Abstract
Objective
The purpose of this study was to evaluate the prognosis according to the number of high risk factors in patients with high risk factors after radical hysterectomy and adjuvant chemoradiation therapy for early stage cervical cancer.
Methods
Clinicopathological variables and clinical outcomes of patients with FIGO (International Federation of Gynecology and Obstetrics) stage IB1 to IIA cervical cancer who had one or more high risk factors after radical hysterectomy and adjuvant chemoradiation therapy were retrospectively analyzed. Patients were divided into two groups according to the number of high risk factors (group 1, single high risk factor; group 2, two or more high risk factors).
Results
A total of 93 patients were enrolled in the present study. Forty nine out of 93 (52.7%) patients had a single high risk factor, and 44 (47.3%) had two or more high risk factors. Statistically significant differences in stage and stromal invasion were observed between group 1 and group 2. However, age, histology, tumor size, and lymphovascular space invasion did not differ significantly between the groups. Distant recurrence occurred more frequently in group 2, and the probability of recurrence and death was higher in group 2.
Conclusion
Patients with two or more high risk factors had worse prognosis in early stage cervical cancer. For these patients, consideration of new strategies to improve survival may be worthwhile. Conduct of further clinical trials is warranted for development of adjuvant treatment strategies individualized to each risk group.
Keywords: Chemoradiation, High-risk factor, Prognosis, Radical hysterectomy, Uterine cervical neoplasms
Introduction
Carcinoma of the uterine cervix is the fourth most common cancer in women worldwide, and the seventh overall, with an estimated 528,000 new cases in 2012, with most of these occurring in the developing world [1]. The definitive treatment for patients with early-stage cervical cancer consists of radiation therapy (RT) or radical hysterectomy with pelvic and /or paraaortic lymph node (LN) dissection [2]. Most patients with early stage cervical cancer undergo surgical treatment. Following surgery, adjuvant therapy is administered to patients with high risk factors such as LN metastases, positive resection margin, and parametrium involvement. Currently, concurrent chemoradiation therapy (CCRT) is the postoperative treatment of choice in patients with high-risk factors after radical hysterectomy [3]. However, many early stage cervical cancer patients with high risk factors may have more than one risk factor and are a heterogeneous group with different prognoses [4,5,6]. For example, when parametrium is involved and pelvic LNs are also positive, the survival rate falls [7,8]. However, for patients with high risk early stage cervical cancer, current treatment strategy consists uniformly of radical hysterectomy followed by CCRT. Therefore, adjuvant treatment may be further individualized to improve survival. Few studies on prognosis according to the number of high risk factors have been reported and strategies for adjuvant therapy for such patients have not been established.
The aim of this study was to evaluate the prognosis according to the number of high risk factors in patients with one or more high risk factor after radical hysterectomy for early stage cervical cancer.
Materials and methods
We identified patients with cervical cancer stage IB to IIA and having one or more high risk factors who underwent type III radical hysterectomy with pelvic and/or paraaortic LN dissection and adjuvant CCRT from February 2002 to December 2011. Patients who received chemotherapy, RT, or CCRT before surgery and patients who received postoperative adjuvant chemotherapy or RT alone were excluded. Small cell neuroendocrine tumors were also excluded. Demographic, clinicopathologic, and follow-up data were obtained from patients' medical records. These data included patient age, International Federation of Gynecology and Obstetrics (FIGO) stage, histologic subtype, tumor size, lymphovascular space invasion, depth of cervical stromal invasion, resection margin status, parametrial involvement, LN metastases, date and site of recurrence, date of death or last follow-up, and patient status at last follow-up. Patients were classified into two groups according to the number of high risk factors (group 1, single high risk factor; group 2, two or more high risk factors). Recurrent lesions were confirmed with biopsy or imaging studies. Local regional recurrence was defined as recurrent disease in the pelvis or retroperitoneal LN and distant metastasis was defined when the recurrent disease is located in the distant organ including liver, lung, bone and brain. This study was approved by the institutional review board (IRB approval number: GCIRB2013-212).
All patients underwent curative CCRT for cervical cancer after radical hysterectomy. The RT field was designed to include the whole pelvis, including the elective area of the pelvic LN area. The external beam RT was performed with higher energy photon (10 or 15 MV) using the four-field box technique based on the three-dimensional conformal RT planning. The external beam RT field was designed to include the whole pelvis and the fractionation was a 1.8 Gy tumor dose daily with five fractions per week to the pelvis areas. The external beam RT dose to the whole pelvis ranged from 30.6 to 61.2 Gy (median, 50.4 Gy), including the booster dose to the parametrial and/or pelvic LNs. Beyond 45 Gy to the whole pelvis, intracavitary radiotherapy with high-dose rate was administered with a dose of 25 to 35 Gy (median, 30 Gy) in two weekly fractions of 3.5 to 5 Gy prescribed at 0.5 cm from the surface of the applicator. Intracavitary radiotherapy was given to all patients with a positive resection margin involvement and to some patients on the basis of pathology results (close resection margin, pelvic LN involvement, and parametrial involvement) according to physicians' preferences. All patients received 5 fluorouracil-cisplatin (FP) combination chemotherapy or weekly cisplatin chemotherapy during RT. The FP chemotherapy regimens consisted of cisplatin 50 mg/m2 by intravenous infusion given on day 1 and 5-fluorouracil at a dose of 1,000 mg/m2 per day for five days given as a continuous infusion on days 1 to 5 three times. The FP chemotherapy was repeated every three weeks. Weekly cisplatin was administered on day 1 at a dose of 40 mg/m2 by intravenous infusion and repeated weekly for 6 weeks.
Recurrence-free survival (RFS) was calculated in months, from the date of surgery to the date of recurrence, censoring, or last follow-up. Overall survival (OS) was defined in months, from the date of surgery to the date of cancer death, censoring, or last follow-up. Patients were categorized into the single high risk factor group and the two or more high risk factors group according to the number of each high risk factor.
The Student's t-test was used for evaluation of between group differences in mean and median values. The chi-square test and Fisher's exact test were used for evaluation of differences in proportions. Survival curve and rate were calculated using the Kaplan-Meier method and differences in survival between groups were compared using the log-rank test for categorical variables and Cox regression model for continuous variables. Multivariate analysis was performed using the Cox regression model with the stepwise conditional method. Differences were regarded as significant when the P-value was less than 0.05. All statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Results
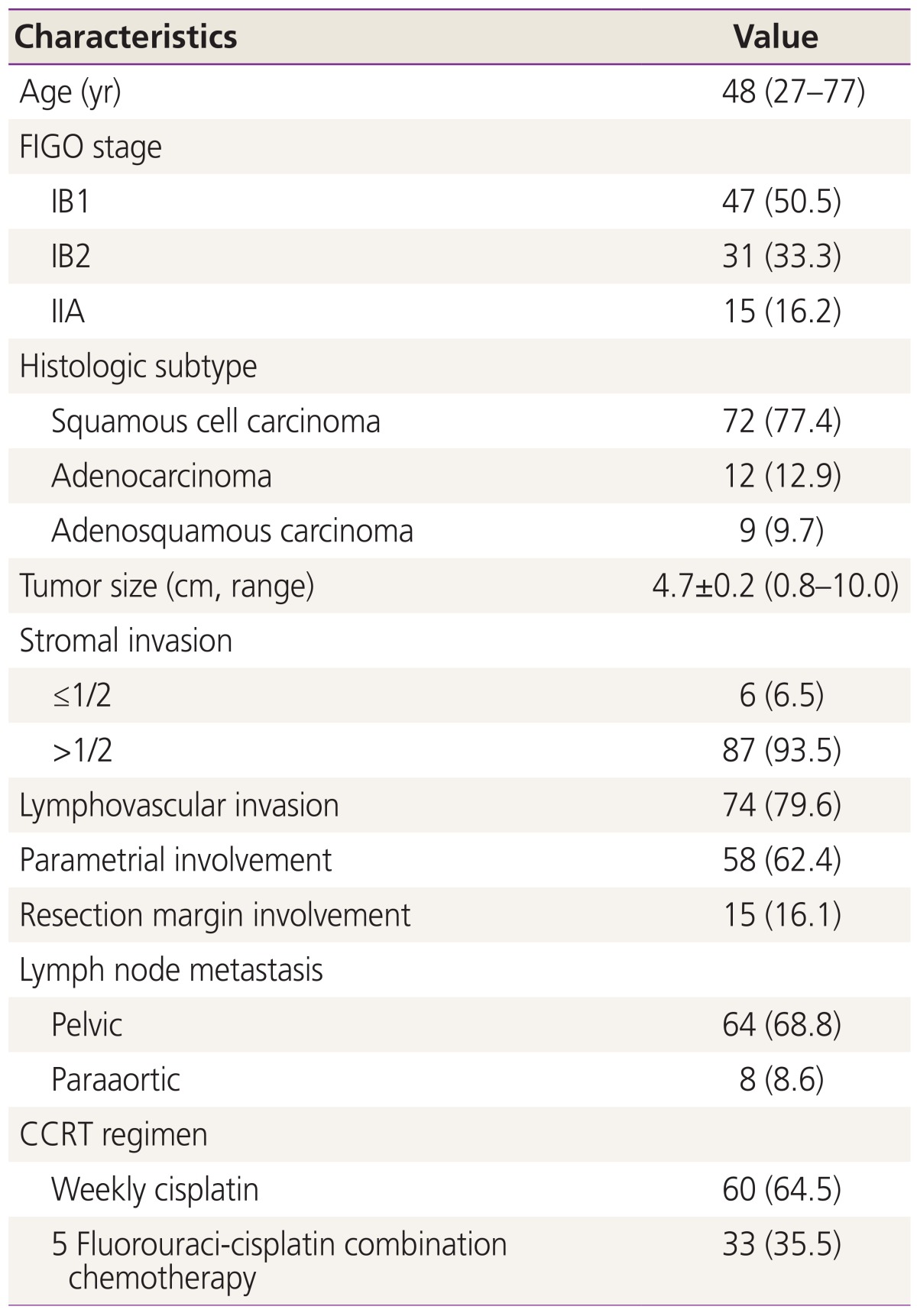
Of 404 patients with FIGO stage IB to IIA cervical cancer treated at the Gachon University Gil Medical Center between 2002 and 2011, 93 patients met our eligibility criteria; they had one or more high risk factors, including LN metastases, parametrial involvement, and positive resection margins. The characteristics of patients are shown in Table 1. All patients with FIGO stage IB1-IIA underwent type III radical hysterectomy with pelvic and/or paraaortic LN dissection and received adjuvant concurrent chemoradiotherapy. Thirty three patients (35.5%) underwent paraaortic LN dissection or sampling. Patients who did not undergo paraaortic LN dissection or sampling showed negative finding on preoperative imaging studies, including pelvic magnetic resonance imaging and positron emission tomography/computed tomography.
Table 1. Patients characteristics (n=93).
Values are presented as median (range) or number (%) unless otherwise indicated.
FIGO, International Federation of Gynecology and Obstetrics; CCRT, concurrent chemoradiation therapy.
Pathologic examination showed that 74 (79.6%) patients had LN metastases. Eight patients had both pelvic and paraaortic LN metastases. Fifteen (16.1%) had positive resection margins, and 58 (62.4%) had parametrial involvement. Forty nine out of 93 (52.7%) patients had a single high risk factor, 33 (35.5%) had two high risk factors, and 11 (11.8%) had all three high risk factors. In group 1, 28 had LN metastases, 19 had parametrial involvement, and 2 had resection margin involvement. In group 2, 8 had positive resection margin with parametrial involvement, 5 had positive resection margin with LN metastases, and 20 had parametrial involvement with LN metastases. The median follow-up period was 50 months (range, 8 to 118 months). Patients were classified into two groups according to the number of high risk factors (group 1, single high risk factor; group 2, two or more high risk factors). All patients received adjuvant CCRT after surgery and patients who had paraaortic LN metatstasis received extended field RT. Sixty patients received cisplatin based combination chemotherapy and 33 patients received weekly cisplatin chemotherapy during RT. There were 23 cases of cancer recurrence and nine cancer deaths during follow-up. The 5-year OS was 86.6% and 5-year RFS was 67.0%.
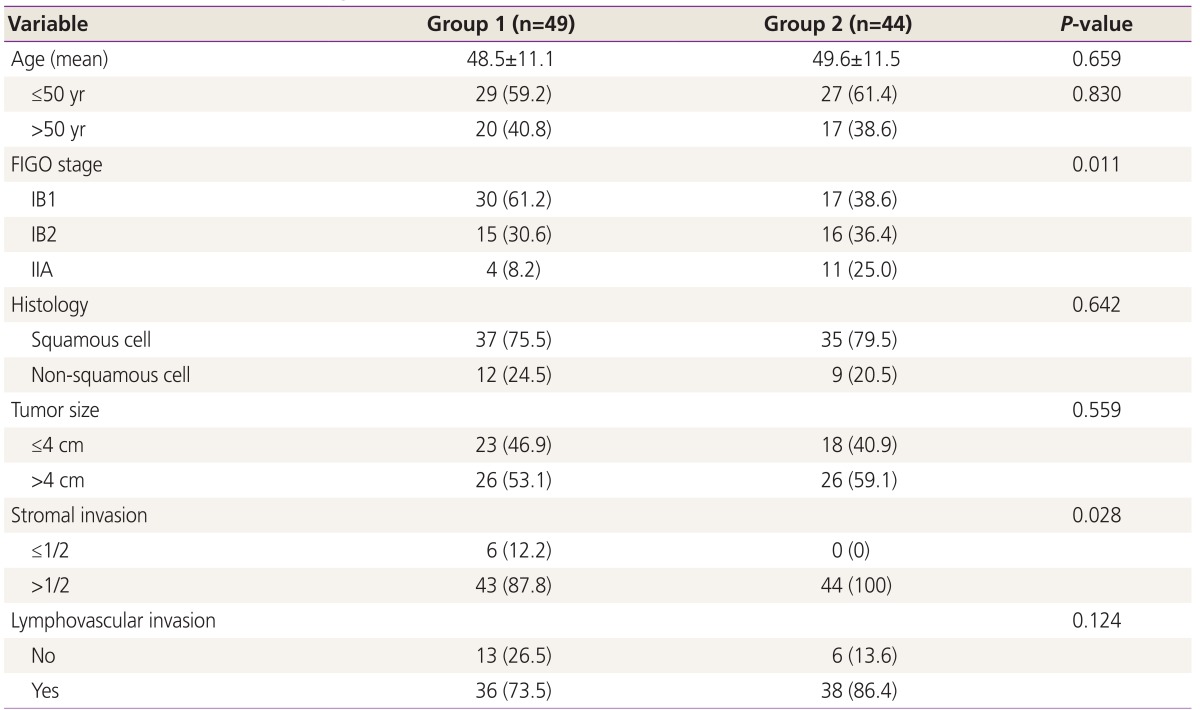
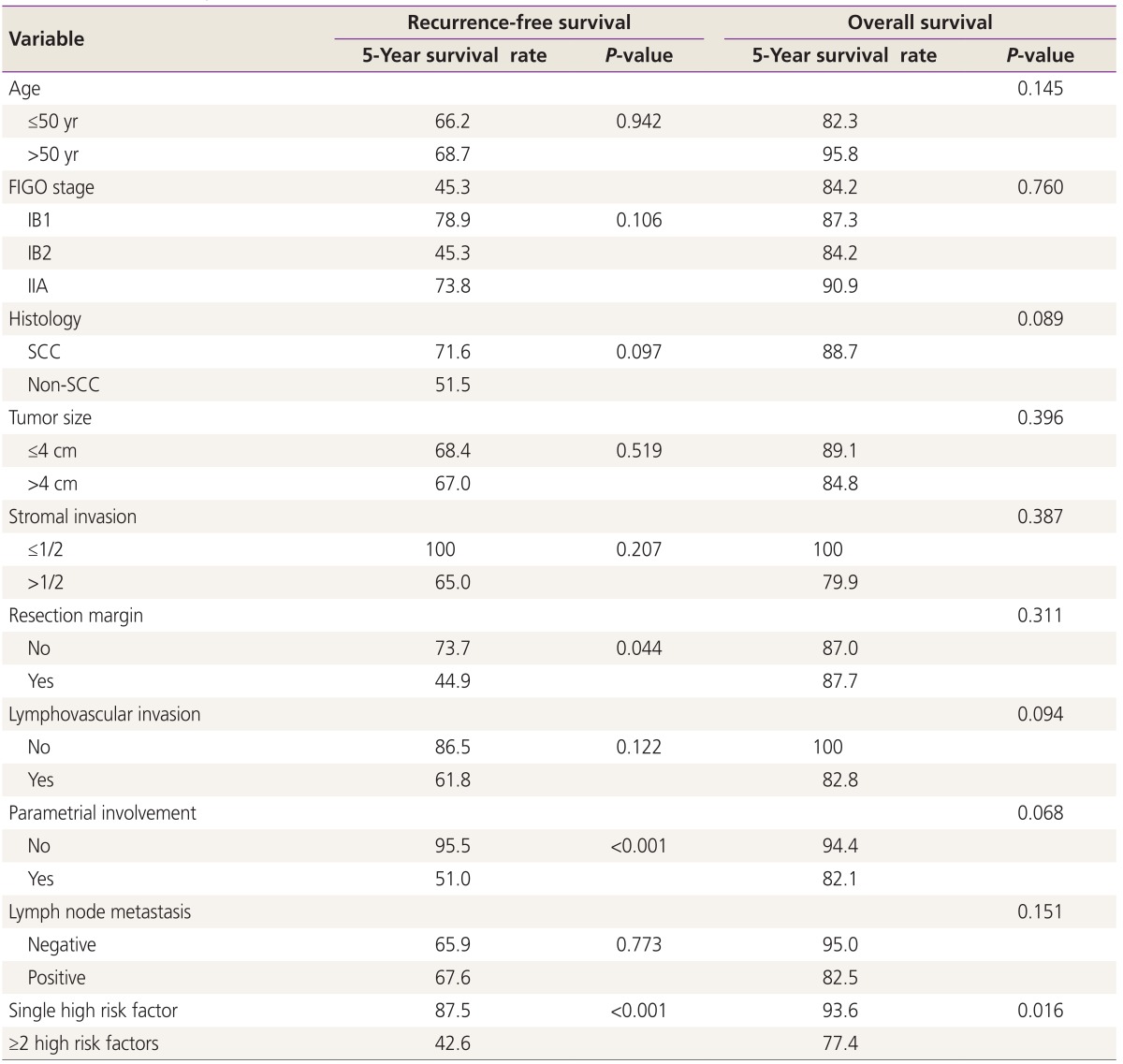
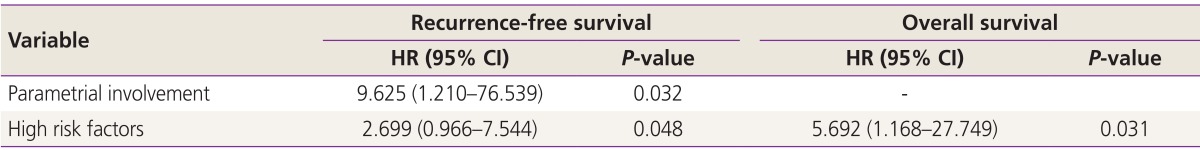
Statistically significant differences in stage and stromal invasion were observed between group 1 and group 2. However, age, histologic subtype, tumor size, and lymphovascular space invasion did not differ significantly among the groups (Table 2). Table 3 and 4 show the results of univariate and multivariate analysis of each variable RFS and OS. The number of high risk factor was statistically significant for both RFS and OS (P<0.001). In the multivariate analysis, parametrial involvement (P=0.032) was statistically significant for RFS (P=0.048), and the number of high risk factors was significant for OS (P=0.031).
Table 2. Distribution of clinicopathologic and intermediate risk factors (n=93).
Table 3. Univariate analysis for recurrence-free survival and overall survival.
FIGO, International Federation of Gynecology and Obstetrics; SCC, squamous cell carcinoma.
Table 4. Multivariate analysis (Cox proportional hazard and stepwise conditional analysis).
HR, hazard ratio; CI, confidence interval.
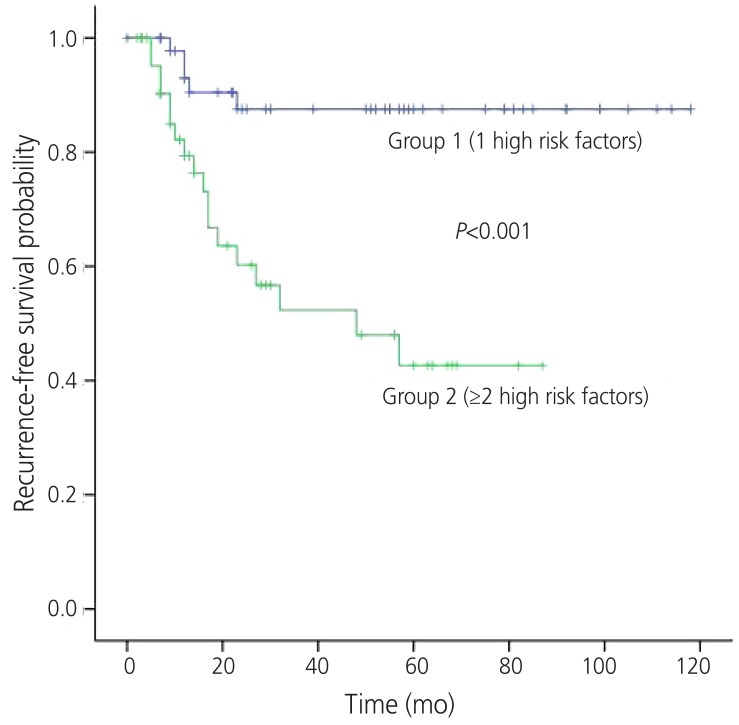
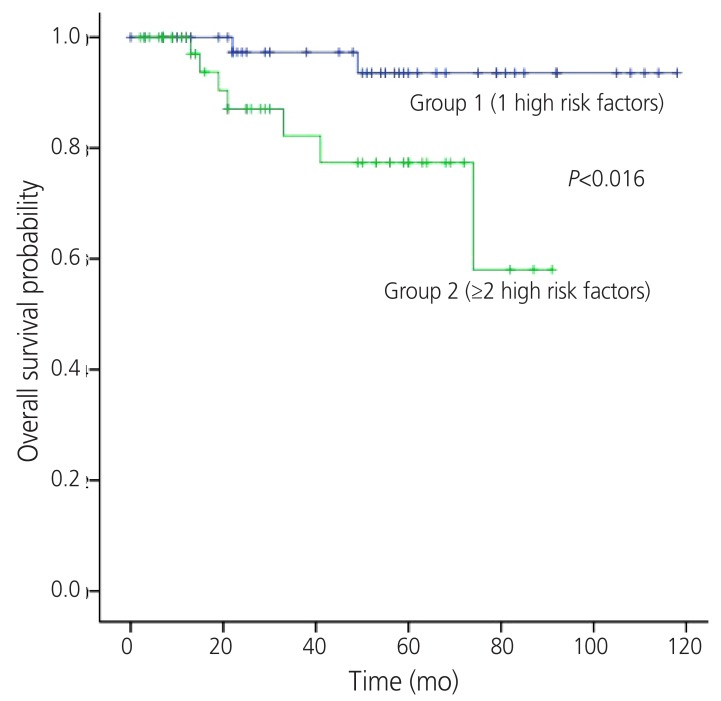
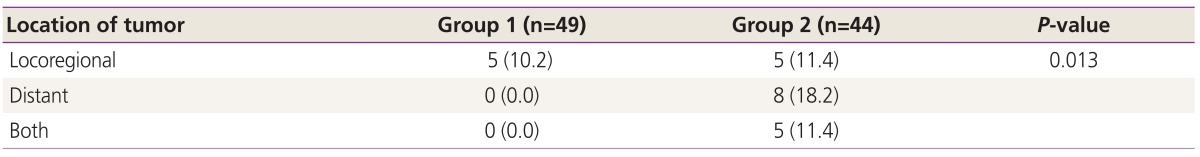
The 5-year RFS rates of group 1 and 2 were 83.4 % and 40.5%, respectively (P<0.001) (Fig. 1). The 5-year OS rates of each group were 92.0% and 79.2%, respectively (P=0.016) (Fig. 2). The location of tumor recurrence according to the number of high risk factors is shown in Table 5. As patients had more high risk factors, distant metastases were increased. No significant difference was observed between chemotherapeutic regimens in patients with recurrence in both groups.
Fig. 1. Recurrence-free survival by the number of high risk prognostic factors.
Fig. 2. Overall survival by the number of high risk prognostic factors.
Table 5. Location of tumor at first recurrence (n=93).
Number in parentheses is the proportion (%) of patients to total patients in each group.
Discussion
We found that RFS and OS differed significantly and the pattern of recurrence differed among these two risk groups. Results of our multivariate analysis showed that the number of high risk factors was significantly prognostic for survival in patients with early-stage cervical cancer who had one or more high risk factors after radical hysterectomy with LN dissection and CCRT. The probability of recurrence and death were higher in patients with multiple high risk factors.
Among various prognostic factors, LN metastasis, parametrial involvement, or positive resection margins are indications for adjuvant CCRT after radical hysterectomy. LN metastasis has the most important prognostic value [9]. However, prognosis of high risk early stage cervical cancer is heterogenous and many patients may have more than one high risk factor, therefore, it may be affected by other clinicopathological risk factors. Several studies have reported that an association of additional risk factors with each high risk factor showed significant correlation with survival. Parametrial involvement and number of positive LN (≥2) are associated with prognosis in patients with positive LN in early stage cervical cancer [10]. In addition, non-squamous cell carcinoma histology, tumor size, and parametrial involvement were associated with survival in patients with LN positive early stage cervical cancer [11]. However, few studies have assessed the clinicopathologic factors related to clinical outcomes in patients according to number of high risk factors.
LN metastasis has been shown to have an obvious impact on prognosis [10,12,13]. The LN status is generally considered to be the most important prognostic factor in cervical cancers treated by radical surgery. In our study, both RFS and OS were not significantly different according to LN metastasis. As an explanation, it may be that CCRT could control recurrence by lymphatic spread. The influence of parametrial involvement on the survival of patients with early stage cervical cancer remains somewhat controversial [14]. Some investigators have reported that parametrial involvement did not show an association with survival in cervical cancer patients with or without LN metastasis [15,16]. In the current study, parametrial involvement was a more important variable in RFS. In particular, in patients with two high risk factors, five patients who had involved resection margin, LN metastasis, and negative parametrium showed no recurrence. However, resection margin status and LN metastasis did not show independent association with survival.
Currently, most patients with high risk factors receive adjuvant RT or CCRT without further evaluation of the risk of recurrence or death. Early stage cervical cancer patients with high risk factors are a heterogenous group with different prognosis according to their clinicopathological risk factors, therefore, other treatment options, such as consolidation chemotherapy may be considered for selected patients [17,18,19].
Patients with multiple-high risk factors also did not show locoregional recurrence but more distant metastasis compared to patients with a single high risk factor, whose majority of recurrence site was locoregional. This appears to be related to failure of systemic control. Indeed, we found that, as the risk factors increased, the rate of both locoregional and distant failure showed a gradual increase.
With regard to results of adjuvant CCRT, an intergroup trial involving the Gynecologic Oncologic Group, the Southerwestern Oncology Group, and the Radiation Therapy Oncology Group reported that postoperative concurrent chemoradiation had 4-year RFS of 81% [9]. In the current study, 5-year RFS of patients with multiple-high risk factors was 42.6% compared to 87.5% of patients with a single high risk factor. The survival difference observed in the current study may be due to the fact that more poor prognostic factors were associated with patients with multiple-high risk factors.
We found that the prognosis and the pattern of recurrence differed according to the number of risk factors, indicating that treatment strategy should be individualized according to risk. In the single risk factor group, most recurrences were located at locoregional sites, indicating that adjuvant RT or CCRT may be appropriate in order to decrease recurrence. However, in the multiple-high risk group, both locoregional recurrence and distant metastasis were common, indicating that systemic adjuvant therapy such as chemotherapy may be appropriate in order to increase survival and to decrease RT-related complications. Currently, there is insufficient evidence regarding the role of consolidation chemotherapy after CCRT or more effective chemotherapeutic regimens during CCRT. According to the Radiation Therapy Oncology Group trial, CCRT could not decrease the para-aortic recurrence after long-term follow up, and more than 50% of patients with recurrence were found to have distant metastasis after CCRT [20]. In a meta-analysis of 18 randomized trials on the effects of chemoradiotherapy for cervical cancer, an additional benefit from adjuvant chemotherapy after CCRT was suggested [21]. Therefore, it is assumed that consolidation chemotherapy after surgery and adjuvant CCRT may have an important effect and serve as a systemic treatment with eradication of micrometastases.
Because this is a retrospective study, selection bias could not be avoided. And there is bias according to the difference of patient number between two groups. However, in order to minimize bias, we enrolled patients who received postoperative CCRT and excluded patients who underwent other types of adjuvant therapy. The result of our study requires validation by another data set in order to determine whether the chosen parameters are valid.
In conclusion, we found that the number of high risk factors was significant for clinical outcomes in patients with high risk factors. For these patients, consideration of new strategies to improve survival may be worthwhile. Conduct of further clinical trials is warranted for development of adjuvant treatment strategies individualized to each risk group.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 3.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 4.Samlal RA, van der Velden J, Schilthuis MS, Gonzalez Gonzalez D, Ten Kate FJ, Hart AA, et al. Identification of high-risk groups among node-positive patients with stage IB and IIA cervical carcinoma. Gynecol Oncol. 1997;64:463–467. doi: 10.1006/gyno.1996.4576. [DOI] [PubMed] [Google Scholar]
- 5.Lai CH, Chang HC, Chang TC, Hsueh S, Tang SG. Prognostic factors and impacts of adjuvant therapy in early-stage cervical carcinoma with pelvic node metastases. Gynecol Oncol. 1993;51:390–396. doi: 10.1006/gyno.1993.1309. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez RD, Soong SJ, Kinney WK, Reid GC, Schray MF, Podratz KC, et al. Identification of prognostic factors and risk groups in patients found to have nodal metastasis at the time of radical hysterectomy for early-stage squamous carcinoma of the cervix. Gynecol Oncol. 1989;35:130–135. doi: 10.1016/0090-8258(89)90029-2. [DOI] [PubMed] [Google Scholar]
- 7.Van Nagel JR, Jr, Donaldson ES, Parker JC, Van Dyke AH, Wood EG. The prognostic significance of cell type and lesion size in patients with cervical cancer treated by radical surgery. Gynecol Oncol. 1977;5:142–151. doi: 10.1016/0090-8258(77)90018-x. [DOI] [PubMed] [Google Scholar]
- 8.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 9.Wertheim MS, Hakes TB, Daghestani AN, Nori D, Smith DH, Lewis JL., Jr A pilot study of adjuvant therapy in patients with cervical cancer at high risk of recurrence after radical hysterectomy and pelvic lymphadenectomy. J Clin Oncol. 1985;3:912–916. doi: 10.1200/JCO.1985.3.7.912. [DOI] [PubMed] [Google Scholar]
- 10.Aoki Y, Sasaki M, Watanabe M, Sato T, Tsuneki I, Aida H, et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol. 2000;77:305–309. doi: 10.1006/gyno.2000.5788. [DOI] [PubMed] [Google Scholar]
- 11.Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Further stratification of risk groups in patients with lymph node metastasis after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol. 2010;117:53–58. doi: 10.1016/j.ygyno.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Uno T, Ito H, Itami J, Yasuda S, Isobe K, Hara R, et al. Postoperative radiation therapy for stage IB-IIB carcinoma of the cervix with poor prognostic factors. Anticancer Res. 2000;20:2235–2239. [PubMed] [Google Scholar]
- 13.Berek JS, Hacker NF. Practical gynecologic oncology. 4th ed. Philadelphia (PA): Lippincott; 2005. [Google Scholar]
- 14.Suprasert P, Srisomboon J, Kasamatsu T. Radical hysterectomy for stage IIB cervical cancer: a review. Int J Gynecol Cancer. 2005;15:995–1001. doi: 10.1111/j.1525-1438.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 15.Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Prognostic factors in node-positive patients with stage IB-IIB cervical cancer treated by radical hysterectomy and pelvic lymphadenectomy. Int J Gynaecol Obstet. 2006;93:130–135. doi: 10.1016/j.ijgo.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Winter R, Haas J, Reich O, Koemetter R, Tamussino K, Lahousen M, et al. Parametrial spread of cervical cancer in patients with negative pelvic lymph nodes. Gynecol Oncol. 2002;84:252–257. doi: 10.1006/gyno.2001.6495. [DOI] [PubMed] [Google Scholar]
- 17.Lahousen M, Haas J, Pickel H, Hackl A, Kurz C, Ogris H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: a randomized, prospective, multicenter trial. Gynecol Oncol. 1999;73:196–201. doi: 10.1006/gyno.1999.5343. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaka T, Kamura T, Yokoyama M, Matsuo N, Nakano H, Sugimori H. Adjuvant chemotherapy after radical hysterectomy for cervical carcinoma: a comparison with effects of adjuvant radiotherapy. Obstet Gynecol. 1998;91:977–981. doi: 10.1016/s0029-7844(98)00079-9. [DOI] [PubMed] [Google Scholar]
- 19.Tattersall MH, Ramirez C, Coppleson M. A randomized trial of adjuvant chemotherapy after radical hysterectomy in stage Ib-IIa cervical cancer patients with pelvic lymph node metastases. Gynecol Oncol. 1992;46:176–181. doi: 10.1016/0090-8258(92)90251-d. [DOI] [PubMed] [Google Scholar]
- 20.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 21.Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]