Abstract
During wound healing of the skin, keratinocytes disassemble hemidesmosomes and reorganize their actin cytoskeletons in order to exert traction forces on and move directionally over the dermis. Nonetheless, the transmembrane hemidesmosome component collagen XVII (ColXVII) is found in actin-rich lamella, situated behind the lamellipodium. A set of actin bundles, along which ColXVII colocalizes with actinin4, is present at each lamella. Knockdown of either ColXVII or actinin4 not only inhibits directed migration of keratinocytes but also relieves constraints on actin bundle retrograde movement at the site of lamella, such that actin bundle movement is enhanced more than 5-fold. Moreover, whereas control keratinocytes move in a stepwise fashion over a substrate by generating alternating traction forces, of up to 1.4 kPa, at each flank of the lamellipodium, ColXVII knockdown keratinocytes fail to do so. In summary, our data indicate that ColXVII-actinin4 complexes at the lamella of a moving keratinocyte regulate actin dynamics, thereby determining the direction of cell movement.—Hiroyasu, S., Colburn, Z. T., Jones, J. C. R. A hemidesmosomal protein regulates actin dynamics and traction forces in motile keratinocytes.
Keywords: cytoskeleton, lamella, lamellipodium, matrix adhesion, migration
The migration of epithelial cells is precisely regulated during development and the repair of wounds, such as those in the skin, and is critical for the dissemination of tumor cells. The mechanisms controlling epithelial cell movement involve cytoskeletal rearrangements and modifications of cell–cell and cell–matrix adhesion complexes. Such changes allow the establishment of cell polarity to ensure directed migration.
For a cell to move, the paradigm is that actin polymerization induces a protrusion at the leading edge of the cell (1). Focal adhesion protein complexes anchor the base of the leading edge to the substrate and integrate matrix anchorage sites with the actin cytoskeleton (1). Contraction of actin filaments, interacting with these anchorage sites, results in the transmission of traction forces (TFs) to the substrate that are necessary for the net forward movement of the cell body (2, 3). Such a mechanism is supported by a number of studies and is consistent with the motility behavior of different cell types as well as their cytoskeleton and focal adhesion protein organizations and functions (4, 5). However, this mechanism fails to take into account the presence of proteins of another cell–matrix adhesion, termed the hemidesmosome, which is present in many types of epithelial cells, most notably those of the skin (keratinocytes) (6, 7).
Hemidesmosomes are assembled by epithelial cells in contact with the basement membrane zone in skin, bladder, cornea, and various mucosae and glands, and tether the epithelium to the connective tissue (8–10). Each hemidesmosome consists of at least 8 distinct proteins including the heterodimeric matrix receptor α6β4 integrin, a 180 kDa type II transmembrane protein termed collagen XVII (ColXVII; 180 kDa bullous pemphigoid antigen), the matrix protein laminin-332, and 2 plakin family members, a 230 kDa molecule termed bullous pemphigoid antigen 1e (BPAG1e) and plectin (8–10). BPAG1e and plectin mediate keratin cytoskeleton anchorage to the cell surface by interacting with the cytoplasmic domains of α6β4 integrin and ColXVII. In turn, the extracellular domains of α6β4 integrin and ColXVII attach cells to laminin-332. Loss of laminin-332, ColXVII, BPAG1e, α6 integrin, β4 integrin, or plectin perturb connective tissue–epithelial cohesion and lead to skin blistering (11).
However, hemidesmosome protein expression is not restricted to stationary cells (12–14). Hemidesmosome protein complexes (HPCs) are present in motile keratinocytes in vitro and in keratinocytes at the front of the epithelium moving over a wound bed in vivo (14). Moreover, up-regulation of hemidesmosome proteins is a hallmark of certain epithelial tumors (15). Indeed, our previous work has provided evidence that HPCs at the base of the leading lamellipodium of a moving keratinocyte act as a scaffold for Rac1 signaling, which activates the phosphatase slingshot to dephosphorylate cofilin and induce local actin polymerization (16–18). Specifically, keratinocytes that exhibit deficiencies in any of the 3 major components of the complex, namely β4 integrin, BPAG1e, or ColXVII, display motility abnormalities in directed migration and a decrease in both Rac1 and cofilin activity (13, 16–20). However, interestingly, expression of constitutively active Rac1 rescues motility deficiencies of BPAG1e knockdown (KD) keratinocytes but not keratinocytes deficient in β4 integrin or ColXVII (17).
We have suggested that the likely explanation for these results is that both β4 integrin and ColXVII have regulatory and/or structural functions in addition to their roles in supporting Rac1 activation in motile cells (16, 17). In this regard, β4 integrin appears to be involved in both binding to and organizing the laminin-rich matrix of a keratinocyte, but the regulatory/structural functions of ColXVII are unclear (20). Thus, in order to define precisely the role of ColXVII in keratinocyte motility, we evaluated cell motility behavior, actin dynamics, adhesion protein localization, and TF generation in vitro in live single keratinocytes and keratinocytes deficient in ColXVII as they move on a gel substrate of 5 kPa stiffness, equivalent to the dermis (21). Our results have uncovered a new role for ColXVII in regulating actin cytoskeleton dynamics and TFs.
MATERIALS AND METHODS
Cell culture
All immortalized human epidermal keratinocyte (iHEK) lines were cultured in defined keratinocyte serum-free medium supplemented with a 1% penicillin/streptomycin mixture (Invitrogen, Carlsbad, CA, USA) and maintained at 37°C. KD or control iHEKs were stably transduced with a lentiviral vector, under puromycin selection, encoding ColXVII-specific short hairpin RNA (shRNA), actinin4-specific shRNA, or scrambled shRNA, respectively, as described elsewhere (18, 22). In some studies, KD keratinocytes were infected with either adenovirus encoding green fluorescent protein (GFP)-tagged N-terminal ColXVII fragment containing amino acid residues 1-517 (ColXVII Nt1-517) or GFP (18). For live cell imaging of actin, cells were infected with adenovirus encoding red fluorescent protein (RFP)-tagged LifeAct (Ibidi USA, Madison, WI, USA).
Preparation of cell substrates
Polyacrylamide gels were prepared as previously described using a ratio of 8% acrylamide/0.25% bis-acrylamide to generate substrates with a stiffness of approximately 5 kPa (23). The gels were activated with sulfo-SANPAH (Life Technologies, Grand Island, NY, USA) and coated with 10 μg/ml human fibronectin (Sigma-Aldrich, St. Louis, MO, USA) to promote cell adhesion (24). For TF microscopy studies, gels were impregnated with 0.1 μm, carboxylate-modified red (560/605) FluoSpheres (Life Technologies).
Antibodies and immunofluorescence probes
Rabbit polyclonal antibodies against actinin1, actinin4, and paxillin (EP2528Y, EPR2533(2), and Y113, respectively) were purchased from Abcam (Cambridge, MA, USA), and the mouse monoclonal antibody against β4 integrin (450-11A) was purchased from BD Biosciences (San Jose, CA, USA). Both rabbit polyclonal IgG and mouse monoclonal IgM antibodies, J17 and 1804b, respectively, directed against the cytoplasmic domain (N terminus) of ColXVII are described elsewhere (25). The mouse monoclonal anti-GFP antibody (RQ2), conjugated to agarose, was purchased from Medical & Biologic Laboratories (Nagoya, Aichi, Japan). Rhodamine-conjugated phalloidin was purchased from Life Technologies. Fluorescein-conjugated goat anti-mouse IgG and IgM antibodies (115-095-166 and 115-095-020, respectively) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Cy5-conjugated goat anti-rabbit IgG (111-495-144) and Alexa Fluor 647–conjugated goat anti-mouse IgG (A-21235) were purchased from Jackson ImmunoResearch Laboratories and Life Technologies, respectively. The horseradish peroxidase–conjugated goat anti-mouse IgG (115-035-166) and anti-rabbit IgG (7074S) antibodies were purchased from Jackson ImmunoResearch Laboratories and Cell Signaling Technology (Beverly, MA, USA), respectively.
Observations of live cells, motility assays, and kymography
Motility assay procedures have been previously described (20). For single cell motility studies, keratinocytes were plated on fibronectin-coated polyacrylamide gels 18 to 24 h before the start of the assay. In all motility assays, cells were imaged every 30 s over 1 h on a heated stage with a Leica DMi8 microscope (Leica Microsystems, Buffalo Grove, IL, USA). Cell motility behavior was analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA, USA). For each cell type, speed and processivity were calculated. Processivity was calculated as follows: processivity = maximum displacement from cell’s origin/path length.
For kymographic analyses of actin retrograde movement, RFP-tagged LifeAct expressing cells were plated onto 35 mm glass-bottomed dishes (MatTek, Ashland, MA, USA) 18 to 24 h before observation with a ×63 objective every 15 s for up to 30 min on a Leica TCS SP8 X confocal microscope (Leica Microsystems). A kymograph was generated from a 1-pixel-wide line drawn in the direction of migration using the Multiple Kymograph plugin (developed by Rietdorf and Seitz, European Molecular Biology Laboratory, Heidelberg, Baden-Württemberg, Germany) for ImageJ (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA). Kymographs were used to calculate actin bundle speed.
Immunoprecipitation, SDS-PAGE, and immunoblot analyses
Extracts of cells on polyacrylamide gels were prepared by placing the coverslips face down on immunoprecipitation buffer and incubating for 2 min at room temperature as described elsewhere (17, 26). GFP protein was immunoprecipitated from the extracts using agarose-conjugated GFP antibodies, and the precipitated protein samples were processed by SDS-PAGE and Western blot analysis as previously detailed (27). The Thermo Scientific PageRuler Plus Prestained Protein Ladder was used (Life Technologies). Immunoblots were visualized using a myECL Imager (Life Technologies).
Immunofluorescence analyses
Cells on gels were processed as detailed elsewhere (28). All samples were observed with a confocal Leica TCS SP8 X or DMi8. Images were exported as TIFF files, and figures were prepared using ImageJ and Adobe Photoshop (Adobe Systems, San Jose, CA, USA). All images of immunofluorescence taken with the confocal Leica TCS SP8 X microscope were processed using the default 3-dimensional deconvolution algorithm built into the LAS X software (Leica Microsystems).
TF microscopy
TF microscopy was performed on cells plated on polyacrylamide gels embedded with fluorescent beads as described above. Live cells were imaged using the Leica TCS SP8 X microscope. After visualization of the cells and beads, cells were detached with trypsin and the beads in the gel were reimaged. For time-lapse TF microscopy, preparations were visualized every 15 s for up to 15 min. Bead images were exported to ImageJ, and bead displacement was used to calculate TFs using the StackReg, Iterative Particle Image Velocimetry, and FTTC plugins following protocols detailed by others (29, 30).
Statistical analysis
All experiments were performed a minimum of 3 times. Statistical significance was determined by Fisher’s test for prevalence data, the Mann-Whitney U test for actin bundle speed data, and Student’s t test for all other data. A value of P < 0.05 was considered significant.
RESULTS
Motility and morphology of keratinocytes on a soft substrate
Keratinocytes populating wounds and metastasizing keratinocytes move over connective tissue elements. Their substrates are relatively soft with a stiffness of around 5 kPa (21). Yet many studies of keratinocyte migration have involved assaying cells moving on stiff substrates such as glass or plastic. Because it is known that substrate stiffness is an important determinant of cell motility (31), we have chosen to study keratinocyte migration under conditions that more closely reflect wound healing and metastasis by analyzing cell movement on a matrix-coated gel substrate with a stiffness of approximately 5 kPa. For these studies, we used iHEK, which express the same complement of cell–extracellular matrix complex proteins and matrix elements as primary keratinocytes (9, 17). Because we use cells infected with lentivirus encoding ColXVII shRNA, iHEK infected with lentivirus encoding scrambled shRNA are used as a control.
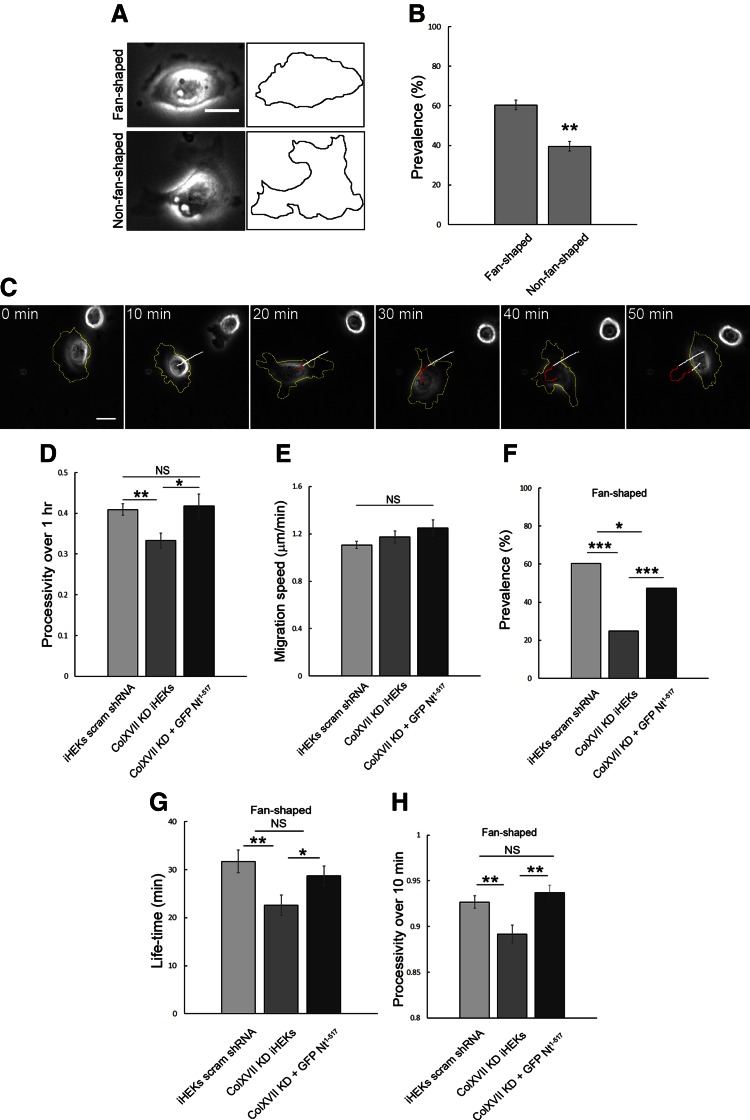
On a 5 kPa stiff substrate, control keratinocytes exhibit distinct morphologic profiles. Over 60% of cells have a fan shape with a single, broad lamellipodium, similar to that of motile fish keratocytes (Fig. 1A, B), and move over the substrate in one direction, lamellipodium first (32, 33). The remainder of the cells appear either elongated or rounded with multiple lamellipodia (i.e., non-fan shaped) (Fig. 1A, B). By following live cells, we have observed conversion of cells between a fan-shaped and a rounded/elongated morphology. For example, in Fig. 1C the cell initially exhibits a fan-shaped morphology and is moving toward the left. The single lamellipodium then retracts, with the retraction being concurrent with a transient inhibition of movement. At the same time, multiple lamellipodia assemble. The cell then pivots, one lamellipodium predominates, and a new leading edge develops, resulting in a change in the direction of cell movement (Fig. 1C).
Figure 1.
Directed migration and shape of keratinocytes are regulated by ColXVII expression. A) Phase images of fan- and non-fan‐shaped control keratinocytes. B) Quantification of prevalence of fan- and non-fan‐shaped control keratinocytes in population (3 experiments, n = 326). C) Still images of migrating iHEKs expressing scrambled shRNA (control cells/iHEK scram shRNA). Yellow line outlines cell; white and red paths indicate movement of cell centroid while cell was fan or non-fan shaped, respectively. D, E) Quantification of processivity and speed of control (3 experiments, n = 168), ColXVII KD (ColXVII KD iHEK) (3 experiments, n = 74), and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD (3 experiments, n = 44) iHEKs. F) Quantification of prevalence of fan-shaped morphology of control (n = 326), ColXVII KD (n = 427), and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD (ColXVII KD + GFP Nt1–517) (n = 130) iHEKs. G) Quantification of lifetime of fan-shaped morphology of control (3 experiments, n = 75), ColXVII KD (3 experiments, n = 75), and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD (3 experiments, n = 65) iHEKs. H) Processivity over 10-min period was determined for control (3 experiments, n = 124), ColXVII KD (3 experiments, n = 103), and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD (3 experiments, n = 90) iHEKs. Scale bars, 20 μm. Values are means ± sem. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001. B, D, E, G, H) Student’s t test, (F) Fisher’s test.
ColXVII loss perturbs keratinocyte motility on soft substrates
Previous studies indicate that HPCs play a role in motility (13, 16–20, 34). Moreover, HPCs appear to act as signaling scaffolds for Rac1 and cofilin (17, 18). In this study, our focus is on the functions of ColXVII, whose positive regulatory role in motility is controversial (18, 35–37). In addition to its role in regulating signaling, we have hypothesized that ColXVII may also play a structural role in regulating directed migration (18). To test this hypothesis, we first assayed the consequences of ColXVII KD on keratinocyte morphology and motility when the cells are plated on a 5 kPa gel substrate. For these studies, we used a previously characterized cloned line of iHEK expressing ColXVII shRNA (18). These KD keratinocytes exhibit approximately 10% ColXVII expression relative to parental iHEKs but show no reduction in α3 and β4 integrin subunit cell-surface expression (18). Consistent with studies of ColXVII KD keratinocytes plated onto glass, ColXVII KD keratinocytes on the gel exhibit aberrant motility behavior, showing a reduction in processivity, a measure of directed migration (Fig. 1D) (18). They exhibit no statistically significant difference in migration speed, however (Fig. 1E). The change in processivity of the KD keratinocytes is reflected in a significant decrease in the prevalence of fan-shaped cells relative to control keratinocyte populations (Fig. 1F). The lifetime of fan-shaped ColXVII KD keratinocytes is also significantly decreased compared to control cells (Fig. 1G). Thus, we restricted processivity analyses of fan-shaped ColXVII KD keratinocytes to a 10-min period (Fig. 1H). In this time frame, even fan-shaped KD cells exhibit a processivity defect which rules out the possibility that loss of processivity induced by ColXVII KD is simply due to the more rapid transition of KD keratinocytes from a fan to a non-fan shape compared to controls (Fig. 1G, H). In addition, the ability of GFP-tagged ColXVII Nt1-517, lacking the laminin-binding and collagen-like extracellular domains of the ColXVII protein, to rescue the shape and motility changes of ColXVII KD keratinocytes indicates that the ColXVII shRNA we used in this and subsequent experiments has no off-target effects (Fig. 1D, F–H). This fragment and its protein interactions have been described in several previous publications (18, 38).
Actin cytoskeleton organization in motile ColXVII KD keratinocytes
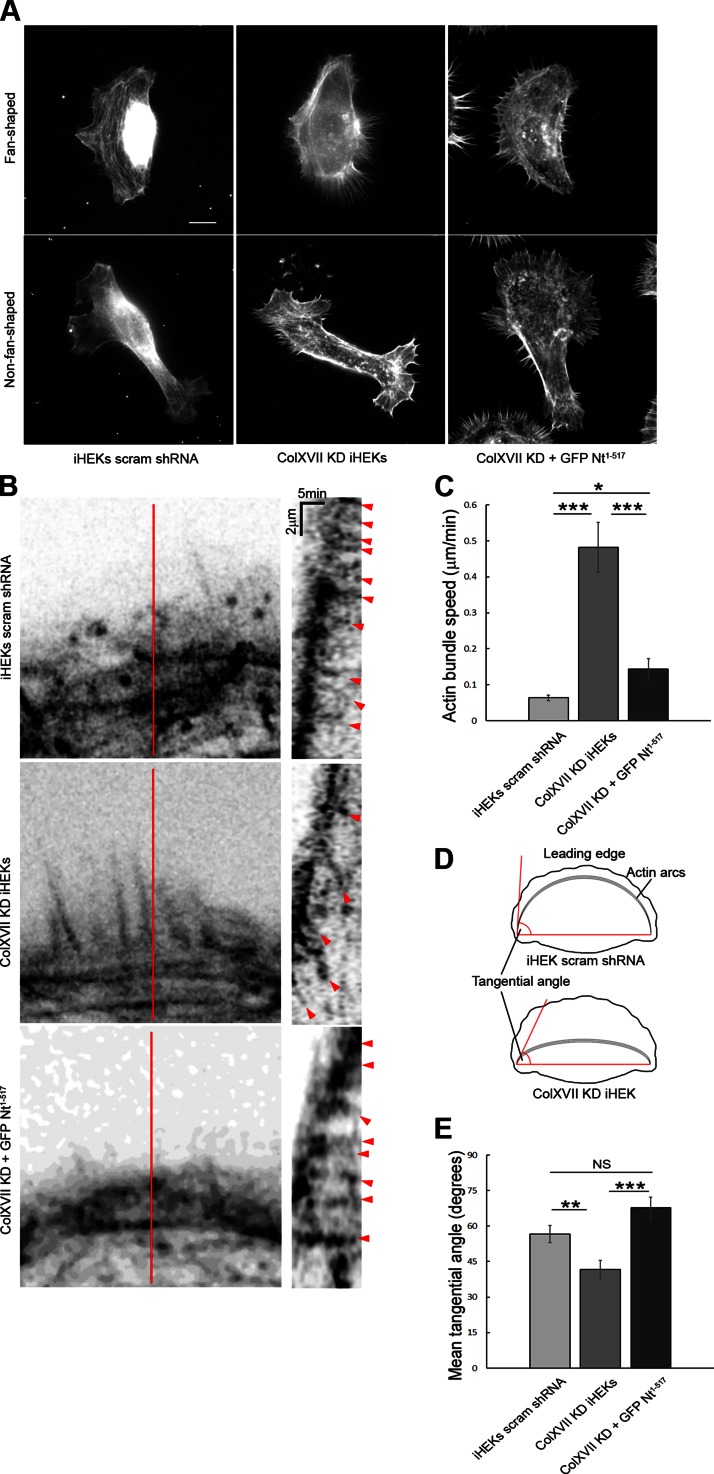
In a moving fish keratocyte, an actin network is assembled in the lamellipodium leading to membrane protrusion and forward movement of the cell (32). Because single, fan-shaped keratinocytes bear a striking resemblance to fish keratocytes, we next investigated the organization and dynamics of the actin cytoskeleton of keratinocytes. In a fixed fan-shaped control keratinocyte, actin filament bundles occur in 1 or more filopodia-like structures aligned perpendicular to the protrusive lamellipodium (Fig. 2A). These filopodia extend from a large bundle or arc of actin filaments close to or at the base of the lamellipodium, the so-called lamella (Fig. 2A). In live fan-shaped control keratinocytes expressing RFP-tagged LifeAct, actin arcs positionally advance, similar to observations of epithelial cells by Burnette and colleagues (2) (Fig. 2B and Supplemental Fig. S1). In brief, in moving keratinocytes, an extensive actin arc composed of multiple actin filaments, organized into bundles, exists along the lamella. Actin filaments appear to add to the exterior aspect of the arc. Bundles comprising the arc do not show extensive retrograde movement as the cell moves forward (Fig. 2B, C and Supplemental Fig. S1). Rather, the movement of the arc appears to be impeded just distal to the lamella. In sharp contrast, in fan-shaped ColXVII KD keratinocytes, actin arcs are less prominent (Fig. 2A). Moreover, actin dynamics are increased in such cells (Fig. 2B, C and Supplemental Fig. S1). On the basis of this finding, we predicted that the angle between the tangent of the arc at its terminus and a segment connecting the 2 actin arc termini (tangential angle) should be decreased in KD keratinocytes compared to controls (Fig. 2D). This is the case (Fig. 2E). Actin arc organization and tangential angle in ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 are comparable to control cells (Fig. 2A, E). Moreover, actin arc dynamics are almost completely restored after expression of GFP-tagged ColXVII Nt1–517 in ColXVII KD keratinocytes (Fig. 2B, C and Supplemental Fig. S1). In fixed non-fan‐shaped cells, regardless of ColXVII expression, actin arcs are either absent or poorly developed (Fig. 2A). In live non-fan-shaped control keratinocytes and ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517, actin arcs developed behind the lamellipodium during the conversion of a cell to a fan shape (Supplemental Fig. S1). In non-fan‐shaped ColXVII KD keratinocytes, actin bundles are more dynamic without obvious actin arc formation behind the lamellipodia during a 15 min observation period (Supplemental Fig. S1).
Figure 2.
ColXVII regulates actin bundle dynamics. A) Actin organization in fan- and non-fan‐shaped control, ColXVII KD, and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD iHEKs. Cells were stained with rhodamine-conjugated phalloidin. Scale bar, 10 μm. B) Actin bundle retrograde movement was recorded in RFP-tagged LifeAct expressing control (n = 12), ColXVII KD (n = 12), and ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 (n = 10). Kymographs in right panels were generated along lines indicated in respective panels at left. Red arrowheads indicate bundle movement. C) Quantification of actin bundle retrograde movement. D, E) Angle between tangent of arc at its terminus and imaginary segment connecting termini of arc was quantified in control (n = 56), ColXVII KD (n = 46), and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD (n = 36) cells. Values are means ± sem. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001. C) Mann-Whitney U test, (E) Student’s t test.
Actin-ColXVII association in moving keratinocytes
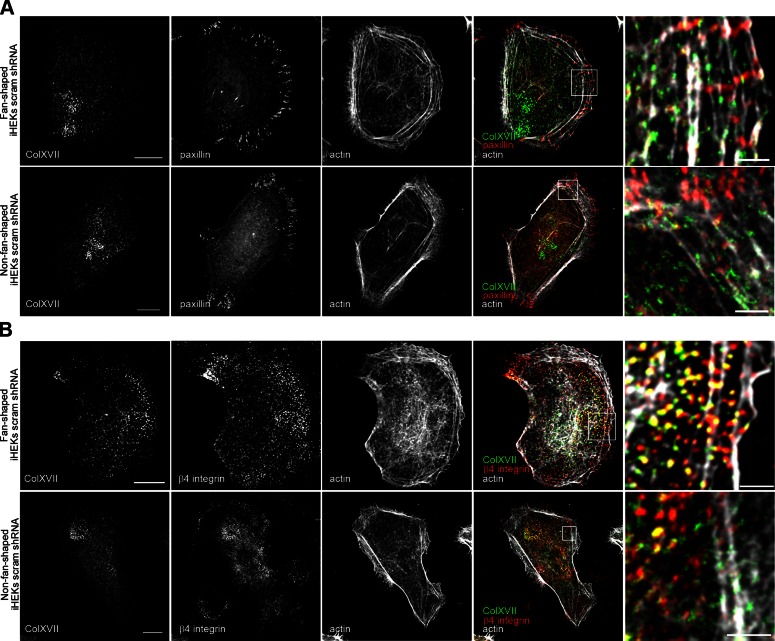
Cell matrix adhesion molecules are key to regulation of cell motility. In this regard, the motility of epithelial cells such as keratinocytes is complicated because they express both focal adhesion proteins and hemidesmosome proteins. In fan-shaped keratinocytes moving on a 5 kPa stiff gel, the focal adhesion protein paxillin is distributed at the flanks of their lamellipodia and localizes at, and perpendicular to, the leading front of such cells (Fig. 3A). In contrast, the hemidesmosome proteins β4 integrin and ColXVII are displayed in a punctate pattern along or just behind the lamella of fan-shaped cells and within the cell body. In the lamella, ColXVII colocalizes with β4 integrin along actin bundles parallel to the leading edge of the cell (Fig. 3B). However, in the same image, we observe ColXVII/β4 integrin puncta that show no clear association with actin behind the lamella and also some β4 integrin puncta that show no obvious association with ColXVII (Fig. 3B). There are also some ColXVII puncta that fail to be stained by the β4 integrin antibody (Fig. 3B). In non-fan‐shaped keratinocytes, β4 integrin and ColXVII show limited colocalization and fail to exhibit association with actin (Fig. 3B).
Figure 3.
Localization of ColXVII, β4 integrin, paxillin, and actin in control keratinocytes. Control keratinocytes were triple stained with rhodamine-conjugated phalloidin and antibodies against ColXVII in combination with paxillin (A) or β4 integrin (B). Fourth column shows overlays of 3 stains. Boxed areas are shown at higher magnification in fifth column for each set of micrographs. Top row of each panel is fan-shaped cell, bottom row non-fan-shaped. Scale bars, 10 μm (left column) and 2 μm (right column).
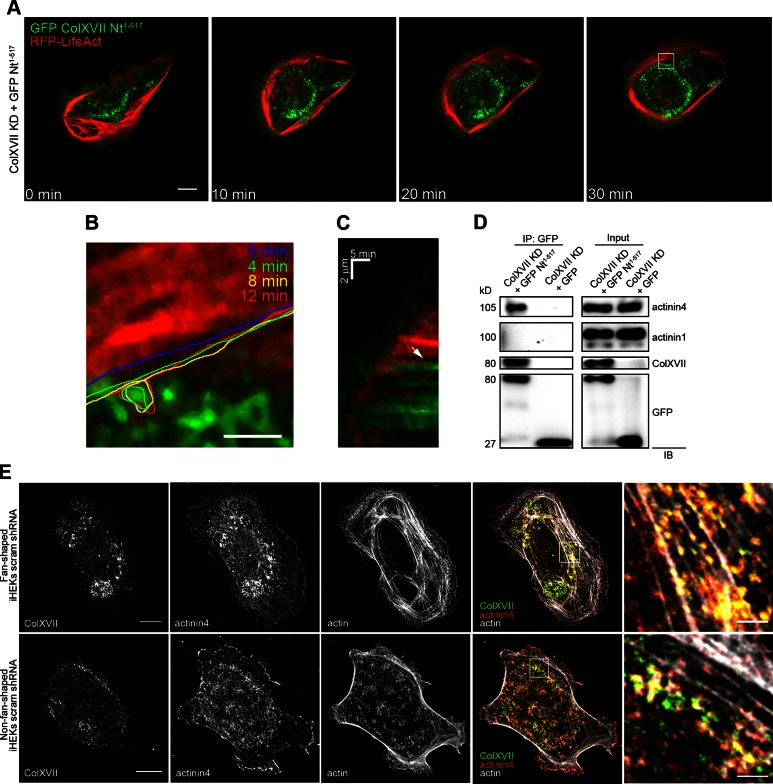
Actin association with ColXVII is also observed in live fan-shaped control keratinocytes expressing RFP-tagged LifeAct and GFP-tagged ColXVII Nt1–517 (Fig. 4A–C and Supplemental Video 1). In the cell imaged in Fig. 4A, ColXVII is recruited to the lamella where it localizes posterior to the actin bundles in the arc (Supplemental Video 1). In this same cell, the retrograde movement of actin bundles appears to be inhibited when they approach ColXVII puncta (Fig. 4B, C).
Figure 4.
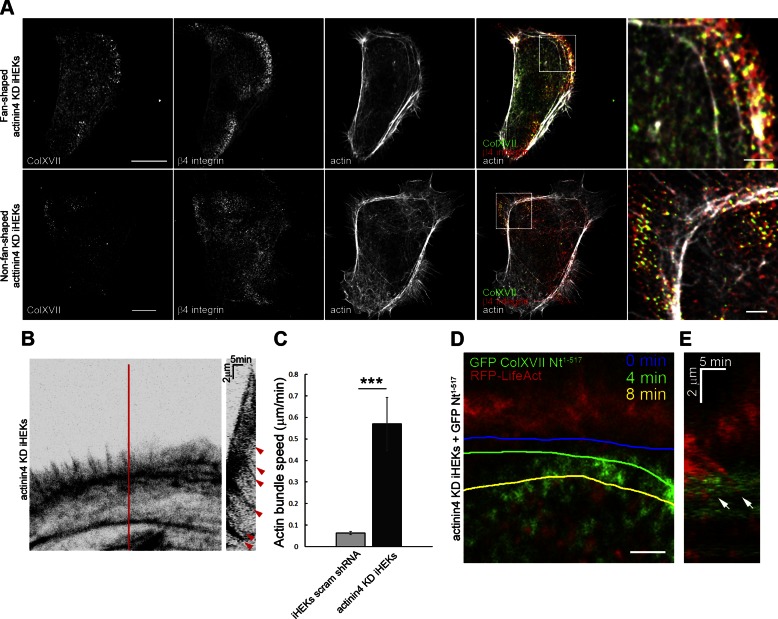
ColXVII interacts with actinin4 and restricts actin dynamics in live keratinocytes. A) Still images taken from time-lapse fluorescent microscopic analyses of a ColXVII KD keratinocyte expressing both GFP-tagged ColXVII Nt1–517 and RFP-tagged LifeAct. Scale bar, 10 μm. B) High magnification of white box in (A). Actin bundle was followed over time; its position is marked by colored lines at indicated time points. Scale bar, 2 μm. C) Kymograph at leading edge of ColXVII KD keratinocyte expressing both GFP-tagged ColXVII Nt1–517 and RFP-tagged LifeAct. White arrow indicates ColXVII-containing structure that inhibits actin bundle retrograde movement. D) Extracts of ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 or GFP alone were subjected to immunoprecipitation using antibodies against GFP (IP: GFP). Extracts (inputs and precipitated proteins) were processed for immunoblotting (IB) using antibodies against either actinin4, actinin1, cytoplasmic domain of ColXVII, or GFP, as indicated. Representative blot of immunoprecipitation experiment is shown. Note that actinin4, but not actinin1, is precipitated by GFP antibodies from extracts of ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517. Immunoblotting of actinin4, actinin1, and GFP antibodies on extracts of ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 or GFP alone are shown as positive controls. Antibodies against ColXVII fail to recognize target in extracts of ColXVII KD keratinocytes expressing GFP alone. E) Control fan-shaped and non-fan-shaped keratinocytes were triple stained with rhodamine-conjugated phalloidin and antibodies against ColXVII and actinin4. Fourth column shows overlays of 3 stains. Boxed areas are shown at higher magnification in fifth column. Scale bars, 10 μm (left column) and 2 μm (right column).
ColXVII has not been demonstrated to interact directly with the cytoskeleton but is known to interact with actinin family members, which directly bind actin (39). Actinin4, but not actinin1, coprecipitates with ColXVII from extracts of ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 (Fig. 4D). In addition, actinin4 colocalizes with ColXVII along actin arcs in the lamella of fan-shaped cells (Fig. 4E). In non-fan‐shaped keratinocytes, actinin4 and ColXVII exhibit some colocalization but do not show association with actin (Fig. 4E). In ColXVII KD keratinocytes, β4 integrin and actinin4 fail to colocalize (Supplemental Fig. S2A). Moreover, actinin4, but not β4 integrin, associates with actin (Supplemental Fig. S2A). It should also be noted that paxillin distribution in ColXVII KD is similar to that observed in control keratinocytes (Fig. 3A and Supplemental Fig. S2B).
Actin dynamics in actinin4 KD keratinocytes
To confirm that the association of ColXVII and actinin4 impacts actin dynamics, we analyzed a previously characterized cloned line of iHEK expressing actinin4 shRNA (22). These KD keratinocytes exhibit approximately 5% actinin4 protein expression relative to control iHEKs (22). Thirty percent of actinin4 KD keratinocytes maintained on a 5 kPa stiff substrate have a fan shape, but in such cells ColXVII and β4 integrin puncta fail to colocalize with actin arcs. Rather, in both fan- and non-fan-shaped actinin4 KD keratinocytes, ColXVII and β4 integrin appear anterior to actin arcs, consistent with our previous data (Fig. 5A) (22). In addition, actin bundle dynamics are increased in actinin4 KD keratinocytes to a similar level to that in ColXVII KD keratinocytes (Fig. 5B, C and Fig. 2B, C). Moreover, in actinin4 KD keratinocytes expressing RFP-tagged LifeAct and GFP-tagged ColXVII Nt1–517, the retrograde movement of actin bundles is not impeded by ColXVII puncta (Fig. 5D, E). Taken together, these data implicate actinin4 as a mediator of ColXVII effects on actin dynamics in motile keratinocytes.
Figure 5.
Actinin4 regulates ColXVII and β4 integrin localization and restricts actin dynamics in live keratinocytes. A) Actinin4 KD keratinocytes were triple stained with rhodamine-conjugated phalloidin and antibodies against ColXVII and β4 integrin. Fourth column shows overlays of 3 stains. Boxed areas are shown at higher magnification in fifth column. Scale bars, 10 μm (left column) and 2 μm (right column). B) Actin bundle retrograde movement was recorded in RFP-tagged LifeAct expressing actinin4 KD keratinocytes (n = 8). Kymograph in right panel was generated along line indicated in panel on left. Red arrowheads indicate bundle movement. C) Quantification of actin bundle retrograde movement. Values are means ± sem. ***P < 0.001. Mann-Whitney U test. D) High-magnification image taken from time-lapse fluorescent microscopic analyses of actinin4 KD keratinocyte expressing both GFP-tagged ColXVII Nt1–517 and RFP-tagged LifeAct. Actin bundle was followed over time; its position is marked by colored lines at indicated time points. Scale bar, 2 μm. E) Kymograph generated from (D). White arrow indicates an actin bundle that moves unimpeded over ColXVII puncta.
Generation of TFs in ColXVII KD keratinocytes
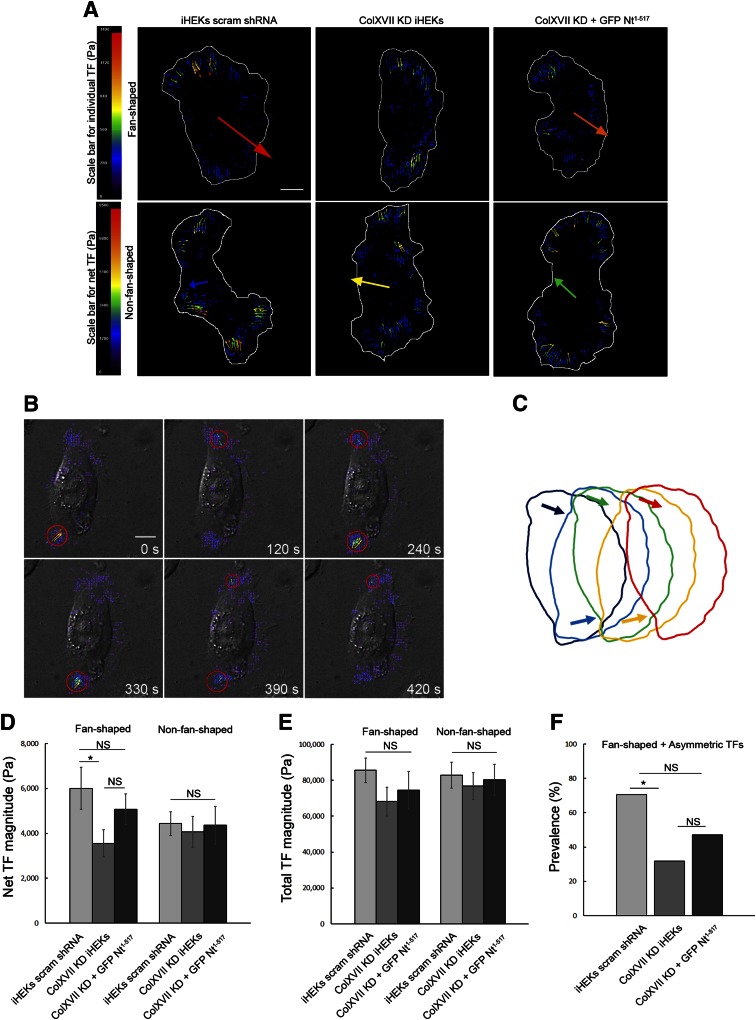
In order to move, cells exert TFs on their substrate via the dynamics of actin bundles linked to adhesion sites (4). Because we observed changes in the actin cytoskeleton resulting from the disruption of ColXVII-actin interactions, we next wondered whether this would impact TFs. Thus, we performed TF microscopy on cells plated onto a fluorescent bead–impregnated gel substrate as described previously (29). We then compared net and total TF magnitudes and their localization. Based on both experimental and computational definitions used by others, we define net TF magnitude as the magnitude of the sum of cellular TF vectors and total TF magnitude as the sum of the magnitudes of cellular TF vectors (4, 40, 41).
In fan-shaped control keratinocytes, TFs localize to the leading front of the lamellipodium and predominate at one of the 2 lamellipodial flanks—that is, the TFs are asymmetrically localized (Fig. 6A). By following live cells as they move over the gel substrate, we also noticed that exertion of forces at the lamellipodial flanks alternates from one to another (Fig. 6B, C and Supplemental Video 2). Despite similar localization of TFs in fan-shaped ColXVII KD keratinocytes, forces tend to be more frequently oriented such that they counteract each other (Fig. 6A). Thus, the net TF magnitude is significantly reduced (Fig. 6D), even though the total magnitude of TFs transmitted to the substrate is only slightly decreased in ColXVII KD keratinocytes (Fig. 6E). We also quantified the prevalence of asymmetric TFs in control and ColXVII KD cells (Fig. 6F). Finally, we saw a partial rescue of TF orientations and net TF magnitude as well as the prevalence of asymmetric TF distribution in ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 (Fig. 6A, D–F).
Figure 6.
ColXVII regulates TFs. A) Quiver plots, indicating local TFs greater than 150 Pa (small arrows) and net TFs (large arrows) in fan- and non-fan-shaped control, ColXVII KD, and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD keratinocytes, as indicated. Cells are outlined in white. Colored scale bars indicate local and net TF magnitudes in Pa. B) Time-lapse TF microscopy performed on a control keratinocyte. The cell was visualized using differential interference contrast microscopy. TF quiver plots were overlaid on cell images at 6 different time points. Only TFs that exceed 150 Pa are shown. Red circles indicate where TFs predominate. C) Model of effect on cell movement of alternating TF localization (arrows) at lamellipodial flanks. Model predicts that cell pivots at each flank and moves in stepwise fashion over substrate. Quantification of net TF magnitude (D) and total TF generation (E) in control (n = 38), ColXVII KD (n = 45), and GFP-tagged ColXVII Nt1–517 expressing ColXVII KD (n = 32) fan- and non-fan-shaped keratinocytes. Only TFs that exceeded 150 Pa were analyzed. F) Prevalence of asymmetric TF localization among fan-shaped cells in (D) and (E) is quantified. Scale bars, 10 μm. Values are means ± sem. NS, not significant, *P < 0.05. D, E) Student’s t test, (F) Fisher’s test.
Non-fan-shaped control keratinocytes generated TFs that tended to counteract each other, resulting in a small reduction in net TF magnitude compared to their fan-shaped counterparts, despite the total TFs being comparable (Fig. 6A,D). Non-fan-shaped ColXVII KD keratinocytes and ColXVII KD keratinocytes expressing GFP-tagged ColXVII Nt1–517 exhibited TFs comparable to non-fan-shaped controls (Fig. 6A, D).
DISCUSSION
In this study, we demonstrated that keratinocytes moving in a directed fashion over a substrate of stiffness equivalent to that of the dermis take on a fan shape, reminiscent of the shape of a motile fish keratocyte (2, 32, 33). Actin bundles are found in an arc at the base of the leading edge or lamella of fan-shaped keratinocytes and stabilize the advancing lamellipodium as the cells migrate. By following live cells, we have observed keratinocytes undergoing morphologic changes. These cells lose their fan shape, assemble multiple lamellipodia, and pivot. One of the lamellipodia predominates and determines the new direction of migration. This behavior is consistent with the branch-and-pivot mechanism in fibroblasts proposed by Welf and colleagues (42).
Our data indicate that the continued leading edge protrusion of a fan-shaped moving keratinocyte involves extension of actin filaments in filopodia-like arrays from the arc of actin bundles. Moreover, the dynamics of the bundles within the actin arc that we have described here are consistent with the treadmill mechanism described by Burnette and colleagues (2) in which actin arcs are replenished at the lamellar front and removed at the rear. In addition, the retrograde movement of actin bundles in keratinocytes appears inhibited in the same fashion that retrograde actin movement is impeded by focal adhesion sites along the base of the lamellipodium in the growth cones of Aplysia (43). In sharp contrast, in keratinocytes, the hemidesmosome protein ColXVII is enriched at sites where actin movement is inhibited, implicating ColXVII as the substrate adhesion molecule that determines actin dynamics. In support of this notion, we show that actin retrograde movement is enhanced in ColXVII KD keratinocytes. Moreover, the loss of ColXVII-mediated inhibition of actin dynamics provides an explanation for the decreased angle between the tangent of the arc at its terminus and a segment connecting the 2 actin arc termini, which we observed in KD keratinocytes.
The data we present here with regard to ColXVII–actin colocalization and the impact of ColXVII loss on actin dynamics indicate that HPCs are involved in indirectly tethering actin bundles to the substrate. The association of HPCs with actin could be mediated by α6β4 integrin because α6β4 integrin binds the cytolinker plectin, which possesses an actin binding domain (6, 7, 9). However, linkage of HPCs and actin via association of α6β4 integrin with plectin appears unlikely because it has been reported that actin binding to plectin is inhibited when plectin interacts with α6β4 integrin (44). Instead, our data implicate actinin4 in linking ColXVII, and hence HPCs, to actin. We base this conclusion on the colocalization of actinin4 with ColXVII along actin bundles in fan-shaped moving keratinocytes; the coimmunoprecipitation of actinin4, but not actinin1, with ColXVII; the similar phenotype of ColXVII and actinin4 KD cells with regard to their actin dynamics; and the inability of ColXVII puncta to impede actin arc movements when actinin4 is knocked down.
Because a truncated ColXVII protein lacking the extracellular domain that binds laminin rescues actin arc dynamics, as evidenced by decreasing actin bundle speed, we can rule out the possibility that ColXVII impedes actin arc dynamics by exclusively mediating adhesion of keratinocytes to their laminin-rich substrate. Rather, based on colocalization of β4 integrin and ColXVII along actin bundles at the lamella of fan-shaped cells, we suggest that ColXVII stabilizes actin interaction at the substrate-attached surface by binding to α6β4 integrin, which in turn interacts with laminin-332 in the matrix. Therefore, the loss of β4 integrin would also be predicted to change actin dynamics and TFs in keratinocytes. However, testing this prediction is complicated by the known effects of β4 integrin KD on expression of other integrin subunits, which interact with and regulate actin cytoskeleton organization (19).
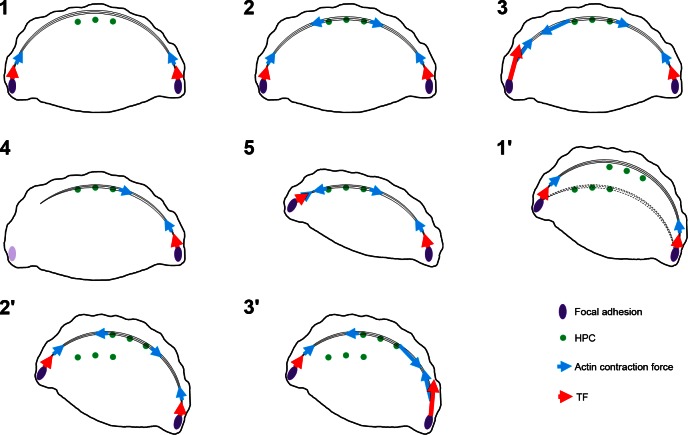
TF microscopy indicates that, in fan-shaped cells, forces are localized at the flanks of the lamellipodium and are directed mainly inward and toward the cell center, similar to data derived from analyses of mammalian cells and fish keratocytes by others (45, 46). There are also forces arrayed along the front edge of the lamellipodium, directed inward, but these TFs are of lesser magnitude than those at the flanks. As these fan-shaped cells move, there is an on/off phenomenon at each flank in a relatively sequential manner, suggesting that the cells move in a stepwise fashion rather than glide over their substrate. This would be consistent with a model in which high TFs are needed to detach focal adhesions at one flank of the cell while the other flank stays attached. The detached flank then protrudes and reattaches; the process then repeats with the opposing flank (Fig. 6C and Fig. 7). In contrast to control keratinocytes, the on/off switching of TFs at the flanks of the single lamellipodium in fan-shaped ColXVII KD keratinocytes does not occur. The TF foci in KD keratinocytes cancel each other and inhibit directed migration, consistent with the motility phenotype of these cells. This leads to the question, how does ColXVII regulate the TF asymmetry in control keratinocytes? We predict that the alternation of TFs at the flanks, which results in pivoting, is due to the asymmetric restriction of actin bundle contraction by indirect ColXVII binding along the length of actin arcs. By mediating the interaction of an actin bundle with the cell surface in a punctate array along the length of the bundle, ColXVII acts like a metal snap (Fig. 7). Moreover, by doing so, it can restrict contraction forces to one side of the arc or the other (Fig. 7). In such a model, sequential contraction at the flanks would be induced by either localization of effector proteins or by the differential activation of such effectors at each flank. For example, localized calcium ion fluxes and/or differential activation of myosin light chain kinase or phosphatase would allow this potential mechanism to occur (47, 48).
Figure 7.
Model of role of HPCs, focal adhesions, and actin in generation of TFs and cell movement (see Discussion for details). In (1), actin bundles undergo retrograde movement until they encounter HPCs (2). Because HPCs anchor actin bundles to the substrate, contraction can be localized (blue arrows) to one flank, resulting in larger TFs at the left flank vs. right [TF magnitudes represented by large and small red arrows in (3)]. In (4), detachment of actin bundles from focal adhesions at the left flank allows the cell to pivot, as shown in (5). In (1′), HPCs disengage from actin bundles and new HPCs reassemble along the lamella. In (2′), these new complexes reengage with actin moving in a retrograde fashion. In (3′), TFs predominate at the right flank due to localized contraction of the actin cytoskeleton to allow the cell to move stepwise over its substrate.
In summary, we have demonstrated that in motile keratinocytes ColXVII binds to and stabilizes actin bundles to facilitate generation of TFs, leading to directed migration. In vivo, this would facilitate the reepithelialization of a healing wound by promoting the directed migration of those cells at the front edge of the wound. We suggest that the latter cells act as leaders for the movement of the entire cellular sheet that epithelializes the wound surface, a possibility that is supported by work in a number of other systems (49). Intriguingly, the data here, together with studies revealing ColXVII interaction with BPAG1e [known to bind intermediate filaments (6, 7, 9)], indicate that ColXVII binds, albeit indirectly, to 2 different cytoskeleton systems. Such interactions allow ColXVII to play multiple regulatory roles in adhesion and motility. In epithelial cells of various tissue types, ColXVII integrates the intermediate filament cytoskeleton in the cytoplasm with laminins in the extracellular matrix, thereby participating in the stabilization of cell–substrate interactions at the site of the hemidesmosome. In contrast, in moving keratinocytes, ColXVII not only modulates Rac1 activation (18) but also, as we now demonstrate, regulates actin dynamics and TFs. That hemidesmosome proteins participate in TF generation has never been described. Because ColXVII is up-regulated in a number of cancers, most notably at the invasive front of squamous cell carcinomas (50), we suggest that actin–ColXVII interaction promotes the motility of certain tumor cells via an impact on both cytoskeleton dynamics and the development of the TFs that cells generate to move in a directed manner through a matrix microenvironment.
Acknowledgments
Research reported in this publication was supported by the U.S. National Institutes of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR054184). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- BPAG1e
bullous pemphigoid antigen 1e
- ColXVII
collagen XVII
- ColXVII
Nt1–517, N-terminal ColXVII fragment containing amino acid residues 1-517
- GFP
green fluorescent protein
- HPC
hemidesmosome protein complex
- iHEK
immortalized human epidermal keratinocyte
- KD
knockdown
- RFP
red fluorescent protein
- shRNA
short hairpin RNA
- TF
traction force
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Pollard T. D., Borisy G. G. (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 2.Burnette D. T., Manley S., Sengupta P., Sougrat R., Davidson M. W., Kachar B., Lippincott-Schwartz J. (2011) A role for actin arcs in the leading-edge advance of migrating cells. Nat. Cell Biol. 13, 371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogh B. S., Multhaupt H. A. B., Couchman J. R. (2014) Protein kinase C, focal adhesions and the regulation of cell migration. J. Histochem. Cytochem. 62, 172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananthakrishnan R., Ehrlicher A. (2007) The forces behind cell movement. Int. J. Biol. Sci. 3, 303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause M., Gautreau A. (2014) Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 15, 577–590 [DOI] [PubMed] [Google Scholar]
- 6.Margadant C., Charafeddine R. A., Sonnenberg A. (2010) Unique and redundant functions of integrins in the epidermis. FASEB J. 24, 4133–4152 [DOI] [PubMed] [Google Scholar]
- 7.Hopkinson S. B., Hamill K. J., Wu Y., Eisenberg J. L., Hiroyasu S., Jones J. C. R. (2014) Focal contact and hemidesmosomal proteins in keratinocyte migration and wound repair. Adv. Wound Care (New Rochelle) 3, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones J. C. R., Hopkinson S. B., Goldfinger L. E. (1998) Structure and assembly of hemidesmosomes. BioEssays 20, 488–494 [DOI] [PubMed] [Google Scholar]
- 9.Borradori L., Sonnenberg A. (1999) Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Invest. Dermatol. 112, 411–418 [DOI] [PubMed] [Google Scholar]
- 10.Walko G., Castañón M. J., Wiche G. (2015) Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 360, 363–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawamura D., Nakano H., Matsuzaki Y. (2010) Overview of epidermolysis bullosa. J. Dermatol. 37, 214–219 [DOI] [PubMed] [Google Scholar]
- 12.Goldfinger L. E., Hopkinson S. B., deHart G. W., Collawn S., Couchman J. R., Jones J. C. (1999) The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci. 112, 2615–2629 [DOI] [PubMed] [Google Scholar]
- 13.Pullar C. E., Baier B. S., Kariya Y., Russell A. J., Horst B. A. J., Marinkovich M. P., Isseroff R. R. (2006) β4 integrin and epidermal growth factor coordinately regulate electric field–mediated directional migration via Rac1. Mol. Biol. Cell 17, 4925–4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Underwood R. A., Carter W. G., Usui M. L., Olerud J. E. (2009) Ultrastructural localization of integrin subunits β4 and α3 within the migrating epithelial tongue of in vivo human wounds. J. Histochem. Cytochem. 57, 123–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo W., Giancotti F. G. (2004) Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5, 816–826 [DOI] [PubMed] [Google Scholar]
- 16.Kligys K., Claiborne J. N., DeBiase P. J., Hopkinson S. B., Wu Y., Mizuno K., Jones J. C. R. (2007) The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization, and motility behavior of keratinocytes. J. Biol. Chem. 282, 32520–32528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamill K. J., Hopkinson S. B., DeBiase P., Jones J. C. R. (2009) BPAG1e maintains keratinocyte polarity through β4 integrin-mediated modulation of Rac1 and cofilin activities. Mol. Biol. Cell 20, 2954–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamill K. J., Hopkinson S. B., Jonkman M. F., Jones J. C. R. (2011) Type XVII collagen regulates lamellipod stability, cell motility, and signaling to Rac1 by targeting bullous pemphigoid antigen 1e to α6β4 integrin. J. Biol. Chem. 286, 26768–26780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kligys K. R., Wu Y., Hopkinson S. B., Kaur S., Platanias L. C., Jones J. C. R. (2012) α6β4 integrin, a master regulator of expression of integrins in human keratinocytes. J. Biol. Chem. 287, 17975–17984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sehgal B. U., DeBiase P. J., Matzno S., Chew T.-L., Claiborne J. N., Hopkinson S. B., Russell A., Marinkovich M. P., Jones J. C. R. (2006) Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J. Biol. Chem. 281, 35487–35498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achterberg V. F., Buscemi L., Diekmann H., Smith-Clerc J., Schwengler H., Meister J.-J., Wenck H., Gallinat S., Hinz B. (2014) The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 134, 1862–1872 [DOI] [PubMed] [Google Scholar]
- 22.Hamill K. J., Hopkinson S. B., Skalli O., Jones J. C. R. (2013) Actinin-4 in keratinocytes regulates motility via an effect on lamellipodia stability and matrix adhesions. FASEB J. 27, 546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenberg J. L., Safi A., Wei X., Espinosa H. D., Budinger G. S., Takawira D., Hopkinson S. B., Jones J. C. (2011) Substrate stiffness regulates extracellular matrix deposition by alveolar epithelial cells. Res. Rep. Biol. 2011, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y. L., Pelham R. J. Jr (1998) Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 298, 489–496 [DOI] [PubMed] [Google Scholar]
- 25.Hopkinson S. B., Riddelle K. S., Jones J. C. R. (1992) Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J. Invest. Dermatol. 99, 264–270 [DOI] [PubMed] [Google Scholar]
- 26.Bae Y. H., Mui K. L., Hsu B. Y., Liu S.-L., Cretu A., Razinia Z., Xu T., Puré E., Assoian R. K. (2014) A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal. 7, ra57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harlow E., Lane D. (1988) Antibodies: A Laboratory Manual. CSHL Press, Cold Spring Harbor, New York [Google Scholar]
- 28.Klatte D. H., Kurpakus M. A., Grelling K. A., Jones J. C. (1989) Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J. Cell Biol. 109, 3377–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisenberg J. L., Beaumont K. G., Takawira D., Hopkinson S. B., Mrksich M., Budinger G. R. S., Jones J. C. R. (2013) Plectin-containing, centrally localized focal adhesions exert traction forces in primary lung epithelial cells. J. Cell Sci. 126, 3746–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng Q., Duchemin-Pelletier E., Deshiere A., Balland M., Guillou H., Filhol O., Théry M. (2012) Spatial organization of the extracellular matrix regulates cell–cell junction positioning. Proc. Natl. Acad. Sci. USA 109, 1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo C.-M., Wang H.-B., Dembo M., Wang Y. L. (2000) Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svitkina T. M., Verkhovsky A. B., McQuade K. M., Borisy G. G. (1997) Analysis of the actin–myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J. Cell Biol. 139, 397–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theriot J. A., Mitchison T. J. (1991) Actin microfilament dynamics in locomoting cells. Nature 352, 126–131 [DOI] [PubMed] [Google Scholar]
- 34.Nanba D., Toki F., Tate S., Imai M., Matsushita N., Shiraishi K., Sayama K., Toki H., Higashiyama S., Barrandon Y. (2015) Cell motion predicts human epidermal stemness. J. Cell Biol. 209, 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Löffek S., Hurskainen T., Jackow J., Sigloch F. C., Schilling O., Tasanen K., Bruckner-Tuderman L., Franzke C. W. (2014) Transmembrane collagen XVII modulates integrin dependent keratinocyte migration via PI3K/Rac1 signaling. PLoS One 9, e87263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tasanen K., Tunggal L., Chometon G., Bruckner-Tuderman L., Aumailley M. (2004) Keratinocytes from patients lacking collagen XVII display a migratory phenotype. Am. J. Pathol. 164, 2027–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franzke C.-W., Has C., Schulte C., Huilaja L., Tasanen K., Aumailley M., Bruckner-Tuderman L. (2006) C-terminal truncation impairs glycosylation of transmembrane collagen XVII and leads to intracellular accumulation. J. Biol. Chem. 281, 30260–30268 [DOI] [PubMed] [Google Scholar]
- 38.Hopkinson S. B., Jones J. C. R. (2000) The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome. Mol. Biol. Cell 11, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez A. M., Otey C., Edlund M., Jones J. C. R. (2001) Interactions of a hemidesmosome component and actinin family members. J. Cell Sci. 114, 4197–4206 [DOI] [PubMed] [Google Scholar]
- 40.Deutsch A., Howard J., Falcke M., Zimmermann W. (2012) Function and Regulation of Cellular Systems, Birkhäuser, Berlin, Germany [Google Scholar]
- 41.Mousavi S. J., Doweidar M. H. (2015) Three-dimensional numerical model of cell morphology during migration in multi-signaling substrates. PLoS One 10, e0122094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welf E. S., Ahmed S., Johnson H. E., Melvin A. T., Haugh J. M. (2012) Migrating fibroblasts reorient directionality by a metastable, PI3K-dependent mechanism. J. Cell Biol. 197, 105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnette D. T., Schaefer A. W., Ji L., Danuser G., Forscher P. (2007) Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of Aplysia neuronal growth cones. Nat. Cell Biol. 9, 1360–1369 [DOI] [PubMed] [Google Scholar]
- 44.Geerts D., Fontao L., Nievers M. G., Schaapveld R. Q. J., Purkis P. E., Wheeler G. N., Lane E. B., Leigh I. M., Sonnenberg A. (1999) Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 147, 417–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnette D. T., Shao L., Ott C., Pasapera A. M., Fischer R. S., Baird M. A., Der Loughian C., Delanoe-Ayari H., Paszek M. J., Davidson M. W., Betzig E., Lippincott-Schwartz J. (2014) A contractile and counterbalancing adhesion system controls the 3D shape of crawling cells. J. Cell Biol. 205, 83–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burton K., Park J. H., Taylor D. L. (1999) Keratocytes generate traction forces in two phases. Mol. Biol. Cell 10, 3745–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eddy R. J., Pierini L. M., Matsumura F., Maxfield F. R. (2000) Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113, 1287–1298 [DOI] [PubMed] [Google Scholar]
- 48.Vicente-Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montell D. J. (2008) Morphogenetic cell movements: diversity from modular mechanical properties. Science 322, 1502–1505 [DOI] [PubMed] [Google Scholar]
- 50.Stelkovics E., Korom I., Marczinovits I., Molnar J., Rasky K., Raso E., Ficsor L., Molnar B., Kopper L., Krenacs T. (2008) Collagen XVII/BP180 protein expression in squamous cell carcinoma of the skin detected with novel monoclonal antibodies in archived tissues using tissue microarrays and digital microscopy. Appl. Immunohistochem. Mol. Morphol. 16, 433–441 [DOI] [PubMed] [Google Scholar]