Abstract
Purpose
Rituximab maintenance therapy has been shown to improve progression-free survival in patients with follicular lymphoma; however, the optimal duration of maintenance treatment remains unknown.
Patients and Methods
Two hundred seventy patients with untreated, relapsed, stable, or chemotherapy-resistant follicular lymphoma were treated with four doses of rituximab monotherapy in weekly intervals (375 mg/m2). Patients achieving at least a partial response were randomly assigned to receive maintenance therapy with one infusion of rituximab every 2 months, either on a short-term schedule (four administrations) or a long-term schedule (maximum of 5 years or until disease progression or unacceptable toxicity). The primary end point was event-free survival (EFS). Progression-free survival, overall survival (OS), and toxicity were secondary end points. Comparisons between the two arms were performed using the log-rank test for survival end points.
Results
One hundred sixty-five patients were randomly assigned to the short-term (n = 82) or long-term (n = 83) maintenance arms. Because of the low event rate, the final analysis was performed after 95 events had occurred, which was before the targeted event number of 99 had been reached. At a median follow-up period of 6.4 years, the median EFS was 3.4 years (95% CI, 2.1 to 5.3) in the short-term arm and 5.3 years (95% CI, 3.5 to not available) in the long-term arm (P = .14). Patients in the long-term arm experienced more adverse effects than did those in the short-term arm, with 76% v 50% of patients with at least one adverse event (P < .001), five versus one patient with grade 3 and 4 infections, and three versus zero patients discontinuing treatment because of unacceptable toxicity, respectively. There was no difference in OS between the two groups.
Conclusion
Long-term rituximab maintenance therapy does not improve EFS, which was the primary end point of this trial, or OS, and was associated with increased toxicity.
INTRODUCTION
Rituximab has been widely investigated for maintenance treatment in patients with follicular lymphoma (FL) because of its efficacy, safety profile, and pharmacokinetic characteristics. Rituximab maintenance improves the duration of remission or progression-free survival (PFS) in the first-line as well as in the relapsed setting.1-4 To date, no individual trial has shown a significant increase in overall survival (OS). A systematic review has shown improved OS only in patients with relapsed or refractory FL.5 Efficacy has been demonstrated after different induction therapies, for example, chemotherapy, rituximab monotherapy, and immunochemotherapy. Various dosing schedules and treatment durations were investigated: rituximab 375 mg/m2 every 2 months,1 every 3 months,3 or four weekly infusions every 6 months.6 None of these schedules has been compared head to head, and the optimal duration for rituximab maintenance therapy is unknown. Different study groups investigated a maintenance duration of 2 years, which is the most widely adopted.3,4
In the previous trial SAKK 35/98, we focused on a chemotherapy-free approach and investigated immunotherapy with single-agent rituximab: rituximab induction followed by maintenance therapy with four infusions every 2 months.1 Compared with no maintenance treatment, rituximab maintenance prolonged median event-free survival (EFS) from 12 to 23 months (P = .02). This benefit was maintained with longer follow-up.2 The present open-label, international, multicenter, randomized phase III trial SAKK 35/03 was designed to assess the potential benefit of prolonging rituximab maintenance therapy to a maximum of 5 years.
PATIENTS AND METHODS
Study Design and Treatment
The trial consisted of two phases: induction therapy followed by a randomized maintenance therapy phase. Patients were registered prior to induction and randomly assigned after achieving at least a partial response to induction therapy.
Patients were eligible for induction if they were older than 18 years and presented with FL of grade 1, 2, 3a, or 3b, with CD20 expression on immunohistochemistry. Patients with untreated, relapsed or progressed, chemotherapy-resistant, or stable disease could be included in the trial if a treatment was indicated according to the treating physician. There was no definition of need for therapy in the protocol. Previous anti-CD20 therapy was allowed, provided patients had responded to this treatment (complete or partial response) and at least 12 months had elapsed since the last anti-CD20 therapy. At least one two-dimensionally measurable lesion on computed tomography (CT) scan with a large transverse diameter of ≥ 11 mm was required. The amount of tumor burden was not an inclusion or exclusion criterion. Patients were required to have a performance status of 2 or less on the WHO scale and adequate cardiac function (ejection fraction ≥ 50%) on echocardiography or a multiple-gated acquisition scan. A history of prior or concomitant malignancies was an exclusion criterion. Presence or history of CNS involvement, serious underlying medical conditions, transformation to high-grade lymphoma, and regular use of corticosteroids were also exclusion criteria. Pretreatment testing for HIV infection or active hepatitis B or hepatitis C virus infection was not requested.
Induction therapy consisted of four weekly intravenous infusions of rituximab at the standard dose of 375 mg/m2. Restaging was performed between 11 and 13 weeks after the start of induction therapy. Patients achieving a partial response or a confirmed or unconfirmed complete response according to the Cheson criteria were eligible to be randomly assigned to the maintenance phase of the trial.7 The short-term maintenance therapy consisted of four rituximab infusions (375 mg/m2) administered intravenously every 2 months, whereas the long-term maintenance therapy consisted of rituximab infusions (375 mg/m2) every 2 months for a maximum of 5 years or until relapse or progression or unacceptable toxicity occurred. The 2-month interval was chosen on the basis of preliminary pharmacokinetic data and on the results of our previous trial SAKK 35/98.1,8
Eligible patients were randomly assigned centrally at the SAKK Coordinating Center in Bern, Switzerland, to short-term rituximab maintenance or long-term rituximab maintenance in a one-to-one ratio. Random assignment was stratified for disease status (untreated v pretreated), bulky disease (≥ 5 cm; yes or no), and center. The study was not masked.
The study protocol (Data Supplement) was approved by the local or national ethics committees according to the law of each country, and the study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before registration.
Evaluation and Response Criteria
Initial staging included physical examination; standard laboratory assessments, including immunoglobulin G (IgG) serum levels; CT scans of the neck, chest, abdomen, and pelvis; and bone marrow biopsy. During maintenance therapy, patients were assessed by clinical examination and complete blood counts every 2 months. CT scans were performed every 6 months during the first 5 years after random assignment. Patients reaching the end of maintenance therapy without an event were assessed by clinical and laboratory examinations and CT scans every 6 months until the occurrence of an event. In the case of an event, regular CT scans were discontinued. If bone marrow was involved at baseline, a biopsy was repeated at restaging and at relapse or progression.
CT scans were assessed by the local investigator. The corresponding author checked all reported tumor measurements for completeness and plausibility.
Statistical Methods
Statistical assumptions were based on our previous trial with short-term rituximab maintenance for FL.1 Sample size calculation allowed detection of a median EFS increase of 2.5 to 4.5 years in the long-term maintenance arm with 80% power and an overall two-sided type I error probability of 5%. Comparisons between the two arms were performed using the log-rank test for survival end points. We planned to register 270 patients and to randomly assign approximately 135 patients. Two interim analyses were planned to allow early discontinuation of the trial for either efficacy or futility, but only one analysis was conducted after 70 events. The first interim analysis was omitted because of too few events. The critical values for early discontinuation had to be obtained using the O’Brien-Fleming boundary shape on the basis of a spending function for the overall type I error probability and a spending function for the overall type II error probability. A minimum of 99 events was required for the final analysis and was expected approximately 5.14 years after the last random assignment.
The primary study end point was EFS from the time of random assignment. An event was defined as disease progression; relapse; unacceptable toxicity (a life-threatening or serious event that was probably, possibly, or definitively related to the trial treatment, eg, worsening of cardiac function with ejection fraction < 50%, any grade 3 or grade 4 nonhematologic toxicity other than infusion-related symptoms, or any grade 4 hematologic toxicity); death from any cause; initiation of any nonprotocol antilymphoma treatment or concomitant corticosteroids introduced because of lymphoma symptoms or concomitant radiotherapy; or secondary malignancy. Secondary end points were PFS, OS, objective response, and adverse reactions during and after maintenance therapy. PFS was defined as time from random assignment to relapse, progression, or death from FL, whichever occurred first.
Survival functions were estimated by using the Kaplan-Meier method and compared between treatment arms using the log-rank test. Multiple Cox proportional hazards regression models were used to investigate the effect of disease status and bulky disease at registration. The assumption of proportional hazard was checked by using the Grambsch-Therneau test. The protocol stated that approaches other than the log-rank test and Cox regression may be applied if this assumption could not be justified. The statistical analysis plan written for the final analysis stated that the weighted log-rank test would be used as proposed by Harrington and Fleming,9 with more weight placed on later time points, for the sensitivity analysis. Fisher’s exact test was used to compare proportions between treatment arms. All randomly assigned patients were included in the analyses on an intention-to-treat basis.
It had been decided to collect the remaining data for the final analysis by the end of April 2013. The final analysis could be performed even if fewer than 99 events were observed. Indeed, the total number of events had reached a plateau, and it seemed unlikely that the targeted number of 99 events could be reached within a reasonable period of time—the planned 5.14 years of follow-up had already been reached in January 2013. By February 2013, 89 events were counted, and considering this number as the final number of events would give a post hoc power of 77%. The decision to perform the final analysis even if the targeted number of events was not reached was made independently from any results generated, and this analysis would still ensure a statistical power close to 80%.
RESULTS
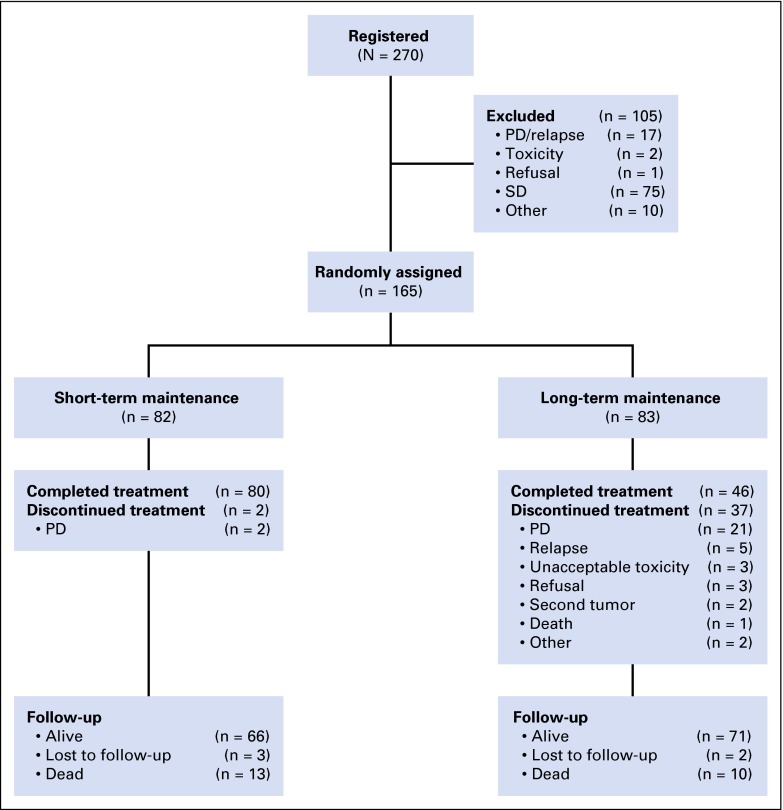
Between August 2004 and September 2007, 270 patients were enrolled from 26 centers in seven countries. Figure 1 shows the trial profile. Of 270 patients, 105 were not randomly assigned, primarily because they did not achieve at least a partial remission after induction. The overall response rate to induction was 62.8% (Table 1). One hundred sixty-five patients were randomly assigned, with 82 in the short-term maintenance arm and 83 in the long-term maintenance arm. The baseline characteristics of randomly assigned patients are included in Table 2. Tumor burden was not formally assessed according to standard criteria when patients entered the trial. More women than men were randomly assigned to the long-term maintenance arm (69% v 55%, respectively). More patients in the long-term than in the short-term maintenance arm had elevated lactate dehydrogenase (LDH) levels (23% v 7%, respectively) and presented with extranodal involvement (45% v 22%, respectively). Otherwise, the two groups were well balanced. Two thirds of the patients were treatment naïve. Approximately 10% had received previous anti-CD20 therapy. Only one patient had grade 3b FL. More than 75% of patients were classified as stages III and IV in both arms using the Ann Arbor Conference classification of disease stages. Nearly 70% of patients in both groups were scored as intermediate or high risk according to the Follicular Lymphoma International Prognostic Index risk score.
Fig 1.
Trial profile. PD, progressive disease; SD, stable disease.
Table 1.
Response to Induction Therapy
| Response | No. (%) of Patients (n = 261) |
|---|---|
| Complete response | 35 (13.4) |
| Unconfirmed complete response | 9 (3.4) |
| Partial response | 120 (46.0) |
| Stable disease | 78 (29.9) |
| Progressive disease | 19 (7.3) |
Table 2.
Patient Characteristics
| Characteristic | Short-Term Maintenance (n = 82) | Long-Term Maintenance (n = 83) |
|---|---|---|
| Sex | ||
| Female | 45 (55) | 57 (69) |
| Male | 37 (45) | 26 (31) |
| Median age, years (range) | 55 (34-81) | 57 (25-81) |
| Disease status | ||
| Untreated | 55 (67) | 58 (70) |
| Relapsed/progressed | 27 (33) | 24 (29) |
| Stable | 0 (0) | 1 (1) |
| Previous chemotherapy | 20 (24) | 21 (25) |
| Previous anti-CD20 | 9 (11) | 7 (8) |
| Previous radiotherapy | 7 (9) | 5 (6) |
| Grade | ||
| 1 and 2 | 69 (84) | 67 (81) |
| 3 | 5 (6) | 5 (6) |
| 3a | 5 (6) | 7 (9) |
| 3b | 0 (0) | 1 (1) |
| Ann Arbor stage | ||
| I and II | 19 (23) | 16 (19) |
| III | 33 (40) | 29 (35) |
| IV | 30 (37) | 37 (45) |
| FLIPI risk | ||
| Low | 26 (32) | 25 (31) |
| Intermediate | 35 (43) | 29 (36) |
| High | 20 (25) | 27 (33) |
| Elevated LDH | 6 (7) | 19 (23) |
| Nodal areas ≥ 4 | 45 (55) | 49 (60) |
| Extranodal sites | 18 (22) | 37 (45) |
| Bulky disease > 5 cm | 22 (27) | 23 (28) |
| B symptoms | 14 (17) | 12 (14) |
| Fever > 38°C | 5 (6) | 2 (2) |
| Drenching sweats | 11 (13) | 10 (12) |
| Weight loss | 4 (5) | 2 (2) |
NOTE. Data are given as No. (%) unless otherwise noted.
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase.
Two patients in the short-term maintenance arm did not complete the maintenance therapy because of progressive disease. Thirty-seven patients in the long-term arm discontinued maintenance therapy before 5 years, mainly as a result of progressive disease or relapse (n = 26). Unacceptable toxicity (n = 3), refusal (n = 3), secondary cancer (n = 2), death unrelated to lymphoma or lymphoma therapy (n = 1), and other (n = 2) were additional causes for early discontinuation in the long-term maintenance arm. The median number of rituximab administrations was four in the short-term maintenance arm and 30 in the long-term maintenance arm.
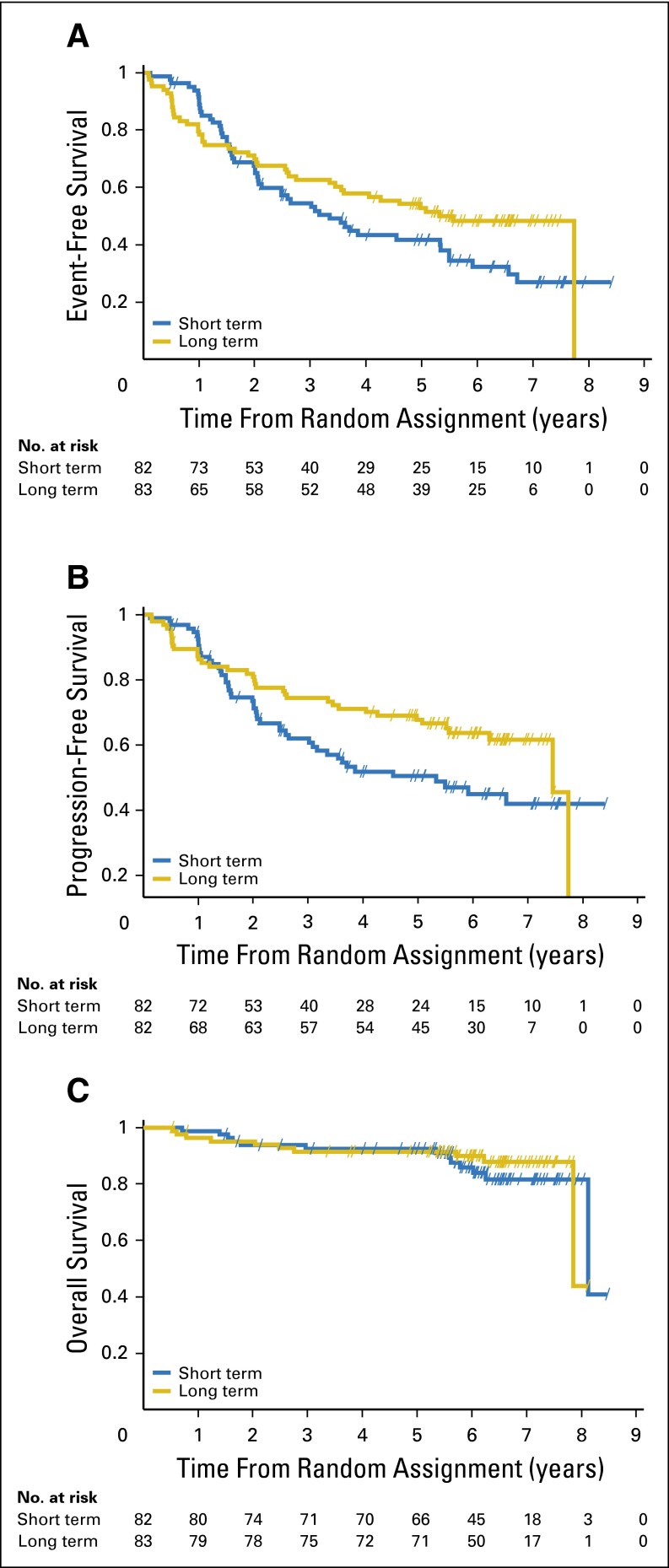
Ninety-five events were recorded, with 52 (63.4%) in the short-term arm versus 43 (51.8%) in the long-term arm (Table 3). The median EFS was 3.4 years (95% CI, 2.1 to 5.3) in the short-term arm and 5.3 years (95% CI, 3.5 to not available) in the long-term arm. Using the prespecified log-rank test, the primary end point of the trial was not met; the EFS difference was not statistically significant (log-rank test P = .14). We observed a difference in disease progression and relapse during the first 8 months after random assignment (three events in the short-term arm v 10 events in the long-term arm) when treatment in both arms was the same, with an early crossing of the EFS curves at 18 months (Fig 2A). When using the weighted log-rank test as proposed by Harrington and Fleming,9 with more weight placed on later time points to reflect interest in late events, a significant difference in EFS between the two arms was shown in favor of the long-term maintenance therapy (P = .015). Median PFS was significantly longer in the long-term arm compared with the short-term arm (7.4 [95% CI, 5.1 to not available] v 3.5 years [95% CI, 2.1 to 5.9], respectively; log-rank test, P = .04; Fig 2B). There was no significant difference in the observed best response and in OS (Fig 2C). Median OS from random assignment was approximately 8 years in both arms. After a median follow-up period of 6.4 years, 23 deaths were recorded, with 13 in the short-term arm and 10 in the long-term arm.
Table 3.
Events for Event-Free Survival
| Event | Short-Term Maintenance (n = 82) | Long-Term Maintenance (n = 83) |
|---|---|---|
| No. of events | 52 | 43 |
| Type of event, No. (%) | ||
| Death | 3 (5.8) | 2 (4.7) |
| PD or relapse | 46 (88.5) | 30 (69.8) |
| Secondary malignancy | 3 (5.8) | 6 (14.0) |
| Unacceptable toxicity | 0 (0) | 5* (11.6) |
Abbreviation: PD, progressive disease.
Including three patients who discontinued trial treatment as a result of unacceptable toxicity and two patients with grade 4 neutropenia that did not lead to permanent treatment discontinuation.
Fig 2.
Survival curves. (A) Event-free survival, (B) progression-free survival, and (C) overall survival.
More adverse events were observed in the long-term rituximab maintenance arm, mostly grades 1 and 2. At least one adverse event was reported in 41 patients (50%) in the short-term arm and in 63 patients (76%) in the long-term arm (P < .001). Seven grade 3 and grade 4 infections were reported in five patients in the long-term arm and one in the short-term arm (P = .21). Six subsequent malignancies developed in the short-term arm (Hodgkin lymphoma, n = 2; breast cancer, n = 2; prostate cancer, n = 1; and melanoma, n = 1) and eight in the long-term arm (breast cancer, n = 3; cutaneous basal cell carcinoma, n = 2; soft tissue sarcoma, n = 1; colorectal cancer, n = 1; and prostate cancer, n = 1). Maintenance treatment was discontinued as a result of unacceptable toxicity in three patients in the long-term maintenance arm and none in the short-term maintenance arm (P = .25). The only possible progressive, multifocal leukoencephalopathy occurred in a patient who was included in the trial without knowing of his HIV infection. We observed no reactivation of viral hepatitis.
The results of the secondary end points—IgG serum levels, lymphocyte subpopulations in the peripheral blood, prognostic value of baseline C-reactive protein, molecular remission, and pharmacoeconomic analysis—are discussed in the Appendix (online only).
DISCUSSION
In this study comparing short-term with long-term rituximab maintenance therapy, a significant difference in EFS, the primary end point, between two maintenance durations was not shown. We observed a difference in early events, favoring the short-term maintenance, in the first 8 months when the treatment in both arms was still identical. These events consisted mainly of disease progression and relapse (three in the short-term arm and 10 in the long-term arm). This led to an early separation of the survival curves favoring the short-term maintenance arm and to a later crossing of the survival curves. Patient characteristics at baseline showed more patients in the long-term arm than in the short-term arm with elevated serum LDH levels (23% v 7%, respectively) and extranodal involvement (45% v 22%, respectively). An elevated serum LDH level is a major adverse prognostic factor. The number of involved extranodal sites is also an unfavorable prognostic factor in FL.10 This imbalance may explain, at least in part, the difference in relapse or progression during the first 8 months of maintenance therapy.
However, a sensitivity analysis emphasizing interest in late events showed a statistically significant increase in EFS with long-term maintenance compared with the 8-month maintenance regimen. By using the weighted log-rank test, the difference in EFS was shown to be significant (P = .015). Long-term rituximab maintenance doubled the median PFS, a secondary end point. Of note, one half of the patients in the long-term maintenance arm were still in remission at 7 years after treatment with rituximab only.
There was no OS difference between the two groups. Longer follow-up will be needed to assess whether long-term rituximab maintenance has an impact on OS. Survival data are still being collected.
These data further substantiate the observation by us and others that the longer the duration of rituximab treatment, the longer the duration of remission.1,11,12 A systematic review and meta-analysis of randomized trials showed an improved OS only in patients with relapsed or refractory FL and not in previously untreated patients.5 Also, at least two randomized studies have shown that the time to the next nonrituximab treatment is the same, whether rituximab is administered continually as maintenance or intermittently, at every progression.11,13 The second strategy is probably more cost effective because it uses less than one half of the rituximab without apparently compromising survival.
There were more patients with at least one adverse event in the long-term rituximab maintenance arm than in the short-term arm (76% v 50%, respectively). Patients in the long-term arm also had clinical examinations every 2 months for a longer period of time. We observed seven infections of grades 3 or 4 in five patients in the long-term rituximab arm. Other studies using induction therapy with rituximab in combination with chemotherapy reported higher infection rates with rituximab maintenance therapy.4 Although long-term rituximab maintenance treatment led to a decrease in IgG serum levels in more than one half of patients as well as to profound B-cell depletion, this did not result in a relevant increase in grade 3 or 4 infections. In a small subset of patients, we showed that the profound B-cell depletion in the long-term maintenance arm improves approximately 1 year after the last rituximab infusion.
A systematic review compared schedule-related toxicities with maintenance rituximab in FL and mantle-cell lymphoma.14 Patients treated with rituximab alone during induction had fewer toxicities compared with those treated with rituximab plus chemotherapy during induction (12% v 35%, respectively; P = .031).
Our trial had several limitations. The study population was heterogeneous (treatment naïve, relapsed, stable, and chemotherapy resistant), patients with low and high tumor burden were eligible for the trial, and the need for therapy was at the discretion of the treating physician, allowing the inclusion of patients suitable for a watch-and-wait strategy. We have no data on how the maintenance treatment and the frequent visits to the outpatient clinic every 2 months over 5 years influenced quality of life.
In different studies, rituximab maintenance therapy was administered for 2 years.4,15 On the basis of our previous trial, we used 8 months of rituximab maintenance therapy as the control arm. The design of our trial does not allow any comparison of the 5-year versus the 2-year rituximab maintenance schedule. In conclusion, this trial of long-term rituximab maintenance did not show a significant difference in the primary end point of EFS, as assessed by the prespecified log-rank test. Long-term rituximab maintenance therapy significantly prolonged PFS, but this did not translate into an OS benefit. Long-term rituximab maintenance is associated with increased toxicity. Combining anti-CD20 antibodies with new immunomodulatory drugs, such as lenalidomide, the Bruton’s tyrosine kinase inhibitor ibrutinib, or the the PI3Kδ inhibitor idelalisib instead of chemotherapy are promising new treatment strategies.16 These future combinations will broaden the spectrum of therapeutic options in patients with FL, allowing the omission or delay of cytostatic chemotherapy.
Supplementary Material
Acknowledgment
We thank the entire Swiss Group for Clinical Cancer Research (SAKK) team and all the monitors, the Roche teams for their support, and the Data Safety Monitoring Committee members, A. Hagenbeek, MD, PhD, L. Trümper, MD, and W. Qian, PhD.
Appendix
Immunoglobulin G Serum Levels
Immunoglobulin G (IgG) serum levels were not available from all patients. At registration, IgG serum levels were the same in both arms (short-term maintenance median IgG, 9.1 g/L; range, 4.7 to 15.8; n = 73; long-term maintenance median IgG, 9.3 g/L; range, 1.2 to 29.4; n = 77). During rituximab maintenance therapy, IgG levels dropped below 5 g/L or below the locally defined lower limit of normal in 22% of patients in the short-term maintenance arm (n = 67) and in 41% of patients in the long-term maintenance arm (n = 78; P = .02). The proportion of patients with low IgG levels increased in the follow-up phase (38% in the short-term maintenance arm [n = 69] and 62% in the long-term maintenance arm [n = 47; P = .01]). There was no correlation between low IgG levels and grade 3 and 4 infections.
Lymphocyte Subpopulations in Peripheral Blood
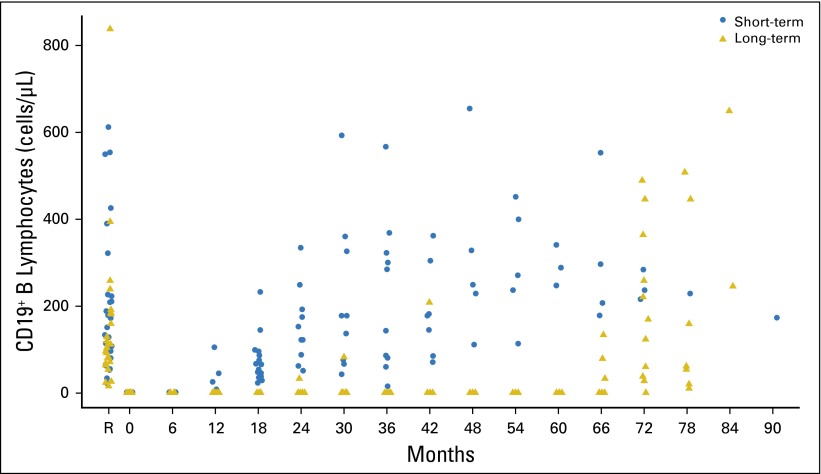
There were 37 and 36 patients in the short-term and long-term maintenance arms, respectively, for whom absolute counts of peripheral blood lymphocyte subpopulations were measured. Severe depletion of B lymphocytes was observed at random assignment (month 0) after induction and persisted during maintenance therapy (Appendix Fig A1). For a few patients in the short-term maintenance arm, B lymphocytes were detectable approximately 6 months after the last rituximab infusion, but they were detectable in all patients 1 year after the last rituximab infusion. In the long-term maintenance arm, B lymphocytes were detectable in a few patients (three of nine) 5.5 years after random assignment and in all 11 patients with data at 6 years. Thus, similar recovery times were observed after short-term and long-term maintenance therapy. No changes in the other lymphocyte subpopulations in the peripheral blood were observed.
Prognostic Value of Baseline C-Reactive Protein
There was no evidence that elevated baseline C-reactive protein (CRP) was associated with impaired prognosis in terms of event-free survival. However, in terms of progression-free survival, a significant effect of baseline CRP was detected: the risk of progression was higher for patients with elevated CRP than for those without elevated CRP (hazard ratio, 1.8; P = .035).
Molecular Remission
For molecular remission and duration of molecular remission, the sample size was too small to carry out a proper statistical analysis.
Pharmacoeconomic Analysis
The pharmacoeconomic analysis could not be performed because of difficulties in obtaining access to data from patient health insurance companies.
Fig A1.
CD19+ lymphocytes (cells/μL). Two outlier values (4,165 and 25,328) at registration are not shown on this graph. Month 0, random assignment; PD, progressive disease; R, registration.
Footnotes
Written on behalf of the Swiss Group for Clinical Cancer Research SAKK.
Supported by a scientific grant from Oncosuisse (OCS 01468-02-2004); the Swiss State Secretariat for Education, Research, and Innovation (SERI); and F. Hoffmann-La Roche, which provided rituximab for the maintenance therapy free of charge.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Christian Taverna, Emanuele Zucca, Michele Ghielmini
Administrative support: Christine Biaggi Rudolf, Corinne Rusterholz
Provision of study materials or patients: Christian Taverna, Giovanni Martinelli, Felicitas Hitz, Walter Mingrone, Thomas Pabst, Lidija Cevreska, Auro del Giglio, Anna Vanazzi, Daniele Laszlo, Johann Raats, Daniel Rauch, Daniel A. Vorobiof, Andreas Lohri, Ingmar A.F.M. Heijnen, Emanuele Zucca, Michele Ghielmini
Collection and assembly of data: All authors
Data analysis and interpretation: Christian Taverna, Stéphanie Rondeau, Ingmar A.F.M. Heijnen, Emanuele Zucca, Michele Ghielmini
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Rituximab Maintenance for a Maximum of 5 Years After Single-Agent Rituximab Induction in Follicular Lymphoma: Results of the Randomized Controlled Phase III Trial SAKK 35/03
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Christian Taverna
Honoraria: Roche, Celgene
Consulting or Advisory Role: Roche, Celgene
Giovanni Martinelli
No relationship to disclose
Felicitas Hitz
Honoraria: Roche, Mundipharma
Consulting or Advisory Role: Roche, Mundipharma
Walter Mingrone
Stock or Other Ownership: Amgen, Exelixis
Research Funding: Puma Biotechnology (Inst)
Travel, Accommodations, Expenses: Roche, Bayer AG, Janssen-Cilag
Thomas Pabst
No relationship to disclose
Lidija Cevreska
No relationship to disclose
Auro del Giglio
No relationship to disclose
Anna Vanazzi
No relationship to disclose
Daniele Laszlo
No relationship to disclose
Johann Raats
No relationship to disclose
Daniel Rauch
No relationship to disclose
Daniel A. Vorobiof
Consulting or Advisory Role: Amgen, Roche, Bristol-Myers Squibb
Andreas Lohri
No relationship to disclose
Christine Biaggi Rudolf
No relationship to disclose
Stéphanie Rondeau
No relationship to disclose
Corinne Rusterholz
No relationship to disclose
Ingmar A.F.M. Heijnen
No relationship to disclose
Emanuele Zucca
Research Funding: Roche (Inst), Mundipharma (Inst), Celgene (Inst), Janssen-Cilag (Inst)
Michele Ghielmini
Honoraria: Roche
Consulting or Advisory Role: Roche, Celgene, Janssen-Cilag, Mundipharma, Gilead Sciences
Research Funding: Roche (Inst)
REFERENCES
- 1.Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 2.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28:4480–4484. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 3.van Oers MH, Klasa R, Marcus RE, et al. Rituximab maintenance improves clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma in patients both with and without rituximab duri9g induction: Results of a prospective randomized phase 3 intergroup trial. Blood. 2006;108:3295–3301. doi: 10.1182/blood-2006-05-021113. [DOI] [PubMed] [Google Scholar]
- 4.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 5.Vidal L, Gafter-Gvili A, Salles G, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: An updated systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2011;103:1799–1806. doi: 10.1093/jnci/djr418. [DOI] [PubMed] [Google Scholar]
- 6.Hainsworth JD, Litchy S, Burris HA, III, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Horning SJ, Coiffier B, et al. NCI Sponsored International Working Group Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 8.Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 9.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika. 1982;69:553–566. [Google Scholar]
- 10.Federico M, Vitolo U, Zinzani PL, et al. Prognosis of follicular lymphoma: A predictive model based on a retrospective analysis of 987 cases. Intergruppo Italiano Linfomi. Blood. 2000;95:783–789. [PubMed] [Google Scholar]
- 11.Hainsworth JD, Litchy S, Shaffer DW, et al. Maximizing therapeutic benefit of rituximab: Maintenance therapy versus re-treatment at progression in patients with indolent non-Hodgkin’s lymphoma—A randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2005;23:1088–1095. doi: 10.1200/JCO.2005.12.191. [DOI] [PubMed] [Google Scholar]
- 12.Kimby E. Background for the positive position for rituximab maintenance. Ann Oncol. 2011;22(suppl 4):iv81–iv82. [Google Scholar]
- 13.Kahl BS, Hong F, Williams ME, et al. Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: Eastern Cooperative Oncology Group protocol e4402. J Clin Oncol. 2014;32:3096–3102. doi: 10.1200/JCO.2014.56.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nabhan C, Ollberding NJ, Villines D, et al. A systematic review of comparative schedule-related toxicities with maintenance rituximab in follicular and mantle cell lymphomas. Leuk Lymphoma. 2014;55:1288–1294. doi: 10.3109/10428194.2013.839787. [DOI] [PubMed] [Google Scholar]
- 15.Ardeshna KM, Qian W, Smith P, et al. Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: An open-label randomised phase 3 trial. Lancet Oncol. 2014;15:424–435. doi: 10.1016/S1470-2045(14)70027-0. [DOI] [PubMed] [Google Scholar]
- 16.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: An open-label, phase 2 trial. Lancet Oncol. 2014;15:1311–1318. doi: 10.1016/S1470-2045(14)70455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.