Abstract
Purpose
Breast implant–associated anaplastic large-cell lymphoma (BI-ALCL) is a rare type of T-cell lymphoma that arises around breast implants. The optimal management of this disease has not been established. The goal of this study is to evaluate the efficacy of different therapies used in patients with BI-ALCL to determine an optimal treatment approach.
Patients and Methods
In this study, we applied strict criteria to pathologic findings, assessed therapies used, and conducted a clinical follow-up of 87 patients with BI-ALCL, including 50 previously reported in the literature and 37 unreported. A Prentice, Williams, and Peterson model was used to assess the rate of events for each therapeutic intervention.
Results
The median and mean follow-up times were 45 and 30 months, respectively (range, 3 to 217 months). The median overall survival (OS) time after diagnosis of BI-ALCL was 13 years, and the OS rate was 93% and 89% at 3 and 5 years, respectively. Patients with lymphoma confined by the fibrous capsule surrounding the implant had better event-free survival (EFS) and OS than did patients with lymphoma that had spread beyond the capsule (P = .03). Patients who underwent a complete surgical excision that consisted of total capsulectomy with breast implant removal had better OS (P = .022) and EFS (P = .014) than did patients who received partial capsulectomy, systemic chemotherapy, or radiation therapy.
Conclusion
Surgical management with complete surgical excision is essential to achieve optimal EFS in patients with BI-ALCL.
INTRODUCTION
Approximately 450,000 breast implants are placed annually for cosmetic or reconstructive purposes in the United States, and an estimated 10 million women worldwide have breast implants.1 The first case of breast implant–associated anaplastic large-cell lymphoma (BI-ALCL) was reported in 1997,2 and subsequent clinicopathologic and epidemiologic studies have shown that BI-ALCL is a distinct entity.3,4 In 2011, the US Food and Drug Administration published a safety communication that cautioned women with breast implants about the increased risk of developing BI-ALCL in the fluid or capsule surrounding the implant and encouraged the reporting of new occurrences.5 BI-ALCL is a rare disease; de Jong et al6 estimated an annual incidence of BI-ALCL of 0.1 to 0.3 per 100,000 women with implants. In recent years, however, the number of cases of BI-ALCL reported in the literature has greatly increased, which suggests that this disease was underdiagnosed in the past.
Limited observations suggest that patients with BI-ALCL often have clinically indolent disease, but there is a subset of patients reported who had progressive disease that sometimes resulted in death, which suggests a broader disease spectrum.7 A number of therapeutic approaches for patients with BI-ALCL have been reported in the literature, highlighting a lack of consensus and the need for definition of the optimal treatment of patients with BI-ALCL.
In this study, we collected data from 87 patients with BI-ALCL who had complete pathologic and staging information, data on therapies used, and clinical follow up. The goal of this study was to assess the efficacy of various therapeutic modalities on disease outcome.
PATIENTS AND METHODS
Study Design
This study was approved by the institutional review board of The University of Texas MD Anderson Cancer Center. BI-ALCL was defined as a T-cell lymphoma that is associated with a breast implant, composed of large, pleomorphic cells that uniformly expressed CD30 and lacked anaplastic lymphoma kinase (ALK) expression or genetic abnormalities involving ALK at chromosome 2q23. The tumor begins on the luminal surface of the fibrous capsule surrounding the implant and may show varying degrees of infiltration of the capsule, the surrounding soft tissue, or the breast parenchyma. Patients with ALCL confined to the skin of the breast (ie, cutaneous ALCL) were excluded. Rare patients with widespread ALK-negative ALCL with a breast mass that did not involve the fibrous capsule around an implant were also excluded.
This study included 37 unpublished occurrences of BI-ALCL that had complete clinical, therapeutic, and follow-up data. In addition, a literature search was performed for all reports of BI-ALCL worldwide published between January 1997 and December 2014. Corresponding authors of all published cases of BI-ALCL were contacted to contribute clinical information, pathology slides, and treatment and follow-up data, and the slides were reviewed to confirm the diagnosis of BI-ALCL (Fig 1).3,4
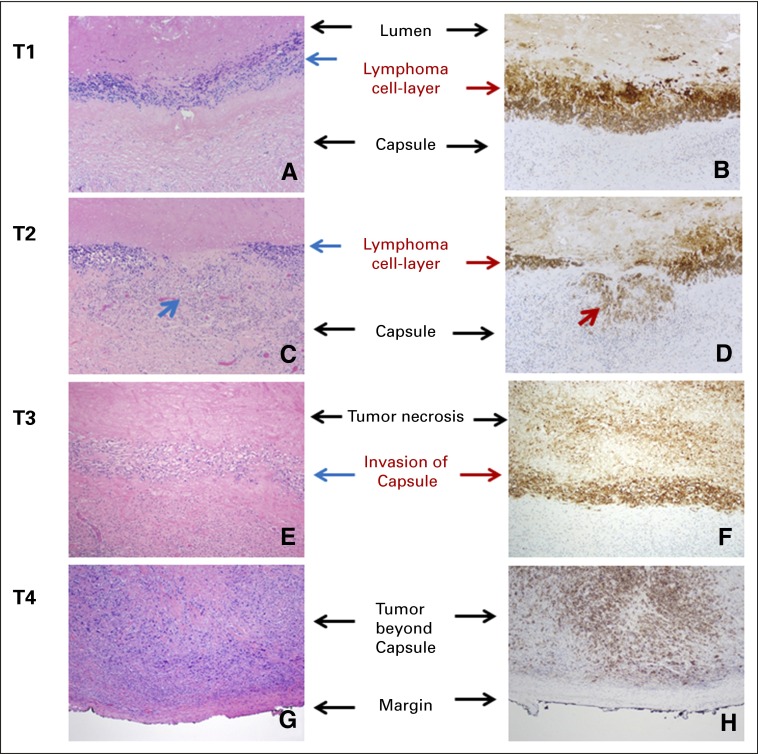
Fig 1.
Pathologic T staging. (A and B) T1: lymphoma cells confined to the effusion or a layer on the luminal side of the capsule; (C and D) T2: lymphoma cells superficially infiltrate the luminal side of the capsule. Arrows indicate the areas of invasion; (E and F) T3: clusters or sheets of lymphoma cells infiltrate into the thickness of the capsule; and (G and H) T4: lymphoma cells infiltrating beyond the capsule, into the adjacent soft tissue or breast parenchyma. Left column, hematoxylin and eosin stain; right column, CD30 immunohistochemistry; magnification, ×100.
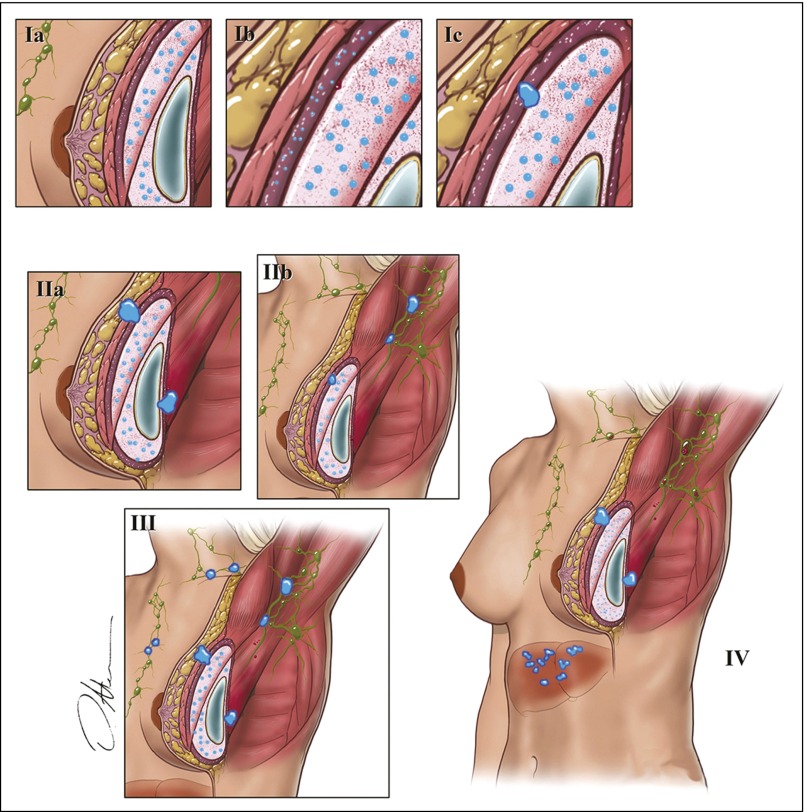
Patients were staged at the time of presentation by using the Ann Arbor system.8 In addition, we designed a surgical and pathologic staging system for BI-ALCL modeled after the American Joint Committee on Cancer TNM system for staging solid tumors (Fig 2; Table 1).9
Fig 2.
This TNM system was modeled after the American Joint Committee on Cancer TNM staging system for solid tumors.
Table 1.
Proposed TNM Staging for Breast Implant–Associated Anaplastic Large-Cell Lymphoma
| TNM or Stage Designation | Description |
|---|---|
| T: tumor extent | |
| T1 | Confined to effusion or a layer on luminal side of capsule |
| T2 | Early capsule infiltration |
| T3 | Cell aggregates or sheets infiltrating the capsule |
| T4 | Lymphoma infiltrates beyond the capsule |
| N: lymph node | |
| N0 | No lymph node involvement |
| N1 | One regional lymph node (+) |
| N2 | Multiple regional lymph nodes (+) |
| M: metastasis | |
| M0 | No distant spread |
| M1 | Spread to other organs/distant sites |
| Stage | |
| IA | T1N0M0 |
| IB | T2N0M0 |
| IC | T3N0M0 |
| IIA | T4N0M0 |
| IIB | T1-3N1M0 |
| III | T4N1-2M0 |
| IV | TanyNanyM1 |
For this study, limited surgery was defined as partial capsulectomy, implant removal or replacement, or excisional biopsy of the capsule or mass. Complete surgery was defined as breast implant removal and total capsulectomy with complete excision of any associated mass and negative margins on final pathologic evaluation. Clinical follow-up data, including details of adjuvant therapy and dates of events after any therapeutic intervention, were collected. Details of chemotherapy agents and number of cycles administered, as well as radiation therapy details, were also obtained.
Statistical Analysis
Because the patients received a number of therapies for their diseases, either concurrently or sequentially, traditional methods of presenting outcome oversimplify the clinical picture. Therefore, for this study, we divided therapies into one of four categories: limited surgery, complete surgery, systemic chemotherapy, or radiation therapy. For event-free survival (EFS), an event included lymphoma persistence, recurrence, progression, relapse, or death. EFS was calculated from the time of therapeutic intervention to the time of an event or last clinical follow-up. Overall survival (OS) was calculated from the time of diagnosis of BI-ALCL to death or last follow-up. Patients who were alive or had no event at last follow-up were censored. The EFS and OS probabilities were estimated by the Kaplan-Meier product-limit method.10 The log-rank test was used to compare survival among the subgroups. We used a Prentice, Williams, and Peterson model to assess the effect of treatment on EFS.11 P < .05 was considered significant, and all tests were two sided. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and R software (The R Foundation for Statistical Computing, Boston, MA).
RESULTS
Study Group
The study group of 87 patients was derived from a search of the literature and from unpublished BI-ALCL occurrences. From a total of 91 patients with BI-ALCL identified in PubMed, we obtained complete clinical data and follow-up data for 50 patients. This study also included 37 patients with unpublished data culled from many participating institutions. The pathologic findings of all 87 patients were centrally reviewed; these patients were included in this analysis of OS and EFS.2-4,6,12-35 The remaining 41 patients had incomplete data and were excluded from analysis.
The clinical and pathologic features of these 87 patients are summarized in Table 2. All patients were women, and the median age was 54 years (range, 28 to 87 years). The median interval from implantation to diagnosis of BI-ALCL was 8 years (range, 2 to 25 years). Fifty-two patients (59.8%) presented with an effusion around the implant (so-called seroma), 17 patients (19.5%) had a breast mass and effusion, 15 patients (17.2%) had a breast mass, and three patients (3.5%) had neither. Fifty-seven patients (65.5%) had lymphoma confined by the fibrous capsule, and 30 patients (34.5%) had lymphoma infiltration beyond the capsule. Axillary lymph nodes were positive for lymphoma in 13 (14.9%) of 87 patients, either at the time of diagnosis or subsequently. The median clinical follow-up time was 30 months (mean, 45 months; range, 3 to 217 months).
Table 2.
Clinicopathologic Features of Patients With Breast Implant–Associated Anaplastic Large-Cell Lymphoma
| Clinical Feature | Patients With BI-ALCL |
|---|---|
| Age, years | |
| Median | 54 |
| Range | 28-87 |
| Laterality | |
| Right | 46 (52.9) |
| Left | 37 (42.5) |
| Bilateral | 4 (4.6) |
| Reason for initial implantation | |
| Cosmetic | 51 (58.6) |
| Breast cancer reconstruction | 36 (41.4) |
| Type of implant (n = 81) | |
| Silicone | 40 (49.4) |
| Saline | 41 (50.6) |
| Texture of implant (n = 48) | |
| Purely textured | 45 (93.7) |
| Purely smooth | 0 (0) |
| Both smooth/textured | 3 (2.3) |
| Interval to lymphoma diagnosis, years | |
| Median | 8 |
| Mean | 9.1 |
| Range | 2-25 |
| Clinical presentation | |
| Effusion only | 52 (59.8) |
| Mass only | 15 (17.2) |
| Effusion and mass | 17 (19.5) |
| No mass, no effusion | 3 (3.4) |
| T stage | |
| T1 | 31 (35.6) |
| T2 | 11 (12.6) |
| T3 | 14 (16.1) |
| T4 | 30 (34.5) |
| N stage | |
| 0 | 74 (85.1) |
| 1 | 13 (14.9) |
| Ann Arbor stage at presentation | |
| IE | 74 (86.2) |
| IIE | 13 (13.8) |
| TNM stage at presentation | |
| IA | 31 (35.6) |
| IB | 10 (11.5) |
| IC | 12 (13.8) |
| IIA | 22 (25.3) |
| IIB | 4 (4.6) |
| III | 8 (9.2) |
| IV | 0 (0) |
| Chemotherapy (n = 51) | |
| CHOP | 44 (86.3) |
| 3 cycles | 11 |
| 4 cycles | 2 |
| 6 cycles | 28 |
| NA | 3 |
| CHOEP | 11 (21.6) |
| 6 cycles | 11 |
| NS | 2 (3.9) |
| ABVD | 2 (3.9) |
| Hyper-CVAD | 1 (1.9) |
| Follow-up, months | |
| Median | 30 |
| Mean | 45 |
| Range | 3-217 |
NOTE. Data are given as No. (%) unless otherwise noted.
Abbreviations: ABVD, adriamycin, bleomycin, vinblastine, and dacarbazine; BI-ALCL, breast implant–associated anaplastic large-cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CHOEP, CHOP plus etoposide; Hyper-CVAD course A, hyperfractionated; cyclophosphamide, vincristine, doxorubicin, and dexamethasone with course B: methotrexate and cytarabine; NA, not available; NS, chemotherapy not specified.
Therapy
Because many of the patients received two or more therapeutic interventions, there were a total of 207 therapeutic interventions. Forty-three patients underwent limited surgery; 74 patients, complete surgical excision; 51 patients, systemic chemotherapy; and 39 patients, radiation therapy. Details of chemotherapy regimens and the number of cycles administered are shown in Table 2. The most common chemotherapy regimen used was cyclophosphamide, doxorubicin, vincristine, and prednisolone. Although some patients achieved a complete remission with chemotherapy alone, 29% of these patients experienced relapse or other events, which suggests that systemic chemotherapy alone was insufficient in a substantial number of patients. Among the 51 patients who received systemic chemotherapy, six (54.5%) of 11 patients without concomitant complete surgical excision had events. Furthermore, nine (22.5%) of 40 patients who received chemotherapy and complete surgical excision had events; all events occurred before the patients underwent a complete surgical excision. Thirty-five patients received only complete surgical excision without chemotherapy or radiation therapy, and four (11.4%) of these patients had events.
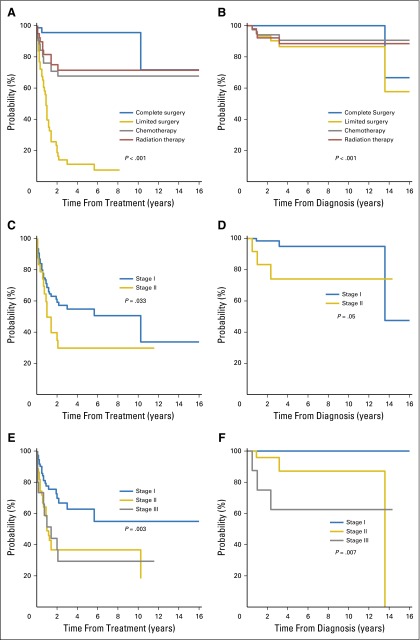
For the entire study group, the OS rate was 94% and 91% at 3 and 5 years, respectively, and the 3-year and 5-year EFS rates were both 49%. Complete surgical excision prolonged OS (P < .001) and EFS (P < .001) compared with the other therapeutic interventions. Eventually, 74 patients underwent a complete surgical excision, and only four patients (5%) had events. The product-limit method was used to estimate the EFS after each treatment approach, because many patients received several therapies. The EFS and OS curves by the type of therapy and staging (cumulative incidence rates at 1, 3, and 5 years) are shown in Figs 3A and 3B. Patients who had complete surgical excision had better OS than did patients who did not have complete surgical excision (P < .001, log-rank test).
Fig 3.
Survival curves according to treatment approaches: event-free survival (A), overall survival (B). Survival curves according to Ann Arbor stage: event-free survival (C), overall survival (D). Survival curves according to proposed TNM staging: event-free survival (E), overall survival (F).
Of 87 patients, 46 had events, which included six deaths.4,13,16,28,35 The clinicopathologic features, therapy, and cause of death of these six patients is summarized in Appendix Table A1 (online only). The age of patients at diagnosis ranged from 43 to 63 years. The indications for implants were cosmetic in four patients and were reconstruction for breast cancer in two patients. All patients had tumors that arose in the capsule around the breast implant. The size of tumors was available in 3 patients and ranged from 1.2 to 7 cm and in two patients the tumor was referred to as large and voluminous but without quantification, and no information was available for the last patient. At diagnosis, two patients had stage I disease and four had stage II disease. One patient had a tumor biopsy and five patients had limited surgery, such as implant removal or partial capsulectomy; all experienced relapse of disease. Patient 4 had complete capsulectomy 1 year later and did not receive adjuvant therapy; this patient was free of disease for 120 months when she was diagnosed with follicular lymphoma and, a few months later, diffuse large B-cell lymphoma; staging of the bone marrow revealed large B-cell lymphoma and chronic lymphocytic leukemia. The cause of death of the patient was widespread diffuse large B-cell lymphoma. None of the other five patients received complete resection of the tumor or complete capsulectomy. Patient 5 did not receive adjuvant therapy and died as a result of respiratory failure secondary to mediastinal adenopathy, tracheal narrowing, and pleural effusion 28 months after diagnosis.28 The other four patients received chemotherapy and radiation therapy; all experienced recurrences and progression of disease, with the bulk of disease in the mediastinum, chest wall, and pleural effusion areas. Other affected areas were lymphadenopathy in axilla, supraclavicular, internal mammary, and peritracheal regions. Patients 2 and 6 also had pulmonary infiltrates. The time of death from diagnosis among patients who died as a result of ALCL ranged from 7 to 38 months. Thus, the five patients who died with BI-ALCL had disease confined to the breast or had local extension to the chest wall, pleura or mediastinum, and lung (in two patients), but no evidence of disseminated ALCL.
Prognosis by Stage
The probability of having events after various therapeutic interventions was analyzed separately by stage. Because more than 80% of the occurrences were Ann Arbor stage I, this system was not adequately refined to divide patients into multiple prognostic groups. Because BI-ALCL appears to progress locally, similar to a solid tumor, we examined whether a newly proposed TNM staging system for BI-ALCL would be more prognostic. The EFS curves with this TNM staging system are shown in Figure 3E. Patients with stage I disease had better EFS than did those with higher stages (P = .003). To estimate the effect of stage on EFS, we applied the Prentice, Williams, and Peterson model. By using the TNM staging system, the rate of events was 2.6-fold higher for stage II disease and was 2.7-fold higher for stage III disease compared with stage I disease.
The event rates by pathologic stage were compared for the different therapeutic approaches (Table 3). Among 43 patients who had limited surgery (not complete excision), the event rates were not different among the different pathologic stages (P = .44). Among the 74 patients who had complete surgical excision, the rate of events was 14.3% for patients with T4 stage and was 0% for patients with T1 and T2 stages (P < .001). Among the 51 patients who received systemic chemotherapy, the rate of events was 60% for patients with T4 stage and was 13.3% for patients with T1 stage (P < .001). Among the 39 patients who had radiation therapy, the rate of events was 56% for patients with T4 stage and was 0% for patients with T1 to T3 stages (P < .001). The pathologic and clinical stages were evenly distributed for patients who received complete or partial surgery, chemotherapy, or radiation therapy (P = .48). Of note, the new TNM staging system appeared to predict OS more accurately than did the Ann Arbor system (P = .007).
Table 3.
Rate of Events After Various Treatment Approaches of Patients With Breast Implant–Associated Anaplastic Large-Cell Lymphoma
| Treatment Approach | Event Rate (%) by Time Point | ||
|---|---|---|---|
| 1 Year | 3 Years | 5 Years | |
| Limited surgery (n = 43) | 60 | 89 | 89 |
| Complete surgery (n = 74) | 4 | 4 | 4 |
| Radiation therapy (n = 39) | 18 | 28 | 28 |
| Chemotherapy (n = 51) | 24 | 32 | 32 |
| Ann Arbor stage | |||
| IE | 31 | 45 | 45 |
| IIE | 50 | 70 | 70 |
| TNM stage | |||
| I | 22 | 37 | 37 |
| II | 51 | 63 | 63 |
| III | 48 | 71 | 71 |
NOTE. The total number of treatment approaches is greater than 87, because some patients received multiple treatment approaches.
DISCUSSION
We present a detailed analysis of the pathologic findings, therapies, and long-term follow-up of 87 patients with BI-ALCL. We show evidence to support a pivotal role for complete surgical excision in the management of patients with this disease. Patients who had complete surgical excision had improved EFS and OS compared with those patients who underwent only limited surgery, chemotherapy, or radiation therapy. Surgical treatment of BI-ALCL requires removal of the implant, total capsulectomy, and complete removal of any disease or mass with negative margins. This approach is also recommended for patients in whom the tumor is only identified in an effusion when no tumor cells are found lining the capsule.
Although most patients with BI-ALCL have a relatively indolent clinical course, reports of deaths attributable to disease emphasize the importance of a timely diagnosis and adequate treatment with appropriate surveillance.13 Patients with BI-ALCL who died had local or regional extension of the disease. There were no patients with BI-ALCL who went on to develop disseminated disease. This pattern of progression suggests that BI-ALCL is a distinct entity and is more similar to solid tumors than to other non-Hodgkin lymphomas. This pattern of disease spread is also better suited to a TNM staging system that is modeled after solid tumor staging; in this study, we show that a newly proposed TNM staging system is more applicable than the Ann Arbor system for predicting prognosis and for evaluating treatment regimens in patients with BI-ALCL.
On the basis of the review of the cases in this study group, it appears that an implant capsule can drain into multiple regional lymph node basins. As a result, there does not seem to be a role for sentinel lymph node biopsy in the management of patients with BI-ALCL; however, if dissemination to lymph nodes occurs, the axillary lymph nodes are most likely to be involved. Because of the focal localization of lymphoma in most cases with lymph node involvement, fine-needle aspiration of enlarged lymph nodes may yield a false-negative result; therefore, excisional biopsy of any suspicious lymph nodes should be performed. Strong consideration should be given to the involvement of a surgical oncologist when dealing with this disease, because complete surgical excision is the optimal treatment for most patients. An incomplete resection or inadequate local surgical control may subject the patient to adjunct treatments that may not be needed, such as chemotherapy or radiation therapy.
More important, greater than 50% of the patients in this study received systemic chemotherapy. A number of regimens were used, and the most common was cyclophosphamide, doxorubicin, vincristine, and prednisolone. Approximately one third of these patients experienced progression of disease or did not achieve response, which suggests that systemic chemotherapy alone was insufficient for these patients. Conversely, only 4% of patients had events if they were treated by complete resection of the tumor, implant, and fibrous capsule. The analysis is complicated by the fact that many patients who had a complete surgical excision also received other therapies, including chemotherapy, either before or after a complete surgical excision. Eleven patients were treated with systemic chemotherapy and never underwent complete excision, and six of these patients died as a result of disease. Also, 40 patients received chemotherapy and underwent complete surgical excision; nine of these patients had events, but all events occurred before these patients received a complete surgical excision. Overall, only 4% of patients who underwent complete surgical excision had events. The observation that a lymphoma may benefit from surgical therapy or resection challenges the current paradigm for the management of lymphomas, which usually involves radiation therapy or systemic chemotherapy. However, BI-ALCL is mostly localized to the breast or chest region, and no bona fide occurrences of systemic disease occurred after the diagnosis of BI-ALCL in this patient cohort. Furthermore, this paradigm is not entirely novel for lymphomas. For example, primary cutaneous ALCL is often a localized disease that can be cured with excision or local radiation therapy.36 However, for the small subset of patients with more advanced disease, the role of chemotherapy requires additional analysis.
Establishing the diagnosis of BI-ALCL can be challenging, and a multidisciplinary approach is essential for the diagnosis and management of these patients. Management requires experts in diagnostic imaging, pathology, hematology/oncology, surgical oncology, radiation oncology, and plastic surgery. In an earlier study, we suggested that ultrasonography is the most effective tool to screen patients for BI-ALCL.21 The capsule surrounding the implant may be thickened and fibrous or deceptively normal in appearance. Cytologic specimens of effusions can be helpful, and adequate fluid should be aspirated to allow for testing. Biopsy or resection specimens involved with BI-ALCL will show large pleomorphic tumor cells that strongly and uniformly express CD30 and are of T-cell lineage, as shown by immunohistochemistry or flow cytometry.4 The T-cell antigens CD3 and CD5 are often negative in BI-ALCL, which is a potential diagnostic pitfall17; however, most cases express T-cell markers CD4 and CD43 (Fig 4).
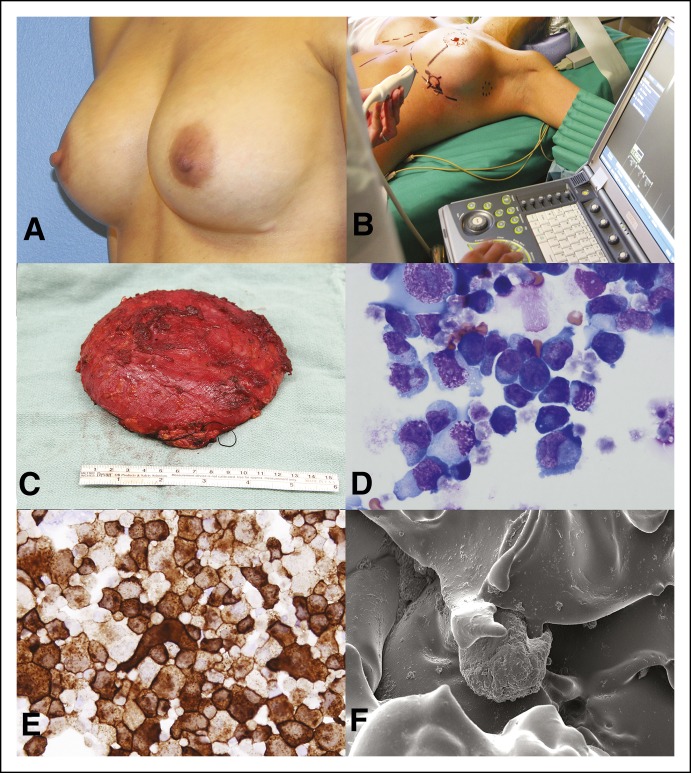
Fig 4.
Patient example and surgical treatment. This woman presented 7 years after bilateral cosmetic breast augmentation with swelling of the left breast and palpable lymphadenopathy (A). She underwent an incisional biopsy of the capsule, drainage of the effusion, and subsequent complete surgical excision that included implant removal and total capsulectomy with lymph node excisional biopsy by ultrasound guidance (B and C). Effusion demonstrated large cells (D: Wright Giemsa, ×1000; E: Anti CD30 immunocytochemistry, ×1000) capsule and excised lymph nodes were negative for lymphoma. The diagnosis rendered was breast implant–associated anaplastic large-cell lymphoma, Ann Arbor stage IE, MD Anderson Cancer Center stage 1A. Scanning electron microscopy demonstrates the textured surface of the involved breast implant with attached cells. (F; magnification, ×1,000) The patient did not receive radiation or chemotherapy and underwent surveillance by positron emission tomography–computed tomography scan every 3 months the first year and every 6 months after the first year. Patient is disease free after 2 years of follow-up.
We acknowledge that this study has limitations. This is a retrospective study in which most patients received therapies at various institutions worldwide. To minimize selection bias, we collected information from all of the reported cases in the world literature and contacted corresponding authors to obtain the details of the therapies that were used. In the literature, only 91 occurrences of BI-ALCL had been reported at the time of writing, and we obtained the pathology slides and complete clinical records of 50 patients. In addition, we included 37 unpublished cases, which represented the total experience of the authors of this manuscript at their respective institutions. Therefore, we do not believe that substantial selection bias was involved in the formation of this patient cohort.
In summary, the data we present show that timely diagnosis and complete surgical excision of lymphoma, implants, and the surrounding fibrous capsule is the optimal approach for the management of patients with BI-ALCL. Patients who receive breast implants need to be advised of the risk, albeit low, of developing BI-ALCL, as well as the common presenting symptoms, such as a mass or delayed onset (> 1 year) of effusion. Future research is warranted to determine if any modifiable risk factors, either in the patient or in the type of breast implant used, exist for this disease.
Supplementary Material
Appendix
Table A1.
Clinicopathologic Features, Therapies, and Causes of Death of Six Patients With Breast Implant–Associated Anaplastic Large-Cell Lymphoma
| Patient | Reference | Age, Years | Implant Indication | Tumor Size, cm | Ann Arbor Stage | Treatments | Time of Death From Diagnosis, Months | Cause of Death |
|---|---|---|---|---|---|---|---|---|
| 1 | Unpublished | 52 | Cosmetic | 7 | II | Limited surgery, systemic chemotherapy, RT | 7 | ALCL, mediastinal mass with progressive bronchial compression |
| 2 | Aladily et al4 | 47 | Breast cancer | 2.5 | II | Systemic chemotherapy, RT | 24 | ALCL, mediastinal mass with progressive bronchial compression, pleural effusion, pneumonia |
| 3 | Carty et al13 | 57 | Cosmetic | Large | I | Limited surgery, systemic chemotherapy, RT | 38 | ALCL, chest wall invasion, pleural infiltration, respiratory failure |
| 4 | Miranda et al16 | 63 | Breast cancer | 1.2 | I | Limited and then complete surgery | 163 | DLBCL, follicular lymphoma, disseminated disease; free of ALCL for 120 months and at death |
| 5 | Ivaldi et al28 | 53 | Cosmetic | Voluminous | II | Limited surgery | 28 | ALCL, axillary and internal mammary lymph nodes, tracheal narrowing, pleural effusion, respiratory failure |
| 6 | Lechner et al35 | 43 | Cosmetic | NA | II | Limited surgery, systemic chemotherapy, RT | 10 | ALCL, mediastinal mass with progressive bronchial compression, pleural effusions |
Abbreviations: ALCL, anaplastic large-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; NA, not available or not applicable; RT, radiation therapy.
Footnotes
Supported in part by Grant No. P30-CA016672 from the Cancer Center Support Grant, National Cancer Institute.
Presented in part at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Mark W. Clemens, L. Jeffrey Medeiros, Roberto N. Miranda
Provision of study materials or patients: Mark W. Clemens, L. Jeffrey Medeiros, Charles E. Butler, Kelly K. Hunt, Michelle A. Fanale, Steven Horwitz, Dennis D. Weisenburger, Elizabeth A. Morgan, Rashmi Kanagal-Shamanna, Vinita Parkash, Aliyah R. Sohani, Judith A. Ferry, NehaMehta-Shah, Ahmed Dogan, Hui Liu, Nora Thormann, Arianna Di Napoli, Stephen Lade, Jorge Piccolini, Ruben Reyes, Travis Williams, Colleen M. McCarthy, Summer E. Hanson, Loretta J. Nastoupil, Rakesh Gaur, Yasuhiro Oki, Ken H. Young, Roberto N. Miranda
Collection and assembly of data: Mark W. Clemens, L. Jeffrey Medeiros, Charles E. Butler, Kelly K. Hunt, Michelle A. Fanale, Steven Horwitz, Dennis D. Weisenburger, Elizabeth A. Morgan, Rashmi Kanagal-Shamanna, Vinita Parkash, Aliyah R. Sohani, Judith A. Ferry, Neha Mehta-Shah, Ahmed Dogan, Hui Liu, Nora Thormann, Arianna Di Napoli, Stephen Lade, Jorge Piccolini, Ruben Reyes, Travis Williams, Colleen M. McCarthy, Summer E. Hanson, Loretta J. Nastoupil, Rakesh Gaur, Yasuhiro Oki, Ken H. Young, Roberto N. Miranda
Data analysis and interpretation: Mark W. Clemens, L. Jeffrey Medeiros, Charles E. Butler, Dennis D. Weisenburger, Jun Liu, Jing Ning, Roberto N. Miranda
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Complete Surgical Excision Is Essential for the Management of Patients With Breast Implant–Associated Anaplastic Large-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Mark W. Clemens
Consulting or Advisory Role: Allergan
L. Jeffrey Medeiros
No relationship to disclose
Charles E. Butler
No relationship to disclose
Kelly K. Hunt
Consulting or Advisory Role: Armada Health
Michelle A. Fanale
Honoraria: Seattle Genetics, Takeda Pharmaceuticals, Research to Practice, Plexus
Consulting or Advisory Role: Spectrum Pharmaceuticals, Acetylon Pharmaceuticals, Clarient, Amgen,
Research Funding: Millennium Pharmaceuticals, Seattle Genetics, Novartis, MedImmuine, Bristol-Myers Squibb, Celgene, Molecular Templates, Genentech, Gilead Sciences
Travel, Accommodations, Expenses: Takeda Pharmaceuticals, Spectrum Pharmaceuticals, Research to Practice, Plexus
Steven Horwitz
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Janssen Pharmaceuticals, Millennium Pharmaceuticals, Rand Corporation, Seattle Genetics, Spectrum Pharmaceuticals
Research Funding: Celgene, Millennium Pharmaceuticals, Infinity Pharmaceuticals, Kirin Pharmaceuticals, Seattle Genetics, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: ADC Therapeutics, Rand Corporation, Janssen Pharmaceuticals
Dennis D. Weisenburger
Consulting or Advisory Role: Allergan
Jun Liu
No relationship to disclose
Elizabeth A. Morgan
No relationship to disclose
Rashmi Kanagal-Shamanna
No relationship to disclose
Vinita Parkash
No relationship to disclose
Jing Ning
No relationship to disclose
Aliyah R. Sohani
No relationship to disclose
Judith A. Ferry
No relationship to disclose
Neha Mehta-Shah
No relationship to disclose
Ahmed Dogan
Consulting or Advisory Role: Cancer Genetics, Janssen Pharmaceuticals, Foundation Medicine
Travel, Accommodations, Expenses: Janssen Pharmaceuticals
Hui Liu
No relationship to disclose
Nora Thormann
No relationship to disclose
Arianna Di Napoli
No relationship to disclose
Stephen Lade
No relationship to disclose
Jorge Piccolini
No relationship to disclose
Ruben Reyes
Employment: Parexel
Travel, Accommodations, Expenses: Roche
Travis Williams
No relationship to disclose
Colleen M. McCarthy
No relationship to disclose
Summer E. Hanson
No relationship to disclose
Loretta J. Nastoupil
Honoraria: Genentech, Celgene
Research Funding: TG Therapeutics, AbbVie, Janssen Biotech
Travel, Accommodations, Expenses: Janssen Biotech
Rakesh Gaur
Research Funding: Amgen, Celegene, Bristol-Myers Squibb, Cyclacel, MedImmune
Yasuhiro Oki
No relationship to disclose
Ken H. Young
No relationship to disclose
Roberto N. Miranda
No relationship to disclose
REFERENCES
- 1.US Food and Drug Administration Breast Implants. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/
- 2.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 3.Roden AC, Macon WR, Keeney GL, et al. Seroma-associated primary anaplastic large-cell lymphoma adjacent to breast implants: An indolent T-cell lymphoproliferative disorder. Mod Pathol. 2008;21:455–463. doi: 10.1038/modpathol.3801024. [DOI] [PubMed] [Google Scholar]
- 4.Aladily TN, Medeiros LJ, Amin MB, et al. Anaplastic large-cell lymphoma associated with breast implants: A report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Anaplastic large-cell lymphoma (ALCL) in women with breast implants: Preliminary FDA findings and analyses. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm239996.htm.
- 6.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 7.Kim B, Roth C, Young VL, et al. Anaplastic large cell lymphoma and breast implants: Results from a structured expert consultation process. Plast Reconstr Surg. 2011;128:629–639. doi: 10.1097/PRS.0b013e31821f9f23. [DOI] [PubMed] [Google Scholar]
- 8.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 9.Sobin LH, Gospadarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. ed 7. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958 ;53:457–481. [Google Scholar]
- 11.Prentice RL, Williams BJ, Peterson V. On the regression analysis of multivariate failure time data. Biometrika. 1981 ;68:373–379. [Google Scholar]
- 12.Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: A study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–1309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 13.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large-cell lymphoma of the breast. Plast Reconstr Surg. 2011;128:112e–118e. doi: 10.1097/PRS.0b013e318221db96. [DOI] [PubMed] [Google Scholar]
- 14.Farkash EA, Ferry JA, Harris NL, et al. Rare lymphoid malignancies of the breast: A report of two cases illustrating potential diagnostic pitfalls. J Hematop. 2009;2:237–244. doi: 10.1007/s12308-009-0043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George EV, Pharm J, Houston C, et al. Breast implant-associated ALK-negative anaplastic large-cell lymphoma: A case report and discussion of possible pathogenesis. Int J Clin Exp Pathol. 2013;6:1631–1642. [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda RN, Lin L, Talwalkar SS, et al. Anaplastic large-cell lymphoma involving the breast: A clinicopathologic study of 6 cases and review of the literature. Arch Pathol Lab Med. 2009;133:1383–1390. doi: 10.5858/133.9.1383. [DOI] [PubMed] [Google Scholar]
- 17.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: Long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popplewell L, Thomas SH, Huang Q, et al. Primary anaplastic large-cell lymphoma associated with breast implants. Leuk Lymphoma. 2011;52:1481–1487. doi: 10.3109/10428194.2011.574755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Largent J, Oefelein M, Kaplan HM, et al. Risk of lymphoma in women with breast implants: Analysis of clinical studies. Eur J Cancer Prev. 2012;21:274–280. doi: 10.1097/CEJ.0b013e328350b0ae. [DOI] [PubMed] [Google Scholar]
- 20.Wu D, Allen CT, Fromm JR. Flow cytometry of ALK-negative anaplastic large-cell lymphoma of breast implant-associated effusion and capsular tissue. Cytometry B Clin Cytom. 2015;88:58–63. doi: 10.1002/cyto.b.21178. [DOI] [PubMed] [Google Scholar]
- 21.Adrada BE, Miranda RN, Rauch GM, et al. Breast implant-associated anaplastic large-cell lymphoma: Sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat. 2014;147:1–14. doi: 10.1007/s10549-014-3034-3. [DOI] [PubMed] [Google Scholar]
- 22.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 23.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 24.Do V, Shifrin DA, Oostendorp L. Lymphoma of the breast capsule in a silicone implant-reconstructed patient. Am Surg. 2010;76:1030–1031. [PubMed] [Google Scholar]
- 25.Olack B, Gupta R, Brooks GS. Anaplastic large cell lymphoma arising in a saline breast implant capsule after tissue expander breast reconstruction. Ann Plast Surg. 2007;59:56–57. doi: 10.1097/SAP.0b013e31804d442e. [DOI] [PubMed] [Google Scholar]
- 26.Alobeid B, Sevilla DW, El-Tamer MB, et al. Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large-cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma. 2009;50:831–833. doi: 10.1080/10428190902795527. [DOI] [PubMed] [Google Scholar]
- 27.Taylor KO, Webster HR, Prince HM. Anaplastic large cell lymphoma and breast implants: Five Australian cases. Plast Reconstr Surg. 2012;129:610e–617e. doi: 10.1097/PRS.0b013e3182450aae. [DOI] [PubMed] [Google Scholar]
- 28.Ivaldi C, Perchenet AS, Jallut Y, et al. Two cases of lymphoma in an implant capsule: A difficult diagnosis, an unknown pathology [in French] Ann Chir Plast Esthet. 2013;58:688–693. doi: 10.1016/j.anplas.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Bautista-Quach MA, Nademanee A, Weisenburger DD, et al. Implant-associated primary anaplastic large-cell lymphoma with simultaneous involvement of bilateral breast capsules. Clin Breast Cancer. 2013;13:492–495. doi: 10.1016/j.clbc.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Weathers WM, Wolfswinkel EM, Hatef DA, et al. Implant-associated anaplastic large-cell lymphoma of the breast: Insight into a poorly understood disease. Can J Plast Surg. 2013;21:95–98. doi: 10.1177/229255031302100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chai SM, Kavangh S, Ooi SS, et al. Anaplastic large-cell lymphoma associated with breast implants: A unique entity within the spectrum of peri-implant effusions. Diagn Cytopathol. 2014;42:929–938. doi: 10.1002/dc.23152. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Wei S. Breast implant-associated anaplastic large cell lymphoma: Review of a distinct clinicopathologic entity. Arch Pathol Lab Med. 2014;138:842–846. doi: 10.5858/arpa.2013-0068-RS. [DOI] [PubMed] [Google Scholar]
- 33.Hart AM, Lechowicz MJ, Peters KK, et al. Breast implant–associated anaplastic large-cell lymphoma: Report of 2 cases and review of the literature. Aesthet Surg J. 2014;34:884–894. doi: 10.1177/1090820X14539503. [DOI] [PubMed] [Google Scholar]
- 34.Acevedo-Banez I, Garcia-Gomez FJ, Jimenez-Granero P, et al. F-FDG-PET/CT in implant-associated anaplastic large-cell lymphoma of the breast. Br J Haematol. 2014;169:1. doi: 10.1111/bjh.13268. [DOI] [PubMed] [Google Scholar]
- 35.Lechner MG, Megiel C, Church CH, et al. Survival signals and targets for therapy in breast implant–associated ALK–anaplastic large-cell lymphoma. Clin Cancer Res. 2012;18:4549–4559. doi: 10.1158/1078-0432.CCR-12-0101. [DOI] [PubMed] [Google Scholar]
- 36.Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: A report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653–3661. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.