Abstract
Purpose
To determine the oncologic efficacy, cardioprotective effectiveness, and safety of dexrazoxane added to chemotherapy that included a cumulative doxorubicin dose of 360 mg/m2 to treat children and adolescents with newly diagnosed T-cell acute lymphoblastic leukemia (T-ALL) or lymphoblastic non-Hodgkin lymphoma (L-NHL).
Patients and Methods
Patients were treated on Pediatric Oncology Group Protocol POG 9404, which included random assignment to treatment with or without dexrazoxane given as a bolus infusion immediately before every dose of doxorubicin. Cardiac effects were assessed by echocardiographic measurements of left ventricular function and structure.
Results
Of 573 enrolled patients, 537 were eligible, evaluable, and randomly assigned to an arm with or without dexrazoxane. The 5-year event-free survival (with standard error) did not differ between groups: 76.7% (2.7%) for the dexrazoxane group versus 76.0% (2.7%) for the doxorubicin-only group (P = .9). The frequencies of severe grade 3 or 4 hematologic toxicity, infection, CNS events, and toxic deaths were similar in both groups (P ranged from .26 to .64). Of 11 second malignancies, eight occurred in patients who received dexrazoxane (P = .17). The mean left ventricular fractional shortening, wall thickness, and thickness-to-dimension ratio z scores measured 3 years after diagnosis were worse in the doxorubicin-alone group (n = 55 per group; P ≤ .01 for all comparisons). Mean fractional shortening z scores measured 3.5 to 6.4 years after diagnosis remained diminished and were lower in the 21 patients who received doxorubicin alone than in the 31 patients who received dexrazoxane (−2.03 v −0.24; P ≤ .001).
Conclusion
Dexrazoxane was cardioprotective and did not compromise antitumor efficacy, did not increase the frequencies of toxicities, and was not associated with a significant increase in second malignancies with this doxorubicin-containing chemotherapy regimen. We recommend dexrazoxane as a cardioprotectant for children and adolescents who have malignancies treated with anthracyclines.
INTRODUCTION
Greater than 75% of children with T-cell malignancy, either T-cell acute lymphoblastic leukemia (T-ALL) or lymphoblastic non-Hodgkin lymphoma (L-NHL), will be cured with multiagent chemotherapy regimens.1-4 Anthracyclines are an important part of these regimens. Of concern are the potential late effects of intensive treatment and its impact on survivor quality of life. In the Childhood Cancer Survivor Study, long-term survivors had a 73% cumulative incidence of chronic health conditions and an all-cause mortality that was 11 times as high as that of their siblings.5-7 Cardiovascular toxicities, including cardiomyopathy, coronary artery disease, arrhythmias, and sudden death related to anthracycline treatment, are among the most common serious adverse events.8-15 Thus, identification of strategies to reduce anthracycline-related cardiac toxicity is important.
Dexrazoxane is a bis-dioxopiperazine compound that readily enters cells and is hydrolyzed to form a potent chelator of heavy metals, especially iron, zinc, and copper. How anthracyclines cause cardiomyopathy is not fully understood, but it may involve the ability to complex with heavy metals (especially iron, derived from cellular ferritin), generating free radicals that damage cardiomyocyte membranes and intracellular structures.16-18 Thus, iron chelation may be how dexrazoxane protects against anthracycline-induced cardiac damage.19 Several preclinical studies have confirmed the protective effects of dexrazoxane,20-22 including early clinical studies in adults with breast cancer.23-25 Evidence for the applicability to treatment for children is growing.
The Dana-Farber Cancer Institute (DFCI) ALL Consortium developed an anthracycline-based regimen that provided superior outcomes in patients with T-ALL and L-NHL on three consecutive protocols.3 On the basis of the DFCI experience with late subclinical cardiotoxicity using this regimen,9,10 a primary objective of the Pediatric Oncology Group (POG) 9404 study was to evaluate the cardioprotective efficacy of dexrazoxane. Additional objectives were to determine whether dexrazoxane affected event-free survival (EFS) or occurrence of serious adverse events.
PATIENTS AND METHODS
Patients
POG 9404 enrolled patients between June 1996 and September 2001. Patients with newly diagnosed T-ALL were eligible if they were 1 to 21 years old, had greater than 25% blasts in the marrow regardless of nodal disease, and had the T-cell immunophenotype confirmed by flow cytometry at a central reference laboratory. Eligibility criteria for patients with L-NHL included biopsy-proven diffuse lymphoblastic lymphoma confirmed by central pathology review, age younger than 22 years, and Murphy stage III or IV disease (< 25% blasts in the marrow, blasts in the CNS, or both). All patients were untreated except for less than 48 hours of corticosteroid administration or emergency mediastinal radiation to treat severe respiratory distress.
The National Cancer Institute and institutional review boards of each participating center approved the protocol. Informed consent or assent was obtained from patients, their parents, or their legal guardians in accordance with local institutional guidelines, Department of Health and Human Services regulations, and the Declaration of Helsinki.
Treatment
Details of treatment are published elsewhere.26 All patients received vincristine, prednisone, methotrexate, mercaptopurine, one dose per week for a total of 20 weeks of Escherichia coli l-asparaginase, and doxorubicin with intrathecal chemotherapy and cranial radiation (18 Gy). Total duration of therapy was 2 years from the date of documented complete remission.
At enrollment, patients were randomly assigned to receive therapy with or without dexrazoxane and with or without high-dose methotrexate. Dexrazoxane was given as a 300-mg/m2 bolus infusion immediately before each doxorubicin dose, beginning with induction and continuing every 3 weeks until the cumulative dose of doxorubicin (360 mg/m2) was reached. If toxicity required decreasing doxorubicin, the dose was modified to maintain a 10:1 dexrazoxane-to-doxorubicin ratio.
Toxicity was graded according to the NCI Common Terminology Criteria for Adverse Effects, version 2.0. All grade 3 or higher events were reported.
Cardiac Assessments
Serum cardiac troponin-T concentrations (cTnT) were measured at diagnosis (before doxorubicin treatment), immediately before the doxorubicin doses of 120, 240, and 330 mg/m2, and 3 weeks after the last dose of doxorubicin. Troponin concentrations were determined at a central laboratory using the Elecsys Troponin-T STAT Immunoassay (Roche Diagnostics; sensitivity, 0.01 ng/mL).
Echocardiograms were obtained before doxorubicin (pretreatment baseline), at week 4 of induction, 3 weeks after completion of doxorubicin therapy (approximately week 34), and 3 and 6 years from diagnosis (ie, 1 and 4 years from the end of chemotherapy). A central laboratory assessed each for left ventricular (LV) fractional shortening, LV dimension, and LV wall thickness. Central investigators who evaluated troponin and echocardiographic measurements were blinded to the patient clinical status and treatment assignment. Results from the central laboratories were not reported to the patient care centers.
Statistical Methods
Group assignment was stratified by disease (T-ALL v NHL) and by the presence of CNS disease at diagnosis. EFS, estimated with the Kaplan-Meier method, was defined as time from diagnosis to first event (induction death, induction failure, relapse, second malignancy, remission death) or last contact for those who were event free. Overall survival (OS) was defined as time from diagnosis to death as a result of any cause or last contact for those who were still alive. Survival curves were compared with log-rank tests. Proportions were compared with Fisher’s exact test. Continuous variables were compared with t tests. Cumulative incidence rates for second malignancies were computed with the cumulative incidence function for competing risks, and treatment regimens were compared with the K-sample test.27
The effect of dexrazoxane on the probability of an elevated troponin concentration during treatment, adjusted for an elevated concentration at baseline as covariate, was estimated with a generalized estimating equation by using the multiple troponin concentrations during treatment as repeated measures with an exchangeable working correlation structure. Echocardiographic measures adjusted for age, growth, and body-surface area were expressed as z scores on the basis of measurements in 285 healthy children.28,29 Mean z scores or change in z scores from baseline to later time points were compared with two-sample t tests.
RESULTS
Patient Characteristics
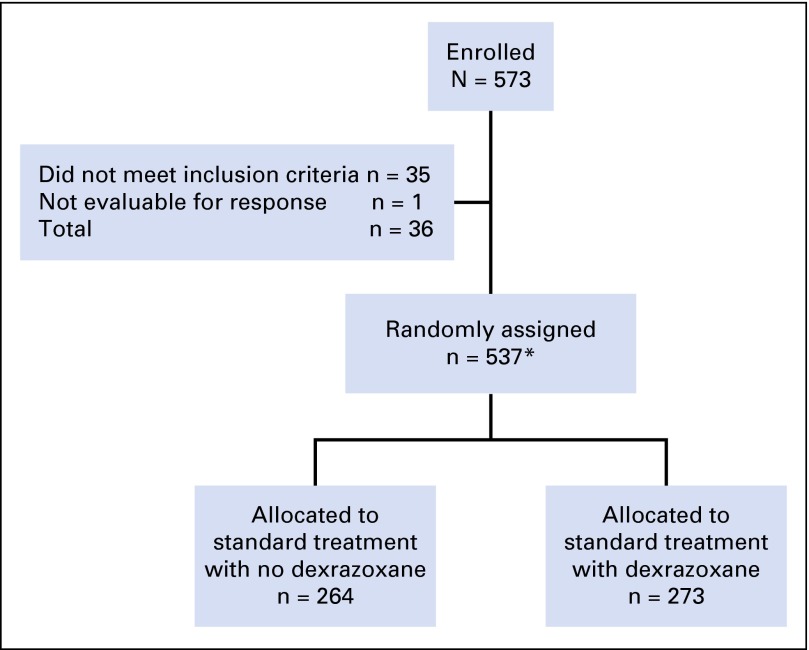
Of 573 patients enrolled, 537 were eligible and evaluable. Of these, 273 received dexrazoxane (Fig 1). Study data were frozen as of September 2011. Patient characteristics, including the number of echocardiograms and troponin concentrations, were similar between treatment groups (Table 1). Characteristics for those with echocardiographic and troponin data were similar to those without cardiac data and to the overall study population (Appendix Tables A1 and A4, online only).
Fig 1.
CONSORT diagram. Summary of patient enrollment for the POG 9404 randomized controlled trial. *In September 2000, based on interim analysis for efficacy, the Data Safety Monitoring Committee closed the methotrexate randomization. The 101 patients enrolled between September 2000 and September 2001, whether or not they received dexrazoxane, all received high-dose methotrexate and are included in this number.
Table 1.
Patient Characteristics by Treatment Group
| Characteristic | No. (%) of Patients | ||
|---|---|---|---|
| Doxorubicin Alone (n = 264) | Doxorubicin + Dexrazoxane (n = 273) | Total (N = 537) | |
| Mean (SD) age, years | 9.7 (4.58) | 9.9 (4.90) | 9.8 (4.74) |
| Median (range) follow-up, years | 8.9 (0.02-14.7) | 9.4 (0.01-15.0) | 9.2 (0.01-15.0) |
| Male sex | 203 (76.9) | 204 (74.7) | 407 (75.8) |
| Ethnicity | |||
| White | 170 (64.4) | 186 (68.1) | 356 (66.3) |
| Nonwhite | 94 (35.6) | 87 (31.9) | 181 (33.7) |
| T-ALL subset | |||
| Median (range) follow-up, years | 9.0 (0.02-14.7) | 9.3 (0.01-14.5) | 9.1 (0.01-14.7) |
| WBC, × 1,000/μL | |||
| < 50 | 71 | 84 | 155 |
| ≥ 50 | 104 | 103 | 207 |
| CNS positive (CNS2/3/bloody tap) | 44 | 56 | 100 |
| L-NHL subset | |||
| Median (range) follow-up, years | 8.5 (0.4-13.9) | 9.5 (0.2-15.0) | 9.2 (0.2-15.0) |
| With stage III disease | 67 | 52 | 119 |
| With stage IV disease | 22 | 33 | 55 |
| With echocardiogram data*† | |||
| Total No. of patients with echocardiograms at least one time point | 218 | 224 | 442 |
| Median (min, max) No. of echocardiograms per patient | 3 (1, 10) | 3 (1, 13) | 3 (1, 13) |
| Total No. of patients with echocardiograms at each time point | |||
| Baseline | 158 | 149 | 307 |
| Approximately week 34 | 61 | 82 | 143 |
| 3 years | 84 | 83 | 167 |
| 3.5 to 6.5 years | 21 | 31 | 52 |
| With troponin data* | |||
| Total No. of patients with at least one troponin measurement | 114 | 125 | 239 |
| Median (min, max) No. of samples per patient | 1 (1, 6) | 1 (1, 6) | 1 (1, 6) |
| Total No. of troponin measures per patient | |||
| 1 | 78 (68.4) | 69 (55.2) | 147 (61.5) |
| 2 | 21 (18.4) | 34 (27.2) | 55 (23.0) |
| 3 | 10 (8.8) | 16 (12.8) | 26 (10.9) |
| 4 | 3 (2.6) | 4 (3.2) | 7 (2.9) |
| 5 | 1 (0.9) | 1 (0.8) | 2 (0.8) |
| 6 | 1 (0.9) | 1 (0.8) | 2 (0.8) |
Abbreviations: L-NHL, lymphoblastic non-Hodgkin lymphoma; SD, standard deviation; T-ALL, T-cell acute lymphoblastic leukemia.
For those patients with more than one echocardiogram for a particular time point, the later one was used as the data for that time point.
Disease Outcomes
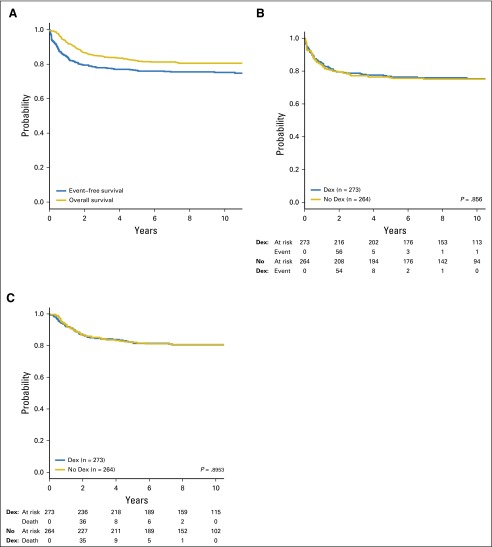
The EFS rates (with standard error [SE]) at 5 and 10 years for all 537 patients were 76.4% (1.9%) and 75.2% (2.6%), respectively (Fig 2A). OS rates (with SE) were 82.1% (1.7%) and 80.6% (2.4%) at 5 and 10 years, respectively (Fig 2A). Neither EFS (Fig 2B) nor OS (Fig 2C) differed between groups (P = .9).
Fig 2.
(A) Event-free survival and overall survival for all eligible, evaluable patients enrolled. (B) Event-free survival by treatment group. (C) Overall survival by treatment group. Dex, dexrazoxane.
The prevalence of adverse events did not differ between treatment groups (Table 2). Relapse was the most common first event in all groups, followed by induction failures. Toxic deaths occurred in less than 2% of patients. The three induction deaths occurred in patients with T-ALL.
Table 2.
Summary of Events and Targeted Toxicities by Treatment Group
| Event Type | No. of Events | P | ||
|---|---|---|---|---|
| Doxorubicin Alone (n = 264) | Doxorubicin + Dexrazoxane (n = 273) | Total (N = 537) | ||
| Induction failure | 13 | 17 | 30 | |
| Relapse | 43 | 38 | 81 | |
| Marrow ± other | 16 | 18 | 34 | |
| CNS ± other | 18 | 13 | 31 | |
| Other | 9 | 7 | 16 | |
| Toxic death | 3 | 6 | 9 | |
| Induction | 1 | 2 | 3 | |
| Remission | 2 | 4 | 6 | |
| Secondary malignant neoplasm | 3: n = 1 each myeloid sarcoma, AMML, DLBL | 8: n = 2 AML, n = 2 astrocytoma, n = 2 GBM, n = 1 medulloblastoma papillary carcinoma (thyroid) | 11 | |
| Total events | 62 | 69 | 131 | |
| Grade 3 or 4 toxicities | ||||
| Infection | 168 | 173 | .64 | |
| Hematologic effects | 237 | 243 | .26 | |
| Mucositis | 52 | 33 | .02 | |
| CNS effects | 23 | 28 | .46 | |
Abbreviations: AML, acute myeloblastic leukemia; AMML, acute myelomonocytic leukemia; DLBL, diffuse large B-cell lymphoma; GBM, glioblastoma multiforme.
Acute Cardiac Toxicity
Five patients had grade 3 or 4 cardiac toxicity while receiving therapy; two had arrhythmias (n = 1 in the dexrazoxane group), and three had decreased LV fractional shortening (all were in the no-dexrazoxane group). All patients recovered and completed chemotherapy, including doxorubicin.
Serum Cardiac Troponin-T
At baseline, troponin was elevated (> 0.01 ng/mL) in seven (3.8%) of 182 patients; five patients (5.2% of 97 total patients) were in the no-dexrazoxane group, and two patients (2.4% of 85 total patients) were in the dexrazoxane group (P = .45). At a median of 21 days (range, 1 to 571 days) after the start of treatment, 385 troponin concentrations were measured in 239 patients; 114 were measured in the no-dexrazoxane group, and 125, in the dexrazoxane group (Table 1). By using the highest troponin concentration obtained after treatment in each patient, a higher proportion of patients not receiving dexrazoxane had elevated concentrations (10 [8.8%] of 114 patients v three [2.4%] of 125 patients; P = .04).
Troponin concentrations both at baseline and during treatment were available for 160 patients. The probability of having an elevated troponin level when using all troponin data collected during treatment was lower in the dexrazoxane-treated group (odds ratio, 0.23; 95% CI, 0.05 to 1.11; P = .067). An elevated troponin concentration at baseline was associated with an increased probability of elevated concentrations during treatment (odds ratio, 5.4; 95% CI, 0.86 to 34.1; P = .072).
Echocardiographic Measures
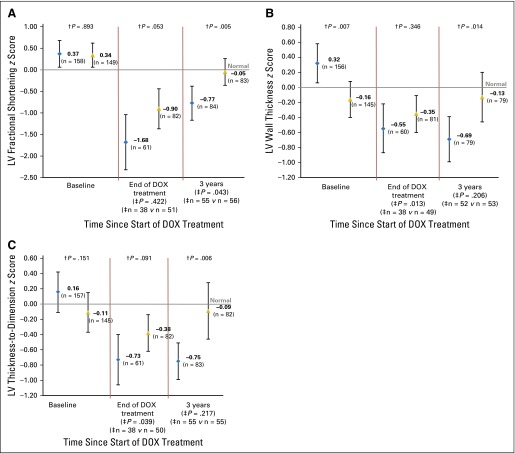
At baseline, mean z scores for LV fractional shortening and LV thickness-to-dimension ratio were similar between treatment groups (Fig 3). The mean z score for LV wall thickness at baseline in the dexrazoxane-treated group was significantly lower than in the no-dexrazoxane group (Fig 3). This difference actually favored the doxorubicin-only group, but, despite this favorable baseline, the LV wall thickness was worse after treatment than it was in the dexrazoxane-treated group. After doxorubicin therapy, mean z scores were lower than age-expected norms29 for all children but did not differ significantly between groups; the mean z score was always closer to normal for the dexrazoxane group. At 3 years, mean z scores for LV fractional shortening, LV wall thickness, and LV thickness-to-dimension ratio in the dexrazoxane-treated patients (−0.05, −0.13, and −0.09, respectively) did not differ significantly from those measures in healthy children, whereas z scores for the no-dexrazoxane group remained significantly reduced (−0.77, −0.69, and −0.75, respectively; Fig 3).
Fig 3.
Estimated mean z scores by treatment group among 307 children with T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma at baseline (n = 307), at end of therapy (n = 143), and at 3 years (n = 167). Comparison of change from baseline at each time point is noted at the bottom of the figure. (A) LV fractional shortening. (B) LV wall thickness. (C) LV thickness-to-dimension ratio. Bars represent 95% CIs; blue diamond, standard treatment only (doxorubicin [DOX]); gold triangle, dexrazoxane plus standard treatment (DRZ + DOX). †P values comparing the two groups at each time point (baseline, end of DOX treatment, and 3 years). ‡P values for differences in change in mean z scores since baseline in DOX- versus DOX- + DRZ-treated patients, for those patients with values at baseline and a second time point. n indicates number of patients with paired studies in each treatment group. Only patients with paired baseline and end-of-therapy or 3-year z scores were included in these analyses.
At 3 years, LV fractional shortening was significantly worse in the no-dexrazoxane group than in the dexrazoxane group (P = .005). The same negative effect was seen for LV wall thickness and LV thickness-to-dimension ratio (P = .01 and P = .006, respectively). A total of 31 patients treated with dexrazoxane and 21 patients treated without dexrazoxane had echocardiograms beyond the 3-year visit. Fractional shortening between groups remained significantly different (−0.24 v −2.03; P < .001). However, z scores for LV wall thickness and LV thickness-to-dimension ratios did not differ (0.27 v 0.13 [P = .81] and 0.70 v 0.03 [P = .33], respectively), although the sample sizes were small.
Toxicity
Rates of infection, hematologic toxicity, and CNS toxicity did not differ between groups (Table 2). Mucositis was more frequent in the no-dexrazoxane group (P = .02).
Second Malignancies
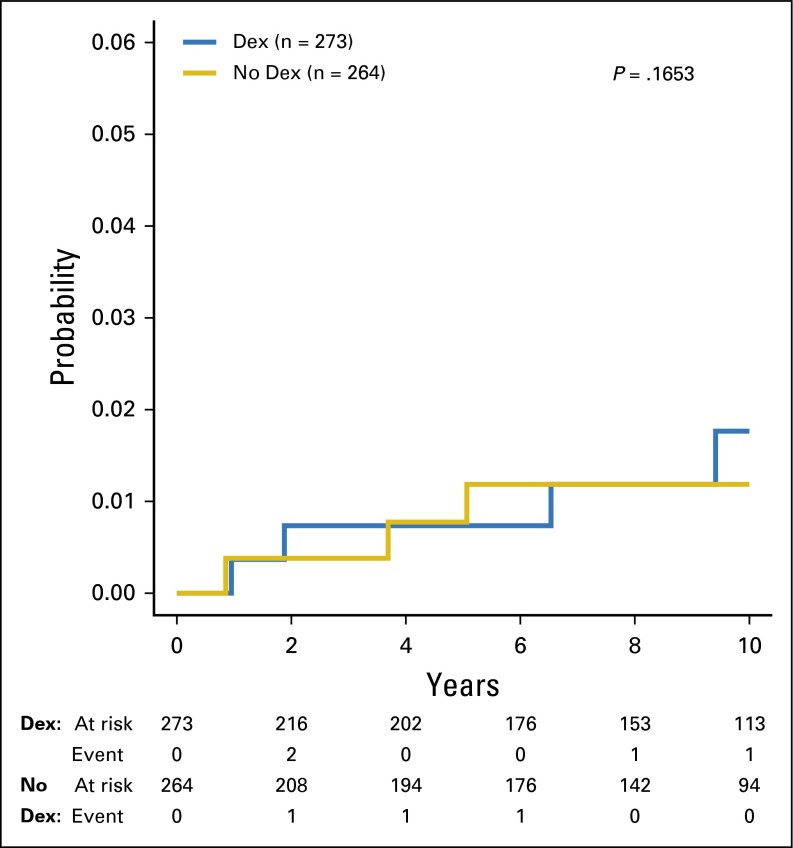
A second malignant neoplasm (SMN) was diagnosed in 11 patients, three of which were in the no-dexrazoxane group (Appendix Table A5). The 5-year cumulative incidence rates (with SE) were 0.8% (0.5%) and 0.7% (0.5%) for the no-dexrazoxane and dexrazoxane groups, respectively (P = .17; Fig 4). At 10 years, rates (with SE) in the two groups were 1.2% (0.7%) and 1.8% (0.9%), respectively. Dexrazoxane was not associated with the occurrence of SMN.
Fig 4.
Cumulative incidence of second malignant neoplasms by treatment group. Dex, dexrazoxane.
DISCUSSION
We observed an estimated mean 10-year EFS rate of 74%, which compares favorably with results for similar groups of unselected patients with T-cell malignancies treated contemporaneously by other investigators.1-4 More important, neither OS nor EFS in our study differed between treatment groups.
Dexrazoxane prevents acute cardiotoxicity in adults with cancer who receive anthracyclines.23-25, 30 However, these studies have only limited applications in pediatric oncology, because few studies included children,31-35 the sample sizes were small, and the follow-up times were short. In a meta-analysis of 10 randomized trials that assessed the efficacy of dexrazoxane as a cardioprotectant,36 three studies included adults and children, and one study included only children. The authors concluded that dexrazoxane prevented heart damage, although they noted that these studies applied different definitions and assessments of cardiac toxicity and heart failure. They were unable to determine whether dexrazoxane in combination with other cancer therapies had adverse effects.
The echocardiographic changes expected with late follow-up of childhood cancer survivors treated with anthracyclines are decreases in LV fractional shortening, mass, and wall thickness, which reflects both decreased LV systolic performance and an inadequate amount of heart muscle for the body-surface area. Continued follow-up reveals pathologic remodeling of the LV, in which the heart walls continue to thin out of proportion to body-surface area and the ventricular dimension, resulting in a restrictive cardiomyopathy, as indicated by the thickness-to-dimension ratio becoming more negative. Long-term survivors with restrictive cardiomyopathy are at increased risk for heart failure.
Patients treated with doxorubicin alone were more likely than those who concomitantly received dexrazoxane to have echocardiographic abnormalities; they demonstrated more negative z scores at the completion of doxorubicin and at 3 and 6 years after diagnosis. Statistically significant differences in LV fractional shortening, wall thickness, and thickness-to–end diastolic dimension ratios suggest that dexrazoxane prevented late cardiac injury by protecting against the pathologic process of LV remodeling, which is associated with the development of cardiomyopathy. In fact, the hearts of patients who received dexrazoxane were echocardiographically normal at 3 years as indicated by the z scores, a finding consistent with a protective effect of dexrazoxane against both acute and late cardiotoxicity.
Serum cTnT elevations represent active cardiomyocyte injury and cell death. When both LV fractional shortening and serum cTnT were measured during anthracycline therapy for childhood ALL in the same study, they were not significantly associated.37 Yet, for the same protocol, cTnT measurements over the first 90 days of doxorubicin were significantly associated with abnormally reduced LV end diastolic posterior wall thickness; reduced LV mass; and, marginally, with reduced LV thickness-to-dimension ratio, suggestive of the start of pathologic LV remodeling 4 years after the ALL diagnosis. Thus, the loss of cardiomyocyte, as documented by elevations of cTnT, during doxorubicin therapy for childhood ALL significantly predict an inadequate LV size to support the body-surface area and result in the start of pathologic remodeling years later.38
Our findings are similar to results of DFCI 95-01, in which 205 patients with high-risk ALL were randomly assigned to receive doxorubicin with or without dexrazoxane. The 5-year EFS rates were 76% and 77%, respectively.39 DFCI ALL Consortium investigators found that, at 5 years from diagnosis, z scores for LV wall thickness and LV thickness-to-dimension ratios were significantly better in patients who received dexrazoxane.40 Thus, among more than 700 patients, there is evidence that dexrazoxane protects the heart without interfering with the antileukemic efficacy of doxorubicin.
Heart failure was not reported among our patients at any time during treatment or follow-up. Of the five patients in whom cardiac dysfunction occurred during treatment (arrhythmias or decreased fractional shortening), only one received dexrazoxane. Interestingly, all five received high-dose methotrexate and had a serious infection when the cardiac toxicity occurred.
Evaluation of the toxicity associated with dexrazoxane in intensive chemotherapy was an additional goal of POG 9404. Rates of targeted toxicities of infection, hematologic abnormalities, and CNS events (ie, seizures), did not differ between treatment groups. The difference in the rate of mucositis was statistically significant but favored the dexrazoxane group, which had fewer events.
Second malignancies are uncommon but well-recognized events after treatment of ALL or lymphoma.41,42 The cumulative incidence of SMNs is 1% to 2% among patients treated between 1985 and 2000.43 Secondary leukemias are associated with etoposide, a topoisomerase II inhibitor, and alkylating agents.41,42,44 In POG 9404, which used neither etoposide nor an alkylating agent, 11 patients had SMNs (n = 4 were myeloid leukemias) as first events, for a cumulative incidence of less than 2%, which compares favorably with other reports. The occurrence of SMNs did not differ significantly between treatment groups. When analyzed separately for acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) and solid tumors, the treatment groups still did not differ.
The above result is in contrast to that reported by Tebbi et al45 of an early, unexpected, increased SMN risk in patients who receive dexrazoxane. In this randomized trial of dexrazoxane for the treatment of Hodgkin lymphoma, eight patients experienced an SMN as a first event (n = 6 had AML or MDS), but only one of the six was in the doxorubicin-alone group. These results suggested that dexrazoxane might be associated with an increased risk of SMN, particularly AML/MDS. This study was unique, because all patients received etoposide, doxorubicin, and low-dose, involved-field or regional-field radiation, and slow-responders received the alkylating agent cyclophosphamide; all of these treatments are associated with SMNs. In addition, all patients were being treated for Hodgkin lymphoma, a diagnosis with a higher risk of SMN.
The DFCI ALL Consortium also has published its experience with SMNs in their patients with high-risk leukemia who received dexrazoxane.46,47 After a median follow up of 6.2 years, the incidence of SMNs did not differ between groups with or without dexrazoxane treatment.6 Among 553 patients treated with dexrazoxane on three consecutive DFCI protocols, the overall 5-year cumulative incidence (with SE) of SMNs was 0.24% (0.24%), with no secondary AML.47 Thus, similar to our findings, dexrazoxane was not associated with an increased incidence of AML/MDS or SMNs.
Using Pediatric Health Information System data, Seif et al48 retrospectively identified 15,532 patients with new diagnoses of malignancy (excluding AML) who received anthracycline as part of treatment. Among 1,046 patients who also received dexrazoxane, three developed secondary AML (0.21%) compared with 77 (0.55%) of 14,126 patients who received an anthracycline without dexrazoxane. Although these data are limited to inpatients and used only International Classification of Diseases, 9th revision, codes to establish diagnoses, this multivariable analysis of a large cohort of pediatric patients showed no association between dexrazoxane and risk of secondary AML.
The cumulative risk of secondary brain tumors did not differ significantly in patients who did or did not receive dexrazoxane, but the observation of five such tumors among patients enrolled in POG 9404, in light of prior analyses, merits discussion. All five patients with secondary brain tumors received cranial irradiation, and four were treated with high-dose methotrexate. Multiple studies report an association between cranial radiotherapy for CNS prophylaxis and increased risk for secondary brain tumors among survivors of ALL. Incidence rates vary from very low, less than 0.5%,45 to very high, 12.8%, as reported in the St Jude Total Therapy XII study.49 Relling et al49 postulated that the high incidence of brain tumors in their study was related to an interaction among radiotherapy, intensive antimetabolite therapy, and the thiopurine methyltransferase phenotype, although subsequent studies have not found an association with thiopurine methyltransferase status.50
Although treatment differences may contribute to the differing observations among these studies, small sample sizes, limited number of events, and varied follow-up lengths also are likely explanations.39,46,47 Although follow-up is still relatively short for late effects, the Cochrane meta-analysis mentioned in the Discussion section also found no difference in the occurrence of SMNs between controls and patients treated with dexrazoxane.36 Even if there were a theoretical, small, increased risk of secondary brain tumors from an interaction between cranial radiotherapy and dexrazoxane, such theoretical concerns are increasingly less relevant in the context of today’s treatment, because the dose and frequency of cranial radiotherapy for CNS prophylaxis in children with ALL has markedly decreased since the 1990s.
Strengths of our large, national, cooperative group study include the randomized design, the central measurement of cardiac end points, and the blinding of cardiology laboratory investigators to treatment allocation throughout follow-up. Thus, the effects of observer variability and bias were minimized.51 In addition, all patients received the same cumulative anthracycline dose.
A potential limitation was the marked lack of echocardiographic data, especially beyond 3 years, for patients lost to cardiac follow-up. Although the small number of troponins and echocardiograms limits the statistical power for some analyses, the subsets of patients with and without cardiac data were similar. To address this issue of late cardiotoxicity and efficacy of dexrazoxane, Children’s Oncology Group investigators are actively recruiting survivors of POG 9404 and Hodgkin lymphoma trials POG 9425 and POG 9426 to undergo long-term follow-up evaluations for clinical and subclinical signs of cardiac effects with clinical assessments, serum biomarkers, and echocardiograms.
In our study, the addition of dexrazoxane to a doxorubicin-containing multiagent chemotherapy regimen was cardioprotective and did not compromise antileukemic efficacy. This cardioprotective effect from doxorubicin-induced pathologic LV remodeling and subclinical cardiomyopathy was sustained at the 3-year follow-up and at up to 6 years in the subset of patients studied at later times. The incidences of toxicity and, importantly, second malignancies were not significantly increased in patients who received dexrazoxane. We recommend dexrazoxane as a cardioprotectant for children and adolescents who have malignancies treated with anthracyclines.
Supplementary Material
Appendix
Table A1.
Comparison of Patient Characteristics by Troponin Data Obtained at Baseline
| Characteristic | No. (%) of Patients | P | ||
|---|---|---|---|---|
| Total Randomly Assigned on Study (N = 537) | Total With Baseline Troponin Data (n = 182) | Total Without Troponin Data (n = 355) | ||
| Mean (SD) age, years | 9.8 (4.74) | 9.8 (4.85) | 9.8 (4.69) | .876 |
| Median (range) follow-up, years | 9.2 (0.01, 15.0) | 9.4 (0.02, 15.0) | 9.0 (0.01, 14.7) | .003 |
| Male sex | 407 (75.8) | 140 (76.9) | 267 (75.2) | .661 |
| Ethnicity | ||||
| White | 356 (66.3) | 123 (67.6) | 233 (65.6) | .651 |
| Nonwhite | 181 (33.7) | 59 (32.4) | 122 (34.4) | |
| T-ALL subset | ||||
| Median (range) follow-up, years | 9.1 (0.01, 14.7) | 9.3 (0.02, 14.5) | 9.0 (0.01, 14.7) | .079 |
| WBC, × 1,000/μL | ||||
| < 50 | 155 (42.8) | 54 (45.4) | 101 (41.6) | .491 |
| ≥ 50 | 207 (57.2) | 65 (54.6) | 142 (58.4) | |
| CNS positive (CNS2/3/bloody tap) | 100 (27.8) | 34 (28.8) | 66 (27.3) | .759 |
| L-NHL subset | ||||
| Median (range) follow-up, years | 9.2 (0.2, 15.0) | 10.0 (0.38, 15.0) | 8.7 (0.24, 13.9) | .006 |
| With stage III disease | 119 (68.4) | 45 (71.4) | 74 (66.7) | .516 |
| With stage IV disease | 55 (31.6) | 18 (28.6) | 37 (33.3) | |
| Mean (SD) No. of echocardiograms per patient | 3.02 (1.61) | 3.35 (1.65) | 2.81 (1.55) | .0002 |
NOTE. Means between groups were compared with a two-sample t test. Medians between groups were compared with the Wilcoxon test.
Abbreviations: L-NHL, lymphoblastic non-Hodgkin lymphoma; SD, standard deviation; T-ALL, T-cell acute lymphoblastic leukemia.
Table A2.
Comparison of Patient Characteristics by Echocardiogram Obtained at Baseline
| Characteristic | No. (%) of Patients | P | ||
|---|---|---|---|---|
| Total Randomly Assigned on Study (N = 537) | Total With Baseline Echocardiograms (n = 307) | Total Without Echocardiograms (n = 230) | ||
| Mean (SD) age, years | 9.8 (4.74) | 9.9 (4.75) | 9.7 (4.74) | .688 |
| Median (range) follow-up, years | 9.2 (0.01, 15.0) | 8.9 (0.02, 15.0) | 9.4 (0.01, 14.2) | .985 |
| Male sex | 407 (75.8) | 237 (77.2) | 170 (73.9) | .379 |
| Ethnicity | ||||
| White | 356 (66.3) | 202 (65.8) | 154 (67.0) | .779 |
| Nonwhite | 181 (33.7) | 105 (34.2) | 76 (33.0) | |
| T-ALL subset | ||||
| Median (range) follow-up, years | 9.1 (0.01, 14.7) | 8.8 (0.02, 14.7) | 9.5 (0.01, 14.2) | .958 |
| WBC, × 1,000/μL | ||||
| < 50 | 155 (42.8) | 104 (47.3) | 51 (35.9) | .033 |
| ≥ 50 | 207 (57.2) | 116 (52.7) | 91 (64.1) | |
| CNS positive (CNS2/3/bloody tap) | 100 (27.8) | 59 (26.9) | 41 (29.1) | .659 |
| L-NHL subset | ||||
| Median (range) follow-up, years | 9.2 (0.2, 15.0) | 8.9 (0.2, 15.0) | 9.4 (0.4, 13.9) | .891 |
| With stage III disease | 119 (68.4) | 59 (67.8) | 60 (69.0) | .871 |
| With stage IV disease | 55 (31.6) | 28 (32.2) | 27 (31.0) | |
NOTE. Means between groups were compared with the two-sample t test. Medians between groups were compared with the Wilcoxon test.
Abbreviations: L-NHL, lymphoblastic non-Hodgkin lymphoma; SD, standard deviation; T-ALL, T-cell acute lymphoblastic leukemia.
Table A3.
Comparison of Characteristics for Patients With Versus Without Echocardiogram Obtained at 3 Years
| Characteristic | No. (%) of Patients | P | ||
|---|---|---|---|---|
| Total (N = 537) | With Echocardiograms (n = 167) | Without Echocardiograms (n = 370) | ||
| Mean (SD) age, years | 9.8 (4.7) | 10.0 (4.7) | 9.7 (4.7) | .488 |
| Male sex | 407 (75.8) | 126 (75.4) | 281 (75.9) | .901 |
| Ethnicity | ||||
| White | 356 (66.3) | 115 (68.9) | 241 (65.1) | .398 |
| Nonwhite | 181 (33.7) | 52 (31.1) | 129 (34.9) | |
| T-ALL subset | ||||
| WBC, × 1,000/μL | ||||
| < 50 | 155 (42.8) | 51 (45.1) | 104 (41.8) | .549 |
| ≥ 50 | 207 (57.2) | 116 (52.7) | 91 (64.1) | |
| CNS positive (CNS2/3/bloody tap) | 100 (27.8) | 33 (29.5) | 67 (27.0) | .631 |
| L-NHL subset | ||||
| With stage III disease | 119 (68.4) | 36 (66.7) | 83 (69.2) | .743 |
| With stage IV disease | 55 (31.6) | 18 (33.3) | 37 (30.8) | |
NOTE. Means between groups were compared with the two-sample t test. Proportions were compared with the Fisher’s exact test.
Abbreviations: L-NHL, lymphoblastic non-Hodgkin lymphoma; SD, standard deviation; T-ALL, T-cell acute lymphoblastic leukemia.
Table A4.
Comparison of Characteristics for Patients With Versus Without Echocardiogram Obtained Both at Baseline and at 3 Years
| Characteristic | No. (%) of Patients | P | ||
|---|---|---|---|---|
| Total (N = 307) | With Echocardiograms (n = 111) | Without Echocardiograms (n = 196) | ||
| Mean (SD) age, years | 9.9 (4.75) | 10.0 (4.7) | 9.9 (4.8) | .835 |
| Male sex | 237 (77.2) | 83 (74.8) | 154 (78.6) | .446 |
| Ethnicity | ||||
| White | 202 (65.8) | 75 (67.6) | 127 (64.8) | .623 |
| Nonwhite | 105 (34.2) | 36 (32.4) | 69 (35.2) | |
| T-ALL subset | ||||
| WBC, × 1,000/μL | ||||
| < 50 | 104 (47.3) | 41 (51.3) | 63 (45.0) | .372 |
| ≥ 50 | 116 (52.7) | 39 (48.8) | 77 (55.0) | |
| CNS positive (CNS2/3/bloody tap) | 59 (26.9) | 22 (27.5) | 37 (26.6) | .887 |
| L-NHL subset | ||||
| With stage III disease | 59 (67.8) | 20 (64.5) | 39 (69.6) | .624 |
| With stage IV disease | 28 (32.2) | 11 (35.5) | 17 (30.4) | |
NOTE. Means between groups were compared with the two-sample t test. Proportions were compared with the Fisher’s exact test.
Abbreviations: L-NHL, lymphoblastic non-Hodgkin lymphoma; SD, standard deviation; T-ALL, T-cell acute lymphoblastic leukemia.
Table A5.
Summary of Second Malignant Neoplasms That Occurred As First Events
| Diagnosis and Treatment Arm | Treatment Regimen | Time Since Start of Treatment (years) | Second Malignancy |
|---|---|---|---|
| Treatment with doxorubicin alone | |||
| L-NHL | DFCI | 0.9 | Myeloid sarcoma |
| T-ALL + CNS | DFCI + HDM | 3.7 | Acute myelomonocytic leukemia |
| T-ALL | DFCI + HDM | 5.1 | NHL, diffuse large B-cell |
| Treatment with doxorubicin plus DRZ | |||
| T-ALL | DFCI + DRZ | 1.0 | Acute myeloid leukemia |
| T-ALL | DFCI + DRZ | 9.4 | Medulloblastoma |
| L-NHL | DFCI + DRZ | 10.6 | Papillary carcinoma, thyroid |
| T-ALL | DFCI + DRZ + HDM | 1.9 | Acute myeloid leukemia |
| T-ALL | DFCI + DRZ + HDM | 11.9 | Glioblastoma |
| T-ALL + CNS | DFCI + DRZ + HDM | 12.1 | Glioblastoma |
| L-NHL | DFCI + DRZ + HDM | 12.0 | Astrocytoma, anaplastic type |
| L-NHL | DFCI + DRZ + HDM | 6.5 | Astrocytoma |
Abbreviations: DFCI, standard with doxorubicin alone without dexrazoxane or high-dose methotrexate; DRZ, dexrazoxane; HDM, high-dose methotrexate; L-NHL, lymphoblastic non-Hodgkin lymphoma; T-ALL, T-cell acute lymphoblastic leukemia.
Footnotes
Listen to the podcast by Dr Chow at www.jco.org/podcasts
Written on behalf of the Children’s Oncology Group Acute Lymphoblastic Leukemia Committee.
Supported in part by the Clinical Trials Evaluation Program of the National Cancer Institute, National Institutes of Health grants No. U10 CA098543, U10 CA098413, U10 CA180886, and U10 CA180899. A complete listing of grant support for research conducted by the Pediatric Oncology Group and Children’s Cancer Study Group before initiation of the Children’s Oncology Group grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm. The Michael Garil Fund provided additional support for cardiology studies.
Results from this manuscript have been presented at the American Society of Clinical Oncology Scientific Sessions, Chicago, IL, June 1-5, 2012, and at the American Society of Pediatric Hematology/Oncology Annual Meeting, Cincinnati, OH May 15-17, 2008.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Barbara L. Asselin, Jeanette Pullen, Michael J. Borowitz, Robert E. Hutchison, Yaddanapudi Ravindranath, Bruce M. Camitta, Steven E. Lipshultz
Collection and assembly of data: Barbara L. Asselin, Meenakshi Devidas, Vivian I. Franco, Jeanette Pullen, Michael J. Borowitz, Robert E. Hutchison, Bruce M. Camitta, Steven E. Lipshultz
Data analysis and interpretation: Barbara L. Asselin, Meenakshi Devidas, Lu Chen, Saro H. Armenian, Bruce M. Camitta, Steven E. Lipshultz
Administrative support: Saro H. Armenian
Provision of study materials or patients: Barbara L. Asselin, Jeanette Pullen, Yaddanapudi Ravindranath, Bruce M. Camitta, Steven E. Lipshultz
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Barbara L. Asselin
Consulting or Advisory Role: Sigma Tau Pharmaceuticals, Jazz Pharmaceuticals
Speakers' Bureau: Jazz Pharmaceuticals
Meenakshi Devidas
No relationship to disclose
Lu Chen
No relationship to disclose
Vivian I. Franco
No relationship to disclose
Jeanette Pullen
No relationship to disclose
Michael J. Borowitz
Research Funding: Amgen, MedImmune, Bristol-Myers Squibb, Becton Dickinson
Robert E. Hutchison
No relationship to disclose
Yaddanapudi Ravindranath
No relationship to disclose
Saro H. Armenian
No relationship to disclose
Bruce M. Camitta
No relationship to disclose
Steven E. Lipshultz
Consulting or Advisory Role: Clinigen Group
Research Funding: Pfizer, Roche
REFERENCES
- 1.Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients: Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84:3122–3133. [PubMed] [Google Scholar]
- 2.Schrappe M, Reiter A, Ludwig WD, et al. German-Austrian-Swiss ALL-BFM Study Group Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: Results of trial ALL-BFM 90. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 3.Goldberg JM, Silverman LB, Levy DE, et al. Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21:3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 4.Seibel NL, Steinherz PG, Sather HN, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood. 2008;111:2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reulen RC, Winter DL, Frobisher C, et al. British Childhood Cancer Survivor Study Steering Group Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 8.Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 10.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 11.Krischer JP, Epstein S, Cuthbertson DD, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: The Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 12.Nysom K, Colan SD, Lipshultz SE. Late cardiotoxicity following anthracycline therapy for childhood cancer. Prog Pediatr Cardiol. 1998;8:121–138. [Google Scholar]
- 13.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 14.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol. 1998;25:10–14. (suppl 10) [PubMed] [Google Scholar]
- 17.Muindi JRF, Sinha BK, Gianni L, et al. Hydroxyl radical production and DNA damage induced by anthracycline-iron complex. FEBS Lett. 1984;172:226–230. doi: 10.1016/0014-5793(84)81130-8. [DOI] [PubMed] [Google Scholar]
- 18.Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: Analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [PubMed] [Google Scholar]
- 19.Hasinoff BB, Hellmann K, Herman EH, et al. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem. 1998;5:1–28. [PubMed] [Google Scholar]
- 20.Herman E, Ardalan B, Bier C, et al. Reduction of daunorubicin lethality and myocardial cellular alterations by pretreatment with ICRF-187 in Syrian golden hamsters. Cancer Treat Rep. 1979;63:89–92. [PubMed] [Google Scholar]
- 21.Lebrecht D, Geist A, Ketelsen UP, et al. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol. 2007;151:771–778. doi: 10.1038/sj.bjp.0707294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agen C, Bernardini N, Danesi R, et al. Reducing doxorubicin cardiotoxicity in the rat using deferred treatment with ADR-529. Cancer Chemother Pharmacol. 1992;30:95–99. doi: 10.1007/BF00686399. [DOI] [PubMed] [Google Scholar]
- 23.Speyer JL, Green MD, Kramer E, et al. Protective effect of the bispiperazinedione ICRF-187 against doxorubicin-induced cardiac toxicity in women with advanced breast cancer. N Engl J Med. 1988;319:745–752. doi: 10.1056/NEJM198809223191203. [DOI] [PubMed] [Google Scholar]
- 24.Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 25.Swain SM. Adult multicenter trials using dexrazoxane to protect against cardiac toxicity. Semin Oncol. 1998;25:43–47. (suppl 10) [PubMed] [Google Scholar]
- 26.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: A randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118:874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 28.Colan SD, Parness IA, Spevak PJ, et al. Developmental modulation of myocardial mechanics: Age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–629. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 29.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985) 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 30.Venturini M, Michelotti A, Del Mastro L, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J Clin Oncol. 1996;14:3112–3120. doi: 10.1200/JCO.1996.14.12.3112. [DOI] [PubMed] [Google Scholar]
- 31.Wexler LH, Weaver-McClure L, Steinberg SM, et al. Randomized trial of recombinant human granulocyte-macrophage colony-stimulating factor in pediatric patients receiving intensive myelosuppressive chemotherapy. J Clin Oncol. 1996;14:901–910. doi: 10.1200/JCO.1996.14.3.901. [DOI] [PubMed] [Google Scholar]
- 32.Holcenberg JS, Tutsch KD, Earhart RH, et al. Phase I study of ICRF-187 in pediatric cancer patients and comparison of its pharmacokinetics in children and adults. Cancer Treat Rep. 1986;70:703–709. [PubMed] [Google Scholar]
- 33.Schiavetti A, Castello MA, Versacci P, et al. Use of ICRF-187 for prevention of anthracycline cardiotoxicity in children: Preliminary results. Pediatr Hematol Oncol. 1997;14:213–222. doi: 10.3109/08880019709009491. [DOI] [PubMed] [Google Scholar]
- 34.BuLock FA, Gabriel HM, Oakhill A, et al. Cardioprotection by ICRF-187 against high dose anthracycline toxicity in children with malignant disease. Br Heart J. 1993;70:185–188. doi: 10.1136/hrt.70.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuler D, Horváth E, Koós R, et al. Safety of dexrazoxane in children with all undergoing anthracycline therapy: Preliminary results of a prospective pilot study. Pediatr Hematol Oncol. 1997;14:93–94. doi: 10.3109/08880019709030891. [DOI] [PubMed] [Google Scholar]
- 36.van Dalen EC, Caron HN, Dickinson HO, et al. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev. 2011;6:CD003917. doi: 10.1002/14651858.CD003917.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 38.Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: Associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 42.Smith MA, Rubinstein L, Anderson JR, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J Clin Oncol. 1999;17:569–577. doi: 10.1200/JCO.1999.17.2.569. [DOI] [PubMed] [Google Scholar]
- 43.Schrappe M, Nachman J, Hunger S, et al. Educational symposium on long-term results of large prospective clinical trials for childhood acute lymphoblastic leukemia (1985-2000) Leukemia. 2010;24:253–254. doi: 10.1038/leu.2009.276. [DOI] [PubMed] [Google Scholar]
- 44.Pui CH, Relling MV. Topoisomerase II inhibitor-related acute myeloid leukemia. Br J Haematol. 2000;109:13–23. doi: 10.1046/j.1365-2141.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 45.Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 46.Barry EV, Vrooman LM, Dahlberg SE, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 47.Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: A report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer. 2011;47:1373–1379. doi: 10.1016/j.ejca.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seif AE, Walker DM, Li Y, et al. Dexrazoxane exposure and risk of secondary acute myeloid leukemia in pediatric oncology patients. Pediatr Blood Cancer. 2015;62:704–709. doi: 10.1002/pbc.25043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–39. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 50.Stanulla M, Schaeffeler E, Möricke A, et al. Thiopurine methyltransferase genetics is not a major risk factor for secondary malignant neoplasms after treatment of childhood acute lymphoblastic leukemia on Berlin-Frankfurt-Münster protocols. Blood. 2009;114:1314–1318. doi: 10.1182/blood-2008-12-193250. [DOI] [PubMed] [Google Scholar]
- 51.Lipshultz SE, Easley KA, Orav EJ, et al. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function: The prospective P(2)C(2) HIV study. Circulation. 2001;104:310–316. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.