Abstract
Background
Given the interconnectivity of Brazil with the rest of the world, Zika virus (ZIKV) infections have the potential to spread rapidly around the world via viremic travellers. The extent of spread depends on the travel volume and the endemicity in the exporting country. In the absence of reliable surveillance data, we did mathematical modelling to estimate the number of importations of ZIKV from Brazil into Europe.
Design
We applied a previously developed mathematical model on importations of dengue to estimate the number of ZIKV importations into Europe, based on the travel volume, the probability of being infected at the time of travel, the population size of Brazil, and the estimated incidence of ZIKV infections.
Results
Our model estimated between 508 and 1,778 imported infections into Europe in 2016, of which we would expect between 116 and 355 symptomatic Zika infections; with the highest number of importations being into France, Portugal and Italy.
Conclusions
Our model identified high-risk countries in Europe. Such data can assist policymakers and public health professionals in estimating the extent of importations in order to prepare for the scale up of laboratory diagnostic assays and estimate the occurrence of Guillain–Barré Syndrome, potential sexual transmission, and infants with congenital ZIKV syndrome.
Keywords: Zika virus, travel, importations, Brazil, Europe
Introduction
In May 2015, an outbreak of Zika virus (ZIKV) infections was first reported in Brazil, and by December 2015, 500,000–1,500,000 ZIKV infections were estimated (1). By October 2015, increasing number of microcephaly cases and other neonatal malformations were thought to be associated with ZIKV infections (2). On 1 February 2016, the clusters of microcephaly and Guillain–Barré Syndrome (GBS) cases in likely association with ZIKV infections were declared a public health emergency of international concern (3). Given the interconnectivity of Brazil with the remainder of the world, ZIKV has the potential to spread rapidly around the world via viremic travellers (4). The extent of spread depends on the travel volume to destination countries and the endemicity in the exporting country (5–7). Because of the mild clinical manifestations of the disease in the vast majority of cases, ZIKV infections in individual travellers are unlikely to lead to cancellation of flights or disruption of holiday/business plans. Furthermore, 80% of all infections are thought to be asymptomatic. The biggest concern is the spread to areas where suitable mosquito vectors exist and importation could trigger further outbreaks. However, given that sexual transmission of ZIKV has been reported, the spread of ZIKV via viremic travellers to areas without the Aedes mosquitoes is equally of concern (8). Sexual transmission to non-travelling contacts in Europe could propagate ZIKV infections in Europe, resulting in a potential upsurge of GBS cases as a result of imported ZIKV infections and putting pregnant women at risk. Therefore it is important to estimate the potential number of travellers returning to Europe with ZIKV infections.
ZIKV infections remain underdiagnosed and underreported because of the non-specific and mild manifestations and lack of widely available diagnostic assays. Therefore, for the time being any estimates on the epidemiological burden remain crude estimates. We based our calculations on the published estimate of 500,000–1,500,000 infections (both symptomatic and asymptomatic) for the year 2015 in Brazil (1). Reliance on reported events of importation will only underestimate the true importation risk as most imported cases will not be detected and reported, unless the clinical manifestations are more severe. In the absence of reliable surveillance data, mathematical modelling is necessary to estimate the number of importations of ZIKV from Brazil into Europe.
Methods
We applied a previously developed mathematical model on exportations to estimate the number of ZIKV importations into Europe (9). This model takes into account the travel volume, the probability of being infected at the time of travel, the population size of Brazil, and the estimated incidence of ZIKV infections (estimated numbers over population size). The model was previously developed to estimate the risk of dengue acquisition in international travellers (10–12), and has also been applied to estimate polio virus importations (13).
The number of travellers departing from Brazilian airports on commercial flights to each of the European countries was obtained from the International Air Transport Association (IATA) for the year 2012. As we only had access to the year 2012 flight data, the travel pattern of outgoing flights in 2015 or 2016 was assumed to not have changed significantly.
We calculated the force of infection, λ(t) from the assumption that there had been 0.5 to 1.5 million ZIKV infections in Brazil. In addition, we assumed that the seasonal distribution of cases followed the same as for dengue, given that both viral infections share the same Aedes vectors, and initial observations have claimed that ZIKV seems to follow the path of dengue (14). As populations of Aedes aegypti and Aedes albopictus are climate sensitive and display a seasonal pattern in Brazil (15–17), ZIKV infections are likely to exhibit the same seasonal pattern as dengue in Brazil.
The steps for the mathematical models are detailed in the Supplement. In brief, we first fitted a continuous function to the time distribution of notified cases from which we estimate the force of infection λ(t). The product of the force of infection by the fraction of susceptible individuals is the number of reported cases.
The individual risk of acquiring the infection from the ZIKV-infected mosquitoes, Risk (t), is given by
| 1 |
where, again, λ(t) is the force of infection or incidence density rate; t1 is, in the case of travellers, the moment they arrive at the endemic area; and t2 is the moment they depart. Note that the concept of risk expressed in equation (1) means the risk for travellers that remain in the ZIKV endemic area for the period between t1 and t2. For locals, t2–t1 is the time interval considered for the risk calculation (e.g. the month-by-month risk calculation).
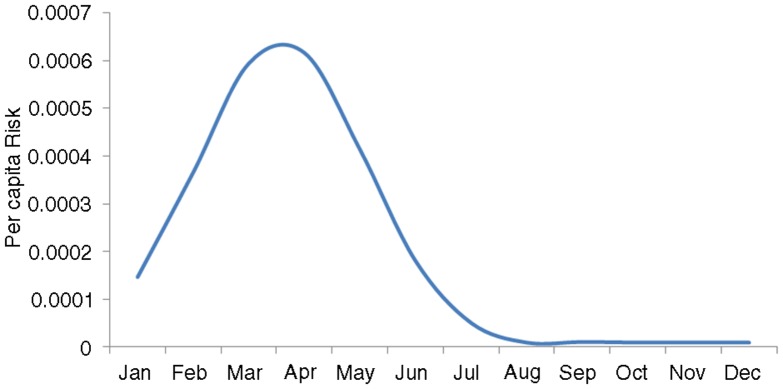
The risk varies with time. As Fig. 1 shows, this risk is highest in the months with the highest number of reported dengue cases (as a consequence of a higher density of infected mosquitoes), at its maximum by the month of April. This would also fit with the observation of the onset of excess microcephaly cases in October 2015 (6–9 months after the high season of January to April).
Fig. 1.
Calculated per capita risk (probability of being infected). Risk(t)=1–exp[−λ(t)t], where λ(t) is the force of infection.
As the function Risk (t) represents the individual risk of acquiring the infection, we can use it as the probability that one passenger flying from a Brazilian airport is infected with the ZIKV. By multiplying the individual probability of being infected by the number of passengers leaving Brazilian airports, we have the total number of expected infections that are flying to European countries.
Our model applies to individuals from Brazil travelling to Europe or travellers having visited Brazil and now returning to Europe.
Results
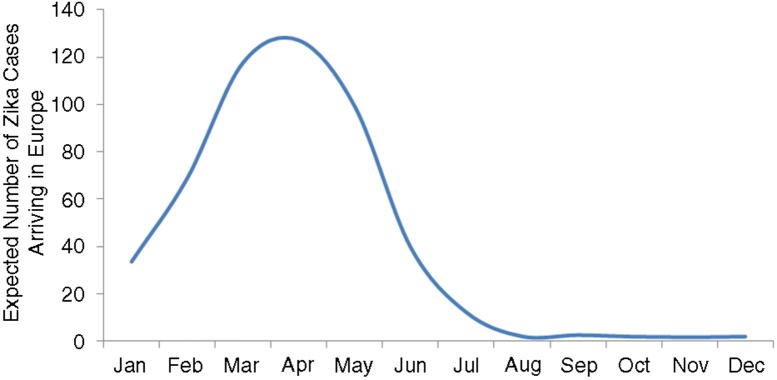
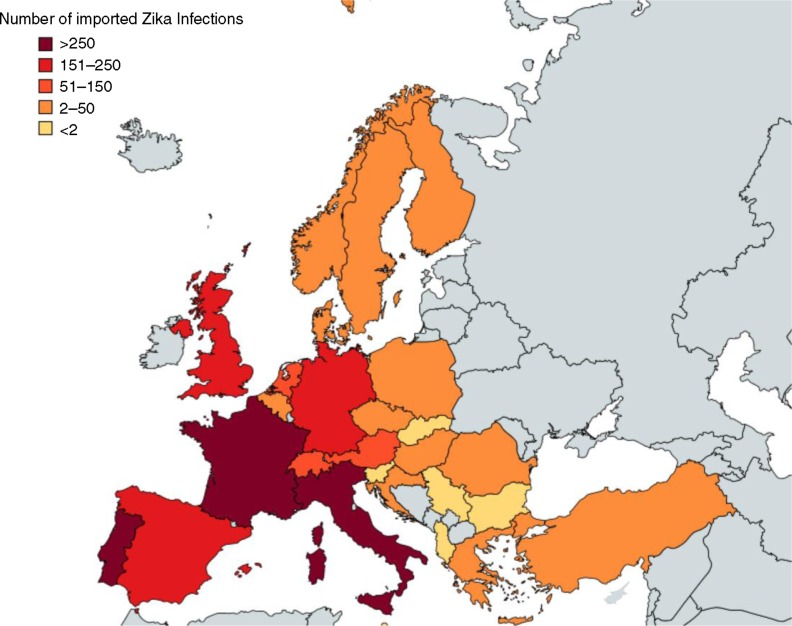
Figures 1 and 2 show the resulting curve for the individual risk of acquiring the infection and the expected number of ZIKA cases arriving in Europe by month, respectively. Table 1 and the Map show the results of the expected number of ZIKV cases exported to European countries from Brazil, based on an estimated lower bound of 500,000 and upper bound of 1.5 million ZIKV infections, respectively, assuming that these ZIKV infections exhibit the same seasonal pattern as dengue infections. In total, our models estimated between 508 and 1,778 imported cases, respectively, into all European countries, with the highest numbers being in France, Portugal, and Italy (Table 1 and Fig. 3). Of these, 80% would likely be asymptomatic; hence, we would expect between 116 and 355 symptomatic ZIKV infections.
Fig. 2.
Expected number of travellers with Zika virus infections arriving in Europe by month, in the year 2015.
Table 1.
Estimated imported ZIKV infections from Brazil to Europe based on the 1.5 million and 500,000 ZIKV infections scenarios in 2015
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual risk for each scenario | 500 K | 0.000146 | 0.000366 | 0.000594 | 0.000617 | 0.000414 | 0.00018 | 5.03E-05 | 9.06E-06 | 1.05E-05 | 9.06E-06 | 9.06E-06 | 9.06E-06 | |
| 1.5 G | 0.000511 | 0.00128 | 0.00208 | 0.00216 | 0.00145 | 0.000631 | 0.000176 | 3.17E-05 | 3.67E-05 | 3.17E-05 | 3.17E-05 | 3.17E-05 | ||
| Albania | Travellers | 9 | 12 | 8 | 9 | 10 | 67 | 1 | 28 | 9 | 13 | 21 | 0 | 187 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.5 G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Austria | Travellers | 1986 | 1840 | 2570 | 2203 | 24000 | 2304 | 2339 | 3235 | 3660 | 2067 | 2245 | 0 | 48449 |
| Expected cases for each scenario | 500 K | 0 | 1 | 2 | 1 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 |
| 1.5 G | 1 | 2 | 5 | 5 | 35 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 51 | |
| Belgium | Travellers | 2733 | 2543 | 2565 | 2647 | 2375 | 2599 | 3194 | 3513 | 2313 | 2296 | 1881 | 2449 | 31108 |
| Expected cases for each scenario | 500 K | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| 1.5 G | 1 | 3 | 5 | 6 | 3 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 22 | |
| Bulgaria | Travellers | 156 | 124 | 67 | 122 | 223 | 159 | 159 | 319 | 69 | 92 | 76 | 158 | 1724 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.5 G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Croatia | Travellers | 229 | 271 | 234 | 354 | 491 | 926 | 675 | 597 | 883 | 551 | 201 | 200 | 5612 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 1.5 G | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | |
| Czech Rep | Travellers | 561 | 901 | 1096 | 1092 | 1945 | 1663 | 1867 | 1805 | 2438 | 1470 | 1193 | 1026 | 17057 |
| Expected cases for each scenario | 500 K | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 1.5 G | 0 | 1 | 2 | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | |
| Denmark | Travellers | 3384 | 2151 | 1878 | 2015 | 2880 | 3274 | 4052 | 4421 | 1971 | 1506 | 1531 | 1725 | 30788 |
| Expected cases for each scenario | 500 K | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| 1.5 G | 2 | 3 | 4 | 4 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 20 | |
| Finland | Travellers | 1405 | 799 | 651 | 687 | 833 | 1026 | 843 | 660 | 374 | 537 | 661 | 0 | 8476 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 1.5 G | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | |
| France | Travellers | 29302 | 28118 | 33352 | 32796 | 35533 | 37932 | 40265 | 32572 | 37959 | 33476 | 27270 | 39248 | 407823 |
| Expected cases for each scenario | 500 K | 4 | 10 | 20 | 20 | 15 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 80 |
| 1.5 G | 15 | 36 | 69 | 71 | 52 | 24 | 7 | 1 | 1 | 1 | 1 | 1 | 279 | |
| Germany | Travellers | 21552 | 20305 | 22563 | 21564 | 21872 | 20226 | 21372 | 24965 | 29920 | 22872 | 21713 | 23703 | |
| Expected cases for each scenario | 500 K | 3 | 7 | 13 | 13 | 9 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1.5 G | 11 | 26 | 47 | 47 | 32 | 13 | 4 | 1 | 1 | 1 | 1 | 1 | ||
| Greece | Travellers | 712 | 590 | 1499 | 1141 | 1484 | 1805 | 2329 | 1836 | 2439 | 1386 | 646 | 690 | 16557 |
| Expected cases for each scenario | 500 K | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 1.5 G | 0 | 1 | 3 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | |
| Hungary | Travellers | 472 | 553 | 531 | 714 | 1190 | 1244 | 1437 | 1617 | 1282 | 788 | 452 | 465 | 10745 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 1.5 G | 0 | 1 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | |
| Italy | Travellers | 36580 | 30257 | 34235 | 33521 | 38120 | 32883 | 35912 | 33365 | 39478 | 34887 | 28373 | 30136 | 407747 |
| Expected cases for each scenario | 500 K | 5 | 11 | 20 | 21 | 16 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 83 |
| 1.5 G | 19 | 39 | 71 | 72 | 55 | 21 | 6 | 1 | 1 | 1 | 1 | 1 | 289 | |
| Malta | Travellers | 97 | 67 | 61 | 65 | 127 | 86 | 102 | 96 | 73 | 51 | 114 | 108 | 1047 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.5 G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Netherland | Travellers | 8055 | 6418 | 3727 | 7209 | 6997 | 7051 | 7669 | 7328 | 7006 | 7103 | 6391 | 6396 | 81350 |
| Expected cases for each scenario | 500 K | 1 | 2 | 2 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 15 |
| 1.5 G | 4 | 8 | 8 | 16 | 10 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 53 | |
| Norway | Travellers | 2423 | 1488 | 1409 | 2034 | 1728 | 1826 | 2479 | 1943 | 1677 | 1305 | 1354 | 2116 | 21782 |
| Expected cases for each scenario | 500 K | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 1.5 G | 1 | 2 | 3 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | |
| Poland | Travellers | 1016 | 760 | 884 | 1635 | 1344 | 1784 | 1828 | 1272 | 1018 | 1160 | 1025 | 971 | 14697 |
| Expected cases for each scenario | 500 K | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 1.5 G | 1 | 1 | 2 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | |
| Portugal | Travellers | 35079 | 29220 | 30250 | 34716 | 34795 | 35512 | 38984 | 34306 | 39754 | 30603 | 28926 | 33905 | 406050 |
| Expected cases for each scenario | 500 K | 5 | 11 | 18 | 21 | 14 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 80 |
| 1.5 G | 18 | 37 | 63 | 75 | 50 | 22 | 7 | 1 | 1 | 1 | 1 | 1 | 278 | |
| Romania | Travellers | 610 | 654 | 482 | 513 | 540 | 468 | 448 | 764 | 698 | 646 | 479 | 545 | 6847 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 1.5 G | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | |
| Serbia | Travellers | 124 | 69 | 83 | 64 | 119 | 115 | 74 | 140 | 57 | 140 | 74 | 75 | 1134 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.5 G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Slovakia | Travellers | 11 | 5 | 8 | 5 | 13 | 14 | 28 | 3 | 20 | 9 | 11 | 3 | 130 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.5 G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Slovenia | Travellers | 261 | 26 | 30 | 117 | 172 | 108 | 103 | 117 | 157 | 61 | 96 | 50 | 1298 |
| Expected cases for each scenario | 500 K | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.5 G | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Spain | Travellers | 23205 | 22487 | 20259 | 21545 | 24095 | 25562 | 28821 | 20111 | 24405 | 21842 | 16803 | 22603 | 271738 |
| Expected Cases for Each scenario | 500 K | 3 | 8 | 12 | 13 | 10 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 54 |
| 1.5 G | 12 | 29 | 42 | 47 | 35 | 16 | 5 | 1 | 1 | 1 | 1 | 1 | 189 | |
| Switzerland | Travellers | 25562 | 11487 | 10804 | 8521 | 8295 | 9701 | 9461 | 11820 | 7604 | 9249 | 8803 | 11189 | 132496 |
| Expected cases for each scenario | 500 K | 4 | 4 | 6 | 5 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| 1.5 G | 13 | 15 | 22 | 18 | 12 | 6 | 2 | 0 | 0 | 0 | 0 | 0 | 90 | |
| Sweden | Travellers | 3617 | 2458 | 2823 | 2082 | 1962 | 2310 | 2379 | 2015 | 1586 | 1428 | 1671 | 1576 | 25907 |
| Expected cases for each scenario | 500 K | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| 1.5 G | 2 | 3 | 6 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | |
| UK | Travellers | 28678 | 22484 | 23246 | 24708 | 24263 | 25302 | 30344 | 24356 | 26938 | 22458 | 22068 | 23657 | 298502 |
| Expected cases for each scenario | 500 K | 4 | 8 | 14 | 15 | 10 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 59 |
| 1.5 G | 15 | 29 | 48 | 53 | 35 | 16 | 5 | 1 | 1 | 1 | 1 | 1 | 206 | |
| Turkey | Travellers | 1717 | 2258 | 2760 | 3890 | 4097 | 4432 | 4321 | 3641 | 5417 | 4394 | 2816 | 3691 | 43434 |
| Expected cases for each scenario | 500 K | 0 | 1 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 8 |
| 1.5 G | 1 | 3 | 6 | 8 | 6 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 28 | |
| Total number of cases for each scenario | 500 K | 508 | ||||||||||||
| 1.5 G | 1778 |
Fig. 3.
Estimated importations of Zika virus infections via travellers from Brazil to Europe in the year 2015, based on a high estimate of 1,500,000 Zika virus infections in Brazil.
Conclusions
Our estimates are consistent with those reported by the European Centre for Disease Control (ECDC). As of 3 March 2016, ECDC had recorded 209 imported cases into 16 European countries, of which 81 were into France, and 32 into Spain (18). Geographical distribution of ZIKV has steadily broadened since the virus was first detected in Brazil in 2015. By March 2016, ZIKV transmission had been reported in 28 countries and territories (19); hence, the exportation risk will be even higher than we reported. However, we were not able to calculate such a risk for the other countries as incidence data for those countries have not yet been published. Given that Brazil so far has been the country most affected with the highest absolute numbers of estimated ZIKV infections, it is justified to focus our model on Brazil as exporting country only, until more data are available from other Latin American countries.
A limitation of our study is that the underlying assumption of our model is the equal distribution of cases throughout the country, and the equal probability of travelling throughout the Brazilian population. However, in early 2015, the geographic concentration of most cases were in Northeast Brazil – but by late 2015 and early 2016, the distribution was already much wider spread with all major cities in Brazil being affected (18, 20–22). Hence our modelled estimates of ZIKV exportations based on travel volume will be a more accurate reflection of the situation in 2016, assuming that the year 2016 will also see between 500,000 and 1,500,000 ZIKV infections.
According to the French Polynesian case control study on ZIKV-related GBS, one would expect 24 GBS cases out of 100,000 ZIKV infections (23). In other words, if these estimates hold true, one would need to have 5,000 imported ZIKV infections to see one case of ZIKV-associated GBS in returning travellers from ZIKV-affected countries. Given the current exportation numbers estimated to be no more than 1,800, the probability of a ZIKV-associated GBS case in Europe in 2015 or 2016 is relatively low. However, the number of ZIKV-affected countries within Latin America, the Caribbean, and beyond is rising, and hence the likelihood of substantial number of returning travellers presenting with GBS is will increase. The true risk of ZIKV-related infections that can lead to central nervous system malformations and microcephaly in pregnant women is currently unknown, especially for sexual transmission (24). However, potentially every single viremic male returning traveller could infect his pregnant or non-pregnant partner, especially in the first 2–4 weeks after ZIKV infection (25–27). Hence, the Centre for Disease Control and travel medicine providers have advised for precautions (abstinence or condoms) to be taken for men returning home from ZIKV-affected countries, particularly in the first few weeks after return (28, 29).
An additional cause of concern is the risk of ZIKV establishing itself in European regions where the presence of A. albopictus is endemic, in particular for Mediterranean countries recording increasingly hotter summers (30), although the ZIKA competence for A. albopictus is not fully known at this stage.
We identified high-risk countries in Europe, and policymakers and clinicians need to be aware of such data. Furthermore, our models can be applied by individual countries or by continents alike and used as an additional tool to estimate the risk of importation based on the main contributing factors such as travel volume and the evolving ZIKA endemicity in exporting countries. Our models help policymakers estimate the extent of importations in order to prepare for the scale up of laboratory diagnostic assays and estimate the occurrence of GBS, potential sexual transmission, and infants with congenital ZIKA syndrome.
Acknowledgements
This work was partially funded for AWS and EM through the European Commission 7th framework, under the Dengue Tools consortium (Grant No. 282589). The funders played no role in the study design, data collection, analysis, or preparation of the manuscript.
Authors' contributions
AWS and EM had the study idea; EM developed the mathematical models and calculated the data for the tables; KK provided the air passenger data; S-H K did the map and contributed to the tables; AWS wrote the paper. All authors contributed to the final paper
Conflict of interest and funding
KK is the founder of BlueDot, a social benefit corporation that models global infectious disease threats. All other authors have no conflict to declare.
Paper context
The Zika virus outbreak in Brazil has gripped the world's attention. We applied mathematical modelling to estimate the extent of Zika virus importations from Brazil to Europe for the year 2016. Our model estimated between 508 and 1,778 imported infections into Europe in 2016, with the highest number of importations being into France, Portugal, and Italy. Such data can assist policymakers to scale up preparatory measures in Europe.
References
- 1.European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus disease epidemic potential association with microcephaly and Guillain-Barreé syndrome. Stockholm: ECDC; 2016. [Google Scholar]
- 2.Zika virus: a new global threat for Editorial. Zika Virus: a new global threat for 2016. Lancet Infect Dis. 387:719–721. [Google Scholar]
- 3.Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387:719–21. doi: 10.1016/S0140-6736(16)00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogoch II, Brady OJ, Kraemer MU, German M, Creatore MI, Kulkarni M, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–6. doi: 10.1016/S0140-6736(16)00080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quam MB, Wilder-Smith A. Importation index of dengue to determine the most probable origin of importation. J Travel Med. 2015;22:72. doi: 10.1111/jtm.12177. [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Quam M, Sessions O, Rocklov J, Liu-Helmersson J, et al. The 2012 dengue outbreak in Madeira: exploring the origins. Euro Surveill. 2014;19:20718. doi: 10.2807/1560-7917.es2014.19.8.20718. [DOI] [PubMed] [Google Scholar]
- 7.Tatem AJ, Huang Z, Das A, Qi Q, Roth J, Qiu Y. Air travel and vector-borne disease movement. Parasitology. 2012;139:1816–30. doi: 10.1017/S0031182012000352. [DOI] [PubMed] [Google Scholar]
- 8.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission – continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–16. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- 9.Lopez LF, Amaku M, Coutinho FA, Quam M, Burattini M, Struchiner C, et al. Modeling importations and exportations of infectious diseases via travelers. Bull Math Biol. 2016;78:185–209. doi: 10.1007/s11538-015-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massad E, Wilder-Smith A, Ximenes R, Amaku M, Lopez LF, Bezerra FA, et al. Risk of symptomatic dengue for foreign visitors to the 2014 FIFA World Cup in Brazil. Mem Inst Oswaldo Cruz. 2014;109:394–7. doi: 10.1590/0074-0276140133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massad E, Rocklov J, Wilder-Smith A. Dengue infections in non-immune travellers to Thailand. Epidemiol Infect. 2013;141:412–17. doi: 10.1017/S0950268812000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massad E, Wilder-Smith A. Risk estimates of dengue in travelers to dengue endemic areas using mathematical models. J Travel Med. 2009;16:191–3. doi: 10.1111/j.1708-8305.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilder-Smith A, Leong WY, Lopez LF, Amaku M, Quam M, Khan K, et al. Potential for international spread of wild poliovirus via travelers. BMC Med. 2015;13:133. doi: 10.1186/s12916-015-0363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–4. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 15.Lowe R, Coelho CA, Barcellos C, Carvalho MS, Catão Rde C, Coelho GE, et al. Evaluating probabilistic dengue risk forecasts from a prototype early warning system for Brazil. Elife. 2016;5 doi: 10.7554/eLife.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavalcanti LP, Coelho IC, Vilar DC, Holanda SG, Escossia KN, Souza-Santos R. Clinical and epidemiological characterization of dengue hemorrhagic fever cases in northeastern, Brazil. Rev Soc Bras Med Trop. 2010;43:355–8. doi: 10.1590/s0037-86822010000400003. [DOI] [PubMed] [Google Scholar]
- 17.Siqueira JB, Jr., Martelli CM, Coelho GE, Simplicio AC, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981–2002. Emerg Infect Dis. 2005;11:48–53. doi: 10.3201/eid1101.031091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ECDC. Epidemiological situation of the Zika outbreak: European Centre for Disease Prevention and Control. Stockholm: ECDC; 2016. http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/Pages/epidemiological-updates.aspx. [Google Scholar]
- 19.WHO. World Health Organization. Geneva: WHO; 2016. Zika virus situation report. www.who.int/emergencies/zika-virus/situation-report/en/ [Google Scholar]
- 20.Garcia E, Yactayo S, Nishino K, Millot V, Perea W, Briand S. Zika virus infection: global update on epidemiology and potentially associated clinical manifestations. Weekly Epidemiol Rec. 2016;91:73–81. [PubMed] [Google Scholar]
- 21.Heukelbach J, Alencar CH, Kelvin AA, De Oliveira WK, Pamplona de Goes Cavalcanti L. Zika virus outbreak in Brazil. J Infect Dev Ctries. 2016;10:116–20. doi: 10.3855/jidc.8217. [DOI] [PubMed] [Google Scholar]
- 22.Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas – region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:55–8. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 23.Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasil P, Pereira JP, Jr., Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro RM, et al. Zika virus infection in pregnant women in Rio de Janeiro – Preliminary report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. http://www.nejm.org/doi/full/10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non–vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–2. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–61. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Fiorentini C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- 28.Vouga M, Musso D, Van Mieghem T, Baud D. CDC guidelines for pregnant women during the Zika virus outbreak. Lancet. 2016;387:843–4. doi: 10.1016/S0140-6736(16)00383-4. [DOI] [PubMed] [Google Scholar]
- 29.Goorhuis A, von Eije KJ, Douma RA, Rijnberg N, van Vugt M, Stijnis C, et al. Zika virus and the risk of imported infection in returned travelers: implications for clinical care. Travel Med Infect Dis. 2016;14:13–15. doi: 10.1016/j.tmaid.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Helmerson JL, Quam M, Wilder-Smith A, Stenlund H, Ebi K, Massad E, et al. Climate change and Aedes vectors: 21st century projections for dengue transmission in Europe. EBiomedicine. 2016 doi: 10.1016/j.ebiom.2016.03.046. doi: http://www.sciencedirect.com/science/article/pii/S2352396416301335. [DOI] [PMC free article] [PubMed] [Google Scholar]