Abstract
The peritumoral physical microenvironment consists of complex topographies that influence cell migration. Cell decision making, upon encountering anisotropic, physiologically relevant physical cues, has yet to be elucidated. By integrating microfabrication with cell and molecular biology techniques, we provide a quantitative and mechanistic analysis of cell decision making in a variety of well-defined physical microenvironments. We used MDA-MB-231 breast carcinoma and HT1080 fibrosarcoma as cell models. Cell decision making after lateral confinement in 2-dimensional microcontact printed lines is governed by branch width at bifurcations. Cells confined in narrow feeder microchannels prefer to enter wider branches at bifurcations. In contrast, in feeder channels that are wider than the cell body, cells elongate along one side wall of the channel and are guided by contact with the wall to the contiguous branch channel independent of its width. Knockdown of β1-integrins or inhibition of cellular contractility suppresses contact guidance. Concurrent, but not individual, knockdown of nonmuscle myosin isoforms IIA and IIB also decreases contact guidance, which suggests the existence of a compensatory mechanism between myosin IIA and myosin IIB. Conversely, knockdown or inhibition of cell division control protein 42 homolog promotes contact guidance–mediated decision making. Taken together, the dimensionality, length scales of the physical microenvironment, and intrinsic cell signaling regulate cell decision making at intersections.—Paul, C. D., Shea, D. J., Mahoney, M. R., Chai, A., Laney, V., Hung, W.-C., Konstantopoulos, K. Interplay of the physical microenvironment, contact guidance, and intracellular signaling in cell decision making.
Keywords: cell migration, microfluidics, confinement
INTRODUCTION
In vivo, cancer cells migrate through complex and confining microenvironments, including tunnel-like regions in the interstitial spaces of the tumor stroma (1–3), circulatory or lymph vessels (4), or the vasculature of target organs (4). These migration tracks are topographically complex, with pores ranging in diameter from <1 µm to 20 µm (∼10–1000 µm2 pore area) (5), and they are often bordered by thick, aligned collagen bundles that create paths of least resistance for cell migration (1). Mounting evidence suggests that cells adopt distinct signaling pathways to optimize cell locomotion in different physical microenvironments (6–8), such as confined vs. unconfined spaces (9–12); however, these studies do not account for the plasticity of the tumor microenvironment itself, which further promotes cell invasion through tracks that dynamically appear and change as disease progresses (13). Models of the tumor microenvironment should therefore include both confinement and anisotropic physical features to reflect the variability of the tumor microenvironment. It is important for our understanding of cell trafficking during cancer metastasis to delineate distinct microenvironment-dependent migration mechanisms that underlie topography-driven cell migration and decision making at tissue and vascular junctures.
In vitro models of cellular-scale migration environments that present track-like spaces include both printed extracellular matrix (ECM) proteins (14, 15) and microchannel devices (16–18). Cells migrating through confined microchannel mazes may locally consume growth factors to create autologous gradients that assist in finding the shortest migration path (19). Similarly, leukocytes that are confined in microchannels and that encounter bifurcations prefer to enter the channel of least hydraulic resistance (20). As such, MDA-MB-231 breast cancer cells migrating inside confined microchannels preferentially choose wider branches at bifurcation points, even when actomyosin contractility is inhibited (21); however, studies indicating this preference were performed using only a single feeder channel width that was smaller than the diameter of the cell. Thus, these studies do not account for the range of pore sizes found in the body (5). It is conceivable that decision-making processes depend on the microenvironment from which cells migrate. Importantly, the molecular constituents that mediate cell decision making are not known.
One possible driver of migration along tracks in the tumor microenvironment and in target organs is contact guidance. Contact guidance, which describes the phenomenon in which cells align to topographic features of a substrate (22), has typically been studied on grooved substrates with pitch less than the width of a cell—significantly smaller than cell-scale topographic cues found in vivo. Contact guidance is a clinically relevant phenomenon, as, for example, the contact-guided migration of breast cancer cells along collagen fibers oriented perpendicular to the tumor interface is an independent prognostic indicator of breast cancer patient survival (23, 24). However, little is known about how contact guidance affects cell decision making during migration in complex microenvironments. Here, we examine decision making and its interplay with contact guidance in tumor cells migrating within engineered Y-shaped microchannels that impose various degrees of confinement (e.g., cell contact with 2 vs. 4 walls of a microchannel). Migration within microchannels is directly compared with migration on laterally confining, 2-dimensional (2D) printed protein lines of the same Y-shaped design. Cells in full contact with their substrate, either because of a narrow feeder channel width or cell spreading on 2D printed lines, preferentially enter wider regions at bifurcations. In contrast, in wider feeder channels, cells respond to a topographic cue on one side of the cell body to be guided by contact with a side wall into the contiguous branch channel independent of branch channel width. That is, as a result of contact guidance in wider feeder channels, the location of the cell before the bifurcation is predictive of the destination branch of the cell. We elucidate the cellular mechanisms that influence cell contact guidance to illustrate how the position of the cell before bifurcation is predictive of cell choice.
MATERIALS AND METHODS
Cell lines and small interfering RNA experiments
MDA-MB-231 human breast carcinoma and HT1080 human fibrosarcoma cells were grown to 70–90% confluency in DMEM with 4.5 g/L glucose, l-glutamine, and sodium pyruvate (Corning Cellgro, Manassas, VA, USA), and supplemented with 10% (v/v) fetal bovine serum (FBS; Life Technologies/Gibco, Carlsbad, CA, USA) and 1% (v/v) penicillin/streptomycin (P/S; 100 U penicillin and 100 µg/ml streptomycin; Gibco). Cells were maintained in a humidified incubator at 37°C, 5% CO2. Cells were subcultured every 3–5 d after detachment from cell culture dishes by adding 0.05% typsin-EDTA (Gibco). During select experiments, cells seeded in the devices were incubated with the following pharmacologic agents or their appropriate vehicle control (VC): 50 µM blebbistatin (VC is DMSO; Sigma-Aldrich, St. Louis, MO, USA) or 20 µM ML141 4-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydropyrazol-2-yl]benzenesulfonamide; VC is DMSO; Santa Cruz Biotechnology, Dallas, TX, USA).
In separate experiments, select proteins involved in cell migration and polarity were knocked down via small interfering RNA (siRNA) transfection with Lipofectamine 2000 (Life Technologies). All siRNAs were from Santa Cruz Biotechnology: control-A (sc-37007), myosin, heavy chain 9, nonmuscle gene (MYH9; sc-61120), myosin, heavy chain 10, nonmuscle gene (MYH10; sc-61122), Ras homolog gene family, member A (RhoA; sc-29471), cell division control protein 42 homolog (Cdc42; sc-29256), and β1-integrin (sc-35674). For knockdown, a total of 200,000 cells were seeded in the well of a 6-well plate and spread overnight in growth medium. Targeting or control siRNA at a concentration of 10 µM in manufacturer-provided dilution buffer was diluted with Lipofectamine 2000 in Opti-MEM transfection medium (Life Technologies) at a ratio of 9.33 µl:13.33–26.67 µl:400 µl (Lipofectamine:siRNA:Opti-MEM). Of the transfection cocktail (60–120 pmol siRNA/well), 200 µl was added to plated cells immersed in 800 µl Opti-MEM. The resulting transfection medium contained 0.06–0.12 pmol siRNA/ml. After incubation for 6 h, 1 ml/well DMEM with 20% FBS and 2% P/S was added to each well. Cells were washed with Dulbecco’s PBS (DPBS), and media was replaced with regular growth medium 24 h after initial siRNA addition. Cells were used in migration experiments or examined via Western blot 48 h after transfection. We performed Western blot analysis as described in Chen et al. (25), Wang et al. (26), and Chen et al. (27). Membranes were stained with either anti-myosin IIA (M8064; Sigma-Aldrich), anti-myosin IIB (clone N-17; Santa Cruz Biotechnology), anti-RhoA (clone 26C4; Santa Cruz Biotechnology), anti-Rac 1 (clone 23A8; EMD Millipore), anti-β1 (clone N-20; Santa Cruz Biotechnology), or anti-Cdc42 (clone P1; Santa Cruz Biotechnology) antibodies. An anti-actin antibody (Ab-5; BD Biosciences, San Jose, CA, USA) was used as a loading control.
Fabrication of a microfluidic device for examination of cell migration in multiple topographic regimes
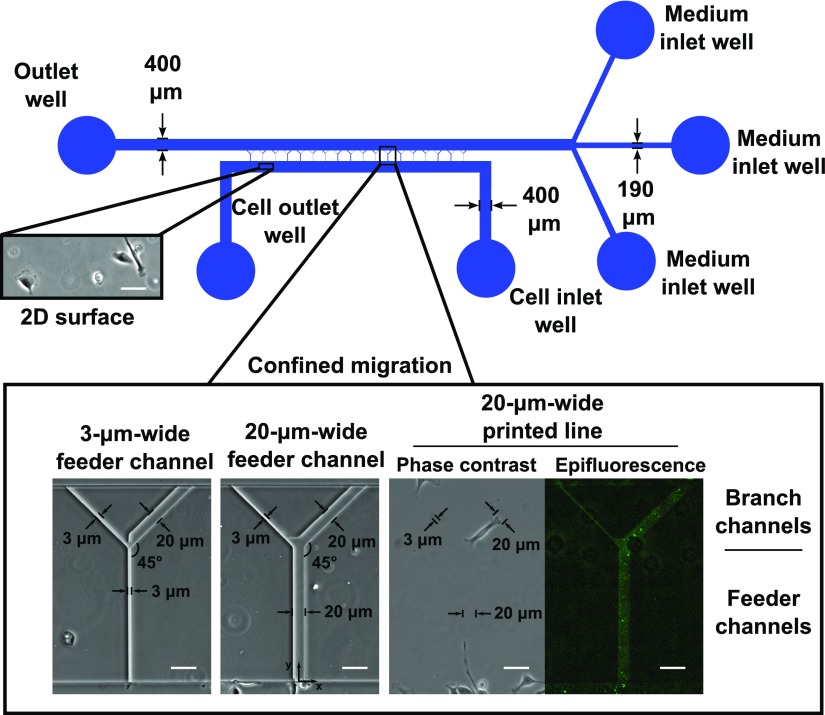
An array of microchannels was fabricated between 2 parallel-running, cell- and medium-containing channels to define the topography of the microenvironment in which cells migrate (Fig. 1). The microchannels were Y-shaped, with 3- or 20-μm-wide by 10-µm-tall feeder channels bifurcating to 10-µm-tall branch channels of prescribed width. End-to-end channel length was 390 µm. Designs were produced in AutoCAD (Autodesk, McLean, VA, USA) and transferred to chrome-on-glass darkfield photolithography masks (Front Range Photomask, Colorado Springs, CO, USA). A scale schematic of the migration device is shown in Fig. 1.
Figure 1.
Design of microfluidic device and microcontact printed surfaces. To-scale schematic of the microfluidic device used for migration studies and as a template to print collagen type I on glass coverslips. Insets show specific regions of the device. Unconfined 2D surfaces were available in the cell seeding region below the microchannel entrances. Microchannels were arrayed between larger cell seeding and medium channels. Microchannels consisted of 3- or 20-µm-wide feeder channels bifurcating to branch channels of various widths. All microchannels were 10 µm in height. Alternatively, collagen type I was printed in the same projected geometry as that of the microchannels. Deposited collagen is shown in the epifluorescence image. Scale bars, 50 µm.
We fabricated molds for the microfluidic devices by using multilayer photolithography. SU-8 3010 negative photoresist (Microchem, Newton, MA, USA) was spun to a thickness of 10 µm on a mechanical grade silicon wafer (University Wafer, South Boston, MA, USA), soft baked, and exposed through a mask defining the Y-shaped microchannels on an EVG620 mask aligner (EVG, St. Florian am Inn, Austria). After a postexposure bake, the photoresist was developed with SU-8 developer, and the patterned wafer was rinsed with isopropanol. A layer of SU-8 3025 was spun on top of the microchannels to a thickness of 50 µm, soft baked, and exposed through a mask defining the cell- and medium-containing channels of the device. The new layer of photoresist was baked postexposure and developed. The completed wafer was hard baked for 10 min at 150°C and treated overnight with vapor phase (tridecafluoro-1,1,2,2,-tetrahydrooctyl)-1-trichlorosilane (Pfaltz & Bauer, Waterbury, CT, USA).
The final microfluidic devices were formed by using replica molding with polydimethylsiloxane (PDMS; Sylgard 184 kit; Dow Corning, Midland, MI, USA). PDMS prepolymer and cross-linker were mixed at a 10:1 ratio, poured over the wafer, degassed, and cured at 85°C for 2 h. PDMS devices were peeled from the wafer mold and diced. Identical PDMS devices were used to create 2D or 3-dimensional (3D) Y-channel topographies for microfluidic template printing or confined migration assays, respectively, as described in the “Microchannel cell migration experiments” and “Microfluidic template printing experiments” sections.
Microchannel cell migration experiments
We cleaned PDMS devices and glass coverslips or slides (75 × 25 mm) with ethanol and deionized water, and these were exposed to oxygen plasma in a Harrick PDC-32G plasma cleaner (Harrick Plasma, Ithaca, NY, USA) at 18 W for 2 min after a 5-min chamber evacuation. PDMS devices were irreversibly bound to the glass slide or coverslip and immediately filled with a solution of 20 µg/ml collagen type I (BD Biosciences). Devices were coated by absorption for 1 h at 37°C. In select experiments, the glass slide was first coated with a thin layer of PDMS (∼25-µm thick) before plasma treatment, device assembly, and collagen coating, which led to the formation of microchannels that consisted of 4 PDMS walls. The device was washed with DPBS after the removal of the coating solution. We harvested MDA-MB-231 or HT1080 cells from tissue culture dishes by adding 0.05% trypsin-EDTA. After quenching by an initial resuspension in growth medium, cells were washed twice in serum-free medium [DMEM with 1% (v/v) P/S, no FBS], and resuspended to a concentration of 1–2 × 106 cells/ml.
Of the cell suspension (corresponding to 50,000–100,000 total cells), 50 µl was next added to the cell inlet well of the device, with 20 µl serum-free media added to the opposite side of the microchannels to prevent convective flow of cells into the channels. Cells were allowed to adhere at the microchannel entrances for 5–10 min before the device was washed with DPBS. We then filled the 4 inlet wells of the device with 100 µl/well growth medium that contained 10% FBS. In select experiments, the medium was supplemented with pharmacologic inhibitors. We imaged migration in both the microchannels and on the 2D portions of the device for up to 24 h on an inverted Nikon Eclipse Ti microscope (Nikon, Tokyo, Japan) with automated controls (NIS-Elements; Nikon) and a ×10/0.45 numerical aperture Ph1 objective using time-lapse microscopy. During the experiments, cells were maintained on a temperature- and CO2-controlled stage top incubator (Okolab, Pozzuoli, Italy or Tokai Hit, Shizuoka-hen, Japan). In some experiments, cells that migrated inside the 20-µm-wide feeder microchannels were fixed, stained for F-actin by using Alexa Fluor 488 phalloidin, and imaged via confocal microscopy, as described in Stroka et al. (11).
Microfluidic template printing experiments
We cleaned PDMS devices and glass coverslips with ethanol and deionized water. Devices were reversibly bound to coverslips by application of gentle pressure to seal the microchannel outlines to glass surfaces. A device was primed with a 5% Alconox solution (Alconox, Inc., White Plains, NY, USA), washed 3× with DPBS, and filled with 20 µg/ml collagen type I that contained 1:500 collagen type I antibody (C2456, clone COL-1; Sigma-Aldrich) and 1:1000 Alexa Fluor 488 (Life Technologies) to mark the printed collagen regions. The collagen solution was absorbed on the coverslip for 1 h at 37°C. The device was then washed 3× with DPBS and was peeled from the coverslip, and the coverslip was washed with DPBS. The slide was immersed in 2% (w/v) Pluronic F-127 solution (P2443; Sigma-Aldrich) for 1 h at room temperature to backfill nonprinted regions. The slide was washed 3× with DPBS and stored for up to 48 h at 4°C before cell seeding. To seed cells, we prepared MDA-MB-231 cells in a manner identical to that of the microchannel experiments and resuspended to 400,000–800,000 cells/ml. A volume of 100 µl (40,000–80,000 total cells) was then pipetted over the printed regions. We filled medium reservoirs surrounding the printed regions with growth medium, and cells were allowed to settle and adhere to the protein patterns for 1–2 h. The solution was then aspirated, and the surface was washed with DPBS to remove nonadherent cells. We filled the medium reservoir with medium and imaged the cells as described in the “Microchannel cell migration experiments” section.
Cell tracking
We manually tracked cell paths every 10 min in ImageJ (National Institutes of Health, Bethesda, MD, USA) using the MTrackJ plugin (28). In addition, in select experiments, we traced cells every 30 min by using the polygon ROI function in ImageJ, and shape factors were measured. All cells that fully entered the microchannels or microcontact printed regions for at least 1 h were tracked at the midpoint between cell poles for the duration of their migration, which began when the cell was fully within the microchannel or on the printed design. For microcontact printing experiments, only cells that seeded on, or entered the base region of, the microcontact printed lines were considered. If the cell exited the channel, tracking was discontinued. If cells reached the branch region (Fig. 1), tracking was discontinued at the maximum distance away from the entrance (i.e., cells that reached the branch region were not tracked if they turned around). Dividing cells were not tracked, and we disregarded the tracks for cells that were blocked at the bifurcation by the presence of an occluding cell. We measured cell positions with respect to the midpoint of the channel entrance (Fig. 1). Feeder channels were oriented at 90° from horizontal, so cells elongated along these channels had a fit elliptical angle of ∼90°.
All cell path and shape calculations were made by using a custom-written MATLAB (MathWorks, Natick, MA, USA) program with cell position and shape data as the input. Averages of measurements in speed or shape factors were calculated by taking the mean of those measurements for 1 cell, and coefficients of variation in those measurements for a given cell were also calculated. Persistence was calculated by dividing the net displacement of the cell by the total distance traveled by the cell over the course of tracking. Shape factors were similarly calculated by using the ImageJ Measure ROI function. Therefore, each point on the plots provided represents the average value of that metric for a given cell or the variance in that metric as measured over the course of tracking for that cell. We grouped individual measurements across biologic repeats to obtain characteristic distributions of cell speed, persistence, and shape factors for each microenvironment and treatment described. For experiments that studied cell speed and shape, with distance into the microchannel or printed line, measurements were binned into 20-µm-long regions along the length of the channel, and the mean and se of those measurements were calculated. Cells were counted as having been contact guided if they entered a branch channel and did not cross the midline of the microchannel (x = 0) within ∼1 cell length of the bifurcation. (The distance was chosen from measurements of cell length and set so that untreated cells would not have elongated to bifurcation before this point.)
Statistics
Before analysis, we grouped cells from experiments for a given set of conditions (e.g., channel geometry, inhibitor, knockdown). A minimum of 3 independent experiments were performed for each condition, unless otherwise stated. All experiments using an inhibitor or knockdown were run side-by-side with cells either treated with the inhibitor vehicle or transfected with nontargeting control siRNA, respectively. For all statistical analysis, data were analyzed for normality by using the D’Agostino-Pearson omnibus normality test. Samples with gaussian distributions were compared via unpaired Student’s t test or 1-way ANOVA with post hoc Tukey’s multiple comparisons test. Samples with nongaussian distributions were compared by using an unpaired Mann-Whitney U test or Kruskal-Wallis nonparametric ANOVA test with Dunn’s multiple comparisons test. We performed calculations by using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). Fractions of cells to branch channels of various widths or fractions of contact-guided cells were analyzed by using the 2-population proportion z test. We calculated 95% confidence intervals for the proportions measured by using the exact method of Clopper and Pearson in GraphPad Prism.
RESULTS
Cell decision making at asymmetric bifurcations is dependent on the degree and type of confinement
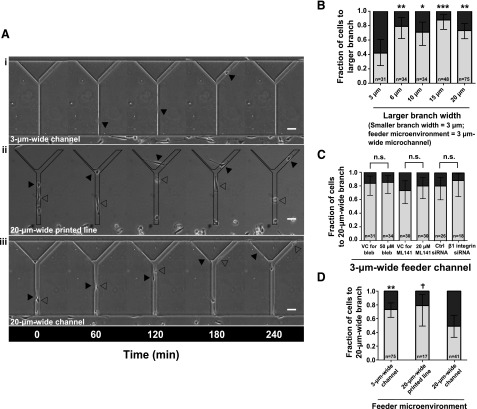
A variety of substrates with cellular-scale feature sizes were engineered to examine how the microenvironment impacts cell decision making. In initial experiments, MDA-MB-231 breast cancer cells migrated within collagen type I–coated PDMS microchannels with straight feeder channels bifurcating to branch channels of various widths (Figs. 1 and 2A, B). Feeder channels were 3 µm wide and 10 µm high. The width of the left branch channel was set at 3 µm, wheras the right branch channel width varied from 3 µm to 6, 10, 15, or 20 µm (Fig. 2B and Supplemental Movie S1). Although cells were equally likely to enter the left or right branch channel at symmetric bifurcations, cells preferentially (≥70%) entered the wider branch channel when the bifurcation was asymmetric (Fig. 2B). When the feeder and the left branch channel widths were set at 3 µm and the wider right branch channel width was set at 20 µm, cell preference for the wide branch channel was not modulated by inhibition of actomyosin contractility via blebbistatin treatment, inhibition of Cdc42 with ML141, or knockdown of β1-integrin (Fig. 2C).
Figure 2.
Cell decision making from various feeder microenvironments. The physical microenvironment affects the decision making of MDA-MB-231 cells at bifurcations. A) Phase contrast images of MDA-MB-231 cells migrating inside Y-shaped microchannels with 3-µm-wide feeder channels bifurcating to 3- and 20-µm-wide branches (i); on Y-shaped printed lines with a 20-µm-wide feeder region bifurcating to 3- and 20-µm-wide branches (ii); and inside Y-shaped microchannels with 20-µm-wide feeder channels bifurcating to 3- and 20-µm-wide branches (iii). All surfaces were coated with collagen type I (20 µg/ml). Arrowheads illustrate the positions of indicated cells at prescribed time points. B) Fraction of cells from a 3-µm-wide feeder microchannel migrating into the larger branch when encountering a bifurcation in which the width of 1 branch was set at 3 µm and the width of the other branch varied from 3 to 6, 10, 15, or 20 µm. C) Fraction of cells entering the 20-µm-wide branch channels from a 3-µm-wide feeder channel upon treatment with 50 µM blebbistatin, 20 µM ML141, or the appropriate controls, or transfection with nontargeting control (ctrl) siRNA or siRNA targeting β1-integrin. D) Fraction of cells from the microenvironments shown in panel A entering the 20-µm-wide branch at bifurcations. In panels B-D, data represent fraction of cells. Error bars show 95% confidence intervals of these fractions. The total number of cells making a decision for each condition is indicated on the plot. Data were collected and pooled over n ≥ 4 independent experiments. Significance was assessed by using the 2-population proportion z test with respect to the design containing symmetric 3-µm-wide branch channels (B), the device with the 20-µm-wide feeder microchannel (C), or to control cells (D). n.s., not significant. †P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001. Scale bars, 50 µm.
To explore cell decision making with other types of physical confinement (e.g., full vs. partial confinement), we engineered Y-shaped microchannels, albeit with wider feeder channels (20 µm instead of 3 µm wide) (Figs. 1 and 2A). Moreover, we generated Y-shaped collagen type I–printed lines, with a 20-µm-wide feeder line bifurcating to branch lines of 3 µm (left) or 20 µm (right) widths (Figs. 1 and 2A). The areas surrounding the patterned features were made inaccessible to cell adhesion by treatment with Pluronic F-127, an amphiphilic block copolymer that forms a stable anti-adhesive coating on cell culture substrates, repelling cell attachment for ∼1 wk (29). The majority of MDA-MB-231 cells (∼75%) that reached the bifurcation migrated onto the wider branch line (Fig. 2D and Supplemental Movie S1). Of interest, MDA-MB-231 cells migrating inside 20-µm-wide feeder microchannels that split into 3- vs. 20-µm-wide branches displayed no bias for the wider branch (Fig. 2D and Supplemental Movie S1). Although cell preference for larger microcontact-printed areas has been demonstrated previously (30), to our knowledge, this is the first demonstration of pore size–independent cell decisions. We thus investigated the mechanisms driving this disparate decision making.
Contact guidance drives cell decision making in feeder microchannels that are wider than the cell body
We observed that cells migrating on Y-shaped collagen type I–printed lines vs. Y-shaped microchannels of identical dimension exhibited different morphologic characteristics. Specifically, MDA-MB-231 cells migrating on printed lines displayed broad lamellopodia, essentially covering the entire width of the feeder line (Supplemental Movie S1). In contrast, the majority (>80%) of MDA-MB-231 cells in 20-µm-wide feeder microchannels became elongated and moved preferentially along 1 side wall (Supplemental Movie S1). These qualitative differences are substantiated by quantitative analysis of projected surface area, aspect ratio, and minor axis length of cells migrating on printed lines vs. microchannels of identical dimension (Supplemental Fig. S1).
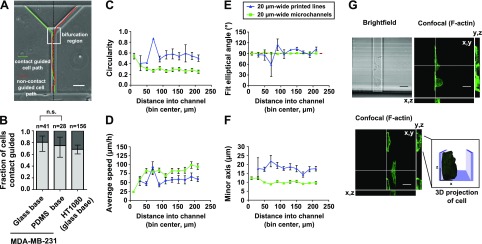
We postulate that cell elongation and migration along a single side wall is predictive of the decision making from 20-µm-wide feeder microchannels. To this end, we analyzed the likelihood of cells switching from 1 side wall to the other before choosing a branch within ∼1 cell length of the bifurcation (Fig. 3A and Supplemental Movie S2). Cells that did not switch walls were termed contact guided; this definition is illustrated schematically in Figure 3A. Contact guidance was indeed a powerful predictor of the destination branch of the cell in MDA-MB-231 breast cancer cells, with ∼80% being contact guided (Fig. 3B). Contact guidance of MDA-MB-231 cells occured regardless of whether the channel base was composed of glass or PDMS (Fig. 3B), and channels with a glass base were used in subsequent experiments. To generalize our observations, we demonstrated that contact guidance was also predictive of decision making by HT1080 fibrosarcoma cells in these microchannels (Fig. 3B). To further illustrate the critical role of contact guidance in decision making, we engineered Y-shaped microchannels in which 1 branch led to a dead end. As shown in Supplemental Movie S2, contact guidance prompted MDA-MB-231 cells to enter and migrate along the side walls of the dead-end branch.
Figure 3.
The distinct role of contact guidance in cell decision making at bifurcations and polarization in microchannels. A) Schematic of cell paths for a contact-guided and a noncontact-guided cell. Cells were scored as contact guided if they did not cross the midline of the channel while in the bifurcation region of the device. The bifurcation region (box) is defined as the area within 1 cell length away from the bifurcation. B) Fraction of contact-guided MDA-MB-231 and HT1080 cells in 20-µm-wide feeder microchannels. In select experiments with MDA-MB-231 cells, the glass slide making up the base of the microchannels was coated with a thin layer (∼25 µm) of PDMS before device assembly and functionalization with collagen type I. Columns show fraction of contact-guided cells, and bars show 95% confidence intervals of the fractions. Comparison between proportions of contact-guided MDA-MB-231 cells migrating on glass or PDMS bases was made using the 2-population proportion z test. C–F) Circularity (C), average speed (D) elliptical angle of fit (E), and minor axis length (F) of MDA-MB-231 cells that go on to enter a branch channel as a function of distance into the feeder channel. Measurements were binned within 20-µm-long regions of the feeder channel. Symbols show the average value in each bin, accompanied by se of each measurement. Symbols are plotted at the center position of each bin. Dashed line (E) shows the angle of the microchannel (90° with respect to the horizontal). All data were collected and pooled over n ≥ 2 independent experiments. G) Representative images of MDA-MB-231 cells migrating in 20-µm-wide, 10-µm-tall feeder microchannels. Cells were stained for F-actin using Alexa Fluor 488 phalloidin. Confocal images taken at the basal surface of the cell are shown in the x-y plane. Cells were imaged at 0.8 µm axial intervals to generate orthogonal reconstructions in the x-z and y-z planes. The locations of the orthogonal reconstructions are indicated by the white lines in the x-y planes. A 3D surface reconstruction of the indicated cell is shown in the inset. n.s., not significant. Scale bars, 50 µm (A) and 20 µm (G).
To examine the spatial pattern of cell polarization during migration along the channel side walls, we quantified MDA-MB-231 cell morphology with respect to position in the feeder microchannels. As cells elongate along a side wall with distance into the feeder channels, depicted by decreased circularity (Fig. 3C), cell speed increases (Fig. 3D). Tight positioning along a side wall was further evidenced by increased alignment of the fit of the elliptical angle to the channel wall and a progressive decrease in minor axis length (Fig. 3E, F). As cells become progressively elongated and aligned along 1 side wall, they reduce exploration of the channel microenvironment, and their decision making is predominantly based on contact guidance. Aligned cells were spindle shaped, spanning approximately 10 µm up the side wall and 10 µm along the microchannel floor. Images of MDA-MB-231 cells elongated along microchannel walls are shown in Fig. 3G to illustrate typical cell morphology in 20-µm-wide feeder microchannels. Collectively, this analysis indicates spatially regulated cell polarization during contact guidance in microchannels. In contrast, no distance-dependent changes in polarization were noted when MDA-MB-231 cells migrated on 20-µm-wide printed lines (Fig. 3D–F).
The critical role of integrin-mediated adhesion in contact guidance
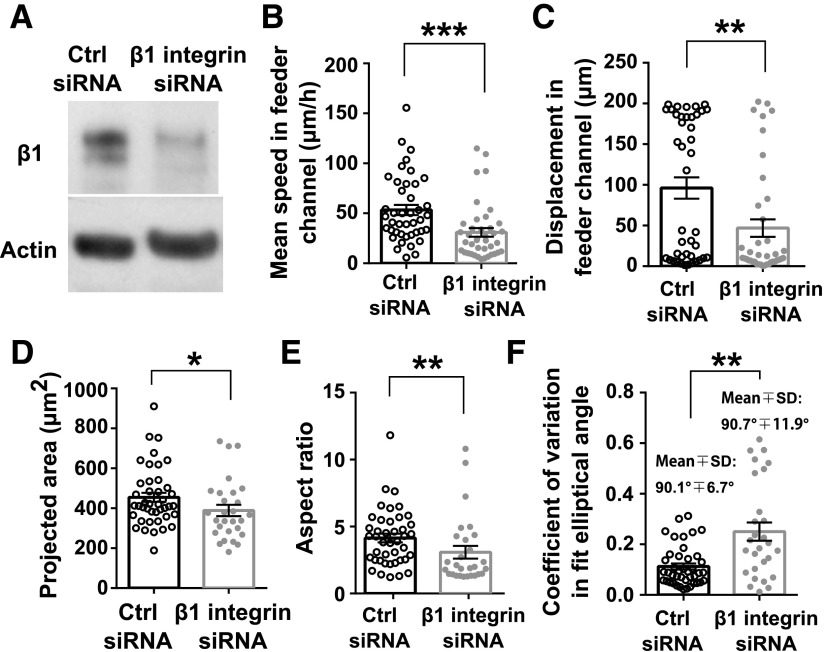
As contact guidance is critical to cell decision making, we wished to delineate the mechanisms responsible for MDA-MB-231 cell migration along a topographic feature on 1 side of the cell. Integrins are transmembrane proteins involved in the binding of cells to extracellular substrates and mechanotransduction (31). β1-Integrin is responsible for cellular binding to collagen type I (32). Knockdown of β1-integrin in MDA-MB-231 cells via siRNA (Fig. 4A) drastically decreased the efficiency of cell migration in 20-µm-wide feeder microchannels compared with cells transfected with nontargeting control siRNA. This decrease was evidenced by a lower average migration speed and markedly shorter net cell displacements from the initial cell position over the time course of tracking in the straight feeder region of the microchannels (Fig. 4B, C). For these calculations, cells transfected with control siRNA were tracked in the feeder region of the microchannels for 328 ± 36 min (means ± sem), whereas β1-integrin knockdown cells were tracked for 358 ± 46 min. We further hypothesized that integrin knockdown would manifest as impaired cell elongation and alignment along the channel side walls; indeed, β1-integrin knockdown significantly decreased the projected spread area of cells in microchannels (Fig. 4D), presumably as a result of the inability of nascent protrusions to bind and mature. Inefficiencies in cell spreading and elongation were further reflected by a significant decrease in cell aspect ratio upon β1-integrin knockdown (Fig. 4E). Importantly, any elongation was not persistent or oriented as there was a significant increase in the variance of the elliptical angle of fit upon knockdown (Fig. 4F). Collectively, β1-integrins are required for efficient contact guidance of MDA-MB-231 cells in feeder microchannels that are wider than the cell body. When integrin expression is reduced, cell alignment to microchannel walls, which is a prerequisite for contact guidance to drive decision making, is inhibited.
Figure 4.
The critical role of integrin-mediated adhesions in the contact guidance of MDA-MB-231 cells. MDA-MB-231 cells were transfected with nontargeting control (ctrl) siRNA or siRNA-targeting β1-integrin. A) β1-integrin knockdown was confirmed via Western blot. B–F) Average speed (B), displacement (C), projected area (D), aspect ratio (E), and coefficient of variation in elliptical angle of fit (F) of cells transfected with ctrl or β1-integrin siRNA. Parameters were quantified in the straight feeder regions of the microchannels. Columns represent population means. Error bars show sem. Data points represent values of each metric for 1 cell for n ≥ 28 cells from n = 4 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 by Mann-Whitney U test.
Inhibition of cell contractility decreases contact guidance–mediated decision making
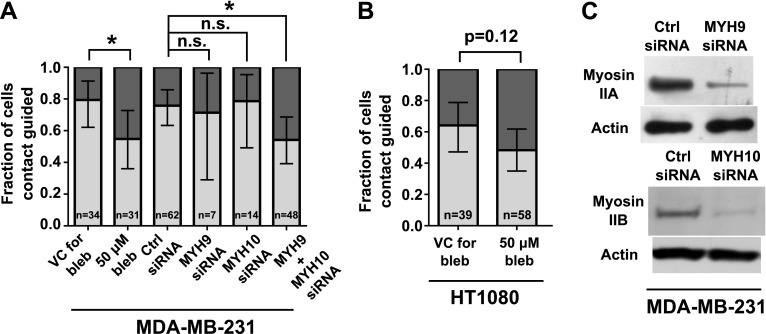
The involvement of β1-integrins in contact guidance suggests a role for actomyosin contractility in the ability of a cell to persistently migrate along a side wall in 20-µm-wide feeder channels. We thus assessed the effects of blebbistatin, a myosin II ATPase inhibitor that interferes with actomyosin contractility (33), on contact guidance–mediated decision making. MDA-MB-231 cells treated with blebbistatin exhibited significantly less guidance by contact at the bifurcation than did control cells (Fig. 5A). A similar trend was observed for HT1080 cells (Fig. 5B).
Figure 5.
Inhibition of cell contractility impairs contact guidance–mediated decision making of MDA-MB-231 and HT1080 cells. MDA-MB-231 or HT1080 cells were treated with 50 µM blebbistatin or VC. In select experiments, MDA-MB-231 cells were transfected with control (ctrl) siRNA or siRNA-targeting myosin IIA (MYH9) and/or myosin IIB (MYH10). A, B) Fractions of contact-guided MDA-MB-231 cells (A) and HT1080 cells (B) during the decision-making process from 20-µm-wide feeder channels. Data show overall fraction of contact-guided cells, and bars show 95% confidence intervals of the fractions. Data were collected and pooled over n ≥ 3 independent experiments. The number of cells assayed for each condition is indicated on the plot. Comparisons between fractions of treated and control cells were analyzed using the 2-population proportion z test. C) Knockdown of myosin isoforms in MDA-MB-231 cells was confirmed via Western blot, with actin as loading control. Panel shows 2 blots from the same cell lysate (collected after concurrent knockdown of myosin IIA and myosin IIB) immunoblotted by using an antibody to either myosin IIA (MYH9) or myosin IIB (MYH10). n.s., not significant. *P < 0.05.
To confirm that actomyosin contractility is a regulator of contact guidance in 20-µm-wide feeder channels, we knocked down nonmuscle myosin isoforms in MDA-MB-231 cells via siRNA (Fig. 5C). Of the 3 nonmuscle myosin II isoforms, MDA-MB-231 cells express myosin IIA and myosin IIB (34). Individual knockdown of myosin IIA or myosin IIB did not affect cell contact guidance at 2 siRNA doses (0.06 or 0.12 pmol siRNA/ml transfection medium; Fig. 5C and Supplemental Fig. S2). In contrast, cells depleted of both myosin isoforms via siRNA (0.06 pmol siRNA per isoform/ml transfection medium; Fig. 5C) had significantly less guidance by contact relative to controls (Fig. 5A). Taken together, we conclude that the cross-linking of actin to myosin is essential for MDA-MB-231 cell contact guidance, with compensation pathways available when this linkage is not fully abrogated.
Inhibition of Cdc42 increases contact guidance–mediated decision making
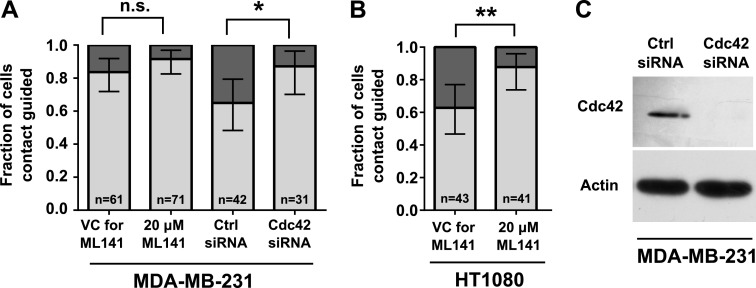
In light of our data showing an association between cell polarization and contact guidance (Fig. 3), and because RhoGTPase Cdc42 is critical to cell polarization (35), we examined its contribution to contact guidance. Cell treatment with the Cdc42 inhibitor ML141 tended to increase the fraction of contact-guided MDA-MB-231 cells at bifurcations, although this difference was not statistically significant (Fig. 6A). It is noteworthy that HT1080 fibrosarcoma cells, which display moderately lower baseline levels of contact guidance than do MDA-MB-231 cells, became significantly more contact guided upon Cdc42 inhibition via ML141 (Fig. 6B).
Figure 6.
Inhibition of Cdc42 increases contact guidance–mediated decision making of MDA-MB-231 and HT1080 cells. MDA-MB-231 or HT1080 cells were treated with ML141 (20 µM) or VC. In separate experiments, MDA-MB-231 cells were transfected with control (ctrl) siRNA or siRNA-targeting Cdc42. A, B) Fractions of contact-guided MDA-MB-231 cells (A) and HT1080 cells (B) during the decision-making process. Data show overall fraction of contact-guided cells, and bars show 95% confidence intervals of the fractions. Data were collected and pooled over n ≥ 3 independent experiments. The number of cells assayed for each condition is indicated on the plot. Comparisons between fractions of treated and control cells were analyzed using the 2-population proportion z test. C) Knockdown of Cdc42 in MDA-MB-231 cells was confirmed via Western blot, with actin as loading control. n.s., not significant. *P < 0.05.
The impact of Cdc42 on the decision making of cells migrating inside 20-µm-wide feeder microchannels was further examined by transiently knocking down Cdc42 in MDA-MB-231 cells, which was confirmed via Western blot (Fig. 6C). This molecular intervention significantly increased the fraction of contact-guided cells at the bifurcation compared with scramble control cells (Fig. 6A). Taken together, these data suggest that interfering with Cdc42 function promotes contact guidance–mediated decision making.
DISCUSSION
Understanding how cells respond to different physical microenvironments is crucial for developing strategies that control, manipulate, promote, or stop cell motility in vivo. Mounting evidence suggests that the local topography of the tumor microenvironment regulates the modes of cell migration (36). Specifically, cell invasion from primary tumors is associated with bundled ECM protein fibers aligned radially to the tumor interface (24). Although studies have elucidated how cells switch between mesenchymal and amoeboid migration modes to navigate fibrillar collagen–rich tissue (6), there is no conceptual framework for understanding cell decision making through tunnel-like anisotropic microenvironments that are prevalent in vivo (1, 2, 4, 13).
We discovered that different types of confinement (2D vs. 3D confinement or partial vs. full confinement) exert distinct effects on cell decision making at bifurcations. Cells migrating from feeder regions in which they fully explore the microenvironment favor entry into wider branches. This is the case for cells migrating on laterally confined 2D printed lines as well as inside narrow microchannels (i.e., full confinement). In contrast, repression of cell lamellipodia at bifurcations, which can be achieved in wider feeder channels as a result of contact guidance and migration along a single side wall, results in pore size–independent decisions at bifurcations. Suppression of secondary protrusions in contact-guided cells may result from the high energy barrier required to break actin bundles aligned along the direction of elongation (37) or increases in membrane tension in elongated cells (38). Taken together, cell decision making at bifurcations is dependent on the dimensionality (2D vs. 3D) as well as the lateral length scales of feeder channels. The novelty of these findings illustrates the power of using microfabrication techniques as model systems to study cell migration. It is noteworthy, however, that such a fine control of microenvironmental topography to elucidate length scale–dependent decisions is not possible in fibroblast-remodeled ECM gels (39) or along bulk-aligned collagen fibers (40).
From the molecular perspective, persistent elongation and orientation along a single side wall in wide feeder channels (i.e., contact guidance) requires β1-integrin expression. Moreover, inhibition of cell contractility via treatment with blebbistatin suppresses contact guidance. Importantly, dual knockdown of myosin IIA and IIB is required to suppress contact guidance. It is noteworthy that individual knockdown of myosin II isoforms failed to alter the fraction of contact-guided cells, which suggests a requirement of universal abrogation of actomyosin contractility and the existence of a compensatory mechanism between myosin IIA and IIB. Along these lines, knockdown of RhoA did not affect contact guidance (Supplemental Fig. S2). We thus postulate that concomitant inhibition of RhoA- and myosin light chain kinase–driven activity is needed to repress contact guidance. This is substantiated by our findings that show that simultaneous inhibition of RhoA/Rho-associated protein kinase via Y-27632 and myosin light chain kinase via ML-7 is needed to suppress the fraction of contact-guided cells (Supplemental Fig. S2). Myosin II is an important regulator of cell shape and mechanosensing (41) in complex topographies. In light of our data demonstrating the importance of actomyosin contractility and β1-integrin in contact guidance, we cannot rule out a potential interplay between these 2 molecular constituents. This scenario is supported by prior work showing that blebbistatin reduces the size of focal adhesions in 2 dimensions (42). On the other hand, contact guidance was promoted when Cdc42 function was disrupted. Consistent with the finding that Cdc42 inhibition increases contact guidance and migration persistence through complex physical spaces, knockdown of Cdc42 has been reported to increase MDA-MB-231 cell migration and invasion in transwell assays (43). It is noteworthy that knocking down or inhibiting Cdc42 function or cell contractility did not significantly change the fraction of cells entering the wide vs. the narrow branches at bifurcations relative to appropriate matched controls. Instead, these interventions affect how cell position before the bifurcation is predictive of cell choice.
Finally, this study highlights the need for characterizing and standardizing the precise physical microenvironmental features in cell migration studies. Such standardization has recently helped explain seemingly contradictory results in 3D ECM gels (44). Confinement to printed lines has been put forth as a model of 3D migration (15), but it is unlikely that this adhesion-dependent mode of migration recapitulates the adhesion-independent 3D migration modalities we (9) and others (12, 45) have observed. Importantly, distinct decision making outcomes are noted when cells are confined within microchannels vs. in laterally confining lines. It is thus imperative to define and decipher how microtopology, particularly on the length scale of interstitial spaces, can potentiate and impact cell migration.
Acknowledgments
The authors thank Dr. Denis Wirtz (The Johns Hopkins University) for providing HT1080 cells. This study was supported by the U.S. National Institutes of Health, National Cancer Institute Grants R01-CA183804 and R01-CA186286 (to K.K.), and by a National Science Foundation Graduate Research Fellowship (to C.D.P.). The authors declare no conflicts of interest.
Glossary
- 2D
2-dimensional
- 3D
3-dimensional
- Cdc42
cell division control protein 42 homolog
- DPBS
Dulbecco’s PBS
- ECM
extracellular matrix
- FBS
fetal bovine serum
- PDMS
polydimethylsiloxane
- P/S
penicillin/streptomycin
- RhoA
Ras homolog gene family, member A
- siRNA
small interfering RNA
- VC
vehicle control
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Alexander S., Koehl G. E., Hirschberg M., Geissler E. K., Friedl P. (2008) Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem. Cell Biol. 130, 1147–1154 [DOI] [PubMed] [Google Scholar]
- 2.Weigelin B., Bakker G.-J., Friedl P. (2012) Intravital third harmonic generation microscopy of collective melanoma cell invasion: principles of interface guidance and microvesicle dynamics. Intravital 1, 32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroka K. M., Konstantopoulos K. (2014) Physical biology in cancer. 4. Physical cues guide tumor cell adhesion and migration. Am. J. Physiol. Cell Physiol. 306, C98–C109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander S., Weigelin B., Winkler F., Friedl P. (2013) Preclinical intravital microscopy of the tumour-stroma interface: invasion, metastasis, and therapy response. Curr. Opin. Cell Biol. 25, 659–671 [DOI] [PubMed] [Google Scholar]
- 5.Wolf K., Alexander S., Schacht V., Coussens L. M., von Andrian U. H., van Rheenen J., Deryugina E., Friedl P. (2009) Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 20, 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedl P., Wolf K. (2010) Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 188, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanz-Moreno V., Gadea G., Ahn J., Paterson H., Marra P., Pinner S., Sahai E., Marshall C. J. (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510–523 [DOI] [PubMed] [Google Scholar]
- 8.Sanz-Moreno V., Marshall C. J. (2010) The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr. Opin. Cell Biol. 22, 690–696 [DOI] [PubMed] [Google Scholar]
- 9.Balzer E. M., Tong Z., Paul C. D., Hung W. C., Stroka K. M., Boggs A. E., Martin S. S., Konstantopoulos K. (2012) Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J. 26, 4045–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung W. C., Chen S. H., Paul C. D., Stroka K. M., Lo Y. C., Yang J. T., Konstantopoulos K. (2013) Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J. Cell Biol. 202, 807–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroka K. M., Jiang H., Chen S. H., Tong Z., Wirtz D., Sun S. X., Konstantopoulos K. (2014) Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y. J., Le Berre M., Lautenschlaeger F., Maiuri P., Callan-Jones A., Heuzé M., Takaki T., Voituriez R., Piel M. (2015) Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 160, 659–672 [DOI] [PubMed] [Google Scholar]
- 13.Friedl P., Alexander S. (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 [DOI] [PubMed] [Google Scholar]
- 14.Maiuri P., Terriac E., Paul-Gilloteaux P., Vignaud T., McNally K., Onuffer J., Thorn K., Nguyen P. A., Georgoulia N., Soong D., Jayo A., Beil N., Beneke J., Lim J. C. H., Sim C. P.-Y., Chu Y.-S., Jiménez-Dalmaroni A., Joanny J. F., Thiery J. P., Erfle H., Parsons M., Mitchison T. J., Lim W. A., Lennon-Duménil A. M., Piel M., Théry M.; WCR Participants (2012) The first World Cell Race. Curr. Biol. 22, R673–R675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle A. D., Wang F. W., Matsumoto K., Yamada K. M. (2009) One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irimia D., Toner M. (2009) Spontaneous migration of cancer cells under conditions of mechanical confinement. Integr. Biol. (Camb.) 1, 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tong Z., Balzer E. M., Dallas M. R., Hung W. C., Stebe K. J., Konstantopoulos K. (2012) Chemotaxis of cell populations through confined spaces at single-cell resolution. PLoS One 7, e29211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroka K. M., Gu Z., Sun S. X., Konstantopoulos K. (2014) Bioengineering paradigms for cell migration in confined microenvironments. Curr. Opin. Cell Biol. 30, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherber C., Aranyosi A. J., Kulemann B., Thayer S. P., Toner M., Iliopoulos O., Irimia D. (2012) Epithelial cell guidance by self-generated EGF gradients. Integr. Biol. (Camb.) 4, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prentice-Mott H. V., Chang C. H., Mahadevan L., Mitchison T. J., Irimia D., Shah J. V. (2013) Biased migration of confined neutrophil-like cells in asymmetric hydraulic environments. Proc. Natl. Acad. Sci. USA 110, 21006–21011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak M., Erickson D. (2014) Mechanical decision trees for investigating and modulating single-cell cancer invasion dynamics. Lab Chip 14, 964–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teixeira A. I., Abrams G. A., Bertics P. J., Murphy C. J., Nealey P. F. (2003) Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 116, 1881–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conklin M. W., Eickhoff J. C., Riching K. M., Pehlke C. A., Eliceiri K. W., Provenzano P. P., Friedl A., Keely P. J. (2011) Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provenzano P. P., Eliceiri K. W., Campbell J. M., Inman D. R., White J. G., Keely P. J. (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen S. H., Dallas M. R., Balzer E. M., Konstantopoulos K. (2012) Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J. 26, 1349–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P., Chen S. H., Hung W. C., Paul C., Zhu F., Guan P. P., Huso D. L., Kontrogianni-Konstantopoulos A., Konstantopoulos K. (2015) Fluid shear promotes chondrosarcoma cell invasion by activating matrix metalloproteinase 12 via IGF-2 and VEGF signaling pathways. Oncogene 34, 4558–4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S. H., Hung W. C., Wang P., Paul C., Konstantopoulos K. (2013) Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci. Rep. 3, 1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meijering E., Dzyubachyk O., Smal I. (2012) Methods for cell and particle tracking. Methods Enzymol. 504, 183–200 [DOI] [PubMed] [Google Scholar]
- 29.Raman P. S., Paul C. D., Stroka K. M., Konstantopoulos K. (2013) Probing cell traction forces in confined microenvironments. Lab Chip 13, 4599–4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang S. S., Guo W. H., Kim Y., Wang Y. L. (2013) Guidance of cell migration by substrate dimension. Biophys. J. 104, 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross T. D., Coon B. G., Yun S., Baeyens N., Tanaka K., Ouyang M., Schwartz M. A. (2013) Integrins in mechanotransduction. Curr. Opin. Cell Biol. 25, 613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grzesiak J. J., Tran Cao H. S., Burton D. W., Kaushal S., Vargas F., Clopton P., Snyder C. S., Deftos L. J., Hoffman R. M., Bouvet M. (2011) Knockdown of the β(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int. J. Cancer 129, 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovács M., Tóth J., Hetényi C., Málnási-Csizmadia A., Sellers J. R. (2004) Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279, 35557–35563 [DOI] [PubMed] [Google Scholar]
- 34.Betapudi V., Licate L. S., Egelhoff T. T. (2006) Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 66, 4725–4733 [DOI] [PubMed] [Google Scholar]
- 35.Wedlich-Soldner R., Altschuler S., Wu L., Li R. (2003) Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231–1235 [DOI] [PubMed] [Google Scholar]
- 36.Kedrin D., Gligorijevic B., Wyckoff J., Verkhusha V. V., Condeelis J., Segall J. E., van Rheenen J. (2008) Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods 5, 1019–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmud G., Campbell C. J., Bishop K. J. M., Komarova Y. A., Chaga O., Soh S., Huda S., Kandere-Grzybowska K., Grzybowski B. A. (2009) Directing cell motions on micropatterned ratchets. Nat. Phys. 5, 606–612 [Google Scholar]
- 38.Houk A. R., Jilkine A., Mejean C. O., Boltyanskiy R., Dufresne E. R., Angenent S. B., Altschuler S. J., Wu L. F., Weiner O. D. (2012) Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell 148, 175–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shieh A. C., Rozansky H. A., Hinz B., Swartz M. A. (2011) Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 71, 790–800 [DOI] [PubMed] [Google Scholar]
- 40.Provenzano P. P., Inman D. R., Eliceiri K. W., Trier S. M., Keely P. J. (2008) Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J. 95, 5374–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey M. T., Tsai I. Y., Russell T. P., Hanks S. K., Wang Y. L. (2006) Cellular responses to substrate topography: role of myosin II and focal adhesion kinase. Biophys. J. 90, 3774–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasapera A. M., Schneider I. C., Rericha E., Schlaepfer D. D., Waterman C. M. (2010) Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol. 188, 877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo Y., Wu Y., Chakraborty C. (2012) Cdc42 negatively regulates intrinsic migration of highly aggressive breast cancer cells. J. Cell. Physiol. 227, 1399–1407 [DOI] [PubMed] [Google Scholar]
- 44.Wolf K., Te Lindert M., Krause M., Alexander S., Te Riet J., Willis A. L., Hoffman R. M., Figdor C. G., Weiss S. J., Friedl P. (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 201, 1069–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tozluoğlu M., Tournier A. L., Jenkins R. P., Hooper S., Bates P. A., Sahai E. (2013) Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat. Cell Biol. 15, 751–762 [DOI] [PubMed] [Google Scholar]