Abstract
Apolipoprotein M (ApoM) transports sphingosine-1-phosphate (S1P) in plasma, and ApoM-deficient mice (Apom−/−) have ∼50% reduced plasma S1P levels. There are 5 known S1P receptors, and S1P induces adherens junction formation between endothelial cells through the S1P1 receptor, which in turn suppresses vascular leak. Increased vascular permeability is a hallmark of inflammation. The purpose of this study was to explore the relationships between vascular leakage in ApoM deficiency and S1P1 function in normal physiology and in inflammation. Vascular permeability in the lungs was assessed by accumulation of dextran molecules (70 kDa) and was increased ∼40% in Apom−/− mice compared to WT (C57Bl6/j) mice. Reconstitution of plasma ApoM/S1P or treatment with an S1P1 receptor agonist (SEW2871) rapidly reversed the vascular leakage to a level similar to that in WT mice, suggesting that it is caused by decreased plasma levels of S1P and reduced S1P1 stimulation. In a carrageenan-induced model of inflammation, Apom−/− mice had increased vascular leakage compared with that in WT mice. Adenoviral overexpression of ApoM in Apom−/− mice decreased the vascular leakage compared to adenoviral overexpression of green fluorescent protein. The study suggests that vascular leakage of albumin-sized particles in ApoM deficiency is S1P- and S1P1-dependent and this dependency exacerbates the response to inflammatory stimuli.—Christensen, P. M., Liu, C. H., Swendeman, S. L., Obinata, H., Qvortrup, K., Nielsen, L B., Hla, T., Di Lorenzo, A., Christoffersen, C. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1.
Keywords: vascular permeability, endothelium, inflammation
Apolipoprotein M (ApoM) is a member of the lipocalin superfamily. The structure holds an 8 stranded antiparallel β-barrel enclosing a hydrophobic binding pocket (1), which can bind small lipophilic molecules, such as retinol (2), myristic acid (1), and sphingosine-1-phosphate (S1P) (3). However, Apom−/− mice have less S1P in plasma and no S1P in HDL and exhibit several physiologic alterations, such as compromised vascular barrier function (3) and increased lymphopoiesis (4), suggesting that S1P is the physiologic ligand that is carried and chaperoned by ApoM. S1P affects several biologic processes, such as lymphocyte trafficking, angiogenesis, endothelial cell migration, and endothelial barrier function (5). In plasma, S1P is transported by HDL particles (∼70%) and albumin (∼30%) (6). S1P exerts its effects by binding to GPCRs (S1P1–5). The S1P receptors expressed on endothelial cells are S1P1, S1P2, and S1P3, with the S1P1 receptor being most abundantly expressed (7–9). Activation of the S1P1 receptor controls blood pressure homeostasis (10) and leads to adherens junction assembly, which in turn enhances the endothelial barrier via cytoskeletal rearrangements (11).
Enhanced vascular permeability is one of the early hallmarks of inflammation (12). Inflammatory mediators induce endothelial barrier impairment by opening adherens junctions, leading to plasma extravasation into the interstitial tissue spaces, induction of leukocyte adhesion molecules, and increased leukocyte cell infiltration (13). Factors that resist endothelial barrier breakdown may accordingly influence inflammatory responses. Recent studies have suggested a role for sphingosine kinases and plasma S1P in suppressing acute systemic inflammation (14–16). Thus, it is suggested that S1P plays a significant role in maintaining a healthy endothelial barrier function.
In plasma, ApoM circulates at a concentration of ∼20 mg/L (0.9 µM) (17) and is mainly associated with HDL (18). Human ApoM-free HDL contains no detectable S1P and is unable to activate S1P1 receptors on endothelial cells in vitro (3). ApoM-deficient mice (Apom−/−) have an ∼50% lower concentration of plasma S1P than do wild-type (WT) mice, and the vascular barrier function appears to be compromised (3). However, it is unknown whether the ApoM/S1P complex plays a significant role in activation of endothelial S1P-receptors in vivo.
The purpose of the present study was to examine the relationship of vascular leakage in Apom−/− mice to plasma S1P and the S1P receptor system in normal physiology and in inflammation.
MATERIALS AND METHODS
Chemicals and reagents
Acetonitrile (RH1016) was obtained from Rathburn Chemicals, Ltd. (Walkerburn, United Kingdom); potassium phosphate (KH2PO4; pH 4.8; 60221), HCl 32% (84421), and NaCl (73575; Fluka Analytical, Brøndby, Denmark); methanol (34885), chloroform (34854), KCN (potassium cyanide; 207810), dimethylamine 40% (426458), RIPA buffer (R0278), and Evans blue (E2129; Sigma-Aldrich, Brøndby, Denmark); FOS-choline-14 (850338P) and d-erythro-sphingosine-1-phosphate (C17 base; 860641; Avanti Polar Lipids, Inc., Alabaster, AL, USA); formamide (1096841000; Merck, Hellerup, Denmark), and trichloroacetic acid 30% (LAB84505; Bie and Berntsen A/S, Søborg, Denmark).
Mice
Mice were housed in individual ventilated cages in a temperature-controlled facility with a 12 h light/dark cycle at the Panum Institute (University of Copenhagen, Denmark) or Weill Cornell Medical College (New York, New York, USA) and were fed a standard chow diet (Altromin 1314; Brogaarden, Gentofte, Denmark) or irradiated diet and water or acidified water ad libitum. Apom−/− mice were backcrossed at least 7 times onto a C57B6/J background (19). WT mice were age- and gender-matched C57Bl6/J mice.
Endothelial-specific S1P1 knockout (S1pr1f/f/Cdh5-Cre-ERT2; S1P1-ECKO) mice were generated as previously described (20). All animal experiments were approved by the Animal Experiments Inspectorate, Danish Veterinary and Food Administration, Ministry of Food, Agriculture and Fisheries, Denmark, or were performed in accordance with the Institutional Animal Care and Use Committee of Weill Cornell Medical College.
Assessment of vascular permeability with dextran molecules
Female mice, 15–18 wk old, were injected in the tail vein with Texas red–labeled dextran 70 kDa (0.1 mg/5 g bodyweight; D1864; Thermo Scientific–Invitrogen, Nærum, Denmark) dissolved in saline and sterile filtered (SLHV013NL; Merck-Millipore, Hellerup, Denmark) (Supplemental Fig. 1). Plasma samples were collected in K2-EDTA containing Microvettes (16444100; Sarstedt, Nümbrecht, Germany). At euthanasia, mice were perfused with saline (20 ml) through the left ventricle for systemic perfusion and through the right ventricle for pulmonary perfusion. Lungs were snap frozen in liquid N2. For quantification of dextran in plasma, diluted samples (1:10) were analyzed with CytoFluor (Thermo Scientific–PerSeptive Biosystems, Framingham, MA, USA) at excitation/emission 360/460 nm and 590/645 nm. For quantification of dextran in lungs, the tissues were homogenized in saline with a polytron (PT 1200; Buch and Holm A/S, Herlev, Denmark) and centrifuged 10 min at 1500 rpm, at room temperature. The supernatants were analyzed with CytoFluor. To verify the size of the dextran molecules, they were diluted (1:2.2) with PBS containing 0.01% EDTA and run on a Superose 6 10/300 GL column (17-5172-01; GE Healthcare, Brøndby, Denmark). Fractions were analyzed with CytoFluor.
Assessment of vascular permeability with Evans blue
Female mice, 17-20 wk old, were injected in the tail vein with 3% Evans blue solution (1 µl/g bodyweight). After 30 min, the mice were euthanized and perfused with saline through the left ventricle of the heart for systemic perfusion (20 ml) and through the right ventricle of the heart for perfusion of the pulmonary circuit (3 ml). The lungs were removed and kept on ice until extraction. For extraction of Evans blue, the lungs were weighed, minced, and incubated at 56°C for 16 h in 1 ml formamide. After incubation, 200 µl supernatant was analyzed with an ELISA-reader (PowerWave XS; Bio-Tek, Winooski, VT, USA), and the amount of Evans blue was determined from optic density 620 nm minus optic density 500 nm. Samples were analyzed in duplicate, and the amount of Evans blue in the tissues was determined with a standard curve (0.12–15 µg/ml) of Evans blue in formamide.
Analysis of S1P1 mRNA expression
RNA was extracted from 20–30 mg lung tissue with Nucleospin RNA/Protein kit (740933, Macherey-Nagel, Düren, Germany) according to the manufacturer’s protocol. The integrity of the RNA was determined with an RNA 6000 Nano Kit (5067-1511; Agilent Technologies, Santa Clara, CA, USA) on a 2100 Bioanalyzer (Agilent Technologies). cDNA was generated from 1 μg RNA with a High Capacity cDNA Reverse Transcription Kit (4368814; Thermo Scientific-Applied Biosystems), and gene expression was determined by quantitative real-time PCR on a LightCycler (Roche, Hvidovre, Denmark).
Primer sequences were as follows: S1P1R forward: 5′-ATGCGGATCGCGCGCGGTGAG-3′, S1P1R reverse: 5′-GCAAAGCCAGGTCAGCGAGC-3′; and β-actin forward: 5′-CTGTCGAGTCGCGTCCACCCG-3′, β-actin reverse: 5′-ACATGCCGGAGCCGTTGTCGAC-3′.
Measurement of S1P1 protein expression
To determine levels of S1P1 receptor protein in the lung, we modified a protocol from Oo et al. (21). Tissue was cut into small pieces, immersed in 1 ml hypotonic buffer (10 mM Tris-HCl (pH 7.8–8.0), 1 mM EDTA), minced with a polytron (PT 1200; Buch & Holm A/S, Herlev, Denmark), and incubated on ice for 10–15 min. After incubation, the tissue was dissociated in a TissueLyser (Qiagen, Copenhagen, Denmark) for 3 min at 30 Hz. Samples were subjected to 3 freeze–thaw cycles by placing them on dry ice and at room temperature, respectively. Extracts were centrifuged at 20,900 g for 15 min at 4°C. The pellet was washed 3 times with 500 µl PBS, resuspended in 300 µl extraction buffer (RIPA buffer+0.5% FOS-choline-14), and rotated overnight at 4°C. Extracts were centrifuged at 10,000 g for 5 min at 4°C, and supernatants were transferred to clean tubes. The supernatants were up-concentrated 3 times by freeze drying and resuspended in water. The total amount of protein in the supernatants was determined with Pierce BCA Protein Assay Kit (23225; Thermo Scientific, Roskilde, Denmark). The concentrated supernatants (38–48 µg) were analyzed on a 16% SDS gel. The gel was subjected to Western blot with primary antibody against the S1P1 receptor (anti-EDG1, ab137467; Abcam, Cambridge, United Kingdom) at 1:200 dilution and secondary antibody (926-32211; LiCor Biosciences, Lincoln, NE, USA) at 1:20,000 dilution.
Electron microscopy
Female mice, 3 WT and 2 Apom−/−, were preanesthetized with inhalation of halothane 3% (Halocarbon Laboratories, River Edge, NJ, USA). Anesthesia was induced by intraperitoneal injection with pentothal natrium 2.5 g (Abbott Scandinavia AB, Solna, Sweden), 55 mg/kg body weight. The lungs were fixed by vascular perfusion through the right ventricle of the heart with 2% v/v glutaraldehyde in 0.05 M sodium phosphate buffer (pH 7.2) for 5 min. After fixation, the lungs were excised and stored in the same fixative. After isolation of suitable specimen blocks, the samples were rinsed 3 times in 0.15 M sodium cacodylate buffer (pH 7.2) and subsequently postfixed in 1% w/v OsO4 in 0.12 M sodium cacodylate buffer (pH 7.2) for 2 h. The specimens were dehydrated in a graded series of ethanol, transferred to propylene oxide, and embedded in Epon, according to standard procedures. Sections, ∼80 nm thick, were cut with an Ultracut E microtome (Reichert-Jung; Leica MicroSystems, Wetzlar, Gemany) and collected on 1-hole copper grids with Formvar-supporting membranes, stained with uranyl acetate and lead citrate, and subsequently examined with a CM 100 transmission electron microscope (Philips, Eindhoven, The Netherlands), operated at an accelerating voltage of 80 kV. Digital images were recorded with an Osis Veleta digital slow scan 2k × 2k CCD camera (Olympus, Tokyo Japan) and the ITEM software package (Irvine, CA, USA).
For each mouse 1–2 samples were examined. For quantification of cell–cell junctions and number of intracellular vesicles, 10 capillaries from each sample were chosen randomly and junctions and vesicles were counted manually. ImageJ 1.45s (National Institutes of Health, Bethesda, MD, USA) was used for measurement of endothelial cell area.
Treatment of mice with plasma from ApoM-TgH or ApoM−/− mice
Female Apom−/− mice (median age 11 wk) were injected with plasma (110 μl i.v.) freshly isolated from Apom−/− or ApoM-TgH mice. Vascular permeability was assessed with Evans blue, as described above, 30 min after injection of plasma.
Treatment of mice with W146 and SEW2871
Female mice, 17–19 wk old, were injected once with SEW2871 (10 mg/kg i.p., S3944; Sigma-Aldrich) or saline 6.5 h before assessment of vascular permeability or twice with W146 (10 mg/kg i.p., W1020; Sigma-Aldrich) 6.5 and 2.5 h before assessment of vascular permeability, respectively. SEW2871 was dissolved to 10 mg/ml in DMSO (1.02931; Merck-Millipore) and diluted to 1 mg/ml in 3% bovine serum albumin (BSA; A7030, Sigma-Aldrich) in PBS. W146 was dissolved to 5 mg/ml in HCl-EtOH (LAB40372; Bie and Berntsen A/S).
Carrageenan-induced paw edema
Male C57Bl/6, Apom−/−, S1pr1f/f/Cdh5-Cre-ERT2, and S1pr1 f/stop/f/ Cdh5-Cre-ERT2 mice 16 wk of age were anesthetized with isoflurane 2% before subplantar injection of 50 μl λ-carrageenan (3% wt/vol; Sigma-Aldrich) or saline into the left paw. The index of the inflammatory response in the paw edema was quantified by measurement of the change in paw volume with a hydroplethysmometer, specially modified for small volumes (Ugo Basile, Milan, Italy). Paw volume was measured immediately before the injection of carrageenan, and 2–192 h thereafter (22).
Generation of adenovirus expressing ApoM
Full-length cDNA of human ApoM (790 bp) was PCR purified and cloned into the Kpn and NotI site of the pAdTrack-CMV vector to generate recombinant adenovirus plasmid (pAd-ApoM) as described (23). The recombinant adenoviral vector was linearized with the PacI restriction enzyme and transfected into packaging competent human embryonic kidney 293 cells to generate high-titer adenovirus particles. Recombinant adenovirus was purified by 2 consecutive CsCl2 ultracentrifugation steps, dialyzed, and titered for further experiments.
Treatment of mice with adenovirus
Male Apom−/− mice at 16 wk of age were injected with 5 × 108 pfu i.v. of Ad-ApoM or Ad-green fluorescent protein (GFP). Paw inflammation was induced by subplantar injection of carrageenan solution (3% w/v) in distillate water, 96 h after adenovirus treatment.
Immunoblot analysis of plasma ApoA1 and ApoM
Blood was collected from Ad-ApoM- or Ad-GFP-injected Apom−/− mice, before and 1, 2, 7, 14, 21, and 28 d after injection of 5 × 108 pfu i.v. of Ad-ApoM. The blood was centrifuged at 2400 g for 10 min, and 1 µl of plasma was analyzed by SDS-PAGE and transferred to PVDF membranes. Monoclonal antibodies against human and mouse ApoM (GeneTex, Irvine, CA, USA) were used for detection of the proteins. Membranes were stripped and reprobed for human or mouse ApoA1 (both from Abcam, Cambridge, MA, USA). Protein loading was visualized by Ponceau red staining.
Isolation of murine HDL
HDL from WT and Apom−/− mice was prepared by the standard KBr density gradient technique (24). Accordingly, plasma was isolated in the presence of EDTA and clarified by centrifugation. Plasma was adjusted to a density of >1.06 with crystalline KBr and centrifuged at 55,000 rpm in a TL-100 Ultracentrifuge in a T100.3 rotor (Beckman Coulter, Jersey City, NJ, USA) for 18 h to separate plasma VLDL-LDL fractions. After collection of LDL, the remaining plasma was adjusted to a density of >1.21 with crystalline KBr and centrifuged at 51,000 rpm, as above, and the HDL fraction was collected sterilely. Because serum albumin also acts as a carrier for S1P, HDL was collected and suspended in 1.21 KBr density solution and recentrifuged as above, to eliminate residual albumin to <0.1% of plasma concentration. The resulting HDL was dialyzed against >2000 vol PBS at 4°C with 3 changes of PBS over 48 h. Each HDL sample was evaluated for ApoM expression by Western blot, with a rabbit mAb (GeneTex) and S1P content by mass spectrophotometry (average, 740 ng S1P/mg HDL).
In vitro endothelial cell barrier
Human umbilical vein endothelial cells (HUVECs) were maintained under standard conditions and analyzed between passages 4 and 8. The barrier function was evaluated by measuring the resistance of a cell-covered electrode by using and endothelial cell impedance system instrument (Applied BioPhysics, Troy, NY, USA). HUVECs were plated on 0.1% gelatin (Sigma-Aldrich)- coated electrodes (8W10E plates) at the density of 6 × 104 cells/well. When confluent, the cells were starved for 2 h in endothelial basal medium (EBM-2; Lonza, Basel, Switzerland) and treated with S1P (10 and 30 nM; S1P was dissolved in 2% fatty acid-free BSA; Sigma-Aldrich) or HDL isolated from WT and Apom−/− mice (10 and 30 μg/ml). The resistance was monitored and expressed as fractional resistance.
Statistical analysis
All statistical analyses were performed with Prism, version 4.03 (GraphPad Software, Inc., La Jolla, CA, USA). Groups of 2 were compared by using Student’s t test. Where appropriate, Welch’s correction for unequal variances was applied. When comparing samples from the same animal before and after treatment, a paired Student’s t test was applied. Groups of more than 2 were compared with 1-way ANOVA with Tukey’s post hoc test or Bonferroni’s test for multiple comparisons. P ≤ 0.05 indicated statistical significance.
RESULTS
ApoM deficiency leads to increased vascular permeability in lungs
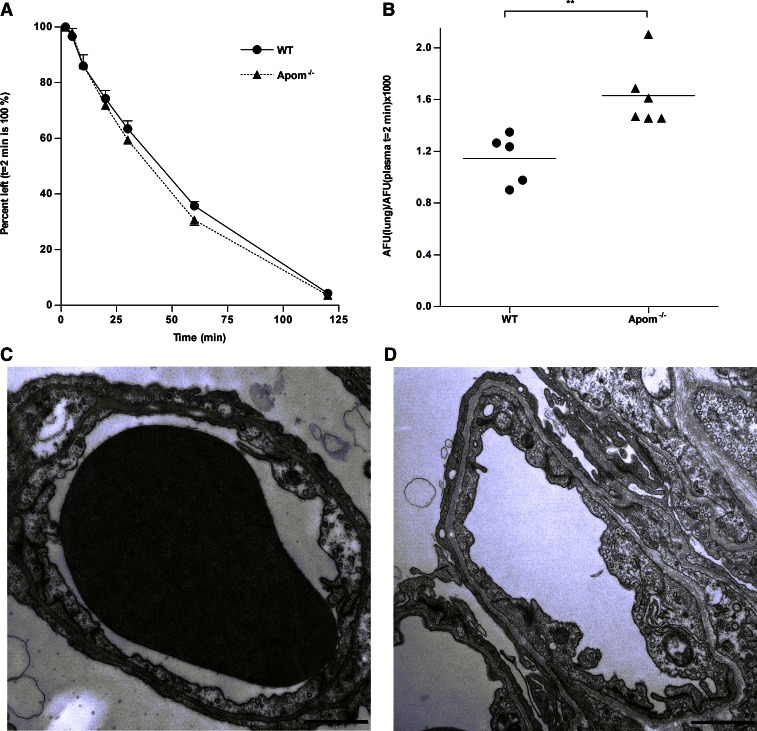
To study vascular permeability, we injected 70 kDa fluorescent dextran intravenously into WT and Apom−/− mice. There was no difference between the two mouse strains in plasma decay of the fluorescent tracer (Fig. 1A). However, Apom−/− mice accumulated significantly higher amounts of 70 kDa dextran in the lungs compared to WT mice (Fig. 1B). These results suggest that in a normal physiologic setting, ApoM-deficiency leads to increased vascular permeability in the lungs.
Figure 1.
Apom−/− mice have increased permeability, but normal vascular morphology of the lungs. Vascular permeability was assessed by injecting WT (n = 5) and Apom−/− (n = 6) mice intravenously with fluorescent dextran molecules (70 kDa). A) Decay of fluorescent dextran in plasma. The amount of fluorescence 2 min after injection was set to 100%. Each point represents means ± sem. B) Accumulation of dextran in lung tissue was calculated as average fluorescence units (AFU) in lung tissue normalized to AFU in plasma of each mouse 2 min after injection. Each point represents an individual mouse, and horizontal lines represent the mean. **P ≤ 0.01, Student's t test. C, D) Transmission electron micrographs of capillaries in lungs of WT (C) and Apom−/− (D) mice. Scale bars, 1 µm.
Apom−/− mice have normal lung vascular morphology
ApoM is expressed during embryonic development (25, 26). Lack of S1P or S1P1 results in impaired vasculogenesis during embryonic life (27, 28). Therefore, the gross morphology of the pulmonary vasculature in Apom−/− mice was assessed by transmission electron microscopy. There were no apparent differences in the morphology of endothelial cell junctions between WT and Apom−/− mice (Fig. 1C, D). Also, on a semiquantitative analysis, there was no indication that the Apom−/− mice have fewer junctions than WT mice (as judged by number of cell–cell junctions per square micrometer endothelial cells; Supplemental Fig. 2). Finally, quantification of intracellular vesicles possibly involved in transendothelial transport of albumin in endothelial cells showed no difference in vesicle count between WT and Apom−/− mice (Supplemental Fig. 2).
ApoM/S1P-enriched plasma can reverse the increased vascular permeability in Apom−/− mice
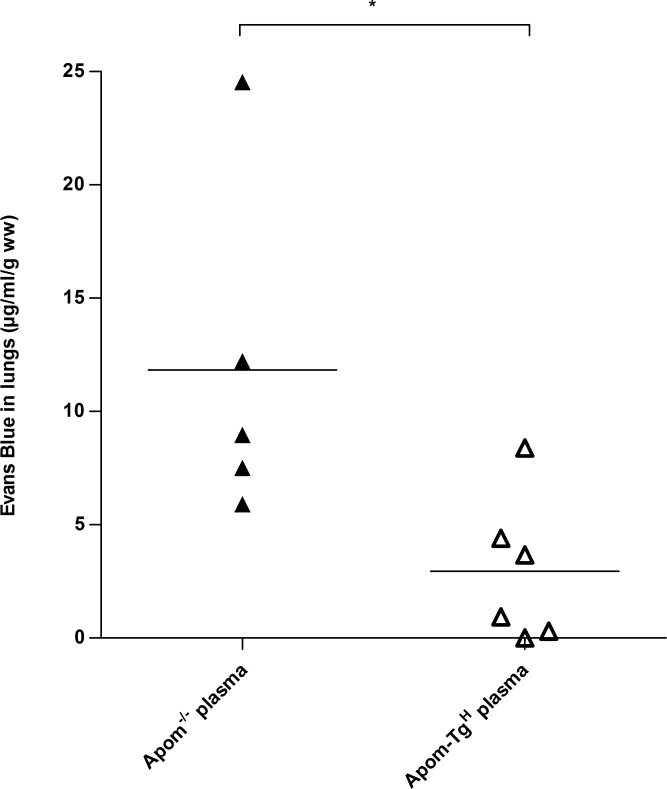
To investigate whether endothelial leakage in the lungs of Apom−/− mice was reversible and directly related to the S1P system, Apom−/− mice were injected with ApoM/S1P-rich plasma (110 μl) from Apom-TgH mice (19) or ApoM-free/S1P-low plasma from Apom−/− mice 1 hour before assessing the pulmonary vascular permeability with Evans blue. Apom−/− mice receiving plasma from Apom-TgH mice accumulated significantly less Evans blue in the lungs compared to Apom−/− mice receiving Apom−/− plasma (2.95 ± 1.32 μg/ml/g vs. 11.83 ± 3.34 μg/ml/g; P = 0.027; Fig. 2). This result illustrated that the increased vascular permeability in the lungs of Apom−/− mice is rapidly reversible by reconstitution of plasma ApoM/S1P.
Figure 2.
Injection of ApoM-containing plasma reverses increased vascular permeability in Apom−/− mice. Apom−/− mice were injected with plasma from Apom-TgH (n = 6) or Apom−/− (n = 5) mice. Vascular permeability in lung tissue was assessed with Evans blue 1 hour after injection. Each point represents an individual mouse and horizontal lines represent the mean. *P ≤ 0.05, Student's t test.
Activation of the S1P1 receptor can reverse the increased vascular permeability in Apom−/− mice
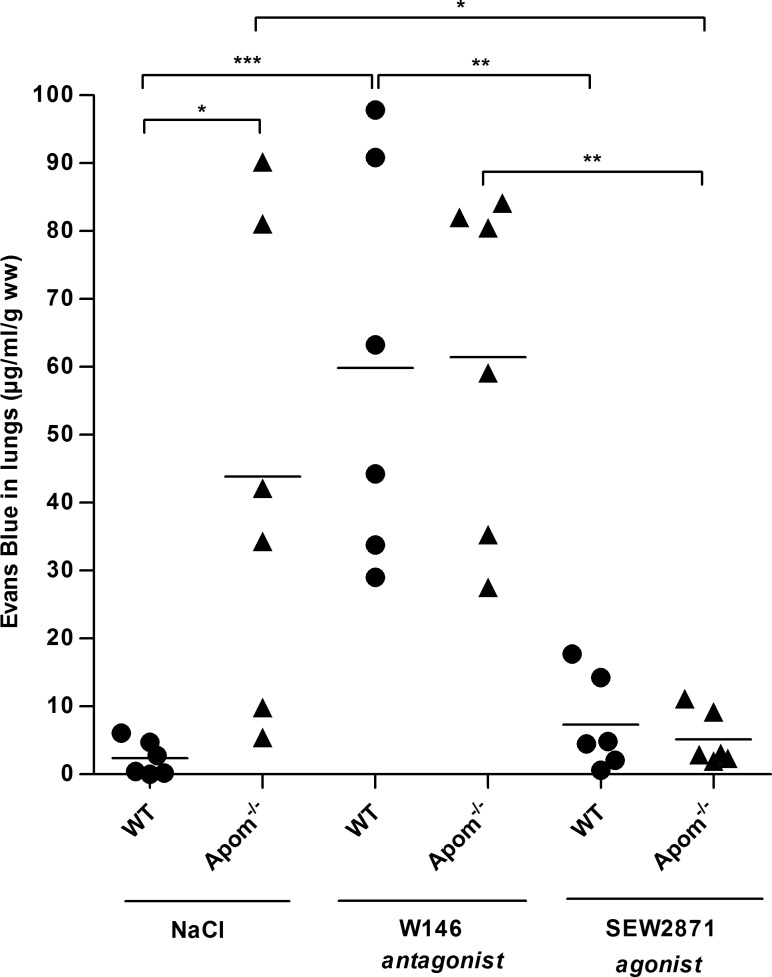
The S1P1 receptor is the most abundantly expressed S1P receptor on endothelial cells and is important for vascular barrier function (8, 11). Accordingly, endothelium-specific S1P1 knockout mice (S1P1-ECKO) had ∼5-fold increased vascular permeability in the lungs (4), suggesting that the decreased S1P/S1P1 signaling confers vascular leakage in vivo. To determine whether the beneficial effects of reconstituting plasma ApoM/S1P in Apom−/− mice is attributable to activation of S1P1, WT and Apom−/− mice were treated with an antagonist (W146) or an agonist (SEW2871) of S1P1. The S1P1 antagonist W146 had no effects on pulmonary Evans blue accumulation in Apom−/− mice, whereas the agonist SEW2871 reversed the vascular leakage in the lungs of Apom−/− mice (Fig. 3). In WT mice, the antagonist W146 increased accumulation of Evans blue in the lungs, whereas the agonist SEW2871 had no effects. This result suggests that the increased pulmonary vascular permeability in Apom−/− mice is caused by diminished S1P/S1P1 receptor signaling.
Figure 3.
Pharmacological activation of S1P1 reverses the increased vascular permeability in Apom−/− mice. WT and Apom−/− mice (n = 6) were treated with 10 mg/kg W146 (S1P1 antagonist) or SEW2871 (S1P1 agonist) or saline 6.5 h before injection of Evans blue for assessment of vascular permeability in lung tissue. *P ≤ 0.05, *P ≤ 0.01, ***P ≤ 0.001, 1-way ANOVA with Tukey’s post hoc test.
Even though we found that Apom−/− mice had ∼30% (P ≤ 0.005) reduced expression levels of S1P1 mRNA compared to those in WT mice (Supplemental Fig. 3), the expression level of S1P1 protein was not different in WT and Apom−/− mice. Hence, the diminished S1P/S1P1 signaling in Apom−/− mice was not attributable to a reduced number of S1P1 receptors on endothelial cells.
Inflammation-induced vascular permeability is increased in Apom−/− mice
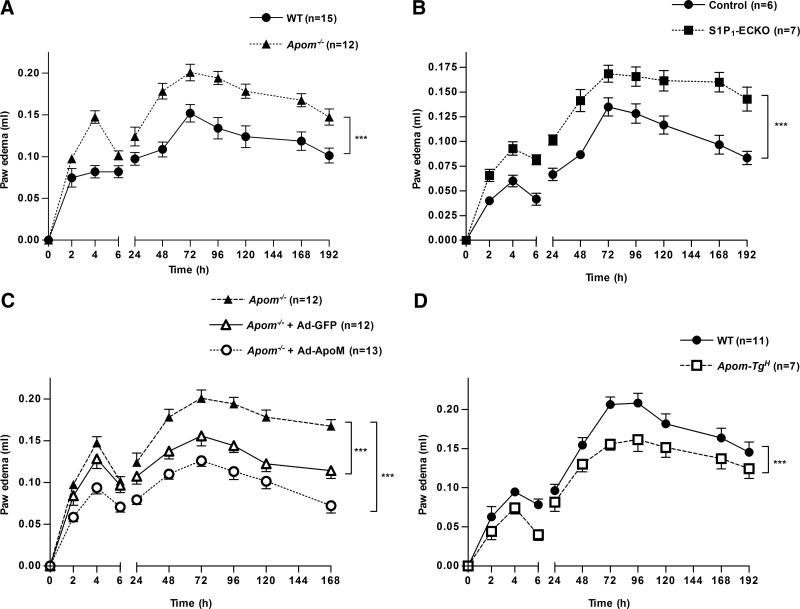
Increased vascular permeability is one of the hallmarks of inflammation. Since Apom−/− mice had increased basal vascular permeability in the lungs, we sought to determine whether the extravasation of plasma proteins and leukocytes during an inflammatory response was also increased in the absence of ApoM. Thus, we used the well-established model of acute inflammation, the carrageenan-induced paw edema (22). In this model, the acute phase of the inflammatory response is dependent on mediators such as histamine, bradykinin, and prostaglandins, whereas the chronic phase results from mononuclear cell infiltration (29). The magnitude of inflammation was markedly increased in Apom−/− vs. WT in both the acute and chronic phases (Fig. 4A). Similar to Apom−/− mice, the S1P1-ECKO mice showed an exaggerated paw inflammation in response to carrageenan compared to their control littermates (Fig. 4B), suggesting that S1P/S1P1 signaling plays an important role in preserving the endothelial cell barrier functions to limit plasma protein and leukocyte extravasation during an inflammatory process.
Figure 4.
ApoM exerts anti-inflammatory functions in the carrageenan-induced paw edema. Mice were injected with 50 µl of carrageenan (3% w/v) into the subplantar area of the left paw. Changes in paw volume were measured at different time-points after carrageenan injection. A) Carrageenan-induced paw edema in Apom−/− and WT mice. B) Carrageenan-induced paw edema in S1P1-ECKO mice and control littermates. C) Carrageenan-induced paw edema in Apom−/− mice 96 h after intravenous injection of Ad-ApoM, Ad-GFP, or vehicle. D) Carrageenan-induced paw edema in Apom-TgH and WT mice. Data represent means ± sem of 3 independent experiments. ***P < 0.001, by 2-way ANOVA, followed by Bonferroni post hoc test to correct for multiple comparisons of time between mouse genotypes.
To further corroborate the role of ApoM in permeability and inflammation, Apom−/− mice were transduced with adenoviral particles designed to express either ApoM or GFP (Ad-ApoM and Ad-GFP, respectively). The expression of ApoM was evaluated by immunoblot analysis of plasma from mice treated with Ad-ApoM and Ad-GFP at different time points after injection (Supplemental Fig. 4). The inflammatory response was markedly reduced in Apom−/− mice injected with Ad-ApoM compared with untreated Apom−/− mice (Fig. 4C). Ad-GFP exerted a moderate suppression of the second, but not the first, phase of inflammation. Nevertheless, both early and late phases of paw edema were significantly reduced in Ad-ApoM vs. Ad-GFP-treated mice. The role of ApoM in restraining inflammatory process was further supported by Apom-TgH mice, expressing 10-fold higher plasma levels of human ApoM (19), which showed a significantly attenuated paw inflammation vs. controls (Fig. 4D).
ApoM-containing HDL increases the endothelial barrier functions in vitro
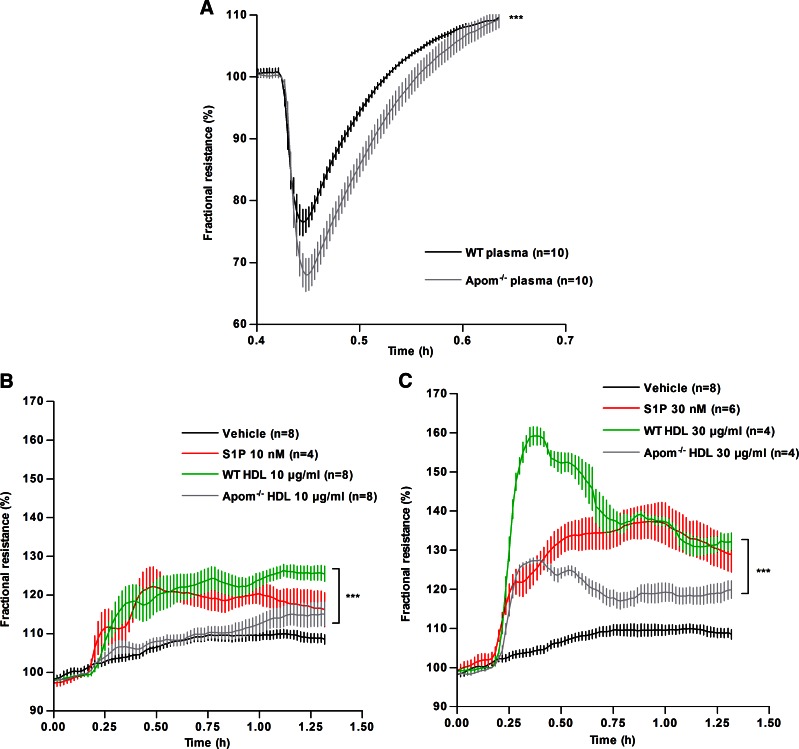
Histamine is one of the key vasoactive molecules, which contribute to vascular permeability (30). To assess the direct effect of ApoM-bound S1P on the endothelial barrier function, changes in transendothelial electrical resistance (TEER) on a confluent monolayer of HUVECs were measured. HUVECs were preincubated with Apom−/− or WT plasma followed by histamine stimulation. HUVECs treated with WT plasma were more resistant to histamine-induced barrier breakdown compared to cells incubated with Apom−/− plasma (Fig. 5A). Further, we assessed the changes in basal TEER in response to purified WT and Apom−/− HDL (10 and 30 μg/ml). S1P (10 and 30 nM) bound to BSA was used as a positive control. WT HDL induced an increase in TEER with a marked greater magnitude compared Apom−/− HDL, especially at 30 μg/ml (Fig. 5B, C). These data suggest that HDL plays an important role in preserving the endothelial barrier integrity through ApoM-bound S1P.
Figure 5.
ApoM-containing HDL increases the endothelial barrier functions in vitro. A) TEER of confluent monolayer of HUVECs was measured and expressed as fractional resistance (%) of the basal values. HUVECs were treated with 5% plasma from WT and Apom−/− mice, stimulated with histamine (3 µM) and TEER was measured over time. B, C) TEER measured in HUVECs treated with WT HDL or Apom−/− HDL [10 µg/ml (B) and 30 µg/ml (C)] or S1P (10 and 30 nM, solubilized in 2% BSA solution) or vehicle as the control. All data represent the results of 3 separate experiments. ***P < 0.001, 2-way ANOVA.
DISCUSSION
The data in the present study suggest that the increased pulmonary vascular permeability of ApoM-deficient mice is not due to gross morphologic abnormalities. Instead, it is related to decreased plasma S1P levels and is rapidly reversible by treatment with ApoM/S1P-enriched plasma or an S1P1 agonist (SEW2871). Furthermore, we found that ApoM improves the endothelial barrier function in both the early and late phases of the inflammatory response.
A priori, we considered the possibility that ApoM deficiency affects the ultrastructure of the pulmonary vasculature, given that S1P deficiency leads to abnormal angiogenesis (27). This idea, however, was not supported by the transmission electron microscopy data. Instead, infusion of ApoM/S1P enriched plasma or treatment with the S1P1 receptor agonist SEW2871 rapidly reversed the vascular leakage in Apom−/− mice. Hence, the data suggest that decreased plasma levels of S1P caused by ApoM deficiency impair the vascular barrier as a result of diminished stimulation of S1P1.
Increased vascular permeability is an important component of the inflammatory response. Here, we report that ApoM deficiency and reduced S1P/S1P1 signaling increases the vascular permeability in carrageenan-induced inflammation. These results, together with recent observations that generalized loss of plasma S1P increases susceptibility to inflammation (15), suggest that the ApoM/S1P axis is an important regulator in inflammation. As further evidence of such a role of the ApoM/S1P/S1P1 axis, we show that endothelial deletion of S1P1, which is the abundant S1P receptor in endothelium, results in increased susceptibility to inflammation-induced vascular leakage in both the mediator and cellular phases of the carrageenan response. As proof of the dependence on our observations on ApoM, we performed rescue experiments by treating Apom−/− mice with recombinant adenovirus overexpressing ApoM. This treatment raised plasma ApoM levels in Apom−/− mice and resulted in a decreased inflammation-induced vascular leakage comparable to WT mice.
ApoM has been described to be a negative acute phase reactant, which decreases during inflammation because of decreased gene transcription and/or increased plasma clearance (31). Humans with sepsis or systemic inflammatory response syndromes (SIRS) have significantly decreased plasma ApoM levels with the degree of decrease reflecting the severity of the disease (32). ApoM carries S1P in plasma (3), and S1P enhances the endothelial barrier via S1P1. Accordingly, it was hypothesized that decreased plasma ApoM levels in sepsis and SIRS would confer decreased plasma S1P levels, which in turn would compromise the vascular barrier function and worsen the sepsis pathology. The studies herein support this hypothesis by showing that ApoM deficiency in mice leads to increased vascular permeability, both in normal conditions and in the presence of inflammation and that this effect is due to decreased S1P/S1P1 signaling. Furthermore, reconstitution of ApoM levels decreased the inflammation-induced vascular permeability in Apom−/− mice. These findings raise the possibility that increasing plasma levels of ApoM and/or S1P could have beneficial effects in patients with conditions involving compromised endothelial barrier function. Hence, clinical studies are warranted to test the potential of ApoM and S1P as therapeutic targets in conditions where endothelial barrier function is compromised, which includes sepsis, viral hemorrhagic fevers, and acute lung injury.
Supplementary Material
Acknowledgments
The authors thank Zhila Nikrozi for preparation of the specimens for electron microscopy and the Core Facility for Integrated Microscopy (Faculty of Health and Medical Sciences, University of Copenhagen) for use of the electron microscopes. This work was supported by grants from The Danish Research Council (to L.B.N.); The Novo Nordisk Foundation (to C.C.); U.S. National Institutes of Health, Heart, Lung and Blood Institute Grant HL89934 (to T.H.) and R01HL126913-01 (to A.D.L.); Foundation Leducq (to T.H.); Harold S. Geneen Charitable Trust Award for Coronary Heart Disease Research (to A.D.L.), and a Ph.D. scholarship from the Faculty of Health and Medical Sciences (University of Copenhagen) (to P.M.C.). The authors declare no conflicts of interest.
Glossary
- Ad-ApoM
adenovirus vector expressing apoM
- ApoM
apolipoprotein M
- Apom−/−
apolipoprotein M-deficient (mouse)
- Apom-TgH
apolipoprotein M-transgenic high (mouse)
- BSA
bovine serum albumin
- GFP
green fluorescent protein
- HUVEC
human umbilical vein endothelial cell
- S1P
sphingosine-1-phosphate
- S1P1–5
sphingosine-1-phosphate receptor 1-5
- SIRS
systemic inflammatory response syndromes
- TEER
transendothelial electrical resistance
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Sevvana M., Ahnström J., Egerer-Sieber C., Lange H. A., Dahlbäck B., Muller Y. A. (2009) Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J. Mol. Biol. 393, 920–936 [DOI] [PubMed] [Google Scholar]
- 2.Ahnström J., Faber K., Axler O., Dahlbäck B. (2007) Hydrophobic ligand binding properties of the human lipocalin apolipoprotein M. J. Lipid Res. 48, 1754–1762 [DOI] [PubMed] [Google Scholar]
- 3.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnström J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., Dahlbäck B. (2011) Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 108, 9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaho V. A., Galvani S., Engelbrecht E., Liu C., Swendeman S. L., Kono M., Proia R. L., Steinman L., Han M. H., Hla T. (2015) HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523, 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kluk M. J., Hla T. (2002) Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta 1582, 72–80 [DOI] [PubMed] [Google Scholar]
- 6.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. (2000) Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352, 809–815 [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez T., Skoura A., Wu M. T., Casserly B., Harrington E. O., Hla T. (2007) Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 27, 1312–1318 [DOI] [PubMed] [Google Scholar]
- 8.Lee M. J., Thangada S., Claffey K. P., Ancellin N., Liu C. H., Kluk M., Volpi M., Sha’afi R. I., Hla T. (1999) Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312 [DOI] [PubMed] [Google Scholar]
- 9.Skoura A., Sanchez T., Claffey K., Mandala S. M., Proia R. L., Hla T. (2007) Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J. Clin. Invest. 117, 2506–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantalupo A., Zhang Y., Kothiya M., Galvani S., Obinata H., Bucci M., Giordano F. J., Jiang X. C., Hla T., Di Lorenzo A. (2015) Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat. Med. 21, 1028–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goddard L. M., Iruela-Arispe M. L. (2013) Cellular and molecular regulation of vascular permeability. Thromb. Haemost. 109, 407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medzhitov R. (2008) Origin and physiological roles of inflammation. Nature 454, 428–435 [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Gorshkova I. A., Berdyshev E., He D., Fu P., Ma W., Su Y., Usatyuk P. V., Pendyala S., Oskouian B., Saba J. D., Garcia J. G., Natarajan V. (2011) Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am. J. Respir. Cell Mol. Biol. 45, 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camerer E., Regard J. B., Cornelissen I., Srinivasan Y., Duong D. N., Palmer D., Pham T. H., Wong J. S., Pappu R., Coughlin S. R. (2009) Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest. 119, 1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng X., Hassoun P. M., Sammani S., McVerry B. J., Burne M. J., Rabb H., Pearse D., Tuder R. M., Garcia J. G. (2004) Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 169, 1245–1251 [DOI] [PubMed] [Google Scholar]
- 17.Axler O., Ahnström J., Dahlbäck B. (2007) An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J. Lipid Res. 48, 1772–1780 [DOI] [PubMed] [Google Scholar]
- 18.Christoffersen C., Nielsen L. B., Axler O., Andersson A., Johnsen A. H., Dahlbäck B. (2006) Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid Res. 47, 1833–1843 [DOI] [PubMed] [Google Scholar]
- 19.Christoffersen C., Jauhiainen M., Moser M., Porse B., Ehnholm C., Boesl M., Dahlbäck B., Nielsen L. B. (2008) Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J. Biol. Chem. 283, 1839–1847 [DOI] [PubMed] [Google Scholar]
- 20.Jung B., Obinata H., Galvani S., Mendelson K., Ding B. S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., Hla T. (2012) Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oo M. L., Chang S. H., Thangada S., Wu M. T., Rezaul K., Blaho V., Hwang S. I., Han D. K., Hla T. (2011) Engagement of S1P₁-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 121, 2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriques M. G., Silva P. M., Martins M. A., Flores C. A., Cunha F. Q., Assreuy-Filho J., Cordeiro R. S. (1987) Mouse paw edema: a new model for inflammation? Braz. J. Med. Biol. Res. 20, 243–249 [PubMed] [Google Scholar]
- 23.Luo J., Deng Z. L., Luo X., Tang N., Song W. X., Chen J., Sharff K. A., Luu H. H., Haydon R. C., Kinzler K. W., Vogelstein B., He T. C. (2007) A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2, 1236–1247 [DOI] [PubMed] [Google Scholar]
- 24.Chapman M. J., Goldstein S., Lagrange D., Laplaud P. M. (1981) A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 22, 339–358 [PubMed] [Google Scholar]
- 25.Faber K., Axler O., Dahlbäck B., Nielsen L. B. (2004) Characterization of apoM in normal and genetically modified mice. J. Lipid Res. 45, 1272–1278 [DOI] [PubMed] [Google Scholar]
- 26.Zhang X. Y., Jiao G. Q., Hurtig M., Dong X., Zheng L., Luo G. H., Nilsson-Ehle P., Ye Q., Xu N. (2004) Expression pattern of apolipoprotein M during mouse and human embryogenesis. Acta Histochem. 106, 123–128 [DOI] [PubMed] [Google Scholar]
- 27.Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S., Proia R. L. (2005) Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 25, 11113–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Wada R., Yamashita T., Mi Y., Deng C. X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S. S., Lee M. J., Liu C. H., Hla T., Spiegel S., Proia R. L. (2000) Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris C. J. (2003) Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 225, 115–121 [DOI] [PubMed] [Google Scholar]
- 30.Di Lorenzo A., Fernández-Hernando C., Cirino G., Sessa W. C. (2009) Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc. Natl. Acad. Sci. USA 106, 14552–14557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feingold K. R., Shigenaga J. K., Chui L. G., Moser A., Khovidhunkit W., Grunfeld C. (2008) Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis 199, 19–26 [DOI] [PubMed] [Google Scholar]
- 32.Kumaraswamy S. B., Linder A., Åkesson P., Dahlbäck B. (2012) Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Crit. Care 16, R60 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.