Abstract
Selective autophagy contributes to intracellular homeostasis by mediating the degradation of cytoplasmic material such as aggregated proteins, damaged or over-abundant organelles, and invading pathogens. The molecular machinery for selective autophagy must ensure efficient recognition and sequestration of the cargo within autophagosomes. Cargo specificity can be mediated by autophagic cargo receptors that specifically bind the cargo material and the autophagosomal membrane. Here we review the recent insights into the mechanisms that enable cargo receptors to confer selectivity and exclusivity to the autophagic process. We also discuss their different roles during starvation-induced and selective autophagy. We propose to classify autophagic events into cargo-independent and cargo-induced autophagosome formation events.
Keywords: autophagy, autophagosome, cargo receptor, isolation membrane, Atg8
Abbreviations: PAS, Phagophore Assembly Site; PI3Kc1, phosphatidylinositol 3-phosphate kinase complex 1; Cvt, cytoplasm-to-vacuole targeting; LIR, LC3-Interacting Region
Graphical abstract
Highlights
-
•
Cargo receptors mediate selective autophagy.
-
•
High-avidity interactions with Atg8 proteins target the receptors to isolation membranes.
-
•
Dependent on the stimulus, cargo receptors act prior or after isolation membrane generation.
Introduction
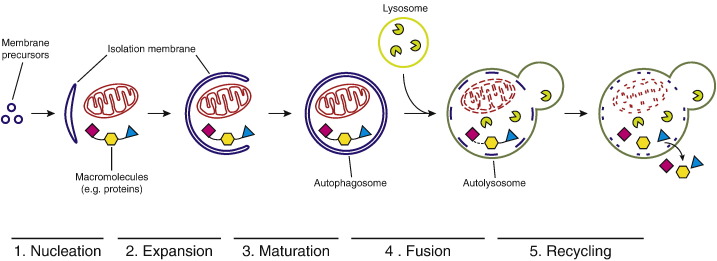
Macroautophagy (hereafter autophagy) is a conserved pathway for the degradation of cytoplasmic material and the recycling of nutrients. During autophagy, a double-membrane organelle called autophagosome is formed in a de novo manner. Autophagosomes originate from a crescent-shaped structure termed the isolation membrane or phagophore (Fig. 1). In yeast isolation, membranes are likely nucleated at a specific site in the perivacuolar region referred to as Phagophore Assembly Site (PAS) [1], [2]. As they grow, isolation membranes sequester a portion of the cytoplasm. When they close to give rise to an autophagosome, this cytoplasmic material is trapped in its lumen and is ultimately degraded upon fusion of the autophagosome with the lysosomal compartment (or the vacuole in yeast) [1].
Fig. 1.
Autophagy delivers cytoplasmic material to the lysosomal compartment for degradation. (1) Membrane donors including Atg9 vesicles nucleate an isolation membrane. (2) The isolation membrane expands and engulfs cytoplasmic cargo material including organelles and macromolecules. (3) The isolation membrane matures into a closed double-membrane autophagosome. (4) The outer autophagosomal membrane fuses with a lysosome (or the vacuole in yeast), leading to the degradation of the inner membrane and the cargo. (5) Components are recycled back into the cytoplasm.
The formation of autophagosomes requires a set of conserved factors that are recruited to the site of autophagosome formation in a hierarchical manner [1]. These factors can be grouped into functional units including the Atg1/ULK1 kinase complex, the class III phosphatidylinositol 3-phosphate kinase complex 1 (PI3Kc1) containing the Atg14/ATG14 subunit, the Atg9/ATG9A cycling system, the WIPIs and the two conjugation systems for Atg12/ATG12 and the ATG8 family members. One of the most downstream effects of the combined action of these proteins is the covalent attachment of the ubiquitin-like ATG8-family proteins to the membrane lipid phosphatidylethanolamine in the isolation membrane.
Autophagy was initially characterized as a bulk degradation pathway induced by glucagon and nutrient deprivation [3], [4], [5], [6], [7]. So-called bulk autophagy serves to recycle building blocks to compensate for the lack of nutrients and is thought to be rather non-selective toward its substrates (referred to as cargos) [8], [9]. It has, however, become clear that autophagy also contributes to intracellular homeostasis in non-starved cells by selectively degrading cargo material such as aggregated proteins, damaged mitochondria, excess peroxisomes and invading pathogens (reviewed in Refs. [10], [11], [12]). The crucial role of selective autophagy for cellular homeostasis is emphasized by the fact that tissue-specific knockout of autophagy genes in mice results in neurodegeneration or liver cancer [13], [14], [15], [16]. Additionally, it has been shown that cells with defective autophagy are unable to clear certain intracellular pathogens (reviewed in Refs. [17], [18]). Emerging evidence has also shown that selective autophagy plays an important role in the homeostasis of intracellular free iron, by controlling the levels of the iron-chelating protein ferritin (referred to as ferritinophagy) [19], [20], [21], [22].
Finally, in yeast, the cytoplasm-to-vacuole targeting (Cvt) pathway exploits the autophagic machinery for the delivery of the Ape1, Ams1 and Ape4 enzymes to the vacuole via autophagosome-like vesicles called Cvt vesicles [23], [24], [25]. The Cvt pathway also mediates the degradation of retrotransposon particles and thereby serves to protect the Saccharomyces cerevisiae genome from retrotransposition [26].
Unlike bulk autophagy, selective autophagy and the Cvt pathway need to meet at least three criteria for an efficient process to happen: first, the cargo has to be specifically recognized; second, the cargo has to be effectively tethered to a nascent autophagosome; and third, non-cargo material has to be excluded from the autophagosome. In fact, Cvt vesicles are smaller in diameter than starvation-induced autophagosomes, and in contrast to autophagosomes, their membrane is tightly apposed to the cargo preventing unrelated material from being engulfed [27], [28], [29], [30].
Selectivity in autophagy is conferred by cargo receptor proteins, which are able to tether a cargo to a nascent autophagosomal by simultaneously binding the cargo and ATG8-family proteins on the isolation membrane (see below). While some cargo receptors bind their cargos directly, in mammalian cells several receptor proteins recognize poly-ubiquitin chains attached to the surface of cargos for selective autophagy [11]. In the following sections, we will discuss the biochemical principles of cargo sorting from other cellular material that often contains the same binding sites. We will further discuss how cargo receptors bind membrane-localized ATG8-family proteins as opposed to their soluble forms. Finally, we will discuss the position of the cargo receptors in the autophagic hierarchy dependent on the stimulus for autophagosome formation.
Principles of Cargo Recognition by Autophagic Cargo Receptors
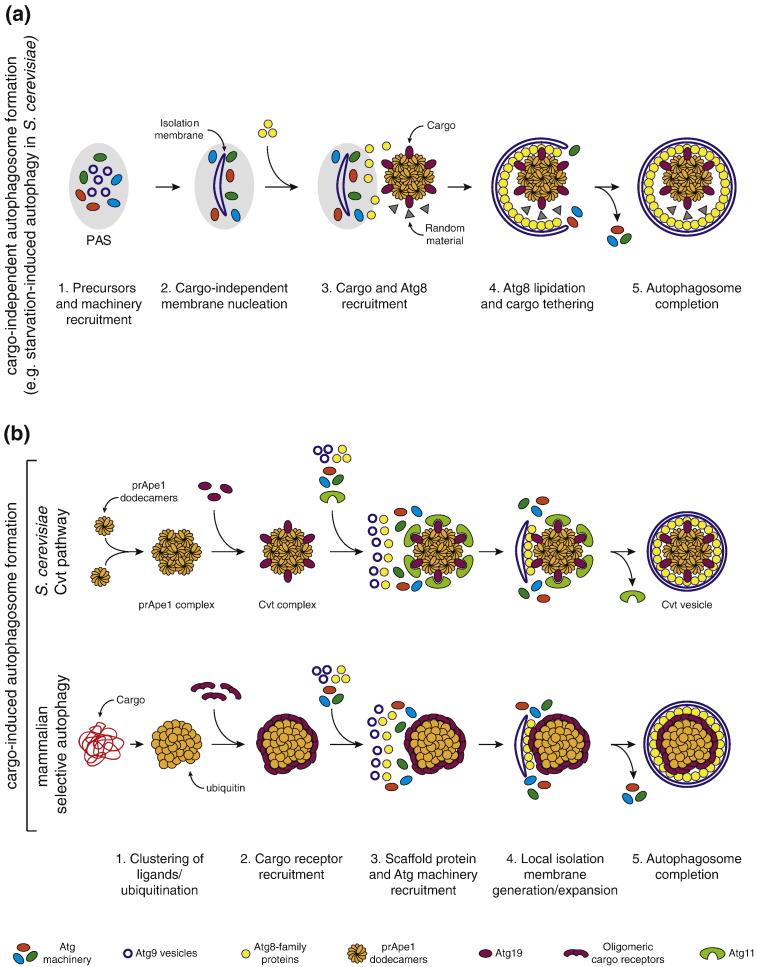
The Cvt pathway is the prototypical example for selective autophagy in S. cerevisiae [31]. prApe1, the major cargo of the Cvt pathway, is synthesized in the cytoplasm as a zymogen with an N-terminal propeptide. prApe1 monomers assemble in the cytoplasm into dodecamers which further aggregate into higher order particles in a propeptide-dependent manner [32], [33], [34]. The cargo receptor Atg19 specifically binds to the prApe1 propeptide with high affinity via its coiled-coil domain [30], [35], [36], [37]. Atg19 can also bind to Ams1 and thereby include it into the so-called Cvt complex [36], [38]. Subsequently, Atg19 mediates the recruitment of the Cvt complex to the PAS via an interaction with Atg11 [36], [39], [40]. Finally, the interaction of Atg19 with Atg8 on the isolation membrane mediates the engulfment of the Cvt complex by a Cvt vesicle (see below) [30], [36]. The propeptide region of prApe1 is essential for the formation of the prApe1 complex and its transport into the vacuole [33], [34], [36]. It has recently been shown that a mutation that prevents the formation of prApe1 dodecamers also prevents its delivery into the vacuole, even though this mutant still retains an intact propeptide. Conversely, the fusion of a propeptide to unrelated oligomeric particles is sufficient to drive their efficient delivery into the vacuole [34]. Intriguingly, these data suggest that several propeptides, and by implication several Atg19 cargo receptors clustered on a larger structure, are required and sufficient for efficient transport into the vacuole via the Cvt pathway.
In mammalian cells, NCOA4 has been recently identified as the cargo receptor for ferritin during a process referred to as ferritinophagy [19], [21], [22]. Excessive intracellular iron is sequestered by ferritin, to prevent harmful oxidative reactions. Ferritin forms complexes consisting of 24 subunits and therefore also represent multimeric particles [41]. Under conditions of low intracellular iron, NCOA4 receptors bind specifically to ferritin heavy chains (FTH1) and mediate the delivery of the ferritin complexes into the lysosomal compartment via selective autophagy [19], [21], [22]. Degradation of ferritin within the lysosomes ultimately leads to the release of chelated iron, which is subsequently transported back into the cytosol [42], [43].
The human cargo receptor p62/SQSTM1 mediates the degradation of ubiquitinated cargo material such as aggregated proteins or cytosolic bacteria [44], [45], [46], [47]. p62 binds ubiquitin with a relatively low affinity. This low affinity is at least in part due to homo-dimerization of the UBA domain, which is mutually exclusive with mono-ubiquitin binding [48], [49], [50], [51]. The affinity of p62 for ubiquitin can be increased by phosphorylation of serine 403 within the UBA domain [52]. The N-terminal PB1 domain of p62 drives homo-oligomerization [53], [54], and in vitro p62 oligomers were shown to assemble into long helical structures [55]. Oligomerization of p62 confers high avidity to the interaction with clustered ubiquitin and, thus, stabilizes binding to the cargo material on which ubiquitin is concentrated [56]. It was proposed that the interaction of multiple UBA domains in oligomeric p62 with clustered ubiquitin counteracts the self-association of UBA domain [50]. Thus, oligomerization of p62 and dimerization of UBA domain might cooperate to achieve selectivity for highly ubiquitinated cargos [50], [56].
The preferential binding of p62 to certain ubiquitin chain types might constitute a further level of regulation during selective autophagy. Indeed, it is known that different ubiquitin chains trigger different cellular processes. While K48-linked chains are recognized by the proteasome and therefore mediate proteasomal degradation of their substrates, K63-linked chains have also been associated with autophagy [57], [58]. Interestingly, it has been shown that oligomeric p62 preferentially binds to linear and K63-linked chains, as well as to mono-ubiquitin, compared to K48-linked chains [44], [56], [59]. Furthermore, it has been reported that binding to ubiquitin chains partially disrupts p62 oligomers in vitro, and that this effect is most evident in the presence of K48-linked chains [56], [60]. Their presence on a substrate might therefore prevent the accumulation of p62 and thereby counteract its degradation by autophagy. This may in turn favor its proteasomal degradation.
The cargo receptor CALCOCO2/NDP52 acts during selective autophagy of intracellular bacteria and damaged mitochondria [61], [62], [63], [64], [65]. NDP52 contains a predicted coiled-coil region that mediates homo-dimerization and a C-terminal ubiquitin binding zinc-finger domain [66], [67], [68]. Recently, it was shown that the isolated zinc-finger domain of NDP52 binds to single-ubiquitin moieties and to poly-ubiquitin chains, regardless of their linkage type. Interestingly, in the context of full-length dimeric NDP52, the zinc-finger domains bound to two different ubiquitin chains rather than to two ubiquitin molecules within the same chain [68]. Thus, similar to p62, full-length NDP52 might preferentially accumulate on cargos that are modified with multiple chains and therefore represent regions with high local ubiquitin concentrations. Furthermore, unlike the isolated zinc-finger domain, full-length NDP52 showed a slightly reduced binding affinity for K48-linked di-ubiquitin chains compared to linear or K63-linked di-ubiquitin chains [68], [69]. Therefore, it might be possible that, in analogy to p62, self-association confers chain specificity to NDP52. Similarly, the UBA domain of the dimeric NBR1 cargo receptor has been reported to bind to single-ubiquitin moieties with a slight preference for K63-linked chains over K48-linked chains [70].
In contrast to the rather weak interactions of p62, NDP52 and NBR1 with single ubiquitin, Atg19 binds with high affinity to its prApe1 cargo. prApe1 is synthesized in the cytoplasm and must be efficiently delivered into the vacuole by the Cvt pathway in order to function. The high affinity binding of Atg19 to prApe1 facilitates the efficient transport of prApe1. The NCOA4 cargo receptor could act according to similar principles. On the other hand, the targets of p62, NDP52 and NBR1 are not normally destined to be transported into the lysosome but become a target for selective autophagy only after their ubiquitination. One way to distinguish ubiquitinated autophagic cargo material from other cellular structures with ubiquitin tags may thus be the presence of clustered ubiquitin on the cargo material that is read out by high-avidity interactions of the cargo receptors with ubiquitin. Indeed, while ubiquitination per se could be sufficient to render any cellular material a substrate for autophagy [71], the higher the local concentration of ubiquitin on a cytoplasmic material, the more efficient the recruitment of cargo receptors will be. An additional level of regulation may be provided at the level of ubiquitin chain linkages. In particular, K48-linked ubiquitin chains may be less preferred targets for receptor binding, allowing proteins marked with these chains to preferentially undergo proteasomal degradation. However, it should be noted that all ubiquitin chain types have been found enriched in insoluble inclusions of autophagy-deficient mice [72]. Therefore, the relative contribution of ubiquitin clustering and of specific ubiquitin chains in cargo receptors recruitment has still to be elucidated.
Mechanisms of Isolation Membrane Targeting by Cargo Receptors
During autophagy and the Cvt pathway in S. cerevisiae, the small ubiquitin-like modifier Atg8 becomes conjugated to the headgroup of phosphatidylethanolamine on the isolation membrane [73]. First, Atg8 undergoes a proteolytic cleavage mediated by Atg4, which exposes the C-terminal glycine employed for the subsequent conjugation reaction [74]. The reaction proceeds similarly to the conjugation of ubiquitin to lysine residues in target proteins and requires Atg7 as the activating enzyme (E1), Atg3 as the conjugating enzyme (E2) and the Atg12–Atg5–Atg16 protein complex as the E3-like enzyme [73], [75], [76]. In mammalian cells, at least six functional Atg8 homologues are found: the three members of the microtubule-associated protein 1 light chain 3 (MAP1LC3) subfamily (LC3A, LC3B and LC3C, respectively) and the three members of the gamma-aminobutyric receptor-associated protein (GABARAP) subfamily (GABARAP, GABARAP-L1 and GABARAP-L2/GATE-16) [77], [78], [79], [80].
Cargo receptors bind to ATG8-family proteins via a conserved LC3-Interacting Region (LIR) motif, also known as Atg8-Interacting Motif (AIM) [46], [81]. The LIR motif consists of the consensus sequence ΘXXΓ, where Θ is an aromatic residue (W/F/Y) and Γ is hydrophobic (L/I/V), while X is any other residue [82], [83]. However, some non-canonical LIR motifs, which do not apparently match this consensus, are also found [30], [84], [85].
The presence of negatively charged residues in the near proximity of the core LIR sequence has been shown to contribute to the binding to ATG8-family proteins [12], [46], [81], [86], [87]. For instance, phosphorylation of a serine adjacent to the core LIR motif in cargo receptor Optineurin increases the affinity toward LC3B by a factor of 5 ([86] and Table 1). However, the overall affinity of the LIR motif for Atg8 family members remains relatively low, that is, in the micromolar range, even upon phosphorylation (Table 1) [30], [84], [86], [87], [88]. Furthermore, the modification of ATG8-family proteins with phosphatidylethanolamine is not essential for the interaction of Atg8-family proteins with cargo receptors [30], [56], [89]. This raises the question as to how cargo receptors can be specifically recruited to isolation membranes in vivo, a question that has recently been addressed for the Atg19 cargo receptor. Atg19 contains multiple LIR motifs in its C-terminal domain [30, 90]. Each LIR motif binds to the same site in Atg8, enabling Atg19 to bind simultaneously to multiple Atg8 proteins. Therefore, multiple LIR motifs in tandem allow Atg19 to select for Atg8-decorated membranes, by establishing high-avidity interactions with concentrated Atg8 [30] (C. Abert et al., unpublished results).
Table 1.
Known dissociation constants for LIR-mediated cargo receptors—ATG8-family proteins interactions
| Protein | LIR seq. | Partner | KD | Method | Reference |
|---|---|---|---|---|---|
| Atg19 | Multiple LIR motifsa | Atg8 | 35 μM | ITC | [30] |
| p62 | DDDWTHL | LC3B | 1.5 μM | ITC | [84] |
| NDP52 | ENEEDILVVTTQGE | LC3C | 1.5 μM | FA | [85] |
| NDP52 | ENEEDILVVTTQGE | LC3A | 15 μM | FA | [85] |
| NBR1 | SEDYIII | LC3B | 2.9 μM | ITC | [88] |
| NBR1 | SEDYIII | GABARAP-L1 | 3 μM | ITC | [88] |
| OPTN | EDSFVEI | LC3B | 67 μM | ITC | [86] |
| pOPTN (pS177) | EDpSFVEI | LC3B | 13 μM | ITC | [86] |
| OPTN | EDSFVEI | LC3B | 40 μM | ITC | [87] |
| NIX (W35) | SSWVEL | LC3B | 91 μM | ITC | [84] |
| NIX (W35) | SSWVEL | LC3A | 28 μM | ITC | [84] |
| NIX (W139) | WVSDWSS | LC3B | 670 μM | ITC | [84] |
| NIX (W139) | WVSDWSS | LC3A | 130 μM | ITC | [84] |
Where applicable, the core LIR sequence matching the consensus ΘxxΓ is underlined.
ITC = isothermal calorimetry; FA = fluorescence anisotropy; SPR = surface plasmon resonance.
Overall affinity of the full-length Atg19 molecule containing multiple LIR motifs.
Mammalian p62 contains only one functional LIR motif [46], [56], but oligomerization clusters the LIR motifs and thereby increases the avidity of p62 toward LC3B concentrated on a surface. In contrast, oligomerization has no effect on the binding of each LIR to free LC3B [56]. Furthermore, introducing multiple LIRs in a non-oligomerizing version of p62 restores avid binding to clustered LC3B, in analogy to the situation in Atg19 [30], [56]. Therefore, Atg19 and p62 both exploit avidity-driven interactions to select for ATG8-decorated membranes, albeit employing different molecular mechanisms to achieve them (tandem LIR motifs versus oligomerization, respectively). Given that many cargo receptors form dimers or multimers and/or contain multiple LIR motifs, avidity-driven interactions with membrane-localized Atg8-family proteins are likely to be a more general property of cargo receptors [30], [49], [56], [84], [91], [92], [93], [94].
Cargo or Isolation Membrane First?
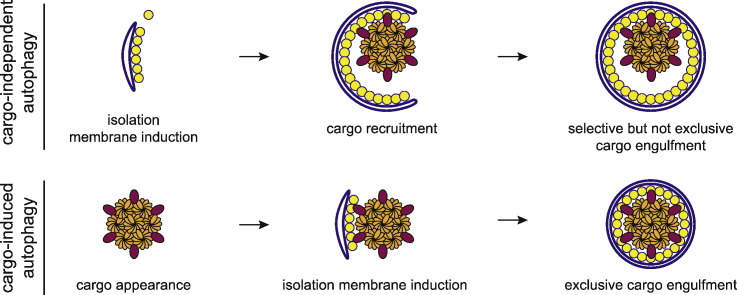
In yeast, the PAS is defined as a peri-vacuolar, dot-like structure in which the prApe1 cargo and Atg proteins co-localize, and it is likely that autophagosomes form there [2]. In mammalian cells, autophagosomes are generated at multiple sites throughout the cytoplasm (for detailed reviews, see Refs. [95], [96]). Selective autophagy requires the efficient orchestration of autophagosome biogenesis and cargo recruitment. How these processes are coordinated is still unclear. In principle, it is possible that the cargo is recruited to pre-existing isolation membranes. Another intriguing possibility is that during selective autophagy, the presence of the cargo induces the formation of isolation membranes [65], [97], [98]. Recent literature insights point in the direction that the nature of the stimulus for autophagosome formation determines whether the generation of the isolation membrane is upstream or downstream of cargo recruitment (Fig. 2).
Fig. 2.
Autophagosome formation during starvation-induced and starvation-independent autophagy. (A) During starvation, autophagosome formation is likely triggered independently of any cargo. (1) Starvation-dependent TOR complex inhibition results in activation of the Atg1/ULK1 complex and hierarchical recruitment of the autophagic machinery (PI3Kc1 complex, WIPIs, Atg9 vesicles, Atg12- and Atg8-conjugation systems) to the site of autophagosome formation. (2) The autophagic machinery nucleates an isolation membrane independently of any bound cargo. (3) Atg8 proteins and cargo-bound cargo receptors are recruited to the PAS. (4) Atg8 becomes membrane attached by lipidation and cargo receptors selectively tether their cargo to the isolation membrane, but engulfment of random cytoplasmic material is not prevented. (5) The isolation membrane matures into a closed autophagosome containing both selective cargos and random material. (B) In non-starved cells, selective autophagosomes and Cvt vesicles are generated in a cargo-dependent manner. (1) Autophagic substrates display a high local concentration of ligands (ubiquitin chains or motifs such as the prApe1 propeptide). (2) Cargo receptors are recruited to the cargo site via high-affinity or high-avidity interactions with the concentrated ligands. (3) Scaffold proteins (i.e., Atg11) and the autophagic machinery are hierarchically recruited to the cargo site via interactions with cargo receptors and/or the cargo. (4) The autophagic machinery is locally activated and drives the nucleation of an isolation membrane in proximity of the cargo. (5) The isolation membrane elongates until a complete autophagosome is formed. High-avidity interactions between cargo receptors and membrane-tethered Atg8-family proteins mediate close apposition of the membrane and the cargo resulting in the exclusion of other cytoplasmic material.
Autophagosome formation can be massively induced by starvation or inhibition of the mechanistic target of rapamycin complex 1 (mTORC1) using rapamycin. This system has been used extensively to study the processes underlying autophagosome formation. Genetic and imaging approaches have shown that the main protein complexes required for this process are recruited in a hierarchical manner. Although there is some discrepancy between the genetic and temporal hierarchies, these analyses have placed the Atg1/ULK1 complex most upstream, followed by the ATG9A system, PI3Kc1, the WIPIs, the ATG12–ATG5–ATG16L complex and finally the ATG8-family proteins [99], [100], [101]. There is substantial evidence that even starvation- or rapamycin-induced autophagy has some degree of selectivity. For instance, in S. cerevisiae, the vacuolar transport of the prApe1 cargo is increased upon starvation and the protein is tethered to the membrane of larger autophagosomes that also contain other cytoplasmic material [29]. In addition, Ams1 can be selectively delivered into the vacuole upon starvation by the Atg34 cargo receptor [102]. Similarly, rapamycin treatment promotes Atg39- and Atg40-mediated autophagic degradation of the endoplasmic reticulum (ER-phagy) [103]. In mammalian cells FAM134B, the functional counterpart of Atg40 mediates ER-phagy during nutrient starvation [104]. Furthermore, acetaldehyde dehydrogenase (Ald6), leucine aminopeptidase III (Lap3), aspartyl aminopeptidase (Ape4), ubiquitinated proteins, proteasomes and mature ribosomes are all preferentially targeted to the vacuole via autophagy during nitrogen starvation in S. cerevisiae [25], [105], [106], [107], [108], [109]. In mammalian cells that were stimulated with Wnt ligand and subsequently starved, the Wnt-signaling effector Dishevelled (Dvl2) forms ubiquitinated aggregates that are selectively incorporated into autophagosomes in a p62-dependent manner [110]. Rapamycin treatment promoted the clearance of aggregated proteins by autophagy in mammalian cell lines and Drosophila melanogaster [111]. In Arabidopsis thaliana starvation induces the autophagic degradation of proteasomes [112].
In starvation-induced autophagy, cargo recruitment is likely downstream of isolation membrane formation and might coincide or follow ATG8-family protein conjugation (Fig. 2a). Indeed, in starved mammalian cells, the accumulation of the p62 cargo receptor to punctate structures followed the accumulation of the other components of the autophagic machinery and coincided with the recruitment of LC3B [100]. However, in an earlier study, it was shown that p62 recruitment to pre-autophagosomal structures does not absolutely depend on the interaction with ATG8-family proteins [113]. Therefore, the actual mechanism of p62 recruitment to the site of autophagosome biogenesis might be more complex.
During autophagic events that are independent of starvation or global mTORC1 inhibition, the sequence of events is likely different. In fact there is increasing evidence that the cargo is upstream of isolation membrane formation. For instance, it was shown that in S. cerevisiae, in the absence of starvation, the deletion of the Cvt pathway cargo prApe1 prevents the recruitment of several core autophagy proteins to the PAS, including Atg1 and Atg8. However, during starvation the localization at the PAS of these factors is not affected by the deletion of APE1 [114]. These data strongly suggest that in the absence of starvation, the Cvt complex has a crucial role in recruiting the autophagic machinery. Recently, it was shown that under normal growth conditions, the activation of the Atg1 kinase is triggered by Atg11 bound to the Atg19 cargo receptor on the Cvt complex [115]. The amount of active auto-phosphorylated Atg1 is strongly reduced in ape1 ∆ cells, as wells as in cells lacking Atg19 and Atg11, or in cells expressing an Atg11 mutant incapable of binding to Atg19. In contrast, the activity of Atg1 under starvation is not affected by the deficiency of these proteins [115]. Collectively, these results suggest that the cargo is required for Atg1 kinase activation, which is one of the most upstream signals during autophagosome formation. In contrast, upon starvation the requirement for the prApe1 cargo is bypassed.
Employing Salmonella typhimurium or transfection reagent-coated latex beads as models for selective autophagy in mammalian cells, it was observed that both these substrates were associated with ubiquitin-positive structures after rupture of the endosomal membrane [116]. Interestingly, the localization of the p62 cargo receptor preceded the recruitment of the machinery for autophagosome formation and in particular that of LC3B, which was recruited downstream of all the other components [116]. Similarly, core components of the autophagy machinery including ULK1 and ATG9A have been found to localize around damaged mitochondria independent of ATG8-family proteins conjugation [117]. Recently, it was reported that cargo receptors recruit the autophagic machinery to damaged mitochondria during mitophagy [65]. The recruitment of the autophagic machinery to mitochondria upon induction of mitochondrial damage was monitored in HeLa cells lacking five cargo receptors (p62, NBR1, Optineurin, NDP52 and TAXBP1/CALCOCO3) [65], [69]. Strikingly, ULK1, WIPI1 and DFCP1 were not efficiently recruited to damaged mitochondria in the cargo receptors KO cells [65]. In addition, TRIM20 and TRIM21 that act as cargo receptors to degrade components of the inflammasome and the interferon response recruit the autophagic machinery to their cargo material [118].
Collectively, these data suggest that during autophagic events that are triggered in the absence of starvation and that are commonly referred to as selective autophagy, the presence of cargo material triggers autophagosome formation (Fig. 2b). Here the usually bulky cargo materials such as protein aggregates, mitochondria, bacterial pathogens or the prApe1 complexes serve as template to recruit the autophagic machinery. The recruitment of this machinery is at least in part mediated by cargo receptors and culminates in local lipidation of ATG8-family proteins. Additionally, ubiquitinated cargos may also contribute directly to the recruitment of the autophagic machinery [116].
These membrane-localized ATG8 proteins in turn are avidly bound by the cargo receptors resulting in close apposition of the autophagosomal membrane and the cargo and therefore exclusion of non-cargo material from its sequestration within autophagosomes (Fig. 2b). This process may occur only at permissive sites that are capable of donating membrane to the process such as ER-related structures in mammalian cells and plants or the PAS in S. cerevisiae [2], [119], [120].
In contrast, during starvation isolation membranes are generated independently of bulky cargo material [29], [114] (Fig. 2a). The high local concentration of lipidated ATG8-family proteins in turn will recruit cargo receptors and/or cargo receptor–cargo complexes to the isolation membranes [30], [56], which in turn bring with them cargo material such as ubiquitinated proteins or prApe1. As a result, cargo material is tethered to the isolation membranes, but the engulfment of random material would not be prevented.
Given advances in the field, it is becoming increasingly confusing to group autophagic events into selective and starvation-induced autophagy, as some degree of selectivity may be a universal property of autophagosome formation. It may therefore be more appropriate to classify autophagic events into cargo-independent and cargo-induced autophagosome formation (Fig. 2).
Future perspectives
The model of cargo-induced autophagosome formation could help to explain how isolation membranes are generated in non-starved cells and how they can be efficiently tethered to cargos. However, several links are still missing in order to have a more thorough understanding of the whole process. First, the hypothesis of high-avidity interactions between cargo receptors and clustered target molecules should be tested for other cargo receptor proteins. Second, a more detailed molecular understanding of the interaction networks between cargo receptors, ubiquitin, the autophagic machinery and membranes will be required.
Acknowledgments
The authors thank Graham Warren for comments on the manuscript. The authors are supported by an ERC grant (No. 260304), by the FWF (No. P25546-B20), by the EMBO Young Investigator Program and by the Uni:docs Program of the University of Vienna.
Conflict of Interest Statement: The authors declare no conflict of interests.
Edited by Guest Editor
References
- 1.Kraft C., Martens S. Mechanisms and regulation of autophagosome formation. Curr. Opin. Cell Biol. 2012;24(4):496–501. doi: 10.1016/j.ceb.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki K. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20(21):5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeshige K. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992;119(2):301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashford T.P., Porter K.R. Cytoplasmic components in hepatic cell lysosomes. J. Cell Biol. 1962;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novikoff A.B., Essner E. Cytolysomes and mitochondrial degeneration. J. Cell Biol. 1962;15:140–146. doi: 10.1083/jcb.15.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Duve C., Wattiaux R. Functions of lysosomes. Annu. Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 7.Mortimore G.E., Schworer C.M. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature. 1977;270(5633):174–176. doi: 10.1038/270174a0. [DOI] [PubMed] [Google Scholar]
- 8.Kopitz J. Nonselective autophagy of cytosolic enzymes by isolated rat hepatocytes. J. Cell Biol. 1990;111(3):941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuma A. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 10.Kraft C., Reggiori F., Peter M. Selective types of autophagy in yeast. Biochim. Biophys. Acta. 2009;1793(9):1404–1412. doi: 10.1016/j.bbamcr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Khaminets A., Behl C., Dikic I. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2015 doi: 10.1016/j.tcb.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Rogov V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell. 2014;53(2):167–178. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Hara T. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu M. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131(6):1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takamura A. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25(8):795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randow F., Youle R.J. Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe. 2014;15(4):403–411. doi: 10.1016/j.chom.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13(10):722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancias J.D. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4 doi: 10.7554/eLife.10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishi-Itakura C. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 2014;127(Pt 18):4089–4102. doi: 10.1242/jcs.156034. [DOI] [PubMed] [Google Scholar]
- 21.Mancias J.D. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowdle W.E. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 2014;16(11):1069–1079. doi: 10.1038/ncb3053. [DOI] [PubMed] [Google Scholar]
- 23.Harding T.M. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 1995:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutchins M.U., Klionsky D.J. Vacuolar localization of oligomeric-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae. J. Biol. Chem. 2001:20491–20498. doi: 10.1074/jbc.M101150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuga M. Aspartyl aminopeptidase is imported from the cytoplasm to the vacuole by selective autophagy in Saccharomyces cerevisiae. J. Biol. Chem. 2011:13704–13713. doi: 10.1074/jbc.M110.173906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K. Selective autophagy regulates insertional mutagenesis by the Ty1 retrotransposon in Saccharomyces cerevisiae. Dev. Cell. 2011;21(2):358–365. doi: 10.1016/j.devcel.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 27.Arstila A.U., Trump B.F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am. J. Pathol. 1968:687–733. [PMC free article] [PubMed] [Google Scholar]
- 28.Baba M. Ultrastructural analysis of the autophagic process in yeast: detection of autophagosomes and their characterization. J. Cell Biol. 1994:903–913. doi: 10.1083/jcb.124.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baba M. Two distinct pathways for targeting proteins from the cytoplasm to the vacuole/lysosome. J Cell Biol. 1997:1687–1695. doi: 10.1083/jcb.139.7.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawa-Makarska J. Nat. Cell Biol.; 2014. Cargo binding to Atg19 unmasks additional Atg8 binding sites to mediate membrane–cargo apposition during selective autophagy; pp. 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch-Day M.A., Klionsky D.J. The CVT pathway as a model for selective autophagy. FEBS Lett. 2010;584(7):1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J. Transport of a large oligomeric protein by the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 1997;137(3):609–618. doi: 10.1083/jcb.137.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morales Quinones M., Winston J.T., Stromhaug P.E. Propeptide of aminopeptidase 1 protein mediates aggregation and vesicle formation in cytoplasm-to-vacuole targeting pathway. J. Biol. Chem. 2012;287(13):10121–10133. doi: 10.1074/jbc.M111.311696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su M.Y. Structure of yeast Ape1 and its role in autophagic vesicle formation. Autophagy. 2015;11(9):1580–1593. doi: 10.1080/15548627.2015.1067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott S.V. Cvt19 is a receptor for the cytoplasm-to-vacuole targeting pathway. Mol. Cell. 2001;7(6):1131–1141. doi: 10.1016/s1097-2765(01)00263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shintani T. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev. Cell. 2002;3(6):825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leber R. Yol082p, a novel CVT protein involved in the selective targeting of aminopeptidase I to the yeast vacuole. J. Biol. Chem. 2001;276(31):29210–29217. doi: 10.1074/jbc.M101438200. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y. Selective transport of alpha-mannosidase by autophagic pathways: structural basis for cargo recognition by Atg19 and Atg34. J. Biol. Chem. 2010;285(39):30026–30033. doi: 10.1074/jbc.M110.143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yorimitsu T., Klionsky D.J. Atg11 links cargo to the vesicle-forming machinery in the cytoplasm to vacuole targeting pathway. Mol. Biol. Cell. 2005;16(4):1593–1605. doi: 10.1091/mbc.E04-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki K., Kamada Y., Ohsumi Y. Studies of cargo delivery to the vacuole mediated by autophagosomes in Saccharomyces cerevisiae. Dev. Cell. 2002;3(6):815–824. doi: 10.1016/s1534-5807(02)00359-3. [DOI] [PubMed] [Google Scholar]
- 41.Arosio P., Ingrassia R., Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta. 2009;1790(7):589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Dong X.P. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455(7215):992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurz T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 2008;129(4):389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seibenhener M.L. Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation. Mol. Cell. Biol. 2004;24(18):8055–8068. doi: 10.1128/MCB.24.18.8055-8068.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjorkoy G. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on Huntingtin-induced cell death. J. Cell Biol. 2005;171(4):603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pankiv S. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007;282(33):24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y.T. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 2009;183(9):5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
- 48.Raasi S. Diverse polyubiquitin interaction properties of ubiquitin-associated domains. Nat. Struct. Mol. Biol. 2005;12(8):708–714. doi: 10.1038/nsmb962. [DOI] [PubMed] [Google Scholar]
- 49.Kirkin V. A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell. 2009;33(4):505–516. doi: 10.1016/j.molcel.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Long J. Dimerisation of the UBA domain of p62 inhibits ubiquitin binding and regulates NF-kappaB signalling. J. Mol. Biol. 2010;396(1):178–194. doi: 10.1016/j.jmb.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Isogai S. Crystal structure of the ubiquitin-associated (UBA) domain of p62 and its interaction with ubiquitin. J. Biol. Chem. 2011;286(36):31864–31874. doi: 10.1074/jbc.M111.259630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsumoto G. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol. Cell. 2011;44(2):279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 53.Lamark T. Interaction codes within the family of mammalian Phox and Bem1p domain-containing proteins. J. Biol. Chem. 2003:34568–34581. doi: 10.1074/jbc.M303221200. [DOI] [PubMed] [Google Scholar]
- 54.Wilson M.I. PB1 domain-mediated heterodimerization in NADPH oxidase and signaling complexes of atypical protein kinase C with Par6 and p62. Mol. Cell. 2003:39–50. doi: 10.1016/s1097-2765(03)00246-6. [DOI] [PubMed] [Google Scholar]
- 55.Ciuffa R. The selective autophagy receptor p62 forms a flexible filamentous helical scaffold. Cell Rep. 2015;11(5):748–758. doi: 10.1016/j.celrep.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 56.Wurzer B. Oligomerization of p62 allows for selection of ubiquitinated cargo and isolation membrane during selective autophagy. Elife. 2015;4 doi: 10.7554/eLife.08941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komander D., Rape M. The ubiquitin code. Annu. Rev. Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 58.Tan J.M. Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum. Mol. Genet. 2008;17(3):431–439. doi: 10.1093/hmg/ddm320. [DOI] [PubMed] [Google Scholar]
- 59.Long J. Ubiquitin recognition by the ubiquitin-associated domain of p62 involves a novel conformational switch. J. Biol. Chem. 2008;283(9):5427–5440. doi: 10.1074/jbc.M704973200. [DOI] [PubMed] [Google Scholar]
- 60.Ciuffa R. Cell Reports. The Authors; 2015. The Selective Autophagy Receptor p62 Forms a Flexible Filamentous Helical Scaffold; pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 61.Heo J.-M. Molecular Cell. Elsevier Inc.; 2015. The PINK1–PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mostowy S. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 2011:26987–26995. doi: 10.1074/jbc.M111.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thurston T.L.M. Nat. Immunol. Nature Publishing Group; 2009. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria; pp. 1215–1221. [DOI] [PubMed] [Google Scholar]
- 64.von Muhlinen N. Mol. Cell. Elsevier Inc.; 2012. LC3C, Bound Selectively by a Noncanonical LIR Motif in NDP52, Is Required for Antibacterial Autophagy; pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lazarou M. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korioth F. Molecular characterization of NDP52, a novel protein of the nuclear domain 10, which is redistributed upon virus infection and interferon treatment. J. Cell Biol. 1995;130(1):1–13. doi: 10.1083/jcb.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sternsdorf T. Cellular localization, expression, and structure of the nuclear dot protein 52. J. Cell Biol. 1997;138(2):435–448. doi: 10.1083/jcb.138.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie X. Molecular basis of ubiquitin recognition by the autophagy receptor CALCOCO2. Autophagy. 2015;11(10):1775–1789. doi: 10.1080/15548627.2015.1082025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tumbarello D.A. The autophagy receptor TAX1BP1 and the molecular motor myosin VI are required for clearance of Salmonella typhimurium by autophagy. PLoS Pathog. 2015;11(10) doi: 10.1371/journal.ppat.1005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walinda E. Solution structure of the ubiquitin-associated (UBA) domain of human autophagy receptor NBR1 and its interaction with ubiquitin and polyubiquitin. J. Biol. Chem. 2014;289(20):13890–13902. doi: 10.1074/jbc.M114.555441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim P.K. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc. Natl. Acad. Sci. U. S. A. 2008;105(52):20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riley B.E. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway: a potential role for protein aggregation in autophagic substrate selection. J. Cell Biol. 2010;191(3):537–552. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ichimura Y. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 74.Kirisako T. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000;151(2):263–276. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanada T. The Atg12-Atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007;282(52):37298–37302. doi: 10.1074/jbc.C700195200. [DOI] [PubMed] [Google Scholar]
- 76.Romanov J. Mechanism and functions of membrane binding by the Atg5–Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31(22):4304–4317. doi: 10.1038/emboj.2012.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kabeya Y. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He H. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J. Biol. Chem. 2003;278(31):29278–29287. doi: 10.1074/jbc.M303800200. [DOI] [PubMed] [Google Scholar]
- 79.Kabeya Y. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004;117(Pt 13):2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 80.Shpilka T. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12(7):226. doi: 10.1186/gb-2011-12-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ichimura Y. Structural basis for sorting mechanism of p62 in selective autophagy. J. Biol. Chem. 2008;283(33):22847–22857. doi: 10.1074/jbc.M802182200. [DOI] [PubMed] [Google Scholar]
- 82.Birgisdottir Å.B., Lamark T., Johansen T. The LIR motif—crucial for selective autophagy. J. Cell Sci. 2013;126(15):3237–3247. doi: 10.1242/jcs.126128. [DOI] [PubMed] [Google Scholar]
- 83.Alemu E.A. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 2012;287(47):39275–39290. doi: 10.1074/jbc.M112.378109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Novak I. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11(1):45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.von Muhlinen N. LC3C, bound selectively by a noncanonical LIR motif in NDP52, is required for antibacterial autophagy. Mol. Cell. 2012;48(3):329–342. doi: 10.1016/j.molcel.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wild P. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333(6039):228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rogov V.V. Structural basis for phosphorylation-triggered autophagic clearance of Salmonella. Biochem. J. 2013;454(3):459–466. doi: 10.1042/BJ20121907. [DOI] [PubMed] [Google Scholar]
- 88.Rozenknop A. Characterization of the interaction of GABARAPL-1 with the LIR motif of NBR1. J. Mol. Biol. 2011;410(3):477–487. doi: 10.1016/j.jmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Shvets E. Dissecting the involvement of LC3B and GATE-16 in p62 recruitment into autophagosomes. Autophagy. 2011;7(7):683–688. doi: 10.4161/auto.7.7.15279. [DOI] [PubMed] [Google Scholar]
- 91.Ying H. Posttranslational modifications, localization, and protein interactions of optineurin, the product of a glaucoma gene. PLoS One. 2010;5(2):e9168. doi: 10.1371/journal.pone.0009168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim B.-W. Structural basis for recognition of autophagic receptor NDP52 by the sugar receptor galectin-8. Nat. Commun. 2013;4:1613. doi: 10.1038/ncomms2606. [DOI] [PubMed] [Google Scholar]
- 93.Monaco C. The RFG oligomerization domain mediates kinase activation and re-localization of the RET/PTC3 oncoprotein to the plasma membrane. Oncogene. 2001;20(5):599–608. doi: 10.1038/sj.onc.1204127. [DOI] [PubMed] [Google Scholar]
- 94.Zhang G., Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J. Biophys. Chem. 2002;277(9):7059–7065. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- 95.Shibutani S.T., Yoshimori T. A current perspective of autophagosome biogenesis. Cell Res. 2014;24(1):58–68. doi: 10.1038/cr.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suzuki K., Ohsumi Y. Current knowledge of the pre-autophagosomal structure (PAS) FEBS Lett. 2010;584(7):1280–1286. doi: 10.1016/j.febslet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 97.Shibutani S.T., Yoshimori T. Autophagosome formation in response to intracellular bacterial invasion. Cell. Microbiol. 2014;16(11):1619–1626. doi: 10.1111/cmi.12357. [DOI] [PubMed] [Google Scholar]
- 98.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Suzuki K. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12(2):209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 100.Koyama-Honda I. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy. 2013;9(10):1491–1499. doi: 10.4161/auto.25529. [DOI] [PubMed] [Google Scholar]
- 101.Itakura E., Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6(6):764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suzuki K. Selective transport of alpha-mannosidase by autophagic pathways: identification of a novel receptor, Atg34p. J. Biol. Chem. 2010;285(39):30019–30025. doi: 10.1074/jbc.M110.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mochida K. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature. 2015;522(7556):359–362. doi: 10.1038/nature14506. [DOI] [PubMed] [Google Scholar]
- 104.Khaminets A. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522(7556):354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 105.Kraft C. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat. Cell Biol. 2008;10(5):602–610. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- 106.Onodera J., Ohsumi Y. Ald6p is a preferred target for autophagy in yeast, Saccharomyces cerevisiae. J. Biol. Chem. 2004;279(16):16071–16076. doi: 10.1074/jbc.M312706200. [DOI] [PubMed] [Google Scholar]
- 107.Kageyama T., Suzuki K., Ohsumi Y. Lap3 is a selective target of autophagy in yeast, Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2009;378(3):551–557. doi: 10.1016/j.bbrc.2008.11.084. [DOI] [PubMed] [Google Scholar]
- 108.Lu K., Psakhye I., Jentsch S. Autophagic clearance of polyQ proteins mediated by ubiquitin–Atg8 adaptors of the conserved CUET protein family. Cell. 2014;158(3):549–563. doi: 10.1016/j.cell.2014.05.048. [DOI] [PubMed] [Google Scholar]
- 109.Waite K.A. Starvation induces proteasome autophagy with different pathways for core and regulatory particle. J. Biol. Chem. 2015 doi: 10.1074/jbc.M115.699124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gao C. Autophagy negatively regulates wnt signalling by promoting dishevelled degradation. Nat. Cell Biol. 2010;12(8):781–790. doi: 10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- 111.Berger Z. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum. Mol. Genet. 2006;15(3):433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 112.Marshall R.S. Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/Ubiquitin receptor RPN10 in Arabidopsis. Mol. Cell. 2015;58(6):1053–1066. doi: 10.1016/j.molcel.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Itakura E., Mizushima N. p62 Targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J. Cell Biol. 2011;192(1):17–27. doi: 10.1083/jcb.201009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shintani T., Klionsky D.J. Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J. Biol. Chem. 2004;279(29):29889–29894. doi: 10.1074/jbc.M404399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kamber R.A., Shoemaker C.J., Denic V. Receptor-bound targets of selective autophagy use a scaffold protein to activate the Atg1 kinase. Mol. Cell. 2015;59(3):372–381. doi: 10.1016/j.molcel.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fujita N. Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J. Cell Biol. 2013;203(1):115–128. doi: 10.1083/jcb.201304188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Itakura E. Structures containing Atg9A and the ULK1 complex independently target depolarized mitochondria at initial stages of Parkin-mediated mitophagy. J. Cell Sci. 2012;125(Pt 6):1488–1499. doi: 10.1242/jcs.094110. [DOI] [PubMed] [Google Scholar]
- 118.Kimura T. TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 2015;210(6):973–989. doi: 10.1083/jcb.201503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Axe E.L. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008;182(4):685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le Bars R. ATG5 defines a phagophore domain connected to the endoplasmic reticulum during autophagosome formation in plants. Nat. Commun. 2014;5:4121. doi: 10.1038/ncomms5121. [DOI] [PubMed] [Google Scholar]