Abstract
Background and purpose
We evaluated the accuracy of three methods of estimating radiation dose to the heart from two-dimensional tangential radiotherapy for breast cancer, as used in Denmark during 1982–2002.
Material and methods
Three tangential radiotherapy regimens were reconstructed using CT-based planning scans for 40 patients with left-sided and 10 with right-sided breast cancer. Setup errors and organ motion were simulated using estimated uncertainties. For left-sided patients, mean heart dose was related to maximum heart distance in the medial field.
Results
For left-sided breast cancer, mean heart dose estimated from individual CT-scans varied from <1 Gy to >8 Gy, and maximum dose from 5 to 50 Gy for all three regimens, so that estimates based only on regimen had substantial uncertainty. When maximum heart distance was taken into account, the uncertainty was reduced and was comparable to the uncertainty of estimates based on individual CT-scans. For right-sided breast cancer patients, mean heart dose based on individual CT-scans was always <1 Gy and maximum dose always <5 Gy for all three regimens.
Conclusions
The use of stored individual simulator films provides a method for estimating heart doses in left-tangential radiotherapy for breast cancer that is almost as accurate as estimates based on individual CT-scans.
Keywords: Breast cancer, Heart dose, Heart disease
Breast cancer is the commonest cancer among women worldwide. It is usually diagnosed at an early stage and modern treatment can involve several modalities including surgery, chemotherapy, radiotherapy, endocrine treatment and anti-HER-2 treatment, depending on the biomarker profile of the tumour. A meta-analysis of data from randomised trials in early breast cancer has shown that, after breast conserving surgery, radiotherapy to the conserved breast halves the rate of breast cancer recurrence and reduces the breast cancer death rate by about a sixth [1]. These effects are largely independent of other treatment modalities. The randomised trials also show, however, that radiotherapy may increase the risk of heart disease [2].
The radiation dose to the heart is usually larger in radiotherapy for left-sided than for right-sided breast cancer, and several studies have found that patients irradiated for left-sided breast cancer have higher risks of heart disease than those irradiated for right-sided breast cancer [3], [4], [5], [6]. None of these studies included detailed information on radiation doses to the heart. A recent case-control study of Danish and Swedish women irradiated for breast cancer during 1958–2001, in which cardiac dose was estimated for individual women, showed that the rate of major coronary events increased linearly with mean dose to the heart by 7.4% per Gy with no apparent threshold below which there was no risk [7]. In the case–control study, radiotherapy planning for most of the women was performed using conventional simulators rather than individual CT planning. Radiation doses to the heart for these women were, therefore, estimated using a “typical patient CT-scan” for which the heart dose from a variety of different breast cancer radiotherapy regimens was near the average. Each radiotherapy regimen in the study was then reconstructed on this “typical patient CT-scan” [8]. This method did not take into account likely variations in cardiac dose from individual variations in patient anatomy, setup errors or organ motion during treatment. It is, however, possible to allow for individual variation in patient anatomy in women who received left-tangential radiotherapy using the amount of heart in the fields on conventional simulator films [9], [10].
The first aim of the present study was, therefore, to evaluate the variability in radiation doses to the heart due to anatomical differences, setup errors, and organ motion from 2D-tangential radiotherapy in Denmark. The second aim was to examine the use of conventional simulator films as a surrogate for individual CT-scans to estimate individual radiation doses to the heart.
Material and methods
Reconstruction of radiotherapy plans
Since 1977, the national guidelines from the Danish Breast Cancer Cooperative Group (DBCG) have included technical breast cancer radiotherapy protocols [11] which are used by all Danish radiotherapy centres. In order to learn how these protocols were implemented in each centre, interviews were conducted (by ELL) with present and former radiographers, oncologists, and physicists.
Three main regimens were used between 1982 and 2002. Based on the nodal status the patient would receive one of the three techniques (referred to as regimens A, B and C) as illustrated in Fig. 1. For the tangential fields, the beam angle, collimator and custom block were based on radiopaque wires marking 3 cm contralateral to the mid-sternal line (regimens A and B) or the mid-axillary and mid-sternal lines (regimen C), as well as the caudal borders of the mammary tissue. A maximum of 3 cm of lung tissue, seen in beam’s eye view, was allowed within the tangential fields. To achieve dose homogeneity, a standard mix of 85% 6 MV photons and 15% 18 MV photons with a 60 degree wedge was used. A photon field angled at 15 degrees contralaterally to the treated breast was used for the periclavicular region for regimens A and B. The prescribed dose was 48 Gy in 24 fractions. Further details on the procedure for plan reconstruction are given in Appendix A.
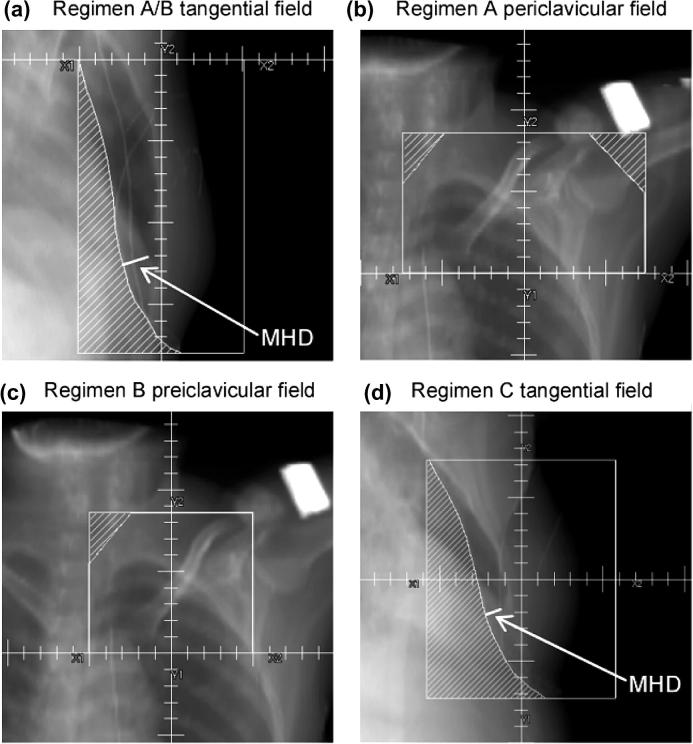
Fig. 1.
Beam’s eye views for the three tangential radiotherapy regimens. Regimens A and B were both targeted at the conserved breast and internal mammary chain (IMC) with opposing tangential fields (panel a) and differed only in the periclavicular region where an anterior field was targeted at the periclavicular and axillary lymph nodes for regimen A (panel b) and the periclavicular lymph nodes only for regimen B (panel c). Regimen C was targeted at the conserved breast only, with opposing tangential fields (panel d). For relating mean heart dose to the amount of the heart in the field, the maximum heart distance (MHD) orthogonal to the field edge was measured for the tangential fields, as illustrated.
CT-planning scans
Today, three-dimensional (3D) CT-based radiotherapy has replaced two-dimensional (2D) conventional simulation in all Danish radiotherapy centres. In 3D CT-based radiotherapy, target volumes and organs at risk are delineated. Radiopaque wires as described above are still in use, and patient position remains the same. Therefore, 2D-based radiotherapy regimens could be reconstructed using CT-scans of recent breast cancer patients. Regimens A and B used identical tangential fields and were therefore reconstructed on the same patients. Twenty patients with left-sided and five patients with right-sided breast cancer were selected from patients treated at Odense University Hospital during 2010 for these reconstructions. Twenty further left-sided and 5 further right-sided patients were selected for reconstructing regimen C. Selection was made at random from all patients with similar characteristics to those for which the specific regimen was used historically.
Dose calculation
The dose distributions from the reconstructed regimens were calculated using the collapsed-cone algorithm in Pinnacle with a 2 mm resolution using a model of an Elekta accelerator, as used for breast cancer radiotherapy in the 1990 s. The heart was delineated on the CT-scans using an automatic heart delineation tool (ABAS – Atlas-based autosegmentation by Elekta). This atlas was based on the heart contours of 15 breast cancer patients whose hearts had been delineated according to published guidelines [12], [13]. The ABAS automatic heart delineations have been verified in a separate study showing good performance when compared with manual delineations [14]. The delineations and dose distributions were imported into Matlab R2007b and analysed using the open-source tool CERR [15] and in-house code.
Setup errors and organ motion
The effects of setup errors and organ motion on heart dose were simulated using a combination of random shifts and blurring of the dose distribution in order to mimic systematic and random errors respectively. Blurring was simulated by a 3D convolution of the dose cube with a Gaussian distribution with standard deviation (σ), representing the total random error. The systematic errors were simulated by shifting the blurred dose distribution 100 times for each patient, with each shift representing a potential treatment course for the patient. The shifts were drawn at random from a Gaussian distribution with a standard deviation (Σ) representing the total systematic error. Calculations were performed for σ = Σ = 1, 2, …, 10 mm. Mean heart dose was calculated for each shift resulting, for each patient, in 100 estimates of the dose that the patient could actually have received.
Estimation of heart dose using simulator films
To examine the potential value of simulator films for estimating individual heart doses for patients with left-sided disease, digitally reconstructed radiographs (DRRs) were generated and the maximum distance that the heart protruded into the medial field (maximum heart distance: MHD) was measured, as illustrated in Fig. 1, panels (a) and (d). The MHD was measured perpendicularly to the field edge at source-skin distance (SSD) = 100 cm. The natural logarithm of the mean heart dose was then related to MHD using linear regression.
Error in estimated mean heart dose
The distribution of the mean heart doses that each patient could actually have received, taking into account setup errors and organ motion, was compared to the mean dose estimated using three different methods, based on increasing amounts of information: (1) Regimen based: the median of the mean heart doses for each regimen, as estimated from the CT-based treatment planning scans for the selected patients, (2) Film-based: individual estimates of mean heart dose calculated from individual measures of MHD, as derived from simulator films, and the linear regression relating the logarithm of mean heart dose to MHD for the sampled patients, and (3) CT-based: individual estimates based on the patient’s own CT-scan.
Results
Mean and maximum heart doses for the three reconstructed regimens, estimated using the individual CT-based planning scans for the patients selected for the study, are presented in Fig. 2. For right-sided patients the mean heart dose was always less than 1 Gy and the maximum dose always less than 5 Gy for all three regimens. For left-sided radiotherapy, the heart doses were higher and there was substantial inter-patient variation: mean doses ranged from below 1 to more than 8 Gy and maximum doses ranged from 5 to 50 Gy. The averages of both the mean and the maximum doses were significantly higher for left-sided than for right-sided treatments (average of mean doses: left-sided 3.50 Gy, right-sided 0.78 Gy, p for difference: <0.0001; average of maximum doses: left-sided 43.3 Gy, right-sided 3.14 Gy, p for difference: <0.0001). Among left-sided patients, the heart doses from regimen A were slightly larger than from regimen B while the doses from regimen C were lower than from regimen B (average of mean doses: 3.84, 3.83, and 2.82 Gy for regimens A, B and C respectively; average of maximum doses: 43.8, 43.8, and 42.3 Gy). The differences between the three regimens were, however, not statistically significant (p for heterogeneity: 0.23 and 0.88 for average mean and maximum doses respectively).
Fig. 2.
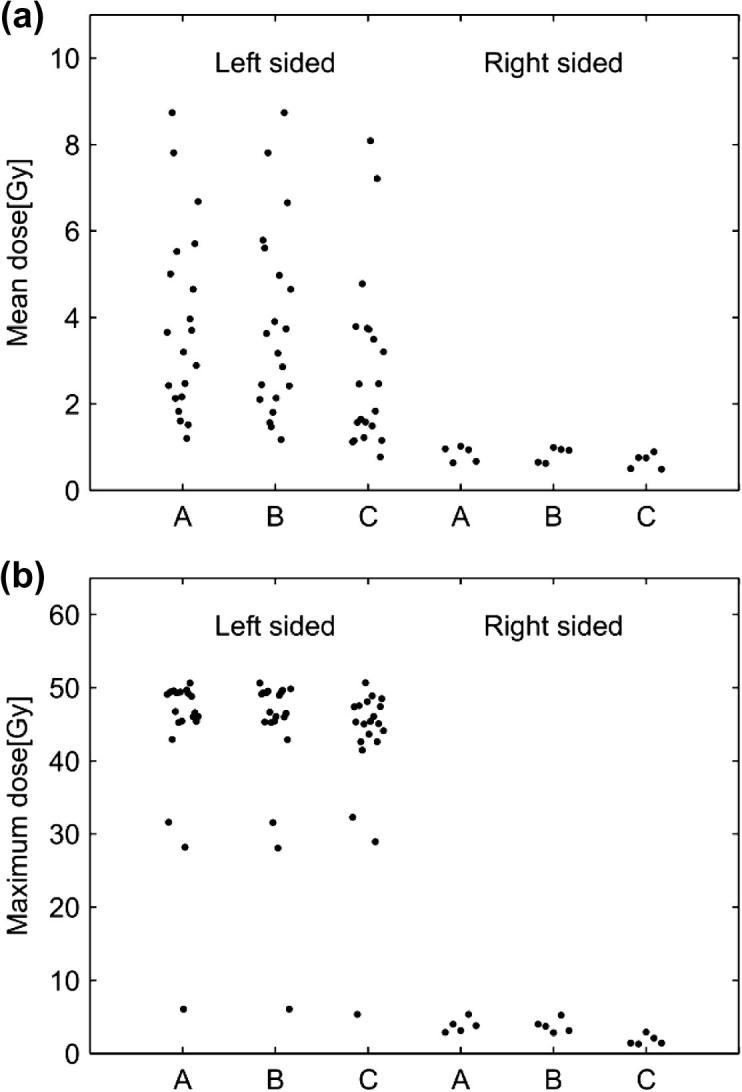
Estimated heart doses for the three reconstructed techniques. Estimates are based on the individual CT-based planning scans for the patients included in the study. Mean dose (a) and maximum dose (b) for the three regimens (A, B and C) for left-sided patients (20 for each regimen) and right-sided patients (5 for each regimen).
Setup errors and organ motion
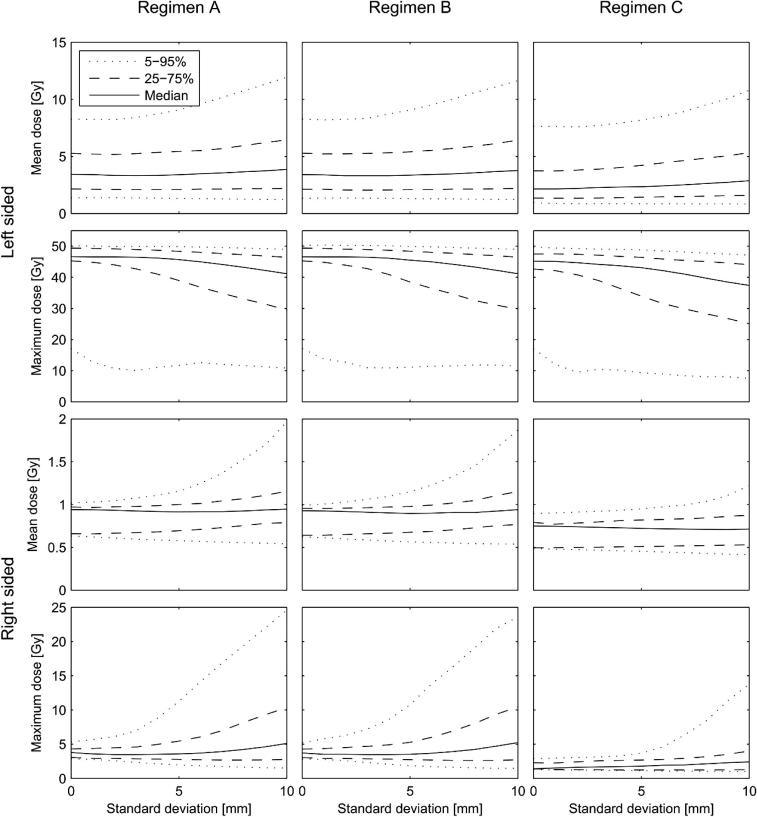
The distributions of the mean heart doses that each of the 40 patients with left-sided disease and 10 patients with right-sided disease could actually have received for different assumptions regarding the magnitude of the standard deviation due to setup errors and organ motion are summarised in Fig. 3. For left-sided patients, bigger standard deviations resulted in substantial increases in the 95th percentiles of the mean heart dose, small increases in the 75th percentiles and the medians and little change in the 25th and 5th percentiles. Bigger standard deviations caused little change in the 95th percentiles of the maximum heart dose for left-sided patients, and reduced the medians and 75th, 25th and 5th percentiles. For right-sided patients, bigger standard deviations increased the 95th percentiles of both mean and maximum heart doses substantially, but had a much smaller effect on the medians.
Fig. 3.
Distribution of the mean heart doses that each patient could actually have received, taking into account setup errors and organ motion, as well as the effect of positional variability derived from the individual CT-scans. The standard deviations of the random (σ) and systematic (Σ) errors were assumed to be equal (σ = Σ) and the simulations were performed with values in the range of 0 to 10 mm. For each patient and each regimen, 100 mean doses received were simulated for each value of the standard deviation considered, giving 100 × 20 doses for left-sided breast cancer at each value of the standard deviation (100 × 5 values for right-sided). The median value of these simulated doses and the 5th, 25th, 75th and 95th percentiles are shown as a function of the standard deviation. A standard deviation of zero corresponds to the distribution of doses shown in Fig. 2.
Estimation of heart dose using simulator films
For any particular values of random and systematic errors, the distribution of individual mean heart doses was positively skewed and so a logarithmic transformation (ln) was applied before further analysis. Patient-specific averages of the simulated values of the ln(mean heart dose) were then calculated. The dependence of these patient-specific averages on MHD is shown in Fig. 4 for three sets of mean heart doses, based on simulated setup and organ motion errors of 0, 5 and 10 mm respectively. For all three error magnitudes ln(mean heart dose) and MHD were highly correlated (R2: 0.87, 0.87 and 0.85 for errors of 0 mm, 5 mm and 10 mm respectively) and the residuals showed no evidence of departure from a linear relationship. The effect of radiotherapy regimen (as indicated by the inclusion in the regression of a categorical variable with three levels) was not significant (p = 0.65, 0.91, and 0.98 for errors of 0 mm, 5 mm and 10 mm respectively).
Fig. 4.
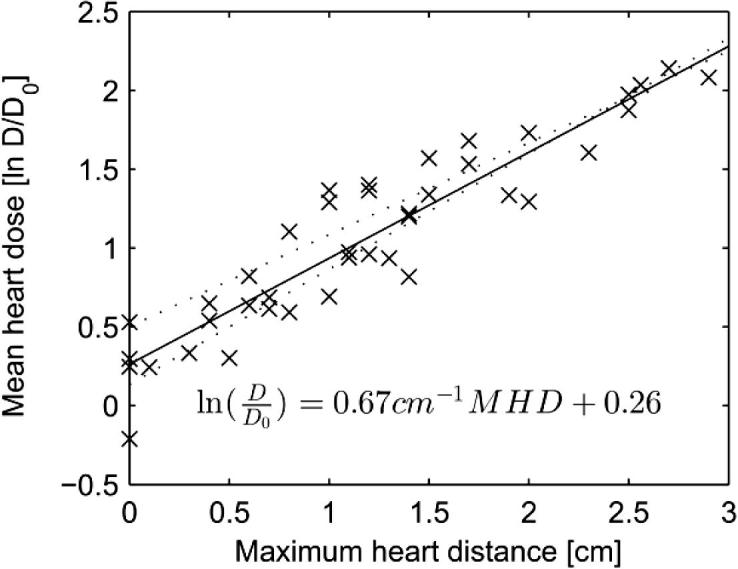
Relation between mean heart dose actually received and maximum heart distance (MHD) for patients with left-sided breast cancer. ln () denotes natural logarithm, D is mean heart dose in Gy and D0 is set at 1 Gy. Crosses are patient-specific averages of simulated values. Crosses, solid regression line and regression equation assume a standard deviation of the random and systematic errors of 5 mm (σ = Σ = 5 mm). The regression lines assuming standard deviations of 0 mm and 10 mm are shown by the lower and upper dotted lines, respectively.
Error in estimated mean heart dose
For patients with left-sided disease, Fig. 5 shows the root mean square error (RMSE) of the estimated mean heart doses compared with estimates of the doses that the patients could actually have received for the three different methods of dose estimation. For estimates based on regimen only, the RMSE was substantial, especially for patients with MHDs in the highest or lowest quintile. For the film-based estimates, the RMSEs were much lower, and comparable to those for the CT-based estimates. For the CT-based estimates, the RMSE values reflect only the effect of setup and organ uncertainties. These were around 0.5 Gy for patients with MHD in the lowest quintile, 1–1.5 Gy for patients with in the middle three quintiles, and around 2 Gy for patients with MHD in the highest quintile.
Fig. 5.
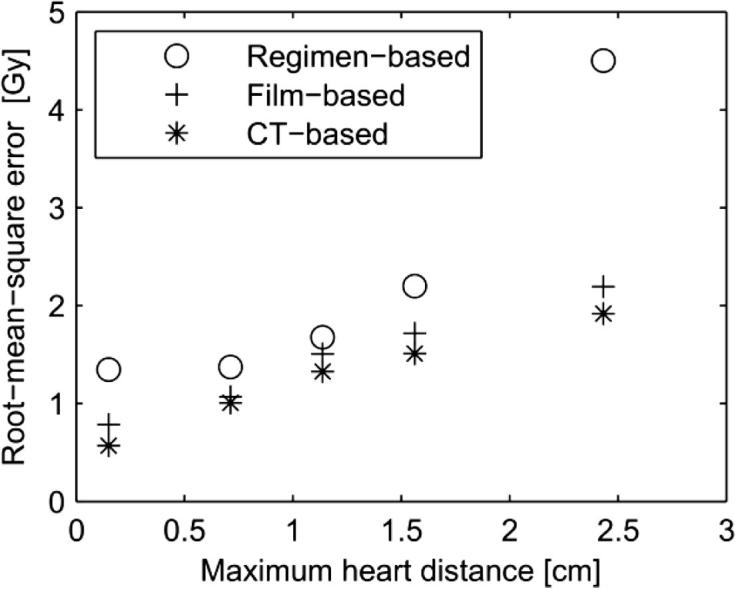
Root-mean-squared error (RMSE) of the estimated mean heart doses for left-sided patients compared with received mean heart doses. Received mean heart doses were calculated from the individual CT-based planning scans for the patients included in the study together with simulated organ motion and setup errors, assuming a standard deviation for both random and systematic errors of 5 mm. Mean heart doses were estimated by three different methods based on increasing amounts of information: (1) regimen-based, (2) film-based (assuming σ = Σ = 5 mm, as shown in Fig. 4), and (3) CT-based (but ignoring any setup uncertainties and organ motion). The 40 left-sided patients were divided in five equally sized groups according to the MHD and for each group the RMSE for each method is shown.
Discussion
This study shows that there is substantial uncertainty in estimating heart doses from left-sided 2D-tangential radiotherapy for Danish breast cancer patients treated from 1982 to 2002 using only information on prescribed dose, laterality (left–right) and regimen. The use of individual simulator films provides an alternative method for which the uncertainty is markedly reduced and is, in fact, close to the uncertainty expected when individual CT-based estimates are used.
Several studies of heart disease after radiotherapy for breast cancer have used left-versus-right comparisons [3], [4], [5], [6] because radiation doses to the heart are usually higher for left-sided than for right-sided radiotherapy due to the anatomical position of the heart. Correa et al. [16] measured proportions of heart and lung tissue in the radiation field for patients given cardiac diagnostic tests after radiotherapy and found that significantly more lung tissue was included in the field for patients with cardiac abnormalities versus patients without cardiac abnormalities. Using details regarding the targets irradiated, Nilsson et al. [17] found an association between areas of expected high radiation dose in the heart and later location of stenosis in the coronary arteries. However, none of these studies attempted to estimate heart doses from the treatments.
Darby et al. [7] showed that the relationship between mean dose to the heart from breast cancer radiotherapy and subsequent risk of ischaemic heart disease was linear. However, the dosimetry methods used in that study [18] did not take into account individual anatomical variations, setup errors, or organ motion during treatment. The present study was, therefore, conducted to estimate the effect of these uncertainties. The results will enable a more precise estimation of dose–response relationships in the future, partly because it will be possible to use the average of 20 estimates for each regimen instead of just one estimate on a representative patient and, most importantly, because the use of simulator films will reduce considerably errors in estimated dose compared to a regimen-specific average value (Fig. 5).
Mean heart dose from left-sided tangential radiotherapy vary substantially because the heart is located near the field border. Therefore small differences in the heart position relative to the tangential fields have a major impact on the heart dose. Two wide tangential regimens (A and B) differed only in the periclavicular region and therefore delivered nearly identical heart doses. Heart doses from tangential radiotherapy with the medial border on the mid-sternal line (regimen C) were slightly lower, but did not differ significantly from doses from the wide tangential regimens. One explanation for this relatively small difference is the constraint of a maximum of 3 cm of lung in field, which tended to limit the heart dose. Compared to previous estimates of heart doses on a representative patient [18], the doses obtained in the present study are on average lower, but they have large inter-patient variation.
In a recently published quality assurance study from the DBCG [19], heart doses were estimated for breast cancer radiotherapy used since 2003 by reconstructing radiotherapy on CT-scans of newly diagnosed breast cancer patients, as performed in our study. Two of the techniques reconstructed in that study are similar to the left-sided regimen C and right sided regimen A/B in our study. Similar doses were indeed found in that study with average mean heart doses of 0.8 Gy for right-sided regimen A/B and 3.4 Gy for left-sided regimen C.
Setup errors and organ motion
During the period considered in this study, all centres used port films for setup verification at the first fraction, and a few centres used additional setup verification halfway through the treatment course. A total standard deviation of 3–4 mm for both random and systematic uncertainties was estimated by combining the results from a published study of the uncertainty of heart position relative to the breast [20] and simulated historical setup uncertainty based on orthogonal images (kV or Mv) of 413 present-day breast cancer patients [21]. These studies were, however, based on electronic imaging devices whereas film-based portal imaging was used in the pre-CT era. Using port films was more time-consuming and less accurate due to the time delay between exposure and the manual evaluation, and a higher total uncertainty in the order of 4–5 mm would be expected with port films. Knowing the exact value is, however, not critical as the effect on the doses for errors of around 5 mm is limited, as shown in Fig. 3.
Estimation of heart dose using simulator films
One other study has evaluated the relationship between maximum heart distance and later heart disease [6]. It found no statistically significant relationship but, with only 139 events in left-sided patients, the study had limited power. In the present study, using MHD as the only explanatory variable in a simple linear model provided a good correlation with the logarithm of mean heart dose, in line with findings in previous studies [9], [10]. Taylor et al. [9] found the relationship between MHD and mean heart dose to be: 2.9 Gy/cm MHD + 4.1 Gy whereas Kong et al. [10] found it to be: 2.8 Gy/cm MHD + 2.2 Gy. In the present study the doses were log transformed to account for the skewed distribution of mean heart doses but, for comparison we performed linear regression analysis based on mean dose in Gy, resulting in the following relationship: 2.43 Gy/cm MHD + 0.42 Gy for σ = Σ = 0 mm. Hence, given the differences in the prescribed dose, radiotherapy techniques and dose calculation algorithms, there is good agreement between these dosimetric studies.
Error in estimated mean heart dose
The simulations of setup error and organ motion enabled the differences between estimated doses and the doses likely to have been received to be studied. When using the regimen-specific median estimate the errors were large for the group of patients receiving the highest doses, with an RMSE of approximate 4.5 Gy (see Fig. 5). Using MHD as predictor reduced this error, especially for the high-dose patients where the RMSE was more than halved. Using individual doses, corresponding to the planned dose in CT-based radiotherapy, resulted in only a slight further reduction of the RMSE. Using MHD or CT-based estimates in preference to regimen-specific estimates would be especially important in studies where distribution of heart doses may not be representative of that in the general irradiated breast cancer population, such as for the cases in a case-control study of radiation-related heart disease.
Conclusions
The mean heart doses from right-sided tangential radiotherapy used in the pre-CT era were less than 1 Gy for all patients in the study, whereas the heart doses for left-sided radiotherapy were higher with large inter-patient variation. Estimates of heart doses for women treated in the pre-CT era based on radiotherapy regimen and laterality only, have large uncertainty for patients who received left-tangential radiotherapy. Stored individual simulator films enable prediction of mean heart dose of left-sided patients with an accuracy close to that expected from individual CT-based estimates.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors would like to thank the following for spending time contributing to details on radiotherapy practise (in alphabetical order): Bente Sommer Kristensen, Dorte Klitgaard, Dorthe Brønnum, Flemming Kjaer-Kristoffersen, Hans Jacob Hansen, Ingelise Jensen, Jens Juul Christensen, Knud Aage Werenberg, Marian Vetke Jensen, Martin Kjellgren, Mette Skovhus Thomsen and Sune Zimmerman.
We gratefully acknowledge funding from Cancer Research UK and the Department of Health, London (project grant 091/0203) to the CTSU, University of Oxford.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.radonc.2016.02.017.
Appendix A. Supplementary data
References
- 1.(EBCTCG) EBCTCG, Darby S, McGale P. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke M., Collins R., Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. Dec 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Darby S., McGale P., Peto R., Granath F., Hall P., Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90,000 swedish women. BMJ. Feb 2003;326:256–257. doi: 10.1136/bmj.326.7383.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in us seer cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 5.Roychoudhuri R., Robinson D., Putcha V., Cuzick J., Darby S., Møller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7 doi: 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borger J.H., Hooning M.J., Boersma L.J. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys. 2007;69:1131–1138. doi: 10.1016/j.ijrobp.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 7.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 8.Taylor C.W., Nisbet A., McGale P., Darby S.C. Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. Int J Radiat Oncol Biol Phys. 2007;69:1484–1495. doi: 10.1016/j.ijrobp.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Taylor C.W., McGale P., Povall J.M. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys. 2009;73:1061–1068. doi: 10.1016/j.ijrobp.2008.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Kong F.M., Klein E.E., Bradley J.D. The impact of central lung distance, maximal heart distance, and radiation technique on the volumetric dose of the lung and heart for intact breast radiation. Int J Radiat Oncol Biol Phys. 2002;54:963–971. doi: 10.1016/s0360-3016(02)03741-0. [DOI] [PubMed] [Google Scholar]
- 11.Overgaard M., Christensen J.J. Postoperative radiotherapy in DBCG during 30 years. techniques, indications and clinical radiobiological experience. Acta Oncol. 2008;47:639–653. doi: 10.1080/02841860802078085. [DOI] [PubMed] [Google Scholar]
- 12.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen M.H., Berg M., Pedersen A.N. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the danish breast cancer cooperative group. Acta Oncol. 2013;52:703–710. doi: 10.3109/0284186X.2013.765064. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzen E.L., Ewertz M., Brink C. Automatic segmentation of the heart in radiotherapy for breast cancer. Acta Oncol. 2014;53:1366–1372. doi: 10.3109/0284186X.2014.930170. [DOI] [PubMed] [Google Scholar]
- 15.Deasy J.O., Blanco A.I., Clark V.H. Cerr: a computational environment for radiotherapy research. Med Phys. 2003;30:979–985. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]
- 16.Correa C.R., Das I.J., Litt H.I. Association between tangential beam treatment parameters and cardiac abnormalities after definitive radiation treatment for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:508–516. doi: 10.1016/j.ijrobp.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson G., Holmberg L., Garmo H., Terent A., Blomqvist C. Radiation to supraclavicular and internal mammary lymph nodes in breast cancer increases the risk of stroke. Br J Cancer. 2009;100:811–816. doi: 10.1038/sj.bjc.6604902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor C.W., ø nnum D.B., Darby S.C. Cardiac dose estimates from danish and swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol. 2011;100:176–183. doi: 10.1016/j.radonc.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorsen L.B.J., Thomsen M.S., Overgaard M., Overgaard J., Offersen B.V. Quality assurance of conventional non-ct-based internal mammary lymph node irradiation in a prospective danish breast cancer cooperative group trial: the DBCG-imn study. Acta Oncol. 2013;52:1526–1534. doi: 10.3109/0284186X.2013.813643. [DOI] [PubMed] [Google Scholar]
- 20.Topolnjak R., Borst G.R., Nijkamp J., Sonke J.J. Image-guided radiotherapy for left-sided breast cancer patients: geometrical uncertainty of the heart. Int J Radiat Oncol Biol Phys. 2012;82:e647–e655. doi: 10.1016/j.ijrobp.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Josipovic M., Korreman S., Zacharatou C. Impact of an off-line correction protocol on setup uncertainty in modern breast cancer radiation therapy. Radiother Oncol. 2009;92 173-173. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.