Abstract
Chironomids (Diptera: Chironomidae), also known as non-biting midges, are one of the most abundant groups of insects in aquatic habitats. They undergo a complete metamorphosis of four life stages of which three are aquatic (egg, larva, and pupa), and the adult emerges into the air. Chironomids serve as a natural reservoir of Aeromonas and Vibrio cholerae species. Here, we review existing knowledge about the mutual relations between Aeromonas species and chironomids. Using 454-pyrosequencing of the 16S rRNA gene, we found that the prevalence of Aeromonas species in the insects’ egg masses and larvae was 1.6 and 3.3% of the insects’ endogenous microbiota, respectively. Aeromonas abundance per egg mass remained stable during a 6-month period of bacterial monitoring. Different Aeromonas species were isolated and some demonstrated the ability to degrade the insect’s egg masses and to prevent eggs hatching. Chitinase was identified as the enzyme responsible for the egg mass degradation. Different Aeromonas species isolated from chironomids demonstrated the potential to protect their host from toxic metals. Aeromonas is a causative agent of fish infections. Fish are frequently recorded as feeding on chironomids. Thus, fish might be infected with Aeromonas species via chironomid consumption. Aeromonas strains are also responsible for causing gastroenteritis and wound infections in humans. Different virulence genes were identified in Aeromonas species isolated from chironomids. Chironomids may infest drinking water reservoirs, hence be the source of pathogenic Aeromonas strains in drinking water. Chironomids and Aeromonas species have a complicated mutual relationship.
Keywords: Aeromonas, chironomid, Chironomus, egg mass, virulence genes, chitinase, Vibrio cholerae
Chironomids
Chironomids (Diptera; Chironomidae), also known as non-biting midges, are the most abundant insects in freshwater habitats (Armitage et al., 1995). Members of the Chironomidae family are opportunistic colonizers of all aquatic environments. They undergo a complete metamorphosis of four life stages: eggs, larvae, pupae, and adults. Females of the genus Chironomus lay egg masses at the interface between water and air, usually glued to water plants or rocks. Each egg mass contains hundreds of eggs wrapped in a gelatinous matrix, composed mainly of glycoprotein and chitin (Broza et al., 2000; Halpern et al., 2003; Laviad et al., 2016). The matrix protects the eggs and is settled by various bacterial species (Halpern et al., 2007; Senderovich et al., 2008; Senderovich and Halpern, 2012, 2013; Failla et al., 2015; Halpern and Senderovich, 2015). The larvae of most Chironomus species pass through four stages that can be diagnosed by body length and diameter of the head box (Failla et al., 2015). Larvae at the first stage are planktonic and attracted to light; when they find a suitable place they settle and become benthic and no longer respond to light (Oliver, 1971). The larvae transform into pupae and the adults emerge into the air. Chironomid adults create aerial swarms for mating, after which the females lay the egg masses (Broza et al., 2005).
Chironomids are found worldwide, from Nepal glaciers at an altitude of 6,600 m to Lake Baikal at a depth of 1,000 m. They have also invaded the sea and are found on all coasts to a depth of 30 m (Wolfram et al., 1999; Epler, 2001). The estimated number of species in the Chironomidae family is 15,000–20,000 (Ali, 1995). Chironomids have the ability to reproduce fast in large numbers, and can compete with other benthic organisms for food (Demoor, 1992). Under certain conditions, such as low dissolved oxygen levels in water, chironomid larvae may be the only insects that can survive at the bottom of the water habitat. They are successful in aquatic environments with low nutrient resources and can tolerate extreme environmental changes. Some species show the ability to survive in extreme conditions of temperature, pH, salinity, organic pollution, and heavy metal loads (Failla et al., 2015).
Chironomids are inhabited by diverse bacterial species. A significant number of their endogenous bacteria are closely related to species known as toxicant degraders (Senderovich and Halpern, 2012, 2013). More details can be found in a recent review of chironomids’ microbiome (Halpern and Senderovich, 2015).
Aeromonas
Members of the genus Aeromonas belong to the family Aeromonadaceae in the order Aeromonadales (Martin-Carnahan and Joseph, 2005). Aeromonas species are Gram-negative rods and facultative anaerobes, and can be isolated from a variety of sources such as water and sewage, from various aquatic environments and clinical tissue samples from human or animals, from food sources such as meat, poultry, seafood and vegetables, and from chironomids (Janda and Abbott, 1998; Halpern et al., 2007; Pérez-Valdespino et al., 2009).
Aeromonas species have been associated with human disease for more than 50 years and are recognized as important causes of intestinal and extra-intestinal illnesses in humans, including gastroenteritis and septicemia in immune-compromised persons, serious wound infections in healthy individuals and in patients undergoing medicinal leech therapy, and a number of less well described illnesses such as peritonitis, meningitis, endocarditis, and infections of the eye, joints, and bones (Janda and Abbott, 1998, 2010; Figueras, 2005; Parker and Shaw, 2011; Igbinosa et al., 2012; Senderovich et al., 2012).
The following species are the most abundant in clinical samples: Aeromonas caviae (29.9%), A. dhakensis (25.5%), A. veronii (22%), and A. hydrophila (18%; Figueras and Beaz-Hidalgo, 2015). Among others, less frequently isolated clinical species are A. schubertii, A. simiae, A. diversa, A. taiwanensis, A. sanarellii, A. media, and A. salmonicida (Janda and Abbott, 2010; Beaz-Hidalgo et al., 2012; Senderovich et al., 2012; Latif-Eugenín et al., 2016).
Aeromonas in Chironomids
Rouf and Rigney (1993) were the first to identify Aeromonas species from chironomid larvae. Following these findings, different Aeromonas species were identified from chironomid egg masses: A. caviae (punctata), A. culicicola, A. dhakensis (aquariorum), A. hydrophila, A. media, A. salmonicida, A. sanarellii, A. schubertii, A. taiwanensis, and A. veronii (Halpern et al., 2007; Senderovich et al., 2008; Figueras et al., 2011; Halpern, 2011; Beaz-Hidalgo et al., 2012; Laviad, 2012; Senderovich and Halpern, 2012, 2013; Halpern and Senderovich, 2015; Laviad et al., 2016; Supplementary Table S1). Except A. culicicola, all these species have a clinical record (see the list in the previous paragraph). Aeromonas was also identified from chironomid egg masses and larvae by culture-independent methods like cloning and 454-pyrosequencing of the 16S rRNA gene (Senderovich and Halpern, 2012, 2013; Supplementary Table S1). Figueras et al. (2011) re-identified 23 A. caviae egg masses isolates from Senderovich et al. (2008) as A. aquariorum. This species was later re-identified as A. dhakensis (Beaz-Hidalgo et al., 2013).
Pathogenicity Potential of Aeromonas Isolates from Chironomids
Aeromonas possess a multifactorial virulence potential which enables them to colonize, invade, and infect different hosts. Among the virulence factors are structural components that act as adhesins (i.e., flagella, outer membrane proteins, etc.), secreted toxins (hydrolytic lipases, proteases, haemolysins, and enteorotoxins), interactions of different types of secretion systems (e.g., Type III secretion system [TTSS]), iron acquisition mechanisms, and quorum-sensing molecules which modulate expression of the virulence genes (Janda and Abbott, 2010; Senderovich et al., 2012).
Some of the chironomid Aeromonas isolates were scanned for the presence of virulence genes (Supplementary Table S2). Abundances of the following genes were studied: pla/lipH3/apl-1/lip (genes for phospholipase); ahpB (gene for elastase); alt, act, ast (cytotoxic and cytotonic enterotoxins genes); fla (gene for flagellin); ascF-ascG, and aexT (TTSS genes). All the studied A. dhakensis isolates were negative for the ast gene, 50% were positive for act, the majority of isolates were positive for ahpB, alt, ascF, pla/lipH3/apl-1/lip, and fla (93, 96.4, 85.7, 82, and 53.5%, respectively) and only one isolate was positive for aexT (Supplementary Table S2; Senderovich et al., 2008; Figueras et al., 2011; Shaked, 2011). A. sanarellii and A. taiwanensis (8 and 3 isolates respectively) that were scanned for virulence genes proved positive for ahpB and fla genes and negative for ast, act, and alt genes. The majority were positive for pla/lipH3/apl-1/lip genes. As for the TTSS genes, all A. sanarellii isolates were negative for the aexT gene and 25% were positive for the ascF gene. Two out of the three A. taiwanensis isolates were positive for both ascF and aexT genes (Beaz-Hidalgo et al., 2012; Supplementary Table S2). The results shown in Supplementary Table S2 demonstrate that Aeromonas isolates from chironomids present pathogenicity potential for humans and animals.
Antibiotic Resistance in Aeromonas Isolates from Chironomids
Resistance to β-lactamase antibiotics is a characteristic of Aeromonas species. The expression of chromosomally encoded β-lactamases is associated with resistance activity against a variety of β-lactam antibiotics like penicillin and cephalosporin (Janda and Abbott, 2010; Carvalho et al., 2012). Beaz-Hidalgo et al. (2012), who studied the antibiotic resistance of chironomid egg masses A. taiwanensis and A. sanarellii isolates, found that all strains were resistant to β-lactam antibiotics: Ampicillin (the first “broad spectrum” penicillin), Cefalotin (a first-generation cephalosporin), and Ertapenem. Most strains (∼75%) were also resistant to Amoxicillin–clavulanic acid (Amoxicillin with β-lactamase inhibitor). All strains showed sensitivity to 12 of the 19 tested antibiotics (Amikacin, Aztreonam, Cefepime, Cefotaxime, Ceftazidime, Ciprofloxacin, Gentamicin, Piperacillin–tozobactam, Tigecycline, Tobramycin, Trimethoprim–sulfamethoxazole, and Imipenem; Beaz-Hidalgo et al., 2012).
Aeromonas Prevalence in Chironomids
The abundance of Aeromonas per chironomid egg mass was monitored over a 6-month period (Shaked, 2011). Egg masses were collected from the Tivon waste stabilization pond (WSP) in northern Israel; abundance was studied by culturable and molecular methods. Aeromonas was isolated and identified from chironomid egg masses by means of a selective m-Aeromonas agar medium. Aeromonas colony-forming units (cfu) were counted per egg mass. Real-time PCR assay was used in parallel by amplifying a fraction of the 16S rRNA gene region with primers specific to the genus Aeromonas (Aer66f/Aer613r), according to Yu et al. (2008). The number of Aeromonas per egg mass obtained by the molecular method was usually ten times higher than the culturable cfu number. Aeromonas numbers stayed steady through almost the entire sampling period, demonstrating that Aeromonas is a stable resident in chironomids (Table 1). Furthermore, 454-pyrosequencing of the 16S rRNA gene showed that Aeromonas sp. comprised 1.6 and 3.3% of the egg masses and the larvae microbiota, respectively (Senderovich and Halpern, 2013).
Table 1.
Abundance of Aeromonas in chironomid egg masses collected from the Tivon WSP.
| Samplinga | Aeromonas (cfu/egg mass) culturable method | No. of Aeromonas/egg mass real-time PCR method |
|---|---|---|
| 1 (0) | 2.17 × 103 | 9.35 × l04 |
| 2 (12) | 2.47 × l03 | 1.30 × l04 |
| 3 (25) | 5.59 × l03 | 1.02 × l04 |
| 4 (40) | 3.61 × l03 | 1.62 × l04 |
| 5 (65) | ND | 1.71 × l04 |
| 8 (112) | ND | 1.06 × l04 |
| 9 (118) | 2.78 × l02 | 1.10 × l04 |
| 10 (122) | 2.92 × l03 | 2.20 × l04 |
| 11 (127) | 3.97 × l03 | 1.41 × l04 |
| 12 (133) | ND | 8.58 × l03 |
| 13 (140) | 4.38 × l03 | 1.97 × l04 |
| 15 (154) | 2.87 × l03 | 5.31 × l03 |
| 16 (160) | 2.33 × l02 | 5.40 × l03 |
| 17 (167) | 4.51 × l03 | 1.88 × 104 |
Chironomid egg masses were sampled between April and September, 2009. Numbers in the table are the average of three egg masses, three repeats for each (data from Shaked, 2011). Comparison of Aeromonas cfu/egg mass and estimated Aeromonas numbers were estimated by real-time PCR technology using the number of copies of the 16S–23S rRNA intergenic region, according to Kong et al. (1999). aNumbers in parentheses indicate day of sampling. Date of the first sampling was April 1, 2009. ND, Not detectable.
Egg Mass Degradation by Aeromonas sp.
The gelatinous matrix that surrounds the egg masses consists mainly of glycoprotein and chitin (Halpern et al., 2003; Laviad et al., 2016). Vibrio cholerae degrade chironomid egg masses and prevent the eggs from hatching (Broza and Halpern, 2001) by secreting Haemagglutinin/Protease (HAP; Halpern et al., 2003).
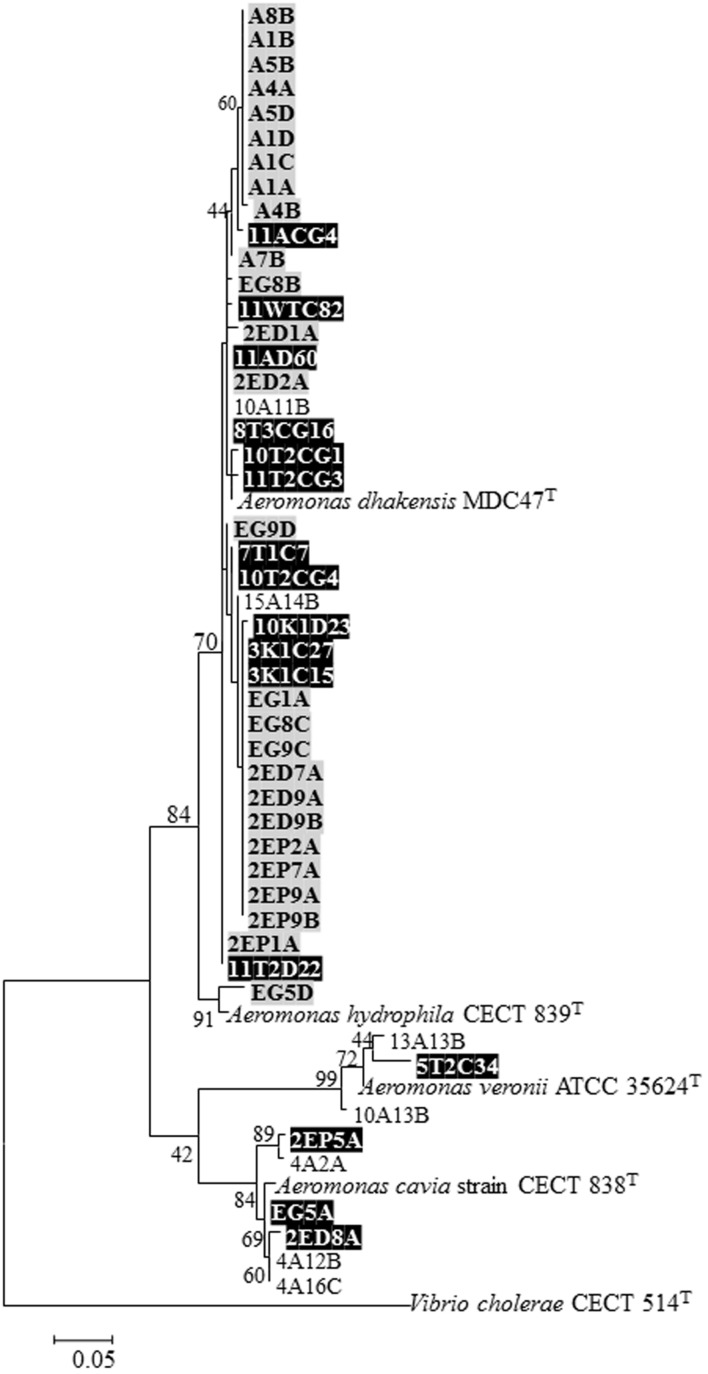
Senderovich et al. (2008) screened 1,100 bacterial isolates (other than V. cholerae) and found that 43 isolates were able to degrade the egg masses. Most of these isolates were identified as Aeromonas species. Laviad et al. (2016) examined the ability of 129 Aeromonas chironomid egg mass isolates to degrade the egg masses. Only 5% of them demonstrated this ability. The egg mass degrading factor of A. dhakensis was identified as a chitinase, and not a protease as was found for V. cholerae (Laviad et al., 2016). In fact, it then became clear that A. dhakensis secreted chitinase constitutively while most Aeromonas strains secrete chitinase inductively (Laviad et al., 2016). Aeromonas species that were found to degrade the egg masses constitutively are presented in Figure 1. Interestingly, most chironomid A. dhakensis isolates (>70%) were chitinase-constitutive, hence could degrade the egg masses without induction in the presence of chitin (Figueras et al., 2011; Laviad et al., 2016).
FIGURE 1.
Neighbor-joining phylogenetic tree derived from rpoD gene sequences representing all Aeromonas isolates that showed the ability to degrade the egg masses constitutively (meaning without chitin induction). The tree shows the relation of egg-mass degrading Aeromonas isolates to known Aeromonas species. The sequence alignments were performed with the CLUSTAL W program in MEGA 6 software. Numbers at nodes indicate bootstrap values (percentages of 1000 replicates). Bar 0.05 substitutions per nucleotide position. Isolates marked black were published in Senderovich et al. (2008) and Figueras et al. (2011). Isolates marked gray were isolated from egg masses and adults in India (Laviad, 2012). Unmarked isolates are from Laviad et al. (2016). All the type strains are from the NCBI data base.
The Ability of Aeromonas to Protect its Host from Toxic Metals
Chironomids are known to be tolerant of extreme environments, including contamination by heavy metals. Chironomid larvae can be bioaccumulators of mercury at high concentrations of the metal (Chetelat et al., 2008). Accumulation of various metals (lead, chromium, cadmium, copper, arsenic, iron, nickel, and manganese) by chironomids was found related to the presence of the metals in the sediment (Desrosiers et al., 2008). The mechanism that enables chironomids to survive in these extreme environments is not completely understood. Senderovich and Halpern (2012, 2013) demonstrated that 40 and 25% of the total bacterial communities in the egg masses and the larvae, respectively, were related to species possessing detoxification abilities. In addition, they screened isolates from egg masses and larvae for their ability to detoxify chromium, copper, lead, and zinc. They also used the Koch postulates to prove that endogenous bacteria can help chironomids to survive in environments with toxic chromium and lead. Some of these metal detoxification isolates belonged to the following Aeromonas species: A. caviae, A. dhakensis, A. hydrophila, A. veronii, and A. taiwanensis. They were found to be resistant to some or all the following metals Pb(NO3)2, K2CrO4, CuSO4, ZnCl2, Ni, and Co in concentrations between 1 and 10 mM (Senderovich and Halpern, 2012, 2013).
Aeromonas Dispersal
Aeromonas species cause disease in a variety of invertebrates and vertebrates including fish, birds, frogs, and domestic animals (Pérez-Valdespino et al., 2009). Eight of 21 disease outbreaks in ornamental fish were linked to Aeromonas species (Hettiarachchi and Cheong, 1994). Aeromonas can cause various fish diseases such as septicemia, ulcerative, hemorrhagic frunculosis, etc. These diseases result in financial loss in the aquaculture sector. The most important species that cause fish diseases are A. salmonicida and A. hydrophila (Beaz-Hidalgo and Figueras, 2012). Chironomid egg masses and larvae are part of some fish species’ diet. Fish species that consume chironomid egg masses and/or larvae may acquire Aeromonas from the insect.
In a survey conducted between 1982 and 1984, Shane et al. (1984) found 20 A. hydrophila isolates in 15 different bird species. A. hydrophila was also found to be the agent of high mortality in waterfowl (Korbel and Kösters, 1989).
Halpern et al. (2008) and Senderovich et al. (2010) suggested that fish that live on a diet that includes chironomids may be infected with V. cholerae (whose natural reservoirs are also chironomids). V. cholerae can be further transferred from the fish to waterbirds that consume fish; likewise Aeromonas may be transmitted from chironomids to waterbirds through fish. Furthermore, chironomids can survive the gut passage in waterbirds (endozoochory; Green and Sanches, 2006) and can even be attached directly to the bird’s feet and feathers (epizoochory; Frisch et al., 2007). Thus, Aeromonas can be transmitted via chironomids to fish and waterbirds.
Concluding Remarks
Chironomid egg masses and larvae inhabit different Aeromonas species. They are found in persistent numbers in the egg masses through all seasons of the year. Their abundance in the insects’ egg masses and larvae is 1.6 and 3.3% from the endogenous microbiota respectively. Aeromonas species degrade chironomid egg masses and can prevent eggs from hatching by secreting chitinase. However, most Aeromonas species produce this enzyme inductively, meaning that it is induced in the presence of chitin (which is one of the egg mass components). About 5% of Aeromonas chironomid isolates degrade the egg masses constitutively—and interestingly, most of these constitutive degrading isolates belong to the species A. dhakensis. Aeromonas species that inhabit chironomids are able to protect their host from toxic metals. So on one hand Aeromonas species may have a role in controlling chironomid populations (by degrading the egg masses), but on the other they may protect the insect from toxic heavy metals.
Aeromonas species identified from chironomids are human or fish pathogens and contain various virulent genes. As chironomids may infest drinking water supply systems, they may disseminate Aeromonas species to humans. It is also probable that Aeromonas species are transmitted from chironomids to fish and waterbirds, and thereby are globally dispersed.
Unresolved Questions and Future Research
-
1.
Most A. dhakensis isolates were chitinase constitutive. Does this ability to degrade chironomid egg masses give A. dhakensis a relative advantage over other Aeromonas species in proliferating in the egg mass niche? In a study in India (Laviad, 2012), this species proved the most abundant of Aeromonas species in egg masses and adults (53.4 and 66.7%, respectively).
-
2.
A whole genome sequence of A. dhakensis strain (Wu et al., 2012) revealed that a gene encoding a metalloprotease is located between two chitinase genes (Laviad et al., 2016). These three genes seem to fit in the same operon, therefore may be transcribed under the same control. In V. cholerae a metalloprotease was found responsible for egg mass degradation. Does this metalloprotease also have a role in egg mass degradation activity in Aeromonas? If so, does it act synergistically with chitinase? Moreover, does chitinase play a part in degradation of the egg masses by V. cholerae?
-
3.
Do Aeromonas species protect their host from toxicants other than heavy metals? For example, Aeromonas was found to degrade carbamyl (an insecticide; Hamada et al., 2015).
-
4.
What are the interactions between the different Aeromonas species that inhabit chironomid egg masses?
-
5.
Both Aeromonas species and V. cholerae are constantly present in the chironomid niche, usually at a rate of at least 5 to 1 in favor of the Aeromonas species (Senderovich and Halpern, 2013). What are the interactions between these two egg mass degrading bacteria species?
Author Contributions
SL and MH analyzed the data and wrote the paper and MH contributed the funding support.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported in part by a grant from the German Research Foundation (DFG, the Deutsche Forschungsgemeinschaft, GZ: HO 930/5-1; MH).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmicb.2016.00736
References
- Ali A. (1995). “Nuisance, economic impact and possibilities for control,” in The Chironomidae: The Biology and Ecology of Non-biting Midges, eds Armitage P. D., Cranston P. S., Pinder L. C. V. (London: Chapman and Hall; ), 339–345. [Google Scholar]
- Armitage P., Cranston P. S., Pinder L. C. V. (eds) (1995). The Chironomidae: The biology and Ecology of Non-biting Midges. London: Chapman and Hall. [Google Scholar]
- Beaz-Hidalgo R., Figueras M. J. (2012). “Molecular detection and characterization of furunculosis and other Aeromonas fish infections,” in Health and Environment in Aquaculture, eds Carvalho E. D., David G. S., Silva R. J. (Rijeka: InTech Open Access Publisher; ), 97–132. [Google Scholar]
- Beaz-Hidalgo R., Martinez-Murcia A., Figueras M. J. (2013). Reclassification of Aeromonas hydrophila subsp. dhakensis Huys et al. 2002 and Aeromonas aquariorum Martinez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst. Appl. Microbiol. 36 171–176. 10.1016/j.syapm.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Beaz-Hidalgo R., Shaked T., Laviad S., Halpern M., Figueras M. J. (2012). Chironomid egg masses harbour the clinical species Aeromonas taiwanensis and Aeromonas sanarellii. FEMS. Microbiol. Lett. 337 48–54. 10.1111/1574-6968.12003 [DOI] [PubMed] [Google Scholar]
- Broza M., Gancz H., Halpern M., Kashi Y. (2005). Adult non-biting midges: possible windborne carriers of Vibrio cholerae non-O1 non-O139. Environ. Microbiol. 7 576–585. 10.1111/j.1462-2920.2005.00745.x [DOI] [PubMed] [Google Scholar]
- Broza M., Halpern M. (2001). Chironomid egg masses and Vibrio cholerae. Nature 412:40 10.1038/35083691 [DOI] [PubMed] [Google Scholar]
- Broza M., Halpern M., Inbar M. (2000). Non-biting midges (Diptera; Chironomidae) in waste stabilization ponds: an intensifying nuisance in Israel. Water Sci. Technol. 42 71–74. [Google Scholar]
- Carvalho M. J., Martínez-Murcia A., Esteves A. C., Correia A., Saavedra M. J. (2012). Phylogenetic diversity, antibiotic resistance and virulence traits of Aeromonas spp. from untreated waters for human consumption. Int. J. Food Microbiol. 159 230–239. 10.1016/j.ijfoodmicro.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Chetelat J., Amyot M., Cloutier L., Poulain A. (2008). Metamorphosis in Chironomids, more than mercury supply, controls methylmercury transfer to fish in high Arctic lakes. Environ. Sci. Technol. 42 9110–9115. 10.1021/es801619h [DOI] [PubMed] [Google Scholar]
- Demoor F. C. (1992). Factors influencing the establishment of aquatic insect invaders. Trans. R. Soc. S. Afr. 48 141–158. 10.1080/00359199209520259 [DOI] [Google Scholar]
- Desrosiers M., Gagnon C., Masson S., Martel L., Babut M. P. (2008). Relationships among total recoverable and reactive metals and metalloids in St. Lawrence River sediment: bioaccumulation by chironomids and implications for ecological risk assessment. Sci. Total Environ. 389 101–114. 10.1016/j.scitotenv.2007.08.019 [DOI] [PubMed] [Google Scholar]
- Epler J. H. (2001). Identification manual for the larval Chironomidae (Diptera) of North and South Carolina. A guide to the taxonomy of the midges of the southeastern United States, including Florida. Special Publication SJ2001-SP13. Palatka, FL: North Carolina Department of Environment and Natural Resources. [Google Scholar]
- Failla A. J., Vasquez A. A., Fujimoto M., Ram J. L. (2015). The ecological, economic and public health impacts of nuisance chironomids and their potential as aquatic invaders. Aquat. Invasions 10 1–15. 10.3391/ai.2015.10.1.01 [DOI] [Google Scholar]
- Figueras M. J. (2005). Clinical relevance of Aeromonas. Rev. Med. Microbiol. 16 145–153. 10.1097/01.revmedmi.0000184410.98677.8a [DOI] [Google Scholar]
- Figueras M. J., Beaz-Hidalgo R. (2015). “Aeromonas infections in humans,” in Aeromonas, Chap. 4 ed. Graf J. (Norfolk: Caister Academic Press; ), 65–108. [Google Scholar]
- Figueras M. J., Beaz-Hidalgo R., Senderovich Y., Laviad S., Halpern M. (2011). Re-identification of Aeromonas isolates from chironomid egg masses as the potential pathogenic bacteria Aeromonas aquariorum. Environ. Microbiol. Rep. 3 239–244. 10.1111/j.1758-2229.2010.00216 [DOI] [PubMed] [Google Scholar]
- Frisch F., Green A. J., Figuerola J. (2007). High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquat. Sci. 69 568–574. 10.1007/s00027-007-0915-0 [DOI] [Google Scholar]
- Green A. J., Sanches M. I. (2006). Passive internal dispersal of insect larvae by migratory birds. Biol. Lett. 2 55–57. 10.1098/rsbl.2005.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. (2011). “Chironomids and Vibrio cholerae,” in Beneficial Microorganisms in Multicultural Life Forms, eds Rosenberg E., Gophna U. (Berlin: Springer; ), 43–56. [Google Scholar]
- Halpern M., Gancz H., Broza B., Kashi Y. (2003). Vibrio cholerae Hemagglutinin/Protease degrades chironomid egg masses. Appl. Environ. Microbiol. 69 4200–4204. 10.1128/AEM.69.7.4200-4204.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M., Landsberg O., Raats D., Rosenberg E. (2007). Culturable and VBNC Vibrio cholerae: interactions with chironomid egg masses and their bacterial population. Microb. Ecol. 53 285–293. 10.1007/s00248-006-9094-0 [DOI] [PubMed] [Google Scholar]
- Halpern M., Senderovich Y. (2015). Chironomid microbiome. Microb. Ecol. 70 1–8. 10.1007/s00248-014-0536-9 [DOI] [PubMed] [Google Scholar]
- Halpern M., Senderovich Y., Izhaki I. (2008). Waterfowl—the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog. 4:e1000173 10.1371/journal.ppat.1000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M., Matar A., Bashir A. (2015). Carbaryl degradation by bacterial isolates from a soil ecosystem of the Gaza Strip. Braz. J. Microbiol. 46 1087–1091. 10.1590/S1517-838246420150177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettiarachchi D. C., Cheong C. (1994). Some characteristics of Aeromonas hydrophila and Vibrio species isolated from bacterial disease outbreaks in ornamental fish culture in Sri Lanka. J. Natl. Sci. Counc. Sri Lanka 22 261–269. [Google Scholar]
- Igbinosa I. H., Igumbor E. U., Aghdasi F., Tom M., Okoh A. I. (2012). Emerging Aeromonas species infections and their significance in public health. Sci. World J. 2012 625023 10.1100/2012/625023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda J. M., Abbott S. L. (1998). Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin. Infect. Dis. 27 332–344. 10.1086/514652 [DOI] [PubMed] [Google Scholar]
- Janda J. M., Abbott S. L. (2010). The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23 35–73. 10.1128/CMR.00039-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong R. Y. C., Pelling A., So C. L., Wu R. S. S. (1999). Identification of oligonucleotide primers targeted at the 16S-23S rDNA intergenic spacers for genus- and species-specific detection of Aeromonas. Mar. Pollut. Bull. 38 802–808. 10.1016/S0025-326X(99)00044-2 [DOI] [Google Scholar]
- Korbel R., Kösters J. (1989). Epidemic deaths of wild birds after Aeromonas hydrophila infection. Tierarztl. Prax. 17 297–298. [PubMed] [Google Scholar]
- Latif-Eugenín F., Beaz-Hidalgo R., Figueras M. J. (2016). First record of the rare species Aeromonas schubertii from mussels: phenotypic and genetic reevaluation of the species and a review of the literature. Arch. Microbiol. 198 333–345. 10.1007/s00203-016-1189-5 [DOI] [PubMed] [Google Scholar]
- Laviad S. (2012). Monitoring Aeromonas Species in Chironomids and Characterization of the Secreted Egg Mass Degrading Factor by A. aquariorum. Haifa: University of Haifa (in Hebrew). [Google Scholar]
- Laviad S., Golan A., Shaked T., Vaizel-Ohayon D., Halpern M., Pick E. (2016). Aeromonas chitinase degrades chironomid egg masses. Environ. Microbiol. Rep. 8 30–37. 10.1111/1758-2229.12347 [DOI] [PubMed] [Google Scholar]
- Martin-Carnahan A., Joseph S. W. (2005). “Aeromonadales ord. nov,” in Bergey’s Manual of Systematic Bacteriology, 2nd Edn Vol. 2 ed. Garrity G. M. (New York, NY: Springer; ), 556–587. [Google Scholar]
- Oliver D. R. (1971). Life history of the Chironomidae. Annu. Rev. Entomol. 16 211–230. 10.1146/annurev.en.16.010171.001235 [DOI] [Google Scholar]
- Parker J. L., Shaw J. G. (2011). Aeromonas spp. clinical microbiology and disease. J. Infect. 62 109–118. 10.1016/j.jinf.2010.12.003 [DOI] [PubMed] [Google Scholar]
- Pérez-Valdespino A., Fernández-Rendón E., Curiel-Quesada E. (2009). Detection and characterization of class 1 integrons in Aeromonas spp. isolated from human diarrheic stool in Mexico. J. Basic Microb. 49 572–578. 10.1002/jobm.200900095 [DOI] [PubMed] [Google Scholar]
- Rouf M. A., Rigney M. M. (1993). Bacterial florae in larvae of the lake fly Chironomus plumosus. Appl. Environ. Microbiol. 59 1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderovich Y., Gershtein Y., Halewa E., Halpern M. (2008). Vibrio cholerae and Aeromonas: do they share a mutual host? ISME J. 2 276–283. 10.1038/ismej.2007.114 [DOI] [PubMed] [Google Scholar]
- Senderovich Y., Halpern M. (2012). Bacterial community composition associated with chironomid egg masses. J. Insect Sci. 12 149 10.1673/031.012.14901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderovich Y., Halpern M. (2013). The protective role of endogenous bacterial communities in chironomid egg masses and larvae. ISME J. 7 2147–2158. 10.1038/ismej.2013.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderovich Y., Izhaki I., Halpern M. (2010). Fish as reservoirs and vectors of Vibrio cholerae. PLoS ONE 5:e8607 10.1371/journal.pone.0008607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderovich Y., Ken-Dror S., Vainblat I., Blau D., Izhaki I., Halpern M. (2012). A molecular study on the prevalence and virulence potential of Aeromonas spp. recovered from patients suffering from diarrhea in Israel. PLoS ONE 7:e30070 10.1371/journal.pone.0030070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaked T. (2011). Evaluation and Assimilation of the Real-Time PCR Method for Detection of Vibrio cholerae in Drinking Water and for Monitoring V. cholerae and Aeromonas spp. Populations in Chironomid Egg Masses. Haifa: University of Haifa (in Hebrew). [Google Scholar]
- Shane S. M., Harrington K. S., Montrose M. S., Roebuck R. G. (1984). The occurrence of Aeromonas hydrophila in avian diagnostic submissions. Avian Dis. 28 804–807. 10.2307/1590253 [DOI] [PubMed] [Google Scholar]
- Wolfram G., Donabaum K., Schagerl M., Kowarc V. A. (1999). The zoobenthic community of shallow salt pans in Austria: preliminary results on phenology and the impact of salinity on benthic invertebrates. Hydrobiologia 40 193–202. 10.1023/A:1017014414599 [DOI] [Google Scholar]
- Wu C. J., Wang H. C., Chen C. S., Shu H. Y., Kao A. W., Chen P. L., et al. (2012). Genome sequence of a novel human pathogen, Aeromonas aquariorum. J. Bacteriol. 194 4114–4115. 10.1128/JB.00621-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. P., Farrell S. K., Robinson B., Chu K. H. (2008). Development and application of real-time PCR assay for quantifying total and aerolysin gene-containing Aeromonas in source, intermediate, and finished drinking water. Environ. Sci. Technol. 42 1191–1200. 10.1021/es071341g [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.