass vaccination campaigns can be performed at fixed-post clinics, which is typically required for injectable vaccines, or can be carried out door-to-door, usually by minimally trained personnel administering non-injectable vaccines [6].
While immunization rates have risen since the introduction of the Expanded Program on Immunization (EPI), there remain significant barriers to more effective vaccination in developing countries (Table 1). We summarize these barriers in the rest of this section.
Table 1.
| Need for increased vaccine effectiveness |
| Need for trained healthcare providers |
| Need for effective supply chain |
| Risk of sharps |
| Vaccine wastage due to multi-dose vials |
| Need for vaccine reconstitution |
| Cost of vaccine/vaccination |
1.1 Need for increased vaccine effectiveness
While many vaccines are extremely effective and offer life-long protection, other vaccines provide only moderate protection rates, especially in developing countries where nutrition levels may be low and individuals may have a compromised immune system due to presence of other infections [9, 10]. Most vaccines need booster doses in order to mount an appropriate immune response; this requires vaccinating the same people multiple times, which can be difficult to execute in places with poor healthcare infrastructure and recordkeeping.
For example, the efficacy of oral polio vaccine (OPV) is known to be sub-optimal in densely populated tropical countries [9] and the immunogenicity of rotavirus vaccine has been shown to be much worse in resource-poor countries in Africa and Asia [11–13]. Measles vaccine can be less efficacious in the presence of vitamin A deficiency in developing countries and vitamin A supplementation along with measles vaccination is often recommended [10].
1.2 Need for trained healthcare providers
Most vaccines are administered by hypodermic needle and syringe injection. A trained healthcare provider is needed to safely administer these injections as well as to safely dispose of the resulting sharps waste. The lack of trained healthcare providers in developing countries can be a significant barrier to attaining high vaccination rates, especially in the case of vaccination campaigns [14].
Smallpox eradication was achieved in part due to the ability to achieve high vaccination coverage using minimally trained personnel administering the vaccine using the scarification technique with a bifurcated needle [15]. Similarly, OPV is being administered orally by minimally trained personnel as part of polio eradication efforts [14], and the anticipated switch to inactivated polio vaccine (IPV) that is given by injection is of great concern to public health officials due to its increased cost and complexity [16].
1.3 Need for effective supply chain
Vaccines must be maintained at the correct temperature (i.e., usually refrigerated) during storage and distribution as well as during use after reconstitution. Heat and freezing temperatures are both detrimental to most vaccines. The resulting need for a cold chain during storage and distribution can be difficult to maintain due to limited infrastructure in developing countries, leading to vaccine wastage [17, 18]. Size and volume of vaccine vials and syringes are thus also important considerations to utilize the supply chain most effectively [19, 20].
The cost of the cold chain is estimated to be $200 to $300 million per year [18] and can even experience failures in industrialized countries with established cold chain systems [17], indicating that developing countries with less-established cold chain systems can be especially susceptible to losses in the cold chain.. As an example of the variation in cold-chain space occupied by a given vaccine presentation, estimates suggest that one dose of a given vaccine in a 10-dose vial occupies 3 cm3 of cold-chain volume, where as one dose of vaccine in a single-dose vial presentation occupies 12.9 cm3 of cold-chain volume [21].
1.4 Risk of sharps
Hypodermic needles need to be handled carefully to prevent needle-stick injuries to healthcare providers and others. Hypodermic needles also create biohazardous sharps waste after use that needs to be disposed of safely to ensure that the needles are not reused intentionally or accidentally. During vaccination campaigns it may be more difficult to safely collect and dispose of needles in developing countries [22, 23]
Both healthcare workers and patients are at risk due to unsafe injection practices. A study estimated that up to 33,800 HIV infections, 1.7 million hepatitis B infections and 315,000 hepatitis C infections are caused every year due to unsafe injection practices [24].
1.5 Vaccine wastage due to multi-dose vials
Many vaccines are available in multi-dose (e.g., ten-dose) vials for injection. On a per-dose basis, multi-dose vials are less expensive than single dose vials, take up less space during transportation and in the cold-chain and create less waste. However, the actual cost savings can be difficult to evaluate based on the amount of vaccine that gets wasted because opened vials need to be used quickly to prevent microbial growth and, if not used in time, must be discarded. Vaccine wastage can be very high in developing countries for some vaccines [25–27].
In general vaccine wastage rates increase as the number of vaccine doses per vial increases and an estimate suggests wastage rates for 10 dose vials could be as high as 25% for liquid vaccines and 40% for lyophilized vaccines [21]. The WHO Vaccine Presentation and Packaging Advisory Group’s guidelines recommend vaccines to be presented in formats to minimize the number of steps and potential for error during administration when possible [20].
1.6 Need for vaccine reconstitution
Some vaccines are lyophilized and need to be reconstituted with a diluent at the time of use for injection, which adds additional challenges in developing countries [28]. Reconstitution adds another step that requires additional reconstitution needles, syringes and vials that also need to be stored and transported in part in the cold chain, further complicating the supply chain. Time and expertise is needed to reconstitute the vaccine since there is room for error if an incorrect diluent is used or mixing is not carried out using sterile devices. Reconstitution errors lead to vaccine wastage, ineffective vaccination or, in some cases, injury to patients.
As an example, measles vaccine contamination by Staphylococcus Aureus from non-sterile diluent has been documented in many countries and accidental injection of other drugs stored in the diluent’s container have resulted in infant deaths [28]. In a recent case in Syria, the use of an incorrect diluent for the reconstitution of measles vaccine caused the death of 15 children [24].
1.7 Cost of vaccine/vaccination
The cost of vaccination is the cost of vaccine plus the logistical costs associated with making the vaccine available for use. Healthcare provider, waste disposal, vaccine transportation, cold-chain and vaccine wastage all contribute to the cost of vaccination [29, 30]. While vaccine manufacturers often sell vaccine at significantly reduced cost for use in developing countries, the logistical costs to vaccinate can remain a significant barrier.
As evidence of the significance of vaccination costs other than the cost of the vaccine itself, a study of the average cost to administer vaccines in Senegal found that logistics comprise approximately 50% of the total average cost of each dose delivered [29]. As another example, the 2015 UNICEF price for measles/rubella vaccine is US$0.578 per dose [31], but the cost to administer a dose of measles and rubella vaccine is estimated at approximately US$1.50 per dose [32].
2. Microneedle patches address challenges to vaccination in developing countries
2.1 Overview of microneedles for vaccination
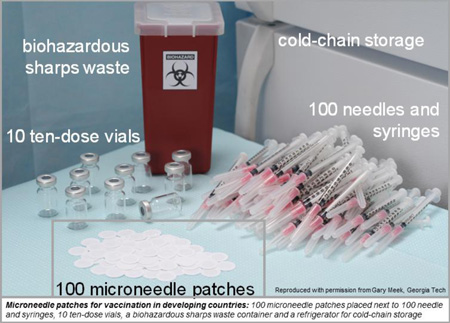
Microneedle patches (MNPs) have been proposed to improve vaccination in developing countries and are the subject of increasing research in academia and industry (Figure 1). Microneedles are less than one millimeter long and deliver vaccines to the skin’s epidermis and dermis, as compared to conventional injection into deeper tissues in the muscle or subcutaneous space by hypodermic needle and syringe. In a MNP, an array of microneedles is attached to a backing such that it can be applied to the skin by hand like a bandage [33, 34].
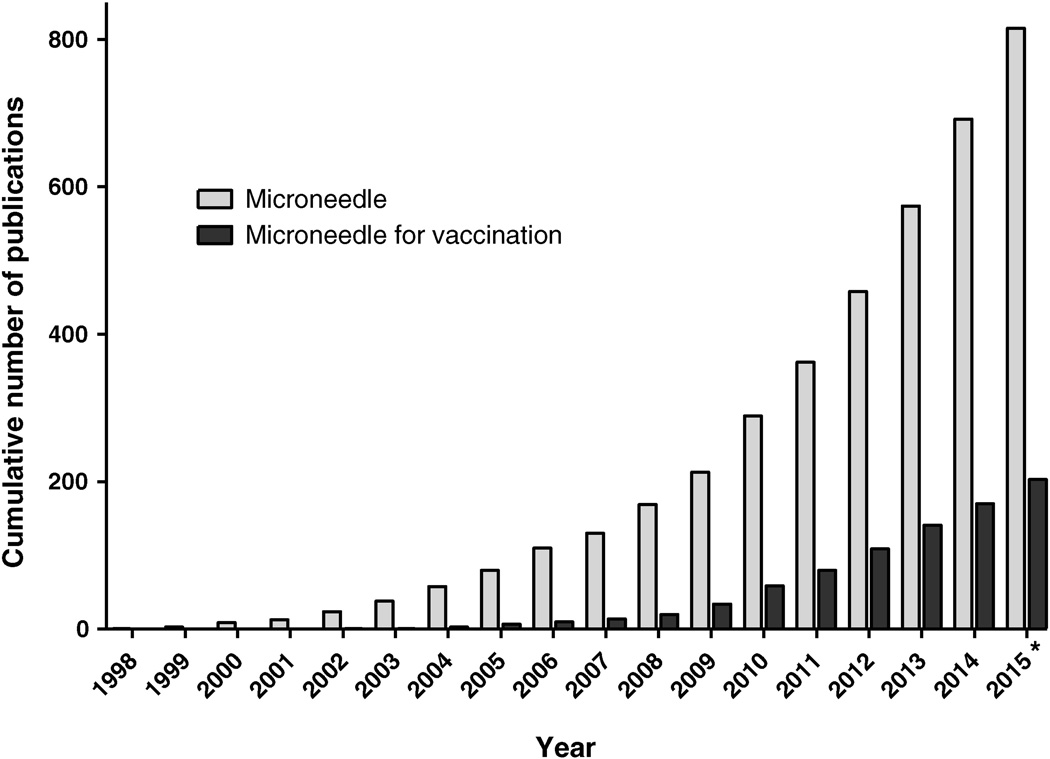
Figure 1.
Cumulative number of publications on microneedles and on microneedles for vaccination. The total number of microneedle publications was determined by searching the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) on 2nd August 2015 using the search terms “microneedle”, “microfabricated needle”, or “nanopatch”. The subset of microneedle publications with focus on vaccination was determined by adding “vaccin*” or “immuniz*” terms to the previous search. Conference proceedings were excluded. *Publications from 2015 only represent those posted on PubMed by 2nd August 2015.
MNPs are typically designed either as solid metal, silicon or polymer microneedles coated with vaccine that releases the vaccine upon dissolution of the coating in the skin or as solid, dissolving microneedles made of water-soluble materials that encapsulate vaccine and releases the vaccine when the microneedles dissolve in the skin. While this review focuses on MNP, microneedles have also been employed for vaccination as solid microneedles used for skin pretreatment followed by application of a topical vaccine formulation for delivery through residual holes in the skin and as hollow microneedles for liquid vaccine formulation delivery into the skin.
In contrast to hypodermic needles that deliver vaccine in a liquid form, MNPs contain the vaccine in a dried solid form which dissolves within the skin upon administration. Each MNP contains a single dose of the vaccine and can be easily applied by pressing down against the skin with the thumb or with the use of an applicator. Upon application of a MNP to the skin, the microneedles penetrate the skin and the patch is left on the skin for a few minutes to allow for dissolution to deliver the payload contained in it. In the case of coated MNP, the coating dissolves but not the microneedles themselves. In the case of dissolving MNPs, the microneedles dissolve within the skin, thus leaving behind only the backing and no biohazardous sharps waste.
MNPs inherently target vaccine delivery to the skin, which is the largest immunological organ in the body and is densely populated by antigen-presenting cells, which play a crucial role in induction of immune responses. As a result, skin vaccination has been shown to be beneficial for many vaccines [35]. However, conventional intradermal injection using a hypodermic needle by the Mantoux technique can be difficult to perform reproducibly [36]. MNPs offer a simple and reliable way to target the skin and have been studied for delivery of many vaccines [33, 34, 37, 38]. Table 2 summarizes the vaccines that have been studied using microneedles; although not otherwise part of this review, hollow microneedles have been included in the table for completeness.
Table 2.
Vaccines studied with microneedles
| Microneedle type | ||
|---|---|---|
| Coated | Dissolving | Hollow |
| Adenovirus [39–42] | Adenovirus [39–42] | Anthrax [43–46] |
| BCG [47, 48] | Amyloid β peptide [49] | Botulism [45, 50] |
| Chikungunya virus [51] | Diphtheria [52–56] | Influenza [55, 57–83] |
| Hepatitis B [84–87] | HIV [88] | Japanese encephalitis [89] |
| Hepatitis C [90] | Influenza [55, 57–83] | Poliovirus [91] |
| Herpes simplex virus [92, 93] | Malaria [41, 52, 94] | Rabies virus [95] |
| HPV [96] | Measles [97] | Staphylococcus aureus [43, 45] |
| Influenza [55, 57–83] | Poliovirus [98] | Yersinia pestis [45, 99] |
| Measles [100] | Tetanus [52] | |
| Modified Vaccinia Ankara [39, 94] | ||
| Rotavirus [101] | ||
| Small Pox [102] | ||
| West Nile virus [51] | ||
2.2 Potential impact of microneedle patches for vaccination in developing countries
In addition to effectively targeting the skin, MNPs offer many other advantages for vaccination, including addressing logistical challenges to vaccine delivery, which are extremely important for vaccination in developing countries. Table 3 summarizes the main advantages that MNPs offer to vaccination in developing countries.
Table 3.
Advantages of microneedle patches for vaccination in developing countries
| Increased vaccine effectiveness |
| Reduced need for trained healthcare providers |
| Simplified supply chain |
| Reduced risk of sharps |
| Reduced vaccine wastage |
| No need for vaccine reconstitution |
| Reduced cost of vaccine/vaccination |
2.3 Increased vaccine effectiveness
2.3.1 Skin vaccination enables dose sparing
Delivering vaccines in the epidermis or dermis puts the antigen in close contact with the skin’s rich population of antigen-presenting cells and can result in lower doses of antigens being used. For example, dose-sparing using the intradermal route has been demonstrated in clinical studies for IPV, seasonal influenza and rabies vaccines [36, 103]. Since MNPs also target the skin for delivery, they could offer improved protection in terms of vaccine dose sparing or a wider range of immune response. In support of that hypothesis, vaccination using MNPs has demonstrated dose-sparing in pre-clinical studies with influenza [63, 78], rotavirus [101] and herpes simplex virus [92], among other vaccines.
2.3.2 Skin vaccination offers improved protection
MNP vaccination has been shown to provide superior immunological responses by other measures as well. Vaccination at the same dose has been shown to produce stronger antibody and/or cellular responses when performed using MNPs compared to hypodermic injection [83, 104, 105], including improved immune responses in very young animals [104]. As a measure of protection, animals vaccinated against influenza using MNPs have been shown to clear virus from the lungs after challenge with live influenza virus better than those vaccinated intramuscularly [67, 105, 106]. Immune response and protection after vaccination have also been shown to last longer after MNP vaccination compared to intramuscular injection [107].
While the mechanisms responsible for the increased immunogenicity of vaccination using MNPs is still under study, evidence suggests that it may be due to vaccine delivery targeted to the unique collection of antigen-presenting cells found in the skin (e.g., Langerhans cells) [75, 76, 94, 108], transport of antigen and antigen-presenting cells from the skin to draining lymph nodes [73], adjuvanted immune response due to cell death caused by the trauma of microneedle insertion into skin [64, 109], and other factors.
2.4 Reduced need for trained healthcare providers
The simple and minimally invasive approach of MNP delivery could allow administration by personnel with minimal training and also offer the possibility of self-administration – with or without the presence of a healthcare provider. This could enable vaccines that currently must be injected by trained healthcare personnel at fixed-post clinics to instead be administered by minimally trained personnel in house-to-house campaigns.
In focus group studies of the public as well as healthcare professionals, MNPs were generally viewed favorably as compared to hypodermic needle injections, suggesting good acceptance of MNPs [110, 111]. In human studies with placebo MNPs, naïve subjects with no prior experience with microneedles were able to successfully administer MNPs when provided with only a brief set of instructions [112, 113]. MNPs for drug delivery have been taken home and used repeatedly by patients without supervision with excellent outcomes [114]. Additional analysis showed that the use of self-administered MNPs could improve vaccination coverage [113] and their use was shown to be cost effective in the majority of scenarios considered in an analysis of influenza vaccination in the United States [111].
2.5 Simplified supply chain
2.5.1 Simplified storage, distribution and disposal
MNPs are much smaller in size than a vaccine vial and needle-syringe system, which could allow MNPs to be stored in a smaller volume and enable simpler storage and distribution [115]. For example, microneedle arrays are typically on the order of 1 cm2 in area and, once assembled onto a patch, could have a representative volume on the order of 1 cm3 [33, 37]. Although packaging, possibly in multi-dose presentations, would increase the product size, it is clear that MNPs have the potential to dramatically reduce the size of vaccines during storage, distribution and disposal.
2.5.2 Reduction or elimination of cold chain
MNPs contain vaccines in a dried form, and suitable excipients can be used in the formulation to make vaccines thermostable. If sufficiently stabilized, MNP could be stored at ambient temperature, eliminating the cold chain completely. If only partial thermostability is achieved, MNPs could be refrigerated during storage at major distribution hubs, but then removed from the cold chain during transportation, storage at village clinics or mass vaccination campaigns.
Influenza vaccine MNPs have been studied extensively for stability at elevated temperatures. A recent study identified formulations stable for at least 6 months at 25 °C and for at least a few weeks at 40 °C [116]. Thermostability has also been studied for MNPs with adenovirus-based vaccines [40] and measles vaccine, which was shown to be stable for at least 4 months at 25 °C and lost less than 10-fold potency after 4 months at 40 °C [97].
2.6 Reduced risk of sharps
MNPs contain microneedles that are a few hundred microns tall and are assembled on a patch backing that is applied to the skin either with thumb pressure or the use of a high-velocity applicator. Casual contact with a MNP is unlikely to result in accidental penetration of microneedles into the skin of an unintended subject, because the MNP needs to be placed flat against the surface of the skin and a significant force needs to be applied for a successful insertion [113]. MNPs could in this way reduce the risks associated with accidental needle stick injury to healthcare providers.
After use, MNPs may offer additional safety advantages. Dissolving MNPs contain microneedles made of water-soluble, biocompatible materials that dissolve in the skin after administration. Thus, they do not leave behind biohazardous sharps waste; only an adhesive backing that can be discarded as non-sharps waste (e.g., similar to a used bandage). This eliminates the risk of injury and disease transmission from used needles. Coated MNPs do not completely eliminate sharps waste. However, used MNPs cannot be reloaded with vaccine absent special coating equipment, making reuse unlikely. Accidental exposure to used MNPs is also expected to be safer than for hypodermic needles because, as mentioned above, it is difficult to get microneedles to penetrate the skin without an intentional, forceful application.
2.7 Reduced vaccine wastage
Each MNP contains a single dose of vaccine and is intended as a single-use product. In comparison to multi-dose vials, single-dose MNPs avoid the problem of vaccine wastage because vaccine in a multi-dose vial must be discarded before all of the doses are used. The single-dose format also avoids patients being turned away without vaccination, as sometimes occurs when an insufficient number of patients need a vaccine on a given day and the vaccinator does not want to open a new vial, knowing that much of the vaccine will be wasted [26].
2.8 No need for vaccine reconstitution
Vaccines are often lyophilized to increase vaccine stability, but this requires vaccine reconstitution before use. MNPs contain vaccine that is administered in a dried form without reconstitution that rapidly dissolves in the skin upon administration. In this way, MNPs can have the increased stability of a dry formulation without the time of clinical personnel and risk of errors associated with reconstitution.
2.9 Reduced cost of vaccine/vaccination
2.9.1 Low-cost manufacturing
In developing countries, a critical concern is the cost of vaccination. Part of that cost is the cost the vaccine itself. The cost-of-goods for a vaccine manufactured in a MNP may be similar to that of conventional vaccine vials or pre-filled syringes, depending in part on the type of MNP technology used. The cost of MNP manufacturing can be low in part because the materials are generally low-cost medical-grade polymers, metals and other excipients that are used in very small amounts, e.g., a representative microneedle array (not including the backing, adhesive and packaging) weighs less than 1 g, and the backing, adhesive and packaging are typically made of conventional pharmaceutical materials used in transdermal patches and other products.
Manufacturing of coated MNPs typically involves a metal, polymer or silicon microneedle structure than can be mass-produced at low cost (e.g., < US$ 0.10), upon which a vaccine is coated by dipping or spraying, allowed to dry and packaged. Manufacturing of dissolving MNPs typically involves a polymer microneedle mold that can be mass produced at low cost (e.g., < US$ 0.10), onto which a vaccine is cast, allowed to dry and packaged. Dipping, spraying, coating and drying are all commonly performed in the pharmaceutical industry, which suggests that MNP manufacturing methods can be compatible with conventional pharmaceutical manufacturing environments and equipment. Much of the cost of MNP manufacturing is the need to perform it under aseptic conditions, which is similar to the cost structure of manufacturing vaccines in vials and syringes.
Terminal sterilization after manufacturing of microneedle patches may be possible, but the sterilization method will need to maintain stability of the vaccine as well as be compatible with the materials that microneedle patches are made of. Although terminal sterilization of vaccine patches has not been studied yet, electron beam and gamma irradiation of a microneedle patch containing a peptide therapeutic was found to unacceptably alter the product [117].
While companies have not released detailed information about their manufacturing methods and costs, 3M offers a solid microneedle device (sMTS) that has undergone FDA-approval and is available for purchase as a stand-alone device with no vaccine or other active. Their proprietary GMP manufacturing and aseptic coating technology has a capacity of up to 10,000 patches per day [118].
2.9.2 Reduced cost of vaccination
In addition to the cost of the vaccine, the complete cost of vaccination should be considered, by accounting for the logistical costs of getting a vaccine delivered to a patient. Thus, even if the cost of a MNP vaccine is greater than a conventional one, those increased costs may be more than offset by reduced logistical costs, including direct costs of vaccine delivery and indirect costs of reduced vaccine safety, efficacy and coverage.
As discussed throughout this section, the costs of vaccination could be reduced through the use of MNPs to increase vaccine effectiveness, reduce the need for trained healthcare providers, simplify the supply chain, reduce the risk of sharps, reduce vaccine wastage and eliminate the need for vaccine reconstitution.
3. Directions for future research and development
MNPs have great potential to improve vaccination in developing countries, but more work needs to be done to realize this potential. Overall, translation of preclinical studies into clinical trials of MNP vaccination is strongly needed, as is commercial manufacturing that can mass produce MNPs at suitable cost. Additional considerations follow.
-
▪
Increased vaccine effectiveness has been shown for a number of vaccines in animal models, but has not yet been established in human subjects, and the mechanisms associated with improved immunogenicity need further elucidation.
-
▪
Initial studies suggest that MNPs can be reliably used by minimally trained personnel, including patients themselves, but more widespread assessment and possible improved MNP designs are needed to assure reliable vaccine delivery.
-
▪
Reduced product size and increased vaccine thermostability are expected to simplify the supply chain, but the true extent of thermostability and the actual impact on healthcare systems have not yet been determined.
-
▪
Reduced risk of sharps is expected, especially for dissolving MNPs. While MNPs reduce this risk associated with hypodermic needles, MNPs may introduce new, unanticipated risks that may only become apparent once they are placed in the hands of diverse users in diverse scenarios and cultures.
-
▪
Reduced vaccine waste and elimination of vaccine reconstitution appear to be inherent capabilities of MNP vaccines, but, again, unintended consequences of these changes may present new challenges.
-
▪
The cost of MNP manufacturing remains a significant uncertainty and an opportunity for advances that bring down costs. Modeling can predict the possible cost savings associated with MNP vaccination balancing cost of goods and costs of vaccine delivery, but commercial and clinical implementation will be needed to determine the true cost, which will vary based on vaccine and use scenario. Identification of terminal sterilization methods that avoid the need for costly aseptic manufacturing could significantly reduce the costs of MNP products.
4. Conclusions
Many lives could be saved by improved vaccination in developing countries. MNPs offer advantages that could improve vaccination through increased vaccine effectiveness, reduced need for trained healthcare providers, simplified supply chain, reduced risk of sharps, reduced vaccine wastage, no need for vaccine reconstitution and reduced cost of vaccine/vaccination. With continued development, especially translation into clinical trials and advanced manufacturing, MNPs have great potential to address the limitations of current vaccination methods and thereby improve vaccination in developing countries.
Acknowledgements
The authors would like to thank Darin Zehrung for a critical reading of the manuscript and providing insight and references concerning the barriers to vaccination in developing countries, and Kimberly Haight and Daniel Pardo for their help in gathering information for this review. This work was supported in part by a grant to Mark Prausnitz from the National Institutes of Health and an ORISE fellowship to Jaya Arya funded by the Centers for Disease Control and Prevention. Mark Prausnitz is an inventor on patents and has a significant financial interest in a company that is developing microneedle-based products (Micron Biomedical).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
References
- 1.WHO. Global Immunization Data July 2014. [July 30 2015]; Available from: http://www.who.int/immunization/monitoring_surveillance/global_immunization_data.pdf.
- 2.CDC. Measles Vaccination. [July 30 2015]; Available from: http://www.cdc.gov/measles/vaccination.html.
- 3.Institute for Health Metrics and Evaluation (IHME) GBD Database. Seattle, WA: IHME, University of Washington; 2014. [Accessed [July 30 2015]]. Available from [ http://www.healthdata.org/search-gbd-data?s=measles]. [Google Scholar]
- 4.LaMontagne DS, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bulletin of the World Health Organization. 2011;89(11):821–830. doi: 10.2471/BLT.11.089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen AK, Fields R, McQuestion M. The future of routine immunization in the developing world: challenges and opportunities. Global Health: Science and Practice. 2014;2(4):381–394. doi: 10.9745/GHSP-D-14-00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linkins RW, et al. Evaluation of house-to-house versus fixed-site oral poliovirus vaccine delivery strategies in a mass immunization campaign in Egypt. Bulletin of the World Health Organization. 1995;73(5):589–595. [PMC free article] [PubMed] [Google Scholar]
- 7.LaFond A, et al. Drivers of routine immunization coverage improvement in Africa: findings from district-level case studies. Health Policy Plan. 2015;30(3):298–308. doi: 10.1093/heapol/czu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favin M, et al. Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4(4):229–238. doi: 10.1016/j.inhe.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Grassly NC, et al. New Strategies for the Elimination of Polio from India. Science. 2006;314(5802):1150–1153. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 10.Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. International Journal of Epidemiology. 2010;39(suppl 1):i48–i55. doi: 10.1093/ije/dyq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhi SA, et al. Effect of Human Rotavirus Vaccine on Severe Diarrhea in African Infants. New England Journal of Medicine. 2010;362(4):289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 12.Vesikari T, et al. Safety and Efficacy of a Pentavalent Human–Bovine (WC3) Reassortant Rotavirus Vaccine. New England Journal of Medicine. 2006;354(1):23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 13.Bhandari N, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. The Lancet. 2014;383(9935):2136–2143. doi: 10.1016/S0140-6736(13)62630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari H, et al. Polio eradication. Efficacy of inactivated poliovirus vaccine in India. Science (New York, N.Y.) 2014;345(6199):922–925. doi: 10.1126/science.1255006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenner F. History of international public health. 6. Geneva: World Health Organization; 1988. Smallpox and its eradication. c1988. [Google Scholar]
- 16.Initiative, W.G.P.E. Geneva, Switzerland: World Health Organization; 2013. Polio Eradicatio & Endgame Strategic Plan 2013–2018. [Google Scholar]
- 17.The Cost of a Broken Vaccine Cold Chain Part Two, Financial Cost. 2014 Wednesday, September 17 [February 25]; Available from: http://www.csafeglobal.com/the-cost-of-a-broken-vaccine-cold-chain-part-two-financial-cost-1. [Google Scholar]
- 18.Das P. Revolutionary vaccine technology breaks the cold chain. The Lancet Infectious Diseases. 2004;4(12):719. doi: 10.1016/s1473-3099(04)01222-8. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Guidelines on the international packaging and shipping of vaccines. 2005
- 20.VPPAG. Generic Preferred Product Profile for Vaccines [Google Scholar]
- 21.Alliance TWBG. Immunization Financing Toolkit: A Resource for Policy-Makers and Program Managers. [August 2 2015];2010 [Google Scholar]
- 22.Miller MA, Pisani E. The cost of unsafe injections. Bull World Health Organ. 1999;77(10):808–811. [PMC free article] [PubMed] [Google Scholar]
- 23.Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promotion International. 2004;19(1):95–103. doi: 10.1093/heapro/dah110. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Injection safety policy and global campaign. [July 30 2015]; Available from: www.who.int/injection_safety/global-campaign/en/.
- 25.Drain PK, Nelson CM, Lloyd JS. Single-dose versus multi-dose vaccine vials for immunization programmes in developing countries. Bulletin of the World Health Organization. 2003;81(10):726–731. [PMC free article] [PubMed] [Google Scholar]
- 26.Haidari LA, et al. One size does not fit all: The impact of primary vaccine container size on vaccine distribution and delivery. Vaccine. 2015;33(28):3242–3247. doi: 10.1016/j.vaccine.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. WHO policy statement: multi-dose vial policy (MDVP): handling of multi-dose vaccine vials after opening. 2014
- 28.Clements CJ, Larsen G, Jodar L. Technologies that make administration of vaccines safer. Vaccine. 2004;22(15–16):2054–2058. doi: 10.1016/j.vaccine.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 29.PATH. Some vaccine costs are hidden below the surface. [March 15 2015]; Available from: http://www.path.org/publications/files/TS_opt_banner_hippo.pdf. [Google Scholar]
- 30.Lydon P, et al. Health system cost of delivering routine vaccination in low-and lower-middle income countries: what is needed over the next decade? Bulletin of the World Health Organization. 2014;92(5):382–384. doi: 10.2471/BLT.13.130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNICEF. MR vaccine supplier prices. [3 August 2015]; Available from: http://www.unicef.org/supply/files/MR.pdf. [Google Scholar]
- 32.Measles and Rubella move fast. [3 August 2015]; Available from: http://www.cdc.gov/globalhealth/measles/pdf/measles-factsheet2015.pdf.
- 33.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64(14):1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettis RJ, Harvey AJ. Microneedle delivery: clinical studies and emerging medical applications. Therapeutic Delivery. 2012;3(3):357–371. doi: 10.4155/tde.12.13. [DOI] [PubMed] [Google Scholar]
- 35.Nestle FO, et al. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zehrung D, Jarrahian C, Wales A. Intradermal delivery for vaccine dose sparing: overview of current issues. Vaccine. 2013;31(34):3392–3395. doi: 10.1016/j.vaccine.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 37.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 38.Vrdoljak A. Review of recent literature on microneedle vaccine delivery technologies. Vaccine: Development and Therapy. 2013 [Google Scholar]
- 39.Vrdoljak A, et al. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. Journal of Controlled Release. 2012;159(1):34–42. doi: 10.1016/j.jconrel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachy V, et al. Langerin negative dendritic cells promote potent CD8(+) T-cell priming by skin delivery of live adenovirus vaccine microneedle arrays. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(8):3041–3046. doi: 10.1073/pnas.1214449110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carey JB, et al. Microneedle-mediated immunization of an adenovirus-based malaria vaccine enhances antigen-specific antibody immunity and reduces anti-vector responses compared to the intradermal route. Sci. Rep. 2014;4 doi: 10.1038/srep06154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erdos G, et al. Dissolvable microneedle arrays deliver live adenovirus to the skin for genetic immunization. Journal of Immunology. 2012;188:1. [Google Scholar]
- 43.Ryan E, et al. Microneedle-mediated transdermal bacteriophage delivery. European Journal of Pharmaceutical Sciences. 2012;47(2):297–304. doi: 10.1016/j.ejps.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikszta JA, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infection and Immunity. 2006;74(12):6806–6810. doi: 10.1128/IAI.01210-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morefield GL, Tammariello RF, Purcell BK, Worsham PL, Chapman J, Smith LA, Ulrich RG. An alternative approach to combination vaccines: intradermal administration of isolated components for control of anthrax, botulism, plague and staphylococcal toxic shock. Journal of immune based therapies and vaccines. Journal of Immune Based Therapies and Vaccines. 2008;6(5) doi: 10.1186/1476-8518-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikszta JA, et al. Protective immunization against inhalational anthrax: A comparison of minimally invasive delivery platforms. Journal of Infectious Diseases. 2005;191(2):278–288. doi: 10.1086/426865. [DOI] [PubMed] [Google Scholar]
- 47.Hiraishi Y, et al. Bacillus Calmette-Guérin vaccination using a microneedle patch. Vaccine. 2011;29(14):2626–2636. doi: 10.1016/j.vaccine.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong YL, et al. Drying a tuberculosis vaccine without freezing. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2591–2595. doi: 10.1073/pnas.0611430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuo K, et al. Vaccine efficacy of transcutaneous immunization with amyloid β using a dissolving microneedle array in a mouse model of Alzheimer's disease. Journal of Neuroimmunology. 2014;266(1–2):1–11. doi: 10.1016/j.jneuroim.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Torrisi BM, et al. Pocketed microneedles for rapid delivery of a liquid-state botulinum toxin A formulation into human skin. Journal of Controlled Release. 2013;165(2):146–152. doi: 10.1016/j.jconrel.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prow TW, et al. Nanopatch-Targeted Skin Vaccination against West Nile Virus and Chikungunya Virus in Mice. Small. 2010;6(16):1776–1784. doi: 10.1002/smll.201000331. [DOI] [PubMed] [Google Scholar]
- 52.Matsuo K, et al. Transcutaneous immunization using a dissolving microneedle array protects against tetanus, diphtheria, malaria, and influenza. Journal of Controlled Release. 2012;160(3):495–501. doi: 10.1016/j.jconrel.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Ding Z, et al. Transcutaneous Immunization Studies in Mice Using Diphtheria Toxoid-Loaded Vesicle Formulations and a Microneedle Array. Pharmaceutical Research. 2011;28(1):145–158. doi: 10.1007/s11095-010-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bal S, et al. Microneedle-Based Transcutaneous Immunisation in Mice with N-Trimethyl Chitosan Adjuvanted Diphtheria Toxoid Formulations. Pharmaceutical Research. 2010;27(9):1837–1847. doi: 10.1007/s11095-010-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding Z, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. Journal of Controlled Release. 2009;136(1):71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 56.Ding Z, et al. Immune Modulation by Adjuvants Combined with Diphtheria Toxoid Administered Topically in BALB/c Mice After Microneedle Array Pretreatment. Pharmaceutical Research. 2009;26(7):1635–1643. doi: 10.1007/s11095-009-9874-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim Y-C, et al. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. Journal of Controlled Release. 2010;142(2):187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alarcon JB, et al. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clinical and Vaccine Immunology. 2007;14(4):375–381. doi: 10.1128/CVI.00387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnou R, et al. Intradermal influenza vaccine for older adults: A randomized controlled multicenter phase III study. Vaccine. 2009;27(52):7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 60.Beran J, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. Bmc Medicine. 2009;7:15. doi: 10.1186/1741-7015-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi HJ, et al. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. Journal of Controlled Release. 2013;166(2):159–171. doi: 10.1016/j.jconrel.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi HJ, et al. Stability of influenza vaccine coated onto microneedles. Biomaterials. 2012;33(14):3756–3769. doi: 10.1016/j.biomaterials.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernando GJP, et al. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. Journal of Controlled Release. 2012;159(2):215–221. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 64.Fernando GJP, et al. Potent Immunity to Low Doses of Influenza Vaccine by Probabilistic Guided Micro-Targeted Skin Delivery in a Mouse Model. Plos One. 2010;5(4):11. doi: 10.1371/journal.pone.0010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holland D, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: A randomized controlled trial. Journal of Infectious Diseases. 2008;198(5):650–658. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 66.Kim YC, et al. Stability Kinetics of Influenza Vaccine Coated onto Microneedles During Drying and Storage. Pharmaceutical Research. 2011;28(1):135–144. doi: 10.1007/s11095-010-0134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim YC, et al. Enhanced Memory Responses to Seasonal H1N1 Influenza Vaccination of the Skin with the Use of Vaccine-Coated Microneedles. Journal of Infectious Diseases. 2010;201(2):190–198. doi: 10.1086/649228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim YC, et al. Cross-protection by co-immunization with influenza hemagglutinin DNA and inactivated virus vaccine using coated microneedles. Journal of Controlled Release. 2013;172(2):579–588. doi: 10.1016/j.jconrel.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kommareddy S, et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine. 2013;31(34):3435–3441. doi: 10.1016/j.vaccine.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 70.Kommareddy S, et al. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. Journal of Pharmaceutical Sciences. 2012;101(3):1021–1027. doi: 10.1002/jps.23019. [DOI] [PubMed] [Google Scholar]
- 71.Koutsonanos DG, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Scientific Reports. 2012;2:10. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levin Y, Kochba E, Kenney R. Clinical evaluation of a novel microneedle device for intradermal delivery of an influenza vaccine: Are all delivery methods the same? Vaccine. 2014;32(34):4249–4252. doi: 10.1016/j.vaccine.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 73.Martin MD, et al. Local Response to Microneedle-Based Influenza Immunization in the Skin. Mbio. 2012;3(2):8. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nougarede N, et al. Nine mu g intradermal influenza vaccine and 15 mu g intramuscular influenza vaccine induce similar cellular and humoral immune responses in adults. Human Vaccines & Immunotherapeutics. 2014;10(9):2713–2720. doi: 10.4161/hv.29695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pearton M, et al. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine. 2010;28(37):6104–6113. doi: 10.1016/j.vaccine.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pearton M, et al. Host Responses in Human Skin After Conventional Intradermal Injection or Microneedle Administration of Virus-Like-Particle Influenza Vaccine. Advanced Healthcare Materials. 2013;2(10):1401–1410. doi: 10.1002/adhm.201300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Puig-Barbera J, et al. Intradermal and virosomal influenza vaccines for preventing influenza hospitalization in the elderly during the 2011–2012 influenza season: A comparative effectiveness study using the Valencia health care information system. Vaccine. 2014;32(42):5447–5454. doi: 10.1016/j.vaccine.2014.07.095. [DOI] [PubMed] [Google Scholar]
- 78.Quan FS, et al. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. Journal of Controlled Release. 2010;147(3):326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Quan FS, et al. Stabilization of Influenza Vaccine Enhances Protection by Microneedle Delivery in the Mouse Skin. Plos One. 2009;4(9):10. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song JM, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Research. 2010;88(2):244–247. doi: 10.1016/j.antiviral.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weldon WC, et al. Effect of Adjuvants on Responses to Skin Immunization by Microneedles Coated with Influenza Subunit Vaccine. Plos One. 2012;7(7):8. doi: 10.1371/journal.pone.0041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan L, et al. Advanced Materials and Nanotechnology for Drug Delivery. Advanced Materials. 2014;26(31):5533–5540. doi: 10.1002/adma.201305683. [DOI] [PubMed] [Google Scholar]
- 83.Zhu QY, et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(19):7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Andrianov AK, et al. Poly[di(carboxylatophenoxy)phosphazene] Is a Potent Adjuvant for Intradermal Immunization. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(45):18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo L, et al. Effective transcutaneous immunization against hepatitis B virus by a combined approach of hydrogel patch formulation and microneedle arrays. Biomedical Microdevices. 2013;15(6):1077–1085. doi: 10.1007/s10544-013-9799-z. [DOI] [PubMed] [Google Scholar]
- 86.Yin D, et al. Hepatitis B DNA Vaccine-Polycation Nano-Complexes Enhancing Immune Response by Percutaneous Administration with Microneedle. Biological and Pharmaceutical Bulletin. 2013;36(8):1283–1291. doi: 10.1248/bpb.b13-00050. [DOI] [PubMed] [Google Scholar]
- 87.Mikszta JA, et al. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nature Medicine. 2002;8(4):415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 88.Pattani A, et al. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. Journal of Controlled Release. 2012;162(3):529–537. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dean CH, et al. Cutaneous Delivery of a Live, Attenuated Chimeric Flavivirus Vaccine Against Japanese Encephalitis (ChimeriVax (TM)-JE) in Non-Human Primates. Human Vaccines. 2005;1(3):106–111. doi: 10.4161/hv.1.3.1797. [DOI] [PubMed] [Google Scholar]
- 90.Gill HS, et al. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17(6):811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Maaden K, et al. Novel Hollow Microneedle Technology for Depth-Controlled Microinjection-Mediated Dermal Vaccination: A Study with Polio Vaccine in Rats. Pharmaceutical Research. 2014;31(7):1846–1854. doi: 10.1007/s11095-013-1288-9. [DOI] [PubMed] [Google Scholar]
- 92.Chen X, et al. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. Journal of Controlled Release. 2010;148(3):327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Kask AS, et al. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine. 2010;28(47):7483–7491. doi: 10.1016/j.vaccine.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Carey JB, et al. Microneedle Array Design Determines the Induction of Protective Memory CD8<sup>+</sup>T Cell Responses Induced by a Recombinant Live Malaria Vaccine in Mice. PLoS ONE. 2011;6(7):e22442. doi: 10.1371/journal.pone.0022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laurent PE, et al. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28(36):5850–5856. doi: 10.1016/j.vaccine.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 96.Corbett HJ, et al. Skin Vaccination against Cervical Cancer Associated Human Papillomavirus with a Novel Micro-Projection Array in a Mouse Model. PLoS ONE. 2010;5(10):e13460. doi: 10.1371/journal.pone.0013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Edens C, et al. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine. 2015;33(37):4712–4718. doi: 10.1016/j.vaccine.2015.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edens C, et al. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine. 2015;33(37):4683–4690. doi: 10.1016/j.vaccine.2015.01.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang J, et al. Protective Immunity in Mice Achieved with Dry Powder Formulation and Alternative Delivery of Plague F1-V Vaccine. Clinical and Vaccine Immunology. 2009;16(5):719–725. doi: 10.1128/CVI.00447-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Edens C, et al. Measles vaccination using a microneedle patch. Vaccine. 2013;31(34):3403–3409. doi: 10.1016/j.vaccine.2012.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moon S, et al. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013;31(34):3396–3402. doi: 10.1016/j.vaccine.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hooper JW, et al. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laurent PE, et al. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28(36):5850–5856. doi: 10.1016/j.vaccine.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 104.Koutsonanos DG, et al. Enhanced immune responses by skin vaccination with influenza subunit vaccine in young hosts. Vaccine. 2015;33(37):4675–4682. doi: 10.1016/j.vaccine.2015.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sullivan SP, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quan FS, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010;84(15):7760–7769. doi: 10.1128/JVI.01849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koutsonanos DG, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pulit-Penaloza JA, et al. A protective role of murine langerin(+) cells in immune responses to cutaneous vaccination with microneedle patches. Sci Rep. 2014;4:6094. doi: 10.1038/srep06094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ruutu MP, et al. Increasing mechanical stimulus induces migration of Langerhans cells and impairs the immune response to intracutaneously delivered antigen. Exp Dermatol. 2011;20(6):534–536. doi: 10.1111/j.1600-0625.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 110.Birchall JC, et al. Microneedles in clinical practice--an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28(1):95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 111.Lee BY, et al. An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine. 2015;33(37):4727–4736. doi: 10.1016/j.vaccine.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Donnelly RF, et al. Hydrogel-Forming Microneedle Arrays Can Be Effectively Inserted in Skin by Self-Application: A Pilot Study Centred on Pharmacist Intervention and a Patient Information Leaflet. Pharm Res. 2014 doi: 10.1007/s11095-014-1301-y. [DOI] [PubMed] [Google Scholar]
- 113.Norman JJ, et al. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32(16):1856–1862. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cosman F, et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2010;95(1):151–158. doi: 10.1210/jc.2009-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toon J. Self-Administration of Flu Vaccine with a Patch May be Feasible. [March 2 2015]; Available from: http://www.news.gatech.edu/2014/02/26/self-administration-flu-vaccine-patch-may-be-feasible-study-suggests. [Google Scholar]
- 116.Mistilis MJ, Bommarius AS, Prausnitz MR. Development of a Thermostable Microneedle Patch for Influenza Vaccination. Journal of Pharmaceutical Sciences. 2015;104(2):740–749. doi: 10.1002/jps.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ameri M, Wang X, Maa YF. Effect of irradiation on parathyroid hormone PTH(1–34) coated on a novel transdermal microprojection delivery system to produce a sterile product--adhesive compatibility. J Pharm Sci. 2010;99(4):2123–2134. doi: 10.1002/jps.21985. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, et al. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm Res. 2012;29(1):170–177. doi: 10.1007/s11095-011-0524-4. [DOI] [PubMed] [Google Scholar]