Abstract
Purpose
Oncotype DX (ODX) is a tumor gene-profiling test that aids in adjuvant chemotherapy decision-making. ODX has the potential to improve quality of care; however, if not equally accessible across racial groups, disparities in cancer care quality may persist or worsen. We examined racial disparities in ODX testing uptake.
Methods
We used data from the Carolina Breast Cancer Study, phase III, a longitudinal, population-based study of 2,998 North Carolina women who received a diagnosis of breast cancer between 2008 and 2014. Our primary analysis used modified Poisson regression to determine the association between race and whether ODX testing was ordered among two strata: node-negative and node-positive breast cancer.
Results
A total of 1,468 women with estrogen receptor–positive, human epidermal growth factor receptor-2–negative, stage I or II breast cancer met inclusion criteria. Black patients had higher-grade and larger tumors, more comorbidities, younger age at diagnosis, and lower socioeconomic status than non-black women. Overall, 42% of women had ODX test results in their pathology reports. Compared with those who did not receive ODX testing, women who received ODX testing tended to be younger and have medium tumor size and grade. Our regression analyses indicated no racial disparities in ODX uptake among node-negative patients. However, racial differences were detected among node-positive patients, with black patients being 46% less likely to receive ODX testing than non-black women (adjusted relative risk, 0.54; 95% CI, 0.35 to 0.84; P = .006).
Conclusion
We did not find racial disparities in ODX testing for node-negative patients for whom ODX testing is guideline recommended and widely covered by insurers. However, our findings suggest that a newer, non–guideline-concordant application of ODX testing for node-positive breast cancer was accessed less by black women than by non-black women, reflecting more guideline concordant care among black women.
INTRODUCTION
This year, an estimated 231,840 women will receive a diagnosis of invasive breast cancer,1 almost 50% with early-stage, hormone receptor–positive disease.2,3 Among these women, some will benefit from chemotherapy in addition to endocrine therapy as part of their adjuvant therapy. Historically, clinicopathologic features such as tumor grade, size, age, and comorbidities, have driven adjuvant chemotherapy decision-making.4,5 However, commercially available genetic technologies, such as Oncotype DX test (ODX; Genomic Health, Redwood City, CA), alleviate some uncertainty associated with the use of only clinicopathologic criteria to estimate adjuvant chemotherapy benefit.
ODX testing became commercially available in 2004 for the management of early-stage, node-negative, estrogen receptor–positive (ER+) breast cancer.6 This 21-tumor gene expression profiling panel categorizes the disease into low-, intermediate-, or high-risk groups on the basis of the 10-year risk of distant recurrence.7 Women with low-risk scores are unlikely to benefit from the addition of chemotherapy to adjuvant endocrine therapy, whereas women with high scores derive considerably more benefit.7 Evidence suggests that providers change adjuvant chemotherapy decision-making in 30% to 40% of patients in the presence of ODX test results,8-12 reducing the overuse of adjuvant chemotherapy.5,13 Furthermore, ODX testing appears to be cost-effective.5,14,15 Level I evidence of chemotherapy decision-making based on ODX in node-negative, ER+ disease awaits results of the randomized TAILORx trial.16
The first private insurer began covering ODX testing for women with ER+, human epidermal growth factor receptor 2–negative (HER2−), early-stage breast cancer in 2005.6 Medicare began covering ODX testing for these patients in 2006.6 Soon thereafter, medical guidelines began incorporating ODX testing for adjuvant chemotherapy decision-making for patients with node-negative breast cancer with ER+, HER2−, stage I or II disease with tumors 0.5 cm or larger.6 Since its initial validation in patients with node-negative disease, several studies have demonstrated the prognostic validity of ODX testing for certain subgroups of women with one to three positive nodes as well.17-19 Investigators in the ongoing RxPONDER trial will evaluate ODX testing for women with early-stage, node-positive, ER+ breast cancer.20 Currently, ODX testing for women with positive lymph nodes is not guideline recommended or widely covered by insurance providers.
By helping clinicians make individualized, evidence-based decisions regarding chemotherapy, ODX testing can contribute to targeted, high-quality care for women with breast cancer. However, if such technologies are not equally accessible by patients, it may unintentionally exacerbate existing disparities in breast cancer care processes and outcomes.21 Because only one third of patients with guideline-eligible disease (ie, node-negative, ER+ disease in stage I or II) receive ODX testing,22 an understanding of who is tested is critical for targeting interventions to increase its use. The few studies in which researchers examined racial differences in ODX testing offered mixed evidence.22-25 Of note, these studies have limited generalizability because they were conducted in academic settings,22,24 in hospitals within a single urban setting,22,23 or among only Medicare beneficiaries.26
We sought to extend previous research by examining racial variation in ODX testing across a diverse patient population. Furthermore, we sought to disentangle the effect of race on ODX testing on the basis of lymph node status because clinical guidelines and insurance coverage vary.
METHODS
Data Source
The Carolina Breast Cancer Study (CBCS phase III) is a population-based study of women with a diagnosis of breast cancer from across 44 North Carolina counties. Because it oversampled black and young women, CBCS phase III is particularly well powered to examine racial health disparities in breast cancer. Between 2008 and 2013, CBCS phase III investigators enrolled 2,998 women, 20 to 74 years old, with invasive breast cancer by means of rapid case ascertainment in collaboration with the North Carolina Cancer Registry. Patients were randomly selected for recruitment in four strata: black women younger than 50 years, black women aged 50 years or older, non-black women younger than 50 years, and non-black women aged 50 years or older. For this study, we used baseline survey, medical record and pathology report abstraction data. This research was approved by the University of North Carolina institutional review board.
Subjects
We included women whose breast cancer was ER+, stage I or II, and HER2−. Patients were excluded if they had multiple tumors or undetermined tumor grade, size, or progesterone receptor (PR) status (Fig 1). Missing data about tumor characteristics was rare for black (1.5%) and non-black (2.1%) women (P = .51). Given our sample sizes, we estimated that with 80% power and a two-sided α of 0.05, we could detect 18.7% and 37.5% changes in relative risk among women with node-negative or node-positive disease, respectively.27
Fig 1.
Composition of sample population with exclusion criteria. ER, estrogen receptor; HER2+, human epidermal growth factor receptor 2–positive; PR, progesterone receptor.
Measures
We used the definition of the Institute of Medicine for health disparity: “the difference in treatment or access not justified by the differences in health status or preferences of the groups.” This definition implies that race is a social construct and, thus, controls for socioeconomic status (SES) variables associated with race may mask existing racial disparities.28,29 As such, SES factors, that is, marital status, education, employment after diagnosis, family income, and insurance type, were not included in our primary model with which we estimated the reduced form effect of race on ODX testing. To examine the residual direct effect of race on ODX testing, we developed a secondary model that included SES covariates.
Dependent variable.
ODX testing information was abstracted from pathology reports. Patients without ODX reports in their pathology records were assumed not to have received the test.
Independent variable.
Race, abstracted from the baseline survey, was patient self-reported. Race was dichotomized as non-black, which included white, Asian, and other, and as black, irrespective of ethnicity.
Covariates.
Covariates included the tumor characteristics (stage, size, grade, and PR status), treatment (lumpectomy v mastectomy and radiation), and clinical characteristics (comorbidities and age). Age at diagnosis was dichotomized as younger than 50 years or 50 years or older. From baseline surveys, we calculated a count of comorbidities from five clinical categories: heart disease, hypertension, obesity, diabetes, and chronic obstructive pulmonary disease. Tumor and treatment characteristics were abstracted from pathology and medical reports, respectively. Tumor stage and radiation therapy were dropped because of multicollinearity with tumor size and surgery type, respectively. Endocrine therapy and adjuvant chemotherapy determined from medical record abstraction were each dichotomized as ever starting therapy or not. Adjuvant chemotherapy was defined as chemotherapy that occurred after the first primary surgery, that is, lumpectomy or mastectomy. Our secondary model also included the SES variables of marital status, which was married or living as married versus other; education less than high school, high school, or college and more; current employment as yes, no, or not reported; annual family income of less than $15,000, $15,000 to $30,000, $30,000 to $50,000, more than $50,000, or not reported; and insurance status as insured versus uninsured.
Analyses
Descriptive statistics were calculated by using population weights. We compared black and non-black women, as well as those who did and did not receive ODX testing using weighted linear regression and weighted χ2 tests for continuous and binary or categorical variables, respectively. We also reported ODX uptake over time by node status, using weighted χ2 tests. Multivariate analyses were based on a modified Poisson regression with sandwich error terms, which estimates relative risk consistently and efficiently with binary outcomes.30,31 Both descriptive and multivariate analyses addressed complex survey design by means of sample weights and design effects with the use of Taylor series approximations. We also accounted for clustering at the provider level. Analyses were conducted by using STATA (StataCorp, College Station, TX). Because racial differences may change as new evidence emerges for ODX testing, we conducted an exploratory analysis to observe qualitative racial differences in ODX uptake overtime by node status.
Factors that influence ODX testing may vary between women with lymph node–positive and those with lymph node–negative breast cancer; therefore, we conducted sensitivity analyses. This uncovered a need to stratify analyses by lymph node status of N0 versus N1. As a result, we present six models: unadjusted, primary, and secondary models within each lymph node stratum.
RESULTS
Characteristics of Patients by Race and ODX Uptake
Overall, non-black women tended to have fewer comorbidities, older age at diagnosis, and higher SES than black women; they also had tumors that were PR-positive and smaller and lower grade than those of black women (Table 1). Fewer black women received ODX testing than non-black women (33.9% v 43.2%, P = .001). However, when stratified by node status, this racial difference was present only among patients with node-positive disease (14.4% v 34.0%, P < .001).
Table 1.
Population-Weighted Sample Characteristics by Race and Lymph Node Status
| Full Sample | Node Positive | Node Negative | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Non-Black | Black | P | Non-Black | Black | P | Non-Black | Black | P |
| N | 859 | 609 | 203 | 180 | 656 | 429 | |||
| Weighted N | 3,895 | 793 | 828 | 218 | 3,067 | 574 | |||
| ODX testing | |||||||||
| Assay included | 43.2 | 33.9 | .001 | 34 | 14.4 | < .001 | 45.7 | 41.4 | .23 |
| Recurrence score | 17.7 (8.3) | 19 (16.2) | .17 | 18.0 (6.4) | 19.2 (13.9) | .61 | 17.7 (8.7) | 19.0 (16.7) | .2 |
| Tumor | |||||||||
| Stage I v II | 65.9 | 55.3 | < .001 | 11.6 | 7 | .16 | 80.6 | 73.7 | .02 |
| Size, cm | < .001 | .22 | .01 | ||||||
| < 2 | 75.5 | 65.9 | 54.8 | 45.5 | 81 | 73.7 | |||
| 2-5 | 22.4 | 32.3 | 43.5 | 53.3 | 16.8 | 24.3 | |||
| > 5 | 2.1 | 1.8 | 1.7 | 1.2 | 2.2 | 2 | |||
| Grade | < .001 | .003 | .004 | ||||||
| 1 | 38.4 | 27.9 | 29.5 | 18.2 | 40.8 | 31.6 | |||
| 2 | 46 | 47.2 | 48.8 | 43.8 | 45.2 | 48.5 | |||
| 3 | 15.6 | 24.9 | 21.7 | 38 | 13.9 | 20 | |||
| PR positive | 89.9 | 80.8 | < .001 | 92.5 | 80.2 | .004 | 89.2 | 81 | < .001 |
| Treatment | |||||||||
| Start ET | 90.7 | 87.1 | .06 | 93.7 | 88.2 | .08 | 89.8 | 86.7 | .15 |
| Start adjuvant CT | 27.5 | 36.7 | < .001 | 56.6 | 66.1 | .09 | 19.6 | 25.5 | .03 |
| Radiation therapy | 68.1 | 68 | .97 | 71.3 | 76.3 | .3 | 67.3 | 64.9 | .49 |
| Lumpectomy* | 62.9 | 61.9 | 49.4 | 49.2 | .97 | 66.5 | 66.7 | .13 | |
| Clinical | |||||||||
| Age at diagnosis, years | 57.7 (8.7) | 55.5 (16.9) | .003 | 56.0 (9.2) | 52.6 (15.0) | < .001 | 58.2 (8.6) | 56.6 (17.2) | .04 |
| Diagnosis year | .49 | .41 | .49 | ||||||
| 2008 | 11.2 | 10.9 | 14.8 | 14.5 | 10.2 | 9.6 | |||
| 2009 | 19.4 | 19.2 | 15.7 | 22.6 | 20.4 | 17.9 | |||
| 2010 | 24.3 | 23 | 19.9 | 19.7 | 25.5 | 24.3 | |||
| 2011 | 23.7 | 28 | 26.4 | 27.3 | 23 | 28.3 | |||
| 2012 or 2013 | 21.4 | 18.8 | 23.2 | 12.2 | 20.9 | 20 | |||
| Comorbidities | |||||||||
| Diabetes | 11 | 25.7 | < .001 | 12.5 | 20.8 | .06 | 10.6 | 27.6 | < .001 |
| COPD | 3.6 | 3.4 | .87 | 3.8 | 3.2 | .77 | 3.6 | 3.5 | .97 |
| Obesity | 11 | 21.9 | < .001 | 10 | 19.5 | .02 | 11.3 | 22.8 | < .001 |
| Heart disease | 6.7 | 7.2 | .73 | 7.8 | 5.5 | .43 | 6.4 | 7.9 | .41 |
| Hypertension | 38.9 | 65.7 | < .001 | 39.4 | 61.4 | < .001 | 38.8 | 67.3 | < .001 |
| Socioeconomic | |||||||||
| Family income, $ | < .001 | < .001 | < .001 | ||||||
| < 15,000 | 7.1 | 25.1 | 8.2 | 30.7 | 6.8 | 23 | |||
| 15,000-30,000 | 12.3 | 25 | 9.4 | 29.6 | 13.1 | 23.3 | |||
| 30,000-50,000 | 17.1 | 19.3 | 18 | 15.7 | 16.8 | 20.6 | |||
| > 50,000 | 56.5 | 25.1 | 60.5 | 17.3 | 55.4 | 28.1 | |||
| Not reported | 7 | 5.4 | 3.9 | 6.7 | 7.9 | 4.9 | |||
| Insurance | |||||||||
| Private | 78.9 | 63.7 | < .001 | 78.7 | 57.2 | < .001 | 79 | 66 | < .001 |
| Medicaid | 6 | 23.5 | < .001 | 6.9 | 28.9 | < .001 | 5.8 | 21.6 | < .001 |
| Medicare | 35.4 | 37.4 | .51 | 28.7 | 32.8 | .47 | 37.2 | 39.1 | .61 |
| Uninsured | 2.9 | 9.7 | < .001 | 2.5 | 14.8 | < .001 | 3 | 7.7 | .001 |
| Married | 71.1 | 39.4 | < .001 | 73.8 | 34.2 | < .001 | 70.3 | 41.4 | < .001 |
| Employment | .12 | .16 | .35 | ||||||
| Unemployed | 53.9 | 58.7 | 50.9 | 61.2 | 54.7 | 57.7 | |||
| Employed | 45.4 | 39.9 | 47.7 | 36.8 | 44.8 | 41.1 | |||
| Not reported | 0.7 | 1.4 | 1.4 | 2 | 0.5 | 1.2 | |||
| Education | < .001 | < .001 | < .001 | ||||||
| HS | 49 | 55.9 | 39 | 60.9 | 51.7 | 54 | |||
| College or higher | 44.8 | 30.6 | 50.4 | 26.9 | 43.3 | 32 | |||
| Less than HS | 6.1 | 13.5 | 10.6 | 12.2 | 4.9 | 14 | |||
NOTE. Data are the mean with SE or percentage unless otherwise specified.
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, chemotherapy; ET, endocrine therapy; HS, high school; ODX, Oncotype DX (Genomic Health, Redwood City, CA); PR, progesterone receptor.
Compared with mastectomy.
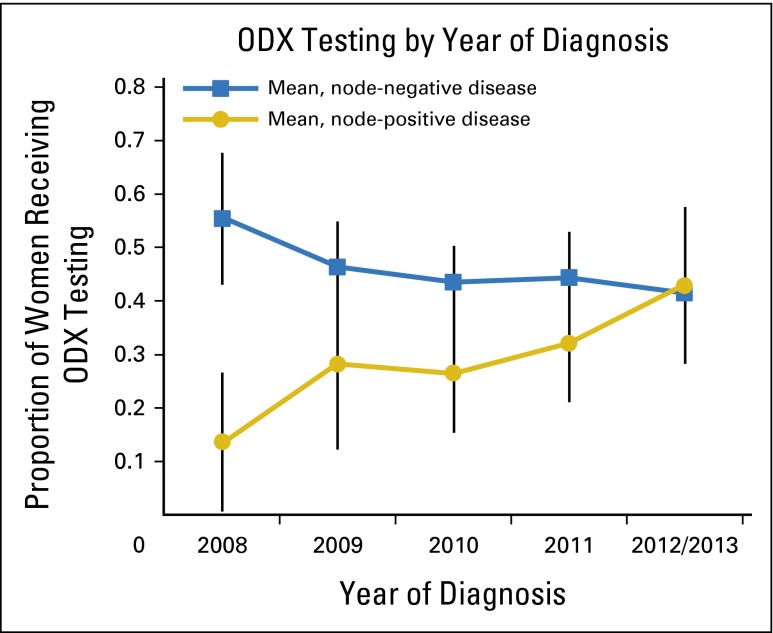
Less than 50% of women who met criteria for ODX testing had test results in their pathology report. Patients with node-positive disease were less likely to receive ODX testing than patients with node-negative disease (30% v 45%, P = .001); however, this difference diminished over time (Fig 2). ODX testing rates increased among women with node-positive disease in 2012 to 2013 compared with 2008, whereas they stayed relatively stable among patients with node-negative disease. Of interest, ODX testing rates among both subpopulations were similar after 2008 (Fig 2). Exploratory analyses suggested that racial differences in ODX test uptake were stable over time within each stratum (data not shown). Patients receiving ODX testing tended to be younger, to have fewer comorbidities, to have tumors smaller than 2 cm and tumors of grade II than patients who did not receive the test. Those receiving ODX testing were also more likely to receive adjuvant chemotherapy than women who did not; both groups were equally likely to start endocrine therapy (Table 2).
Fig 2.
Proportion of women with estrogen receptor–positive, human epidermal growth factor receptor 2–negative, stage I or II disease who underwent Oncotype DX (ODX; Genomic Health, Redwood City, CA) testing, as reported over time, by lymph node status with population weightings.
Table 2.
Sample Characteristics by ODX Use and Lymph Node Status
| Characteristic | Full Sample | Node Positive | Node Negative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No ODX | ODX | P | No ODX | ODX | P | No ODX | ODX | P | |
| n | 900 | 568 | 302 | 81 | 598 | 487 | |||
| Weighted n | 2,734 | 1,953 | 734 | 313 | 2,001 | 1640 | |||
| Tumor | |||||||||
| Size, cm | .04 | .18 | < .001 | ||||||
| < 2 | 74 | 73.6 | 49.4 | 61.1 | 83.1 | 75.9 | |||
| 2-5 | 22.9 | 25.8 | 48.7 | 38.1 | 13.5 | 23.4 | |||
| > 5 | 3 | 0.7 | 2 | 0.8 | 3.4 | 0.7 | |||
| Grade | .003 | .03 | < .001 | ||||||
| 1 | 40.7 | 31 | 26.6 | 28.4 | 45.9 | 31.5 | |||
| 2 | 41.3 | 53.1 | 43.2 | 58.4 | 40.6 | 52 | |||
| 3 | 18 | 16 | 30.2 | 13.2 | 13.5 | 16.5 | |||
| PR positive | 86.5 | 91.1 | .03 | 89.7 | 90.4 | .86 | 85.3 | 91.2 | .01 |
| Treatment | |||||||||
| Start ET | 87.1 | 94.1 | <.001 | 90.6 | 97 | .12 | 85.9 | 93.6 | < .001 |
| Start adjuvant CT | 30.7 | 26.7 | .18 | 70.4 | 30.9 | < .001 | 16.1 | 25.9 | < .001 |
| Radiation therapy | 69.4 | 66.4 | .38 | 77.2 | 61.1 | .01 | 66.5 | 67.4 | .82 |
| Lumpectomy* | 38.7 | 35.4 | .43 | 55.8 | 38.6 | .01 | 32.4 | 34.8 | .56 |
| Clinical | |||||||||
| Age at diagnosis, years | 57.6 (10.9) | 56.9 (9.9) | .28 | 54.0 (12.0) | 58.3 (8.1) | .004 | 58.9 (10.1) | 56.6 (10.3) | .002 |
| Diagnosis year | > .99 | .08 | .41 | ||||||
| 2008 | 10.9 | 11.5 | 18.2 | 6.7 | 8.2 | 12.5 | |||
| 2009 | 19 | 19.8 | 17.6 | 16.1 | 19.5 | 20.5 | |||
| 2010 | 24.6 | 23.4 | 20.8 | 17.6 | 26 | 24.5 | |||
| 2011 | 24.6 | 24.3 | 25.7 | 28.5 | 24.1 | 23.5 | |||
| 2012 or 2013 | 20.9 | 21.0 | 17.2 | 29.8 | 21.9 | 18.9 | |||
| Comorbidities | |||||||||
| Diabetes | 15.5 | 10.6 | .03 | 15.7 | 10.6 | .39 | 15.4 | 10.6 | .06 |
| COPD | 4.9 | 1.8 | .01 | 5.3 | 0 | .08 | 4.8 | 2.1 | .05 |
| Obesity | 13 | 12.6 | .86 | 14.3 | 6.5 | .12 | 12.5 | 13.8 | .59 |
| Heart disease | 8.9 | 3.9 | .005 | 8.5 | 4.8 | .4 | 9 | 3.7 | .008 |
| Hypertension | 45.3 | 40.8 | .19 | 41.8 | 49.3 | .28 | 46.7 | 39.2 | .07 |
| Socioeconomic | |||||||||
| Family income, $ | < .001 | .002 | .006 | ||||||
| < 15,000 | 12.2 | 7.2 | 15.9 | 6.1 | 10.9 | 7.4 | |||
| 15,000-30,000 | 15.6 | 12.9 | 14.2 | 12.2 | 16.1 | 13 | |||
| 30,000-50,000 | 17.4 | 17.6 | 21 | 9.4 | 16 | 19.2 | |||
| > 50,000 | 46.2 | 58.1 | 43.2 | 70.7 | 47.3 | 55.7 | |||
| Not reported | 8.6 | 4.1 | 5.7 | 1.7 | 9.7 | 4.6 | |||
| Insurance | |||||||||
| Private | 74.6 | 79.1 | .13 | 72.8 | 79 | .39 | 75.3 | 79.1 | .26 |
| Medicaid | 10.3 | 6.7 | .03 | 11.9 | 9.1 | .55 | 9.7 | 6.3 | .04 |
| Medicare | 37.3 | 33.5 | .24 | 29 | 30.5 | .82 | 40.3 | 34.1 | .09 |
| Uninsured | 4.7 | 3 | .06 | 6.1 | 2.7 | .08 | 4.2 | 3.1 | .3 |
| Married | 62.7 | 70 | .02 | 62.1 | 73.6 | .06 | 62.8 | 69.3 | .06 |
| Employment Status | .11 | .35 | .009 | ||||||
| Unemployed | 57.4 | 50.9 | 53.1 | 53 | 59 | 50.5 | |||
| Employed | 41.7 | 48.5 | 46.1 | 43.8 | 40 | 49.3 | |||
| Not reported | 0.9 | 0.7 | 0.8 | 3.2 | 1 | 0.2 | |||
| Education | .07 | .56 | .09 | ||||||
| HS | 51 | 49.1 | 41.8 | 47.9 | 54.4 | 49.3 | |||
| College or higher | 40.3 | 45.4 | 46.1 | 44.1 | 38.2 | 45.6 | |||
| Less than HS | 8.7 | 5.6 | 12.2 | 8 | 7.4 | 5.1 | |||
NOTE. Data are the mean with SE or percentage unless otherwise specified.
Abbreviations: COPD, chronic obstructive pulmonary disease; CT, chemotherapy; ET, endocrine therapy; HS, high school; ODX, Oncotype DX (Genomic Health, Redwood City, CA); PR, progesterone receptor.
Compared with mastectomy.
Race and Other Characteristics Independently Associated With Receiving the ODX Test
Among patients with node-negative disease, race was not associated with receiving ODX testing across models (Table 3). Tumor characteristics were independently associated with ODX testing; higher tumor grade, PR positivity, and tumor size of 2 to 5 cm were associated with a greater likelihood of a woman receiving ODX testing among those with node-negative disease. SES factors among patients with node-negative disease were not associated with ODX test performance. Year of diagnosis was not associated with a patient's receiving ODX testing among women with node-negative disease.
Table 3.
Modified Poisson Regression Results for ODX Test Use for Patients With Node-Negative Breast Cancer
| Characteristic | Primary Model | Secondary Model | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| Black v non-black* | 0.91 | 0.76 to 1.094 | .33 | 0.95 | 0.78 to 1.15 | .58 |
| Tumor size v < 2 cm, cm | ||||||
| 2-5 | 1.30 | 1.083 to 1.56 | .005 | 1.31 | 1.10 to 1.56 | .003 |
| > 5 | 0.31 | 0.089 to 1.045 | .059 | 0.31 | 0.091 to 1.019 | .054 |
| Grade v 1 | ||||||
| 2 | 1.41 | 1.16 to 1.72 | .001 | 1.41 | 1.15 to 1.72 | .001 |
| 3 | 1.42 | 1.098 to 1.83 | .007 | 1.45 | 1.13 to 1.86 | .003 |
| PR positive v negative | 1.48 | 1.085 to 2.029 | .014 | 1.50 | 1.099 to 2.051 | .011 |
| Mastectomy v lumpectomy | 1.031 | 0.86 to 1.23 | .74 | 1.05 | 0.88 to 1.25 | .58 |
| No. of comorbidities | 0.91 | 0.83 to 1.011 | .080 | 0.95 | 0.86 to 1.060 | .38 |
| Age at diagnosis < 50 v > 50 years | 1.13 | 0.95 to 1.33 | .16 | 1.074 | 0.90 to 1.28 | .42 |
| Diagnosis year v 2008 | ||||||
| 2009 | 0.86 | 0.66 to 1.12 | .26 | 0.84 | 0.65 to 1.10 | .22 |
| 2010 | 0.84 | 0.66 to 1.072 | .16 | 0.78 | 0.60 to 1.00 | .052 |
| 2011 | 0.85 | 0.66 to 1.10 | .23 | 0.8 | 0.62 to 1.038 | .093 |
| 2012 or 2013 | 0.79 | 0.58 to 1.074 | .13 | 0.74 | 0.54 to 1.011 | .058 |
| Family income v < $15,000, $ | ||||||
| 15,000-30,000 | 0.97 | 0.66 to 1.43 | .89 | |||
| 30,000-50,000 | 1.12 | 0.77 to 1.62 | .56 | |||
| > 50,000 | 1.017 | 0.68 to 1.52 | .94 | |||
| Not reported | 0.64 | 0.39 to 1.069 | .088 | |||
| Uninsured v insured | 0.84 | 0.55 to 1.28 | .42 | |||
| Married v unmarried | 1.11 | 0.91 to 1.37 | .30 | |||
| Employment v unemployed | ||||||
| Employed | 1.068 | 0.89 to 1.29 | .48 | |||
| Not reported | 0.46 | 0.91 to 2.13 | .32 | |||
| Education v HS or v HS or higher | ||||||
| College or higher | 1.082 | 0.92 to 1.27 | .33 | |||
| Less than HS | 0.87 | 0.56 to 1.35 | .53 | |||
| Constant† | 0.30 | 0.19 to 0.47 | <.001 | 0.26 | 0.15 to 0.45 | < .001 |
NOTE. N = 1,049. The number of primary sampling units (ie, provider) used to account for provider-level clustering was 455. Population-weighted sample size = 3,641. Primary model covariates were tumor size, tumor grade, PR status, surgery type, number of comorbidities, age at diagnosis, and year of diagnosis. Additional secondary model covariates were family income, health insurance status, marital status, employment status, and education level.
Abbreviations: HS, high school; ODX, Oncotype DX (Genomic Health, Redwood City, CA); PR, progesterone receptor; RR, risk ratio.
Results for the crude model were RR, 0.90; 95% CI, 0.77 to 1.067; P = .23.
Results for the crude model were RR, 0.46; 95% CI, 0.41 to 0.51; P = < .001.
Among patients with node-positive disease, black women were significantly less likely to receive ODX testing than non-black women (adjusted risk ratio [aRR], = 0.54, 95% CI, 0.35 to 0.84; P = .006; Table 4). Furthermore, women younger than 50 years with positive nodes were significantly less likely to receive ODX testing than women aged 50 years or older (aRR, 0.49; 95% CI, 0.32 to 0.75; P = .001, aRR, 0.52; 95% CI, 0.33 to 0.80; P = .003). Women were significantly more likely to receive ODX testing in 2012 to 2013 compared with 2008 (aRR, 3.2; 95% CI, 1.15 to 8.76; P = .03, aRR, 3.1; 95% CI, 1.088 to 8.86; P = .034). These findings were consistent across models. However, race was not statistically associated with receiving ODX testing in our secondary model (aRR, 0.68; 95% CI, 0.39 to 1.19; P = .172).
Table 4.
Modified Poisson Regression Results for the Association Between Race and Covariates and ODX Testing in Patients With Node-Positive Breast Cancer
| Characteristic | Primary Model | Secondary Model | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| Black v non-black* | 0.54 | 0.35 to 0.84 | .006 | 0.68 | 0.39 to 1.19 | .17 |
| Tumor size v < 2 cm, cm | ||||||
| 2-5 | 0.82 | 0.54 to 1.24 | .35 | 0.90 | 0.59 to 1.39 | .64 |
| > 5 | 0.86 | 0.13 to 5.57 | .88 | 1.30 | 0.20 to 8.26 | .79 |
| Grade v 1 | ||||||
| 2 | 1.19 | 0.77 to 1.83 | .44 | 1.24 | 0.76 to 2.04 | .39 |
| 3 | 0.57 | 0.26 to 1.24 | .16 | 0.56 | 0.27 to 1.16 | .12 |
| PR positive v negative | 1.02 | 0.60 to 1.75 | .94 | 1.08 | 0.58 to 2.03 | .80 |
| Mastectomy v lumpectomy | 0.84 | 0.56 to 1.27 | .41 | 0.93 | 0.62 to 1.39 | .71 |
| No. of comorbidities | 0.87 | 0.68 to 1.11 | .25 | 0.88 | 0.71 to 1.08 | .23 |
| Age at diagnosis < 50 v > 50 years | 0.49 | 0.32 to 0.75 | .001 | 0.52 | 0.33 to 0.80 | .003 |
| Diagnosis year v 2008 | ||||||
| 2009 | 2.40 | 0.85 to 6.75 | .097 | 1.99 | 0.71 to 5.55 | .19 |
| 2010 | 2.061 | 0.81 to 5.26 | .13 | 1.65 | 0.60 to 4.53 | .33 |
| 2011 | 2.53 | 0.97 to 6.56 | .057 | 2.00 | 0.80 to 5.015 | .14 |
| 2012 or 2013 | 3.17 | 1.15 to 8.76 | .026 | 3.10 | 1.088 to 8.86 | .034 |
| Family income v < $15,000, $ | ||||||
| 15,000-30,000 | 1.497 (0.790) | .446 | ||||
| 30,000-50,000 | 0.952 (0.558) | .934 | ||||
| > 50,000 | 2.516 (1.350) | .087 | ||||
| Not reported | 0.802 (0.791) | .791 | ||||
| Uninsured v insured | 0.73 | 0.24 to 2.25 | .58 | |||
| Married v unmarried | 0.93 | 0.59 to 1.48 | .77 | |||
| Employment v unemployed | ||||||
| Employed | 0.82 | 0.53 to 1.25 | .35 | |||
| Not reported | 3.53 | 1.37 to 9.10 | .009 | |||
| Education v HS or v HS or higher | ||||||
| College or higher | 0.62 | 0.40 to 0.96 | .031 | |||
| Less than HS | 0.74 | 0.27 to 2.033 | .56 | |||
| Constant† | 0.22 | 0.071 to 0.71 | .011 | 0.16 | 0.032 to 0.83 | .029 |
NOTE. N = 360. The number of primary sampling units (ie, provider) used to account for provider-level clustering was 232. Population-weighted sample size = 1,047. Primary model covariates were tumor size, tumor grade, progesterone receptor status, surgery type, number of comorbidities, age at diagnosis, and year of diagnosis. Additional secondary model covariates were family income, health insurance status, marital status, employment status, and education level.
Abbreviations: HS, high school; ODX, Oncotype DX (Genomic Health, Redwood City, CA); PR, progesterone receptor; RR, risk ratio.
Results for the crude model were RR, 0.42; 95% CI, 0.27 to 0.66; P = < .001.
Results for the crude model were RR, 0.34; 95% CI, 0.27 to 0.43; P = < .001.
DISCUSSION
Between 2008 and 2013, less than 50% of women in North Carolina who were eligible for ODX testing—that is, those with early-stage, ER+, HER2−, node-negative breast cancer—received ODX testing, and about one third with node-positive breast cancer received ODX testing. However, uptake of ODX testing increased over time among patients with node-positive disease. To our knowledge, our article is the first to describe racial differences in ODX testing among a diverse subgroup of women with node-positive disease, a group in whom ODX testing is becoming more common than before, despite unfavorable clinical guidelines and a lack of universal insurance coverage.
Overall, black women were more likely to begin adjuvant chemotherapy than non-black women. This is likely because black women had larger, higher-grade breast cancers than those of non-black women, consistent with CBCS phase I and II findings.32 Among patients with node-negative disease, the rate of starting adjuvant chemotherapy was higher among those who received ODX testing than those who did not. Of note, this pattern was reversed among patients with node-positive disease. Among women with node-negative disease, patients were less likely to receive ODX testing if they have preferences against adjuvant chemotherapy because test results may not influence their treatment decision.33,34 However, among patients with node-positive disease, guidelines recommend that women receive adjuvant chemotherapy. Therefore, ODX testing may be most commonly offered to women who are not ideal candidates for chemotherapy and for whom providers wish to forgo chemotherapy,34 a situation that potentially accounts for different chemotherapy initiation patterns by node status. Studies to examine the cost-effectiveness of ODX should be designed to stratify analyses by nodal status because different patterns of ODX test and adjuvant chemotherapy use may occur across these strata.
Use of ODX for Patients With Node-Negative Disease
Tumors of medium size (2 to 5 cm), tumors of medium or higher grade, and those PR positive were associated with an increased likelihood of ODX testing. This suggests that, among eligible women, ODX may offer extra information to make decisions about forgoing chemotherapy, particularly among women with certain tumor characteristics.24 Perhaps patients with especially favorable or unfavorable tumor characteristics were more likely to forgo ODX testing because they and their provider already had enough information to make an informed decision about adjuvant chemotherapy without ODX results.
We found differences in neither racial nor SES in ODX test usage among patients with node-negative disease. Perhaps this is the result of the wide coverage by private insurers, Medicaid, and Medicare and the availability of ODX testing for node-negative breast cancer.6 For eligible women who are uninsured or who lack adequate coverage for ODX testing, Genomic Health, the maker of ODX, provides a financial-assistance program.35 This may partially explain why race and SES were not associated with use of ODX testing among patients with node-negative disease.
Use of ODX for Patients With Node-Positive Disease
In contrast, among patients with node-positive breast cancer, black women were significantly less likely to receive ODX testing than non-black women. In sensitivity analyses, this trend persisted across the year of breast cancer diagnosis (data not shown). Unlike testing in node-negative disease, ODX testing in node-positive disease is not widely covered by insurers because it is not recommended in guidelines. Because ODX is not widely covered for women with node-positive disease, concerns about perceived costs may be a barrier to ODX testing despite the fact that Genomic Health provides financial assistance. Because SES and race are correlated, perceived costs could explain potential racial differences in those who receive ODX testing. This may partially explain the attenuated association of race with ODX testing in the secondary model that included SES covariates.
In addition, results from a qualitative study indicated that North Carolina providers often order ODX testing for patients with node-positive disease through the ongoing Southwest Oncology Group RxPONDER trial.34 Low recruitment of black women into the RxPONDER study may contribute to racial differences.36,37 Future investigators should test whether an association exists among race, trial participation, and access to new genetic technologies.
Current medical guidelines do not recommend ODX testing in patients with node-positive, early-stage, ER+ breast cancer. Therefore, lower rates of ODX testing among black women in our sample reflect their receipt of more guideline-concordant care than non-black women with node-positive breast cancer. Thus, differential receipt of ODX testing does not necessarily reflect a racial disparity in the quality of care. This paradox illustrates challenges that will accompany the measurement of disparities in the early adoption of new genetic technologies into clinical practice. Pending results from the RxPONDER trial will provide additional evidence necessary for us to determine whether ODX testing among patients with node-positive disease is appropriate.34
In the node-positive group, women younger than 50 years were less likely to receive ODX testing than those older than 50 years. Younger women tend to be healthier and, therefore, often tolerate chemotherapy better than older women; as a result, providers may be more likely to start chemotherapy in younger women, according to guidelines.34,38 An alternative may be that providers may order ODX testing for older adults to justify their not giving adjuvant chemotherapy to those who are frail or who have multiple comorbidities.
Finally, later year of breast cancer diagnosis was correlated with an increased likelihood of ODX testing in women with node-positive disease. This is likely a result of the accrual of evidence for ODX testing among subgroups of these patients over time. The first major studies whose data suggested the prognostic validity of ODX testing in node-positive disease were reported in 2008 for women receiving chemotherapy and endocrine therapy19 and in 2010 for women receiving endocrine therapy alone.17,18 In 2010, a randomized study demonstrated the predictive validity of ODX testing among postmenopausal women with node-positive breast cancer.16 This may explain why the year of diagnosis was a strong predictor of the use of ODX testing in women with node-positive disease. Of note, we did not see this trend in patients with node-negative disease, likely because ODX testing had already been added to clinical guidelines in 2008 for these patients.6 Future researchers should investigate how racial differences in ODX uptake change as new evidence for the technology emerges.
Limitations
This study has several limitations. First, we were unable to account for patient preferences that likely influence ODX testing and decisions regarding adjuvant chemotherapy. Preferences related to the treatment of early-stage breast cancer may differ by race.39 If so, patient preferences may be a mediating variable between race and ODX testing. Furthermore, we were unable to assess patient, provider, and organizational attitudes about ODX testing. Second, use of ODX testing was determined by abstracting pathology reports. Although it is possible that ODX testing was not added to medical and pathology records, our rates of ODX uptake were similar to those reported in other studies.22,23 Third, our study was powered to reveal 10% unadjusted racial differences; however, when we included covariates, our secondary models were underpowered. Because of power limitations, we were unable to examine racial differences in subsequent chemotherapy uptake by lymph node status. However, data from two studies suggest that racial disparities in use of adjuvant chemotherapy do not occur among all women, that is, those with node-positive and those with node-negative disease, who receive ODX testing.22,40 Also, our study lacked power to examine racial differences in uptake of ODX testing by node status over time beyond the exploratory analyses. Of note, with its oversampling of black women, CBCS phase III presented the best opportunity currently available to examine racial disparities surrounding ODX test uptake. Fourth, our models did not include organizational or provider characteristics that may have influence ODX test use. Organizational factors, such as being examined at a municipal versus tertiary medical centers23 or at community cancer centers versus a comprehensive cancer center,25 may decrease the likelihood of ODX testing. Planned data linkages will facilitate the exploration of multilevel factors in the future. Fifth, we were unable to disaggregate race by ethnicity or country of origin. Finally, our inclusion of SES required us to redefine family income to include a not-reported category. This represents a challenge in the measurement of the independent effects of SES because nonrandom underreporting is common.41In conclusion, this study contributes to our understanding of racial variation in ODX test uptake, particularly by revealing differences across lymph node status. Racial disparities were not observed among women with node-negative disease for whom ODX testing is guideline recommended and widely covered by insurance. This is heartening because genetic technologies are being incorporated into clinical guidelines for cancer care. In the converse, the observed racial difference in patients with node-positive disease suggests that newer, non–guideline-recommended applications of genetic technologies may be used less by those from racial minority groups than by others. This may be the result of disparities in clinical trial participation, less insurance coverage, or unexplained provider and organizational differences in the use of genetic technology where racial minority groups access care. Future investigators should explicitly examine the association of these factors with ODX testing.
Acknowledgment
We thank Andrew Olshan and Mary Beth Bell for facilitating the use of CBCS phase III data and for supporting this work, and Chiu Kit Tse for programming and data-management support. Presented in part at the AACR Science of Health Disparities Conference, San Antonio, TX, November 9-12, 2014.
GLOSSARY TERMS
- Oncotype DX classifier:
a classifier used to estimate risk of recurrence for patients with node-negative estrogen receptor–positive primary breast cancer receiving tamoxifen. Components of this classifier are expression levels based on reverse transcription polymerase chain reaction optimized for use with paraffin-embedded, formalin-fixed tissue of selected genes that were initially identified as prognostic based on published microarray studies. The completely specified classifier was externally validated based on archived specimens from independent studies.
Footnotes
Supported in part by the University of North Carolina Lineberger Cancer Control Education Program Grant No. R25 CA57726 (to M.C.R.), the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence in Breast Cancer Grant No. P50-CA58223 (to M.A.T, and L.A.C), Veterans Affairs Health Services Research and Development Senior Research Career Scientist Grant No. RCS 91-408 (to M.W.), National Institutes of Health Building Interdisciplinary (BIRCWH) K12 Program and North Carolina Translational and Clinical Sciences Institute Research Careers in Women's Health Grant No. UL1TR001111 (to S.B.D.), Agency for Healthcare Research and Quality (AHRQ) Grant No. K99 HS022189 (to M.A.D.), National Institutes of Health BIRCWH Grant No. 5K12HD001441-12 (to K.E.R.-H.), and AHRQ Comparative Effectiveness Research Career Development Award Grant No. 1-K-12 HS019468-01 and American Cancer Society Mentored Research Scholar Award Grant No. MRSG-13-17-01-CPPB (to S.B.W.). The Carolina Breast Cancer Study phase III was funded by National Institutes of Health Grant No. 8389741. Lisa A. Carey received research funding from Genentech and GlaxoSmithKline.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Megan C. Roberts, Morris Weinberger, Stacie B. Dusetzina, Michaela A. Dinan, Katherine E. Reeder-Hayes, Stephanie B. Wheeler
Collection and assembly of data: Lisa A. Carey
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Racial Variation in the Uptake of Oncotype DX Testing for Early Stage Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Megan C. Roberts
No relationship to disclose
Morris Weinberger
No relationship to disclose
Stacie B. Dusetzina
No relationship to disclose
Michaela A. Dinan
Consulting or Advisory Role: Salix
Katherine E. Reeder-Hayes
No relationship to disclose
Lisa A. Carey
No relationship to disclose
Melissa A. Troester
No relationship to disclose
Stephanie B. Wheeler
No relationship to disclose
REFERENCES
- 1.American Cancer Society Breast cancer overview. http://www.cancer.org/cancer/breastcancer/overviewguide/breast-cancer-overview-key-statistics.
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Toole MJ, Kidwell KM, Van Poznak C. Oncotype dx results in multiple primary breast cancers. Breast Cancer. 2014;8:1–6. doi: 10.4137/BCBCR.S13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder EE, Hay SB, Moore K. Factors influencing treatment recommendations in node-negative breast cancer. J Oncol Pract. 2011;7:26–30. doi: 10.1200/JOP.2010.000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed SD, Dinan MA, Schulman KA, et al. Cost-effectiveness of the 21-gene recurrence score assay in the context of multifactorial decision making to guide chemotherapy for early-stage breast cancer. Genet Med. 2013;15:203–211. doi: 10.1038/gim.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trosman JR, Van Bebber SL, Phillips KA. Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract. 2010;6:238–242. doi: 10.1200/JOP.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 8.Asad J, Jacobson AF, Estabrook A, et al. Does Oncotype DX recurrence score affect the management of patients with early-stage breast cancer? Am J Surg. 2008;196:527–529. doi: 10.1016/j.amjsurg.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Albanell J, González A, Ruiz-Borrego M, et al. Prospective transGEICAM study of the impact of the 21-gene recurrence score assay and traditional clinicopathological factors on adjuvant clinical decision making in women with estrogen receptor-positive (ER+) node-negative breast cancer. Ann Oncol. 2012;23:625–631. doi: 10.1093/annonc/mdr278. [DOI] [PubMed] [Google Scholar]
- 10.Biroschak JR, Schwartz GF, Palazzo JP, et al. Impact of Oncotype DX on treatment decisions in ER-positive, node-negative breast cancer with histologic correlation. Breast J. 2013;19:269–275. doi: 10.1111/tbj.12099. [DOI] [PubMed] [Google Scholar]
- 11.de Boer RH, Baker C, Speakman D, et al. The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer. Med J Aust. 2013;199:205–208. doi: 10.5694/mja12.11334. [DOI] [PubMed] [Google Scholar]
- 12.Henry LR, Stojadinovic A, Swain SM, et al. The influence of a gene expression profile on breast cancer decisions. J Surg Oncol. 2009;99:319–323. doi: 10.1002/jso.21244. [DOI] [PubMed] [Google Scholar]
- 13. Chavez-MacGregor M, Niu J, Smith B, et al: Oncotype Dx use and its relationship with chemotherapy administration in the general population. Presented at the American Associate for Cancer Research San Antonio Breast Cancer Symposium, San Antonio, TX, December 9-13, 2014. [Google Scholar]
- 14.Hornberger J, Cosler LE, Lyman GH. Economic analysis of targeting chemotherapy using a 21-gene RT-PCR assay in lymph-node-negative, estrogen-receptor-positive, early-stage breast cancer. Am J Manag Care. 2005;11:313–324. [PubMed] [Google Scholar]
- 15.Lyman GH, Cosler LE, Kuderer NM, et al. Impact of a 21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: an economic analysis based on prognostic and predictive validation studies. Cancer. 2007;109:1011–1018. doi: 10.1002/cncr.22506. [DOI] [PubMed] [Google Scholar]
- 16.Sparano JA. TAILORx: trial assigning individualized options for treatment (Rx) Clin Breast Cancer. 2006;7:347–350. doi: 10.3816/CBC.2006.n.051. [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Barlow WE, Shak S, et al. Breast Cancer Intergroup of North America Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28:1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein LJ, Gray R, Badve S, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26:4063–4071. doi: 10.1200/JCO.2007.14.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemp Clin Trials. 2013;34:1–9. doi: 10.1016/j.cct.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agency for Healthcare Research and Quality: National Healthcare Disparities Report, 2013. AHRQ publication no. 14-0006, Rockville, MD,2014.
- 22.Lund MJ, Mosunjac M, Davis KM, et al. 21-Gene recurrence scores: racial differences in testing, scores, treatment, and outcome. Cancer. 2012;118:788–796. doi: 10.1002/cncr.26180. [DOI] [PubMed] [Google Scholar]
- 23.Guth AA, Fineberg S, Fei K, et al. Utilization of Oncotype DX in an inner city population: race or place. Int J Breast Cancer. 2013;2013:653805. doi: 10.1155/2013/653805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFrank JT, Salz T, Reeder-Hayes K, et al. Who gets genomic testing for breast cancer recurrence risk. Public Health Genomics. 2013;16:215–222. doi: 10.1159/000353518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassett MJ, Silver SM, Hughes ME, et al. Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol. 2012;30:2218–2226. doi: 10.1200/JCO.2011.38.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinan MA, Mi X, Reed SD, et al. Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the Medicare population, 2005-2009. JAMA Oncol. 2015;1:158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 27.Rothman KJ. Episheet: spreadsheets for the analysis of epidemiologic data. http://www.krothman.org/episheet.xls.
- 28.Hebert PL, Sisk JE, Howell EA. When does a difference become a disparity? Conceptualizing racial and ethnic disparities in health. Health Aff (Millwood) 2008;27:374–382. doi: 10.1377/hlthaff.27.2.374. [DOI] [PubMed] [Google Scholar]
- 29.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94:666–668. [PMC free article] [PubMed] [Google Scholar]
- 30.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 31.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22:661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]
- 32.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 33.Spellman E, Sulayman N, Eggly S, et al. Conveying genomic recurrence risk estimates to patients with early-stage breast cancer: oncologist perspectives. Psychooncology. 2013;22:2110–2116. doi: 10.1002/pon.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts MC, Bryson A, Weinberger M, et al. Barriers and facilitators for the use of oncotype DX among oncologists: A qualitative study.; Presented at Academy Health Annual Research Meeting, Minneapolis, MN, June 14-16, 2015. [Google Scholar]
- 35.Genomic Health, Inc Getting tested with the Oncotype DX breast cancer assay. http://breast-cancer.oncotypedx.com/en-US/Patient-Invasive/GettingTested.aspx.
- 36.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 37.Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bremnes RM, Andersen K, Wist EA. Cancer patients, doctors and nurses vary in their willingness to undertake cancer chemotherapy. Eur J Cancer. 1995;31A:1955–1959. doi: 10.1016/0959-8049(95)00513-7. [DOI] [PubMed] [Google Scholar]
- 39.Hayman JA, Fairclough DL, Harris JR, et al. Patient preferences concerning the trade-off between the risks and benefits of routine radiation therapy after conservative surgery for early-stage breast cancer. J Clin Oncol. 1997;15:1252–1260. doi: 10.1200/JCO.1997.15.3.1252. [DOI] [PubMed] [Google Scholar]
- 40.Roberts MC, Weinberger M, Dusetzina SB, et al. Racial variation in adjuvant chemotherapy initiation among breast cancer patients receiving Oncotype DX testing. Breast Cancer Res Treat. 2015;153:191–200. doi: 10.1007/s10549-015-3518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenker N, Raghunathan TE, Chiu P, et al. Multiple imputation of missing income data in the National Health Interview Survey. J Am Stat Assoc. 2006;101:924–933. [Google Scholar]