Abstract
Purpose
Fluorouracil plus leucovorin (FU + LV) adjuvant chemotherapy reduced the risk of recurrence and death across all time points in a pooled analysis of 20,898 patients with colon cancer from 18 randomized studies. The impact of oxaliplatin added to FU + LV on the time course of recurrence and survival remains unknown.
Patients and Methods
A total of 12,233 patients enrolled to the randomized trials C-07, C-08, N0147, MOSAIC (Adjuvant Treatment of Colon Cancer), and XELOXA (Adjuvant XELOX) were pooled to examine the impact of oxaliplatin and tumor-specific factors on the time course of recurrence and death. For each end point, continuous-time risk was modeled over 6 years post treatment in all oxaliplatin-treated patients and patients concurrently randomized to FU + LV with or without oxaliplatin; the latter analyses supported time-dependent treatment comparisons.
Results
Addition of oxaliplatin significantly reduced the risk of recurrence within the first 14 months post treatment for patients with stage II disease and within the first 4 years for patients with stage III disease. Oxaliplatin also significantly reduced risk of death from 2 to 6 years post treatment for patients with stage III disease, with no differences in timing of outcomes between treatment groups (ie, oxaliplatin did not simply postpone recurrence or death compared with FU + LV alone). Patients with stage II disease receiving oxaliplatin did not exhibit a significant reduction in risk of death in the first 6 years post treatment. Recurrence risk peaked near 14 months for both treatments, and risk of recurrence and death increased with increased tumor and nodal burden.
Conclusions
These analyses support the addition of oxaliplatin to fluoropyrimidine-based adjuvant therapy in patients with stage III disease and underscore the need for adequate surveillance of patients with colon cancer during the first 3 years after adjuvant therapy.
INTRODUCTION
More than 1,350,000 people are diagnosed with colorectal cancer, and approximately 700,000 men and women die of the disease worldwide annually.1 The benefit of curative resection followed by adjuvant chemotherapy with infusional fluorouracil, leucovorin, oxaliplatin (FOLFOX) is well established in stage III disease.2,3 Patients with early-stage colon cancer also experience improved survival due to improved screening and staging.4
In a 2009 pooled analysis of 20,898 patients from 18 randomized trials conducted from 1978 to 1999, it was established that cure is probable in a subset of patients who receive adjuvant chemotherapy in addition to surgery.5 This analysis demonstrated that the risk of recurrence is highest in the first 2 years for both treated and untreated patients and that the risk of death peaks approximately 2 years after treatment. The benefit achieved by the addition of adjuvant fluorouracil plus leucovorin (FU + LV) was sustained over the full follow-up period (8 years), supporting the notion that FU–based adjuvant treatment is curative. Furthermore, patients with stage III disease showed a greater improvement from adjuvant treatment than patients with stage II disease, which suggests that the pattern of recurrence (with regard to time course of failure) depends on tumor and nodal stages. Trials testing oxaliplatin-based regimens were excluded from the analyses, as patient follow-up on key endpoints was immature. Therefore, the questions of whether the addition of oxaliplatin to FU + LV alters the time course of recurrence or death, and whether it does so directly or via other factors, such as tumor and nodal stage, remained unanswered.
The ACCENT (Adjuvant Colon Cancer End Points) database6,7 now contains mature outcome data for an additional 12,223 patients from five contemporary randomized trials including oxaliplatin-based treatment arms (MOSAIC [Adjuvant Treatment of Colon Cancer],2,3 NSABP [National Surgical Adjuvant Breast and Bowel Project] C-07,8 C-08,9 XELOXA [Adjuvant Capecitabine and Oxaliplatin],10 and N014711). The MOSAIC study recruited 2,246 patients with stage II or III colon cancer and was the pivotal trial leading to the approval of oxaliplatin in stage III disease.2,3 In patients with stage III disease, 6-year overall survival (OS) improved from 68.7% to 72.9% (hazard ratio [HR], 0.80; P = .023), and in the subgroup of patients with stage IIIC disease (n = 460), the OS improvement was even greater at 11.8%.3 Both NSABP C-07 (N = 2,409; OS HR, 0.80; P < .004)8 and N016968 (XELOXA) studies (N = 1,886; disease-free survival HR, 0.80; P = .005)10 had strikingly corroborating results. NSABP trial C-08 demonstrated no significant improvement in disease-free survival from the addition of bevacizumab (N = 2,672; P = .15; HR, 0.89),9 whereas N0147 similarly demonstrated no improvement in PFS from the addition of cetuximab in KRAS wild-type patients (N = 1,760; P = .33; HR, 1.18).11
The findings from these individual published trials do not address the manner in which patient and tumor characteristics affect the time course of recurrence and death beyond adjuvant treatment with oxaliplatin. We performed a pooled analysis of the modern-era trials described above to evaluate whether patterns of recurrence or death over time differ according to tumor characteristics (eg, tumor or nodal stages) or between patients who received oxaliplatin-based therapy versus FU + LV alone.
PATIENTS AND METHODS
Database and Analysis Populations
The ACCENT database contains patient-level information on more than 30,000 patients enrolled to 25 adjuvant trials since 1977.6,7 This analysis focuses on patients from five modern trials evaluating oxaliplatin-based regimens where mature follow-up is available (Appendix Table A1, online only). Across these trials, median length of follow-up among surviving patients was 6 years. All analyses were repeated in two sets of patients: (1) patients from all five trials who received oxaliplatin-based regimens (ie, excluding those who received FU + LV alone), and (2) patients from the THREE trials with concurrent randomization to chemotherapy with or without oxaliplatin (MOSAIC, C-07, and XELOXA). The former set of analyses were intended to provide the maximum sample size and power to summarize the time course of outcomes for patients treated with FOLFOX/intravenous oxaliplatin plus oral capecitabine (heretofore referred to as FOLFOX), and the latter set of analyses were intended to compare the time course of outcomes between pooled concurrently randomized treatment groups.
Outcome variables included time to recurrence and overall survival, where the latter was defined as the time from randomization to death due to any cause. Both variables were right-censored at the earlier of loss of follow-up or 6 years for consistency, because of differences in follow-up among studies. Analyses were performed overall and according to disease groups of interest: stage of disease (II, III), tumor stage (T1/T2, T3, T4), nodal stage (N0, N1a, N1b, N2a, N2b), tumor location (left, right, transverse/flexures), and tumor grade (low, high). As nodal stage was only collected on the level of N1 versus N2 for the XELOXA trial, patients were randomly assigned nodal substages (ie, N1a v N1b) at rates matching patients with stage III disease from the other ACCENT studies. Patients with other missing factors of interest or outcome data were excluded only from those analyses where the missing data were required, resulting in an overall sample size of 12,233 contributing patients.
Statistical Methods
Patient characteristics were summarized descriptively within each analysis population (FOLFOX alone and concurrently randomized FOLFOX v FU + LV) and statistically compared between pooled treatment groups using χ2 tests, as all variables were categorized. The method of Müller and Wang12 was used to plot the continuous-time hazard of recurrence from the time of randomization up to 6 years, both in the pooled FOLFOX and concurrently randomized populations, and additionally stratified by patient factors of interest (eg, tumor and nodal stages). Unlike the more common Kaplan-Meier survival curves or Cox proportional hazards regression models, which are constrained to be nonincreasing and satisfy constant hazard ratios over time, respectively, this method allows for straightforward visualization of instantaneous risk of recurrence or death over time, including both increases and decreases, with no imposed distributional assumptions. Time-dependent treatment comparisons of FOLFOX versus FU + LV were similarly calculated as continuous-time log hazard ratios and plotted over time by stage of disease with 95% confidence bands using the method of Gilbert et al,13 where a pointwise interval excluding zero indicated a statistically significant treatment effect at that time point. Together, these methods allow potentially different risk patterns (such as changes in the timing of recurrences) to be directly evaluated without imposed modeling assumptions and compared between treatments or other patient characteristics.
All analyses described above were repeated to additionally evaluate the influence of treatment and disease characteristics on the continuous time hazard of death. Effects of the addition of oxaliplatin on early (< 2 years), mid (2 to 4 years), and late (> 4 years) recurrence and death were quantified using logistic regression (early and mid) and Cox proportional hazard (late) models. Annual rates of recurrence and death were reported for up to 5 years of follow-up with exact binomial 95% confidence intervals, while cumulative rates of recurrence and death were reported annually using Kaplan-Meier methods. Both rates were computed within pooled treatment groups and stratified by stage of disease.
RESULTS
A total of 8,993 patients treated with oxaliplatin-containing regimens from all five trials were included in the oxaliplatin-only analyses of continuous-time recurrence and death patterns, whereas 6,468 patients from the three trials with concurrent randomization (MOSAIC, C-07, and XELOXA) were included in treatment effect analyses of recurrence and death patterns (Table 1). Patients were predominantly older than 50 years and white. Most patients were performance status 0, and most had stage III low-grade tumors. The concurrently randomized analysis population did not differ substantially between treatment groups (Table 1), although patients receiving FOLFOX were slightly more likely to have right-sided tumors (P = .058) and worse performance status (P = .038).
Table 1.
Patient and Tumor Characteristics, FOLFOX-Only and FOLFOX v FU + LV Trials
| Variable | FOLFOX-Only Trials | FOLFOX v FU + LV Trials | |||||
|---|---|---|---|---|---|---|---|
| FU + LV | FOLFOX | P | |||||
| No. | % | No. | % | No. | % | ||
| Age, years | .94 | ||||||
| < 50 | 1,993 | 22 | 621 | 19 | 622 | 19 | |
| 50+ | 7,000 | 78 | 2,619 | 81 | 2,606 | 81 | |
| Sex | .64 | ||||||
| Male | 4,737 | 53 | 1,769 | 55 | 1,782 | 55 | |
| Female | 4,256 | 47 | 1,471 | 45 | 1,446 | 45 | |
| Race | .16 | ||||||
| White | 7,885 | 88 | 2,951 | 91 | 2,897 | 90 | |
| Black | 544 | 6 | 105 | 3 | 113 | 4 | |
| Other | 533 | 6 | 182 | 6 | 216 | 7 | |
| NA | 31 | 0 | 2 | 0 | 2 | 0 | |
| PS | .04 | ||||||
| 0 | 7,165 | 80 | 2,716 | 84 | 2,640 | 82 | |
| 1+ | 1,816 | 20 | 520 | 16 | 581 | 18 | |
| NA | 12 | 0 | 4 | 0 | 7 | 0 | |
| Location | .05 | ||||||
| S, left | 3,540 | 46 | 1,155 | 51 | 1,067 | 47 | |
| S, right | 2,851 | 37 | 735 | 32 | 792 | 35 | |
| S, trans | 1,358 | 18 | 375 | 17 | 390 | 17 | |
| NA | 1,244 | 14 | 975 | 30 | 979 | 31 | |
| Grade | .39 | ||||||
| Low | 7,031 | 79 | 2,596 | 83 | 2,633 | 84 | |
| High | 1,847 | 21 | 528 | 16 | 504 | 16 | |
| NA | 115 | 1 | 116 | 6 | 91 | 3 | |
| Stage | .86 | ||||||
| Stage II | 1,453 | 16 | 798 | 25 | 802 | 25 | |
| Stage III | 7,540 | 84 | 2,442 | 75 | 2,426 | 75 | |
| T-stage | .61 | ||||||
| T1/T2 | 1,073 | 12 | 313 | 10 | 289 | 9 | |
| T3 | 6,848 | 77 | 2,494 | 77 | 2,511 | 78 | |
| T4 | 1,028 | 11 | 427 | 13 | 425 | 13 | |
| NA | 44 | 0 | 6 | 0 | 3 | 0 | |
| N-stage | .93 | ||||||
| N0 | 1,454 | 16 | 797 | 25 | 802 | 25 | |
| N1a | 2,153 | 24 | 769 | 24 | 749 | 23 | |
| N1b | 2,473 | 28 | 812 | 25 | 825 | 26 | |
| N2a | 1,669 | 19 | 518 | 16 | 525 | 16 | |
| N2b | 1,241 | 14 | 341 | 11 | 324 | 10 | |
| NA | 3 | 0 | 3 | 0 | 3 | 0 | |
| Total | 8,993 | 100 | 3,240 | 100 | 3,228 | 100 | — |
Abbreviations: FOLFOX, infusional fluorouracil, leucovorin, oxaliplatin; FU + LV, fluorouracil plus leucovorin; NA, not applicable; PS, performance status; S, single.
Risk of Recurrence Over Time
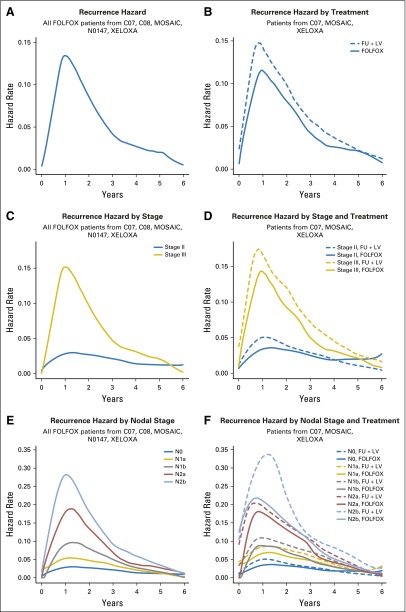
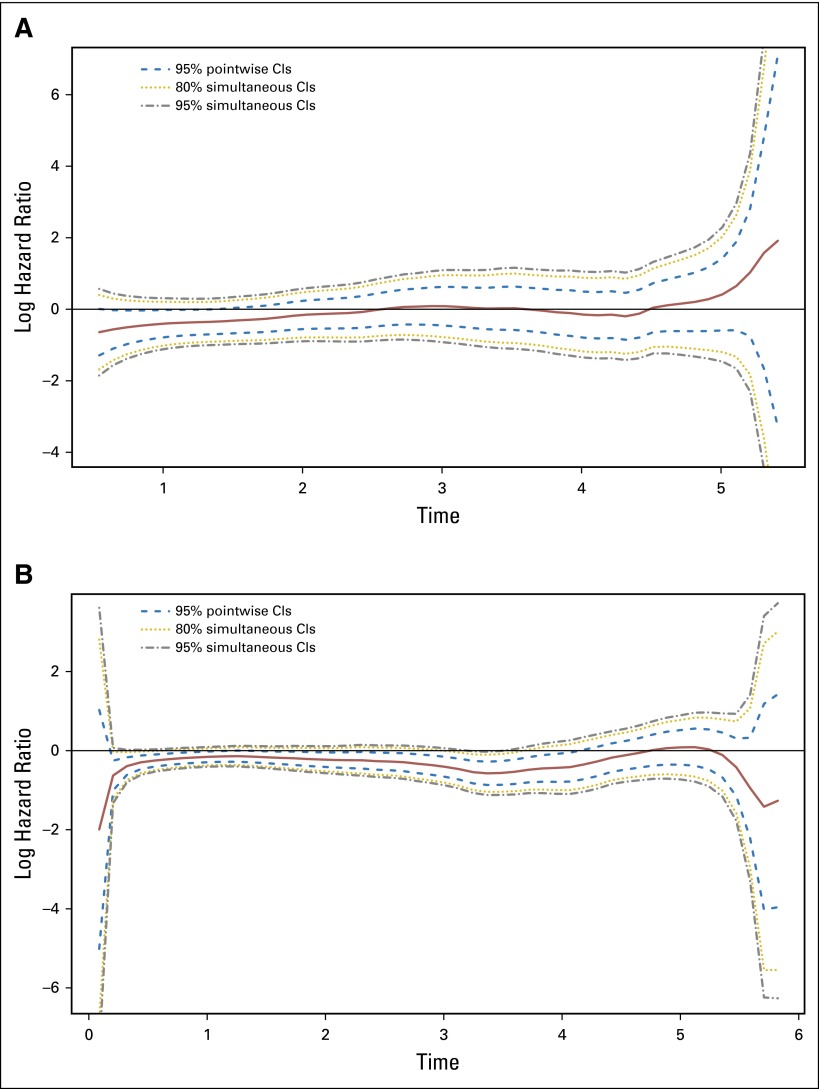
The continuous-time recurrence patterns for patients treated with FOLFOX only and those concurrently randomized to FU + LV versus FOLFOX are shown side-by-side, overall and by disease characteristics (Fig 1). Among FOLFOX-treated patients (Fig 1A), the hazard of recurrence peaked around 14 months post treatment, then diminished to near zero approximately 6 years post treatment. Patients treated with FOLFOX showed a lower risk of recurrence at all time points (Fig 1B). Figure 2 plots the estimated hazard ratio of recurrence for FOLFOX versus FU + LV as a continuous function of time by stage of disease. Here, statistical significance is indicated where the pointwise 95% confidence band excludes the zero effect threshold (log hazard ratio = 0), which occurs from shortly after randomization until approximately 4 years post treatment for patients with stage III disease and within the first 14 months postrandomization for stage II.
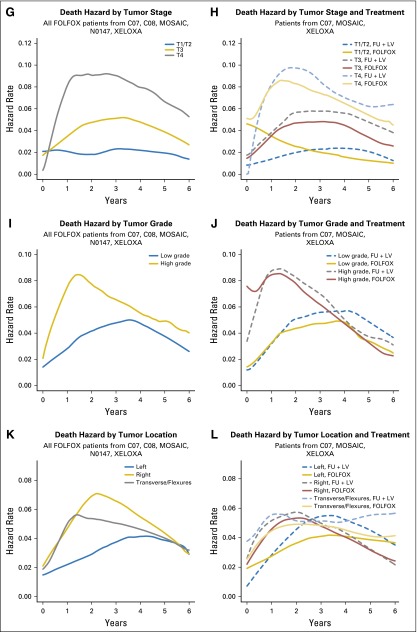
Fig 1.
Hazard of recurrence over time for (A) infusional fluorouracil, leucovorin, oxaliplatin (FOLFOX)–treated patients, and (B) FOLFOX versus fluorouracil plus leucovorin (FU + LV)–treated patients. Paired hazard curves for the same cohorts and stratified by stage (C, D), nodal stage (E, F), tumor stage (G, H), tumor grade (I, J), and tumor location (K, L). MOSAIC, Adjuvant Treatment of Colon Cancer. XELOXA, Adjuvant XELOX.
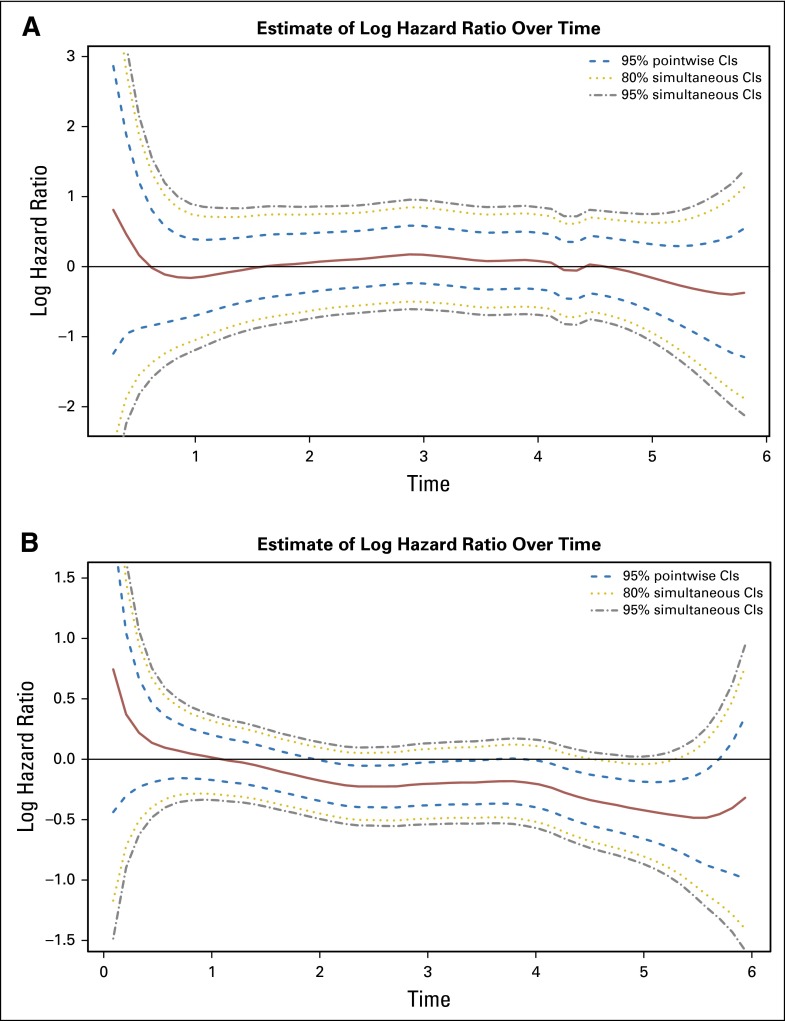
Fig 2.
Time-dependent log hazard ratios for recurrence, infusional fluorouracil, leucovorin, oxaliplatin (FOLFOX) versus fluorouracil plus leucovorin (FU + LV), for (A) patients with stage II disease and (B) patients with stage III disease.
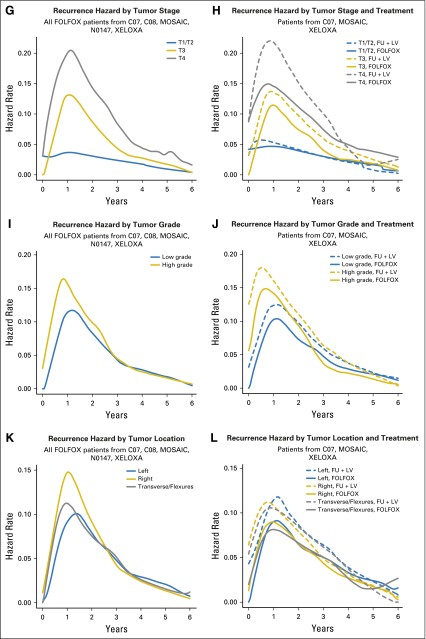
Although the risk of recurrence over time peaks around 14 months, the peak is much more prominent for patients with stage III disease than patients with stage II disease (Fig 1C). Specifically, the risk of recurrence for patients with stage III disease does not diminish to meet the peak recurrence risk of patients with stage II disease until approximately 4 years post treatment. The addition of oxaliplatin diminished the risk of recurrence over time to a greater degree, more uniformly over time, and for a longer period of time for patients with stage III disease (Fig 1D). Higher risk of recurrence over time was associated with higher nodal stage (Fig 1E-1F), with oxaliplatin demonstrating more benefit in patients with more advanced nodal stage disease (Fig 1F). Figures 1E and 1G suggest that the risk of recurrence over time among FOLFOX-treated patients is higher for increased nodal and T stage, up until about 6 years. The pattern of recurrence for T1 and T2 tumors (Fig 1G) is unique in that these tumors have an initially low risk of recurrence that further diminishes over time, whereas patients with T3 and T4 stage disease have a risk of recurrence that peaks around 14 months before diminishing. The addition of oxaliplatin to FU is associated with benefit for patients with both T3 and T4 stage tumors; however, little benefit is evident for patients with T1 and T2 stage tumors (Fig 1H).
Among patients treated with FOLFOX, low-grade tumors appear to recur later and less often at every time point than high-grade tumors (Fig 1I), until approximately 3 years, and thereafter the recurrence risk between low-grade and high-grade tumors was similar. The addition of oxaliplatin reduced the recurrence risk over time for both low- and high-grade tumors for the first 5 years posttreatment (Fig 1J). Patients with right-sided tumors show the highest risk of early recurrence, followed by patients with transverse colon or flexure tumors, whereas patients with left-sided tumors have lower recurrence risk within 18 months post treatment (Fig 1K). Oxaliplatin showed benefit but did not change the time course of recurrence within any tumor location group (Fig 1L).
Risk of Death Over Time
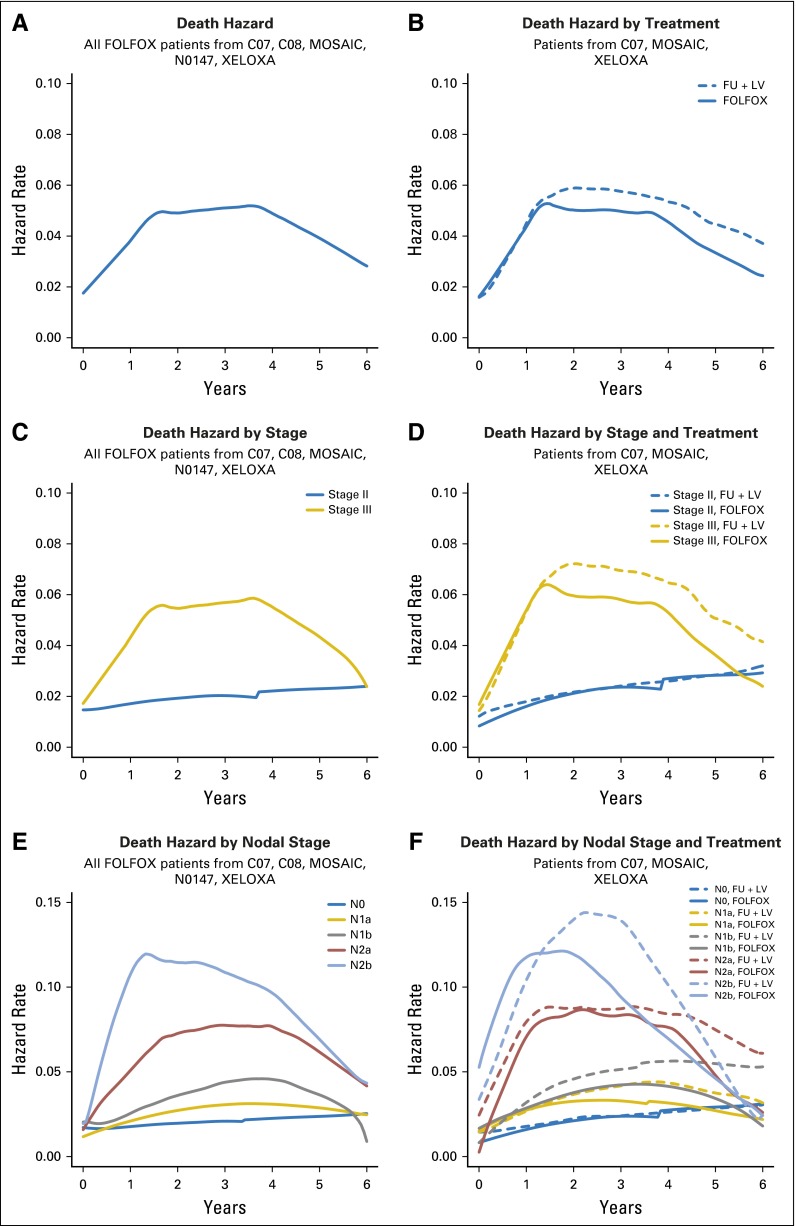
Continuous-time risk of death is displayed side by side for patients treated with FOLFOX only and those concurrently randomized to FU + LV versus FOLFOX, overall and by factors of interest, in Figure 3. Among FOLFOX-treated patients (Fig 3A), the hazard of death increases from 0 to nearly 2 years post treatment, plateaus until nearly 4 years post treatment, and then decreases slightly to year 6. When pooled treatment groups from concurrently randomized patients are compared, it is evident that addition of oxaliplatin reduces the time-dependent hazard of death from 1.5 years to 6 years post treatment. The statistical significance of this treatment effect among patients with stage III disease is confirmed by the continuous-time log-hazard ratio for death (Fig 4B), where the 95% confidence bands exclude the zero effect threshold from approximately years 2 to nearly 6 post treatment. Patients with stage II disease, however, did not show a significant reduction in the risk of death due to oxaliplatin at any time point.
Fig 3.
Hazard of death over time for (A) infusional fluorouracil, leucovorin, oxaliplatin (FOLFOX)–treated patients, and (B) FOLFOX versus fluorouracil plus leucovorin (FU + LV)–treated patients. Paired hazard curves for the same cohorts and stratified by stage (C, D), nodal stage (E, F), tumor stage (G, H), tumor grade (I, J), and tumor location (K, L). MOSAIC, Adjuvant Treatment of Colon Cancer.; XELOX, intravenous oxaliplatin plus oral capecitabine; XELOXA, Adjuvant XELOX.
Fig 4.
Time-dependent log hazard ratios for death, infusional fluorouracil, leucovorin, oxaliplatin (FOLFOX) versus fluorouracil plus leucovorin (FU + LV), for (A) patients with stage II disease and (B) patients with stage III disease.
Patients with stage III disease have a higher risk of death across the entire time period than patients with stage II disease, with the greatest difference in death risk observed from about 1.5 years to 4 years (Fig 3C). Although the addition of oxaliplatin to FU + LV–based chemotherapy results in a lower risk of death than treatment with FU + LV alone beyond 1.5 years, this was observed only for patients with stage III disease (Fig 3D). Risk of death is increased for patients with increased nodal stage and peaks earlier for more advanced stages (Fig 3E). Oxaliplatin appears to have increased benefit in patients with increased nodal stages and no benefit for node-negative patients (Fig 3F). Risk of death is higher for increased tumor stage, with the risk of death peaking later for patients with lower tumor stage (Fig 3G). Although we observe some reversal of treatment effect over time for patients with T1/ T2 tumors (Fig 3H), we note that sample sizes in non-T3 patient groups are low relative to the T3 sample size (Appendix Table A2), precluding strong conclusions.
Patients with high-grade tumors had increased risk of earlier death (peak at approximately 18 months v 3.5 years for low-grade tumors; Fig 3I), with an improvement with the addition of oxaliplatin beginning at 1 year post treatment for patients with high- or low-grade tumors (Fig 3J). Patients with multiple or right-sided tumors (including tumors located in the transverse colon and flexures) had higher risk of death at earlier time points compared with patients with left-sided tumors (Fig 3K). The benefit of oxaliplatin was realized later and for shorter duration in patients with left-sided tumors versus those with transverse or right-sided tumors (Fig 3L).
Effect of Oxaliplatin on Early, Mid, and Late Recurrences and Deaths
Adjuvant FOLFOX reduced the risk of early recurrences (< 2 years) by 23% (odds ratio [OR], 0.77; 95% CI, 0.68 to 0.87; P < .001), mid recurrences (2 to 4 years) by 26% (OR, 0.74; 95% CI, 0.61 to 0.89; P = .001), and late (> 4 years) recurrences by 15% (HR, 0.85; 95% CI, 0.64 to 1.13; P = .27). The addition of oxaliplatin does not impact the risk of early death (OR, 0.98; 95% CI, 0.82 to 1.18; P = .86), but does decrease the risk of mid deaths by 16% (OR, 0.84; 95% CI, 0.71 to 0.99; P = .04) and late deaths by 20% (HR, 0.80; 95% CI, 0.67 to 0.95; P = .01).
Annual and Cumulative Risk of Recurrence and Death by Treatment
The cumulative rates of recurrence and death up to 5 years are provided by treatment and stage of disease (Table 2). The addition of oxaliplatin to FU + LV significantly reduced the cumulative risk of recurrence each year from year 1 through year 5 post treatment (demonstrated by nonoverlapping confidence intervals) for patients with stage III disease, and from year 2 to 5 for patients with stage II disease. Annual rates of recurrence reached significance in years 1 and 4 in patients with stage III disease treated with oxaliplatin compared with FU + LV (Appendix Table A2). The cumulative risk of death was significantly improved with the addition of oxaliplatin for patients with stage III disease beginning at year 3 and continuing through year 5. Most recurrences happen within the first 3 years of completing adjuvant therapy (eg, of the 26.3% patients who received FOLFOX who recurred within 5 years, 22.2% [approximately 80%] did so by 5 years).
Table 2.
Treatment and Stage-Specific Cumulative Rates (%) of Recurrence and Death, by Year, With 95% CIs
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Recurrence | |||||
| All | |||||
| FU | 10.9 (9.8 to 12.0)* | 21.3 (19.9 to 22.7)* | 26.5 (24.9 to 28.0)* | 29.9 (28.3 to 31.5)* | 31.8 (30.2 to 33.4)* |
| FOLFOX | 7.5 (6.9 to 8.0)* | 17.6 (16.7 to 18.3)* | 22.2 (21.3 to 23.1)* | 24.5 (23.6 to 25.4)* | 26.3 (25.3 to 27.2)* |
| Stage II | |||||
| FU | 4.1 (2.7 to 5.4) | 9.5 (7.4 to 11.5)* | 12.2 (9.9 to 14.5)* | 13.9 (11.4 to 16.3)* | 15.3 (12.7 to 17.8)* |
| FOLFOX | 2.1 (1.4 to 2.9) | 5.8 (4.5 to 7.0)* | 8.2 (6.8 to 9.7)* | 9.4 (7.9 to 11.0)* | 10.3 (8.7 to 11.9)* |
| Stage III | |||||
| FU | 13.1 (11.8 to 14.5)* | 25.2 (23.5 to 27.0)* | 31.2 (29.3 to 33.1)* | 35.2 (33.2 to 37.1)* | 37.3 (35.3 to 39.2)* |
| FOLFOX | 8.5 (7.8 to 9.1)* | 19.8 (18.9 to 20.7)* | 24.9 (23.9 to 25.9)* | 27.5 (26.4 to 28.5)* | 29.4 (28.4 to 30.5)* |
| Death | |||||
| All | |||||
| FU | 2.9 (2.3 to 3.4) | 8.3 (7.3 to 9.2) | 13.6 (12.4 to 14.8) | 18.5 (17.1 to 19.8) | 22.3 (20.8 to 23.7)* |
| FOLFOX | 2.7 (2.3 to 3.0) | 7.3 (6.8 to 7.9) | 11.8 (11.2 to 12.5) | 16.4 (15.6 to 17.2) | 20.0 (19.1 to 20.8)* |
| Stage II | |||||
| FU | 1.3 (0.5 to 2.0) | 3.8 (2.5 to 5.1) | 5.5 (3.9 to 7.0) | 8.3 (6.3 to 10.2) | 10.2 (8.1 to 12.3) |
| FOLFOX | 1.5 (0.9 to 2.2) | 3.4 (2.4 to 4.3) | 5.7 (4.4 to 6.9) | 7.6 (6.2 to 9.0) | 9.4 (7.8 to 10.9) |
| Stage III | |||||
| FU | 3.4 (2.7 to 4.1) | 9.7 (8.5 to 10.9) | 16.3 (14.8 to 17.8)* | 21.8 (20.2 to 23.5)* | 26.3 (24.5 to 28.0)* |
| FOLFOX | 2.9 (2.5 to 3.3) | 8.1 (7.5 to 8.7) | 13.0 (12.2 to 13.8)* | 18.2 (17.3 to 19.0)* | 22.1 (21.1 to 23.0)* |
Abbreviations: FOLFOX, infusional fluorouracil, leucovorin, oxaliplatin; FU, fluorouracil.
Indicates significant difference, where the 95% CIs do not overlap
DISCUSSION
Colon cancer remains a leading cause of cancer-related death. In this paper, we address the questions of whether the addition of oxaliplatin, or specific disease factors (eg, tumor and nodal stage), alters the time course of disease recurrence and death compared with FU + LV alone. Pooling individual data from 12,223 patients in the ACCENT database from modern oxaliplatin-containing trials, we found that the addition of oxaliplatin to FU + LV results in a significant and sustained reduction in the risk of recurrence and death for patients with stage III disease. Importantly, these reductions appear to be curative, as annualized risk of each end point diminishes to 4% or less by 5 years post treatment. Continuous time risk of recurrence and death was generally increased for patients with increased tumor and nodal stage and tumor grade, whereas tumor location was less impactful. Patients with more advanced tumors receive early and greater benefit from the addition of oxaliplatin, with reduced risk over time. The effect of oxaliplatin on preventing recurrences was smallest among patients with N0 disease (stage II), consistent with randomized controlled trials.3 Limiting adjuvant therapy in patients with stage II disease would limit late chemotherapy toxicity, including potential deaths attributed to late toxicity, as has been reported in long-term follow-up studies in lung cancer14 and germ cell tumors.15 The ACCENT database does not have such long-term follow-up data to examine this in colon cancer.
One limitation of this analysis is restriction to patients enrolled in clinical trials, who are well understood to represent a specific population of patients with colon cancer with fewer comorbidities than patients who are not enrolled in clinical trials. We also note that to facilitate our pooled comparison of oxaliplatin-based agents versus FU + LV alone, we did not further distinguish between patients treated with FOLFOX and intravenous oxaliplatin plus oral capecitabine, or between patients who received both oxaliplatin-based chemotherapy plus a biologic agent versus those who received oxaliplatin-based chemotherapy alone. The addition of a biologic agent, either bevacizumab9 or cetuximab,11 did not demonstrate an improvement in recurrence-free survival and would therefore have had a minimal impact on this analysis.
Clinical implications of these findings relate to colon cancer surveillance guidelines. More intensive surveillance in the first 3 years after resection is recommended across multiple guidelines,16-18 consistent with our observations that the greatest risk of recurrence occurs within the first 3 years post treatment. However, most surveillance guidelines do not distinguish between stage II and III colon cancer.16-19 In the ACCENT database, we observe that although the greatest risk of recurrence for stage II colon cancer is around 14 months, the risk is still much less than stage III colon cancer. In fact, the risk of recurrence for stage III colon cancer patients does not diminish to the risk observed in patients with stage II disease until about 4 years after surgery, at which point surveillance is generally less intensive. These findings suggest that less intensive surveillance strategies could be considered from the outset for patients with stage II colon cancer. Our data also support recommendations that surveillance can be greatly reduced more than 5 years from completion of therapy as the recurrence rates fall to near 0% in patients with stage II and stage III disease.
In summary, based on a large pooled analysis of individual patient data from five clinical trials, we found that oxaliplatin significantly reduces the risk of recurrence and death within the first 6 years post treatment, with the greatest benefit observed in patients with higher-risk cancers. The time course risk of recurrence for stage II colon cancer is significantly reduced compared with patients with stage III disease, with potential implications for surveillance strategies. These data support the hypothesis that the addition of oxaliplatin to fluoropyrimidine therapy provides sustained benefit over time, preventing recurrences that would ultimately lead to deaths in this large patient population.
Appendix
The following participated in the study: The ACCENT (Adjuvant Colon Cancer End Points) Group: D.J. Sargent, E. Green, A. Grothey, S.R. Alberts, Q. Shi, L.A. Renfro (Mayo Clinic, Rochester, MN), G. Yothers, M.J. O’Connell, N. Wolmark (NSABP [National Surgical Adjuvant Breast and Bowel Project] Biostatistical and Operations Centers, Pittsburgh, PA), A. de Gramont (CTD-INCa GERCOR, Assistance Publique des Hôpitaux de Paris, UPMC Paris VI, Paris, France), R. Gray, D. Kerr (QUASAR [Quick and Simple and Reliable] Collaborative Group, Birmingham and Oxford, United Kingdom), D.G. Haller (Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA), K. Guthrie (SWOG [Southwest Oncology Group] Statistical Center, Seattle, WA), M. Buyse (IDDI, [International Drug Development Institute], Louvain-la-Neuve, Belgium), R. Labianca (Ospedali Riuniti, Bergamo, Italy), J.F. Seitz (University of the Mediterranean, Marseilles, France), C.J. O’Callaghan (NCIC-CTG [National Cancer Institute of Canada Clinical Trials Group], Queens University, Kingston, Ontario, Canada), G. Francini (University of Siena, Siena, Italy), P.J. Catalano (ECOG [Eastern Cooperative Oncology Group] Statistical Center, Boston, MA), C.D. Blanke (Oregon Health Sciences University, Portland, OR), T. Andre (Hôpital Saint Antoine, Paris, France), R.M. Goldberg (Ohio State University Comprehensive Cancer Center, Columbus, OH), A. Benson (Northwestern University, Chicago, IL), C. Twelves (University of Bradford, West Yorkshire, United Kingdom), F. Sirzen (Roche, Basel, Switzerland), L. Cisar (Pfizer, New York, NY), E. Van Cutsem (University Hospital Gasthuisberg, Gasthuisberg, Belgium), and L. Saltz (Memorial Sloan-Kettering Cancer Center, New York, NY).
Table A1.
ACCENT Trials and Number of Patients (With Complete Treatment and Outcome Data) Used for Continuous Time Analyses
| Trial | Years | Treatment Arms | No. |
|---|---|---|---|
| MOSAIC | 1998-2001 | FU + LV v FOLFOX | 2,241 |
| XELOXA | 2003-2004 | FU + LV v XELOX | 1,793 |
| N0147* | 2004-2009 | mFOLFOX6 v mFOLFOX6 + Cetuximab | 3,153 |
| C-07 | 2000-2002 | FU + LV v FOLFOX | 2,434 |
| C-08 | 2004-2006 | mFOLFOX6 v mFOLFOX6 + bevacizumab | 2,612 |
| Total ACCENT | 12,233 |
Abbreviations: ACCENT, Adjuvant Colon Cancer End Points; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; FU, fluorouracil; LV, leucovorin; mFOLFOX6, modified FOLFOX 6 as infusional/bolus fluorouracil, leucovorin, and oxaliplatin; MOSAIC, Adjuvant Treatment of Colon Cancer; XELOX, intravenous oxaliplatin plus oral capecitabine; XELOXA, Adjuvant XELOX.
All patients enrolled to N0147 who received FOLFOX were included, regardless of KRAS status.
Table A2.
Treatment and Stage-Specific Annual Rates (%) of Recurrence and Death, by Year With 95% CIs
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Recurrence | |||||
| All | |||||
| FU | 11.0 (9.9 to 12.1)* | 10.5 (9.4 to 11.6) | 5.2 (4.4 to 6.0) | 3.4 (2.8 to 4.1)* | 2.0 (1.5 to 2.6) |
| FOLFOX | 7.5 (7.0 to 8.1)* | 10.2 (9.5 to 10.8) | 4.7 (4.3 to 5.2) | 2.4 (2.1 to 2.7)* | 1.8 (1.5 to 2.2) |
| Stage II | |||||
| FU | 4.1 (2.8 to 5.7) | 5.4 (3.9 to 7.3) | 2.7 (1.7 to 4.2) | 1.7 (0.9 to 2.9) | 1.4 (0.7 to 2.6) |
| FOLFOX | 2.1 (1.4 to 3.0) | 3.7 (2.8 to 4.8) | 2.5 (1.7 to 3.5) | 1.2 (0.7 to 1.9) | 0.9 (0.4 to 1.6) |
| Stage III | |||||
| FU | 13.2 (11.9 to 14.6)* | 12.2 (10.9 to 13.5) | 6.0 (5.1 to 7.0) | 3.9 (3.2 to 4.8)* | 2.1 (1.6 to 2.9) |
| FOLFOX | 8.5 (7.9 to 9.2)* | 11.4 (10.7 to 12.2) | 5.1 (4.6 to 5.7) | 2.6 (2.3 to 3.1)* | 2.0 (1.7 to 2.4) |
| Death | |||||
| All | |||||
| FU | 2.9 (2.3 to 3.5) | 5.4 (4.7 to 6.3) | 5.4 (4.6 to 6.2) | 4.9 (4.1 to 5.7) | 3.8 (3.2 to 4.6) |
| FOLFOX | 2.7 (2.4 to 3.0) | 4.7 (4.3 to 5.2) | 4.5 (4.1 to 5.0) | 4.7 (4.3 to 5.2) | 3.6 (3.2 to 4.1) |
| Stage II | |||||
| FU | 1.3 (0.6 to 2.3) | 2.5 (1.6 to 3.9) | 1.7 (0.9 to 2.8) | 2.7 (1.7 to 4.1) | 2.1 (1.2 to 3.4) |
| FOLFOX | 1.6 (1.0 to 2.3) | 1.8 (1.2 to 2.7) | 2.3 (1.6 to 3.2) | 2.0 (1.3 to 2.8) | 1.8 (1.1 to 2.6) |
| Stage III | |||||
| FU | 3.4 (2.7 to 4.2) | 6.4 (5.4 to 7.4) | 6.6 (5.6 to 7.6)* | 5.6 (4.7 to 6.6) | 4.4 (3.6 to 5.3) |
| FOLFOX | 2.9 (2.5 to 3.3) | 5.3 (4.8 to 5.8) | 5.0 (4.5 to 5.5)* | 5.3 (4.8 to 5.9) | 4.0 (3.5 to 4.5) |
Abbreviations: FOLFOX, infusional fluorouracil, leucovorin, oxaliplatin; FU, fluorouracil.
Indicates significant differences, where the 95% CIs do not overlap.
Footnotes
Supported by Grant No. CA25224 from the National Cancer Institute.
Presented at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Manish A. Shah, Lindsay A. Renfro, Daniel J. Sargent
Financial support: Daniel J. Sargent
Administrative support: Manish A. Shah, Daniel J. Sargent
Collection and assembly of data: Manish A. Shah, Lindsay A. Renfro, Greg Yothers, Daniel J. Sargent
Data analysis and interpretation: Manish A. Shah, Lindsay A. Renfro, Carmen J. Allegra, Thierry André, Aimery de Gramont, Hans-Joachim Schmoll, Daniel G. Haller, Steven R. Alberts, Daniel J. Sargent
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis of 12,223 Patients From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Manish A. Shah
Consulting or Advisory Role: Eli Lilly/ImClone Systems, Genentech
Research Funding: Sanofi (Inst), Genentech (Inst)
Lindsay A. Renfro
No relationship to disclose
Carmen J. Allegra
No relationship to disclose
Thierry André
Honoraria: Sanofi
Consulting or Advisory Role: Roche
Aimery de Gramont
Consulting or Advisory Role: Roche
Speakers’ Bureau: Sanofi
Hans-Joachim Schmoll
Consulting or Advisory Role: Roche, Bayer Healthcare Pharmaceuticals, GlaxoSmithKline
Research Funding: Roche
Travel, Accommodations, Expenses: Roche, Bayer, GlaxoSmithKline
Daniel G. Haller
Consulting or Advisory Role: Genentech
Speakers’ Bureau: Celgene, Taiho Pharmaceutical
Expert Testimony: Celgene
Steven R. Alberts
No relationship to disclose
Greg Yothers
Employment: Mountainview Pediatrics (I)
Consulting or Advisory Role: Pharmacyclics
Daniel J. Sargent
No relationship to disclose
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] 2013. International Agency for Research on Cancer 2013.
- 2.André T, Boni C, Mounedji-Boudiaf L, et al. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 4.Shi Q, Andre T, Grothey A, et al. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: Evidence of stage migration from the ACCENT database. J Clin Oncol. 2013;31:3656–3663. doi: 10.1200/JCO.2013.49.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 7.Sargent D, Shi Q, Yothers G, et al. Adjuvant Colon Cancer End-points (ACCENT) Group Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: Data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer. 2011;47:990–996. doi: 10.1016/j.ejca.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 9.Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 11.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics. 1994;50:61–76. [PubMed] [Google Scholar]
- 13.Gilbert PB, Wei LJ, Kosorok MR, et al. Simultaneous inferences on the contrast of two hazard functions with censored observations. Biometrics. 2002;58:773–780. doi: 10.1111/j.0006-341x.2002.00773.x. [DOI] [PubMed] [Google Scholar]
- 14.Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol. 2010;28:35–42. doi: 10.1200/JCO.2009.23.2272. [DOI] [PubMed] [Google Scholar]
- 15.Fosså SD, Gilbert E, Dores GM, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007;99:533–544. doi: 10.1093/jnci/djk111. [DOI] [PubMed] [Google Scholar]
- 16.Labianca R, Nordlinger B, Beretta GD, et al. ESMO Guidelines Working Group Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi64–vi72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 17.Meyerhardt JA, Mangu PB, Flynn PJ, et al. American Society of Clinical Oncology Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–4470. doi: 10.1200/JCO.2013.50.7442. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, III, Venook AP, Bekaii-Saab T, et al. National Comprehensive Cancer Network Colon cancer, version 3.2014. J Natl Compr Canc Netw. 2014;12:1028–1059. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 19. Cancer Care Ontario: Colorectal Cancer Follow-up Care Pathway. Disease Pathway Management Secretariat. Version 2013.05. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=298461. [Google Scholar]