Abstract
Meniscus injuries remain a significant challenge due to the poor healing potential of the inner avascular zone. Following a series of studies and clinical trials, tissue engineering is considered a promising prospect for meniscus repair and regeneration. As one of the key factors in tissue engineering, cells are believed to be highly beneficial in generating bionic meniscus structures to replace injured ones in patients. Therefore, cell-based strategies for meniscus tissue engineering play a fundamental role in meniscal regeneration. According to current studies, the main cell-based strategies for meniscus tissue engineering are single cell type strategies; cell coculture strategies also were applied to meniscus tissue engineering. Likewise, on the one side, the zonal recapitulation strategies based on mimicking meniscal differing cells and internal architectures have received wide attentions. On the other side, cell self-assembling strategies without any scaffolds may be a better way to build a bionic meniscus. In this review, we primarily discuss cell seeds for meniscus tissue engineering and their application strategies. We also discuss recent advances and achievements in meniscus repair experiments that further improve our understanding of meniscus tissue engineering.
1. Introduction
As a worldwide medical problem, treatment of meniscus injuries has long been a research focus [1–6]. In adult, the distribution of meniscus neurovascular is complex with heterogeneity [7]. The outside region (red-red zone) is full of neurovascular tissues, while the inside region (white-white zone) lacks neurovascular tissue, and the region (red-white zone) between the former two areas displays a transitional characteristic from both the red-red and white-white regions. The neurovascular distribution is often associated with the prognosis of patients with meniscus tear. It is usually difficult to repair the injuries in the white-white zone [8]. The meniscus performs important functions in load bearing, shock absorption, joint lubrication, and joint stability [9–13]. Injury to this structure can greatly influence joint motion and daily living [14, 15]. According to one report, the incidence of meniscus injury resulting in meniscectomy is 61/100,000, of which the medial meniscus represents 81% and the lateral meniscus, 19% [1]. And it will be higher in athletes [16–18].
Meniscectomy is an effective way to relieve pain and joint swelling. However, follow-up surveys show that this type of surgery may damage knee stability and accelerate the development of osteoarthritis, so that many patients undergoing meniscus resection eventually have to accept a total knee arthroplasty [19–22]. Therefore, this choice may be beneficial for short-term purposes but may cause more long-term damage. An arthroscopic partial meniscectomy may be a better method to reduce joint damage [23, 24]. To maintain the stability of the knee, the surgeon may perform a minimally invasive arthroscopic meniscorrhaphy to repair a lacerated meniscus, which results in improved function compared to a meniscectomy and allows earlier joint motion [25–27]. In young patients, to better protect articular cartilage and restore knee function, an “ideal” solution would be meniscus replacement or regeneration [28]. Meniscal allograft transplantation (MAT) can improve knee function and result in good clinical outcomes; however, further evidence is necessary to determine whether it is chondroprotective [29, 30]. Furthermore, it is difficult to resolve resource, shape matching, and ethical issues. Tissue engineering using natural or synthetic matrices as a scaffold to guide tissue repair or regeneration in three dimensions shows promising prospects for meniscus regeneration [31].
In meniscus tissue engineering, a scaffold is the basis for regenerating a new structure. Scaffold materials are typically selected from polymeric synthetic materials, such as polyglycolic acid (PGA) and poly-L-lactic acid (PLA), and natural biological products, such as silk, collagen type I, and proteoglycans [32–35]. The production process has ranged from “traditional” molding to electrospinning and more recently to three-dimensional (3D) printing technology [32, 33, 36, 37]. Research regarding scaffold preparation has made great progress. Scientists at Columbia University successfully built a meniscus with polycaprolactone (PCL) via 3D printing technology, which was loaded with connective tissue growth factor (CTGF) and transforming growth factor- (TGF-) β3. It was shown to induce internal stem cell migration and differentiation to regenerate a new meniscus [37]. This treatment has been successful in sheep. Stone et al. [38, 39] developed a collagen type I scaffold isolated from bovine tendon that has been used clinically. Growth factors such as TGF and fibroblast growth factors (FGF) play important roles in regulating cell growth and cell differentiation and are regarded as key elements in tissue engineering [40–42]. The growth factors were also used to induce cells to differentiate from stem cells to obtain more cell resources for meniscus tissue engineering [34, 43–45]. Moreover, the studies also revealed that a hypoxic environment is able to slow down cell dedifferentiation process, and mechanical stimulation can improve collagen and glycosaminoglycan (GAG) secretion [44, 46–48]. A bioreactor, which simulates the knee microenvironment, can provide a better platform for meniscus tissue engineering research [49, 50].
The study has shown that a cell-based tissue-engineered meniscus achieves better repair results than cell-free ones [51]. Therefore, current research on meniscus tissue engineering has focused on the use of cells. As one of the three elements of tissue engineering, the choice of cell seed is particularly important. In early research, cells used in meniscus tissue engineering were mainly of a single cell type, and it was difficult to copy the native tissue features. Inspired by the coexistence of cells in the knee and their interaction, researchers carried out cell cocultures to build a tissue-engineered meniscus with better mechanical and cartilaginous properties. This method also solved cell supply issues to some extent. After further understanding the complex structure of the meniscus, combined with the theory of cell coculture, zonal recapitulation was proposed to mimic the organizational heterogeneity of the meniscus. To increase cell interactions and reduce the interference of nonbiological components, research was also conducted to build a tissue-engineered meniscus with cell self-assembly alone without any scaffolds. In this review, we review the reports on cell-based meniscus repair and discuss cell application strategies from single cell type, cell coculture, zonal recapitulation, and scaffold-free cell self-assembly, respectively (Table 1).
Table 1.
Cell application in meniscus tissue engineering.
| Strategy | Cell application | Cell resource | Author |
|---|---|---|---|
| Single cell type | MC | Human (18–84 years) | Baker et al., 2009 [58] |
| New Zealand white rabbits | Gunja and Athanasiou, 2010 [41] | ||
| Allogeneic rabbit cell | Kang et al., 2006 [32] | ||
| New Zealand white rabbits | Esposito et al., 2013 [62] | ||
| AC | Swine | Yoo et al., 2011 [63] | |
| Autologous sheep chondrocytes | Kon et al., 2008 [64] | ||
| Autologous and allogenic swine chondrocytes | Weinand et al., 2006 [66] | ||
| SMC | Adult equine | Fox et al., 2010 [70] | |
| Canine | Warnock et al., 2014 [71] | ||
| SC | Rat bone marrow | Yamasaki et al., 2005 [77] | |
| Femoral and tibial bone marrow of calves | Nerurkar et al., 2011 [78] | ||
| Femoral and tibial bone marrow of calves | Baker et al., 2011 [44] | ||
| Human iliac crest (27–55 years) | Petri et al., 2012 [48] | ||
| Adult human synovial membranes | Sakimura et al., 2006 [81] | ||
|
| |||
| Cell coculture | MC and AC | Knee joint of rabbit | Gunja and Athanasiou, 2009 [87] |
| MC and MSCs | Human meniscus and human MSCs | Cui et al., 2012 [92] | |
| MC and BMSC | Human meniscus and iliac crest of patients | Diao et al., 2013 [91] | |
| MC and BMSC | Human meniscus and human MSCs | Matthies et al., 2013 [93] | |
| MC and SMSC | The knees of pigs | Tan et al., 2010 [95] | |
|
| |||
| Zonal recapitulation | AC and FBC | Human donors (<35 years) | Mandal et al., 2011 [33] |
| AC and MC | Stifle joint of calves | Higashioka et al., 2014 [97] | |
|
| |||
| Scaffold-free | SMC | Synovial villi from canine with stifle osteoarthritis | Warnock et al., 2014 [72] |
| SMSC | Porcine synovial membranes | Moriguchi et al., 2013 [102] | |
| MC and AC | Knee joints of calves | Huey and Athanasiou, 2011 [104] | |
| MC and AC | Knee joints of calves | Hadidi et al., 2015 [106] | |
MC: meniscus cell, AC: articular chondrocyte, MSC: mesenchymal stem cells, BMSC: bone marrow mesenchymal stem cells, SMC: synovial membrane cell, SC: stem cell, SMSC: synovial membrane mesenchymal stem cells, FBC: fibroblast cells, LPA: phospholipid lysophosphatidic acid, FBS: fetal bovine serum, and ADSC: adipose-derived stem cells.
2. Single Cell Type with a Scaffold
As a “classical” strategy for a tissue-engineered meniscus, this method has been used in many studies and as the basis for further cell-based developments. The cells, which include fibrochondrocytes, chondrocytes, synoviocytes, or stem cells, are all involved in building a construct with scaffolds. Herein, we discuss the application of these cells one by one to find an appropriate seed for meniscus tissue engineering.
3. Meniscus Fibrochondrocytes
The structure of the meniscus is complex with heterogeneity (Figure 1). Meniscal extracellular matrix (ECM) mainly comprised collagen fibers and proteoglycans. Most of collagen fibers bundles oriented circumferentially; only few of them presented a radial alignment. The oriented structure of the fibrillar collagens is closely associated with meniscus biomechanical properties. Therefore, it is important to restore the microarchitecture of the collagen fibers for successfully repairing or regenerating the meniscus [52]. The populations of meniscus cells can be divided into three distinct groups: fibrochondrocytes are cells with a round or oval shape mostly located in the inner two-thirds of the meniscus; they express both collagen I and collagen II. Fibroblast-like cells are elongated and found mainly in the outer one-third, the ECM of which is mainly collagen I. Cells of the superficial zone are fusiform in shape and located below the tissue surface [53–55].
Figure 1.
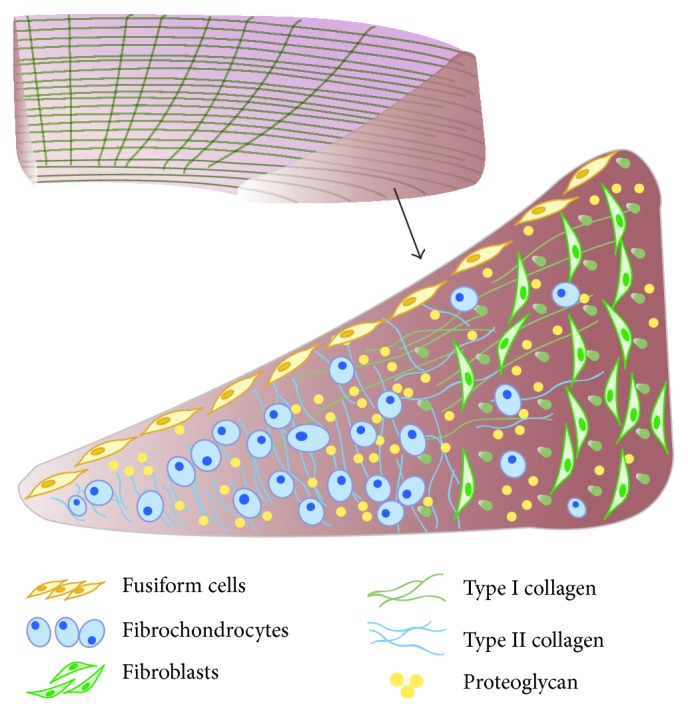
Schematic diagram of meniscus internal ultrastructure. Most of collagen fibers bundles oriented circumferentially; only few of them presented a radial alignment. Cells of the superficial zone are fusiform in shape and located below the tissue surface; the cells in the outer one-third region or vascular zone are mainly elongated fibroblasts, and collagen I accounts for >90%; for inner two-thirds region or avascular zone, round or oval shaped fibrochondrocytes are interspersed, the ratio of collagen I and collagen II is about 2 : 3.
Currently, most studies focusing on cells isolated from the meniscus involve fibrochondrocytes [56, 57]. One advantage of a substitute built with in situ derived cells is better histocompatibility. Baker et al. [58] conducted an experiment in which meniscus cells isolated from 10 human donors who underwent knee surgery were expanded to passage two in monolayer culture and then seeded onto PCL fiber-aligned biodegradable nanofibrous scaffolds and cultured for 10 weeks. The results showed that all constructs seeded with meniscus cells increased in dry weight, DNA, collagen, and GAG contents in a time-dependent manner, and the mechanical properties of the meniscus-derived cell-laden constructs strongly correlated with collagen content, but not donor age. Correlation analysis between collagen content in constructs and the mechanical properties suggests that increasing the collagen deposition of constructs may further enhance their mechanical properties. Similarly, this indicated that autologous cells derived from the patients themselves could represent a potential cell resource for meniscus tissue engineering. However, the number of primary cells isolated from the meniscus was not sufficient to satisfy the needs for tissue engineering. To overcome this, an approach often used is to expand primary cells to passage two or three in monolayer culture until the cell number is sufficient. However, cellular phenotypes and gene expression levels can be changed during passaging, indicating that cells are in a dedifferentiation process [59]. Other studies have shown that the addition of fibroblast growth factor (FGF) into the culture solution or as a coating on the scaffold can inhibit cell dedifferentiation and promote secretion of the ECM [41, 60, 61]. Culturing cells in hypoxic conditions also have a similar effect [46, 47]. Kang et al. [32] used fibrochondrocytes seeded on PGA/PLGA scaffolds to repair rabbit models with a total meniscectomy in vivo. After 6 and 10 weeks, a neomeniscus formed with the appearance of fibrocartilaginous tissue. Moreover, the histological and immunohistochemical structures in the neomeniscus were similar to those of the native meniscus. After 36-week transplantation, histochemical and immunohistochemical structures of neomeniscus were more aligned than those of 10 weeks. Similarly, in the anterior areas, biomechanical properties in neomeniscus were higher than those of native meniscus. However, biomechanical properties of neomeniscus at middle and posterior sites were lower than those of native meniscus. Esposito et al. [62] preseeded fibrochondrocytes on PLDLA/PCL-T scaffolds for three weeks, at which point the constructs were implanted to replace the medial meniscus in rabbits. The constructs showed good compatibility with surrounding tissues, and fibrocartilaginous tissue with mature collagen fibers appeared in histology results after 24 weeks. These experiments indicate the feasibility of regenerating meniscus with meniscus cells using tissue engineering.
4. Articular Chondrocytes
The limited supply of allogeneic or autologous meniscus cells limits their application for meniscus tissue engineering, and monolayer expansion can lead to significant changes in cellular phenotype [59]. Cartilaginous cells, such as articular chondrocytes, are regarded as a promising cell source. Fibrochondrocytes and chondrocytes are both from cartilage tissue, and a study showed their similar cell membrane markers and high expression of collagen II, the major component of the ECM [55]. These similarities in cellular phenotype and molecular biology make it possible for chondrocytes to be used as cell seeds for meniscus tissue engineering. Yoo et al. [63] embedded PLGA scaffolds implanted with chondrocytes subcutaneously in nude mice for seven days to evaluate the fibrocartilage formation ability of the construct. Histology results showed fibrocartilaginous neotissue generation between meniscus discs, demonstrating the feasibility of chondrocytes as cell seeds for meniscus tissue engineering. Moreover, significantly more cartilaginous tissue and complete healing of the meniscus defects was observed in a platelet-rich plasma- (PRP-) treated hyaluronic acid/PCL scaffold seeded with autologous chondrocytes in a total meniscectomy sheep model; the author believed that seeding of the scaffolds with autologous chondrocytes provided additional benefits for fibrocartilaginous tissue repair [64, 65]. However, harvesting autologous cells could cause additional trauma to patients. Weinand et al. [66] compared the repair capacity of autologous and allogeneic cells for meniscus lesions and found that the remediation results between the two cell types showed no significant difference. This experiment demonstrated the essentially equal potential of autologous and allogeneic chondrocytes for meniscus tissue engineering and will likely broaden the possible cell sources for cartilaginous tissue engineering if ethical issues can be resolved. Extensive research on chondrocytes and their successful application in cartilage repair will be beneficial for their application in meniscus tissue engineering.
5. Synoviocytes
As the most abundant tissue in the knee joint, the synovial membrane can affect the development of osteoarthritis by producing microRNAs and other factors [67]. Synoviocytes are considered a resource for meniscus tissue engineering due to their chondrogenic behavior. Studies have shown that synoviocytes can form a meniscus-like matrix in vitro, and FGF promotes collagen II formation and a chondrocytic cell phenotype [68, 69]. Fox et al. [70] investigated the feasibility of fibroblast cells derived from synovial tissue to generate a meniscus construct in vitro. Equine synoviocytes were seeded onto a PGA/PLLA scaffold and cultured with growth factors in a rotating bioreactor. Reverse transcription polymerase chain reaction (RT-PCR) results indicated that fibroblast cells exhibit fibrochondral characteristics, and growth factors improve the expression of collagen II and GAGs. As previously mentioned, bioreactors, which provide mechanical stimulation, have a positive effect on cell differentiation, cell viability, ECM production, and compressive biomechanical properties. As research showed culturing a synoviocyte-seeded PGA scaffold in a rotating bioreactor resulted in much better cell and matrix characteristics than when cultured in static conditions [71]. Furthermore, the research has shown that synoviocytes derived from osteoarthritic joints can produce meniscal fibrocartilage ECM components at levels similar to those of normal synoviocytes [72]. These achievements demonstrate the feasibility of synoviocytes as a potential cell source for meniscus tissue engineering.
6. Stem Cells
Stem cells exhibit features of multidirectional differentiation. Stem cells can be divided into embryonic stem cells and adult stem cells. The latter, such as bone marrow mesenchymal stem cells (BMSC) and adipose-derived stem cells (ADSC), have been a focus of research due to their wide distribution and species diversity [73, 74]. Studies have shown that stem cells can differentiate into cartilaginous cells with abundant matrix; therefore, stem cells can be used as cell seeds for meniscus tissue engineering [75, 76]. Yamasaki et al. [77] built a construct similar to a native meniscus using rat BMSC and a decellularized meniscus scaffold. Nerurkar et al. [78] and Baker et al. [44] built a meniscus substitute with bovine BMSC and aligned PCL electrospun scaffolds and found that dynamic culture conditions and mechanical stimulation were beneficial for stem cells infiltration and proliferation, collagen deposition, gene expression, and reinforcing mechanical properties. The ECM generated in the structure was sufficient and resembled the collagen and GAG content in the native meniscus. It is noteworthy that the duration of preculture may play a role in BMSC responding to the mechanical stimulation, because the condition of preculture can provide a sufficient time for cell colonization and ECM deposition. In addition, human adult BMSC were also investigated to generate a meniscus substitute. The results showed that BMSC improved the tensile strength of collagen scaffold, and mechanical stimulation enhanced the mechanical properties of the structure [34, 48].
Many MSCs exist in synovial membranes and can be induced to differentiate into chondrocytes under appropriate culture conditions [79, 80]. Sakimura et al. [81] found synergistic effects of TGF-β1 and Insulin-like Growth Factor-1 (IGF-1) on synovial MSC in cartilaginous differentiation and GAG production for repairing meniscus defects. Some researchers believe that MSCs in synovial tissue can migrate to damaged areas to promote restoration, just like the scaffold loaded with CTGF and TGF-β3 can regenerate a meniscus via stem cell recruiting [37]. Injections of MSC derived from the synovial membrane have been used to repair meniscus defects in rat, rabbit, and pig models, resulting in effective new fibrocartilage generation [82–84]. Due to their quantity, ease of acquisition and multidirectional differentiation, synovial MSC may represent good prospects in future meniscus tissue engineering.
Direct injection of stem cells into the knee joint for the treatment of meniscus defects and osteoarthritis has been assessed in clinical trials [85]. However, tissue engineering is a better method to infuse a large number of stem cells. Not only does a scaffold provide a specific space for cell attachment and proliferation to maintain high levels of stem cells in a particular area, but also loading growth factors induce stem cell homing to the defect to improve repair and regeneration results.
7. Cell Coculture
According to previous research, with a tissue-engineered meniscus based on a single cell type, it is difficult to copy microstructural features of the native meniscus. Cell coculture, culturing two cell types, is a strategy used to improve the biochemical and biomechanical properties of a tissue-engineered meniscus through cellular interactions and mitigates the need for cell expansion [86]. There are two types of cell coculture systems: direct and indirect. The former is a mixed cell culture, which works through direct cell interaction, while the latter involves separating the cell culture in one system and works through the transfer of growth factors; this is also known as paracrine secretion.
Research has shown that collagen dominates the tensile response while GAG is important for compressive properties [43]. One study found that meniscus fibrochondrocytes alone were not able to produce enough collagen type II and GAG, while pure chondrocytes were unable to deposit sufficient collagen type I to sustain tensile strength. Could coculturing two types of somatic cells have a synergistic effect? Gunja and Athanasiou [87] built a construct by coculturing meniscus cells and chondrocytes on PLLA scaffolds and found that coculturing not only increased ECM secretion but also generated a meniscus structure with better mechanical properties. The compressive properties in cell-seeded constructs approached those of the native meniscus. However, much work needs to be done which enhances the tensile properties in the cell-seeded constructs towards those of the native tissue.
MSCs have the potential to differentiate along the chondrogenic pathway in appropriate conditions, as well as deliver trophic effects to differentiated cells [88–90]. In a coculture system, somatic cells provide inductive stimulation for stem cell differentiation, while stem cells supply various growth factors to maintain cellular phenotype and regulate cell proliferation. Previous studies reported that coculture of MSC with chondrocytes enhances ECM production and inhibits hypertrophy [91], so why not with meniscus cells? Cui et al. [92] cocultured meniscus cells with MSCs at different ratios and found that coculturing not only promoted levels of collagen type I and GAG production but also prevented cell hypertrophy, with meniscus cells and MSC at a 75 : 25 ratio showing the best results. The studies also found that coculturing meniscus cells isolated from osteoarthritic patients with BMSC in a pallet contribute to similar results as normal meniscus cells [93, 94]. These studies strengthen the combined application of meniscus cells and MSCs as a cell source for meniscus tissue engineering. Tan et al. [95] cocultured meniscus cells with synovium-derived stem cells on small intestine submucosa to engineer a meniscus substitute construct and found that this coculture-based tissue construct exhibited higher GAG and collagen II levels, resulting in a concomitant increase in mechanical properties. MSCs derived from synovial tissue secrete IGF-1 with anti-inflammatory effects, which is beneficial in preventing osteoarthritis [96]. Thus, cell coculture not only enriches the choice of cell seeds but also serves as an effective method to maintain cellular phenotype and improve repair effects and may represent a promising strategy for meniscus tissue engineering.
8. Zonal Recapitulation
Compositional differences in the meniscus inner and outer regions make it difficult to fabricate a bionic meniscus. To generate a tissue-engineered meniscus successfully, mimicking its complex internal architecture is important. The heterogeneity of cell types and matrix components in the inner and outer regions makes it nearly impossible for meniscus reconstruction and complete regeneration via tissue engineering methods, especially when relying on a method to build a construct with a single cell type and scaffold. Hence, the idea of seeding different cell types on different regions of a scaffold to create a biomimetic substitute in both structure and content has been developed. This strategy simultaneously creates an indirect coculture system when effectively applying the cells' different physiological characteristics. Mandal et al. [33] have designed a multilayer meniscus scaffold fabricated with silk to mimic native meniscus structure; human articular chondrocytes and dermal fibroblast cells were seeded at the periphery and center of the scaffold, respectively. Scanning electron microscopic (SEM) results reveled that the top two layers showed circular pores, while the bottom third layer had laminar channels. After culturing for four weeks in vitro, the results showed that the cell-seeded construct improved mechanical properties, increased cell populations, enhanced ECM deposition, and maintained a chondrocytic phenotype. Thus, the author suggested that a construct with multiple layers and porosity can act as an effective medium to direct cell orientation and neotissue formation, resembling the structural and mechanical anisotropy of the native meniscus. Higashioka et al. [97] used agar without cell adhesion to build an anisotropic graft with zonal variations; the inner one-third was seeded with 100% chondrocytes and the outer region was generated by coculturing chondrocytes with meniscus cells. The two zones of the structure integrated well and exhibited significantly different mechanical and biochemical properties after culturing. This is a case that combined zonal recapitulation with cell coculture. With the application of 3D printing technology, it may be more conducive to regenerate a construct that is more biomimetic of the native meniscus. Zonal recapitulation is a more suitable method to mimic the complex internal architecture of the meniscus and may be a better cell application strategy for meniscus tissue engineering.
9. Scaffold-Free Tissue Engineering
In “traditional” meniscus tissue engineering, the scaffold is an important factor. Biodegradability, biocompatibility, and certain mechanical strength are necessary for a scaffold [39]. However, it is difficult to copy the complex internal structure of the meniscus with the technologies available today. Some researchers have proposed generating a new meniscus relying on self-assembling cells in a specific artificial environment, simulating the tissue generation process; this is also referred to as scaffold-free culture [98]. High-density cells form a tissue through close interactions in a specific environment, which not only reduces the obstruction of the scaffold but also is more conducive to crosslinking collagen fibers in the ECM to promote mechanical strength [99]. This method can also be divided into single cell type self-assembly and coculture self-assembly.
10. Single Cell Type Self-Assembly
Single cell type self-assembly is mostly used to regenerate the inner part of the meniscus, in which fibrochondrocytes represent the main cell type. A series of experiments were conducted to explore the possibility of generating a neotissue via cell self-assembly. Ballard et al. [100] compared the properties of bioscaffolds generated by meniscus fibrochondrocytes and synoviocytes and determined they had similar collagen and GAG content. He also found that treating synovial neotissue with chondrogenic growth factors (bFGF, TGF-β1, and IGF-1) or mechanical stimulation showed greater fibrocartilage-like matrix content and better biomechanical properties [101]. In addition, he demonstrated the fibrochondrogenic potential of synoviocytes from osteoarthritic joints, making them a potential cell source for meniscal tissue engineering [72]. These studies confirm the feasibility of a synovial bioscaffold as a replacement therapy for meniscus defects. Moreover, Moriguchi et al. [102] used an allogeneic synovial MSC bioscaffold to repair swine meniscus defects. The defects implanted with the bioscaffold were consistently repaired by fibrocartilaginous tissue with good tissue integration and cartilage protection after six months. These results suggest that a scaffold-free tissue-engineered construct could represent a promising cell-based implant to repair meniscus lesions.
11. Cells Coculture Self-Assembly
Cell coculture not only offers more options to generate a meniscus construct but also promotes biochemical and biomechanical properties of the tissue-engineered meniscus. Hoben et al. [103] compared constructs with self-assembling chondrocytes, meniscus fibrochondrocytes, and cocultures of fibrochondrocytes and chondrocytes and found that the coculture resulted in a mixed collagen I and collagen II matrix similar to the native meniscus. To achieve a scaffold-free construct with better mechanical properties mimicking the native meniscus, Huey and Athanasiou [104] combined the self-assembly process with the catabolic enzyme chondroitinase-ABC and TGF-β1 and resulted in a mature neotissue with a higher radial and compressive modulus. This study revealed that self-assembly approach can produce a tissue-engineered construct that is similar to the biochemical and biomechanical characteristics of native meniscus. Hadidi and Athanasiou [105] applied the phospholipid lysophosphatidic acid (LPA) to enhance the mechanical properties of a construct generated by chondrocytes and meniscus cells via a self-assembling process. Moreover, this research further revealed that LPA mainly depended on inducing cytoskeletal reorganization and cell-matrix traction to enhance the mechanical properties. He also investigated the appropriate number of cells to create a scaffold-free structure for meniscus tissue engineering and found that coculturing chondrocytes and meniscus cells at a ratio of 1 : 1 with lower seeding density resulted in beneficial effects on self-assembling fibrocartilage [106]. These studies have shown the feasibility of generating an effective substitute without a scaffold, and the results demonstrated favorable biomechanical properties of the neotissue. From these achievements, we conclude that cells are the most important factor for scaffold-free culturing, and obtaining a sufficient number of cells is the key for applying this technology.
In the future, it will be vital to solve the meniscus regeneration problem, regardless of how the meniscus is created, especially the injuries in the white-white areas of the meniscus, because the role vessels play in maintaining the meniscus in this areas is inadequate. This dilemma might be addressed by the following approaches. In the one side, creating some vascular channels to the scaffolds or direct injection of some stem cells may enhance the meniscus regeneration to some extent; on the other side, loading some growth factors or PRP to the scaffolds may also be beneficial to form a tissue-engineered meniscus. However, some drawbacks exist in the application of constructs. For scaffolds with a single cell type or cell coculture, the mechanical properties are sufficient if the construct is only needed for avascular region regeneration, but it will be difficult for fixation and ideal results, whereas if the construct is needed for whole regeneration, it will be difficult to satisfy the required radial tensile strength. For zonal recapitulation, the mechanical difference between the inner and outer regions is large, and the transition zone will tear easily. For scaffold-free culturing, improving the mechanical properties remains important.
Considering all the current advances, we can envisage an ideal tissue-engineered meniscus in the future: regarding the meniscus internal structure as a blueprint and using the meniscus ECM/cells mixture as material for 3D printing technology, it will be possible to build a tissue-engineered meniscus with zonal heterogeneity, while growth factors within the construct will not only promote cell proliferation and maintain cellular phenotype but also induce recruiting of stem cells in vivo to promote meniscus regeneration. However, for a cell-scaffold construct in vivo, cell supply issues and how to mobilize the migration of stem cells are problems that still need to be resolved.
12. Conclusions
Meniscus tissue engineering is a main focus but remains difficult. It will be essential to create a structure mimicking the architectural and mechanical properties of the native meniscus. Because the natural complex internal architecture is still not absolutely clear, a method based on zonal recapitulation may be more promising with the further development of 3D printing technology. However, scaffold-free cell self-assembly methods to generate a functional meniscus graft with good mechanical properties may be a possibility if an appropriate environment can be created. In conclusion, three main parts, scaffolds, cells and growth factors, need further clarification in order to create an ideal tissue-engineered meniscus. We still have a long way to go.
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (2012AA020502 and 2015AA020303), the National Natural Science Foundation of China (81472092), and the Natural Science Foundation of Shanxi Province, China (2013011057-3).
Disclosure
Wei Niu, Weimin Guo, and Shufeng Han are the coauthors, while Shuyun Liu and Quanyi Guo were the corresponding authors, because Dr. Weimin Guo has done a lot of work in revising the paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Wei Niu, Shufeng Han, Shuyun Liu, and Weimin Guo contributed equally to this work.
References
- 1.Baker B. E., Peckham A. C., Pupparo F., Sanborn J. C. Review of meniscal injury and associated sports. The American Journal of Sports Medicine. 1985;13(1):1–4. doi: 10.1177/036354658501300101. [DOI] [PubMed] [Google Scholar]
- 2.Perrone D. Upon a case of right internal meniscus injur, meniscectomy. Medicina, Cirurgia, Farmacia. 1946;8:20–23. [PubMed] [Google Scholar]
- 3.Pazzi M. Indications for partial meniscectomy. Rivista Degli Infortuni e Delle Malattie Professionali. 1959;46:691–700. [PubMed] [Google Scholar]
- 4.Grana W. A., Connor S., Hollingsworth S. Partial arthroscopic meniscectomy: a preliminary report. Clinical Orthopaedics and Related Research. 1982;164:78–83. [PubMed] [Google Scholar]
- 5.Garrett J. C., Stevensen R. N. Meniscal transplantation in the human knee: a preliminary report. Arthroscopy. 1991;7(1):57–62. doi: 10.1016/0749-8063(91)90079-d. [DOI] [PubMed] [Google Scholar]
- 6.Liu C., Toma I. C., Mastrogiacomo M., Krettek C., Von Lewinski G., Jagodzinski M. Meniscus reconstruction: today's achievements and premises for the future. Archives of Orthopaedic and Trauma Surgery. 2013;133(1):95–109. doi: 10.1007/s00402-012-1624-2. [DOI] [PubMed] [Google Scholar]
- 7.Clark C. R., Ogden J. A. Development of the menisci of the human knee joint. Morphological changes and their potential role in childhood meniscal injury. The Journal of Bone & Joint Surgery—American Volume. 1983;65(4):538–547. [PubMed] [Google Scholar]
- 8.Arnoczky S. P., Warren R. F. Microvasculature of the human meniscus. The American Journal of Sports Medicine. 1982;10(2):90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 9.Seedhom B. B., Dowson D., Wright V. Proceedings: functions of the menisci. A preliminary study. Annals of the Rheumatic Diseases. 1974;33, article 111 doi: 10.1136/ard.33.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaspers P., de Lange A., Huiskes R., van Rens T. J. The mechanical function of the meniscus, experiments on cadaveric pig knee-joints. Acta Orthopædica Belgica. 1980;46:663–668. [PubMed] [Google Scholar]
- 11.Renström P., Johnson R. J. Anatomy and biomechanics of the menisci. Clinics in Sports Medicine. 1990;9(3):523–538. [PubMed] [Google Scholar]
- 12.Zimny M. L., Albright D. J., Dabezies E. Mechanoreceptors in the human medial meniscus. Acta Anatomica. 1988;133(1):35–40. doi: 10.1159/000146611. [DOI] [PubMed] [Google Scholar]
- 13.Markolf K. L., Mensch J. S., Amstutz H. C. Stiffness and laxity of the knee—the contributions of the supporting structures. A quantitative in vitro study. The Journal of Bone & Joint Surgery—American Volume. 1976;58(5):583–594. [PubMed] [Google Scholar]
- 14.Ficat P. Role of the meniscus in traumatology of the knee. Toulouse Médical. 1953;54(3):147–156. [PubMed] [Google Scholar]
- 15.Quintero Esguerra J., Malagon Castro V. Traumatic lesions of the meniscus of the knee. Medicina y Cirugía. 1952;16(5):181–187. [PubMed] [Google Scholar]
- 16.Kujala U. M., Kvist M., Osterman K. Knee injuries in athletes. Review of exertion injuries and retrospective study of outpatient sports clinic material. Sports Medicine. 1986;3(6):447–460. doi: 10.2165/00007256-198603060-00006. [DOI] [PubMed] [Google Scholar]
- 17.DeHaven K. E. Meniscus repair in the athlete. Clinical Orthopaedics and Related Research. 1985;198:31–35. [PubMed] [Google Scholar]
- 18.Cox C. L., Deangelis J. P., Magnussen R. A., Fitch R. W., Spindler K. P. Meniscal tears in athletes. Journal of Surgical Orthopaedic Advances. 2009;18(1):2–8. [PubMed] [Google Scholar]
- 19.Baratz M. E., Fu F. H., Mengato R. Meniscal tears: The effect of meniscectomy and of repair on intraarticular contact areas and stress in the human knee. A preliminary report. The American Journal of Sports Medicine. 1986;14(4):270–275. doi: 10.1177/036354658601400405. [DOI] [PubMed] [Google Scholar]
- 20.Horsky I., Huraj E., Sr., Huraj E., Jr., Sklovsky A. Degenerative changes in the knee joint after meniscectomy. Acta Chirurgiae orthopaedicae et Traumatologiae Čechoslovaca. 1987;54:517–521. [PubMed] [Google Scholar]
- 21.Pengas I. P., Assiotis A., Nash W., Hatcher J., Banks J., McNicholas M. J. Total meniscectomy in adolescents: a 40-year follow-up. The Journal of Bone and Joint Surgery—British Volume. 2012;94(12):1649–1654. doi: 10.1302/0301-620x.94b12.30562. [DOI] [PubMed] [Google Scholar]
- 22.Salata M. J., Gibbs A. E., Sekiya J. K. A systematic review of clinical outcomes in patients undergoing meniscectomy. The American Journal of Sports Medicine. 2010;38(9):1907–1916. doi: 10.1177/0363546510370196. [DOI] [PubMed] [Google Scholar]
- 23.Northmore-Ball M. D., Dandy D. J., Jackson R. W. Arthroscopic, open partial, and total meniscectomy. A comparative study. The Journal of Bone & Joint Surgery—British Volume. 1983;65(4):400–404. doi: 10.1302/0301-620X.65B4.6874710. [DOI] [PubMed] [Google Scholar]
- 24.DeHaven K. E. Meniscus repair—open vs. Arthroscopic. Arthroscopy. 1985;1:173–174. doi: 10.1016/s0749-8063(85)80006-2. [DOI] [PubMed] [Google Scholar]
- 25.Steenbrugge F., Verdonk R., Verstraete K. Long-term assessment of arthroscopic meniscus repair: a 13-year follow-up study. The Knee. 2002;9(3):181–187. doi: 10.1016/s0968-0160(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 26.Majewski M., Stoll R., Müller W., Friederich N. F. Rotatory stability of the knee after arthroscopic meniscus suture repair: a 5-to-17-year follow-up study of isolated medial and lateral meniscus tears. Acta Orthopaedica Belgica. 2009;75(3):354–359. [PubMed] [Google Scholar]
- 27.Steenbrugge F., Corteel J., Verdonk R., Verstraete K. Long-term assessment of arthroscopic meniscus repair. Revue de Chirurgie Orthopedique et Reparatrice de l'Appareil Moteur. 2003;89(8):699–706. [PubMed] [Google Scholar]
- 28.Giuliani J. R., Burns T. C., Svoboda S. J., Cameron K. L., Owens B. D. Treatment of meniscal injuries in young athletes. The Journal of Knee Surgery. 2011;24(2):93–100. doi: 10.1055/s-0031-1280877. [DOI] [PubMed] [Google Scholar]
- 29.Smith N. A., MacKay N., Costa M., Spalding T. Meniscal allograft transplantation in a symptomatic meniscal deficient knee: a systematic review. Knee Surgery, Sports Traumatology, Arthroscopy. 2014;23(1):270–279. doi: 10.1007/s00167-014-3310-0. [DOI] [PubMed] [Google Scholar]
- 30.Vundelinckx B., Vanlauwe J., Bellemans J. Long-term subjective, clinical, and radiographic outcome evaluation of meniscal allograft transplantation in the knee. The American Journal of Sports Medicine. 2014;42(7):1592–1599. doi: 10.1177/0363546514530092. [DOI] [PubMed] [Google Scholar]
- 31.Scotti C., Hirschmann M. T., Antinolfi P., Martin I., Peretti G. M. Meniscus repair and regeneration: review on current methods and research potential. European Cells & Materials. 2013;26:150–170. doi: 10.22203/ecm.v026a11. [DOI] [PubMed] [Google Scholar]
- 32.Kang S.-W., Son S.-M., Lee J.-S., et al. Regeneration of whole meniscus using meniscal cells and polymer scaffolds in a rabbit total meniscectomy model. Journal of Biomedical Materials Research—Part A. 2006;78(3):659–671. doi: 10.1002/jbm.a.30904. [DOI] [PubMed] [Google Scholar]
- 33.Mandal B. B., Park S.-H., Gil E. S., Kaplan D. L. Multilayered silk scaffolds for meniscus tissue engineering. Biomaterials. 2011;32(2):639–651. doi: 10.1016/j.biomaterials.2010.08.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pabbruwe M. B., Kafienah W., Tarlton J. F., Mistry S., Fox D. J., Hollander A. P. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31(9):2583–2591. doi: 10.1016/j.biomaterials.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Warnock J. J., Spina J., Bobe G., et al. Culture of canine synoviocytes on porcine intestinal submucosa scaffolds as a strategy for meniscal tissue engineering for treatment of meniscal injury in dogs. Veterinary Journal. 2014;199(1):49–56. doi: 10.1016/j.tvjl.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 36.Walsh C. J., Goodman D., Caplan A. I., Goldberg V. M. Meniscus regeneration in a rabbit partial meniscectomy model. Tissue Engineering. 1999;5(4):327–337. doi: 10.1089/ten.1999.5.327. [DOI] [PubMed] [Google Scholar]
- 37.Lee C. H., Rodeo S. A., Fortier L. A., Lu C., Erisken C., Mao J. J. Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Science Translational Medicine. 2014;6(266) doi: 10.1126/scitranslmed.3009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone K. R., Rodkey W. G., Webber R., McKinney L., Steadman J. R. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. The American Journal of Sports Medicine. 1992;20(2):104–111. doi: 10.1177/036354659202000202. [DOI] [PubMed] [Google Scholar]
- 39.Stone K. R., Steadman J. R., Rodkey W. G., Li S.-T. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. The Journal of Bone & Joint Surgery—American Volume. 1997;79(12):1770–1777. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Gunja N. J., Uthamanthil R. K., Athanasiou K. A. Effects of TGF-β1 and hydrostatic pressure on meniscus cell-seeded scaffolds. Biomaterials. 2009;30(4):565–573. doi: 10.1016/j.biomaterials.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunja N. J., Athanasiou K. A. Additive and synergistic effects of bFGF and hypoxia on leporine meniscus cell-seeded PLLA scaffolds. Journal of Tissue Engineering and Regenerative Medicine. 2010;4(2):115–122. doi: 10.1002/term.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freymann U., Endres M., Goldmann U., Sittinger M., Kaps C. Toward scaffold-based meniscus repair: effect of human serum, hyaluronic acid and TGF-ß3 on cell recruitment and re-differentiation. Osteoarthritis and Cartilage. 2013;21(5):773–781. doi: 10.1016/j.joca.2013.02.655. [DOI] [PubMed] [Google Scholar]
- 43.Nerurkar N. L., Han W., Mauck R. L., Elliott D. M. Homologous structure-function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials. 2011;32(2):461–468. doi: 10.1016/j.biomaterials.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker B. M., Shah R. P., Huang A. H., Mauck R. L. Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Engineering—Part A. 2011;17(9-10):1445–1455. doi: 10.1089/ten.tea.2010.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okuno M., Muneta T., Koga H., et al. Meniscus regeneration by syngeneic, minor mismatched, and major mismatched transplantation of synovial mesenchymal stem cells in a rat model. Journal of Orthopaedic Research. 2014;32(7):928–936. doi: 10.1002/jor.22614. [DOI] [PubMed] [Google Scholar]
- 46.Tan G.-K., Dinnes D. L. M., Myers P. T., Cooper-White J. J. Effects of biomimetic surfaces and oxygen tension on redifferentiation of passaged human fibrochondrocytes in 2D and 3D cultures. Biomaterials. 2011;32(24):5600–5614. doi: 10.1016/j.biomaterials.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 47.Adesida A. B., Mulet-Sierra A., Laouar L., Jomha N. M. Oxygen tension is a determinant of the matrix-forming phenotype of cultured human meniscal fibrochondrocytes. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039339.e39339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petri M., Ufer K., Toma I., et al. Effects of perfusion and cyclic compression on in vitro tissue engineered meniscus implants. Knee Surgery, Sports Traumatology, Arthroscopy. 2012;20(2):223–231. doi: 10.1007/s00167-011-1600-3. [DOI] [PubMed] [Google Scholar]
- 49.Setton L. A., Guilak F., Hsu E. W., Vail T. P. Biomechanical factors in tissue engineered meniscal repair. Clinical Orthopaedics and Related Research. 1999;(367, supplement):S254–S272. doi: 10.1097/00003086-199910001-00025. [DOI] [PubMed] [Google Scholar]
- 50.Aufderheide A. C., Athanasiou K. A. Comparison of scaffolds and culture conditions for tissue engineering of the knee meniscus. Tissue Engineering. 2005;11(7-8):1095–1104. doi: 10.1089/ten.2005.11.1095. [DOI] [PubMed] [Google Scholar]
- 51.Haddad B., Pakravan A. H., Konan S., Adesida A., Khan W. A systematic review of tissue engineered meniscus: cell-based preclinical models. Current Stem Cell Research & Therapy. 2013;8(3):222–231. doi: 10.2174/1574888x11308030007. [DOI] [PubMed] [Google Scholar]
- 52.McDevitt C. A., Webber R. J. The ultrastructure and biochemistry of meniscal cartilage. Clinical Orthopaedics and Related Research. 1990;(252):8–18. [PubMed] [Google Scholar]
- 53.Cheung H. S. Distribution of type I, II, III and v in the pepsin solubilized collagens in bovine menisci. Connective Tissue Research. 1987;16(4):343–356. doi: 10.3109/03008208709005619. [DOI] [PubMed] [Google Scholar]
- 54.Nakata K., Shino K., Hamada M., et al. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clinical Orthopaedics and Related Research. 2001;(391, supplement):S208–S218. [PubMed] [Google Scholar]
- 55.Verdonk P. C. M., Forsyth R. G., Wang J., et al. Characterisation of human knee meniscus cell phenotype. Osteoarthritis and Cartilage. 2005;13(7):548–560. doi: 10.1016/j.joca.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 56.Peretti G. M., Gill T. J., Xu J.-W., Randolph M. A., Morse K. R., Zaleske D. J. Cell-based therapy for meniscal repair: a large animal study. American Journal of Sports Medicine. 2004;32(1):146–158. doi: 10.1177/0095399703258790. [DOI] [PubMed] [Google Scholar]
- 57.Martinek V., Ueblacker P., Bräun K., et al. Second generation of meniscus transplantation: in-vivo study with tissue engineered meniscus replacement. Archives of Orthopaedic and Trauma Surgery. 2006;126(4):228–234. doi: 10.1007/s00402-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 58.Baker B. M., Nathan A. S., Huffman G. R., Mauck R. L. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthritis and Cartilage. 2009;17(3):336–345. doi: 10.1016/j.joca.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gunja N. J., Athanasiou K. A. Passage and reversal effects on gene expression of bovine meniscal fibrochondrocytes. Arthritis Research & Therapy. 2007;9, article R93 doi: 10.1186/ar2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adesida A. B., Grady L. M., Khan W. S., Hardingham T. E. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Research & Therapy. 2006;8, article R61 doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adesida A. B., Grady L. M., Khan W. S., Millward-Sadler S. J., Salter D. M., Hardingham T. E. Human meniscus cells express hypoxia inducible factor-1α and increased SOX9 in response to low oxygen tension in cell aggregate culture. Arthritis Research & Therapy. 2007;9, article R69 doi: 10.1186/ar2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esposito A. R., Moda M., Cattani S. M., et al. PLDLA/PCL-T scaffold for meniscus tissue engineering. BioResearch Open Access. 2013;2(2):138–147. doi: 10.1089/biores.2012.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo J. J., Bichara D. A., Zhao X., Randolph M. A., Gill T. J. Implant-assisted meniscal repair in vivo using a chondrocyte-seeded flexible PLGA scaffold. Journal of Biomedical Materials Research Part: A. 2011;99(1):102–108. doi: 10.1002/jbm.a.33168. [DOI] [PubMed] [Google Scholar]
- 64.Kon E., Chiari C., Marcacci M., et al. Tissue engineering for total meniscal substitution: animal study in sheep model. Tissue Engineering—Part A. 2008;14(6):1067–1080. doi: 10.1089/ten.tea.2007.0193. [DOI] [PubMed] [Google Scholar]
- 65.Kon E., Filardo G., Tschon M., et al. Tissue engineering for total meniscal substitution: animal study in sheep model—results at 12 months. Tissue Engineering Part A. 2012;18(15-16):1573–1582. doi: 10.1089/ten.tea.2011.0572. [DOI] [PubMed] [Google Scholar]
- 66.Weinand C., Peretti G. M., Adams S. B., Jr., Bonassar L. J., Randolph M. A., Gill T. J. An allogenic cell-based implant for meniscal lesions. The American Journal of Sports Medicine. 2006;34(11):1779–1789. doi: 10.1177/0363546506290666. [DOI] [PubMed] [Google Scholar]
- 67.de Lange-Brokaar B. J. E., Ioan-Facsinay A., van Osch G. J. V. M., et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis and Cartilage. 2012;20(12):1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 68.Warnock J. J., Fox D. B., Stoker A. M., Cook J. L. Evaluation of in vitro growth factor treatments on fibrochondrogenesis by synovial membrane cells from osteoarthritic and nonosteoarthritic joints of dogs. American Journal of Veterinary Research. 2011;72(4):500–511. doi: 10.2460/ajvr.72.4.500. [DOI] [PubMed] [Google Scholar]
- 69.Spina J., Warnock J., Duesterdieck-Zellmer K., Baltzer W., Ott J., Bay B. Comparison of growth factor treatments on the fibrochondrogenic potential of canine fibroblast-like synoviocytes for meniscal tissue engineering. Veterinary Surgery. 2014;43(6):750–760. doi: 10.1111/j.1532-950x.2014.12170.x. [DOI] [PubMed] [Google Scholar]
- 70.Fox D. B., Warnock J. J., Stoker A. M., Luther J. K., Cockrell M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Research in Veterinary Science. 2010;88(2):326–332. doi: 10.1016/j.rvsc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 71.Warnock J. J., Fox D. B., Stoker A. M., et al. Culture of equine fibroblast-like synoviocytes on synthetic tissue scaffolds towards meniscal tissue engineering: a preliminary cell-seeding study. PeerJ. 2014;2, article e353 doi: 10.7717/peerj.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Warnock J. J., Bobe G., Duesterdieck-Zellmer K. F. Fibrochondrogenic potential of synoviocytes from osteoarthritic and normal joints cultured as tensioned bioscaffolds for meniscal tissue engineering in dogs. PeerJ. 2014;2, article e581 doi: 10.7717/peerj.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horwitz E. M. Stem cell plasticity: the growing potential of cellular therapy. Archives of Medical Research. 2003;34(6):600–606. doi: 10.1016/j.arcmed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 74.Ringe J., Kaps C., Burmester G.-R., Sittinger M. Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Die Naturwissenschaften. 2002;89(8):338–351. doi: 10.1007/s00114-002-0344-9. [DOI] [PubMed] [Google Scholar]
- 75.Schaefer D. J., Klemt C., Zhang X. H., Stark G. B. Tissue engineering with mesenchymal stem cells for cartilage and bone regeneration. Der Chirurg. 2000;71:1001–1008. doi: 10.1007/s001040070002. [DOI] [PubMed] [Google Scholar]
- 76.Baker B. M., Mauck R. L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28(11):1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yamasaki T., Deie M., Shinomiya R., et al. Meniscal regeneration using tissue engineering with a scaffold derived from a rat meniscus and mesenchymal stromal cells derived from rat bone marrow. Journal of Biomedical Materials Research Part A. 2005;75(1):23–30. doi: 10.1002/jbm.a.30369. [DOI] [PubMed] [Google Scholar]
- 78.Nerurkar N. L., Sen S., Baker B. M., Elliott D. M., Mauck R. L. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomaterialia. 2011;7(2):485–491. doi: 10.1016/j.actbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.De Bari C., Dell'Accio F., Tylzanowski P., Luyten F. P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and Rheumatism. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 80.Shirasawa S., Sekiya I., Sakaguchi Y., Yagishita K., Ichinose S., Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. Journal of Cellular Biochemistry. 2006;97(1):84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 81.Sakimura K., Matsumoto T., Miyamoto C., Osaki M., Shindo H. Effects of insulin-like growth factor I on transforming growth factor β1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells Tissues Organs. 2006;183(2):55–61. doi: 10.1159/000095509. [DOI] [PubMed] [Google Scholar]
- 82.Hatsushika D., Muneta T., Nakamura T., et al. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthritis and Cartilage. 2014;22(7):941–950. doi: 10.1016/j.joca.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 83.Hatsushika D., Muneta T., Horie M., Koga H., Tsuji K., Sekiya I. Intraarticular injection of synovial stem cells promotes meniscal regeneration in a rabbit massive meniscal defect model. Journal of Orthopaedic Research. 2013;31(9):1354–1359. doi: 10.1002/jor.22370. [DOI] [PubMed] [Google Scholar]
- 84.Horie M., Sekiya I., Muneta T., et al. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. STEM CELLS. 2009;27(4):878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 85.Vangsness C. T., Jr., Farr J., II, Boyd J., Dellaero D. T., Mills C. R., LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a Randomized, Double-Blind, Controlled Study. The Journal of Bone & Joint Surgery—American Volume. 2014;96(2):90–98. doi: 10.2106/jbjs.m.00058. [DOI] [PubMed] [Google Scholar]
- 86.Hendriks J., Riesle J., van Blitterswijk C. A. Co-culture in cartilage tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. 2007;1(3):170–178. doi: 10.1002/term.19. [DOI] [PubMed] [Google Scholar]
- 87.Gunja N. J., Athanasiou K. A. Effects of co-cultures of meniscus cells and articular chondrocytes on PLLA scaffolds. Biotechnology and Bioengineering. 2009;103(4):808–816. doi: 10.1002/bit.22301. [DOI] [PubMed] [Google Scholar]
- 88.Boeuf S., Richter W. Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors. Stem Cell Research & Therapy. 2010;1, article 31 doi: 10.1186/scrt31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu L., Leijten J. C. H., Georgi N., Post J. N., Van Blitterswijk C. A., Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Engineering Part A. 2011;17(9-10):1425–1436. doi: 10.1089/ten.tea.2010.0517. [DOI] [PubMed] [Google Scholar]
- 90.Wu L., Prins H.-J., Helder M. N., van Blitterswijk C. A., Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte Co-Cultures are independent of culture conditions and cell sources. Tissue Engineering Part: A. 2012;18(15-16):1542–1551. doi: 10.1089/ten.tea.2011.0715. [DOI] [PubMed] [Google Scholar]
- 91.Diao H. J., Yeung C. W., Yan C. H., Chan G. C., Chan B. P. Bidirectional and mutually beneficial interactions between human mesenchymal stem cells and osteoarthritic chondrocytes in micromass co-cultures. Regenerative Medicine. 2013;8(3):257–269. doi: 10.2217/rme.13.22. [DOI] [PubMed] [Google Scholar]
- 92.Cui X., Hasegawa A., Lotz M., D'Lima D. Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnology and Bioengineering. 2012;109(9):2369–2380. doi: 10.1002/bit.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matthies N.-F., Mulet-Sierra A., Jomha N. M., Adesida A. B. Matrix formation is enhanced in co-cultures of human meniscus cells with bone marrow stromal cells. Journal of Tissue Engineering and Regenerative Medicine. 2013;7(12):965–973. doi: 10.1002/term.1489. [DOI] [PubMed] [Google Scholar]
- 94.Chowdhury A., Bezuidenhout L. W., Mulet-Sierra A., Jomha N. M., Adesida A. B. Effect of interleukin-1β treatment on co-cultures of human meniscus cells and bone marrow mesenchymal stromal cells. BMC Musculoskeletal Disorders. 2013;14, article 216 doi: 10.1186/1471-2474-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan Y., Zhang Y., Pei M. Meniscus reconstruction through coculturing meniscus cells with synovium-derived stem cells on small intestine submucosa-a pilot study to engineer meniscus tissue constructs. Tissue Engineering—Part A. 2010;16(1):67–79. doi: 10.1089/ten.tea.2008.0680. [DOI] [PubMed] [Google Scholar]
- 96.Ryu J.-S., Jung Y.-H., Cho M.-Y., et al. Co-culture with human synovium-derived mesenchymal stem cells inhibits inflammatory activity and increases cell proliferation of sodium nitroprusside-stimulated chondrocytes. Biochemical and Biophysical Research Communications. 2014;447(4):715–720. doi: 10.1016/j.bbrc.2014.04.077. [DOI] [PubMed] [Google Scholar]
- 97.Higashioka M. M., Chen J. A., Hu J. C., Athanasiou K. A. Building an anisotropic meniscus with zonal variations. Tissue Engineering—Part A. 2014;20(1-2):294–302. doi: 10.1089/ten.tea.2013.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelm J. M., Fussenegger M. Scaffold-free cell delivery for use in regenerative medicine. Advanced Drug Delivery Reviews. 2010;62(7-8):753–764. doi: 10.1016/j.addr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Demirbag B., Huri P. Y., Kose G. T., Buyuksungur A., Hasirci V. Advanced cell therapies with and without scaffolds. Biotechnology Journal. 2011;6(12):1437–1453. doi: 10.1002/biot.201100261. [DOI] [PubMed] [Google Scholar]
- 100.Ballard G. A., Warnock J. J., Bobe G., et al. Comparison of meniscal fibrochondrocyte and synoviocyte bioscaffolds toward meniscal tissue engineering in the dog. Research in Veterinary Science. 2014;97:400–408. doi: 10.1016/j.rvsc.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Warnock J. J., Bobe G., Duesterdieck-Zellmer K. F., et al. Growth factor treated tensioned synoviocyte neotissues: towards meniscal bioscaffold tissue engineering. Veterinary Journal. 2014;200(1):22–30. doi: 10.1016/j.tvjl.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 102.Moriguchi Y., Tateishi K., Ando W., et al. Repair of meniscal lesions using a scaffold-free tissue-engineered construct derived from allogenic synovial MSCs in a miniature swine model. Biomaterials. 2013;34(9):2185–2193. doi: 10.1016/j.biomaterials.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 103.Hoben G. M., Hu J. C., James R. A., Athanasiou K. A. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Engineering. 2007;13(5):939–946. doi: 10.1089/ten.2006.0116. [DOI] [PubMed] [Google Scholar]
- 104.Huey D. J., Athanasiou K. A. Maturational growth of self-assembled, functional menisci as a result of TGF-β1 and enzymatic chondroitinase-ABC stimulation. Biomaterials. 2011;32(8):2052–2058. doi: 10.1016/j.biomaterials.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hadidi P., Athanasiou K. A. Enhancing the mechanical properties of engineered tissue through matrix remodeling via the signaling phospholipid lysophosphatidic acid. Biochemical and Biophysical Research Communications. 2013;433(1):133–138. doi: 10.1016/j.bbrc.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hadidi P., Yeh T. C., Hu J. C., Athanasiou K. A. Critical seeding density improves the properties and translatability of self-assembling anatomically shaped knee menisci. Acta Biomaterialia. 2015;11:173–182. doi: 10.1016/j.actbio.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


