Abstract
Complement activation, an integral arm of innate immunity, may be the critical link to the pathogenesis of idiopathic pulmonary fibrosis (IPF). Whereas we have previously reported elevated anaphylatoxins—complement component 3a (C3a) and complement component 5a (C5a)—in IPF, which interact with TGF-β and augment epithelial injury in vitro, their role in IPF pathogenesis remains unclear. The objective of the current study is to determine the mechanistic role of the binding of C3a/C5a to their respective receptors (C3aR and C5aR) in the progression of lung fibrosis. In normal primary human fetal lung fibroblasts, C3a and C5a induces mesenchymal activation, matrix synthesis, and the expression of their respective receptors. We investigated the role of C3aR and C5aR in lung fibrosis by using bleomycin-injured mice with fibrotic lungs, elevated local C3a and C5a, and overexpression of their receptors via pharmacologic and RNA interference interventions. Histopathologic examination revealed an arrest in disease progression and attenuated lung collagen deposition (Masson’s trichrome, hydroxyproline, collagen type I α 1 chain, and collagen type I α 2 chain). Pharmacologic or RNA interference–specific interventions suppressed complement activation (C3a and C5a) and soluble terminal complement complex formation (C5b-9) locally and active TGF-β1 systemically. C3aR/C5aR antagonists suppressed local mRNA expressions of tgfb2, tgfbr1/2, ltbp1/2, serpine1, tsp1, bmp1/4, pdgfbb, igf1, but restored the proteoglycan, dcn. Clinically, compared with pathologically normal human subjects, patients with IPF presented local induction of C5aR, local and systemic induction of soluble C5b-9, and amplified expression of C3aR/C5aR in lesions. The blockade of C3aR and C5aR arrested the progression of fibrosis by attenuating local complement activation and TGF-β/bone morphologic protein signaling as well as restoring decorin, which suggests a promising therapeutic strategy for patients with IPF.—Gu, H., Fisher, A. J., Mickler, E. A., Duerson, F., III, Cummings, O. W., Peters-Golden, M., Twigg, H. L., III, Woodruff, T. M., Wilkes, D. S., Vittal, R. Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis.
Keywords: C5b-9, TGF-β1, BMP, decorin, IPF
Idiopathic pulmonary fibrosis (IPF) is a fatal, scarring lung disease with mortality rates that are increasing worldwide (1) and that are comparable to other malignancies, such as cancer. The complex signaling pathways and markedly unpredictable interpatient heterogeneity of IPF contribute to the disappointing outcomes of many clinical trials. Complement activation, a key component of the innate immune system, is triggered in response to multiple types of tissue injury (2–10). Previous studies have reported immune complexes in IPF that could activate complement (11), whereas more recent studies have reported the presence of complement activation products in patients with IPF and that the alternative pathway, fragment Ba, was clinically relevant as an indicator of disease severity (12). The link between innate immunity and lung fibrosis, however, has been an area of ongoing controversy. The complement activation pathway has been largely unexplored and may answer questions posed by the prevailing model of IPF that postulates that epithelial injuries trigger and augment mesenchymal activation.
The active anaphylatoxins, complement component 3a (C3a) and complement component 5a (C5a), are sequentially generated when complement is activated. The split product, C5b, complexes with components C6–C9 to form the terminal complement complex, C5b-9, that leads to cell lysis. Typically, the complement cascade is controlled by membrane-bound complement regulators, CD46 and CD55, that are ubiquitously expressed in human respiratory epithelium. We reported elevated levels of local and systemic C3a and C5a in patients with IPFs (13), and this is associated with the loss of local complement regulators. We demonstrated the interaction of C3a and C5a with a key fibrotic mediator, TGF-β1, and these each cause the loss of CD46 and CD55, which is associated with epithelial injury (13). Of interest, whereas TGF-β1 induced normal primary human lung epithelial cells to overexpress C3a receptor (C3aR) and C5a receptor (C5aR), C3a, in turn, stimulated these cells to express TGF-β1 (13). Biochemical and pharmacologic studies indicate that both C3a and C5a bind to and activate GPCRs, which then transduce signals via heterotrimeric G proteins (14, 15).
C3aR is expressed ubiquitously, including in the lung (16–21). Although its functions in the lung are unclear, C3aR stimulates the expression of IL-1β in monocytes (22) and in retinal pigmented epithelium (23). C3aR-mediated effects on nonlung parenchymal cells include: (1) epithelial mesenchymal transition (24), (2) glomerular and tubulointerstitial injury (25), and (3) induction of genes that are responsible for novel signaling pathways and extracellular matrix components, cytoskeletal organization, and clearance of apoptotic bodies (26). C5a binds to ≥2 GPCRs: C5aR1 (CD88) and C5aR2 (C5L2). C5aR1 is expressed in many cells, including in lung alveolar and airway epithelium as well as the endothelium (27, 28). C5aR1-mediated divergent signaling leads either to apoptosis or cell survival, via ERK (29), Akt activation (30), PKC-mediated IL-8 release by the airway epithelium (31), or fibrosis in other organs (32, 33) compared with C5aR2, which is implicated in acute inflammation (34, 35) and balances the biologic responses to C5a (36, 37). Whereas the pathogenesis of acute lung injury has been associated with the destructive effects of C3aR-dependent (38) and C5aR-dependent signaling (5, 38, 39) and with the extracellular histone–dependent signaling of C5aR1/2 (35), the protective effects due to the blockade of C3aR and C5aR1 in airway hyper-responsiveness and inflammation (40) as well as in renal injury/fibrosis are known (33, 41). In this article, because C5aR2 or C5L2 are implicated more in inflammation, we focus on the role of C5aR1, which we hereafter refer to as C5aR, in lung fibrogenesis. It is not known what role the binding of C3a or C5a to their respective receptors, C3aR and C5aR1, plays in driving the pathogenesis of IPF; the cellular and molecular signaling that underlie this unexplored pathway is unknown. To address these questions, we analyzed lung tissues and bronchoalveolar lavage fluid (BALF) from patients with IPF. We then performed a series of experiments using a significantly scarred murine model that had high levels of complement activation at baseline, employing complementary pharmacologic and genetic interventions.
MATERIALS AND METHODS
Human studies
Frozen lung explants and plasma from patients with IPF were obtained through the Lung Tissue Research Consortium of the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (Bethesda, MD, USA; http://www.nhlbi.nih.gov/research/resources/ltrc). Patient demographics of these samples were previously reported by Gu et al. (13). BALF from control participants was obtained by H.L.T. Mean age of the 9 control participants was 36.9 ± 3.0 yr (mean ± sem), with a range of 35–52 yr. Of the total cohort of control participants, 66.7% were white, and the remaining participants were African American; men comprised 66.7% of the total cohort. BALF from patients with IPF was kindly donated by the late Galen S. Toews, M.D. (University of Michigan). Patient demographics are provided in Table 1. All protocols were approved by the institutional review boards of Indiana University School of Medicine and the University of Michigan.
Table 1.
Baseline and pulmonary function characteristics of patients with IPF
| Characteristic | Value |
|---|---|
| Age (yr) | 64.2 ± 1.7 (48–80) |
| Male (%) | 65.2 |
| Race (%) | |
| White and African American | 100 |
| Hispanic | — |
| Asian | — |
| Smoking status | |
| Current smoker (%) | 8.7 |
| Former smoker (%) | 30.4 |
| Nonsmoker (%) | 56.5 |
| Smoking (packs/yr)a | 34.2 ± 5.2 (5–66) |
| Pulmonary function test | |
| FEV1% predicted | 86.0 ± 4.8 (48–138) |
| FVC% predicted | 72.0 ± 4.3 (36–108) |
| DLCO% predicteda | 46.0 ± 3.5 (14–94) |
| CPI | 51.9 ± 2.6 (36–76) |
Values are means ± sem, n = 23; values in parentheses indicate range. CPI, composite physiologic index; DLCO%, percentage carbon monoxide lung diffusing capacity; FEV1%, percentage forced expiratory volume in 1 s; FVC%, percentage forced vital capacity. aData missing, n = 1.
Animal studies
The Animal Care and Use Committee at the Indiana University School of Medicine and at the University of Michigan approved the animal protocols used in this study. C57-BL6 mice (6–8 wk of age; The Jackson Laboratory, Bar Harbor, ME, USA) were instilled with bleomycin (0.025 U intratracheal) as previously described (42, 43) with minor modifications. Antagonists against C3aR (C3aRA-SB290157) were purchased from EMD Millipore (Billerica, MA, USA), and C5aR (C5aRA; PMX-205) was generously donated by T.S.W.
Cell culture conditions and reagents
Normal primary human fetal lung IMR-90 fibroblasts and normal adult lung fibroblasts were obtained from the Institute of Medical Research (Camden, NJ, USA), and fibroblasts derived from the lungs of patients with IPF were provided by D.W. All fibroblasts were grown in 10% fetal bovine serum that contained DMEM, 100 U/ml penicillin/streptomycin and fungizone (Invitrogen, Carlsbad, CA, USA). Cells were seeded at 65–70% confluence and incubated in 5% CO2/95% air. Cells were serum starved by using 0.01% serum for 36 h before specific treatments. These studies used recombinant human C3a and C5a (100 nM; Complement Technology, Tyler, TX, USA) and platelet-derived TGF-β1 (2 ng/ml; Roche Diagnostics, Jena, Germany). All other reagents were from Sigma-Aldrich (St. Louis, MO, USA).
Western blotting and immunofluorescence
Cell lysates of IMR-90 cells or fibroblasts derived from control participants or from patients who were diagnosed with IPF were lysed, and equal protein concentrations were subjected to immunoblotting as previously described (42, 44–46). Densitometric analyses were performed with ImageJ 1.32j (NIH). Formalin-fixed IMR-90 cells and IPF lung tissues were subjected to immunofluorescent staining for α-smooth muscle actin (α-SMA), C3aR or C5aR (1:100), or their corresponding IgG, then counterstained with DAPI, using protocols described previously (42, 44–46).For in vivo delivery of RNA interference (RNAi), single-duplex small interference RNA (siRNA) sequences that targeted C3ar and C5ar (Sigma-Aldrich) or nontargeting control siRNA (Dharmacon Technologies, Pittsburgh, PA, USA) were used.
Murine fibrosis PCR microarrays
Total RNA was isolated from cells by using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and was reverse transcribed by using qScript cDNA SuperMix (Quanta BioSciences, Foster City, CA, USA). Murine lung mRNA was used to generate cDNA. The Mouse Fibrosis PCR Array–RT2 Profiler PCR Arrays (v 3.0; Qiagen) were used according to manufacturer instructions, and array data were analyzed by using PCR Array Data Analysis software (Qiagen). The semiquantitative real-time PCR data for each target gene were expressed as 2−ΔΔCt relative quantitation vs. endogenous control, with error bars representing the sem.
ELISA
Acellular BALF derived from normal participants and from patients with IPF or mice treated with antagonists or siRNA specific to C3aR/C5aR was used to measure the soluble form of C5b-9 with Terminal Complement Complex C5b-9 Bioassay ELISA kit (human or mouse; U.S. Biologic Life Sciences, Salem, MA, USA) per manufacturer protocol. The soluble forms of C3a and C5a were measured in the BALF by using the mouse complement fragment 3a and 5a ELISA kits (My Biosource, San Diego, CA, USA), respectively, per manufacturer protocol. Active TGF-β1 levels were measured in plasma that was collected from mice treated with antagonists or siRNA specific to C3aR/C5aR by using Mouse TGF-β1 Platinum ELISA (eBioscience, San Diego, CA, USA) per manufacturer protocol.
Immunostaining
Normal and IPF lung tissue biopsies were paraffin-embedded and formalin fixed. Tissue sections were immunostained against C3aR and C5aR (1:600; Novus Biologicals, Littleton, CO, USA) and the corresponding rabbit IgG using the protocol published previously (13).
Statistical analyses
Statistical analysis was performed by using Student’s t test and 1-way ANOVA, with Bonferroni post hoc test using Prism, version 4.03 for Windows (GraphPad Software, La Jolla, CA, USA), unless otherwise stated. Statistical significance was defined at P < 0.05.
RESULTS
C3a and C5a induce mesenchymal activation and expression of their respective receptors
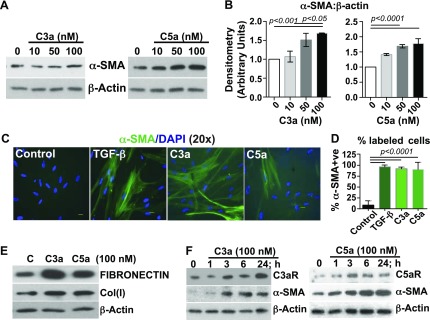
We have previously reported that patients who are diagnosed with IPF have elevated levels of local and systemic C3a and C5a (13). To determine the functional significance of C3a and C5a in the lungs of patients with IPF, we used normal primary human fetal lung IMR-90 fibroblasts to investigate the role of C3a and C5a in mesenchymal activation. IMR-90 cells dose-dependently induced myofibroblast differentiation as indicated by the expression of α-SMA (Fig. 1A, B). The extent of myofibroblast induction was comparable to that of TGF-β1 (Fig. 1C, D), and C3a and C5a also induced the extracellular matrix proteins, cellular fibronectin and collagen type I (Fig. 1E). We further observed a concomitant induction of the expression of their respective receptors parallel with mesenchymal activation (Fig. 1F). These data support a role for C3a and C5a and their respective receptors in mesenchymal activation.
Figure 1.
C3a and C5a induce mesenchymal activation in normal primary human fetal lung fibroblast cultures. IMR-90 cells were grown to 65–70% confluence and were serum starved for ∼ 36 h. A) Cells were treated with varying doses of human recombinant C3a or C5a (10, 50, or 100 nM × 24 h). Cell lysates were immunoblotted with antibodies recognizing α-SMA and β-actin (loading control). B) Immunoblots described in panel A were analyzed by densitometry. Values are expressed as means ± sem of triplicate experiments; statistics: 1-way ANOVA, Bonferroni. C) Mesenchymal activation observed in panel A was confirmed by immunofluorescence staining with anti–α-SMA and DAPI. D) Triplicate results of the immunofluorescent labeling were quantitated by assessing the percent positively labeled cells. Values are expressed as means ± sem; statistics: 1-way ANOVA, Bonferroni. E) Cell lysates from panel A were immunoblotted against fibronectin, collagen type I [Col(I)], and β-actin (loading control). F) Temporal analyses of the respective receptors for C3a and C5a and mesenchymal induction were observed at the indicated time points. Immunoblotting results indicate concomitant induction of C3aR and C5aR with α-SMA. Results are representative of 3 independent experiments. Original magnification, ×20. Scale bars, 100 μm.
Pharmacologic blockade of receptors specific to C3a and C5a during the fibrotic phase of bleomycin-induced lung injury attenuates lung fibrosis
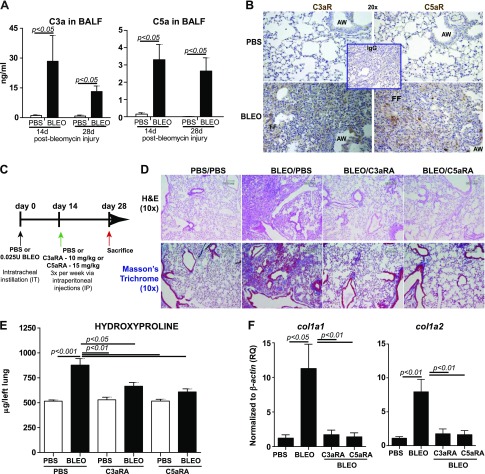
To define the role of C3a and C5a in binding to their respective receptors in a significantly scarred murine lung, we used the bleomycin model of lung fibrosis. Several key fibrogenic responses in mammalian tissues, including TGF-β up-regulation (47, 48) and mesenchymal activation (44, 49, 50), are well simulated in this animal model. We first determined whether C3a and C5a levels are up-regulated in the fibrotic phase of bleomycin-induced lung injury. BALF analyses in Fig. 2A demonstrate that C3a and C5a levels in the injured lungs were increased on d 14 and 28. These data support a relative increase in their respective receptor activity in a fibrotic lung on d 28 (Fig. 2B). Because patients with IPF present with established fibrosis, we simulated this clinical condition and examined the effects of the targeted blockade of C3aR and C5aR in a significantly scarred lung. By using the treatment regimen indicated in Fig. 2C, C3aR and C5aR were blocked at d 14—a time point at which the lungs were significantly fibrotic. Histopathologic assessment was used to examine both lung architecture (hematoxylin and eosin; Fig. 2D) and connective tissue distribution (Masson’s blue trichrome; Fig. 2D). Bleomycin-injured lungs were severely scarred, whereas the blockade of C3aR and C5aR resulted in near normal–appearing lung architecture. We then determined collagen deposition quantitatively by using the standard hydroxyproline assay on entire left lung (Fig. 2E). Of note, at 28 d postbleomycin injury, with 14 d of C3aR or C5aR inhibition, the inhibitor-treated groups had significantly lower levels of collagen compared with the diseased control group. mRNA analyses in Fig. 2F show that bleomycin-induced col1a1 (collagen type I α 1 chain) and col1a2 (collagen type I α 2 chain) gene expressions were suppressed by the blocking of both C3aR and C5aR. Collectively, these results demonstrate that inhibiting C3aR or C5aR effectively arrests the progression of significant fibrosis.
Figure 2.
Pharmacologic blockade of receptors specific to C3a and C5a (C3aR and C5aR) arrest the progression of bleomycin-mediated lung fibrosis. C57-BL/6 mice were subjected to an intratracheal instillation of PBS or bleomycin (0.025 U) on d 0. A) C3a and C5a were analyzed in the BALF collected at the indicated time points after bleomycin injury. Values are given as means ± sem (n = 4 mice per group); unpaired Student's t test. B) Expressions of C3aR and C5aR were assessed in the PBS or bleomycin-instilled mice at d 28 by using immunostained using 3,3′-diaminobenzidine (brown) with corresponding secondary IgG. Nuclei were counterstained by using hematoxylin. C) In the clinically relevant therapeutic model, wherein at d 14, (period of significant collagen deposition), antagonists against C3aR (C3aRA, SB290157; 300 μg/20 g mouse) or C5aR (C5aRA, PMX-205; 200 μg/20 g mouse) were administered intraperitoneally 3 times/wk for 2 wk. Mice were euthanized at d 28. D) Histopathologic exam using hematoxylin and eosin (H&E) and trichrome staining showed that bleomycin-induced fibrosis and collagen deposition were attenuated by the inhibitors. E, F) Analysis of hydroxyproline (E) and col1a1 and col1a2 (F) mRNA expression in the lung. Values are given as means ± sem (n = 10–12 mice/group); 1-way ANOVA, Bonferroni. Results are representative of 3 independent experiments. AW, airway; BLEO, bleomycin; FF, fibroblastic foci. Original magnification, ×20 (B), ×10 (D). Scale bar, 100 μm.
Blockade of C3aR and C5aR suppresses complement activation and terminal complement complex in fibrotic lungs
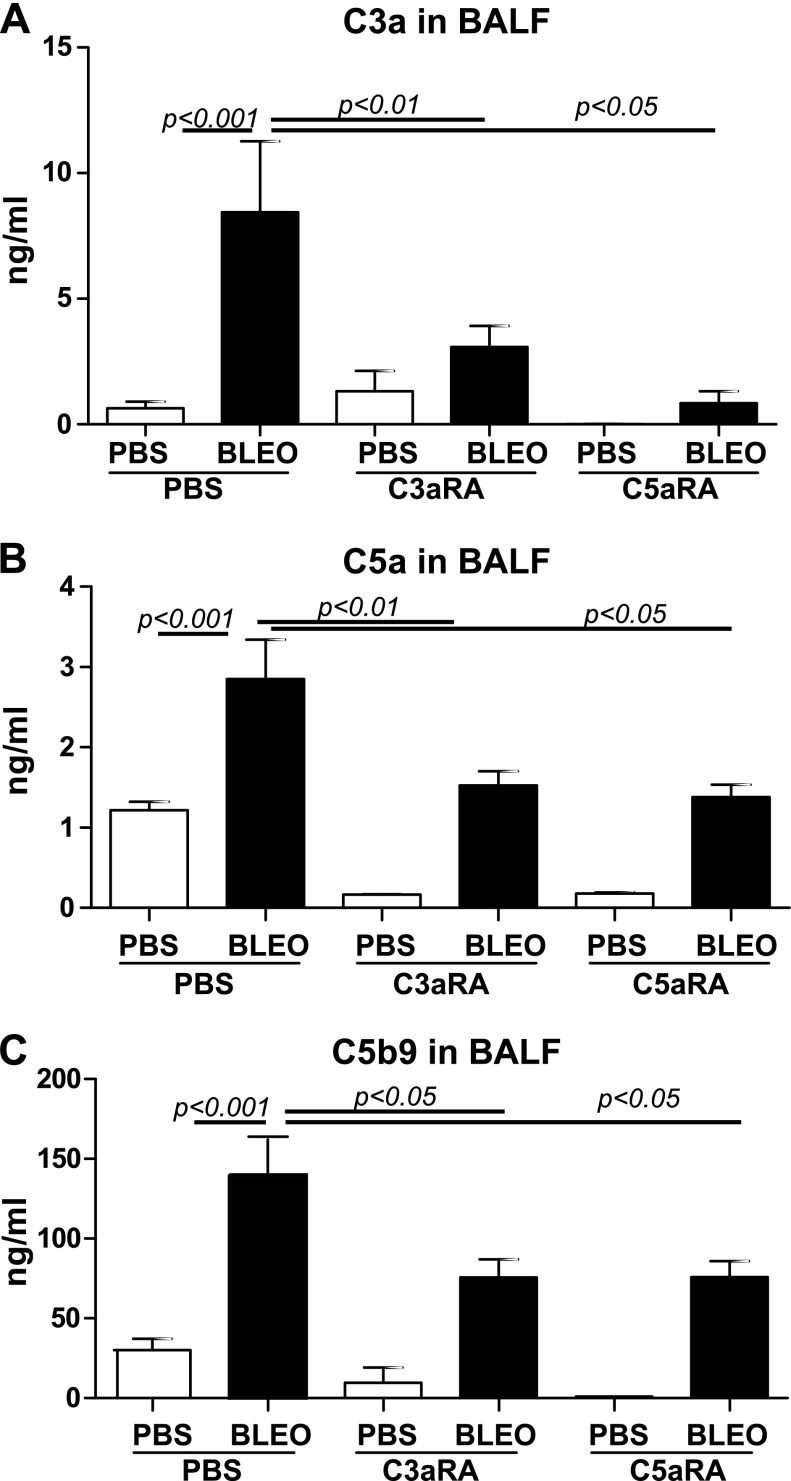
We next investigated the local effects of blocking C3aR and C5aR on the complement components, C3a and C5a, and the terminal complement complex, C5b-9. Increased levels of C3a and C5a in BALF were reported in transfusion-related acute lung injury (51) and in chronic rejection post–lung transplantation (52). Bleomycin-induced C3a (Fig. 3A) and C5a (Fig. 3B) were suppressed by the blockade of C3aR or C5aR. Elevated tissue deposition of C5b-9 was reported during the acute rejection phase post–lung transplantation (53). Bleomycin-induced soluble C5b-9 was suppressed with the blockade of both receptors. Of interest, C5b-9 levels in the bleomycin-injured groups that were treated with inhibitors were still higher than the PBS control group that was treated with inhibitors. These results support a role for complement activation in the pathogenesis of IPF.
Figure 3.
Pharmacologic blockade of receptors specific to C3a and C5a suppresses complement activation in bleomycin-mediated lung fibrosis. BALF collected from Fig. 2 were analyzed for C3a (A), C5a (B), and C5b-9 (C) levels in the lung by ELISA. Values are given as means ± sem (n = 5–6 mice per group); 1-way ANOVA, Newman-Keuls (A) and Bonferroni (B, C). BLEO, bleomycin.
Terminal complement complex, C5b-9, is up-regulated locally and systemically in patients with IPF
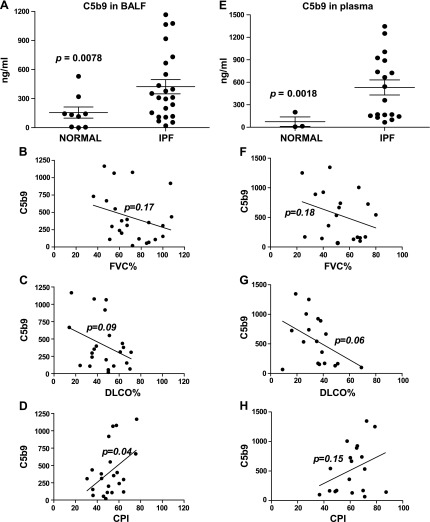
To investigate the clinical relevance of C5b-9 in the pathogenesis of IPF, we examined local and systemic C5b-9 in patients with IPF. We detected increased soluble C5b-9 levels in BALF and in plasma collected from patients with IPF compared with that from control subjects (Fig. 4A, E). We then studied the correlation between local and systemic soluble C5b-9 levels at presentation with clinical markers of disease severity. Whereas local soluble C5b-9 levels at presentation did not correlate with forced vital capacity (FVC) % (Fig. 4B; r = −0.29; 95% CI, −0.63 to 0.13; P = 0.17) or carbon monoxide lung diffusing capacity (DLCO) % (Fig. 4C; r = −0.36; 95% CI, −0.68 to 0.07; P = 0.09), C5b-9 levels were significantly correlated with composite physiologic index (CPI) (54) (Fig. 4D; r = 0.44; 95% CI, 0.02 to 0.72; P = 0.04). However, systemic soluble C5b-9 levels did not correlate with FVC% (Fig. 4F; r = −0.3149; 95% CI, −0.6729 to 0.1627; P = 0.18), DLCO% (Fig. 4G; r = −0.4370; 95% CI, −0.7572 to 0.05261; P = 0.06), or CPI (Fig. 4H; r = 0.3426; 95% CI, −0.1323 to 0.6896; P = 0.15). These results provide a clinical association to our findings in the preclinical murine model.
Figure 4.
Local and systemic C5b-9, a soluble TCC, in patients with IPF. A, E) Patients who were diagnosed with IPF or normal volunteers were analyzed for C5b-9 levels by ELISA in BALF (A) or plasma (E). Values are given as means ± sem; unpaired t test. B–D, F–H) Correlation between BALF (B–D) and plasma (F–H) analyses of C5b-9 and percentage forced vital capacity (FVC%) (B, F), percentage carbon monoxide lung diffusing capacity (DLCO%) (C, G), and composite physiologic index (CPI) (D, H) in patients with IPF. Statistics are provided in the text.
Antagonists specific to C3aR and C5aR may attenuate preexisting fibrosis via up-regulation of TGF-β family ligands, receptors, and modulators
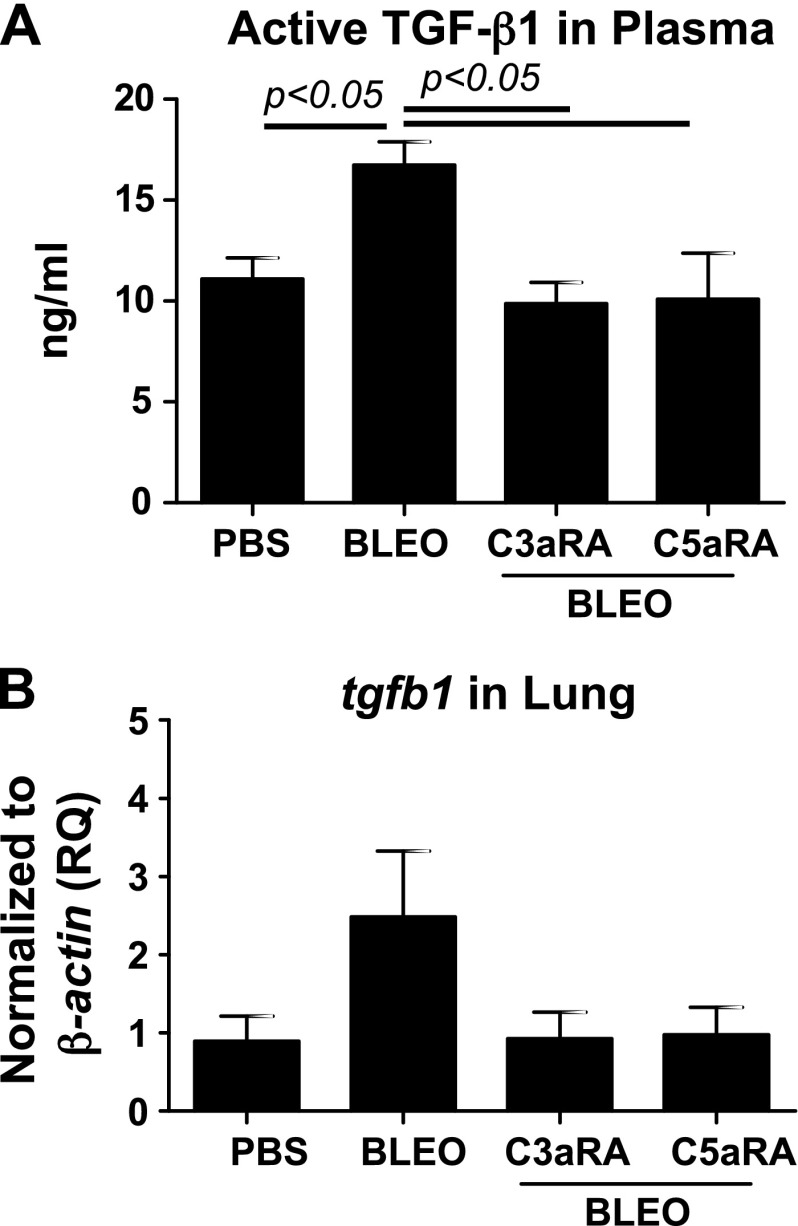
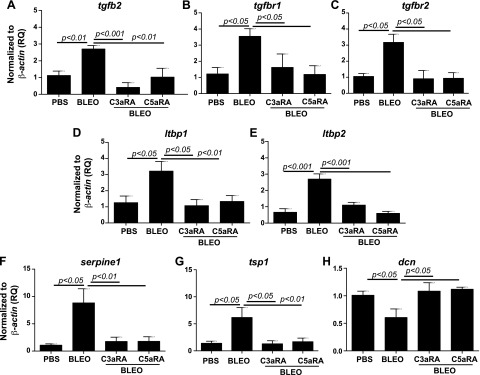
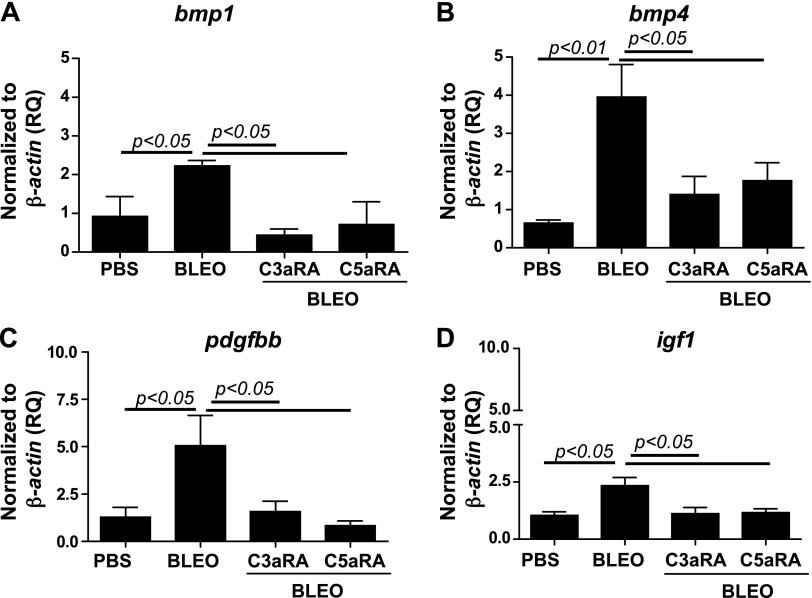
We have previously reported that C3a induces TGF-β, which, in turn, up-regulates C3aR and C5aR in normal primary human lung epithelial cells (13). To further investigate mechanisms that underlie the interaction of C3aR and C5aR and TGF-β in the pathogenesis of lung fibrosis, we first asked whether blockade of C3aR and C5aR affects active TGF-β1 levels. Figure 5A indicates that the blockade of C3aR and C5aR suppressed bleomycin-induced active TGF-β1 in the plasma and that no significant changes were observed in tgfb1 mRNA expression locally (Fig. 5B). We employed a TGF-β/bone morphologic protein (BMP)–specific, 80-gene PCR array to further analyze lung mRNA expression in the related members. A comparison of PBS or bleomycin-instilled lung tissue that either received or did not receive treatment with C3aR- and C5aR-specific inhibitors revealed significant changes that suggested the direct involvement of TGF-β and the upstream role of C3aR- and C5aR-mediated profibrotic signaling in lung fibrosis. Figure 6A–E shows that the bleomycin injury–mediated induction of tgfb2, tgfbr1/2, and ltbp1/2 transcripts was suppressed by blockade of C3aR and C5aR. These data suggest that the profibrotic effects resulting from the activation of C3aR and C5aR may be mediated, in part, via TGF-β ligands and receptors. Inhibition of C3aR and C5aR suppresses bleomycin-induced serpine1 expression (Fig. 6F). Figure 6G demonstrates that tsp1 (thrombospondin-1), an extracellular protein that is critical to normal lung homeostasis, and activation of latent TGF-β (55, 56) was suppressed by C3aR- and C5aR-specific inhibitors in the murine bleomycin-injured lungs. Decorin, a small secreted chondroitin/dermatan sulfate proteoglycan within the family of small leucine-rich proteoglycans was reported to affect the formation of collagen fibrils (57). Our analyses in Fig. 6H shows the restoration of dcn transcripts as a result of C3aR- and C5aR-specific blockade compared with suppressed levels in bleomycin-injured mice. BMPs are secreted signaling molecules that comprise a subfamily of the TGF-β superfamily. We show that both bmp1 and bmp4 were suppressed in inhibitor-treated injured mice compared with bleomycin-injured control mice (Fig. 7A, B). Collectively, these results indicate that the TGF-β modulators, decorin and thrombospondin-1, and members of the BMP family, BMP1/4, are regulated by C3a and C5a in binding to their respective receptors.
Figure 5.
Suppression of TGF-β1 activity in the plasma as a result of pharmacologic blockade of C3aR and C5aR in bleomycin-induced lung fibrosis. A) Plasma collected from Fig. 2 was analyzed for active TGF-β1 by ELISA. B) mRNA expression for tgfb1 was analyzed in the lung by quantitative RT-PCR. Values are given as means ± sem (n = 5–6 mice per group); 1-way ANOVA, Newman-Keuls (A). BLEO, bleomycin; RQ, relative quantitation.
Figure 6.
Regulation of genes belonging to the TGF-β superfamily (ligands, receptors, and modulators) resulting from pharmacologic blockade of C3aR and C5aR in bleomycin-induced lung fibrosis. RNA was isolated from the right lungs, and cDNA was subjected to real-time PCR reactions by using the Mouse TGF-β BMP Signaling PCR array (Qiagen). Specific genes analyzed were isoform tgfb2 (A), receptors tgbr1 (B) and tgbr2 (C), binding proteins ltbp1 (D) and ltbp2 (E), and modulators serpine1 (F), tsp1 (G), and dcn (H). Values are given as means ± sem (n = 5–6 per group); 1-way ANOVA, Bonferroni (A, C, E, F), Newman-Keuls (B, D, G, H). BLEO, bleomycin; RQ, relative quantitation.
Figure 7.
Suppression of genes belonging to the BMP superfamily and profibrotic mediators that interact with TGF-β as a result of pharmacologic blockade of C3aR and C5aR in bleomycin-induced lung fibrosis. RNA was isolated from the right lungs, and cDNA was subjected to real-time PCR reactions by using the Mouse TGF-β BMP Signaling PCR array (Qiagen). Specific genes analyzed were bmp1 (A), bmp4 (B), pdgfbb (C), and igf1 (E). Values are given as means ± sem (n = 5–6 per group); 1-way ANOVA, Newman-Keuls (A, B), Bonferroni (C, D). BLEO, bleomycin; RQ, relative quantitation.
Other signaling pathways that interact with TGF-β
Platelet-derived growth factor (PDGF) is a potent mediator of lung fibrosis (58), and PDGF receptor tyrosine kinase is targeted by a current U.S. Food and Drug Administration–approved drug for IPF, nintedanib (59). IGF-1 exacerbates lung fibrosis (60). Figure 7C, D demonstrates that inhibition of C3aR and C5aR suppresses bleomycin-induced pdgfbb and igf1. These results suggest that blockade of C3aR and C5aR suppresses two significant profibrotic mediators that interact with TGF-β.
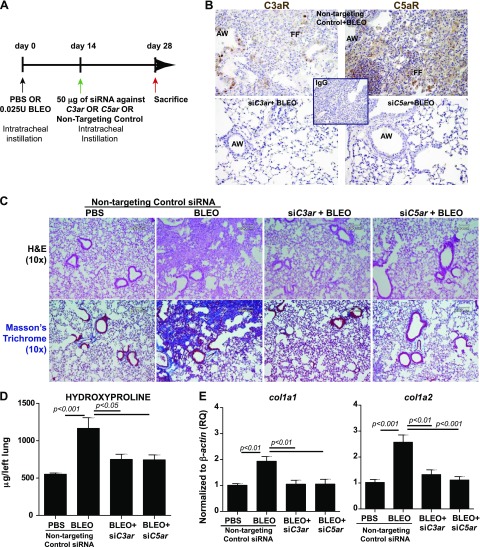
RNAi-mediated gene silencing of C3aR and C5aR mitigates progression of fibrosis by suppressing complement activation locally and active TGF-β systemically
We next examined the effects of the targeted suppression of C3ar and C5ar expression by using an RNAi approach in a clinically relevant model of lung fibrosis. Bleomycin-injured mice with significantly scarred lung tissue were subjected to intratracheal instillation of siRNA specific to C3ar or C5ar or of nontargeting control siRNA (Fig. 8A). Figure 8B shows that gene silencing of C3ar or C5ar suppressed the protein expressions of these receptors compared with mice that received nontargeting control siRNA. Figure 8C further supports our hypotheses that lack of expression of these receptors mediates a profound antifibrotic effect, with significant suppression of fibrosis and collagen deposition (Fig. 8C). This was confirmed by measuring hydroxyproline synthesis (Fig. 8D) and mRNA expression of col1a1 and col1a2 (Fig. 8E) in whole-lung homogenates. We observed the suppression of local C3a, C5a, and soluble C5b-9 in bleomycin-injured mice as measured in BALF (Table 2). Table 2 also presents data that show significant suppression of TGF-β1 activity systemically with knockdown of C3ar and C5aR. These results suggest that the therapeutic blockade of C3aR and C5aR arrests fibrotic progression with suppression of associated complement activation and expression of the profibrotic cytokine TGF-β.
Figure 8.
RNAi-mediated gene silencing of C3aR and C5aR arrests the progression of bleomycin-induced lung fibrosis. A) C57-BL/6 mice were subjected to an intratracheal instillation of PBS or bleomycin (0.025 U) on d 0, followed by intratracheal instillation of 50 μg RNAi at d 14. Tissues were harvested on d 28. B) Protein expressions of C3aR and C5aR were assessed by immunostaining in bleomycin-injured mice instilled with siRNA specific to C3ar or C5ar or nontargeting controls at d 28 to confirm efficacy by 3,3′-diaminobenzidine (brown) with corresponding secondary IgG. Nuclei were counterstained by using hematoxylin. Abbreviations: AW: Airway, FF: Fibroblastic foci. C) Histopathologic exam using hematoxylin and eosin (H&E) and trichrome staining showed that bleomycin-induced fibrotic lung and collagen deposition was attenuated by silencing C3ar and C5ar. D, E) Analysis of hydroxyproline (D) and col1a1 and col1a2 (E) mRNA expression in the lung. Values are given as means ± sem (n = 5–7 per group); 1-way ANOVA, Bonferroni. AW, airway; BLEO, bleomycin; FF, fibroblastic foci. Compared with bleomycin: ***P < 0.001; **P < 0.01; *P < 0.05. Results are representative of 2 independent experiments. Original magnification, ×20 (B), ×10 (C). Scale bar, 100 μm.
Table 2.
Local complement activation and systemic TGF-β1 activity in mice subjected to RNAi-mediated gene silencing of C3ara and C5ara
| Analyte (ng/ml) | NT-PBS | NT-BLEO | siC3ara+BLEO | siC5ara+BLEO |
|---|---|---|---|---|
| C3a, l | 0.4 ± 0.4* | 16.1 ± 4.9 | 3.9 ± 1.4* | 6.9 ± 3.1* |
| C5a, l | 0.7 ± 0.2** | 2.1 ± 0.4 | 1.0 ± 0.2* | 0.9 ± 0.2* |
| C5b-9, l | 105.7 ± 25.7* | 337.3 ± 91.8 | 89.9 ± 15.3** | 91.0 ± 31.9** |
| Active TGF-β1, p | 10.2 ± 0.7* | 15.9 ± 0.9 | 10.6 ± 2.0* | 8.6 ± 0.6** |
Analytes were measured either in the BALF (l) or in the plasma (p). Data are given as means ± sem in each group (n = 5–7). BLEO, bleomycin; NT, nontargeting siRNA. Statistics: 1-way ANOVA; post hoc Bonferroni; compared with NT-BLEO group: *P < 0.05; **P < 0.01.
Expression profile of C3aR and C5aR in patients with IPF
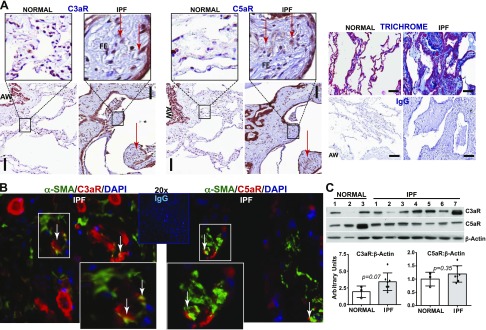
To investigate the clinical relevance of C3aR and C5aR in a human fibrotic lung disease, we compared normal lung tissue with lung tissue from patients with IPF. Whereas C3aR and C5aR are known to be ubiquitously expressed in the respiratory epithelium, these receptors were overexpressed in the fibroblastic foci of the remodeled IPF lung tissue, as determined by immunostaining (Fig. 9A). In addition, the architecture of the tissues was confirmed by using Masson’s trichrome staining for collagen deposition. Figure 9B shows that fibroblastic foci in the IPF lung tissue sections were identified by using α-SMA, a mesenchymal marker, which was coimmunostained for C3aR or C5aR; there is some costaining observed in both cases. Lung fibroblasts derived from lungs of pathologically normal participants and from patients who were diagnosed with IPF were immunoblotted for C3aR and C5aR (Fig. 9C). Although some of the fibroblasts that were derived from the IPF lung tissue expressed increased levels of C3aR and C5aR, no overall significant difference was observed (Fig. 9C). These data support a key role for C3aR and C5aR in the pathogenesis of IPF.
Figure 9.
C3aR and C5aR expression profiles in the lungs of patients with IPF. A) Comparative immunohistochemical staining was performed on paraffin-embedded human IPF lung biopsy explants obtained during lung transplantation and tissue resected from normal (non-IPF) lung tissue using C3aR or C5aR. Trichrome staining shows areas of fibrotic foci with rabbit IgG antibodies. (3,3′-diaminobenzidine, brown; hematoxylin, blue). Representative lesions are presented subsequent to examining lung biopsies from 5 different normal subjects and patients with IPF. B) Double immunofluorescent staining of IPF lung tissue sections with C3aR or C5aR with α-SMA shows costaining in the inset. C) Fibroblasts cultured ex vivo from normal and IPF lungs were immunoblotted for C3aR, C5aR, and β-actin. Densitometric analyses of C3aR and C5aR compared with β-actin are expressed as means ± sem; unpaired t test. AW, airway; FF, fibroblastic foci. Original magnification, ×40. Scale bar, 600 μm.
DISCUSSION
Complement activation is an understudied, yet phylogenetically ancient, innate immune response and is critical to the fighting of infections (61). The current understanding of complement activation on the pathogenesis of IPF is unknown, but exploiting it therapeutically may be the missing link in the development of effective treatments for patients with IPF. C3a and C5a mediate both tissue injury (33, 62) and immune activation (8, 52). Our previous study demonstrated evidence of complement activation in patients with IPF and its role in lung epithelial injury in vitro (13). In the current study, we addressed the profibrotic mechanistic role of C3a and C5a in binding to their receptors in IPF pathogenesis. Whereas our in vitro studies demonstrate that C3a and C5a induced mesenchymal activation and up-regulation of their respective receptor expressions, our in vivo proof-of-concept studies demonstrate that the blockade of their receptors, C3aR and C5aR, arrests the progression of bleomycin-induced lung fibrosis and suppresses local complement activation and systemic TGF-β1 activity. To the best of our knowledge, this is the first study that shows soluble levels of C5b-9 in BALF of patients with IPF. Despite the modest number of samples, it is significantly associated with at least 1 of 3 parameters of disease progression, defined as a relative decline of 10% in FVC, a relative decline of 15% in DLCO, or an increase of 5% in CPI. Finally, pharmacologic or RNAi-mediated blockade of C3aR and C5aR led to attenuation the local transcript expressions of TGF-β–related ligands, receptors, and modulators.
Previous studies have reported C3a- and C5a-mediated mesenchymal transition of glomerular endothelial (63) and tubular epithelial cells (25) as well as the subsequent expression of α-SMA, TGF-β, and fibronectin in vitro. Consistent with findings in the literature, we have reported that C3a and C5a, known to be potent inflammatory mediators, induced α-SMA expression and matrix synthesis. Of interest, a robust network of contractile actin fibers at 72 h was comparable to the effects of TGF-β1. Our findings also indicate a concomitant induction of the respective receptors with mesenchymal activation. Cumulatively, our data suggest that C3a and C5a, in binding to their receptors, induce fibroblast differentiation and matrix synthesis.
Whereas complement proteins C3a, C5, or C5a have been implicated in chronic pancreatitis (64), chronic rejection post–lung transplantation (8, 52), tubulointerstitial injury (41), and tubulointerstitial fibrosis (33), there has been only one report on the role of C5 in bleomycin-injured pulmonary fibrosis (62). Addis-Leiser et al. (62) showed that inhibition of C5, a convertase that can release C5a, before bleomycin-induced lung injury prevented lung fibrosis; however, the results in their report are not clinically relevant due to the early preventive treatment regimen. Moreover, Bao et al. (41) showed that, although lack of C5aR was inconsequential in the context of complement-induced tubulointerstitial injury during renal transplantation, C3aR deficiency conferred a protective effect. In the current report, to compare the relative contribution of C3aR and C5aR, we use a clinically relevant therapeutic model (42, 45) wherein the mice were significantly scarred at the time of intervention. We observed a significant increase in both the ligands (C3a and C5a) and the respective receptors (C3aR and C5aR) in the scarred lung. Increased receptor expression is consistent with localization of C3aR transcripts by in situ hybridization during the development of murine lupus nephritis (41) and by immunostaining in renal tubulointerstitial injury (25). The transcriptional factors that regulate C3aR expression were defined as AP-1 and Ets by Schaefer et al. (65) and are critical to understanding their function. Reports link C3aR and C5aR signaling to p-AKT, p-Foxo1, and other contributing effects, including those that involve the cAMP-PKA-CREB pathway and/or cross-talk with NF-κB, JAK/STAT5, and ERK pathways, all of which have been implicated in the pathogenesis of lung fibrosis (66). Collagen expression and secretion by lung fibroblasts are known to be up-regulated in both clinical IPF (45) and in the bleomycin model of lung fibrosis (42, 44–46, 50). Specifically, increased mRNA expressions of α 1 and 2 chains of collagen type I, the major lung collagen, has been widely reported (45). In the current study, both the antagonists of C3aR and C5aR and the targeted RNAi sequences markedly reduced collagen deposition as well as col1a1 and col1a2 mRNA expression in the lung. Because collagen deposition dictates lung architecture and lung function, our findings clearly suggest a role for both the ligands (C3a and C5a) and their respective receptors in the regulation of collagen synthesis and deposition in IPF pathogenesis.
Because complement activation is an innate immune response, complement split products, C3a, C4a, and C5a, are generated during the early phase of inflammation. It is possible that the generation of these split products may be a result of impaired clearance of tissue debris (67). Previous reports show that protease-activated receptor 2 deficiency in allergic lung inflammation (51) and systemic neutralization of C5 pretransplantation in chronic rejection post–lung transplantation (52) lowered local and systemic C3a and C5a. In our study, we show that whereas blockade of both C3aR and C5aR suppresses local C3a and C5a, blocking the specific C5aR antagonist is relatively more effective; however, this was not recapitulated by the RNAi approach. One possibility for this may be because of the potential nonspecific nature of C3aRA (68). Whereas complement deposition in the tissue was reported in lupus nephritis (69) and in acute rejection phase post–lung transplantation (53), to our knowledge, this is the first study to demonstrate elevated local soluble C5b-9—the terminal complement complex (TCC)—in a murine lung fibrosis model, and that blockade of C3aR and C5aR has protective effects. To our knowledge, this is also the first report to demonstrate the presence of significant levels of TCC in the lungs of patients with IPF.
Our previous in vitro studies demonstrated interaction between complement activation and TGF-β1, which together augment epithelial injury. Specifically, we reported that C3a stimulates normal human primary lung epithelial cells to express TGF-β1, which, in turn, induces C3aR and C5aR expression (13). Li et al. (70) demonstrated that blockade of C3aR ameliorates inflammatory and fibrotic signals in type 2 diabetic nephropathy by suppressing the activation of TGF-β/smad3 and IKBα pathway. Boor et al. (33) reported that complement C5 mediates experimental tubulointerstitial fibrosis and that the cortical mRNA of all PDGF isoforms and of TGF-β1 (i.e., central mediators of renal disease) were significantly reduced in C5−/− mice. Addis-Leiser et al. (62) have shown that local mRNA and active TGF-β1 were significantly suppressed in C5−/− mice in experimental lung fibrosis. Whereas these experimental studies were preventive in nature, our study shows that the therapeutic blockade of both C3aR and C5aR in significantly scarred lungs effectively suppresses active TGF-β1 levels systemically. The therapeutic utility of this finding is that the efficacy of C3aR and C5aR blockade can be tested in an easily accessible clinical tissue, that is, in the plasma. Subsequent analyses of local transcript expressions of TGF-β receptors, ligands, and modulators confirmed our direct observations of the suppression of active TGF-β1 in the plasma and are discussed here. Polosukhin et al. (71) demonstrated increased expression of TGF-β1 and TGF-β2 in bleomycin-mediated airway remodeling. Our previous report showed that collagen type I–induced tolerance down-regulated tgfbr1/2 and ltbp1 in bleomycin-mediated pulmonary fibrosis (45). Latent complexes of TGF-β1 bind with the latent TGF-β1 binding proteins 1 and 2 (LTBP1/2; ltbp1/2), which expose them to the matrix for activation (72). Serpine-1, a member of the serine proteinase inhibitor superfamily and the principal inhibitor of tissue plasminogen activator and urokinase, is associated with collagen accumulation and myofibroblast survival (73), which protects fibroblasts from apoptosis (74) and alveolar injury (75). Dysregulated angiogenic responses in patients with IPF were associated with increases in systemic tsp1 (76), and thrombospondin-1 is implicated in the activation of latent TGF-β1 (77). Consistent with previous findings, the current study shows suppression of the genes discussed here. Decorin plays a critical role in ECM modification (57) and reduces the fibrotic response to bleomycin by inhibiting the profibrotic molecule TGF-β (78–80). The decorin core protein is known to bind with TGF-β and sequester it to the ECM where it is unavailable for substrate binding (81). Accordingly, our findings suggest that blockade of C3aRA and C5aRA restores the transcript levels of decorin and protects against bleomycin-induced fibrotic responses. BMP1 is a profibrotic enzyme that is responsible for the cleavage and maturation of growth factors and ECM proteins, such as lysyl oxidase (43, 82), and BMP4 is a potent inducer of epithelial-to-mesenchymal transition (83–85). Profibrotic TGF-β responses require the cooperation of PDGF receptor tyrosine kinases (86), and the sequential expression of IGF-1 and TGF-β1 synergistically aggravate fibrosis (87). Our findings suggest complementary roles for BMP1/4, PDGF, and IGF-1, in addition to the effects of TGF-β1 and associated receptors, ligands, and modulators in conferring the profibrotic effects demonstrated by C3a and C5a on binding to their receptors C3aR and C5aR.
Our observations in clinical samples from patients with IPF indicate that whereas local C3a (13), C5a (13), and C5b-9 (Fig. 4) levels are elevated, receptors specific to C3a and C5a were localized to fibroblastic foci. However, analyses of fibroblasts grown ex vivo from the lungs of these patients presented some heterogeneity, which masked the significance of our observations. To our knowledge, this is the first report to address the expression pattern of these proteins in the lungs of patients with IPF.
Our study has some potential limitations. First, although we have shown elevated levels of TCC C5b-9 in IPF tissues, we have not shown correlations with each of the lung function parameters. This may be alleviated by increasing the number of samples used. Of note, this is the first report to demonstrate soluble C5b-9 levels in patients with IPF. Second, although we have reported the effects of blocking the anaphylatoxin receptors C3aR and C5aR in the progression of fibrosis, we have not demonstrated any functional effect on immune responses, that is, T-cell activation which might lead to higher IL-17A levels, and B-cell activation which might lead to greater antibody production. Although this was not within the scope of the current study, we had previously reported that C3a and IL-17 are part of a feed-forward loop that may enhance the loss of CD46 and CD55 because exogenous C3a enhanced IL-17 production from alloantigen- or autoantigen (type V collagen)-reactive lymphocytes (52). Furthermore, the potential roles of the signaling molecules identified in this study and their effects on C3a- or C5a-mediated dysregulated tissue repair is unknown.
CONCLUSIONS
Our results suggest that blocking C3a and C5a from binding to their respective receptors, C3aR and C5aR, arrests the progression of fibrosis by attenuating complement activation and complement deposition. In addition, key fibrotic mediators that were induced in this experimental model, primarily the TGF-β1 superfamily along with PDGF, IGF-1, and BMP1/4, are efficiently suppressed with concomitant restoration of decorin. Finally, we present evidence that complement activation represents an important unexplored pathway and a critical link to immune responses and fibrogenesis in the pathogenesis of IPF.
Acknowledgments
This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grant R01-HL109288 (to R.V.). D.S.W. is a cofounder of ImmuneWorks, Inc., a biotechnology company involved in developing therapeutics for various forms of lung diseases. The remaining authors declare no conflicts of interest.
Glossary
- BALF
bronchoalveolar lavage fluid
- BMP
bone morphologic protein
- C3a
complement component 3a
- C3aR
C3a receptor
- C5a
complement component 5a
- C5aR
C5a receptor
- col1a1
collagen type I α 1 chain
- col1a2
collagen type I α 2 chain
- CPI
composite physiologic index
- dcn
decorin
- DLCO
carbon monoxide lung diffusing capacity
- FVC
forced vital capacity
- IPF
idiopathic pulmonary fibrosis
- ltbp
latent transforming growth factor binding protein
- PDGF
platelet-derived growth factor
- RNAi
RNA interference
- serpine1
serpin peptidase inhibitor, clade E (plasminogen activator inhibitor type 1), member 1
- siRNA
small interfering RNA
- α-SMA
α-smooth muscle actin
- TCC
terminal complement complex
- tsp1
thrombospondin 1
REFERENCES
- 1.Hutchinson J. P., McKeever T. M., Fogarty A. W., Navaratnam V., Hubbard R. B. (2014) Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann. Am. Thorac. Soc. 11, 1176–1185 [DOI] [PubMed] [Google Scholar]
- 2.Ballanti E., Perricone C., Greco E., Ballanti M., Di Muzio G., Chimenti M. S., Perricone R. (2013) Complement and autoimmunity. Immunol. Res. 56, 477–491 [DOI] [PubMed] [Google Scholar]
- 3.Syed S. N., Konrad S., Wiege K., Nieswandt B., Nimmerjahn F., Schmidt R. E., Gessner J. E. (2009) Both FcgammaRIV and FcgammaRIII are essential receptors mediating type II and type III autoimmune responses via FcRgamma-LAT-dependent generation of C5a. Eur. J. Immunol. 39, 3343–3356 [DOI] [PubMed] [Google Scholar]
- 4.Atkinson C., Qiao F., Song H., Gilkeson G. S., Tomlinson S. (2008) Low-dose targeted complement inhibition protects against renal disease and other manifestations of autoimmune disease in MRL/lpr mice. J. Immunol. 180, 1231–1238 [DOI] [PubMed] [Google Scholar]
- 5.Bosmann M., Grailer J. J., Ruemmler R., Russkamp N. F., Zetoune F. S., Sarma J. V., Standiford T. J., Ward P. A. (2013) Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 27, 5010–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duehrkop C., Banz Y., Spirig R., Miescher S., Nolte M. W., Spycher M., Smith R. A., Sacks S. H., Rieben R. (2013) C1 esterase inhibitor reduces lower extremity ischemia/reperfusion injury and associated lung damage. PLoS One 8, e72059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leach H. G., Chrobak I., Han R., Trojanowska M. (2013) Endothelial cells recruit macrophages and contribute to a fibrotic milieu in bleomycin lung injury. Am. J. Respir. Cell Mol. Biol. 49, 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan M. A., Maasch C., Vater A., Klussmann S., Morser J., Leung L. L., Atkinson C., Tomlinson S., Heeger P. S., Nicolls M. R. (2013) Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc. Natl. Acad. Sci. USA 110, 6061–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M. A., Nicolls M. R. (2013) Complement-mediated microvascular injury leads to chronic rejection. Adv. Exp. Med. Biol. 735, 233–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westall G. P., Snell G. I., McLean C., Kotsimbos T., Williams T., Magro C. (2008) C3d and C4d deposition early after lung transplantation. J. Heart Lung Transplant. 27, 722–728 [DOI] [PubMed] [Google Scholar]
- 11.Dreisin R. B., Schwarz M. I., Theofilopoulos A. N., Stanford R. E. (1978) Circulating immune complexes in the idiopathic interstitial pneumonias. N. Engl. J. Med. 298, 353–357 [DOI] [PubMed] [Google Scholar]
- 12.Meliconi R., Senaldi G., Sturani C., Galavotti V., Facchini A., Gasbarrini G., Vergani D. (1990) Complement activation products in idiopathic pulmonary fibrosis: relevance of fragment Ba to disease severity. Clin. Immunol. Immunopathol. 57, 64–73 [DOI] [PubMed] [Google Scholar]
- 13.Gu H., Mickler E. A., Cummings O. W., Sandusky G. E., Weber D. J., Gracon A., Woodruff T., Wilkes D. S., Vittal R. (2014) Crosstalk between TGF-β1 and complement activation augments epithelial injury in pulmonary fibrosis. FASEB J. 28, 4223–4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerard C., Gerard N. P. (1994) C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu. Rev. Immunol. 12, 775–808 [DOI] [PubMed] [Google Scholar]
- 15.Siciliano S. J., Rollins T. E., Springer M. S. (1990) Interaction between the C5a receptor and Gi in both the membrane-bound and detergent-solubilized states. J. Biol. Chem. 265, 19568–19574 [PubMed] [Google Scholar]
- 16.Tornetta M. A., Foley J. J., Sarau H. M., Ames R. S. (1997) The mouse anaphylatoxin C3a receptor: molecular cloning, genomic organization, and functional expression. J. Immunol. 158, 5277–5282 [PubMed] [Google Scholar]
- 17.Crass T., Raffetseder U., Martin U., Grove M., Klos A., Köhl J., Bautsch W. (1996) Expression cloning of the human C3a anaphylatoxin receptor (C3aR) from differentiated U-937 cells. Eur. J. Immunol. 26, 1944–1950 [DOI] [PubMed] [Google Scholar]
- 18.Peake P. W., O’Grady S., Pussell B. A., Charlesworth J. A. (1999) C3a is made by proximal tubular HK-2 cells and activates them via the C3a receptor. Kidney Int. 56, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 19.Schraufstatter I. U., Trieu K., Sikora L., Sriramarao P., DiScipio R. (2002) Complement c3a and c5a induce different signal transduction cascades in endothelial cells. J. Immunol. 169, 2102–2110 [DOI] [PubMed] [Google Scholar]
- 20.Monsinjon T., Gasque P., Ischenko A., Fontaine M. (2001) C3A binds to the seven transmembrane anaphylatoxin receptor expressed by epithelial cells and triggers the production of IL-8. FEBS Lett. 487, 339–346 [DOI] [PubMed] [Google Scholar]
- 21.Drouin S. M., Kildsgaard J., Haviland J., Zabner J., Jia H. P., McCray P. B. Jr., Tack B. F., Wetsel R. A. (2001) Expression of the complement anaphylatoxin C3a and C5a receptors on bronchial epithelial and smooth muscle cells in models of sepsis and asthma. J. Immunol. 166, 2025–2032 [DOI] [PubMed] [Google Scholar]
- 22.Asgari E., Le Friec G., Yamamoto H., Perucha E., Sacks S. S., Köhl J., Cook H. T., Kemper C. (2013) C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 122, 3473–3481 [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Kondo N., Cano M., Ebrahimi K., Yoshida T., Barnett B. P., Biswal S., Handa J. T. (2014) Nrf2 signaling modulates cigarette smoke-induced complement activation in retinal pigmented epithelial cells. Free Radic. Biol. Med. 70, 155–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X., Fukuda N., Matsuda H., Endo M., Wang X., Saito K., Ueno T., Matsumoto T., Matsumoto K., Soma M., Kobayashi N., Nishiyama A. (2013) Complement 3 activates the renal renin-angiotensin system by induction of epithelial-to-mesenchymal transition of the nephrotubulus in mice. Am. J. Physiol. Renal Physiol. 305, F957–F967 [DOI] [PubMed] [Google Scholar]
- 25.Tang Z., Lu B., Hatch E., Sacks S. H., Sheerin N. S. (2009) C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J. Am. Soc. Nephrol. 20, 593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun M. C., Reins R. Y., Li T. B., Hollmann T. J., Dutta R., Rick W. A., Teng B. B., Ke B. (2004) Renal expression of the C3a receptor and functional responses of primary human proximal tubular epithelial cells. J. Immunol. 173, 4190–4196 [DOI] [PubMed] [Google Scholar]
- 27.Haviland D. L., McCoy R. L., Whitehead W. T., Akama H., Molmenti E. P., Brown A., Haviland J. C., Parks W. C., Perlmutter D. H., Wetsel R. A. (1995) Cellular expression of the C5a anaphylatoxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J. Immunol. 154, 1861–1869 [PubMed] [Google Scholar]
- 28.Floreani A. A., Heires A. J., Welniak L. A., Miller-Lindholm A., Clark-Pierce L., Rennard S. I., Morgan E. L., Sanderson S. D. (1998) Expression of receptors for C5a anaphylatoxin (CD88) on human bronchial epithelial cells: enhancement of C5a-mediated release of IL-8 upon exposure to cigarette smoke. J. Immunol. 160, 5073–5081 [PubMed] [Google Scholar]
- 29.Nishiura H., Nonaka H., Revollo I. S., Semba U., Li Y., Ota Y., Irie A., Harada K., Kehrl J. H., Yamamoto T. (2009) Pro- and anti-apoptotic dual functions of the C5a receptor: involvement of regulator of G protein signaling 3 and extracellular signal-regulated kinase. Lab. Invest. 89, 676–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y., Hu X. B. (2014) C5a stimulates the proliferation of breast cancer cells via Akt-dependent RGC-32 gene activation. Oncol. Rep. 32, 2817–2823 [DOI] [PubMed] [Google Scholar]
- 31.Wyatt T. A., Heires A. J., Sanderson S. D., Floreani A. A. (1999) Protein kinase C activation is required for cigarette smoke-enhanced C5a-mediated release of interleukin-8 in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 21, 283–288 [DOI] [PubMed] [Google Scholar]
- 32.Das D., Barnes M. A., Nagy L. E. (2014) Anaphylatoxin C5a modulates hepatic stellate cell migration. Fibrogenesis Tissue Repair 7, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boor P., Konieczny A., Villa L., Schult A. L., Bücher E., Rong S., Kunter U., van Roeyen C. R., Polakowski T., Hawlisch H., Hillebrandt S., Lammert F., Eitner F., Floege J., Ostendorf T. (2007) Complement C5 mediates experimental tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 18, 1508–1515 [DOI] [PubMed] [Google Scholar]
- 34.Li R., Coulthard L. G., Wu M. C., Taylor S. M., Woodruff T. M. (2013) C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 27, 855–864 [DOI] [PubMed] [Google Scholar]
- 35.Bosmann M., Grailer J. J., Ruemmler R., Russkamp N. F., Zetoune F. S., Sarma J. V., Standiford T. J., Ward P. A. (2013) Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 27, 5010–5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao H., Neff T. A., Guo R. F., Speyer C. L., Sarma J. V., Tomlins S., Man Y., Riedemann N. C., Hoesel L. M., Younkin E., Zetoune F. S., Ward P. A. (2005) Evidence for a functional role of the second C5a receptor C5L2. FASEB J. 19, 1003–1005 [DOI] [PubMed] [Google Scholar]
- 37.Chen N. J., Mirtsos C., Suh D., Lu Y. C., Lin W. J., McKerlie C., Lee T., Baribault H., Tian H., Yeh W. C. (2007) C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446, 203–207 [DOI] [PubMed] [Google Scholar]
- 38.Sun S., Wang H., Zhao G., An Y., Guo Y., Du L., Song H., Qiao F., Yu H., Wu X., Atkinson C., Jiang S., Tomlinson S., Zhou Y. (2011) Complement inhibition alleviates paraquat-induced acute lung injury. Am. J. Respir. Cell Mol. Biol. 45, 834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L., Guo R. F., Gao H., Sarma J. V., Zetoune F. S., Ward P. A. (2009) Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 23, 3808–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baelder R., Fuchs B., Bautsch W., Zwirner J., Köhl J., Hoymann H. G., Glaab T., Erpenbeck V., Krug N., Braun A. (2005) Pharmacological targeting of anaphylatoxin receptors during the effector phase of allergic asthma suppresses airway hyperresponsiveness and airway inflammation. J. Immunol. 174, 783–789 [DOI] [PubMed] [Google Scholar]
- 41.Bao L., Wang Y., Haas M., Quigg R. J. (2011) Distinct roles for C3a and C5a in complement-induced tubulointerstitial injury. Kidney Int. 80, 524–534 [DOI] [PubMed] [Google Scholar]
- 42.Vittal R., Fisher A., Gu H., Mickler E. A., Panitch A., Lander C., Cummings O. W., Sandusky G. E., Wilkes D. S. (2013) Peptide-mediated inhibition of MK2 ameliorates bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Bio. 49, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grgurevic L., Macek B., Healy D. R., Brault A. L., Erjavec I., Cipcic A., Grgurevic I., Rogic D., Galesic K., Brkljacic J., Stern-Padovan R., Paralkar V. M., Vukicevic S. (2011) Circulating bone morphogenetic protein 1-3 isoform increases renal fibrosis. J. Am. Soc. Nephrol. 22, 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hecker L., Vittal R., Jones T., Jagirdar R., Luckhardt T. R., Horowitz J. C., Pennathur S., Martinez F. J., Thannickal V. J. (2009) NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 15, 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vittal R., Mickler E. A., Fisher A. J., Zhang C., Rothhaar K., Gu H., Brown K. M., Emtiazdjoo A., Lott J. M., Frye S. B., Smith G. N., Sandusky G. E., Cummings O. W., Wilkes D. S. (2013) Type V collagen induced tolerance suppresses collagen deposition, TGF-β and associated transcripts in pulmonary fibrosis. PLoS One 8, e76451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vittal R., Fan L., Greenspan D. S., Mickler E. A., Gopalakrishnan B., Gu H., Benson H. L., Zhang C., Burlingham W., Cummings O. W., Wilkes D. S. (2012) IL-17 induces type V collagen overexpression and EMT via TGF-beta dependent pathways in obliterative bronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L401–L414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang K., Flanders K. C., Phan S. H. (1995) Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 147, 352–361 [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao J., Shi W., Wang Y. L., Chen H., Bringas P. Jr., Datto M. B., Frederick J. P., Wang X. F., Warburton D. (2002) Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 282, L585–L593 [DOI] [PubMed] [Google Scholar]
- 49.Zhang H. Y., Gharaee-Kermani M., Zhang K., Karmiol S., Phan S. H. (1996) Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 148, 527–537 [PMC free article] [PubMed] [Google Scholar]
- 50.Vittal R., Horowitz J. C., Moore B. B., Zhang H., Martinez F. J., Toews G. B., Standiford T. J., Thannickal V. J. (2005) Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am. J. Pathol. 166, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Boer J. D., Van’t Veer C., Stroo I., van der Meer A. J., de Vos A. F., van der Zee J. S., Roelofs J. J., van der Poll T. (2014) Protease-activated receptor-2 deficient mice have reduced house dust mite-evoked allergic lung inflammation. Innate Immun. 20, 618–625 [DOI] [PubMed] [Google Scholar]
- 52.Suzuki H., Lasbury M. E., Fan L., Vittal R., Mickler E. A., Benson H. L., Shilling R., Wu Q., Weber D. J., Wagner S. R., Lasaro M., Devore D., Wang Y., Sandusky G. E., Lipking K., Pandya P., Reynolds J., Love R., Wozniak T., Gu H., Brown K. M., Wilkes D. S. (2013) Role of complement activation in obliterative bronchiolitis post-lung transplantation. J. Immunol. 191, 4431–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang X., Nguyen T. T., Tian W., Sung Y. K., Yuan K., Qian J., Rajadas J., Sallenave J. M., Nickel N. P., de Jesus Perez V., Rabinovitch M., Nicolls M. R. (2015) Cyclosporine does not prevent microvascular loss in transplantation but can synergize with a neutrophil elastase inhibitor, elafin, to maintain graft perfusion during acute rejection. Am. J. Transplant. 15, 1768–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam A. P., Herazo-Maya J. D., Sennello J. A., Flozak A. S., Russell S., Mutlu G. M., Budinger G. R., DasGupta R., Varga J., Kaminski N., Gottardi C. J. (2014) Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 190, 185–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crawford S. E., Stellmach V., Murphy-Ullrich J. E., Ribeiro S. M., Lawler J., Hynes R. O., Boivin G. P., Bouck N. (1998) Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93, 1159–1170 [DOI] [PubMed] [Google Scholar]
- 56.Azuma A., Li Y. J., Abe S., Usuki J., Matsuda K., Henmi S., Miyauchi Y., Ueda K., Izawa A., Sone S., Hashimoto S., Kudoh S. (2005) Interferon-beta inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-beta and thrombospondin. Am. J. Respir. Cell Mol. Biol. 32, 93–98 [DOI] [PubMed] [Google Scholar]
- 57.Shimizukawa M., Ebina M., Narumi K., Kikuchi T., Munakata H., Nukiwa T. (2003) Intratracheal gene transfer of decorin reduces subpleural fibroproliferation induced by bleomycin. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L526–L532 [DOI] [PubMed] [Google Scholar]
- 58.Homma S., Nagaoka I., Abe H., Takahashi K., Seyama K., Nukiwa T., Kira S. (1995) Localization of platelet-derived growth factor and insulin-like growth factor I in the fibrotic lung. Am. J. Respir. Crit. Care Med. 152, 2084–2089 [DOI] [PubMed] [Google Scholar]
- 59.Wollin L., Wex E., Pautsch A., Schnapp G., Hostettler K. E., Stowasser S., Kolb M. (2015) Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 45, 1434–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi J. E., Lee S. S., Sunde D. A., Huizar I., Haugk K. L., Thannickal V. J., Vittal R., Plymate S. R., Schnapp L. M. (2009) Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am. J. Respir. Crit. Care Med. 179, 212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gasque P. (2004) Complement: a unique innate immune sensor for danger signals. Mol. Immunol. 41, 1089–1098 [DOI] [PubMed] [Google Scholar]
- 62.Addis-Lieser E., Köhl J., Chiaramonte M. G. (2005) Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J. Immunol. 175, 1894–1902 [DOI] [PubMed] [Google Scholar]
- 63.Li L., Chen L., Zang J., Tang X., Liu Y., Zhang J., Bai L., Yin Q., Lu Y., Cheng J., Fu P., Liu F. (2015) C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism 64, 597–610 [DOI] [PubMed] [Google Scholar]
- 64.Sendler M., Beyer G., Mahajan U. M., Kauschke V., Maertin S., Schurmann C., Homuth G., Volker U., Volzke H., Halangk W., Wartmann T., Weiss F. U., Hegyi P., Lerch M. M., Mayerle J. (2015) Complement component 5 mediates development of fibrosis, via activation of stellate cells, in 2 mouse models of chronic pancreatitis. Gastroenterology 149, 765–76.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaefer M., Konrad S., Thalmann J., Rheinheimer C., Johswich K., Sohns B., Klos A. (2005) The transcription factors AP-1 and Ets are regulators of C3a receptor expression. J. Biol. Chem. 280, 42113–42123 [DOI] [PubMed] [Google Scholar]
- 66.Kwan W. H., van der Touw W., Paz-Artal E., Li M. O., Heeger P. S. (2013) Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 210, 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rutkowski M. J., Sughrue M. E., Kane A. J., Ahn B. J., Fang S., Parsa A. T. (2010) The complement cascade as a mediator of tissue growth and regeneration. Inflamm. Res. 59, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coulthard L. G., Woodruff T. M. (2015) Is the complement activation product C3a a proinflammatory molecule? Re-evaluating the evidence and the myth. J. Immunol. 194, 3542–3548 [DOI] [PubMed] [Google Scholar]
- 69.Nisihara R. M., Magrini F., Mocelin V., Messias-Reason I. J. (2013) Deposition of the lectin pathway of complement in renal biopsies of lupus nephritis patients. Hum. Immunol. 74, 907–910 [DOI] [PubMed] [Google Scholar]
- 70.Li L., Yin Q., Tang X., Bai L., Zhang J., Gou S., Zhu H., Cheng J., Fu P., Liu F. (2014) C3a receptor antagonist ameliorates inflammatory and fibrotic signals in type 2 diabetic nephropathy by suppressing the activation of TGF-β/smad3 and IKBα pathway. PLoS One 9, e113639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Polosukhin V. V., Degryse A. L., Newcomb D. C., Jones B. R., Ware L. B., Lee J. W., Loyd J. E., Blackwell T. S., Lawson W. E. (2012) Intratracheal bleomycin causes airway remodeling and airflow obstruction in mice. Exp. Lung Res. 38, 135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buscemi L., Ramonet D., Klingberg F., Formey A., Smith-Clerc J., Meister J. J., Hinz B. (2011) The single-molecule mechanics of the latent TGF-β1 complex. Curr. Biol. 21, 2046–2054 [DOI] [PubMed] [Google Scholar]
- 73.Eitzman D. T., McCoy R. D., Zheng X., Fay W. P., Shen T., Ginsburg D., Simon R. H. (1996) Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J. Clin. Invest. 97, 232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang W. T., Akhter H., Jiang C., MacEwen M., Ding Q., Antony V., Thannickal V. J., Liu R. M. (2015) Plasminogen activator inhibitor 1, fibroblast apoptosis resistance, and aging-related susceptibility to lung fibrosis. Exp. Gerontol. 61, 62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhandary Y. P., Shetty S. K., Marudamuthu A. S., Ji H. L., Neuenschwander P. F., Boggaram V., Morris G. F., Fu J., Idell S., Shetty S. (2013) Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1. Am. J. Pathol. 183, 131–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smadja D. M., Nunes H., Juvin K., Bertil S., Valeyre D., Gaussem P., Israel-Biet D. (2014) Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol. Biol. (Paris) 62, 391–394 [DOI] [PubMed] [Google Scholar]
- 77.Zhou Y., Hagood J. S., Murphy-Ullrich J. E. (2004) Thy-1 expression regulates the ability of rat lung fibroblasts to activate transforming growth factor-beta in response to fibrogenic stimuli. Am. J. Pathol. 165, 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hao J., Ju H., Zhao S., Junaid A., Scammell-La Fleur T., Dixon I. M. (1999) Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J. Mol. Cell. Cardiol. 31, 667–678 [DOI] [PubMed] [Google Scholar]
- 79.Kolb M., Margetts P. J., Sime P. J., Gauldie J. (2001) Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1327–L1334 [DOI] [PubMed] [Google Scholar]
- 80.Kolb M., Margetts P. J., Galt T., Sime P. J., Xing Z., Schmidt M., Gauldie J. (2001) Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am. J. Respir. Crit. Care Med. 163, 770–777 [DOI] [PubMed] [Google Scholar]
- 81.Jahanyar J., Joyce D. L., Southard R. E., Loebe M., Noon G. P., Koerner M. M., Torre-Amione G., Youker K. A. (2007) Decorin-mediated transforming growth factor-beta inhibition ameliorates adverse cardiac remodeling. J. Heart Lung Transplant. 26, 34–40 [DOI] [PubMed] [Google Scholar]
- 82.Ge G., Greenspan D. S. (2006) BMP1 controls TGFbeta1 activation via cleavage of latent TGFbeta-binding protein. J. Cell Biol. 175, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen L., Acciani T., Le Cras T., Lutzko C., Perl A. K. (2012) Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am. J. Respir. Cell Mol. Biol. 47, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molloy E. L., Adams A., Moore J. B., Masterson J. C., Madrigal-Estebas L., Mahon B. P., O’Dea S. (2008) BMP4 induces an epithelial-mesenchymal transition-like response in adult airway epithelial cells. Growth Factors 26, 12–22 [DOI] [PubMed] [Google Scholar]
- 85.Câmara J., Jarai G. (2010) Epithelial-mesenchymal transition in primary human bronchial epithelial cells is Smad-dependent and enhanced by fibronectin and TNF-alpha. Fibrogenesis Tissue Repair 3, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrianifahanana M., Wilkes M. C., Gupta S. K., Rahimi R. A., Repellin C. E., Edens M., Wittenberger J., Yin X., Maidl E., Becker J., Leof E. B. (2013) Profibrotic TGFbeta responses require the cooperative action of PDGF and ErbB receptor tyrosine kinases. FASEB J. 27, 4444–4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andonegui G., Ni A., Léger C., Kelly M. M., Wong J. F., Jalloul A., Winston B. W. (2012) Sequential expression of IGF-IB followed by active TGF-β1 induces synergistic pulmonary fibroproliferation in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L788–L798 [DOI] [PMC free article] [PubMed] [Google Scholar]