Abstract
Pendrin (SLC26A4) is a Cl−/anion exchanger expressed in the epithelium of inflamed airways where it is thought to facilitate Cl− absorption and HCO3− secretion. Studies using pendrin knockout mice and airway epithelial cells from hearing-impaired subjects with pendrin loss of function suggest involvement of pendrin in inflammatory lung diseases, including cystic fibrosis (CF), perhaps by regulation of airway surface liquid (ASL) volume. Here we identified small-molecule pendrin inhibitors and demonstrated their efficacy in increasing ASL volume. A cell-based, functional high-throughput screen of ∼36,000 synthetic small molecules produced 3 chemical classes of inhibitors of human pendrin. After structure-activity studies, tetrahydropyrazolopyridine and pyrazolothiophenesulfonamide compounds reversibly inhibited pendrin-facilitated Cl− exchange with SCN−, I−, NO3−, and HCO3− with drug concentration causing 50% inhibition down to ∼2.5 μM. In well-differentiated primary cultures of human airway epithelial cells from non-CF and CF subjects, treatment with IL-13, which causes inflammation with strong pendrin up-regulation, strongly increased Cl−/HCO3− exchange and the increase was blocked by pendrin inhibition. Pendrin inhibition significantly increased ASL depth (by ∼8 μm) in IL-13-treated non-CF and CF cells but not in untreated cells. These studies implicate the involvement of pendrin-facilitated Cl−/HCO3− in the regulation of ASL volume and suggest the utility of pendrin inhibitors in inflammatory lung diseases, including CF.—Haggie, P. M., Phuan, P.-W., Tan, J.-A., Zlock, L., Finkbeiner, W. E., Verkman, A. S. Inhibitors of pendrin anion exchange identified in a small molecule screen increase airway surface liquid volume in cystic fibrosis.
Keywords: ASL, drug discovery, high-throughput screening, inflammatory airway disease
The gene encoding pendrin (PDS, SLC26A4) was identified by positional cloning in subjects with Pendred syndrome, an autosomal-recessive disorder associated with congenital hearing loss and thyroid goiter (1). Pendrin consists of 780 amino acids with a predicted cytoplasmic amino terminus and 12 transmembrane spanning regions (1, 2). Functional studies show that pendrin mediates electroneutral exchange of Cl− with various anions including I−, HCO3−, OH−, NO3−, SCN− (thiocyanate), and HCO2− (formate) (2–8). In addition to the inner ear and thyroid, pendrin is expressed in the kidney, airways, and adrenal gland (1, 4, 6, 7, 9–12).
Several roles of pendrin have been suggested from human molecular genetics, animal models, and cell culture studies. In the inner ear, pendrin is expressed in the apical membrane of epithelial cells in sensory (saccule, utricle, and ampullae) and nonsensory (endolymphatic sac) domains (13, 14). Radiologic studies in children with Pendred syndrome or DFNB4 nonsyndromic hearing loss as well as studies in transgenic mouse models indicate that pendrin is a key determinant of inner-ear luminal fluid volume and composition. However, the mechanisms linking pendrin loss of function to hearing impairment remain unclear. In the thyroid pendrin is expressed on the apical membrane of follicular cells, where it facilitates I− efflux into the follicle colloid for thyroid hormone synthesis (2, 15). In the kidney pendrin is expressed in the apical plasma membrane of type B and non-A/non-B intercalated cells, where it facilitates Cl− absorption and HCO3− secretion, although kidney abnormalities are rare in Pendred syndrome (4, 6, 16).
There is evidence for involvement of pendrin in airway diseases. Pendrin up-regulation is seen in: 1) airway epithelial cultures chronically exposed to cytokines, including IL-4, IL-13, and IL-17A; 2) rodent models of inflammatory lung disease, including allergen (ovalbumin)-induced asthma, chronic obstructive pulmonary disease, infection, and industrial toxin exposure; and 3) humans with rhinovirus infection, asthma, cystic fibrosis (CF), rhinitis, and chronic rhinosinusitis (10, 11, 17–25). Pendrin knockout is also associated with reduced lung inflammation in a murine model of Bordetella pertussis lung infection (26). IL-13, the cytokine used commonly in in vitro and animal models of airway inflammation, is elevated in CF, asthma, chronic rhinosinusitis, viral and certain bacterial infections, and chronic obstructive pulmonary disease, and in response to cigarette smoke (27–34). In murine models of asthma, pendrin knockout reduces pulmonary pathology, airway hyperreactivity, and immune cell infiltration, while pendrin overexpression increases airway hyperreactivity (10, 11).
The mechanisms linking pendrin expression to airway pathology remain incompletely understood. Pendrin may regulate airway surface liquid (ASL) volume, which could secondarily affect mucociliary clearance, bacterial colonization, and other mucosal immune responses. ASL volume is increased in nasal epithelial cultures from DFNB4 subjects with pendrin loss-of-function mutations compared to controls (35). Tracheal epithelial cultures from pendrin knockout mice showed increased ASL volume after IL-13 stimulation compared to cultures from wild-type mice (11). In another study, lung pathology in response to Bordetella pertussis infection was reduced by acetazolamide, suggesting the involvement of ion transport by pendrin in lung inflammation (26). Consideration of electrochemical driving forces in airway epithelia predicts that pendrin can facilitate Cl− absorption and HCO3− secretion; pendrin inhibition can increase steady-state ASL volume because some HCO3− entering the ASL becomes protonated and is removed as CO2. Pendrin may also be involved in mucus production, a hallmark of airway disease, though the evidence is conflicting. Forced expression of pendrin in cell culture models and the murine lung is associated with elevated mucus production (10), and tissue and cell cultures from DFNB4 subjects show reduced mucus production (35). However, mucus production was not altered in pendrin knockout mice in an asthma model (11). Potential compensatory effects of chronic pendrin loss of function in mice and humans confound clear-cut definition of the roles of pendrin in airway biology.
Here we established a high-throughput screen to identify small-molecule inhibitors of pendrin anion exchange, with the goals of developing useful research tools to elucidate the roles of pendrin and as potential therapies for human disease. Compounds emerging from the screen were characterized and used to define the role of pendrin in regulation of ASL properties in primary cultures of airway epithelial cells from non-CF and CF humans.
MATERIALS AND METHODS
Cells for high-throughput screening
Fischer rat thyroid (FRT) cells were cultured in Kaign's modified Ham's F12 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, 18 mg/ml myoinositol, and 45 mg/ml ascorbic acid. For high-throughput screening, FRT cells were stably transfected with EYFP-H148Q/I152L/F46L (EYFP-HIF; in pcDNA3.1/Hygro+), isolated using 0.25 mg/ml hygromycin B, and then transfected with pcDNA3.1+ encoding human pendrin (a gift of W. Namkung, Yonsei University, Seoul, South Korea) with clonal cell lines selected using 0.5 mg/ml G418.
Human bronchial epithelial cell cultures
Bronchial tissues were obtained from non-CF (without significant airway disease) and CF subjects after lung transplantation or from lungs donated for transplantation but subsequently found to be unsuitable for that purpose. Non-CF and CF human bronchial epithelial (HBE and CFBE, respectively) cell cultures were grown at an air–liquid interface as described in detail elsewhere (36). At 21 d after seeding, cells typically formed a tight epithelium (RTE > 1000 Ω cm2). In some experiments the culture medium was supplemented with 10 ng/ml recombinant human IL-13 (Peprotech, Rocky Hill, NJ, USA) for 7 d starting at d 21 of culture. As needed, the apical cell surface was rinsed with PBS to remove excess mucus 24 h or more before ASL measurements. Pendrin transcript levels in human primary cells were measured by TaqMan quantitative PCR using the Hs00166504_m1 probe set (Thermo Fisher Scientific, Grand Island, NY, USA) at the University of California, San Francisco (UCSF), Helen Diller Cancer Center Genome Analysis Core. Human tissues were acquired and used with approval from the UCSF Committee on Human Research.
Screening procedures
High-throughput screening was performed using a semiautomated screening platform (Beckman Coulter, Fullerton, CA, USA) configured as described elsewhere (37). FRT cells expressing human pendrin and EYFP-HIF were plated in 96-well black-walled, clear-bottom tissue culture plates (Corning, Corning, NY, USA) at a density of 20,000 cells/well, and cultured to confluence over 48 h. For screening, plates were washed twice with PBS (in mM: 137 NaCl, 2.7 KCl, 1.1 KH2PO4, 8.1 Na2HPO4, 0.9 CaCl2, 0.5 MgCl2) before addition of 100 μl PBS containing test compounds. Screening was done with ∼36,000 compounds from ChemDiv (San Diego, CA, USA), TimTec (Newark, DE, USA), and Asinex (Winston-Salem, NC, USA). After 10 min incubation with test compound, plates were transferred to a Tecan Infinite M1000 plate reader (Tecan, Morrisville, NC, USA) for fluorescence assay of pendrin activity. The assay duration was 10 s with initial fluorescence intensity recorded for 2 s before addition of 100 µl of NaSCN-substituted PBS (137 mM NaCl replaced with NaSCN). All assay plates contained negative controls (DMSO). In some experiments Cl− exchange for I− or NO3− was measured similarly using NaI- and NaNO3-substituted PBS. Control experiments to measure basal ion transport rates were done in FRT cells expressing EYFP-HIF without pendrin.
Assays of Cl−/HCO3− exchange
Cytoplasmic pH (pHi) was measured to assay pendrin-mediated Cl−/HCO3− exchange in pendrin-expressing FRT cells and primary cell cultures using the pH-sensitive fluorescent probe BCECF. Cells were loaded for 20 min using 10 μM BCECF-AM (Invitrogen, Carlsbad, CA, USA) in HCO3−-containing buffer (in mM: 120 NaCl, 5 KCl, 1 CaCl2, 1 MgSO4, 10 glucose, 5 HEPES, 25 NaHCO3−; pH 7.4) at 27°C in a tissue culture incubator. Cells were washed and transferred to the stage of a TE2000 microscope (Nikon, Melville, NY, USA) equipped with a C9100 EM-CCD (Hamamatsu, San Jose, CA, USA), XCite light source (Excelitas, Waltham, MA, USA), Nikon ×20 NA 0.75 S Fluor objective, Uniblitz shutter (Vincent Associates, Rochester, NY, USA), and filters for BCECF fluorescence (Chroma, Bellows Fall, VT, USA), where they were purged with 95% O2/5% CO2. Pendrin-mediated Cl−/HCO3− exchange was initiated by apical addition of Cl−-free HCO3−-containing buffer (Na gluconate replacing NaCl; pH 7.4; 95% O2/5% CO2 equilibrated) to generate a 100 mM gluconate gradient driving cytoplasmic Cl− efflux. BCECF pH-sensitive fluorescence (490 nm excitation, 535 nm emission) was acquired at 0.5 Hz, with control experiments confirming that BCECF pH-insensitive fluorescence was not altered during exchange protocols. In some experiments pHi changes in the absence of HCO3− were measured using buffers in which NaCl or Na gluconate replaced NaHCO3−. To relate BCECF fluorescence to pH, a calibration curve over the quasilinear range of BCECF sensitivity (pH 6.6–7.7) was generated as previously described (38).
Short-circuit current measurements
IL-13-treated HBE cells were cultured on Snapwell clear permeable supports (12 mm diameter, 0.4 μm polyester membrane; Costar; Corning). Short-circuit current (Isc) was measured using symmetrical HCO3−-buffered solutions (in mM: 120 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 5 HEPES, 25 NaHCO3; pH 7.4) as previously described (36), with ion transport modulators added to both apical and basolateral bathing solutions. Cells were equilibrated with 95% O2/5% CO2 and maintained at 37°C during data acquisition. Statistical analysis was by Student’s t test.
SLC26A3 functional assay
SLC26A3-mediated ion transport was measured in COS7 fibroblasts transiently transfected to express EYFP-HIF and human SLC26A3 (Origene, Rockville, MD, USA). COS7 cells were cultured in DMEM-H21 supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin and transfected using Lipofectamine 2000 (Invitrogen). SLC26A3-mediated transport was measured from EYFP-HIF fluorescence after addition of a NaSCN-substituted PBS solution to generate a 70 mM SCN− gradient. Control experiments were done using COS7 cells expressing EYFP-HIF alone.
ASL depth and pH measurements
ASL depth was measured by confocal imaging of rhodamine B–dextran fluorescence (20 μl of 1 mg/ml in PBS) applied 4 to 6 h before measurement. Scanning confocal microscopy was done using an upright Nikon EZ-C1 confocal microscope equipped with a LUMplan FL N ×60/1.00w objective (Olympus, Waltham, MA, USA). For measurements the transwell filter containing cells with fluorescently stained ASL was inverted, with the immersion objective contacting a droplet of PBS added onto the upper-facing basal surface of the transwell filter. In some experiments ASL depth was measured after treatment with 25 µM PDSinh-A01 in the culture medium for 4 to 6 h. ASL depth was measured using a multipoint histogram method in which each confocal z stack was fitted to a quadratic function, as described and validated previously (39).
ASL pH was measured using the pH-sensitive fluorophore BCECF conjugated to 10 kDa dextran (Invitrogen) as described elsewhere (40), which was added 4 to 6 h before measurement (20 μl of 0.5 mg/ml in PBS). Cell inserts were incubated in HCO3−-containing buffer (5% CO2 environment) and the ASL was imaged using a SMZ stereoscopic microscope (Nikon) equipped with a C9100 EM-CCD, Exfo XCite light source, and BCECF filters (440/×10, 490/×20, 535/25M; Chroma). In some experiments ASL pH was measured after PDSinh-A01 treatment, as described for ASL depth measurements. To relate BCECF fluorescence to pH, a calibration curve over the quasilinear range of BCECF sensitivity was generated.
RESULTS
Pendrin inhibitors identified by high-throughput screening
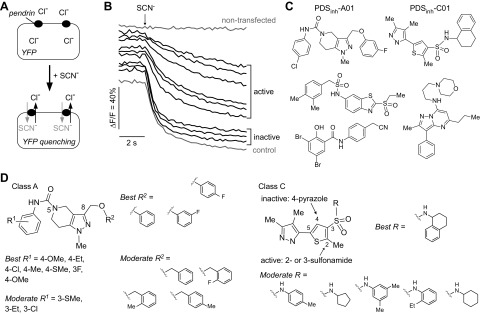
High-throughput screening to identify pendrin inhibitors was done using FRT cells stably expressing human pendrin and the halide-sensitive fluorescent protein EYFP-HIF. FRT cells were chosen for screening because of their low intrinsic permeability to anions that are transported by pendrin. As depicted in Fig. 1A, pendrin activity was assayed from the kinetics of EYFP-HIF fluorescence in response to addition of a SCN−-containing solution to drive pendrin-mediated Cl−/SCN− exchange. Pendrin inhibition reduces the rate of EYFP-HIF quenching. Examples of fluorescence curves from inactive and active compounds are shown in Fig. 1B, together with data from a negative control (DMSO vehicle). Because the only described pendrin inhibitor is the weakly active compound niflumic acid [<40% inhibition at 100 μM (7)], no positive control was included in screening plates.
Figure 1.
High-throughput screen for identification of inhibitors of human pendrin. A) Schematic of assay showing extracellular addition of SCN− results in pendrin-mediated Cl−/SCN− exchange and YFP quenching. B) Representative time course of YFP fluorescence quenching in cells expressing YFP alone (nontransfected, top) and YFP with human pendrin, showing curves for active and inactive compounds (and no test compound, control). C) Chemical structures of pendrin inhibitors identified from screen. D) Structural determinants of activity of class A (left) and C (right) compounds.
Screening of ∼36,000 compounds produced several classes of compounds that inhibited pendrin by >50% at 25 μM (Fig. 1C). On the basis of their potency, 2 compound classes (A and C) were further characterized. A total of 188 commercially available class A (tetrahydropyrazolopyridine) analogs and 160 class C (pyrazolothiophenesulfonamide) analogs were tested, with structural determinants of activity summarized in Fig. 1D. Class A compounds contained a core tetrahydropyrazolopyridine heterocycle with a substituted aniline linked to the core ring at the 5-position via a carbamate linker and with a substituted-methoxy linked at the 8-position of the core. Analogs with different substitution on the aniline ring (R1) were explored. Para-substituted aniline gave the best activity, whereas ortho-substitution (including methyl, methoxy, halo, and trifluoromethyl substituents) abolished activity. The electronic property of the substituent on the aniline appeared to be less crucial, with electron-donating and neutral groups giving highest activity. Several R2 groups on the 8-methoxy were also explored. In general, analogs with R2 substituted with phenyl rings were more active than with benzyl rings. Halide-substituted benzyl rings greatly reduced activity. Class C inhibitors are composed of a thiophene with a sulfonamide group and a pyrazole heterocycle linked at the 3 and 5 positions, respectively. Changing the pyrazole from the 5 to the 4 position of the thiophene ring abolished activity. The sulfonamide group at the 2 or 3 position was tolerated. Substitution on the sulfonamide influenced activity, with primary sulfonamide bearing a bicyclic tetrahydronaphthalene ring giving best activity, and monocyclic ring or alkyl-substituted anilines generally reducing activity. Secondary sulfonamide, alkyl amines, and electron-rich anilines had no activity. The most potent pendrin compound from each class, PDSinh-A01 and PDSinh-C01 (Fig. 1C, top), were further studied.
Characterization of pendrin inhibitors
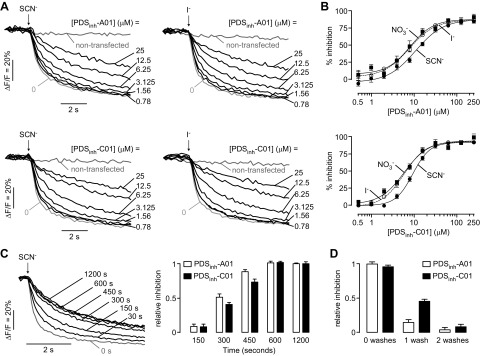
Concentration-dependence measurements were done for inhibition of pendrin-mediated Cl− exchange for SCN− and I− by PDSinh-A01 and PDSinh-C01, with representative original curves shown in Fig. 2A. The data are summarized in Fig. 2B, showing similar micromolar drug concentration causing 50% inhibition (IC50) values for inhibition of Cl−/SCN−, Cl−/I−, and Cl−/NO3− exchange by both compounds. The kinetics of PDSinh-A01 and PDSinh-C01 action were measured for Cl−/SCN− exchange using inhibitors at 10 μM, a concentration producing partial inhibition (Fig. 2C). Near-zero inhibition was seen for both inhibitors within 30 s of compound addition, with ∼50% inhibition observed by 5 min, suggesting an intracellular site of action. Washout studies using compound at 25 μM showed full reversibility (Fig. 2D).
Figure 2.
Characterization of pendrin inhibitors. A) Original fluorescence quenching curves for inhibition of pendrin-mediated Cl−/SCN− (left) and Cl−/I− (right) exchange by PDSinh-A01 (top) and PDSinh-C01 (bottom). B) Concentration-dependent inhibition of pendrin-mediated Cl−/SCN−, Cl−/I−, and Cl−/NO3− exchange by PDSinh-A01 (top) and PDSinh-C01 (bottom). IC50 values for Cl−/SCN− exchange for both compounds were ∼9 μM. PDSinh-A01 and PDSinh-C01 inhibited Cl−/I− and Cl−/NO3− exchange with IC50 of ∼8 µM and ∼5 μM, respectively. C) Kinetics of inhibition of pendrin-mediated Cl−/SCN− exchange in which 10 μM PDSinh-A01 was added for indicated times (left) and summary (right) for PDSinh-A01 and PDSinh-C01. D) Reversibility of PDSinh-A01 and PDSinh-C01. Data shown as means ± sem (n = 4).
Pendrin inhibitors block Cl−/HCO3− exchange
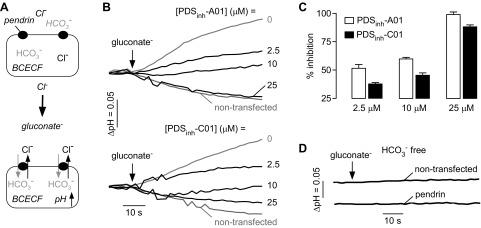
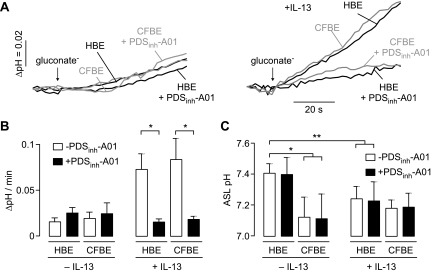
Cl−/HCO3− exchange is the relevant pendrin function in the airways during inflammatory responses (10, 11, 26, 35). Pendrin-mediated Cl−/HCO3− exchange was measured in pendrin-expressing FRT cells using BCECF to report cytoplasmic pH (pHi) (Fig. 3A). BCECF-labeled cells were incubated in a Cl−-containing HCO3−-buffered solution, and Cl−/HCO3− exchange was initiated by application of a gluconate-containing HCO3−-buffered solution to drive Cl− efflux and HCO3− influx, producing cytoplasmic alkalinization with a rate of ∼0.1 pH units/min (Fig 3B). Cytoplasmic alkalinization was strongly inhibited by 25 μM PDSinh-A01 or PDSinh-C01, with the fluorescence responses similar to those in cells not expressing pendrin (Fig. 3B, top). Fifty percent inhibition was seen at ∼2.5 μM PDSinh-A01 (Fig. 3C). In control experiments done in the absence of HCO3−, application of the gluconate-containing solution to cells did not alter pHi in pendrin-expressing or nontransfected cells, indicating that observed pH changes are HCO3− dependent, as expected (Fig. 3D).
Figure 3.
Inhibition of pendrin-mediated Cl−/HCO3− exchange. A) Schematic of assay showing replacement of extracellular Cl− for gluconate in BCECF-loaded FRT cells expressing human pendrin causes Cl− efflux and HCO3− influx, resulting in cytoplasmic alkalinization. B) Representative BCECF fluorescence data showing inhibition of Cl−/HCO3− exchange by PDSinh-A01 and PDSinh-C01. C) Concentration-dependent inhibition of pendrin-mediated Cl−/HCO3− exchange by PDSinh-A01 and PDSinh-C01. Data shown as means ± sem (n = 3–9 cultures). D) Control study done in nontransfected and pendrin expressing cells in absence of HCO3−. BCECF ratio imaging indicated initial cytoplasmic pH of ∼7.3 in all cultures studied.
Specificity of PDSinh-A01 in human airway epithelial cell cultures
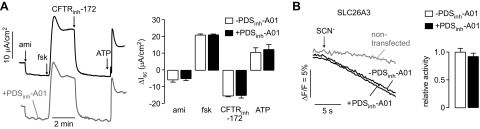
Further experiments were done with PDSinh-A01 because of its greater inhibition potency for Cl−/HCO3− exchange than PDSinh-C01. To investigate PDSinh-A01 specificity, the major ion transport pathways in HBE cells were studied by Isc analysis. Cells were treated with IL-13 to increase Ca2+-activated Cl− conductance (CaCC) activity (41). For Isc measurements, cells were sequentially treated with amiloride to inhibit the epithelial sodium channel (ENaC), forskolin to activate the cystic fibrosis transmembrane conductance regulator (CFTR), CFTRinh-172 to inhibit CFTR, and ATP to activate CaCC. In the absence of PDSinh-A01, ion channel modulators produced the anticipated changes in Isc (Fig. 4A). Pretreatment of cells for 30 min with 25 µM PDSinh-A01 did not alter the Isc responses, indicating that PDSinh-A01 did not affect the activity of ENaC, CFTR, CaCC, the various other transporters (including K+ channels and NKCC1 cotransporter) required to support their activity, or tight junction conductance.
Figure 4.
PDSinh-A01 selectivity studies. A) Isc in IL-13-treated HBE cells in response to agonists and inhibitors that target key ion transport processes: 20 μM amiloride; 20 μM forskolin; 10 μM CFTRinh-172; 100 mM ATP. Experiments were done in absence (left, top) and presence (left, bottom) of PDSinh-A01 (25 μM, 30 min pretreatment). (right) Summary of ΔIsc data (means ± sem, n = 3, differences not significant). B) PDSinh-A01 (25 μM, 30 min) does not inhibit thiocyanate transport by human SLC26A3 as assayed by YFP fluorescence responses after application of 70 mM SCN− gradient. Fluorescence responses (left) and data summary (right). Data shown as means ± sem (n = 17–25 cells). P value differences are not significant.
Experiments were also done to investigate PDSinh-A01 effect on SLC26A3 [CLD (congenital chloride diarrhea)/DRA (down-regulated in adenoma)], the protein most similar to pendrin in sequence (∼50% identity). SLC26A3 activity was measured using a SCN− transport assay that produced yellow fluorescent protein (YFP) fluorescence quenching (Fig. 4B). YFP fluorescence quenching in SLC26A3-expressing cells in response to an SCN− gradient was not significantly inhibited by PDSinh-A01.
Pendrin mediates Cl−/HCO3− exchange in IL-13-treated airway epithelial cell cultures
Pendrin-mediated Cl−/HCO3− exchange was measured in BCECF-labeled HBE and CFBE cells under basal conditions and after pendrin up-regulation by IL-13, a cytokine that is elevated in multiple airway disease states, including CF. In control cultures (no added IL-13) pH changes in response to apical application of a gluconate gradient to drive Cl− efflux and HCO3− influx were not altered by PDSinh-A01, suggesting little or no pendrin activity under basal conditions (Fig. 5A, left). However, treatment of cells with IL-13, which strongly up-regulates pendrin expression (10, 11, 35), produced a robust intracellular alkalinization in response to a gluconate gradient, which was inhibited by 25 μM PDSinh-A01 (Fig. 5A, right; data summary in Fig. 5B). Similar results were found in HBE and CFBE cultures. Quantitative PCR analysis indicated that pendrin transcript levels were increased by 30- to 35-fold in the HBE and CFBE cultures (data not shown), similar to fold increases previously reported in similar cell systems (24, 25, 35).
Figure 5.
PDSinh-A01 inhibits Cl−/HCO3− exchange in IL-13-treated primary human bronchial epithelial cell cultures but does not change ASL pH. A) BCECF fluorescence in HBE and CFBE cells in response to extracellular Cl− replacement by gluconate in control (left) and IL-13-treated (right) cells, with PDSinh-A01 (25 μM, 30 min) as indicated. B) Data shown as means ± sem (n = 3–4 cultures) *P < 0.05 comparing without vs. with PDSinh-A01. C) PDSinh-A01 (25 μM, 4 h) does not alter ASL pH, with data shown for HBE and CFBE cells under control and IL-13-treated conditions. Data shown as means ± sem (n = 3–7 cultures). *P < 0.005; **P < 0.05. BCECF ratio imaging indicated initial cytoplasmic pH of ∼7.3 in all cultures studied.
To investigate the possible involvement of pendrin in ASL pH regulation, fluorescence ratio imaging was done of the ASL in HBE and CFBE cultures after staining with BCECF-dextran. In the absence of IL-13 treatment, ASL pH was ∼0.3 pH units more acidic in CFBE than HBE cultures (Fig. 5C; pH ∼7.4 vs. ∼7.1), in agreement with prior studies showing relative ASL acidity in CF (42, 43). PDSinh-A01 did not alter ASL pH under basal conditions. Treatment of HBE cell cultures with IL-13 produced a significant decrease in ASL pH by ∼0.2 pH units, although no change in ASL pH was seen in IL-13-treated CF cultures. Incubation of IL-13-treated HBE and CFBE cell cultures with PDSinh-A01 did not alter ASL pH. These studies indicate that pendrin is not a key determinant of ASL pH under basal or IL-13-stimulated conditions, suggesting that alternative pathways contribute to ASL pH homeostasis.
Pendrin inhibition increases ASL volume in IL-13-treated HBE and CFBE cultures
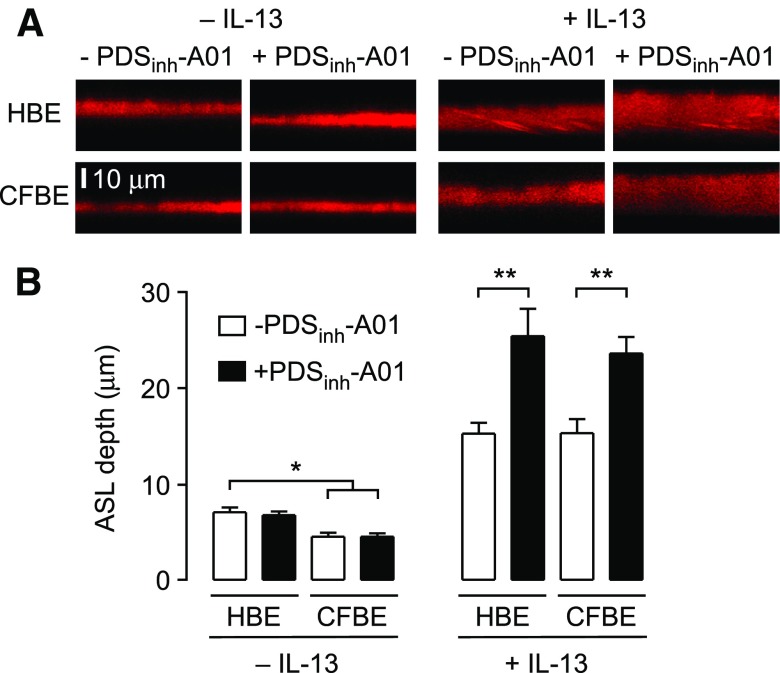
ASL depth was measured in HBE and CFBE cell cultures under basal conditions and with chronic IL-13-treatment. CFBE cell cultures showed reduced ASL depth compared to HBE cell cultures in the absence of IL-13-treatment (∼8 µm vs. ∼5 μm; Fig. 6A, left; summary in Fig. 6B), in agreement with prior studies (39, 44, 45). Under these basal conditions, treatment of HBE or CFBE cell cultures with 25 µM PDSinh-A01 did not significantly change ASL depth. Treatment of HBE and CFBE cell cultures with IL-13 produced a significant increase in ASL depth to ∼16 μm. Remarkably, treatment of IL-13-treated HBE and CFBE cell cultures with PDSinh-A01 produced a significant further increase in ASL depth of ∼8 μm (Fig. 6A, right; summary in Fig. 6B). Pendrin inhibition thus causes ASL hydration in IL-13-treated HBE and CFBE cell cultures.
Figure 6.
PDSinh-A01 increases ASL depth in IL-13-treated primary HBE cell cultures. A. Reconstructions of confocal z-stacks showing rhodamine dextran-labeled ASL in HBE and CFBE cells, without and with IL-13-treatment. PDSinh-A01 was used at 25 μM. B. Summary of ASL depth. Data shown as means ± sem (n = 6–12 cultures). *P < 0.05 compared to HBE cultures (no IL-13); **P < 0.05 compared to IL-13-treated cultures. IL-13 increased ASL depth for all conditions compared to no IL-13-treatment.
DISCUSSION
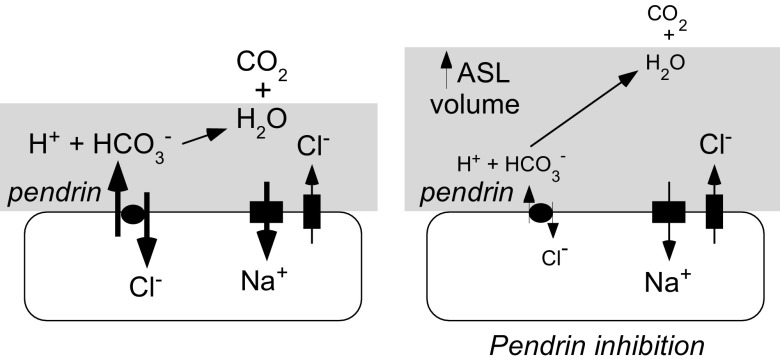
A pendrin-selective inhibitor discovered by high-throughput screening, PDSinh-A01, fully blocked, with low micromolar potency, pendrin-mediated exchange of Cl− for HCO3−, I−, and SCN−. The best previously described pendrin inhibitor, niflumic acid, produces <40% inhibition at 100 μM and has poor specificity (7). PDSinh-A01 increased ASL volume in IL-13-treated airway cell cultures from both non-CF and CF humans, providing pharmacologic evidence for pendrin as a key regulator of ASL hydration in airway cell cultures exposed to an inflammatory stimulant. Because pendrin mediates electroneutral Cl−/HCO3− exchange, pendrin activity will produce net osmol movement into the cell because some HCO3− in the ASL becomes protonated and liberates CO2; pendrin inhibition therefore increases ASL depth in the steady state, as depicted in Fig. 7. Increased airway hydration by pendrin inhibition provides a novel strategy for treatment of CF, and possibly other inflammatory lung diseases, and validates pendrin as a drug target in the lung (46). Although pendrin activity and increased ASL hydration were seen only in cell cultures after IL-13 treatment, we note that asthmalike symptoms (cough, wheezing, airway hyperresponsiveness) and increased IL-13 are prevalent in subjects with CF (29), and that IL-13 is common to several airway diseases. Further, alternative CF-relevant inflammatory pathways also increase pendrin expression in freshly isolated airway epithelial cells from CF subjects (23). There is considerable interest in identifying ion transport modulators to increase ASL depth in CF, with ENaC inhibitors and activators of CaCC under development (47). The current study confirms that pendrin is an alternative target for ASL rehydration.
Figure 7.
Schematic showing increased ASL depth after pendrin inhibition.
In CF, defective Cl− and HCO3− transport into the ASL alters salt and water transport, promoting ASL volume depletion. There is a considerable body of evidence linking ASL volume dysregulation in CF to impaired immune functions and CF disease pathogenesis (48–50). Rehydration of the airway surface is thus considered to be a useful therapeutic approach in CF. Evidence from confocal microscopy, transmission electron microscopy, and fluorescence photobleaching indicate that the ASL consists of 2 discrete layers (51, 52): a superficial mucus layer that contains large gel-forming mucins (MUC5AC and MUC5B) that trap particulates for subsequent mucociliary clearance, and, in contact with cells and cilia, a periciliary layer (PCL) that contains large membrane-bound mucins (MUC1 and MUC4) (52). Classically, the ASL was considered to consist of a mucus gel supported on a waterlike liquid layer surrounding cilia (gel-on-liquid model). More recently, Button and colleagues (52) showed that tethered mucins in the PCL form a mesh to prevent entry of gel-forming mucins and support ciliary beating, and they proposed a gel-on-brush model to describe the ASL. Photobleaching studies support this concept and have established that the fluid phase viscosity of the mucus layer and PCL are similar in non-CF subjects (7–10 times more viscous than saline), but significantly elevated in both regions in CF cultures (25–30 times more viscous than saline) (51). In addition to forming a permeability barrier, tethered mucins in the PCL generate an osmotic pressure to regulate PCL hydration (52). The osmotic potential exerted by CF mucus (>8% solids), but not healthy mucus (∼2% solids), was demonstrated to be sufficient to dehydrate the PCL and collapse cilia (52), and to restrict mobility of bacteria and foster formation of bacterial biofilm precursor macrocolonies (53). Restoration of CFTR expression in CF primary epithelial cells by viral transduction significantly increases ASL depth, ciliary beat frequency, and mucus transport, supporting the idea that loss of CFTR function mediates ASL dehydration (54). Therefore, the bulk of available evidence links reduced ASL volume to increased propensity for infection, with reduced CFTR ion transport leading to reduced mucin hydration in the mucus layer followed by PCL dehydration, ciliary collapse, mucostasis, and bacterial colonization. The current study validates pendrin as a novel target for therapeutic intervention in CF by increasing ASL hydration.
An additional conclusion of the current study is that under basal, noninflammatory conditions (no added IL-13) pendrin does not influence ASL hydration status in HBE and CFBE cell cultures. This observation is in agreement with the absence of pendrin function and low pendrin transcript expression found here and in a prior study (24). A prior study that investigated ASL depth homeostasis in airway cell cultures from subjects with pendrin loss-of-function mutations showed that ASL depth was increased by approximately 2-fold compared to cultures derived from control subjects, both without and with IL-13 (35). A possible explanation for the difference in results is that pendrin loss of function in DFNB4 subjects produces compensatory changes in gene expression or protein function that may alter ASL depth homeostasis. Tracheal epithelial cell cultures from wild-type and pendrin knockout mice showed that pendrin reduced ASL depth only in IL-13-treated cells (11), in agreement with the current study.
CFTR mutation also results in acidification of the ASL (43) and submucosal gland secretions (40). The activity of several antimicrobial constituents in the ASL, including lysozyme and lactoferrin, is reduced by acidic pH (43). Antimicrobial activity was reduced in tracheas from non-CF pigs after ASL acidification (by increasing CO2), whereas ASL alkalinization in tracheas from CF pigs (by addition of NaHCO3) elevated antimicrobial activity (43). These studies provided an additional mechanism by which altered ASL properties in CF could promote microbial infection. A possible deleterious action of pendrin inhibition could be reduced ASL pH resulting from reduced HCO3− delivery to the ASL; however, our studies indicate that PDSinh-A01 does not alter ASL pH in HBE and CFBE cultures, both in the presence and absence of IL-13, suggesting that alternative pathways regulate ASL pH over the time course of experiments. Treatment of HBE cultures with IL-13 reduced ASL pH, albeit to a lesser degree than that observed in CFBE cultures. This observation is consistent with studies of pH in exhaled breath condensate from subjects with asthma (55), which is associated with elevated IL-13 levels. Although asthma is generally considered to be result from exposure to allergens, recent studies have revealed an association between asthma severity and bacterial infections in the respiratory tract (56). As seen in CF, decreased ASL pH in IL-13-stimulated airways may contribute to bacterial infection, though further work is needed to prove this concept.
Increased pendrin expression has been described in various human airway diseases including asthma, chronic obstructive pulmonary disease, bacterial and viral infections, and sinusitis. The present study shows that pendrin inhibition increases ASL depth in IL-13-treated cell cultures from non-CF subjects. In contrast to CF, there are no data directly linking ASL dehydration to pathogenesis of other lung diseases in which pendrin is up-regulated. However, studies of mucus properties in chronic bronchitis and asthma are suggestive of ASL dehydration (57–59), and hence pendrin inhibition may be efficacious in a variety of pulmonary diseases.
There are several prior reports on the biologic properties of the class A tetrahydropyrazolopyridine core scaffold identified in this study. Tetrahydropyrazolopyridines were discovered as novel allosteric inhibitors of mutant isocitrate dehydrogenase 1 (IDH1) and were shown to affect proliferation of primary IDH1 mutant acute myeloid leukemia cells (60). Virtual screening identified compounds bearing a tetrahydropyrazolopyridines ring as potential novel antagonist for activin, a TGF-β protein (61), and for the G-protein-coupled NK3 receptor (62). There are no prior reports on the biologic effects of the pyrazolothiophenesulfonamide scaffold as found in class C inhibitors. A similar pyrazolothiophenesulfonamide was identified from virtual screening as potential novel DNA gyrase inhibitors (63). Active tetrahydropyrazolopyridines and pyrazolothiophenesulfonamide analogs have favorable druglike properties, including the presence of multiple hydrogen bond acceptors, molecular weight of 418 and 382 Da, aLogP values of 3.1 and 5.0, and topological polar surface areas of 58.2 and 70.6 Å2 for class A and C, respectively. These values are consistent with the Lipinski et al. (64) and Veber et al. (65) guidelines for orally bioavailable drugs. Further medicinal chemistry to generate targeted analogs of both inhibitor classes could lead to identification of compounds with greatly improved potency.
Expression of pendrin outside of the airways warrants consideration in the potential use of pendrin inhibitors to treat airway disorders. Hearing loss in Pendred syndrome is believed to result from inner-ear malformation that occurs during fetal development and would thus not be anticipated to be a complication of pharmacologic pendrin inhibition. Additional manifestations of Pendred syndrome, including thyroid goiter and vestibular dysfunction [which presents in mice but rarely in humans (66)], show limited penetrance, suggesting the existence of alternative ion conductance pathways or compensatory mechanisms, though studies using pendrin inhibitors will be needed. Finally, kidney manifestations are rare in Pendred syndrome, suggesting that pendrin inhibition is unlikely to alter renal function.
Acknowledgments
The authors thank W. Namkung (Yonsei University, Seoul, South Korea) for providing cDNA encoding human pendrin. This work was supported by U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases Grants P30 DK072517, DK101373, NIH National Institute of Biomedical Imaging and Bioengineering Grant R37 EB000415, NIH National Institute of Allergy and Infectious Diseases Grant AI111634, and by a Research Development Program grant from the Cystic Fibrosis Foundation. The University of California, San Francisco Helen Diller Cancer Center Genome Analysis Core is supported by NIH National Cancer Institute Grant P30CA082103.
Glossary
- ASL
airway surface liquid
- CaCC
calcium-activated chloride channel
- CF
cystic fibrosis
- CFBE
human cystic fibrosis bronchial epithelial
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial sodium channel
- EYFP-HIF
enhanced yellow fluorescent protein–H148Q/I152L/F46L
- FRT
Fischer rat thyroid
- HBE
human bronchial epithelial
- IC50
drug concentration causing 50% inhibition
- Isc
short-circuit current
- PCL
periciliary layer
- UCSF
University of California, San Francisco
- YFP
yellow fluorescent protein
REFERENCES
- 1.Everett L. A., Glaser B., Beck J. C., Idol J. R., Buchs A., Heyman M., Adawi F., Hazani E., Nassir E., Baxevanis A. D., Sheffield V. C., Green E. D. (1997) Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat. Genet. 17, 411–422 [DOI] [PubMed] [Google Scholar]
- 2.Royaux I. E., Suzuki K., Mori A., Katoh R., Everett L. A., Kohn L. D., Green E. D. (2000) Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology 141, 839–845 [DOI] [PubMed] [Google Scholar]
- 3.Scott D. A., Wang R., Kreman T. M., Sheffield V. C., Karniski L. P. (1999) The Pendred syndrome gene encodes a chloride–iodide transport protein. Nat. Genet. 21, 440–443 [DOI] [PubMed] [Google Scholar]
- 4.Soleimani M., Greeley T., Petrovic S., Wang Z., Amlal H., Kopp P., Burnham C. E. (2001) Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am. J. Physiol. Renal Physiol. 280, F356–F364 [DOI] [PubMed] [Google Scholar]
- 5.Scott D. A., Karniski L. P. (2000) Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am. J. Physiol. Cell Physiol. 278, C207–C211 [DOI] [PubMed] [Google Scholar]
- 6.Royaux I. E., Wall S. M., Karniski L. P., Everett L. A., Suzuki K., Knepper M. A., Green E. D. (2001) Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc. Natl. Acad. Sci. USA 98, 4221–4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedemonte N., Caci E., Sondo E., Caputo A., Rhoden K., Pfeffer U., Di Candia M., Bandettini R., Ravazzolo R., Zegarra-Moran O., Galietta L. J. (2007) Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J. Immunol. 178, 5144–5153 [DOI] [PubMed] [Google Scholar]
- 8.Shcheynikov N., Yang D., Wang Y., Zeng W., Karniski L. P., So I., Wall S. M., Muallem S. (2008) The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J. Physiol. 586, 3813–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett L. A., Morsli H., Wu D. K., Green E. D. (1999) Expression pattern of the mouse ortholog of the Pendred’s syndrome gene (PDS) suggests a key role for pendrin in the inner ear. Proc. Natl. Acad. Sci. USA 96, 9727–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao I., Kanaji S., Ohta S., Matsushita H., Arima K., Yuyama N., Yamaya M., Nakayama K., Kubo H., Watanabe M., Sagara H., Sugiyama K., Tanaka H., Toda S., Hayashi H., Inoue H., Hoshino T., Shiraki A., Inoue M., Suzuki K., Aizawa H., Okinami S., Nagai H., Hasegawa M., Fukuda T., Green E. D., Izuhara K. (2008) Identification of pendrin as a common mediator for mucus production in bronchial asthma and chronic obstructive pulmonary disease. J. Immunol. 180, 6262–6269 [DOI] [PubMed] [Google Scholar]
- 11.Nakagami Y., Favoreto S. Jr., Zhen G., Park S. W., Nguyenvu L. T., Kuperman D. A., Dolganov G. M., Huang X., Boushey H. A., Avila P. C., Erle D. J. (2008) The epithelial anion transporter pendrin is induced by allergy and rhinovirus infection, regulates airway surface liquid, and increases airway reactivity and inflammation in an asthma model. J. Immunol. 181, 2203–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazo-Fernandez Y., Aguilera G., Pham T. D., Park A. Y., Beierwaltes W. H., Sutliff R. L., Verlander J. W., Pacak K., Osunkoya A. O., Ellis C. L., Kim Y. H., Shipley G. L., Wynne B. M., Hoover R. S., Sen S. K., Plotsky P. M., Wall S. M. (2015) Pendrin localizes to the adrenal medulla and modulates catecholamine release. Am. J. Physiol. Endocrinol. Metab. 309, E534–E545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wangemann P. (2011) The role of pendrin in the development of the murine inner ear. Cell. Physiol. Biochem. 28, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wangemann P. (2013) Mouse models for pendrin-associated loss of cochlear and vestibular function. Cell. Physiol. Biochem. 32, 157–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp P., Pesce L., Solis-S J. C. (2008) Pendred syndrome and iodide transport in the thyroid. Trends Endocrinol. Metab. 19, 260–268 [DOI] [PubMed] [Google Scholar]
- 16.Wall S. M., Lazo-Fernandez Y. (2015) The role of pendrin in renal physiology. Annu. Rev. Physiol. 77, 363–378 [DOI] [PubMed] [Google Scholar]
- 17.Kuperman D. A., Lewis C. C., Woodruff P. G., Rodriguez M. W., Yang Y. H., Dolganov G. M., Fahy J. V., Erle D. J. (2005) Dissecting asthma using focused transgenic modeling and functional genomics. J. Allergy Clin. Immunol. 116, 305–311 [DOI] [PubMed] [Google Scholar]
- 18.Lewis C. C., Yang J. Y., Huang X., Banerjee S. K., Blackburn M. R., Baluk P., McDonald D. M., Blackwell T. S., Nagabhushanam V., Peters W., Voehringer D., Erle D. J. (2008) Disease-specific gene expression profiling in multiple models of lung disease. Am. J. Respir. Crit. Care Med. 177, 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita K., Morimoto Y., Endoh S., Uchida K., Fukui H., Ogami A., Tanaka I., Horie M., Yoshida Y., Iwahashi H., Nakanishi J. (2010) Identification of potential biomarkers from gene expression profiles in rat lungs intratracheally instilled with C(60) fullerenes. Toxicology 274, 34–41 [DOI] [PubMed] [Google Scholar]
- 20.Oh J.-H., Yang M.-J., Heo J.-D., Yang Y.-S., Park H.-J., Park S.-M., Kwon M.-S., Song C.-W., Yoon S., Yu I. J. (2012) Inflammatory response in rat lungs with recurrent exposure to welding fumes: a transcriptomic approach. Toxicol. Ind. Health 28, 203–215 [DOI] [PubMed] [Google Scholar]
- 21.Ishida A., Ohta N., Suzuki Y., Kakehata S., Okubo K., Ikeda H., Shiraishi H., Izuhara K. (2012) Expression of pendrin and periostin in allergic rhinitis and chronic rhinosinusitis. Allergol. Int. 61, 589–595 [DOI] [PubMed] [Google Scholar]
- 22.Yick C. Y., Zwinderman A. H., Kunst P. W., Grünberg K., Mauad T., Dijkhuis A., Bel E. H., Baas F., Lutter R., Sterk P. J. (2013) Transcriptome sequencing (RNA-Seq) of human endobronchial biopsies: asthma versus controls. Eur. Respir. J. 42, 662–670 [DOI] [PubMed] [Google Scholar]
- 23.Clarke L. A., Sousa L., Barreto C., Amaral M. D. (2013) Changes in transcriptome of native nasal epithelium expressing F508del-CFTR and intersecting data from comparable studies. Respir. Res. 14, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams K. M., Abraham V., Spielman D., Kolls J. K., Rubenstein R. C., Conner G. E., Cohen N. A., Kreindler J. L. (2014) IL-17A induces Pendrin expression and chloride–bicarbonate exchange in human bronchial epithelial cells. PLoS One 9, e103263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshadri S., Lu X., Purkey M. R., Homma T., Choi A. W., Carter R., Suh L., Norton J., Harris K. E., Conley D. B., Kato A., Avila P. C., Czarnocka B., Kopp P. A., Peters A. T., Grammer L. C., Chandra R. K., Tan B. K., Liu Z., Kern R. C., Schleimer R. P. (2015) Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 136, 1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scanlon K. M., Gau Y., Zhu J., Skerry C., Wall S. M., Soleimani M., Carbonetti N. H. (2014) Epithelial anion transporter pendrin contributes to inflammatory lung pathology in mouse models of Bordetella pertussis infection. Infect. Immun. 82, 4212–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J. S., Rosengart M. R., Kondragunta V., Zhang Y., McMurray J., Branch R. A., Choi A. M. K., Sciurba F. C. (2007) Inverse association of plasma IL-13 and inflammatory chemokines with lung function impairment in stable COPD: a cross-sectional cohort study. Respir. Res. 8, 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asquith K. L., Horvat J. C., Kaiko G. E., Carey A. J., Beagley K. W., Hansbro P. M., Foster P. S. (2011) Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog. 7, e1001339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCuaig S., Martin J. G. (2013) How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir. Med. 1, 137–147 [DOI] [PubMed] [Google Scholar]
- 30.Gour N., Wills-Karp M. (2015) IL-4 and IL-13 signaling in allergic airway disease. Cytokine 75, 68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milonski J., Zielinska-Blizniewska H., Majsterek I., Przybyłowska-Sygut K., Sitarek P., Korzycka-Zaborowska B., Olszewski J. (2015) Expression of POSTN, IL-4 and IL-13 in chronic rhinosinusitis with nasal polyps. DNA Cell Biol. 34, 342–349 [DOI] [PubMed] [Google Scholar]
- 32.May R. D., Fung M. (2015) Strategies targeting the IL-4/IL-13 axes in disease. Cytokine 75, 89–116 [DOI] [PubMed] [Google Scholar]
- 33.Donovan C., Bourke J. E., Vlahos R. (In press) Targeting the IL-33/IL-13 axis for respiratory viral infections. Trends Pharmacol. Sci. S0165-6147(16)00005-5. [DOI] [PubMed] [Google Scholar]
- 34.Comandini A., Rogliani P., Nunziata A., Cazzola M., Curradi G., Saltini C. (2009) Biomarkers of lung damage associated with tobacco smoke in induced sputum. Respir. Med. 103, 1592–1613 [DOI] [PubMed] [Google Scholar]
- 35.Lee H. J., Yoo J. E., Namkung W., Cho H.-J., Kim K., Kang J. W., Yoon J.-H., Choi J. Y. (2015) Thick airway surface liquid volume and weak mucin expression in pendrin-deficient human airway epithelia. Physiol. Rep. 3, e12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namkung W., Finkbeiner W. E., Verkman A. S. (2010) CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol. Biol. Cell 21, 2639–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. (2002) Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin–induced intestinal fluid secretion. J. Clin. Invest. 110, 1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haggie P. M., Verkman A. S. (2007) Cystic fibrosis transmembrane conductance regulator–independent phagosomal acidification in macrophages. J. Biol. Chem. 282, 31422–31428 [DOI] [PubMed] [Google Scholar]
- 39.Song Y., Namkung W., Nielson D. W., Lee J.-W., Finkbeiner W. E., Verkman A. S. (2009) Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: dependence on Na+ and Cl− channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1131–L1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song Y., Salinas D., Nielson D. W., Verkman A. S. (2006) Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am. J. Physiol. Cell Physiol. 290, C741–C749 [DOI] [PubMed] [Google Scholar]
- 41.Huang F., Zhang H., Wu M., Yang H., Kudo M., Peters C. J., Woodruff P. G., Solberg O. D., Donne M. L., Huang X., Sheppard D., Fahy J. V., Wolters P. J., Hogan B. L., Finkbeiner W. E., Li M., Jan Y. N., Jan L. Y., Rock J. R. (2012) Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 109, 16354–16359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coakley R. D., Grubb B. R., Paradiso A. M., Gatzy J. T., Johnson L. G., Kreda S. M., O’Neal W. K., Boucher R. C. (2003) Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA 100, 16083–16088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pezzulo A. A., Tang X. X., Hoegger M. J., Alaiwa M. H., Ramachandran S., Moninger T. O., Karp P. H., Wohlford-Lenane C. L., Haagsman H. P., van Eijk M., Bánfi B., Horswill A. R., Stoltz D. A., McCray P. B. Jr., Welsh M. J., Zabner J. (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui H., Grubb B. R., Tarran R., Randell S. H., Gatzy J. T., Davis C. W., Boucher R. C. (1998) Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 45.Tarran R., Trout L., Donaldson S. H., Boucher R. C. (2006) Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J. Gen. Physiol. 127, 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nofziger C., Dossena S., Suzuki S., Izuhara K., Paulmichl M. (2011) Pendrin function in airway epithelia. Cell. Physiol. Biochem. 28, 571–578 [DOI] [PubMed] [Google Scholar]
- 47.Donaldson S. H., Galietta L. (2013) New pulmonary therapies directed at targets other than CFTR. Cold Spring Harb. Perspect. Med. 3, a009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boucher R. C. (2007) Evidence for airway surface dehydration as the initiating event in CF airway disease. J. Intern. Med. 261, 5–16 [DOI] [PubMed] [Google Scholar]
- 49.Boucher R. C. (2007) Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu. Rev. Med. 58, 157–170 [DOI] [PubMed] [Google Scholar]
- 50.Ghosh A., Boucher R. C., Tarran R. (2015) Airway hydration and COPD. Cell. Mol. Life Sci. 72, 3637–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derichs N., Jin B. J., Song Y., Finkbeiner W. E., Verkman A. S. (2011) Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 25, 2325–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Button B., Cai L.-H., Ehre C., Kesimer M., Hill D. B., Sheehan J. K., Boucher R. C., Rubinstein M. (2012) A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsui H., Wagner V. E., Hill D. B., Schwab U. E., Rogers T. D., Button B., Taylor R. M. II, Superfine R., Rubinstein M., Iglewski B. H., Boucher R. C. (2006) A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 103, 18131–18136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L., Button B., Gabriel S. E., Burkett S., Yan Y., Skiadopoulos M. H., Dang Y. L., Vogel L. N., McKay T., Mengos A., Boucher R. C., Collins P. L., Pickles R. J. (2009) CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 7, e1000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carraro S., Folesani G., Corradi M., Zanconato S., Gaston B., Baraldi E. (2005) Acid–base equilibrium in exhaled breath condensate of allergic asthmatic children. Allergy 60, 476–481 [DOI] [PubMed] [Google Scholar]
- 56.Huang Y. J., Nariya S., Harris J. M., Lynch S. V., Choy D. F., Arron J. R., Boushey H. (2015) The airway microbiome in patients with severe asthma: associations with disease features and severity. J. Allergy Clin. Immunol. 136, 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fahy J. V., Dickey B. F. (2010) Airway mucus function and dysfunction. N. Engl. J. Med. 363, 2233–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson W. H., Coakley R. D., Button B., Henderson A. G., Zeman K. L., Alexis N. E., Peden D. B., Lazarowski E. R., Davis C. W., Bailey S., Fuller F., Almond M., Qaqish B., Bordonali E., Rubinstein M., Bennett W. D., Kesimer M., Boucher R. C. (2015) The relationship of mucus concentration (hydration) to mucus osmotic pressure and transport in chronic bronchitis. Am. J. Respir. Crit. Care Med. 192, 182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rubin B. K. (2014) Secretion properties, clearance, and therapy in airway disease. Transl. Respir. Med. 2, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okoye-Okafor U. C., Bartholdy B., Cartier J., Gao E. N., Pietrak B., Rendina A. R., Rominger C., Quinn C., Smallwood A., Wiggall K. J., Reif A. J., Schmidt S. J., Qi H., Zhao H., Joberty G., Faelth-Savitski M., Bantscheff M., Drewes G., Duraiswami C., Brady P., Groy A., Narayanagari S. R., Antony-Debre I., Mitchell K., Wang H. R., Kao Y. R., Christopeit M., Carvajal L., Barreyro L., Paietta E., Makishima H., Will B., Concha N., Adams N. D., Schwartz B., McCabe M. T., Maciejewski J., Verma A., Steidl U. (2015) New IDH1 mutant inhibitors for treatment of acute myeloid leukemia. Nat. Chem. Biol. 11, 878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J., Mishra R. K., Schiltz G. E., Makanji Y., Scheidt K. A., Mazar A. P., Woodruff T. K. (2015) Virtual high-throughput screening to identify novel activin antagonists. J. Med. Chem. 58, 5637–5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geldenhuys W. J., Kuzenko S. R., Simmons M. A. (2010) Virtual screening to identify novel antagonists for the G protein–coupled NK3 receptor. J. Med. Chem. 53, 8080–8088 [DOI] [PubMed] [Google Scholar]
- 63.Brvar M., Perdih A., Renko M., Anderluh G., Turk D., Solmajer T. (2012) Structure-based discovery of substituted 4,5′-bithiazoles as novel DNA gyrase inhibitors. J. Med. Chem. 55, 6413–6426 [DOI] [PubMed] [Google Scholar]
- 64.Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 [DOI] [PubMed] [Google Scholar]
- 65.Veber D. F., Johnson S. R., Cheng H. Y., Smith B. R., Ward K. W., Kopple K. D. (2002) Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 [DOI] [PubMed] [Google Scholar]
- 66.Everett L. A., Belyantseva I. A., Noben-Trauth K., Cantos R., Chen A., Thakkar S. I., Hoogstraten-Miller S. L., Kachar B., Wu D. K., Green E. D. (2001) Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Hum. Mol. Genet. 10, 153–161 [DOI] [PubMed] [Google Scholar]