Abstract
Purpose
Little is known about the breast cancer risk among childhood cancer survivors who did not receive chest radiotherapy. We sought to determine the magnitude of risk and associated risk factors for breast cancer among these women.
Patients and Methods
We evaluated cumulative breast cancer risk in 3,768 female childhood cancer survivors without a history of chest radiotherapy who were participants in the Childhood Cancer Survivor Study.
Results
With median follow up of 25.5 years (range, 8 to 39 years), 47 women developed breast cancer at a median age of 38.0 years (range, 22 to 47 years) and median of 24.0 years (range, 10 to 34 years) from primary cancer to breast cancer. A four-fold increased breast cancer risk (standardized incidence ratio [SIR] = 4.0; 95% CI, 3.0 to 5.3) was observed when compared with the general population. Risk was highest among sarcoma and leukemia survivors (SIR = 5.3; 95% CI, 3.6 to 7.8 and SIR = 4.1; 95% CI, 2.4 to 6.9, respectively). By the age of 45 years, the cumulative incidence of breast cancer in sarcoma and leukemia survivors was 5.8% (95% CI, 3.7 to 8.4) and 6.3% (95% CI, 3.0 to 11.3), respectively. No other primary cancer diagnosis was associated with an elevated risk. Alkylators and anthracyclines were associated with an increased breast cancer risk in a dose-dependent manner (P values from test for trend were both < .01).
Conclusions
Women not exposed to chest radiotherapy who survive childhood sarcoma or leukemia have an increased risk of breast cancer at a young age. The data suggest high-dose alkylator and anthracycline chemotherapy increase the risk of breast cancer. This may suggest a possible underlying gene-environment interaction that warrants further study.
INTRODUCTION
The improvement in survival of childhood cancer achieved over the last half century is one of the great successes in modern medicine. Over 80% of children diagnosed with cancer before the age of 21 years will be cured.1 There are now over 388,500 childhood cancer survivors in the United States.2 Unfortunately, many survivors have a significantly elevated risk of premature mortality.3,4 Subsequent malignant neoplasms (SMNs) are the leading cause of late mortality among survivors, excluding recurrence of the primary cancer.3,5 Breast cancer is the most frequent SMN among female childhood cancer survivors.6-10 It occurs at a relatively young age in this population and the cumulative incidence increases with age.6 Evidence to date suggests female survivors exposed to chest radiotherapy are the women at high risk for these breast cancers; there is a well-established, dose-response association between chest radiotherapy and breast cancer risk.9-16 Among these women, the cumulative incidence of breast cancer by the age of 50 years is 30%.10 Thus, for women exposed to chest radiotherapy, early initiation of breast cancer surveillance with yearly mammography and breast magnetic resonance imaging is recommended.15,17,18
In 2004, investigators from the Childhood Cancer Survivor Study (CCSS) reported on 20 breast cancer cases in women not exposed to chest radiotherapy. This study found an elevated breast cancer risk, with standardized incidence ratios (SIRs) of 6.7 and 7.6 among bone and soft tissue sarcoma survivors, respectively.6 The small number of cases limited the ability to explore potential risk factors, but suggested some childhood cancer survivors may be at risk for breast cancer due to factors other than chest radiotherapy. In the current study, we evaluated breast cancer risk in women never exposed to chest radiotherapy and examined factors associated with this risk.
PATIENTS AND METHODS
Study Population
The CCSS is a retrospective cohort of 14,358 5-year childhood cancer survivors treated at 26 centers in North America.19,20 Eligibility criteria included: diagnosis of leukemia, CNS malignancy, Hodgkin lymphoma (HL), non-HL, neuroblastoma, soft tissue sarcoma, kidney tumor, or bone cancer; diagnosis between January 1, 1970 and December 31, 1986; less than 21 years of age at diagnosis; and alive 5 years from diagnosis date. Institutional review boards at the participating centers approved the CCSS study. CCSS participants provided informed consent. The cohort methodology has been previously described.19-21
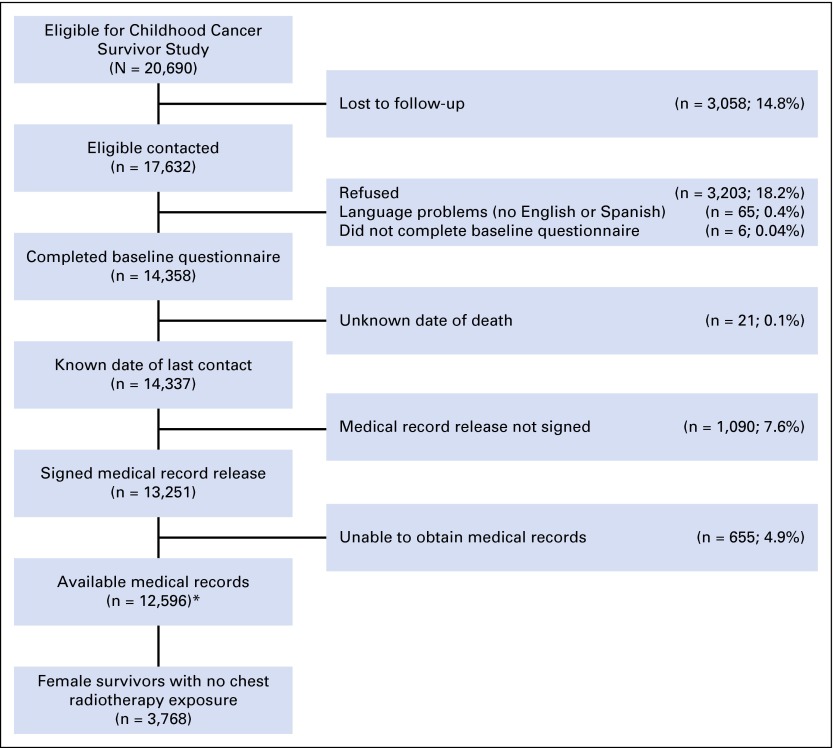
We restricted our analysis to 3,768 female participants who received no chest radiotherapy within 5 years of their childhood cancer diagnosis. We defined chest radiotherapy to include treatment with any of the following fields: mantle, mediastinal (including involved field), hemithorax (or anterior fields on one side of chest), whole-lung irradiation, spinal including posterior thoracic/paravertebral, abdominal (with extension above diaphragm), and total body irradiation. Figure 1 depicts the sample used for this analysis.
Fig 1.
Study flow diagram. (*) Dataset used for all analyses involving treatment data.
Ascertainment of Treatment Information
Therapeutic exposures were ascertained through abstraction of medical records of each participant by use of a standardized protocol.20 This abstraction included diagnosis, chemotherapy, radiation therapy, and surgeries. Cumulative doses of anthracyclines, alkylators, and other chemotherapy agents were determined.19,22,23
Identification and Confirmation of Subsequent Breast Cancer
Breast cancer cases, including invasive cancers as well as ductal carcinoma in situ (DCIS), were ascertained through self- or proxy-report and the National Death Index. The CCSS pathologist reviewed pathology reports to confirm all cases. If the pathology report could not be obtained, medical records or death certificates were reviewed.
Statistical Analysis
Childhood cancer survivors were considered at risk for breast cancer beginning from entry into the CCSS cohort (5 years after their childhood cancer diagnosis) until either a recurrence, a confirmed diagnosis of breast cancer, an SMN other than breast cancer, death, or date of most recent contact. Individuals with a recurrence of their primary diagnosis or with an SMN other than breast cancer were censored at the date of diagnosis with the recurrence or neoplasm because we lacked complete medical records and knowledge of radiation exposure in these cases.
Calculation of SIRs and absolute excess risks.
SIRs were calculated as the ratio of the observed numbers of women with breast cancer to the expected numbers in the general population (including both invasive breast cancer and DCIS). SEER Program data were used to ascertain the number of breast cancers expected in a general population female cohort with the same age and calendar-year distribution as eligible CCSS participants.24 For both the CCSS cohort and SEER Program data, we included only the first primary breast cancer diagnosis. Absolute excess risk was calculated as the difference between the number of observed and expected events divided by the number of person-years follow-up, and is expressed per 10,000 person-years.
Risk factor analysis.
We used Poisson regression analysis for SIRs, with age as the time scale to assess for variables that modify the risk of a first breast cancer diagnosis. Relative SIRs (rSIRs) and 95% CIs are presented. A multivariate model was constructed including the main risk factors of interest, cumulative anthracycline dose, cyclophosphamide equivalent dose (CED),22,23 and age at primary cancer diagnosis, adjusting for race/ethnicity (white, non-Hispanic, and other) and attained age. We explored inclusion of the primary cancer diagnosis (sarcoma/leukemia v other cancers); including it was not significant and did not meaningfully change the results. A test for trend for cumulative anthracycline dose and CED was performed by specifying the ordinal variables using orthogonal polynomial contrasts and testing the statistical significance of the linear contrast.
Calculation of cumulative incidence rates.
Cumulative incidences of breast cancer overall and by primary diagnoses were calculated with a nonparametric estimate, using age as the time scale. Individuals were considered to be at breast cancer risk beginning 5 years after their primary diagnosis, and death was treated as a competing risk.25,26 These estimates represent the cumulative proportion of survivors expected to experience a primary breast cancer according to attained age.
We used SAS software, version 9.2 (SAS Institute, Cary, NC), and Stata software, version 13.0 (StataCorp, College Station, TX).
RESULTS
Characteristics of Survivors With Breast Cancer
With a median follow up of 25.5 years (range, 8 to 39 years), 47 women developed primary breast cancer (41 invasive breast cancer cases; six DCIS cases). The median time from primary cancer to breast cancer was 24.0 years (range, 10 to 34 years). The median age at breast cancer diagnosis was 38.0 years (range, 22 to 47 years). The characteristics of the female childhood cancer survivors are presented in Table 1. Eighty-five percent of women with breast cancer were sarcoma (55%) or leukemia (30%) survivors. Twenty-six percent of women with breast cancer were subsequently deceased at last point of contact. Seven women (15%) with breast cancer had bilateral breast cancer; two women (4%) had synchronous breast cancer, and five women (11%) developed metachronous breast cancer (> 6 months from the first breast cancer diagnosis).
Table 1.
Cancer Diagnosis, Clinical Characteristics, and Patient Demographic Data of Female Survivors of Childhood Cancer Without a History of Chest Radiotherapy Exposure
| Characteristic | Total, N = 3,768 | Breast Cancer, n = 47 | No Breast Cancer, n = 3,721 |
|---|---|---|---|
| Primary childhood cancer, No. (%) | |||
| Leukemia | 1,556 (41.3) | 14 (29.8) | 1,542 (41.4) |
| Acute lymphoblastic leukemia | 1,411 | 10 | 1,401 |
| Acute myelogenous leukemia | 121 | 4 | 117 |
| Others | 24 | 0 | 24 |
| CNS tumor | 474 (12.6) | 2 (4.3) | 472 (12.6) |
| Hodgkin lymphoma | 63 (1.7) | 1 (2.1) | 62 (1.7) |
| Non-Hodgkin lymphoma | 155 (4.1) | 1 (2.1) | 154 (4.1) |
| Kidney tumor | 408 (10.8) | 1 (2.1) | 407 (10.9) |
| Neuroblastoma | 325 (8.6) | 2 (4.2) | 323 (8.7) |
| Sarcoma | 787 (20.9) | 26 (55.3) | 761 (20.5) |
| Soft tissue sarcoma | 418 | 10 | 408 |
| Ewing sarcoma | 99 | 6 | 93 |
| Osteosarcoma | 251 | 10 | 241 |
| Other sarcoma | 19 | 0 | 19 |
| Race/ethnicity, No. (%) | |||
| White, non-Hispanic | 3,295 (87.4) | 42 (89.4) | 3,253 (87.4) |
| Black, non-Hispanic | 152 (4.0) | 3 (6.4) | 149 (4.0) |
| Hispanic | 192 (5.1) | 2 (4.2) | 190 (5.1) |
| Other | 116 (3.1) | 0 (0.0) | 116 (3.1) |
| Unknown | 13 (0.4) | 0 (0.0) | 13 (0.4) |
| Age at diagnosis of primary cancer, years | |||
| Median | 5.0 | 14.0 | 5.0 |
| Range | 0-20 | 3-20 | 0-20 |
| Attained age, years | |||
| Median | 31 | 43 | 31 |
| Range | 8-58 | 29-56 | 8-58 |
| Duration of follow up, years | |||
| Median | 25.5 | 26.0 | 25.5 |
| Range | 8.3-38.9 | 9.9-34.3 | 8.3-38.9 |
| Age at breast cancer diagnosis, years | |||
| Median | 38.0 | ||
| Range | 22-47 | ||
| Chemotherapy for primary cancer, No. (%) | |||
| Alkylators | 1,649 (43.8) | 30 (63.8) | 1,619 (43.5) |
| Cyclophosphamide | 1,567 | 29 | 1,538 |
| Other | 467 | 13 | 454 |
| Cyclophosphamide equivalent dose, mg/m2, No. (%) | |||
| 0 | 2,116 (59.0) | 17 (38.6) | 2,099 (59.3) |
| 1-5,999 | 624 (17.4) | 4 (9.1) | 620 (17.5) |
| 6,000-17,999 | 675 (18.8) | 16 (36.4) | 659 (18.6) |
| ≥ 18,000 | 169 (4.7) | 7 (15.9) | 162 (4.6) |
| Anthracycline cumulative dose, mg/m2, No. (%) | |||
| 0 | 2,321 (63.4) | 14 (31.8) | 2,307 (63.8) |
| 1-249 | 541 (14.8) | 4 (9.1) | 537 (14.9) |
| ≥ 250 | 799 (21.8) | 26 (59.1) | 773 (21.3) |
| Platinum chemotherapy, No. (%) | 181 (4.8) | 2 (4.3) | 179 (4.8) |
| Antimetabolites, No. (%) | 1,962 (52.1) | 24 (51.1) | 1,938 (52.1) |
| Plant alkaloids, No. (%) | 2,774 (73.6) | 36 (76.6) | 2,738 (73.6) |
| Epipodophyllotoxins, No. (%) | 260 (6.9) | 2 (4.3) | 258 (6.9) |
| Radiation for primary cancer, No. (%) | |||
| Any* | 1,912 (50.7) | 22 (46.8) | 1,890 (50.8) |
| Pelvic | 1,892 (50.2) | 22 (46.8) | 1,870 (50.2) |
| Vital status, No. (%) | |||
| Alive at last point of contact | 3,520 (93.4) | 35 (74.5) | 3,485 (93.6) |
| Deceased at last point of contact | 248 (6.6) | 12 (25.5) | 236 (6.3) |
Includes all nonchest radiotherapy.
Cumulative Incidence Rates
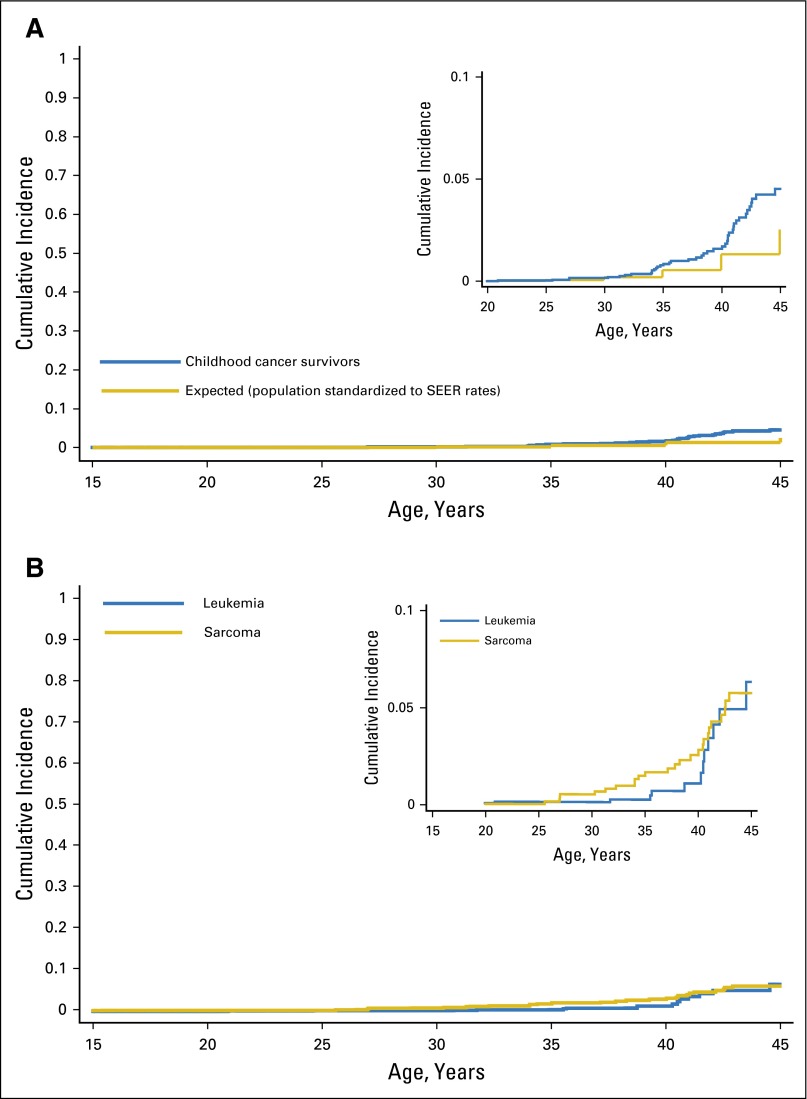
The cumulative incidence of breast cancer by the age of 45 years was 4.5% (95% CI, 3.2 to 6.2) (Fig 2A), and was highest among sarcoma and leukemia survivors at 5.8% (95% CI, 3.7 to 8.4) and 6.3% (95% CI, 3.0 to 11.3), respectively (Fig 2B). Among all primary cancers except sarcoma and leukemia, the cumulative incidence by the age of 45 years was 1.8% (95% CI, 0.6 to 4.0).
Fig 2.
(A) Cumulative incidence of breast cancer in childhood cancer survivors without a history of chest radiotherapy. (B) Cumulative incidence of breast cancer in childhood sarcoma and leukemia survivors without a history of chest radiotherapy.
SIR Analysis
Breast cancer risk was four-fold higher in childhood cancer survivors than the general population (Table 2; SIR = 4.0; 95% CI, 3.0 to 5.3). Notable high-risk groups included survivors of primary sarcoma (SIR = 5.3; 95% CI, 3.6 to 7.8) or leukemia (SIR = 4.1; 95% CI, 2.4 to 6.9), those who were older at primary cancer diagnosis (ages 10 to 20 years; SIR = 4.8; 95% CI, 3.5 to 6.5), and those who were treated with anthracycline and alkylator chemotherapy (SIR = 8.6; 95% CI, 5.7 to 12.8). Sarcoma and leukemia survivors treated with both anthracyclines and alkylators had a 9.8-fold increased breast cancer risk (95% CI, 6.5 to 14.7). In contrast, an increased risk among sarcoma and leukemia survivors who did not receive alkylator or anthracycline chemotherapy was not observed (SIR = 1.2; 95% CI, 0.4 to 3.3). When we examined breast cancer risk among women diagnosed with a primary cancer other than sarcoma or leukemia, we did not observe a significantly increased risk (SIR = 1.9; 95% CI, 0.9 to 4.2).
Table 2.
Breast Cancer Risk Compared With the General Population
| Characteristic | No. Person-Years at Risk | O | E | SIR | 95% CI | AER per 10,000 Person-Years | 95% CI |
|---|---|---|---|---|---|---|---|
| Whole cohort | 72,493 | 47 | 11.8 | 4.0 | 3.0 to 5.3 | 4.9 | 3.0 to 6.7 |
| Age at primary diagnosis, years | |||||||
| 0-9 | 51,750 | 6 | 3.3 | 1.8 | 0.8 to 4.1 | 0.5 | −0.4 to 1.4 |
| 10-20 | 20,743 | 41 | 8.5 | 4.8 | 3.5 to 6.5 | 15.4 | 9.4 to 21.5 |
| Attained age, years | |||||||
| 0-19 | 1,803 | 0 | 0.0 | NA | NA | ||
| 20-39 | 54,109 | 12 | 3.4 | 3.5 | 2.0 to 6.2 | 1.6 | 0.3 to 2.8 |
| 40-49 | 14,999 | 32 | 6.8 | 4.7 | 3.3 to 6.6 | 16.8 | 9.4 to 24.2 |
| 50-59 | 1,582 | 3 | 1.5 | 1.9 | 0.6 to 6.0 | 9.5 | −12.0 to 30.9 |
| Time since diagnosis, years | |||||||
| 5-14.9 | 35,625 | 7 | 0.6 | 11.0 | 5.3 to 23.1 | 1.8 | 0.3 to 3.3 |
| 15-24.9 | 28,171 | 17 | 4.8 | 3.6 | 2.2 to 5.8 | 4.3 | 1.5 to 7.2 |
| ≥ 25 | 8,669 | 23 | 6.4 | 3.6 | 2.4 to 5.4 | 19.1 | 8.3 to 30.0 |
| Primary cancer diagnosis | |||||||
| Leukemia | 29,896 | 14 | 3.4 | 4.1 | 2.4 to 6.9 | 3.5 | 1.1 to 6.0 |
| ALL | 27,156 | 10 | 3.0 | 3.3 | 1.8 to 6.3 | 2.6 | 0.3 to 4.9 |
| AML | 2,377 | 4 | 0.4 | 9.8 | 3.6 to 26.1 | 15.1 | −1.3 to 31.6 |
| Others | 361 | 0 | 0.5 | NA | NA | ||
| Sarcoma | 15,262 | 26 | 4.8 | 5.3 | 3.6 to 7.8 | 13.9 | 7.4 to 20.5 |
| Ewing | 1,844 | 6 | 0.6 | 10.0 | 4.5 to 22.4 | 29.3 | 3.2 to 55.3 |
| Soft tissue | 8,086 | 10 | 2.1 | 4.8 | 2.6 to 9.1 | 9.8 | 2.1 to 17.4 |
| Osteosarcoma | 4,941 | 10 | 2.0 | 5.1 | 2.8 to 9.5 | 16.2 | 3.6 to 28.7 |
| Other sarcoma | 391 | 0 | 0.2 | NA | NA | ||
| CNS tumor | 8,042 | 2 | 1.4 | 1.4 | 0.4 to 5.6 | 0.7 | −2.7 to 4.2 |
| Lymphoma* | 4,379 | 2 | 1.3 | 1.6 | 0.4 to 6.3 | 1.6 | −4.7 to 7.9 |
| Embryonal tumors* | 14,914 | 3 | 0.8 | 3.6 | 1.2 to 11.3 | 1.5 | −0.8 to 3.8 |
| Whole cohort | |||||||
| CED, mg/m2 | |||||||
| 0 | 41,443 | 17 | 6.6 | 2.6 | 1.6 to 4.2 | 2.5 | 0.6 to 4.5 |
| 1-5,999 | 11,342 | 4 | 1.4 | 2.8 | 1.1 to 7.5 | 2.3 | −1.2 to 5.7 |
| 6,000-17,999 | 12,524 | 16 | 2.0 | 7.9 | 4.8 to 12.9 | 11.2 | 4.9 to 17.4 |
| ≥ 18,000 | 3,551 | 7 | 0.7 | 9.4 | 4.5 to 19.7 | 17.7 | 3.1 to 32.3 |
| Anthracycline, mg/m2 | |||||||
| 0 | 45,660 | 14 | 7.2 | 2.0 | 1.2 to 3.3 | 1.5 | −0.1 to 3.1 |
| 1-249 | 9,778 | 4 | 1.0 | 4.0 | 1.5 to 10.7 | 3.1 | −0.9 to 7.1 |
| ≥ 250 | 15,039 | 26 | 3.1 | 8.3 | 5.7 to 12.2 | 15.2 | 8.6 to 21.9 |
| Among leukemia/sarcoma | |||||||
| CED, mg/m2 | |||||||
| 0 | 22,404 | 12 | 4.4 | 2.8 | 1.5 to 4.8 | 3.4 | 0.4 to 6.4 |
| 1-5,999 | 9,563 | 4 | 1.2 | 3.3 | 1.2 to 8.7 | 2.9 | −1.2 to 7.0 |
| 6,000-17,999 | 8,387 | 15 | 1.5 | 10.0 | 6.1 to 16.7 | 16.1 | 7.0 to 25.1 |
| ≥ 18,000 | 2,581 | 6 | 0.6 | 10.3 | 4.6 to 22.9 | 20.9 | 2.3 to 39.5 |
| Anthracycline, mg/m2 | |||||||
| 0 | 24,752 | 8 | 4.4 | 1.8 | 0.9 to 3.6 | 1.5 | −0.8 to 3.7 |
| 1-249 | 7,186 | 4 | 0.8 | 5.0 | 1.8 to 13.1 | 4.5 | −1.0 to 9.9 |
| ≥ 250 | 11,695 | 25 | 2.6 | 9.5 | 6.4 to 14.0 | 19.2 | 10.8 to 27.5 |
ΝΟΤΕ. Breast cancer includes both invasive breast cancer (n = 41) and ductal carcinoma in situ.
Abbreviations: AER, absolute excess risk; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CED, cyclophosphamide equivalent dose; E, expected; NA, not applicable; O, observed; SIR, standardized incidence ratio.
Lymphoma = Hodgkin lymphoma or non-Hodgkin lymphoma; embryonal tumors = kidney tumors or neuroblastoma.
Risk Factor Analysis for Subsequent Breast Cancer
By univariate analysis, breast cancer risk was not significantly different among white, non-Hispanic, and minority survivors. In contrast, survivors diagnosed with their primary cancer between the ages of 10 and 20 years had a higher risk than those diagnosed between 0 and 9 years (rSIR = 2.6; 95% CI, 1.1 to 6.2). Sarcoma and leukemia survivors had a 2.4-fold elevated breast cancer risk compared with all other survivors (95% CI, 1.1 to 5.4). Exposures to alkylator and anthracycline chemotherapy were associated with breast cancer development in a dose-dependent fashion (P values both < .01). Of note, this association persisted when restricting the analysis to only survivors of sarcoma or leukemia (n = 2,343). The number of cases in those who were not survivors of leukemia or sarcoma (n = 7) precludes a dose-response analysis; only two of these seven survivors received any alkylating agents.
To exclude the effect of scatter radiation (radiation from fields other than chest) on breast cancer risk, we examined the risk from exposure to any radiation for the primary cancer and found no association. Pelvic radiation, associated with a protective effect against breast cancer in women exposed to chest radiotherapy,10-12 was not associated with a reduced breast cancer risk in this cohort.
Multivariable analysis (Table 3) revealed that exposure to high doses of alkylator (CED > 18,000 mg/m2; rSIR = 3.0; 95% CI, 1.2 to 7.7) or anthracycline chemotherapy (> 250 mg/m2; rSIR = 3.8; 95% CI, 1.7 to 8.3) was significantly associated with breast cancer development. When we restricted the analysis to only sarcoma or leukemia survivors, alkylators and anthracyclines were associated with breast cancer development in a dose-dependent fashion (test for trend, P values both < .01).
Table 3.
Multivariate Risk Factor Analysis
| Whole Cohort | Leukemia/Sarcoma | |||||
|---|---|---|---|---|---|---|
| Variable | No. of Patients With Breast Cancer | Relative SIRs (95% CI) | P | No. of Patients With Breast Cancer | Relative SIRs (95% CI) | P |
| Cyclophosphamide equivalent dose, mg/m2 | ||||||
| 0 | 15 | — | .044 | 10 | — | .045 |
| 1-5,999 | 4 | 0.6 (0.2 to 2.0) | 4 | 0.7 (0.2 to 2.3) | ||
| 6,000-17,999 | 16 | 1.6 (0.7 to 3.5) | 15 | 1.9 (0.8 to 4.5) | ||
| ≥ 18,000 | 7 | 3.0 (1.2 to 7.7) | 6 | 3.4 (1.2 to 9.7) | ||
| Anthracycline dose, mg/m2 | ||||||
| 0 | 12 | — | .004 | 6 | — | .005 |
| 1-249 | 4 | 2.6 (0.8 to 8.7) | 4 | 4.3 (1.1 to 16.6) | ||
| ≥ 250 | 26 | 3.8 (1.7 to 8.3) | 25 | 5.1 (1.9 to 13.7) | ||
| Age at primary cancer diagnosis, years | ||||||
| 0-9 | 6 | — | .077 | 4 | — | .147 |
| 10-20 | 36 | 2.3 (0.9 to 5.8) | 31 | 2.3 (0.7 to 7.0) | ||
| Ethnicity | .849 | .944 | ||||
| White, non-Hispanic | 37 | — | 31 | — | ||
| Minorities | 5 | 1.1 (0.4 to 2.8) | 4 | 1.0 (0.4 to 3.0) | ||
| Current age, years | .380 | .661 | ||||
| 5-29 | 4 | — | 4 | — | ||
| 30-34 | 11 | 1.3 (0.4 to 4.1) | 7 | 0.8 (0.2 to 2.7) | ||
| 35-39 | 8 | 0.6 (0.2 to 1.9) | 7 | 0.5 (0.1 to 1.7) | ||
| 40+ | 19 | 0.8 (0.3 to 2.5) | 17 | 0.7 (0.2 to 2.2) | ||
NOTE. Survivors with complete data on all risk factors were included.
Abbreviation: SIR, standardized incidence ratio.
DISCUSSION
Overall, we observed that female childhood cancer survivors without a history of chest radiotherapy have a four-fold increased breast cancer risk compared with similar-age women in the general population. Breast cancer risk was particularly increased in sarcoma and leukemia survivors, with a 5.3-fold and 4.1-fold greater risk, respectively; among sarcoma and leukemia survivors exposed to both anthracycline and alkylator chemotherapy, risk was increased to almost 10-fold. To our knowledge, this is the largest study to date focused on breast cancer risk in female childhood cancer survivors who were not exposed to chest radiotherapy for their primary cancer.
A clinically relevant and novel finding observed in this study was the association of two classes of chemotherapy (anthracyclines and alkylators) and breast cancer risk. The association between anthracyclines or alkylators and breast cancer is suggestive of a dose-response relationship. We were limited in this study in our ability to assess the association between chemotherapy exposures and breast cancer risk in the primary cancer diagnoses other than leukemia and sarcoma, given the small number of cases of breast cancer among them (n = 7).
Although increased breast cancer risk has been noted among leukemia and sarcoma survivors, prior studies have not been able to assess the association of chemotherapeutic exposures among these survivors.6,27,28 Further, although the association of breast cancer among sarcoma and leukemia survivors has been observed in the British CCSS, this risk was only observed among survivors exposed to radiation therapy, not among survivors who never received radiation.27,28
Breast cancer presented in women as young as 22 years with a median age at breast cancer diagnosis of 38 years, which is much younger than the general population incidence. Fifteen percent of women presented with bilateral disease. This is a comparable proportion to that observed in previous reports of breast cancer in survivors exposed to chest radiotherapy15; it is higher than the incidence of bilateral disease observed in the general population.29
Prior work suggests an association between chemotherapy and solid SMNs, but these studies largely involved patients treated with radiotherapy.11,30-37 For example, in a case-control study, Inskip et al11 reported a potential independent association between breast cancer and anthracyclines (odds ratio = 1.86; 95% CI, 0.99 to 3.48), adjusted for an exposure to chest radiotherapy. Henderson et al31,32 reported that exposure to alkylators or anthracyclines was independently associated with the development of secondary sarcomas in childhood cancer survivors treated with high doses of radiotherapy. This is consistent with earlier studies in the Late Effects Study Group.36,37 Further, Veiga et al35 reported that alkylator chemotherapy was independently associated with thyroid cancer development among survivors in the CCSS who received low doses of radiotherapy to the neck field.
There is a paucity of data suggesting a relationship between alkylators or anthracyclines and solid tumors developing in survivors not exposed to therapeutic radiation (or in areas distant from the radiation field). In a case-control study of HL survivors who developed lung cancer, Travis et al33 reported that alkylator chemotherapy without radiotherapy was associated with increased lung cancer risk (relative risk = 4.2; 95% CI, 2.1 to 8.8). Similarly, Swerdlow et al34 reported that in HL survivors exposed to chemotherapy alone, there was a significant association between chemotherapy exposure and the development of lung cancer and non-HL.
To our knowledge, this is the first large study that has identified an association between subsequent breast cancer development in a nonirradiated field and exposure to anthracyclines or alkylators. Moreover, our data suggest a possible dose-response relationship. Our findings are consistent with early laboratory-based studies suggesting that anthracycline and alkylator exposure are associated with mammary tumor development in vivo.38-41 These studies confirmed the in vitro observations that anthracycline agents are potent in producing malignant transformation in mammalian cell systems by their binding interaction with DNA and subsequent disruption of the template functions of DNA.42 Alkylators, by alkylating DNA to disrupt cancer growth, may also cause DNA damage in normal tissue such that replication is impaired, placing those exposed at risk for solid tumors.43
Our findings suggest potential gene-environment interactions causing SMN. Sarcoma (with the exception of Ewing sarcoma [EWS]) and leukemia are cancers known to be associated with Li-Fraumeni syndrome (LFS).44,45 Eighty-five percent of breast cancers diagnosed in this cohort occurred among sarcoma and leukemia survivors. Additionally, two women with subsequent breast cancer were survivors of CNS tumors, which are also established to be associated with LFS. For survivors of LFS-associated tumors, it is possible that there are gene-chemotherapy interactions that result in increased breast cancer risk, given our observed dose-response association with alkylator and anthracycline chemotherapy exposure in this cohort. Although this hypothesis is supported by the relatively young ages at which women developed their breast cancer and the high incidence of bilateral disease, we lack the ability to evaluate this in detail. Further analyses of family pedigrees and genetic testing are warranted to provide additional insight into an individual’s cancer predisposition and the prevalence of familial cancer predisposition syndromes in childhood cancer survivors’ SMN risk. In addition, we observed 11 subsequent breast cancer cases in survivors of primary cancers not known to be associated with LFS, including six EWS survivors. Breast cancer after EWS has been described previously in the CCSS and in an analysis of SEER program data.46,47 However, to date, no known cancer predisposition syndrome has been described that includes EWS. This study may lead to further exploration of an association between familial breast cancer and EWS.
Our study has limitations that need to be considered when interpreting the results. Our ability to evaluate the independent risks of primary diagnosis and therapeutic exposures was limited by the relatively small number of patients with the outcome of interest. For example, many sarcoma patients are treated with high doses of both alkylators and anthracyclines, and the role of exposure to each of these two agents cannot be easily dissected. Our exploratory analyses suggest there may be an association between these agents and a diagnosis of sarcoma or leukemia, but we are underpowered to explore this relationship.
In addition, family history data, important for defining familial cancer risk, are not available in sufficient detail in the CCSS to allow for meaningful analysis. We were unable to calculate scatter radiotherapy dose from therapeutic radiation to nonchest areas, although we did examine exposure to any nonchest radiotherapy in our analyses and found no impact on risk of breast cancer.
Last, we were not able to quantify exposure to diagnostic radiation (ie, computed tomography scans). Exposure to diagnostic and surveillance radiographic studies has not been quantified for the CCSS; the cumulative diagnostic radiation exposure may have influenced the breast cancer risk in this cohort.48-50 This is of particular interest in sarcoma patients, whose highest recurrence risk is in the lungs and who undergo multiple chest x-rays and/or chest tomography as part of routine surveillance after completion of therapy.
Female sarcoma and leukemia survivors exposed to high doses of anthracyclines or alkylators may benefit from early breast cancer surveillance, even in the absence of a history of exposure to chest radiotherapy. The declining mortality rate in breast cancer in the United States over the past few decades has been attributed to both large-scale screening leading to the identification of earlier-stage disease and to an improvement in systemic treatment strategies,51 seen in both the general and high-risk breast cancer populations.15,17,52,53
For all childhood cancer survivors, particularly sarcoma and leukemia survivors, our data highlight the potential value of clinicians obtaining a detailed family history of cancer and considering genetic counseling and possible genetic testing where warranted. In women who are found to have an increased familial risk or genetic predisposition to breast cancer, clinicians should counsel them to initiate early breast cancer surveillance as outlined in the National Comprehensive Cancer Network Clinical Practice Guidelines.54 Last, further study aimed at identifying underlying genetic factors in sarcoma and leukemia survivors that might lead to the development of solid tumors, such as breast cancer, and the potential interactions between these factors and anthracycline and alkylator chemotherapy, is warranted.
Footnotes
See accompanying editorial on page 891
Supported by grants from the National Cancer Institute (U24CA55727 [G.T.A.], K07CA134935 [T.O.H.], R01CA136783 [C.S.M.], K05CA160724, and R01CA134722 [K.C.O.]) and the Meg Berté Owen Foundation. Support to St Jude Children’s Research Hospital was also provided by the American Lebanese-Syrian Associated Charities. Support to Memorial Sloan Kettering was provided by the core grant P30 CA008748.
Presented at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30 to June 3, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Tara O. Henderson, Chaya S. Moskowitz, Angela R. Bradbury, Joseph Phillip Neglia, Kenan Onel, Smita Bhatia, Louise C. Strong, Marilyn Stovall, Wendy M. Leisenring, Leslie L. Robison, Gregory T. Armstrong, Lisa R. Diller, Kevin C. Oeffinger
Financial support: Tara O. Henderson, Kevin C. Oeffinger
Administrative support: Tara O. Henderson, Kevin C. Oeffinger
Provision of study materials or patients: Tara O. Henderson, Leslie L. Robison, Gregory T. Armstrong, Kevin C. Oeffinger
Collection and assembly of data: Tara O. Henderson, Joseph Phillip Neglia, Smita Bhatia, Louise C. Strong, Marilyn Stovall, Dana Barnea, Elena Lorenzi, Sue Hammond, Wendy M. Leisenring, Leslie L. Robison, Gregory T. Armstrong, Lisa R. Diller, Kevin C. Oeffinger
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Breast Cancer Risk in Childhood Cancer Survivors Without a History of Chest Radiotherapy: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Tara O. Henderson
Other Relationship: Seattle Genetics
Chaya S. Moskowitz
Consulting or Advisory Role: BioClinica
Joanne F. Chou
No relationship to disclose
Angela R. Bradbury
Research Funding: Myriad Genetics, Hill-Rom (I)
Travel, Accommodations, Expenses: Hill-Rom (I)
Joseph Philip Neglia
No relationship to disclose
Chau T. Dang
Consulting or Advisory Role: Pfizer
Research Funding: Genentech, GlaxoSmithKline
Kenan Onel
No relationship to disclose
Danielle Novetsky Friedman
No relationship to disclose
Smita Bhatia
No relationship to disclose
Louise C. Strong
No relationship to disclose
Marilyn Stovall
No relationship to disclose
Lisa B. Kenney
No relationship to disclose
Dana Barnea
No relationship to disclose
Elena Lorenzi
No relationship to disclose
Sue Hammond
No relationship to disclose
Wendy M. Leisenring
Research Funding: Merck
Leslie L. Robison
No relationship to disclose
Gregory T. Armstrong
No relationship to disclose
Lisa R. Diller
Stock or Other Ownership: Novartis (I), Amgen (I), Celgene (I), Gilead (I)
Kevin C. Oeffinger
No relationship to disclose
REFERENCES
- 1. Howlader N, Noone AM, Krapcho M, et al (eds): SEER Cancer Statistics Review, 1975–2011, Bethesda, MD, National Cancer Institute, http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 2.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24:653–663. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh JM, Nekhlyudov L, Goldie SJ, et al. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152:409–417. doi: 10.1059/0003-4819-152-7-201004060-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia S, Yasui Y, Robison LL, et al. Late Effects Study Group High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 14.De Bruin ML, Sparidans J, van’t Veer MB, et al. Breast cancer risk in female survivors of Hodgkin’s lymphoma: Lower risk after smaller radiation volumes. J Clin Oncol. 2009;27:4239–4246. doi: 10.1200/JCO.2008.19.9174. [DOI] [PubMed] [Google Scholar]
- 15.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–455. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supradiaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: A National Cohort Study. J Clin Oncol. 2012;30:2745–2752. doi: 10.1200/JCO.2011.38.8835. [DOI] [PubMed] [Google Scholar]
- 17.Saslow D, Boetes C, Burke W, et al. American Cancer Society Breast Cancer Advisory Group American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 18.Mulder RL, Kremer LC, Hudson MM, et al. International Late Effects of Childhood Cancer Guideline Harmonization Group Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14:e621–e629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 20.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DM, Liu W, Kutteh WH, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–1223. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Altekruse SF, Kosary CL, Krapcho M, et al (eds): Cancer Statistics Review SEER 1975–2007. Bethesda, MD, National Cancer Institute, http://seer.cancer.gov/archive/csr/1975_2007/ [Google Scholar]
- 25.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–112. [Google Scholar]
- 26.STATA . STATA Survival Analysis and Epidemiological Tables Reference Manual. College Station, TX, : STATA Press; 2011. [Google Scholar]
- 27.Reulen RC, Taylor AJ, Winter DL, et al. Long-term population-based risks of breast cancer after childhood cancer. Int J Cancer. 2008;123:2156–2163. doi: 10.1002/ijc.23743. [DOI] [PubMed] [Google Scholar]
- 28.Reulen RC, Frobisher C, Winter DL, et al. British Childhood Cancer Survivor Study Steering Group Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA. 2011;305:2311–2319. doi: 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 29.Hartman M, Czene K, Reilly M, et al. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007;25:4210–4216. doi: 10.1200/JCO.2006.10.5056. [DOI] [PubMed] [Google Scholar]
- 30.Henderson TO, Oeffinger KC, Whitton J, et al. Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study. Ann Intern Med. 2012;156:757–766. doi: 10.1059/0003-4819-156-11-201206050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson TO, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: A report from the childhood cancer survivor study. Int J Radiat Oncol Biol Phys. 2012;84:224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 34.Swerdlow AJ, Higgins CD, Smith P, et al. Second cancer risk after chemotherapy for Hodgkin’s lymphoma: A collaborative British cohort study. J Clin Oncol. 2011;29:4096–4104. doi: 10.1200/JCO.2011.34.8268. [DOI] [PubMed] [Google Scholar]
- 35.Veiga LH, Bhatti P, Ronckers CM, et al. Chemotherapy and thyroid cancer risk: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2012;21:92–101. doi: 10.1158/1055-9965.EPI-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker MA, D’Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 37.Newton WA, Jr, Meadows AT, Shimada H, et al. Bone sarcomas as second malignant neoplasms following childhood cancer. Cancer. 1991;67:193–201. doi: 10.1002/1097-0142(19910101)67:1<193::aid-cncr2820670132>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 38.Price PJ, Suk WA, Skeen PC, et al. Transforming potential of the anticancer drug adriamycin. Science. 1975;187:1200–1201. doi: 10.1126/science.1167703. [DOI] [PubMed] [Google Scholar]
- 39.Solcia E, Ballerini L, Bellini O, et al. Mammary tumors induced in rats by adriamycin and daunomycin. Cancer Res. 1978;38:1444–1446. [PubMed] [Google Scholar]
- 40.Kelly MG, O’Gara RW, Yancey ST, et al. Induction of tumors in rats with procarbazine hydrochloride. J Natl Cancer Inst. 1968;40:1027–1051. [PubMed] [Google Scholar]
- 41.Walker SE, Anver MR. Accelerated appearance of neoplasms in female NZB/NZW mice treated with high-dose cyclophosphamide. Arthritis Rheum. 1979;22:1338–1343. doi: 10.1002/art.1780221204. [DOI] [PubMed] [Google Scholar]
- 42.Marquardt H, Philips FS, Sternberg SS. Tumorigenicity in vivo and induction of malignant transformation and mutagenesis in cell cultures by adriamycin and daunomycin. Cancer Res. 1976;36:2065–2069. [PubMed] [Google Scholar]
- 43.Weisburger JH, Griswold DP, Prejean JD, et al. The carcinogenic properties of some of the principal drugs used in clinical cancer chemotherapy. Recent Results Cancer Res. 1975;52:1–17. doi: 10.1007/978-3-642-80940-8_1. [DOI] [PubMed] [Google Scholar]
- 44.Li FP, Fraumeni JF., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969;71:747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- 45.Malkin D. Li-fraumeni syndrome. Genes Cancer. 2011;2:475–484. doi: 10.1177/1947601911413466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginsberg JP, Goodman P, Leisenring W, et al. Long-term survivors of childhood Ewing sarcoma: Report from the childhood cancer survivor study. J Natl Cancer Inst. 2010;102:1272–1283. doi: 10.1093/jnci/djq278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sultan I, Rihani R, Hazin R, et al. Second malignancies in patients with Ewing Sarcoma Family of Tumors: A population-based study. Acta Oncol. 2010;49:237–244. doi: 10.3109/02841860903253538. [DOI] [PubMed] [Google Scholar]
- 48.Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. Br J Radiol. 2008;81:362–378. doi: 10.1259/bjr/01948454. [DOI] [PubMed] [Google Scholar]
- 49.Shah NB, Platt SL. ALARA: is there a cause for alarm? Reducing radiation risks from computed tomography scanning in children. Curr Opin Pediatr. 2008;20:243–247. doi: 10.1097/MOP.0b013e3282ffafd2. [DOI] [PubMed] [Google Scholar]
- 50.Schenkman L. Radiology. Second thoughts about CT imaging. Science. 2011;331:1002–1004. doi: 10.1126/science.331.6020.1002. [DOI] [PubMed] [Google Scholar]
- 51. American Cancer Society: Cancer Facts & Figures 2015, Atlanta, GA, American Cancer Society. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index. [Google Scholar]
- 52.Nelson HD, Tyne K, Naik A, et al. Screening for Breast Cancer: Systematic Evidence Review Update for the US Preventive Services Task Force. Rockville, MD, : 2009. Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 53.Moyer VA, U.S. Preventive Services Task Force Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:271–281. doi: 10.7326/M13-2747. [DOI] [PubMed] [Google Scholar]
- 54. NCCN Guidelines for Genetic/Familial High-Risk Assessment: Breast and Ovarian. NCCN Guidelines, Version 2. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.