Abstract
Background
Rattus norvegicus (brown rat) and Rattus rattus (black rat) are known carriers of bacteria, viruses, and parasites of zoonotic and veterinary importance. Moreover, rats may play a role in the transmission of muscle larvae of the zoonotic nematode Trichinella spiralis to farm animals. We aimed to study the intestinal and intramuscular helminths in wild rats from three different environments to assess the relevance of rats as carrier of zoonotic parasites for public health.
Materials and methods
Wild brown rats (117 individuals) and black rats (44 individuals) were captured at farms, in suburban and in rural environments in the Netherlands. Intestinal helminths were isolated and identified morphologically. Artificial digestion was used to isolate muscle larvae.
Results and discussion
Morphological analysis of rat intestinal contents yielded six nematode species (Syphacia muris, Heterakis spumosa, Aonchotheca murissylvatici, Trichuris muris, Nippostrongylus brasiliensis, and Strongyloides sp.), three cestode species (Hymenolepis diminuta, H. nana and Hymenolepis (=Rodentolepis) fraterna), and four trematode species (Plagiorchis muris, Plagiorchis proximus, Echinostoma chloropodis, and Notocotylus imbricatus).
Black rats at farms displayed the lowest intestinal helminth species variation (six species) and carried overall on average 0.93 species simultaneously. In comparison, brown rats at farms carried seven helminth species and 1.91 species simultaneously. Brown rats from suburban environments displayed the highest species variation (11 species) at 1.82 simultaneous helminth species. Absence of trematodes from rats at farms may suggest limited exchange of rats between farms and surrounding wet rural environments. We report four species of veterinary (Syphacia muris) or zoonotic relevance (Hymenolepis diminuta, Hymenolepis nana and Plagiorchis muris). We did not find Trichinella muscle larvae, consistent with long-term prevalence in Dutch wild rats.
Keywords: brown rat, Rattus norvegicus, black rat, Rattus rattus, intestinal helminths, Hymenolepis, Syphacia, Trichinella
Rattus norvegicus (brown rat) and Rattus rattus (black rat) are known carriers of bacteria, viruses, and parasites of zoonotic and veterinary importance (1, 2). In the Netherlands, wild rats from livestock farms have been studied to elucidate their role in spreading of Coxiella burnetii (3) and methicillin-resistant Staphylococcus aureus (4). Some decades earlier, Trichinella prevalence was monitored in Dutch wild rats, mice, voles, badgers, martens, and muskrats (5–7). However, a comprehensive recent parasitological survey in wild rats has not been published in the Netherlands. We were particularly interested in zoonotic parasites to assess the relevance of rats for veterinary public health.
Wild brown rats (117 individuals) and black rats (44 individuals) were captured at different farm types, in urban environments and in rural environments in the Netherlands. For each rat, intestinal helminths and presence of muscle larvae (Trichinella spp.) were analysed. Parasitological data were analysed statistically to evaluate helminth species variation and simultaneous helminth infections in black rats and brown rats from different environments.
The aim of the present study was to identify the intestinal and intramuscular helminths in wild rats, to evaluate parasite species distribution in three different environments, and to assess the relevance of rats carrying zoonotic parasites for public health.
Materials and methods
Wild rats
The highest concentrations of animal husbandry in the Netherlands are found in the two southern provinces Noord-Brabant (Brabant for short) and Limburg; therefore, most rats were captured in 43 six-digit postal codes of this part of the country. Brown rats were captured at 12 farms and black rats at 14 farms. All animals were captured based on convenience sampling by a pest control agency using live traps (Killgerm, Inc., Turnhout, Belgium) baited with food.
Location type
Six-digit postal code areas were checked using Google Maps in the Internet with satellite view, and used in combination with other database entries, to divide trapping locations into three categories: farms (pigs, cattle, goats, and poultry), rural environments (locations outside town boundaries, including ditch banks, agricultural use), and suburban environments (outskirts of rural towns and small cities, including ditch banks).
Age
The age of brown rats was estimated using weight categories adapted from Davis (8). Characteristic indicators for sexual maturity of rats are scrotal testis for males and vaginal perforation and pregnancy for females. We defined weight categories for brown rat males: juvenile (<100 g), young adult (101–200 g), and adult (>200 g), and for females: juvenile (<100 g), young adult (101–175 g), and adult (>175 g).
For black rats, the age categories were defined as follows: for males: juvenile (<100 g), young adult (100–150 g), adult (>150 g), and for females: juvenile (<90 g), young adult (90–130 g), and adult (>130 g).
Season
We used meteorological boundaries for season: spring (March–May), summer (June–August), autumn (September–November), and winter (December–February).
Samples for analysis
After direct transportation to the National Institute for Public Health and the Environment (RIVM, Bilthoven, the Netherlands), rat species, sex and weight, location and location type of capture were recorded. The rats were anaesthetised with isoflurane and were euthanised by cardiac puncture.
Upon death, the intestinal tract including the stomach was taken from each rat for parasitological examination. Diaphragm and muscles of both hind legs of 132 wild rats were separated for Trichinella analysis by artificial digestion. All biological materials were stored either at +4°C for immediate evaluation or at −20°C until further use. The Institute's Animal Ethical Committee has approved all experiments using wild rodents prior to the study (RIVM/DEC permits 200900164 and 201200208).
Isolation of muscle helminths
The diaphragm and muscle tissue of both hind legs were separated from each rat and analysed by artificial digestion according to EU regulation 2075/2005 (9). Since the diaphragm is a predilection site for Trichinella in most mammals, Trichinella ML can be expected in this muscle, even at low levels of infection. For analysis, 3.6–11.8 g muscle tissue per rat (depending on age and size of the animal) was pooled up to a maximum of 115 g in total. Subsequently, the pooled muscle tissue was minced and digested in aqueous pepsin-HCl for 30–60 min at 46°C. After two successive sedimentation steps, the resulting suspension was transferred to petri dishes and examined microscopically for isolated larvae. Muscle samples of 20 animals that had been frozen before were analysed using a validated sequential sieving method to detect Trichinella larvae (10).
Isolation of intestinal helminths
Intestines of the brown rats and black rats were separated individually into small (duodenum up to ileum) and large intestine (including caecum and colon) and their contents were firmly squeezed into 40 ml of PBS using forceps, and subsequently were homogenised by vigorous shaking. The resulting suspension was poured over a 63 µm mesh size sieve and the retentate was extensively washed with tap water to remove debris. The washed retentate was flushed into a 14 cm diameter petri dish and the suspension was meticulously examined to collect all intestinal parasites under a dissection microscope. We were confident that the squeezing technique was efficient, judging the large amount of villus epithelium in the isolates. The stomach was opened to screen for parasites macroscopically and microscopically. Isolated parasites were counted, sexed, and identified morphologically. Subsequently, the parasites were stored in labelled, screw-capped glass tubes containing 70% ethanol until further use.
Generalised linear model analysis of helminth data
Prevalence was determined for all isolated helminth species, stratified by rat host species and location type where rats were captured. Numbers of simultaneously occurring helminth species infesting each individual rat were analysed in relation to the categorical predictors: ‘host species (brown/black)’, ‘host sex (male/female)’, ‘host age (juvenile/young adult/adult)’, ‘season (spring/summer/autumn/winter)’, ‘year’, ‘province (Brabant/Limburg/Friesland/Others)’, ‘location type (farm/rural/suburban)’. In addition, the square root of the cumulative helminth abundance was included as numerical, as indicator for exposure. In formula: nHelminth species ~ host species+host sex+host age+season+(year+province+location type)2+√(cumulative abundance). The square sign indicates interaction between the factors year, province, and location type. The square root transformation of variable ‘cumulative abundance’ is based on visual inspection of the scatterplot for helminth species against cumulative abundance (Fig. 5b). Since the number of species is a non-negative count (n≥0), a Poisson regression with logarithmic link function was applied. Subsequently, the optimal model fit was evaluated by backward and forward model selection in ‘R’ to select the model with the lowest AIC-value (Akaike's Information Criterion).
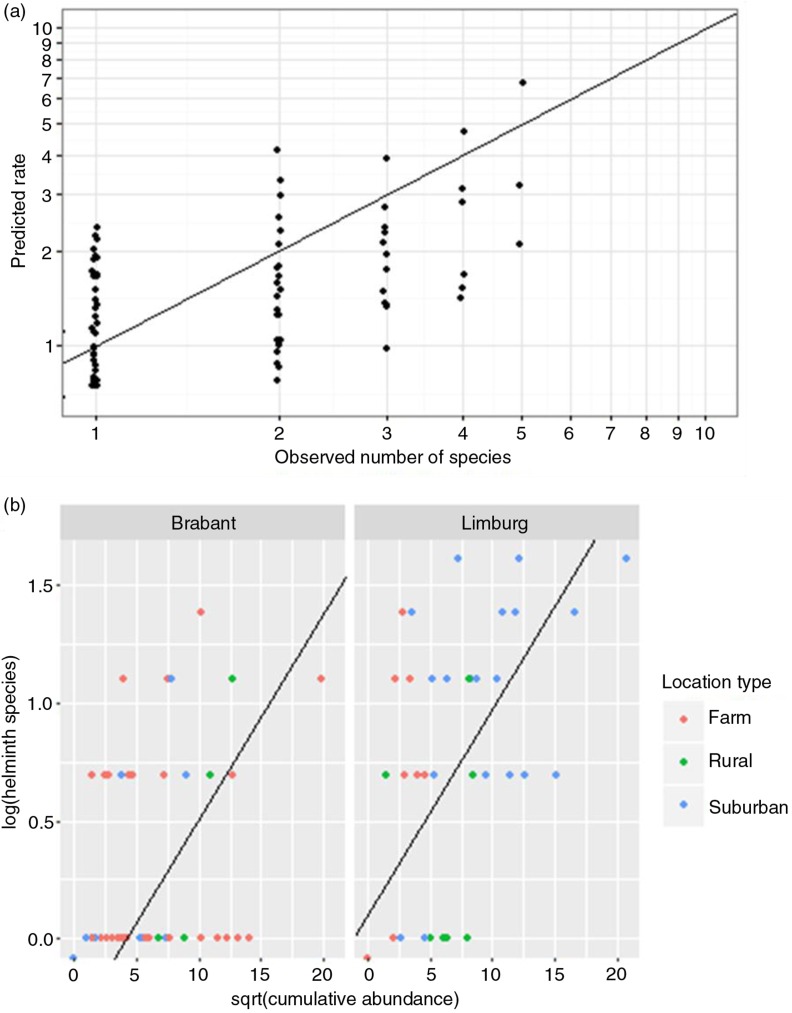
Fig. 5.
The number of simultaneous helminth species correlates with cumulative helminth abundance. (a) The numbers of helminth species predicted by the generalised linear model (Predicted rate) correlate with observed number of helminth species (p(Chi)2=0.9678). Note that the model predicts fractions, whereas helminths are counted in discrete numbers. Data points are presented jittered, to minimise overlap. (b) Simultaneous helminth infections correlated positively with cumulative abundance. The dots show observed data; the line shows model fit to the data, which differs between provinces. Note that in the province of Limburg, data are segregated by location type. Since the model compares √(cumulative abundance) with log transformed number of helminths, count value 0 is placed below the horizontal axis.
Results
Wild rats
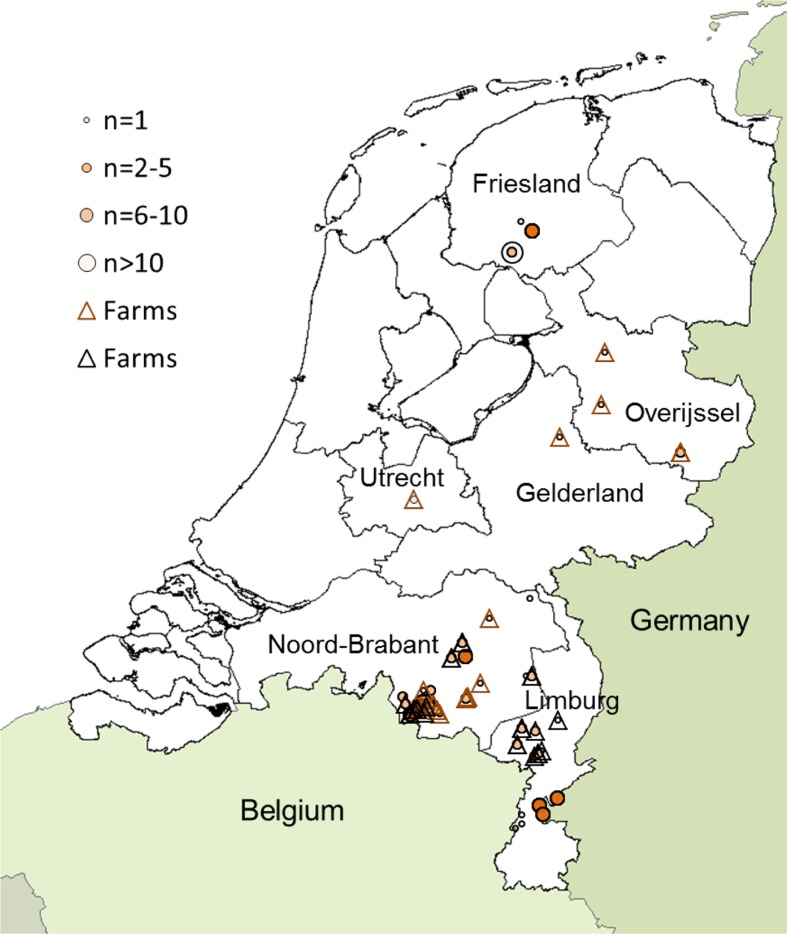
From December 2009 until December 2010, 108 wild brown rats and black rats were captured in the provinces Limburg and Noord-Brabant at farms, in rural and in urban environments. Five brown rats were captured in February 2010 from a goat farm in the province of Overijssel, two brown rats were captured in March and April in the province of Gelderland (of which one from a goat farm). One brown rat was captured in April from a goat farm in the province of Utrecht. In November and December 2011, an additional 25 brown rats were captured in suburban and rural environments in the province of Limburg. Finally, 23 brown rats were captured in rural environments in Friesland between May 2012 and August 2013 (Fig. 1).
Fig. 1.
Geographical origin of captured wild rats. Dots indicate number of rats that were captured. Triangles indicate farms where brown (brown triangle) and black rats (black triangle) were captured.
In the two southern provinces of Brabant and Limburg, 133 rats were captured, divided over 43 six-digit postal codes. Table 1 provides further geographical and temporal details.
Table 1.
Geographical, temporal, and biological data of captured rats
| Location of capture | Time of capture | Brown rat | Black rat | Overall | |||
|---|---|---|---|---|---|---|---|
| Province | n =161 | Year | n =161 | Sex | n =117 | n =44 | n =161 |
| Friesland | 23 (14.3) | 2009 | 9 (5.6) | Female | 54 (46.2) | 20 (45.5) | 74 (46.0) |
| Overijssel | 4 (2.5) | 2010 | 98 (60.9) | Male | 59 (50.4) | 23 (52.3) | 82 (50.9) |
| Gelderland | 2 (1.2) | 2011 | 30 (18.6) | Unknown | 4 (3.4) | 1 (2.3) | 5 (3.1) |
| Utrecht | 1 (0.6) | 2012 | 6 (3.7) | Age | |||
| Noord-Brabant | 82 (50.9) | 2013 | 18 (11.2) | Adult | 70 (59.8) | 17 (38.6) | 87 (54.0) |
| Limburg | 49 (30.4) | Young adult | 34 (29.1) | 7 (15.9) | 41 (25.5) | ||
| Season | Juvenile | 8 (6.8) | 16 (36.4) | 24 (14.9) | |||
| Location type | Spring | 25 (15.5) | Unknown | 5 (4.3) | 4 (9.1) | 9 (5.6) | |
| Farm | 71 (44.1) | Summer | 51 (31.7) | Weight | |||
| Rural | 50 (31.1) | Autumn | 39 (24.2) | Female | 206±78 g | 132±60 g | – |
| Suburban | 40 (24.8) | Winter | 46 (28.6) | Male | 244±95 g | 119±68 g | – |
Counts are given per variable, with prevalence (%) between brackets.
Rat species
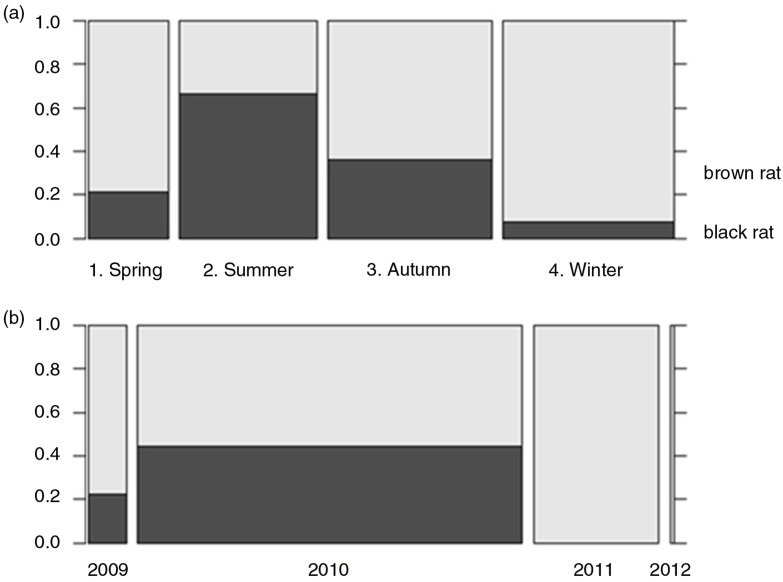
In total, 117 brown rats and 44 black rats were captured. Black rats were captured only in Noord-Brabant and Limburg, which is the prime distribution area for this species in the Netherlands (Fig. 1). In the provinces of Brabant and Limburg, the majority of black rats (93.2%) were caught on farms, whereas only 25.8% of brown rats were captured from farms, consistent with habitat preferences of the two species. Proportionally, most black rats were captured in summer and least in winter (Fig. 2a); the majority of black rats was captured in 2010 (Fig. 2b). Overall, slightly more males (50.9%) than females (46%) were captured and the majority of rats were adults (54.0%) or young adults (25.5%); brown rat males (244±95 g) weighed more than females (206±78 g), whereas black rat females (132±60 g) were heavier than males (119±68 g) (Table 1).
Fig. 2.
Distribution of rat species from the southern provinces per season (a) and per year (b). The width of the blocks is proportional to the number of rats per factor indicated underneath. The percentage of rat species is indicated at right vertical axis.
Helminth species
Morphological analysis of intestinal contents of the 117 brown rats and 44 black rats, yielded six nematode species (Syphacia muris, Heterakis spumosa, Aonchotheca murissylvatici, Trichuris muris, Nippostrongylus brasiliensis, and Strongyloides sp.), which were identified morphologically using Anderson et al. (11).
Three cestode species (Hymenolepis diminuta, H. nana, and Hymenolepis (=Rodentolepis) fraterna) were identified morphologically and confirmed by molecular analyses (data not shown).
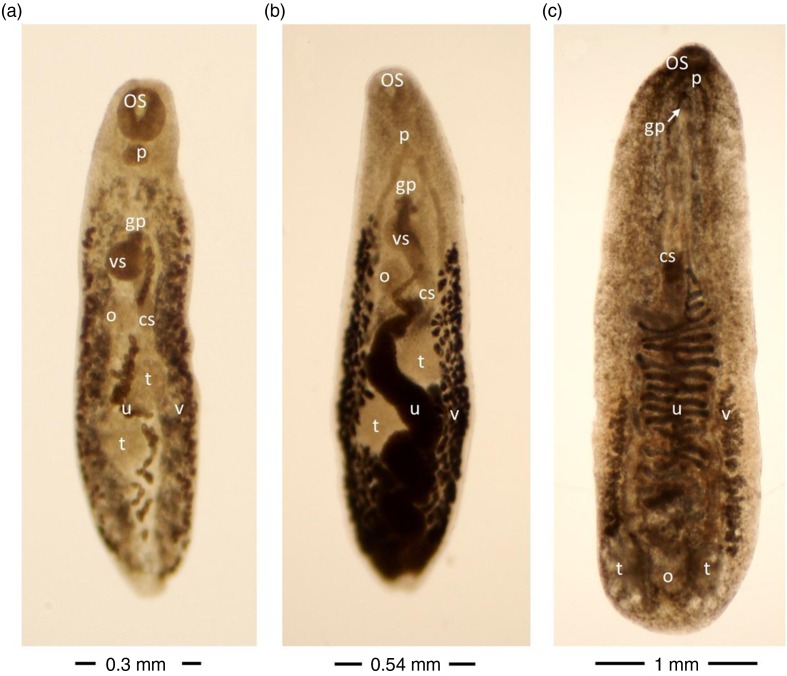
Finally, four trematode species (Plagiorchis muris Fig. 3a), Plagiorchis proximus (Fig. 3b), Echinostoma sp. and Notocotylus imbricatus (Fig. 3c) (Tables 2–4) were identified using morphological examination. Seven specimens of juvenile Echinostoma sp. were isolated, displaying a conspicuous collar bearing 47 spines around the oral sucker. The arrangement of the collar spines was consistent with that of a group of seven Echinostoma spp. (E. chloropodis, E. corvi, E. hystricosum, E. necopinum, E. rousseloti, E. sarcinum and E. travassosi) (Fig. 3.9 in (12)).
Fig. 3.
Plagiorchis muris (a), Plagiorchis proximus (b) and Notocotylus imbricatus (c) isolated from wild rats. Unstained trematodes (a and b ventral view, c dorsal view) as seen with enhanced contrast microscopy. cs cirrus sac, gp genital pore, o ovarium, os oral sucker, p pharynx, t testis, u uterus, vs ventral sucker, v vitellaria.
Table 2.
Prevalence of helminth species in rats, differentiated by location type and host (brown rat and black rat)
| Farm (n=71) | Rural environment (n=50) | Suburban environment (n=40) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown (n=30) | Black (n=41) | Brown (n=49) | Black | Brown (n=38) | Black | ||||||||||||
| Helminth species | % | n | maxa | % | n | maxa | % | n | maxa | n=1 | maxa | % | n | maxa | n=2 | maxa | |
| 1 | Aonchotheca murissylvatici | 13.3 | 4 | 160 | 9.8 | 4 | 10 | 16.3 | 8 | 48 | 1 | 4 | 34.2 | 13 | 74 | 1 | 4 |
| 2 | Syphacia muris (v) | 36.7 | 11 | 1,700 | 56.1 | 23 | 134 | 28.6 | 14 | 159 | 0 | – | 21.1 | 8 | 200 | 0 | 0 |
| 3 | Heterakis spumosa | 53.3 | 16 | 173 | 4.9 | 2 | 1 | 28.6 | 14 | 70 | 0 | – | 50.0 | 19 | 7 | 0 | 0 |
| 4 | Strongyloides sp. | 13.3 | 4 | 198 | 0.0 | 0 | 0 | 6.1 | 3 | 2 | 0 | – | 5.3 | 2 | 0 | 0 | 0 |
| 5 | Nippostrongylus brasiliensis | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 14.3 | 7L,F | 63 | 0 | – | 36.8 | 14L | 0 | 0 | 0 |
| 6 | Trichuris muris | 3.3 | 1 | 0 | 2.4 | 1 | 1 | 0.0 | 0 | 0 | 0 | – | 2.6 | 1 | 0 | 0 | 0 |
| 7 | Hymenolepis diminuta (z) | 50.0 | 15 | 31 | 2.4 | 1 | 1 | 10.2 | 5 | 3 | 0 | – | 10.5 | 4 | 7 | 0 | 0 |
| 8 | Rodentolepis fraterna | 10.0 | 3 | 1 | 19.5 | 8 | 37 | 12.2 | 6 | 58 | 0 | – | 5.3 | 2 | 0 | 0 | 0 |
| 9 | Hymenolepis nana (z) | 3.3 | 1 | 1 | 0.0 | 0 | 0 | 4.1 | 2 | 1 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| Prevalence hymenolepidid speciesb | 63.3 | 19 | – | 22.0 | 9 | – | 26.5 | 13 | – | 0 | – | 15.8 | 6 | – | 0 | – | |
| 10 | Echinostoma sp. | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 5.3 | 2L | 0 | 0 | 0 |
| 11 | Notocotylus imbricatus | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 10.5 | 4L | 0 | 0 | 0 |
| 12 | Plagiorchis proximus | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 7.9 | 3L | 0 | 0 | 0 |
| 13 | Plagiorchis muris | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 4.1 | 2F | 1 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| Prevalence trematode speciesb | 0.0 | 0 | – | 0.0 | 0 | – | 0.0 | 0 | – | 0 | – | 23.7 | 9 | – | 0 | 0 | |
| Total number of species | 8 | 6 | 9 | 1 | 11 | 1 | |||||||||||
| Average #helminths species/rat | 1.91 | 0.93 | 1.00 | 1 | 1.82 | 0.50 | |||||||||||
Parasites of veterinary (v) or zoonotic (z) importance are indicated
only in rats captured in Limburg and in one rat captured in Friesland
only in rats captured in Friesland
only in rats captured in Limburg
highest recorded abundance
Total number of rats carrying hymenolepidid or trematode species.
Table 4.
Prevalence of helminth species captured in the province of Limburg, differentiated to location type and host (brown rat and black rat)
| Limburg | Farm (n=13) | Rural environment (n=9) | Suburban environment (n=24) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown (n=7) | Black (n=6) | Brown (n=9) | Black | Brown (n=24) | Black | ||||||||||||
| Helminth species | % | n | maxa | % | n | maxa | % | n | maxa | n=0 | maxa | % | n | maxa | n=0 | maxa | |
| 1 | Aonchotheca murissylvatici | 14.3 | 1 | 1 | 33.3 | 2 | 10 | 11.1 | 1 | 6 | 0 | 0 | 37.5 | 9 | 76 | 0 | 0 |
| 2 | Syphacia muris (v) | 14.3 | 1 | 1 | 50.0 | 3 | 47 | 0.0 | 0 | 0 | 0 | 0 | 12.5 | 3 | 40 | 0 | 0 |
| 3 | Heterakis spumosa | 71.4 | 5 | 33 | 0.0 | 0 | 0 | 33.3 | 3 | 70 | 0 | 0 | 70.8 | 17 | 116 | 0 | 0 |
| 4 | Strongyloides sp. | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 11.1 | 1 | 1 | 0 | 0 | 8.3 | 2 | 63 | 0 | 0 |
| 5 | Nippostrongylus brasiliensis | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 66.7 | 6 | 63 | 0 | 0 | 58.3 | 14 | 289 | 0 | 0 |
| 6 | Trichuris muris | 14.3 | 1 | 1 | 16.7 | 1 | 1 | 0.0 | 0 | 0 | 0 | 0 | 4.2 | 1 | 2 | 0 | 0 |
| 7 | Hymenolepis diminuta (z) | 71.4 | 5 | 31 | 16.7 | 1 | 1 | 11.1 | 1 | 1 | 0 | 0 | 4.2 | 1 | 6 | 0 | 0 |
| 8 | Rodentolepis fraterna | 0.0 | 0 | 0 | 33.3 | 2 | 2 | 0.0 | 0 | 0 | 0 | 0 | 8.3 | 2 | 1 | 0 | 0 |
| 9 | Hymenolepis nana (z) | 14.3 | 1 | 1 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 |
| Prevalence hymenolepidid speciesb | 85.7 | 6 | – | 50.0 | 3 | – | 11.1 | 1 | – | 0 | – | 12.5 | 3 | – | 0 | – | |
| 10 | Echinostoma sp. | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 8.3 | 2 | 4 | 0 | 0 |
| 11 | Notocotylus imbricatus | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 16.7 | 4 | 8 | 0 | 0 |
| 12 | Plagiorchis proximus | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 12.5 | 3 | 5 | 0 | 0 |
| 13 | Plagiorchis muris | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 | 0.0 | 0 | 0 | 0 | 0 |
| Prevalence trematode speciesb | 0.0 | 0 | – | 0.0 | 0 | – | 0.0 | 0 | – | 0 | 0 | 37.5 | 9 | – | 0 | 0 | |
| Total number of species | 6 | 5 | 5 | 0 | 11 | 0 | |||||||||||
| Average #helminths species/rat | 2.12 | 1.00 | 1.33 | – | 2.42 | 0.50 | |||||||||||
highest recorded abundance
Total number of rats carrying hymenolepidid or trematode species.
Table 3.
Prevalence of helminth species captured in the province of Brabant, differentiated to location type and host (brown rat and black rat)
| Brabant | Farm (n=55) | Rural environment (n=15) | Suburban environment (n=17) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown (n=19) | Black (n=36) | Brown (n=15) | Black | Brown (n=15) | Black | ||||||||||||
| Helminth species | % | n | maxa | % | n | maxa | % | n | maxa | n=0 | maxa | % | n | maxa | n=2 | maxa | |
| 1 | Aonchotheca murissylvatici | 15.8 | 3 | 160 | 5.6 | 2 | 4 | 16.7 | 3 | 48 | 0 | 26.7 | 4 | 74 | 1 | 4 | |
| 2 | Syphacia muris (v) | 52.6 | 10 | 1,700 | 55.6 | 20 | 134 | 22.2 | 4 | 159 | 0 | – | 33.3 | 5 | 200 | 0 | 0 |
| 3 | Heterakis spumosa | 52.6 | 10 | 173 | 5.6 | 2 | 1 | 22.2 | 4 | 1 | 0 | – | 13.3 | 2 | 7 | 0 | 0 |
| 4 | Strongyloides sp. | 21.1 | 4 | 198 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 5 | Nippostrongylus brasiliensis | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 6 | Trichuris muris | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 7 | Hymenolepis diminuta (z) | 36.8 | 7 | 4 | 0.0 | 0 | 0 | 11.1 | 2 | 1 | 0 | – | 20.0 | 3 | 7 | 0 | 0 |
| 8 | Rodentolepis fraterna | 15.8 | 3 | 1 | 16.7 | 6 | 37 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 9 | Hymenolepis nana (z) | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| Prevalence hymenolepidid speciesb | 52.6 | 10 | – | 16.7 | 6 | – | 11.1 | 2 | – | 0 | – | 20.0 | 3 | – | 0 | – | |
| 10 | Echinostoma sp. | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 11 | Notocotylus imbricatus | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 12 | Plagiorchis proximus | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| 13 | Plagiorchis muris | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 0 | 0 | 0 | – | 0.0 | 0 | 0 | 0 | 0 |
| Prevalence trematode speciesb | 0.0 | 0 | – | 0.0 | 0 | – | 0.0 | 0 | – | 0 | – | 60.0 | 9 | – | 0 | 0 | |
| Total number of species | 6 | 4 | 4 | 0 | 4 | 1 | |||||||||||
| Average #helminths species/rat | 1.92 | 0.83 | 0.93 | – | 0.87 | 0.50 | |||||||||||
highest recorded abundance
Total number of rats carrying hymenolepidid or trematode species.
Notocotylus spp. were morphologically identified using relevant literature (13–15). N. imbricatus is clearly distinct from other Notocotylus species parasitising rodents in Europe (16, 17).
Artificial digestion of the diaphragm and both hind legs of each wild rat revealed no Trichinella (or any other) muscle larvae, and thus the best estimate for the prevalence is zero (binomial 95% CI: 0–0.03%).
Helminth variation per rat species
Overall, six helminth species were isolated from black rats, which occurred almost exclusively on farms, whereas in total 13 different species were isolated from brown rats originating from all three environments, and without exception, maximum species abundance was much higher in brown rats than in black rats (Table 1). However, these data differed between provinces. Overall, brown rats from farms carried eight helminth species, rats from rural environment carried nine species, and rats from suburban environment carried 11 different helminth species, to which rats from the province of Limburg contributed substantially (due to three species of trematodes and N. brasiliensis). Black rats from farms carried six helminth species (Tables (2–4)).
Helminth prevalence in relation to location type
N. brasiliensis was absent from both black and brown rats at farms, whereas it was prevalent in brown rats from rural and suburban environment in Limburg (Table 2), and it was demonstrated in one rat captured in rural environment in Friesland (Table 2).
Syphacia muris prevalence was high (>21%) in rats from all environments, at comparably high abundance (300–500), with an exceptional peak of 1,700 S. muris in one brown rat from a farm. H. spumosa was highly prevalent in brown rats from all environments (28.8–53.3%), but far less in black rats (4.9%).
Overall, Hymenolepis spp. prevalence in rats from farms (39.4%, n=71) was significantly higher than in rats from other environments combined (21.1%, n=90) (p=0.0001, Fisher's exact test). Hymenolepis species prevalence in brown rats captured at farms was 63.3%, which is significantly higher than in both rural (26.5%, p=0.0088, Fisher's exact test) and urban environments (15.8%, p<0.0001, Fisher's exact test). In the province of Brabant, Hymenolepis spp. prevalence in rats from farms (52.6%, n=55) was higher than in other environments combined (15.6%, n=32), although not significant (p=0.1987). In the province of Limburg, Hymenolepis spp. prevalence in rats from farms (69.2%, n=13) was significantly higher than in other environments combined (12.1%, n=33) (p=0.0003, Fisher's exact test). Data for the other provinces were too few to draw conclusions.
Three different trematode species, which depend on aquatic intermediate hosts to complete their lifecycle, were mostly demonstrated in rats from suburban environments, which were captured at or nearby banks of small streams in Limburg. Additionally, a fourth trematode species was demonstrated in one out of four rats captured in Friesland. Trematode species were absent from both black and brown rats at farms.
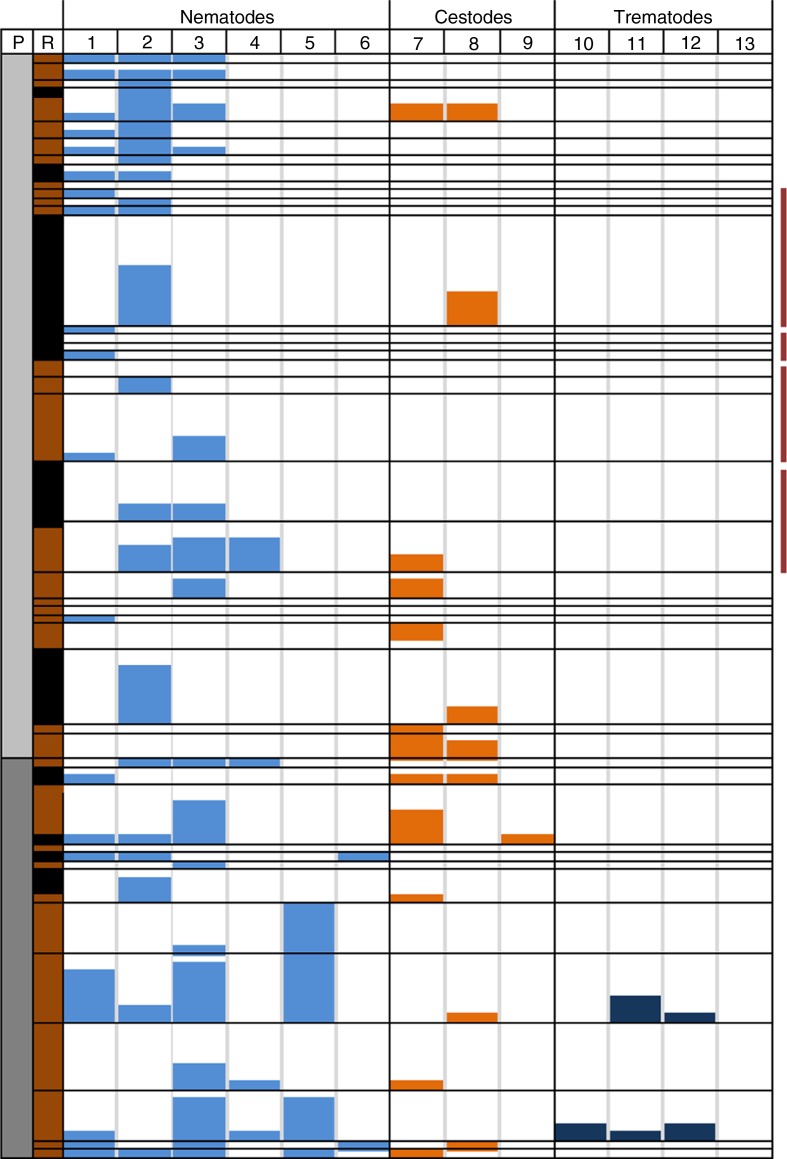
Helminth profile per six-digit postal code area
Figure 4 shows that the absence/presence pattern of helminth species is structured for most six-digit postal codes areas, especially the ones where more than one rat was captured. Season may influence species variation, for example, most of the pig farms were sampled in summer (except three rats captured in April/May and two rats captured in December), whereas cattle farms were sampled in autumn (September–October, except two rats captured in December). Postal codes exhibiting the highest helminth variation as a result of trematode prevalence were all sampled in November–December in Limburg.
Fig. 4.
Helminth profile per six-digit postal code area (anonymised) from the provinces of Brabant and Limburg, where both brown and black rats were captured. Forty-three six-digit postal code areas were sampled and arranged top-down in increasing order. ‘P’ represents province where rats were captured: grey for Brabant and dark grey for Limburg. ‘R’ represents rat species: black for black rats, brown for brown rats. Helminth species numbers in the second row correspond with species given in Table II. Neighbouring postal code areas are indicated by vertical purple bars at the right.
Each row represents a single postal code and row height indicates the number of rats captured. Helminth prevalence is depicted as histogram per postal code area and bar height corresponds to number of helminth positive rats; lowest rows represent one rat.
Generalised linear model analysis of simultaneous helminth infections
Since most brown rats and all black rats were captured in the two southern provinces of Brabant and Limburg, we excluded the other provinces from the final generalised linear model (GLM) analysis, although the same results were obtained with and without rats from the other provinces. Factors ‘season’ and ‘location’ correlated strongly (Table 5), as did ‘location type’ and ‘province’, and therefore could not be tested separately in the model. We chose ‘location’ rather than ‘season’, in view of ecological expectations. The best model fit, using the GLM approach, was obtained with the function nHelminth species ~ √(cumulative abundance)+province (AIC=321.17).
Table 5.
Numbers of captured rats per season, stratified for location type and year
| Location type | Year | ||||||
|---|---|---|---|---|---|---|---|
| Season | Farm | Rural | Suburban | 2009 | 2010 | 2011 | 2012 |
| Autumn | 25 | 6 | 4 | 0 | 28 | 7 | 0 |
| Spring | 7 | 4 | 4 | 0 | 14 | 0 | 1 |
| Summer | 21 | 7 | 3 | 0 | 31 | 0 | 0 |
| Winter | 7 | 6 | 28 | 8 | 10 | 23 | 0 |
The baseline (intercept) was rats in Brabant. In comparison with this baseline, factors ‘√(cumulative abundance)’ (p=7.16×10−11) and ‘Limburg’ (p=2.54×10−3) contributed significantly to the model. The model predicts on average 1.6 times more helminth species in rats in Limburg than in Brabant, at equal cumulative abundance. The model fit was excellent (p(Chi)2=0.9678) (Fig. 5a) and the normal Q–Q plot (depicting residuals between observed and predicted values) only deviated at both extremities (results not shown). According to the model, √(cumulative abundance) predicts 1.09 times increase of simultaneously carried helminth species, which means that, for example, a four-fold increase in cumulative abundance, results in (=2) times 1.09 (=2.18) more helminth species (Fig. 5b). Due to non-randomised sampling, factors ‘province’ and ‘location type’ correlated significantly (p=1.33×10−4, Fisher's exact test). Although factor ‘province’ is maintained in the best model, the determining factor might actually be location type.
Factors ‘year’ (p>0.4720), ‘location type’ (p>0.1633), ‘host’ (p=0.1709), ‘age’ (p>0.6351), ‘sex’ (p=0.9587), and ‘season’ (p>0.4926) did not contribute significantly.
Discussion
The aim of the present paper was to study the intestinal and intramuscular helminths in wild rats and to evaluate parasite species distribution in three different environments, to assess the relevance of rats carrying zoonotic parasites for public health.
Rats were captured as a convenience sample, which caused that neither the effect of season and location type nor the effect of province and host could be separated in our GLM analysis. For example, most black rats (38 out of 44) were captured in Brabant, most of which (36 out of 44) at farms. Moreover, factors ‘province’ and ‘location type’ correlated significantly (p=1.33×10−4, Fisher's exact test). Although random sampling would have been a preferable method and probably could have prevented these complications, it is far more difficult and expensive and therefore less realistic to perform.
The vast majority of black rats was captured only at farms in the southern provinces of Brabant and Limburg, most in summer (22) and autumn (14) and least in winter (3) and spring (5), following typical farm population dynamic, where rat numbers start to increase in spring and start decreasing in autumn to reach the lowest number in winter (18, 19).
Heterakis spumosa, a typically soil-transmitted helminth species, was demonstrated in only 4.9% of black rats, whereas the prevalence in Brown rats at farms was more than 10 times higher (52.6%). The extent to which black and brown rats have soil contact, may explain differences in the number of helminth species seen in our study. In a study on black rats in an old-growth forest in California, only 5% of the nests of black rats were underground burrows (20). In contrast, brown rats may use buildings during winter, but breed in fields and moist environments during summer (21), sometimes occupying two burrows, for example, one in a ditch bank and a second 875 m away in a barn (22), or use underground burrows at the premises of the farm (23).
In the present study, N. brasiliensis was prevalent in brown rats from rural and suburban ditch banks in Limburg. Also trematode species, which depend on aquatic intermediate hosts to complete their life cycle, were demonstrated in rats captured along ditch banks in Limburg. The probability of exchange of these specific rats carrying trematodes and N. brasiliensis between rural environments and farms was considered minute, since these rats were captured some 20 km separated from the nearest farm included in this study. Interestingly, despite presence of ditches and occasionally small streams in rural environments surrounding farms, neither N. brasiliensis nor trematode species were seen in farm rats. This may suggest that rats from farms in our study do not forage in surrounding rural areas, which may explain absence of N. brasiliensis from rats at farms. Alternatively, intermediate hosts relevant for trematodes recorded in our study (lymnaeid snails) may be absent from ditches in the direct surroundings of farms, rendering rats at farms not prone to trematode infections, but that does not explain absence of N. brasiliensis from farm rats.
In the Netherlands, six-digit postal codes (four numbers plus two letters) refer to one street, which may be up to 1,500 m in length in rural areas, or sections of one street between junctions, which may be 50–100 m in length in suburban areas, where additionally even- and odd numbered sides have different letter-codes. The differing helminth profiles observed in (neighbouring) six-digit postal code areas may suggest that postal codes coincided with separate rat territories, although this hypothesis should be put to the test by conducting more research, controlling temporal and geographic variation. However, literature seems to provide supportive data for this hypothesis. The maximum distance that brown rats travel (primarily within their territories) within farm boundaries and margins of fields surrounding a farm was 26–131 m in two studies using radio tracking of rats (24, 25). Also black rats display comparable limited motility, with maximum distances of 97–179 m in their natural woodland environment (20, 26). At farms, with a constant supply of food, motility of black rats (<56 m) is even less than that of brown rats (25).
We found two representatives of the genus Plagiorchis in the intestines of brown rats. P. muris, which was previously recorded in wood mouse (Apodemus sylvaticus) (27), and has been described as zoonotic species on a few occasions in Asia, however without any clinical signs (28, 29); final hosts are mice (Mus musculus and Microtus spp.), brown rat (R. norvegicus), pigeon (Columba livia), and humans (30). The second was P. proximus, which was previously recorded from the duodenum of the American muskrat (Fiber zibethicus) (31).
Notocotylus imbricatus, which is a cosmopolitan digenean species, was found in five individual brown rats. This trematode usually parasitises in ducks (Anatidae and Spatula clypeata) (13, 14), although it was also found in an Australian water rat (Hydromys chrysogaster) (15). N. imbricatus is clearly distinct from other Notocotylus species parasitising rodents in Europe (16, 17). To our best knowledge, none of these trematodes was previously recorded in rats from the Netherlands.
Syphacia muris, which is primarily a rat-to-rat transmitted species was most prevalent in black rats at farms, although not significantly different from brown rats at farms (p=0.1494, Fisher's exact test), but significantly higher compared with brown rats from rural (p=0.0103, Fisher's exact test) and suburban environment (p=0.0025, Fisher's exact test). S. muris gravid females actively leave their host's intestine and deposit the eggs around their host's anus, after which (auto)infection and transmission between rats takes place through grooming and social behaviour. S. muris is of veterinary importance in relation to experimental rodent colonies (32). In a survey in 2006, respondents from 35 US research institutions reported S. muris in 17% of mouse colonies and 42% of rat colonies (33). Although S. muris is considered non-pathogenic, S. muris infections have been shown to exert significant negative effect on digestibility of nutrients and consequently growth of laboratory rats, which could interfere with experiment outcomes (34).
In our study, Hymenolepis spp. prevalence in rats from farms (39.4%, n=71) was significantly higher than in rats from other environments combined (21%, n=90, p=0.0001, Fisher's exact test). Hymenolepis spp. prevalence in brown rats captured at farms was 63.3%, which is significantly higher than in both rural (26.5%, p=0.0020, Fisher's exact test) and suburban environments (15.8%, p<0.0001, Fisher's exact test). In a study in Brown rats captured at nine farms in the United Kingdom, the prevalence of H. diminuta was significantly lower (21.8%, n=243) than in brown rats at 12 farms in the present study (63.3%, n=30, p=0.0002, Fisher's exact test). The prevalence of H. nana in that study was 11% (n=243), which is higher than the prevalence (3.3%) in the present study (35). R. fraterna, the third hymenolepidid species recorded in our study (prevalence 10%), was not demonstrated in British rats (35). Both H. nana and H. diminuta are zoonotic cestode helminths, although the latter is infrequently seen in humans. H. diminuta is transmitted to humans by ingestion of Tribolium confusum (flour beetle, intermediate host) with infested cereals, or by the faecal–oral route. H. nana is transmitted through faecal–oral contact (eggs), or by accidental ingestion of intermediate hosts harbouring cysticercoids. Hymenolepis spp. infections in humans are mostly asymptomatic, although weakness, headache, abdominal pain, and diarrhoea may occur (36).
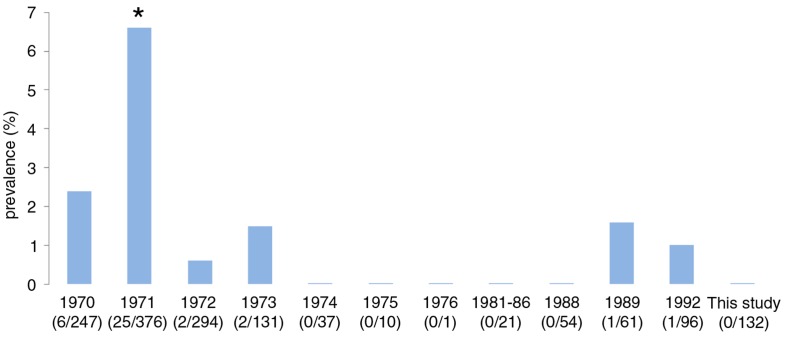
Artificial digestion of the diaphragm and both hind legs of each wild rat revealed no Trichinella muscle larvae (binomial 95% CI: 0–0.03%). This does not differ significantly from historic Trichinella spp. prevalence reported in the years 1970, (2.4%, p=8.5×10−2, Fisher's exact test) and 1972–1992, (0–1.6%, p>0.20, Fisher's exact test) (6). Only in the year 1971, a significantly higher Trichinella prevalence was recorded (6.6%, p=4.3×10−3, Fisher's exact test) (Fig. 6).
Fig. 6.
Historic Trichinella prevalence in wild rats in The Netherlands during the period 1970–1992 (adapted from (6) and (7)) and results from this study. The observed prevalence did not differ significantly from year to year, except for the year 1971 (Fisher's exact test). *Indicates significance.
In conclusion, we demonstrate considerable differences in helminth species variation between environments. The lowest intestinal helminth species variation (six species) and simultaneous parasite infections (0.93) were recorded in black rats. In comparison, brown rats at farms displayed the lowest species variation (eight helminth species), but at the same time the highest number of simultaneous infections (1.91), compared with brown rats from other environments. Absence of trematodes from rats at farms may suggest limited exchange of rats between farms and the surrounding rural environments. We report four species of veterinary (Syphacia muris) or zoonotic relevance (Hymenolepis diminuta, Hymenolepis nana and Plagiorchis muris). We did not find Trichinella muscle larvae, consistent with long-term prevalence in Dutch wild rats.
Acknowledgements
The study was supported by the Netherlands Food and Consumer Product Safety Authority (NVWA), the Netherlands. The authors are grateful to Johan van Rooij and Van Eck Bedrijfshygiene BV (Eindhoven) for collecting the rats and to the Dutch farmers for participating in the project. We thank Chantal Reusken, Ankje de Vries, Marjolein van der Plaats, Marieke Opsteegh, and Miriam Maas for their help with dissection of the rats.
Conflict of interest and funding
The authors declare that they do not have conflicts of interest.
References
- 1.Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol. 2009;35:221–70. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- 2.Reperant LA, Hegglin D, Tanner I, Fischer C, Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. 2009;136:329–37. doi: 10.1017/S0031182008005428. [DOI] [PubMed] [Google Scholar]
- 3.Reusken C, van der Plaats R, Opsteegh M, de Bruin A, Swart A. Coxiella burnetii (Q fever) in Rattus norvegicus and Rattus rattus at livestock farms and urban locations in the Netherlands; could Rattus spp. represent reservoirs for (re)introduction? Prev Vet Med. 2011;101:124–30. doi: 10.1016/j.prevetmed.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 4.van de Giessen AW, van Santen-Verheuvel MG, Hengeveld PD, Bosch T, Broens EM, Reusken CB. Occurrence of methicillin-resistant Staphylococcus aureus in rats living on pig farms. Prev Vet Med. 2009;91:270–3. doi: 10.1016/j.prevetmed.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Kampelmacher EH, Ruitenberg EJ, Berkvens J. [Studies on the occurrence of Trichinella spiralis in man in the Netherlands] Ned Tijdschr Geneeskd. 1966;110:1927–9. [PubMed] [Google Scholar]
- 6.van Knapen F, Frachimont J, Kremers A. Onderzoek naar het voorkomen van Trichinella spiralis bij een aantal in het wild levende knaagdieren (Rodentia: Muridae) en marterachtigen (Carnivora: Mustelidae) in Nederland. [in Dutch with abstract in English]. Rapport nr. 188802003. Bilthoven, The Netherlands: National Institute for Public Health and the Environment (RIVM); 1993. [Google Scholar]
- 7.Franchimont JH, van knapen F, Cremers AFT. Trichinellosis in wildlife in the Netherlands. Proc ICT. 1993;8:561–4. [Google Scholar]
- 8.Davis D. The weight of wild brown rats at sexual maturity. J Mammal. 1949;30:125–30. [PubMed] [Google Scholar]
- 9.European-Commission. Regulation EC No 2075/2005 of the European Parliament and of the Council of 5 December 2005 laying down specific rules on official controls for Trichinella in meat. Off J EC. 2005;L338:60–82. [Google Scholar]
- 10.Franssen F, Deksne G, Esíte Z, Havelaar A, Swart A, van der Giessen J. Trend analysis of Trichinella in a red fox population from a low endemic area using a validated artificial digestion and sequential sieving technique. Vet Res. 2014;45:120–31. doi: 10.1186/s13567-014-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RC, Chabaud AG, Willmott S, editors. Keys to the nematode parasites of vertebrates. Archival volume. Wallingford, UK: CAB International; 2009. [Google Scholar]
- 12.Kanev I, Fried B, Radev V. Collar spine models in the genus Echinostoma (Trematoda: Echinostomatidae) Parasitol Res. 2009;105:921–7. doi: 10.1007/s00436-009-1475-0. [DOI] [PubMed] [Google Scholar]
- 13.Dönges J. Developmental history and morphological evaluation of notocotilids (Trematoda) [In German] Z Parasietenkd. 1962;22:43–67. [PubMed] [Google Scholar]
- 14.Pike AW. Observations on the life cycles of Notocotylus triserialis Diesing, 1839, and N. imbricatus (Looss, 1893) sensu Szidat, 1935. J Helminthol. 1969;43:145–65. doi: 10.1017/s0022149x00003989. [DOI] [PubMed] [Google Scholar]
- 15.Cribb T. Notocotylidae (Digenea) from the Australian water rat Hydromys chrysogaster Geoffroy, 1804 (Muridae) Syst Parasitol. 1991;18:227–37. [Google Scholar]
- 16.Simon-Vincente F, Mascoma S, Lopez-Roman R, Tenora F, Gallego J. Review of Notocotylus species (Trematoda: Notocotylidae) parasitizing rodents in Europe. Folia Parasitol. 1985;32:21–33. [Google Scholar]
- 17.Schuster R. [Echinostoma echinatum, Notocotylus noyeri and Quinqueserialis quinqueserialis as unusual parasites of Rattus norvegicus] Angew Parasitol. 1986;27:221–5. [PubMed] [Google Scholar]
- 18.Davis D. The survival of wild brown rats on a Maryland farm. Ecology. 1948;29:437–48. [Google Scholar]
- 19.McGuire B, Pizzuto T, Bemis WE, Getz LL. General ecology of a rural population of Norway rats (Rattus norvegicus) based on intensive live trapping. A Midl Nat. 2006;155:221–36. [Google Scholar]
- 20.Whisson D, Quinn J, Collins K. Home range and movements of roof rats (Rattus rattus) in an old-growth riparian forest, California. J Mammol. 2007;88:589–94. [Google Scholar]
- 21.Macdonald D, Mathews F, Berdoy M. The behaviour and ecology of Rattus norvegicus: from Opportunism to Kamikaze Tendencies. In: Singleton GR, Hinds LA, Leirs H, Zhang Z, editors. Ecology-based rodent management. Canberra, Australia: ACIR Monograph 59; 1999. [Google Scholar]
- 22.Macdonald D, Fenn M. Rat ranges in arable areas. Proc Zool Soci London. 1995;236:253–7. [Google Scholar]
- 23.Akande O. Report/Master of Science Programme in Veterinary Medicine for International Students, Faculty of Veterinary Medicine and Animal Science. Swedish University of Agricultural Sciences, Report no. 72. 2008. A study on wild rat behaviour and control on a pig farm. [Google Scholar]
- 24.Landreth H. Ecology of Norway rats, Rattus norvegicus, on a deserted farm in western Oklahoma. Proc Okl Acad Sci. 1972;52:45–8. [Google Scholar]
- 25.Gómez Villafañe I, Muschetto E, Busch M. Movements of Norway rats (Rattus norvegicus) in two poultry farms, Exaltación de la Cruz, Buenos Aires, Argentina. Mastozool Neotrop. 2008;15:203–8. [Google Scholar]
- 26.Hooker S, Innes J. Ranging behaviour of forest-dwelling ship rats, Rattus rattus, and effects of poisoning with brodifacoum. N Z J Zool. 1995;22:291–304. [Google Scholar]
- 27.Rogan MT, Craig PS, Hide G, Heath S, Pickles A, Storey DM. The occurrence of the trematode Plagiorchis muris in the wood mouse Apodemus sylvaticus in North Yorkshire, UK. J Helminthol. 2007;81:57–62. doi: 10.1017/S0022149X07214105. [DOI] [PubMed] [Google Scholar]
- 28.Hong S, Woo H, Chai J. A human case of Plagiorchis muris (Tanabe, 1922: Digenea) infection in the Republic of Korea: freshwater fish as possible source of infection. J Parasitol. 1996;82:647–9. [PubMed] [Google Scholar]
- 29.Asada J, Otagaki H, Morita D, Takeuchi T, Sakai Y, Konishi T, et al. A case report on human infection with Plagiorchis muris in Japan. [In Japanese, with abstract in English] Jpn J Parasitol. 1962;11:512–16. [Google Scholar]
- 30.Blankespoor H. Host-parasite relationships of an avian trematode, Plagiorchis noblei Park, 1936. Retrospective Theses and Dissertations, 1970, Paper 4817. Available from: http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=5816&context=rtd [cited 13 May 2016]
- 31.Barker F. Parasites of the American Muskrat (Fiber zibethicus) J Parasitol. 1915;1:184–97. [Google Scholar]
- 32.Easterbrook JD, Kaplan JB, Glass GE, Watson J, Klein SL. A survey of rodent-borne pathogens carried by wild-caught Norway rats: a potential threat to laboratory rodent colonies. Lab Anim. 2008;42:92–8. doi: 10.1258/la.2007.06015e. [DOI] [PubMed] [Google Scholar]
- 33.Carty AJ. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J. 2008;49:272–6. doi: 10.1093/ilar.49.3.272. [DOI] [PubMed] [Google Scholar]
- 34.Plachy V, Litvinec A, Langrová I, Horáková B, Sloup V, Jankovská I, et al. The effect of Syphacia muris on nutrient digestibility in laboratory rats. Lab Anim. 2016;50:39–44. doi: 10.1177/0023677215577038. [DOI] [PubMed] [Google Scholar]
- 35.Webster JP, Macdonald DW. Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology. 1995;111:247–55. doi: 10.1017/s0031182000081804. [DOI] [PubMed] [Google Scholar]
- 36.CDC. Hymenolepiasis. Centers for Disease Control and Prevention. 2016. Available from: http://www.cdc.gov/dpdx/hymenolepiasis/index.html [cited 4 February 2016]