Abstract
Purpose
To examine patterns of health and symptoms associated with the initiation of adjuvant endocrine therapy (ET) for primary breast cancer treatment.
Patients and Methods
The mind-body study (MBS) observational cohort participants provided self-reported data on physical and mental health, ET-related symptoms, as well as depression, fatigue, and sleep obtained at enrollment (after primary treatment, prior to initiation of ET) and 6 and 12 months later. Longitudinal trajectories of outcome variables among three patient groups (no ET, aromatase inhibitor [AI], or tamoxifen) were compared by using linear mixed models.
Results
Two-thirds of the 186 women initiated ET, which was evenly split between AI and tamoxifen, and no significant differences were observed in self-reported measures among the groups at baseline or in covariate-adjusted analyses. Physical health scores were below normative levels initially and improved over time, but the AI group had a significantly lower score at 12 months (P = .05); mental health scores were within the normal range, were similar in each group, and did not change over time. The no-ET group showed either stable or declining symptom severity, whereas the ET groups often showed increased severity over time, and the AI group reported more severe musculoskeletal (P = .02), hot flash (P = .02), and cognitive problems (P = .006) at one or both of the follow-up time points compared with the no-ET group. The tamoxifen group had higher levels of hot flashes (P = .002), cognitive problems (P = .016), and bladder problems (P = .02) than the no-ET group.
Conclusion
Attention should be given to the increased symptom burden associated with ET, and better efforts should be made to address patient-reported outcomes.
INTRODUCTION
At the time of the 2000 NIH consensus conference on breast cancer,1 the standard adjuvant endocrine therapy (ET) for all women with hormone receptor–positive disease was tamoxifen, a treatment in use for greater than 2 decades that had extensive data on efficacy and toxicity.2 The Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial in 20023 showed a significant disease-free survival benefit in postmenopausal women who received anastrozole compared with tamoxifen. Additional studies supported these findings, which included reduced risks for some serious tamoxifen-associated adverse events (ie, endometrial cancer and thromboembolic events) among aromatase inhibitor (AI) –treated patients.3 However, initial clinical trial toxicity reports failed to fully capture symptoms associated with AI therapy, which only emerged later in studies that included patient-reported outcomes (PROs).4-6 Most notable were greater vaginal dryness and pain with intercourse4,7 and musculoskeletal or joint pain.7,8
Currently, AIs are the predominant adjuvant ET prescribed for postmenopausal women, although the 2014 ASCO guideline9 provides only a moderate recommendation for AI therapy as the first choice, and suggests that the more serious ET-related toxicities should be considered as part of treatment decision making. Clinicians need to provide patients with accurate information on ET-associated symptoms, because these may affect quality of life (QOL) and influence treatment adherence.8 To our knowledge, there are few if any prospective studies that directly compare the QOL and symptoms of patients with breast cancer who initiated ET with those of patients with breast cancer who did not receive adjuvant ET after primary treatment (surgery, radiation, adjuvant chemotherapy). Use of a no-ET breast cancer comparison group provides a reference for the recovery from post-treatment symptoms associated with primary cancer therapy and exposes the contribution of ET to persistent symptoms and the pattern of recovery.
The Mind-Body Study (MBS) was a prospective, observational cohort study that enrolled patients with newly diagnosed breast cancer shortly after the completion of primary treatment but prior to the initiation of ET, if planned.10-12 Patients enrolled in the MBS were comprehensively evaluated at study entry and at 6 and 12 months thereafter. In this report, we describe the QOL and pattern of symptoms in women who initiated ET along with patients with breast cancer who did not receive ET.
PATIENTS AND METHODS
Study Participants and Procedures
The design, eligibility/exclusion, recruitment, and procedures used in the MBS were described in earlier publications.10-12 Participants were recruited from the Los Angeles community between 2007 and 2010. Eligibility requirements included female sex; age 21 to 65 years; newly diagnosed with stage 0, I, II, or IIIA breast cancer; completed primary treatments within the past 3 months; had not started ET; available for 12 months of follow-up; and English language proficient. Individuals with major risk factors for pre-existing cognitive impairment and factors that could influence the assessment of inflammation were excluded (related to major MBS research questions). Patients who used supplemental estrogen or progesterone (other than low-dose vaginal estrogen) were also excluded.11 Consenting women attended three separate in-person assessments at baseline (T1) before the initiation of ET, if prescribed, and 6 months (T2) and 12 months (T3) later. Assessments included self-administered questionnaires, neuropsychological testing, and blood tests at each time point.11 The University of California, Los Angeles, institutional review board approved the study, and all participants provided written informed consent.
Demographics, Clinical Information, and Self-Report Assessments
Demographic and medical information was obtained from self-reports and medical record abstraction. A broad range of psychosocial factors, behaviors, and symptoms were assessed; however, this report only examines QOL and symptoms that were relevant to the ET exposures. We administered the RAND 36-item short-form health survey (SF-36) as a measure of health-related QOL,13-15 and we reported scores from the physical component scale (PCS) and mental component scale (MCS). A score of 50 is normative for the general population, and 10 points equals one standard deviation; lower scores indicate poorer QOL. We also described the results from the Breast Cancer Prevention Trial (BCPT) symptom scales,16,17 which was designed to detect symptoms relevant to ET.16,18,19 These scales have been widely used, including in psychosocial intervention studies, in patients with breast cancer and survivors, and they have sound psychometrics.20 Each multi-item scale provides an average severity score for the degree of symptomatic bother; scores are 0, which equals not at all; 1, slightly; 2, moderately; 3, quite a bit; and 4, extremely.20 The Beck Depression Inventory II (BDI-II) was used to assess depressive symptoms during the 2 weeks preceding the study visit.21 Higher scores denote more severe symptoms, and scores indicate minimal (0 to 9), mild (10 to 18), moderate (19 to 29), and severe (≥ 30) depression. Fatigue was assessed with the Multidimensional Fatigue Symptom Inventory (MFSI)22,23 which was developed to measure fatigue in patients with cancer. Only the total score is reported, and higher scores indicate a greater severity of fatigue. The Pittsburgh Sleep Quality Index (PSQI),24 a validated measure of sleep disturbance, was used to examine sleep difficulties. Higher scores indicate greater sleep difficulty, and the cut point of > 5 indicates poor sleep quality.
Statistical Methods
Analysis of variance and Pearson χ2 tests were used to compare baseline demographic and medical variables among the three groups, which were classified by whether ET was administered at T2 (no ET, tamoxifen, AI). Linear mixed models were used to compare the longitudinal trajectories of PRO variables among the three groups. These models included fixed effects for group and time (T1, T2, T3), interactions between group and time, and random intercepts for participants. The covariance structure was selected on the basis of the Bayesian information criterion. We controlled for demographic and medical characteristics that differed between groups at baseline; because the full set included redundant variables, this set was reduced to avoid collinearity. For each model, we obtained P values for the overall group-by-time interaction; for differences among group means at T1, T2, and T3; and for pairwise comparisons between the no-ET group and the other two groups at T2 and T3. The P values for pairwise comparisons were adjusted for multiple comparisons with the Hochberg method.25 Analyses were conducted with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Participants
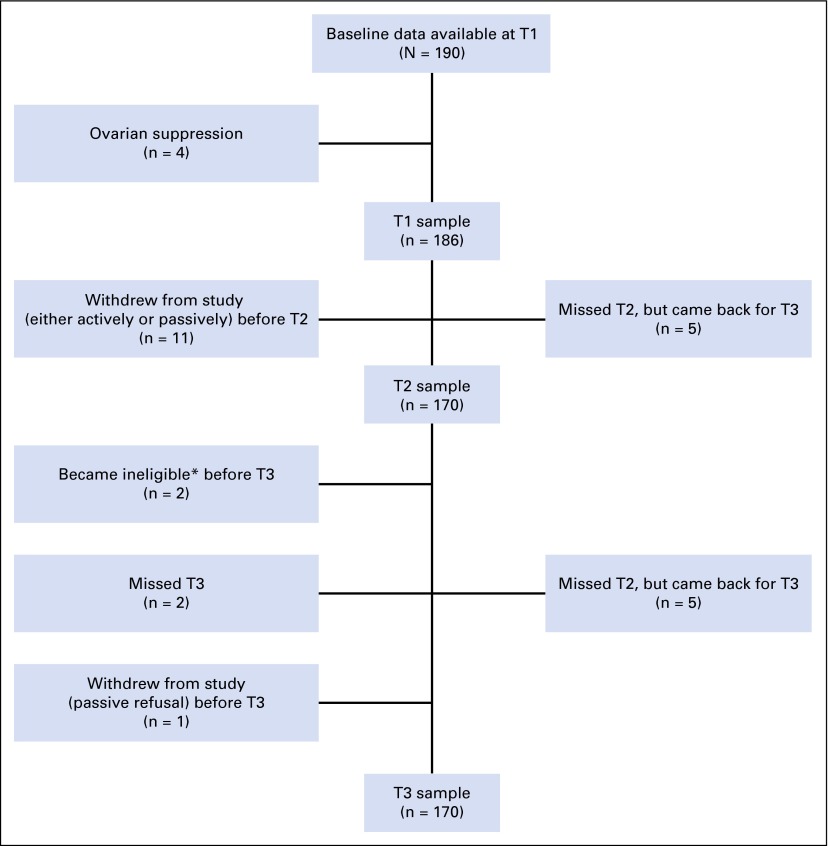
Figure 1 shows the flow of participants during the 12 months after study enrollment. A total of 190 women had questionnaire data available at baseline (T1). Four women who received ovarian suppression therapy were excluded, because the sample was too small to examine separately. Of the remaining 186, 16 patients were lost from the study by 12 months (T3), and some women who missed their 6-month assessment (T2) participated at T3.
Fig 1.
Diagram of patient participation. *Reasons for ineligibility: One participant experienced a recurrence, and another was diagnosed with thyroid cancer. T2, 6-month follow-up; T3, 12-month follow-up.
Table 1 provides the demographic and medical characteristics at enrollment by ET group. The mean age of the sample was 52 years, and age was statistically significantly different (P < .001) among the groups. Patients in the AI group were the oldest, which was consistent with use only in postmenopausal women. There were no other significant demographic differences except for education (P = .04). There were some differences in breast cancer treatments, including type of surgery (mastectomy performed less frequently in the AI group, P = .03); stage (more patients with stage 0 disease in the no-ET group); months since last treatment; and prior adjuvant chemotherapy and radiation therapy before initiation of ET. A noncollinear set of baseline demographic and medical variables (age, education, time since last treatment, and chemotherapy) were controlled for in subsequent mixed models that examined longitudinal patterns of PROs after the initiation of ET.
Table 1.
Baseline Demographic and Medical Characteristics by Endocrine Therapy Group
| Characteristic | No. (%) of Patients | P* | |||
|---|---|---|---|---|---|
| Total (N = 186) | No Endocrine Therapy at T2 (n = 60) | Tamoxifen Initiated by T2 (n = 66) | Aromatase Inhibitor Initiated by T2 (n = 60) | ||
| Mean (SD) age at T1, years | 52.0 (8.2) | 51.6 (8.6) | 46.9 (6.9) | 58.2 (3.8) | < .001 |
| Mean (SD) months from diagnosis to T1 | 5.9 (2.6) | 6.0 (2.9) | 5.9 (2.4) | 5.8 (2.6) | .89 |
| Mean (SD) months from last treatment to T1 | 1.2 (1.0) | 1.9 (1.1) | 1.0 (0.9) | 0.7 (0.6) | < .001 |
| Ethnicity† | |||||
| White, non-Hispanic | 148 (80) | 47 (78) | 48 (73) | 53 (88) | .09 |
| Hispanic | 19 (10) | 5 (8) | 10 (15) | 4 (7) | |
| Black | 5 (3) | 3 (5) | 2 (3) | 0 (0) | |
| Asian | 8 (4) | 2 (3) | 5 (8) | 1 (2) | |
| Other | 6 (3) | 3 (5) | 1 (2) | 2 (3) | |
| Marital status | |||||
| Married | 121 (65) | 40 (67) | 40 (61) | 41 (68) | .63 |
| Not married | 65 (35) | 20 (33) | 26 (39) | 19 (32) | |
| Education | |||||
| Post college | 96 (52) | 30 (50) | 30 (45) | 36 (60) | .04 |
| College | 55 (30) | 16 (27) | 28 (42) | 11 (18) | |
| No college degree | 35 (19) | 14 (23) | 8 (11) | 13 (22) | |
| Employment status | |||||
| Full or part time | 122 (66) | 40 (67) | 44 (67) | 38 (63) | .90 |
| Not employed | 64 (34) | 20 (33) | 22 (33) | 22 (37) | |
| Annual household income, $ | |||||
| ≥ 100,000 | 110 (60) | 31 (52) | 45 (69) | 34 (58) | .15 |
| < 100,000 | 73 (40) | 28 (47) | 20 (31) | 25 (42) | |
| Surgery | |||||
| Mastectomy | 63 (34) | 27 (45) | 23 (35) | 13 (22) | .03 |
| Lumpectomy | 123 (66) | 33 (55) | 43 (65) | 47 (78) | |
| Treatment | |||||
| Chemotherapy and radiation | 74 (40) | 21 (35) | 23 (35) | 30 (50) | < .001 |
| Chemotherapy only | 20 (11) | 4 (7) | 12 (18) | 4 (7) | |
| Radiation only | 64 (34) | 16 (27) | 25 (38) | 23 (38) | |
| Neither | 28 (15) | 19 (32) | 6 (9) | 3 (5) | |
| Stage at diagnosis | |||||
| 0 | 25 (13) | 15 (25) | 9 (14) | 1 (2) | .02 |
| I | 86 (46) | 22 (37) | 33 (50) | 31 (52) | |
| II | 58 (31) | 17 (28) | 19 (29) | 22 (37) | |
| III | 17 (9) | 6 (10) | 5 (8) | 6 (10) | |
| Menstrual status at diagnosis and change | |||||
| Postmenopausal before and after treatment | 100 (54) | 31 (52) | 13 (20) | 56 (93) | < .001 |
| Premenopausal at diagnosis | 86 (46) | 29 (48) | 52 (80) | 4 (7) | |
| Continued menstruating | 48 | 19 | 28 | 1 | |
| Stopped menstruating | 37 | 10 | 24 | 3 | |
| Missing data | 1 | 0 | 1 | 0 | |
Abbreviations: SD, standard deviation; T1, baseline time point; T2, 6-month follow-up time point.
P values are from an analysis of variance for continuous variables and from the Pearson χ2 tests for categoric variables. P values ≤ .05 are statistically significant.
P value is for the comparison of white versus nonwhite ethnicities.
Health-Related QOL and Symptoms
Table 2 lists the T1 PROs before the initiation of ET. There were no group differences in the PCS or MCS scores. The PCS score for all patients was approximately 0.5 standard deviations below the population mean of 50, which reflected a lower physical QOL. There were no significant differences in fatigue, depressive symptoms, or BCPT symptoms among the three groups. Sleep problems were increased in all three groups and did not differ significantly.
Table 2.
Baseline Patient-Reported Outcomes and Symptoms by Endocrine Therapy Group
| Outcome or symptom | Mean (SD) Score | P* | |||
|---|---|---|---|---|---|
| Total (N = 186) | No Endocrine Therapy at T2 (n = 60) | Tamoxifen Initiated by T2 (n = 66) | Aromatase Inhibitor Initiated by T2 (n = 60) | ||
| SF-36 | |||||
| PCS | 45.4 (9.3) | 45.6 (9.6) | 46.3 (8.0) | 44.1 (10.4) | .41 |
| MCS | 49.4 (9.1) | 50.2 (8.4) | 48.6 (10.0) | 49.7 (8.9) | .58 |
| MFSI total | 11.1 (19.2) | 10.2 (19.9) | 11.9 (17.5) | 11.0 (20.4) | .88 |
| PSQI | 7.6 (3.6) | 7.6 (4.0) | 7.4 (3.6) | 7.9 (3.0) | .68 |
| BDI-II | 8.8 (6.8) | 9.0 (7.1) | 8.8 (6.6) | 8.5 (7.0) | .93 |
| BCPT symptom scale | |||||
| Hot flashes | 1.1 (1.1) | 0.8 (1.1) | 1.2 (1.2) | 1.3 (1.1) | .11 |
| Nausea | 0.2 (0.3) | 0.1 (0.3) | 0.2 (0.3) | 0.2 (0.3) | .90 |
| Bladder control problems | 0.3 (0.5) | 0.4 (0.6) | 0.2 (0.4) | 0.4 (0.6) | .05 |
| Vaginal problem | 0.9 (1.2) | 0.7 (0.9) | 0.8 (1.2) | 1.1 (1.3) | .11 |
| Musculoskeletal pain | 1.2 (0.9) | 1.3 (1.0) | 1.1 (0.9) | 1.4 (0.9) | .10 |
| Cognitive problems | 1.3 (1.0) | 1.1 (0.9) | 1.3 (0.9) | 1.4 (1.0) | .39 |
| Weight problems | 1.1 (1.0) | 1.2 (1.1) | 1.0 (0.7) | 1.2 (1.0) | .25 |
| Arm movement problems | 0.5 (0.7) | 0.5 (0.6) | 0.5 (0.7) | 0.4 (0.7) | .83 |
Abbreviations: BCPT, Breast Cancer Prevention Trial; BDI-II, Beck Depression Inventory II; MCS, mental component scale; MFSI, Multidimensional Fatigue Symptom Inventory; PCS, physical component scale; PSQI, Pittsburgh Sleep Quality Index; SF-36, 36-question short-form health survey; T2, 6-month follow-up time point.
P values are from an analysis of variance. P values ≤ .05 are statistically significant.
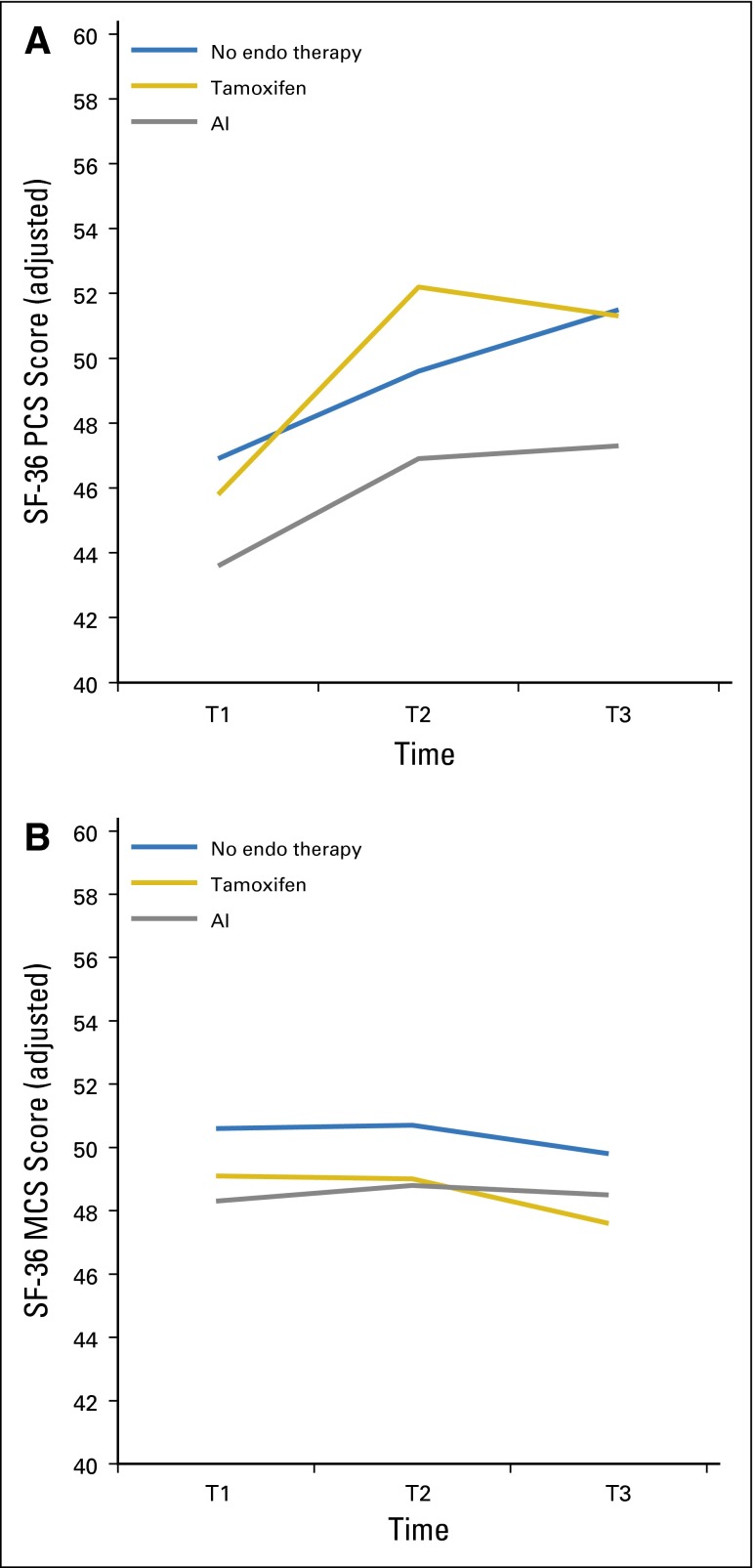
Figure 2A shows the pattern of change in the PCS score in the 12 months after primary therapy by ET group. (Appendix Table A1, online only, lists statistical results for significant model covariates, for all of the longitudinal models. Appendix Table A2 lists the results for the paired comparisons at each time point, with P values.) All women demonstrated rapid improvements in PCS scores by T2, and scores plateaued after that time, as we had previously observed in an independent sample.26 The model results indicated a trend toward a group-by-time interaction (P = .07) over the 12 months, and simple-effects analysis revealed significant adjusted group mean differences at T2 (P = .02) and T3 (P = .05); the AI group had lower scores than the no-ET group (P = .05). Figure 2B shows the adjusted means for the MCS scores over the 12 months. There were no significant differences between groups in change over time or at specific time points for the MCS.
Fig 2.
Longitudinal trajectories of adjusted mean 36-item Short-Form Health Survey (SF-36) summary scores at baseline (T1) and at 6 months (T2) and 12 months (T3) of follow-up. Means were adjusted for age, education, time since last treatment, and chemotherapy. (A) Physical component scale (PCS) score; P = .07 for overall group-by-time interaction; P = .21 for group differences at T1; P = .02 for group differences at T2; and P = .05 for group differences at T3. (B) Mental component scale (MCS) score; P = .92 for overall group-by-time interaction; P = .51 for group differences at T1; P = .56 for group differences at T2; and P = .52 for group differences at T3. AI, aromatase inhibitor; endo, endocrine.
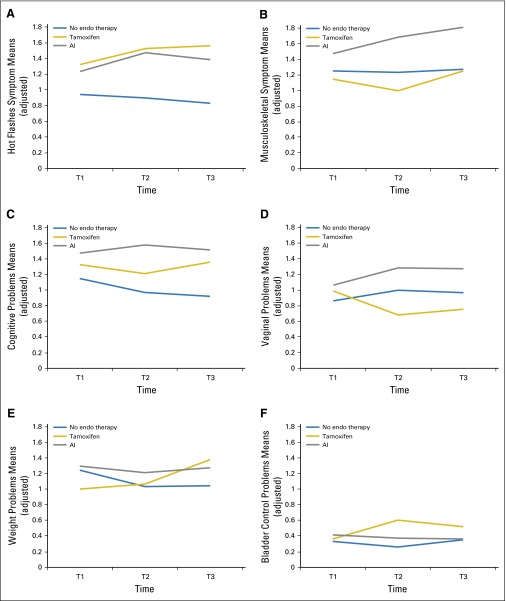
Figure 3A shows the trajectories of hot flash symptom severity, and adjusted group means are significantly higher in the two ET groups at T2 (P = .009) and at T3 (P = .003). Pairwise comparisons indicated statistically significant differences at T2 and T3 between tamoxifen and the no-ET group (P = .008 and P = .002 for respective time points) and between AI and no ET (P = .02 and P = .02 for respective time points; Appendix Table A2).
Fig 3.
Longitudinal trajectories of adjusted mean Breast Cancer Prevention Trial (BCPT) severity scores at baseline (T1) and at 6 months (T2) and 12 months (T3) of follow-up: (A) hot flashes; (B) musculoskeletal complaints; (C) cognitive complaints; (D) vaginal symptoms; (E) weight problems; and (F) bladder problems. Means were adjusted for age, education, time since last treatment, and chemotherapy. AI, aromatase inhibitor; endo, endocrine.
Figure 3B examines the pattern of musculoskeletal symptoms. There was a trend toward a group-by-time interaction (P = .09) and significant differences in adjusted group means at T2 (P = .002) and at T3 (P = .009). After multiplicity adjustment, musculoskeletal symptom scores were significantly higher for AI versus no ET at T3 (P = .02). Altogether, the results indicated that the scores for the tamoxifen and no-ET groups remain essentially unchanged over the 12 months of follow-up, whereas the AI group scores worsened.
Figure 3C shows the trajectories for the cognitive problems score. There were statistically significant differences at T2 and T3 (P = .009 and P = .007, respectively), and the severity of complaints was greater in the ET-treated patients. This is supported by multiplicity-adjusted pairwise comparisons of tamoxifen versus no ET and AI versus no ET at T3 (both P < .02) and for AI versus no ET at T2 (P = .004). These results reflect a decline in cognitive complaints in the no-ET group over time, whereas cognitive complaints persisted or did not recover in the two ET groups.
Vaginal problems (dryness and pain with intercourse) are shown in Figure 3D, with evidence of group differences at T2 (P = .05) as a result of worsening in patients in the AI group and improvement in patients in the tamoxifen group. Figures 3E and 3F describe weight problems and bladder control problems, which both showed significant differences in change over time among groups (P = .04 and P = .01, respectively, for the group-by-time interaction). Tamoxifen-treated patients demonstrated increases in both weight problems and bladder control problems over time, whereas the AI and no-ET groups remained the same. The longitudinal trajectories for the BCPT symptom scales for arm problems and nausea after ET initiation demonstrated low scores at T1 and no changes over time (data not shown).
In terms of covariate effects on the BCPT symptoms, chemotherapy was significantly associated with higher levels of arm problems, cognitive problems, hot flashes, musculoskeletal problems, and vaginal problems. Older age was significantly associated with increased bladder control problems, hot flashes, and vaginal problems (Appendix Table A1).
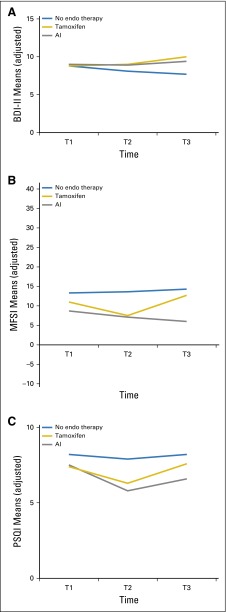
Trajectory model results are shown in Figure 4 for depression, fatigue, and sleep. Figure 4A shows the model results for the BDI-II scores, which showed no significant differences among the ET groups. There were also no significant group-by-time differences for the MFSI scores (Fig 4B). In Figure 4C, we noted persistently elevated PSQI scores for all three groups of patients, with no overall group difference over time (P = .12) but with a significant group difference at T2 (P = .03). Chemotherapy was significantly associated with all three of these symptoms (Appendix Table A1). Repetition of the models with a larger set of control variables (adding stage, surgery type, and menopausal status) gave similar results (results not shown).
Fig 4.
Longitudinal trajectories of adjusted mean depressive symptom, fatigue, and sleep problem scores at baseline (T1) and at 6 months (T2) and 12 months (T3) of follow-up. Means were adjusted for age, education, time since last treatment, and chemotherapy. (A) Depressive symptoms, measured by the Beck Depression Inventory II (BDI-II). (B) Fatigue severity, measured by the Multidimensional Fatigue Symptom Inventory (MFSI); P = .11 for overall group-by-time interaction; P = .53 for group differences at T1; P = .23 for group differences at T2; and P = .09 for group differences at T3. (C) Sleep problems, measured by the Pittsburgh Sleep Quality Index (PSQI; adjusted means) by type of endocrine (endo) therapy at three time points. AI, aromatase inhibitor.
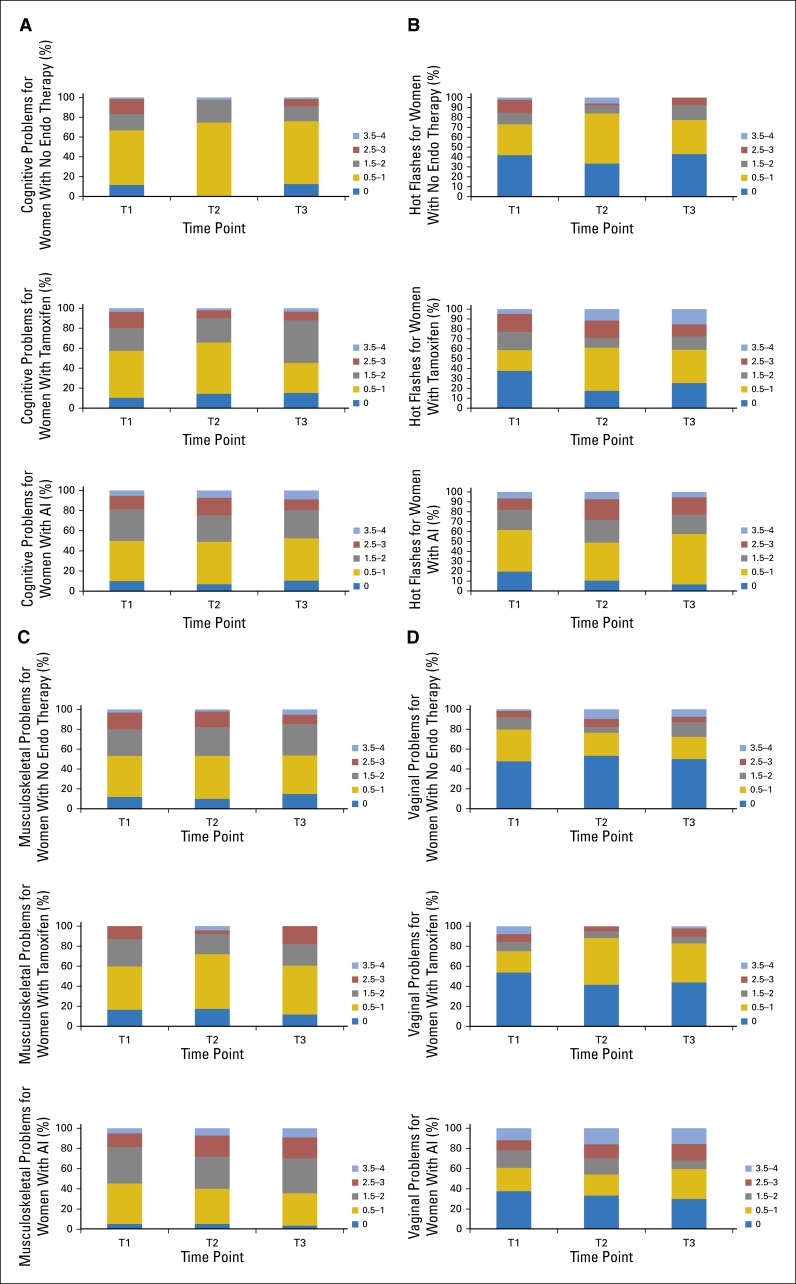
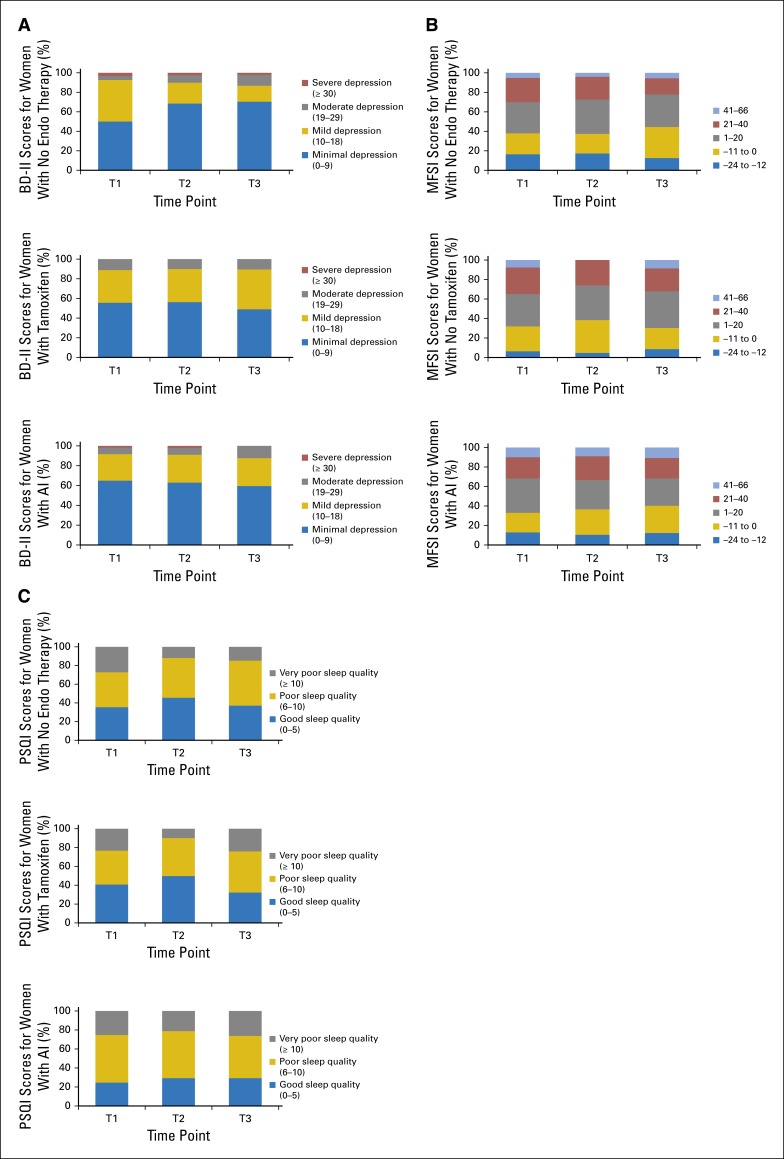
To better understand symptom burden, we show the unadjusted distribution of scores for hot flashes, musculoskeletal symptoms, cognitive problems, and vaginal problems at each time point in Appendix Figure A1. For each symptom and treatment group, it is important to note the changes in severity distributions of those who were not bothered by symptoms (score of 0) and extremely bothered by symptoms (score of 3.5 to 4). As an example, for hot flashes, women who did not receive ET have a decreased proportion in the most severe symptom severity category and an increased proportion in the zero-symptom severity category. In contrast, for both tamoxifen and AI, the zero-severity category diminished, and the most severe categories expanded. Distribution changes at the severity extremes occurred for the other symptoms. Appendix Figure A2 provides distributions for depression, fatigue, and sleep scores. For example, for the PSQI scores, the proportion of women with good sleep in all of the ET groups remained stable across time points, and most increases occurred in the ET groups at T3 in women whose sleep quality moved from poor (score of 6 to 10) to very poor (score of ≥ 10). These distributions are important for understanding the variation in response to these powerful estrogen receptor–targeted therapies, because the mean scores may mask the suffering of a substantial minority of patients.
DISCUSSION
The MBS was designed to examine the impact of ET initiation on cognitive function after primary breast cancer treatment, a situation for which little data are available. Surprisingly, there are limited data on the relative impact of ET on QOL and symptoms in this same setting, which represents a gap in information on the post-treatment effects that may persist or be exacerbated by ET initiation. The comprehensive approach to data collection in the MBS permitted examination of an array of common treatment-associated symptoms at the end of primary treatment and of the pattern of recovery in the subsequent 12 months.
In this study, we documented significantly reduced physical health–related QOL at the end of primary therapy compared with population norms, which improved significantly in the subsequent 12 months but which was significantly poorer in the AI group at T3, with a suggestion of plateau in recovery compared with the tamoxifen and no-ET groups. In contrast, there were no significant changes in mental health–related QOL over time by group, and the scores were close to population norms. The increased severity of symptoms (hot flashes, musculoskeletal pain, vaginal problems) that we found in association with ET are not novel in themselves; however, the comparison of symptoms in the ET groups to women with breast cancer who do not initiate ET is noteworthy. Many ET-related symptoms also overlap with chemotherapy toxicities that have been previously described in cross-sectional studies27,28; however, in this prospective longitudinal examination, the no-ET group experienced stable or declining severity of many symptoms.
For the ET-related symptoms, recovery is affected by prior exposure to chemotherapy as well as by older age. Bladder control problems, hot flashes, and vaginal problems are more severe in older women, and ET exacerbates these symptoms, with some variability according to the age of the woman and the specific ET. Adjuvant chemotherapy contributes significantly to greater symptoms in the year after primary treatment and, combined with ET, is likely responsible for the failure of some chemotherapy-related symptoms to resolve. This can best be seen by looking at the trajectory of the no-ET group, in whom symptoms (eg, cognitive problems, hot flashes) were almost always lower and frequently were declining during the 12 months of follow-up.
Although the QOL and symptom impact of adjuvant ET has been examined within the context of several clinical trials that compared adjuvant tamoxifen with an AI,4,5,29 most studies described the comparative differences in toxicity of the two classes of ET without a relevant no-ET breast cancer comparison group. This is one of the few prospective observational studies to examine the impact of adjuvant ET in the naturalistic setting, when ET is prescribed after completion of primary treatment. Important findings from this study include the level of symptom burden at the end of primary treatment, as we have reported in another independent sample,28 and—especially noted here—sleep disturbance, fatigue, and cognitive problems and their persistence in the following year for most women. MBS scores for sleep and fatigue are similar to those in patients reported by Ancoli-Israel et al,30 who used the same measures. In that study, the patients with breast cancer had significantly worse scores than those in a concurrent healthy-woman control group at post-treatment and at 1 year of follow-up.30
Although we have focused our analysis on the impact of ET initiation on increased symptom severity, it is important to appreciate the pattern of stability or subtle decline in severity that we observed for the women who did not initiate ET (eg, for hot flashes, weight problems, musculoskeletal symptoms, and cognitive problems). This suggests that the resolution of some post-treatment symptoms may be retarded with ET initiation. In a previous study with a post-treatment cohort that was not restricted by upper age (mean age, 56.9 years),26 we examined recovery by exposure to adjuvant chemotherapy or not, during which ET was evenly distributed in the two groups. In that study, we were not able to account for the contribution that initiation of ET might have played in reported symptoms or for the patterns associated with AI and tamoxifen. In this analysis from the MBS, we confirmed the increased cognitive complaints after ET initiation that we previously described in a more detailed analysis at 6 months12 and found that increased cognitive problems persisted at 12 months post treatment in comparison to those who did not receive ET.
Limitations of this study include its observational nature, which precludes inference of causality; the relatively modest sample size; and the specific inclusion and exclusion criteria of the parent study. Thus, the selective nature of the patient sample could underestimate the severity of some symptoms in an older and sicker group of women. In addition, the MBS participants were volunteers who completed a demanding research study and were living in a large metropolitan city; they may not be representative of all groups of women who receive breast cancer treatment. Nevertheless, this report is among the first to show the pattern of symptoms associated with the two major forms of ET, how they differ from each other, and how initiation of ET leads to persistence or worsening of some post-primary treatment symptoms. Better management of ET-associated symptoms needs to be a focus of survivorship care to relieve suffering and to ensure that long-term persistence and adherence to ET is achieved.
Supplementary Material
Acknowledgment
We thank the women who volunteered for this study and shared their experiences after primary breast cancer treatments. In addition, we thank our research staff, who helped with the recruitment of participants and collection of data.
Appendix
Table A1.
Mixed-Model (P value) Results for Control Variables
| Dependent variable | P*† | |||
|---|---|---|---|---|
| Age | Months Since Last Treatment | Education | Chemotherapy | |
| BCPT arm problems | .002 | .005 | ||
| BCPT bladder control problems | .001 | < .001 | ||
| BCPT cognitive problems | < .001 | |||
| BCPT hot flashes | .02 | .006 (−) | .009 | |
| BCPT musculoskeletal problems | .02 | .05 | ||
| BCPT nausea | ||||
| BCPT vaginal problems | .007 | .01 (−) | .02 | |
| BCPT weight problems | .09 (−) | |||
| BDI-II | .02 | |||
| MFSI | .09 | .05 | ||
| MCS‡ | ||||
| PCS‡ | .004 (−) | .08 (−) | ||
| PSQI | .03 | |||
Abbreviations: BCPT, Breast Cancer Prevention Trial; BDI-II, Beck Depression Inventory II; MCS, mental component scale; MFSI, Multidimensional Fatigue Symptom Inventory; PCS, physical component scale; PSQI, Pittsburgh Sleep Quality Index.
P values < .10 are provided. All other P values were > .10.
All associations were positive, except where indicated by (−). The reference value for education was the highest level, so a negative association meant that higher scores were associated with less education.
For all variables except MCS and PCS, a higher score meant a worse outcome.
Table A2.
P Values for Pairwise Comparisons Between Tamoxifen and No Therapy and Between Aromatase Inhibitor and No Therapy at T2 and at T3, Adjusted for Multiple Comparisons With the Hochberg Method
| Outcome Measure | P* | |||
|---|---|---|---|---|
| At T2 | At T3 | |||
| Tamoxifen v No Therapy | AI v No Therapy | Tamoxifen v No Therapy | AI v No Therapy | |
| BDI-II | .63 | .63 | .22 | .25 |
| MFSI | .92 | .24 | .08 | .08 |
| MCS | .36 | .36 | .51 | .51 |
| PCS | .15 | .15 | .93 | .05 |
| PSQI | .51 | .02 | .15 | .08 |
| BCPT cognitive problems | .19 | .004 | .016 | .006 |
| BCPT hot flashes | .008 | .02 | .002 | .02 |
| BCPT musculoskeletal problems | .19 | .06 | .91 | .02 |
| BCPT bladder control problems | .02 | .41 | .36 | .93 |
| BCPT vaginal problems | .26 | .26 | .35 | .35 |
| BCPT weight problems | .90 | .82 | .18 | .28 |
Abbreviations: AI, aromatase inhibitor; BCPT, Breast Cancer Prevention Trial; BDI-II, Beck Depression Inventory II; MCS, mental component scale; MFSI, Multidimensional Fatigue Symptom Inventory; PCS, physical component scale; PSQI, Pittsburgh Sleep Quality Index.
P values are for pairwise comparisons, adjusted for multiple comparisons. P values ≤ .05 are statistically significant.
Fig A1.
Marginal score distributions for selected Breast Cancer Prevention Trial (BCPT) scales by group (treatment with no endo, tamoxifen, or an AI) and by time points (baseline [T1], 6 months of follow-up [T2], or 12 months of follow-up [T3]): (A) cognitive problems, (B) hot flashes, (C) musculoskeletal problems, and (D) vaginal problems. AI, aromatase inhibitor; endo, endocrine.
Fig A2.
Marginal score distributions for Beck Depression Inventory II (BDI-II; A), Multidimensional Fatigue Symptom Inventory (MSFI; B), and Pittsburgh Sleep Quality Index (PSQI; C) by group (no endo therapy, tamoxifen, or an AI) and by time points (baseline [T1], 6 months of follow-up [T2], or 12 months of follow-up [T3]). AI, aromatase inhibitor; endo, endocrine.
Footnotes
Supported by funding from the National Institutes of Health, National Cancer Institute grants No. P30 CA16042 (to C.M.C. and P.A.G.) and No. R01 CA 109650 (to P.A.G.) and by the Breast Cancer Research Foundation (to P.A.G., who is a member of the Scientific Advisory Board of the Breast Cancer Research Foundation).
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Patricia A. Ganz, Julienne E. Bower
Financial support: Patricia A. Ganz
Administrative support: Patricia A. Ganz
Collection and assembly of data: Patricia A. Ganz, Laura Petersen
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study (MBS)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock or Other Ownership: Xenon (I), Intrinsic LifeSciences (I), SIlarus Therapeutics (I), Merganser Biotech (I), Teva, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: Keryx (I), Merganser Biotech (I), Silarus Therapeutics (I), Informed DNA
Research Funding: Keryx (I)
Patents, Royalties, Other Intellectual Property: Related to iron metabolism and the anemia of chronic disease (I); Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I), Keryx (I)
Laura Petersen
No relationship to disclose
Julienne E. Bower
No relationship to disclose
Catherine M. Crespi
No relationship to disclose
REFERENCES
- 1.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: Adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2001;(1):CD000486. doi: 10.1002/14651858.CD000486. [DOI] [PubMed] [Google Scholar]
- 3.Baum M, Budzar AU, Cuzick J, et al. ATAC Trialists’ Group Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 4.Fallowfield L, Cella D, Cuzick J, et al. Quality of life of postmenopausal women in the arimidex, tamoxifen, alone or in combination (ATAC) adjuvant breast cancer trial. J Clin Oncol. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Fallowfield LJ, Bliss JM, Porter LS, et al. Quality of life in the intergroup exemestane study: A randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol. 2006;24:910–917. doi: 10.1200/JCO.2005.03.3654. [DOI] [PubMed] [Google Scholar]
- 6.van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A randomised phase 3 trial. Lancet. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 7.Morales L, Neven P, Timmerman D, et al. Acute effects of tamoxifen and third-generation aromatase inhibitors on menopausal symptoms of breast cancer patients. Anticancer Drugs. 2004;15:753–760. doi: 10.1097/00001813-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chim K, Xie SX, Stricker CT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer. 2013;13:401. doi: 10.1186/1471-2407-13-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. (suppl) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: Examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105:791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz PA, Petersen L, Castellon SA, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: An observational cohort study. J Clin Oncol. 2014;32:3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SF-36 Health Survey: Manual and Interpretation Guide. , Boston, MA,: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 14.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 16.Ganz PA, Day R, Ware JE, Jr, et al. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 17.Cella D, Land SR, Chang CH, et al. Symptom measurement in the Breast Cancer Prevention Trial (BCPT) (P-1): Psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat. 2008;109:515–526. doi: 10.1007/s10549-007-9682-9. [DOI] [PubMed] [Google Scholar]
- 18.Day R, Ganz PA, Costantino JP, et al. Health-related quality of life and tamoxifen in breast cancer prevention: A report from the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Clin Oncol. 1999;17:2659–2669. doi: 10.1200/JCO.1999.17.9.2659. [DOI] [PubMed] [Google Scholar]
- 19.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: The NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 20.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97:448–456. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 21. Beck AT, Steer RA, Brown GK: Manual for the Beck Depression Inventory II. (ed 2). San Antonio, TX, Psychological Corporation, 1996. [Google Scholar]
- 22.Stein KD, Martin SC, Hann DM, et al. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 23.Stein KD, Jacobsen PB, Blanchard CM, et al. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, III, Monk TH, et al. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 25.Dmitrienko ATA, Bretz F, editors. Multiple Testing Problems in Pharmaceutical Statistics. Boca Raton, FL,: Chapman & Hall/CRC Press; 2010. Multiple testing methodology. [Google Scholar]
- 26.Ganz PA, Kwan L, Stanton AL, et al. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29:1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz PA, Rowland JH, Desmond K, et al. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 28.Ganz PA, Kwan L, Stanton AL, et al. Quality of life at the end of primary treatment of breast cancer: First results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 29.Fallowfield L. Quality of life issues in relation to the aromatase inhibitor. J Steroid Biochem Mol Biol. 2007;106:168–172. doi: 10.1016/j.jsbmb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: A 1-year longitudinal study. Support Care Cancer. 2014;22:2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.