Abstract
Purpose
Compared with photon radiation (XRT), proton beam radiation therapy (PBRT) reduces dose to normal tissues, which may lead to better neurocognitive outcomes. We compared change in intelligence quotient (IQ) over time in pediatric patients with brain tumors treated with PBRT versus XRT.
Patients and Methods
IQ scores were available for 150 patients (60 had received XRT, 90 had received PBRT). Linear mixed models examined change in IQ over time since radiation therapy (RT) by RT group, controlling for demographic/clinical characteristics. Craniospinal and focal RT subgroups were also examined.
Results
In the PBRT group, no change in IQ over time was identified (P = .130), whereas in the XRT group, IQ declined by 1.1 points per year (P = .004). IQ slopes did not differ between groups (P = .509). IQ was lower in the XRT group (by 8.7 points) versus the PBRT group (P = .011). In the craniospinal subgroup, IQ remained stable in both the PBRT (P = .203) and XRT groups (P = .060), and IQ slopes did not differ (P = .890). IQ was lower in the XRT group (by 12.5 points) versus the PBRT group (P = .004). In the focal subgroup, IQ scores remained stable in the PBRT group (P = .401) but declined significantly in the XRT group by 1.57 points per year (P = .026). IQ slopes did not differ between groups (P = .342).
Conclusion
PBRT was not associated with IQ decline or impairment, yet IQ slopes did not differ between the PBRT and XRT groups. It remains unclear if PBRT results in clinically meaningful cognitive sparing that significantly exceeds that of modern XRT protocols. Additional long-term data are needed to fully understand the neurocognitive impact of PBRT in survivors of pediatric brain tumors.
INTRODUCTION
Cranial radiation therapy (RT) is associated with declines of two to four intelligence quotient (IQ) points per year among pediatric patients with brain tumors.1-3 Risk for intellectual decline increases with younger age at RT, higher RT dose, and larger RT fields.4-7 Advancements in photon RT (XRT), including intensity-modulated RT (IMRT) and three-dimensional conformal RT, provide more precise radiation dose delivery to target areas.8 Still, XRT entrance and exit doses irradiate surrounding healthy tissue.9 In contrast, proton beam RT (PBRT) deposits the maximum dose at the desired depth of tissue penetration, thereby depositing less entrance dose and no exit dose, and minimizing irradiation of healthy surrounding tissue.9 In this way, there is optimism that PBRT may protect against cognitive sequelae in pediatric patients with brain tumors.
Many publications herald the potential neuroprotective benefit PBRT offers pediatric patients with brain tumors.9-12 Yet, to our knowledge, only three published studies present actual cognitive outcomes data on PBRT-treated pediatric patients with brain tumors. In patients with medulloblastoma/primitive neuroectodermal tumors (PNETs) (n = 5), authors reported no difference in mean IQ between baseline and follow-up (1 to 3 years after PBRT).13 Similarly, no difference between baseline and follow-up IQ scores was identified in patients with ependymoma (n = 14), with 1 to 5 years follow-up after PBRT.14 Finally, a recent report found no difference between baseline and follow-up IQ scores (1 to 8 years after PBRT) in patients with low-grade glioma (n = 12).15 Considering the small samples and lack of comparison groups, these early reports leave much unknown regarding the neurocognitive impact of PBRT in pediatric patients with brain tumors.
In the current study, we examined change in IQ over time in pediatric patients with brain tumors treated with PBRT and compared the change in IQ over time between these patients and a similar XRT cohort. We hypothesized we would observe IQ decline in both treatment cohorts; however, we expected greater score decline in the XRT group due to the relative sparing of healthy brain tissue anticipated in the PBRT group. To our knowledge, this is the first study comparing neurocognitive outcomes between PBRT and XRT cohorts and provides the largest report to date on cognitive outcomes in pediatric patients with brain tumors treated with PBRT.
PATIENTS AND METHODS
Patients
With Institutional Review Board approval, records were examined for pediatric patients with brain tumors from Texas Children’s Hospital who were treated with PBRT from 2007 to 2012 at the MD Anderson Proton Therapy Center. We also examined records for a historical comparison group comprising pediatric patients with brain tumors treated with XRT from 2002 to 2007. This time frame reduced potential treatment selection bias likely after PBRT became available in 2007. Patients were 18 years old or younger at RT, with only a single course of RT. High-grade gliomas, brainstem gliomas, and atypical teratoid/rhabdoid tumors were excluded because they were not consistently treated with PBRT and are associated with limited long-term survival. All patients were English or Spanish speaking.
Measures
Eligible neurocognitive evaluation records included an IQ score derived from the age-appropriate version of one of the following tests: Leiter International Performance Scale16 (18.7%), Wechsler Scales of Intelligence17-19 (70.8%), or the Woodcock-Johnson Tests of Cognitive Ability20 (10.5%). IQ correlations across these tests are reported to range from 0.71 to 0.86.21,22 IQ scores provide a measure of global intellectual functioning as standard scores with a mean (M) of 100 and a standard deviation (SD) of 15.
Socioeconomic status (SES) was derived from the percent of households in poverty within the home ZIP code of a patient. Performance status was derived from the Lansky or Karnofsky scales (depending on patient age) measured at the first clinic visit after diagnosis, with scores ranging from zero (dead) to 100 (fully active, normal).23,24
Statistical Analyses
Summary statistics were stratified by treatment group and compared using independent, two-sample t tests, Wilcoxon rank-sum tests, or χ2 tests. A general linear mixed model compared change in IQ scores over time between treatment groups. The model included fixed effects for treatment group, time, and a group-by-time interaction term, as well as a random intercept and slope. Baseline characteristics significant at P < .05 in univariable analysis were included in a multiple regression model. Regression coefficients were assessed at P < .05. Because the theoretical neurocognitive sparing from PBRT is expected to differ if a patient receives focal RT versus craniospinal irradiation (CSI), we also examined CSI and focal RT groups in separate models.
RESULTS
We identified 205 eligible patients. Eligible neurocognitive evaluation records were available for 150 patients (XRT group, n = 60; PBRT group, n = 90), resulting in an overall inclusion rate of 73.2%. IQ scores were unavailable for deceased (n = 13) or blind patients (n = 7) or for reasons not specified in the medical record (n = 35). Patients without IQ scores did not differ significantly from those included in analyses by RT type, age at diagnosis, age at RT, sex, race, or histology. Serial neurocognitive surveillance is standard clinical procedure at this institution for pediatric patients with brain tumors. Most patients (86.0%) completed the same test battery across serial evaluations. Test type did not differ between groups. Patients in the XRT group had more evaluations (XRT: M, 3.6 evaluations, SD, 2.0; PBRT: M, 2.3 evaluations, SD, 1.2) and longer intervals between RT and last evaluation (XRT: M, 5.4 years, SD, 3.3; PBRT: M, 2.7 years, SD, 1.9), with P < .001 for both. This difference was expected because patients receiving XRT were off treatment longer than those receiving PBRT. The interval between RT and first evaluation did not differ between groups (XRT: M, 0.9 years, SD, 1.4; PBRT: M, 0.7 years, SD, 0.9). Individual patients had one to seven available IQ scores.
The XRT cohort was treated with contemporary RT techniques comparable to those of the PBRT cohort. XRT treatment plans included three-dimensional conformal (8.3%), IMRT (45.0%), and three-dimensional conformal plus IMRT tumor bed (TB)/margin boost (46.7%). The majority of PBRT-treated patients received passive scatter (90.0%) versus scanning beam proton therapy (10.0%). All CSI-treated patients received a TB + margin boost, with an additional posterior fossa (PF) boost administered to 0.0% of patients receiving PBRT and to 35.0% of patients receiving CSI XRT (median PF dose, 36.0 Gy; range, 34.2 to 45.0 Gy).
Patient characteristics are summarized in Table 1. The distribution of histologies differed between treatment groups (P = .002), with a greater percentage of medulloblastoma/PNET and ependymoma tumors in the XRT group. More patients in the XRT group received craniotomy (P = .046), ventriculoperitoneal (VP) shunt (P = .013), and Lansky/Karnofsky performance scores of 80 or lower at first clinic visit after diagnosis (P = .030). Total RT dose to the tumor was higher in the XRT group (P = .010). RT groups did not differ by sex, race or ethnicity, SES, tumor location (infratentorial/supratentorial), or history of CSI. Among CSI-treated patients, there was no difference between PBRT and XRT groups on median tests of CSI dose or TB boost dose (Table 1). For analysis, CSI-treated patients were categorized as receiving standard-dose (30.6 to 39.6 Gy) or reduced-dose CSI (18.0 to 25.2 Gy).
Table 1.
Patient Characteristics by RT Group (N = 150)
| Characteristic* | XRT Group | PBRT Group | P |
|---|---|---|---|
| Total | 60 (40.0) | 90 (60.0) | |
| Sex Female Male | 27 (45.0) 33 (55.0) | 36 (40.0) 54 (60.0) | .543 |
| Race/ethnicity White Black Hispanic/Latino Other race† | 22 (37.3) 8 (13.6) 25 (42.4) 4 (6.8) | 46 (52.3) 13 (14.8) 23 (26.1) 6 (6.8) | .204 |
| Histology Glioma Medulloblastoma/PNET Ependymoma Germ cell tumor Other‡ | 8 (13.3) 28 (46.7) 13 (21.7) 3 (5.0) 8 (13.3) | 20 (22.2) 34 (37.8) 4 (4.4) 17 (18.9) 15 (16.7) | .002 |
| Tumor location Infratentorial Supratentorial | 32 (54.2) 27 (45.8) | 36 (40.4) 53 (59.6) | .206 |
| CSI Yes No | 31 (51.7) 29 (48.3) | 51 (56.7) 39 (43.3) | .547 |
| Craniotomy Yes No | 58 (96.7) 2 (3.3) | 78 (86.7) 12 (13.3) | .046 |
| VP shunt Yes No | 30 (50.0) 30 (50.0) | 27 (30.0) 63 (70.0) | .013 |
| Performance score ≤ 80§ Yes No | 30 (57.7) 22 (42.3) | 32 (38.6) 51 (61.4) | .030 |
| Total RT dose to tumor, Gy, median (range) | 54.0 (30.6-59.4) | 54.0 (30.0-60.0) | .010‖ |
| CSI dose, Gy, median (range) | 23.4 (21.0-39.6) | 23.4 (21.0-39.6) | .911‖ |
| Tumor bed boost dose, Gy, median (range) | 55.8 (44.4-55.8) | 54.0 (30.0-55.8) | .152‖ |
| Age at diagnosis, years, mean ± SD (range) | 7.8 ± 4.0 (0.6-17.9) | 8.6 ± 4.3 (1.1-17.8) | .301 |
| Age at RT, years, mean ± SD (range) | 8.1 ± 3.9 (1.2-18.0) | 9.2 ± 4.1 (1.7-18.2) | .108 |
| Tumor diameter, cm, mean ± SD (range) | 4.6 ± 1.5 (1.5-8.1) | 4.2 ± 1.6 (1.3-9.6) | .155 |
| SES¶, mean ± SD (range) | 15.2 ± 11.3 (0.9-46.0) | 12.6 ± 11.6 (0.8-53.9) | .182 |
NOTE: Data are given as No. (%) unless otherwise indicated.
Abbreviations: CSI, craniospinal irradiation; PBRT, proton beam radiation therapy; PNET, primitive neuroectodermal tumor; RT, radiation therapy; SD, standard deviation; SES, socioeconomic status; VP, ventriculoperitoneal; XRT, photon radiation therapy.
Data were missing for race (n = 3), tumor location (n = 2), performance score (n = 15), and tumor diameter (n = 7). All patients who received CSI also received a tumor bed boost (XRT group, n = 31; PBRT group, n = 51).
The other race category includes Asian (n = 8) and American Indian (n = 2).
The other histology category includes craniopharyngioma (n = 13), choroid plexus carcinoma (n = 4), meningioma (n = 2), ganglioneuroblastoma (n = 1), neuronal glial (n = 1), epidermoid (n = 1), and undetermined (n = 1).
Performance score was obtained from the Lansky/Karnofsky rating at the first clinic visit after diagnosis.
║Nonparametric comparison was used rather than a means test because of the skewed distribution of values.
SES is reported as the percent of households in poverty within the home ZIP code of a patient.
In univariate regressions adjusting for time since RT (Appendix Table A1, online only), lower IQ scores were associated with XRT (P < .001), Black and Hispanic race or ethnicity (v white, P = .023 and P = .008, respectively), younger age at RT (P = .029), infratentorial tumor location (P = .024), medulloblastoma/PNET histology (P = .036), larger tumor diameter (P = .011), history of VP shunt (P = .001), Leiter IQ test (P = .001), a Lansky/Karnofsky performance score of 80 or lower at the first clinic visit after diagnosis (P = .001), lower SES (P = .004), longer time since RT (< .001), and history of PF boost (P = .007). Sex, history of craniotomy, total RT dose to the tumor, CSI, and CSI dose were not associated with IQ after adjusting for time since RT.
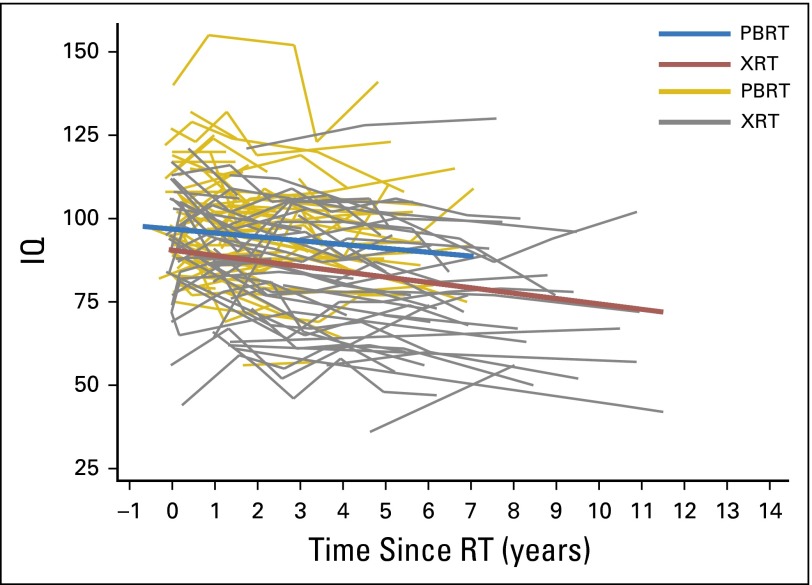
In our first multivariable model (Table 2), we examined if the PBRT group experienced significant IQ decline over time and if PBRT and XRT groups differed in IQ change over time. All variables found to be significantly associated with RT group or IQ in univariate analyses were included as covariates. Although nonsignificant in univariate analyses, CSI history was also included as a covariate, given the known cognitive risk associated with CSI. In this model, no statistically significant IQ decline was found in the PBRT group (95% CI, −1.6 to 0.2; P = .130). In contrast, the XRT group lost a statistically significant 1.1 IQ points per year, on average (95% CI, −1.8 to −0.4; P = .004). Even so, there was not a statistically significant difference in the change in IQ over time between the PBRT and XRT groups (−0.7 v −1.1 points per year, respectively; P = .509). Overall, IQ scores were significantly lower in the XRT group compared with the PBRT group by 8.7 points on average (P = .011). (Without a significant difference in IQ slopes between groups, this difference in IQ scores is best described as a persistent difference between groups rather than a difference at a distinct point in time, eg, baseline). Scores on the Woodcock-Johnson tests were higher than Leiter scores (P < .001). No other statistically significant associations were identified in the model. Exclusion of patients who received a PF boost did not change the overall results of this model (data not shown). Figure 1 presents regression lines of change in IQ over time since RT for both RT groups, on the basis of average values for all variables included in this model.
Table 2.
Linear Mixed Effects Model of IQ Change Over Time by RT Group (n = 123)*
| Parameter | Beta† | 95% CI | P |
|---|---|---|---|
| Time since RT, years | −0.7 | −1.6 to 0.2 | .130 |
| RT group (XRT) × time since RT, years | −0.4 | −1.6 to 0.8 | .509 |
| RT group, XRT | −8.7 | −15.5 to −2.0 | .011 |
| Age at RT, years | −0.1 | −0.9 to 0.8 | .905 |
| Total RT dose to tumor, Gy | −0.1 | −0.9 to 0.7 | .810 |
| CSI | 4.7 | −6.5 to 15.8 | .414 |
| VP shunt | −4.7 | −10.9 to 1.6 | .144 |
| Tumor location, supratentorial‡ | −1.3 | −10.2 to 7.7 | .778 |
| Tumor diameter, cm‡ | −1.3 | −3.5 to 0.8 | .227 |
| Histology | .276 | ||
| Glioma | — | ||
| Medulloblastoma/PNET | −7.7 | −21.4 to 6.0 | .271 |
| Ependymoma | 7.3 | −6.9 to 21.6 | .312 |
| Germ cell tumor | −2.6 | −16.7 to 11.5 | .716 |
| Other§ | 5.7 | −5.2 to 16.5 | .306 |
| Performance score ≤ 80‡║ | −6.7 | −13.9 to 0.4 | .063 |
| SES‡ | −18.3 | −46.1 to 9.5 | .197 |
| Race/ethnicityঠ| .146 | ||
| White | — | ||
| Black | −10.2 | −19.0 to -1.4 | .023# |
| Hispanic/Latino | −2.2 | −10.1 to 5.6 | .579 |
| Other | 0.5 | −11.0 to 12.0 | .935 |
| IQ test type | .000 | ||
| Leiter | — | ||
| Woodcock-Johnson | 12.1 | 5.2 to 19.1 | .001 |
| Wechsler | 2.1 | −3.6 to 7.8 | .465 |
Abbreviations: —, reference group; CSI, craniospinal irradiation; IQ, intelligence quotient; PNET, primitive neuroectodermal tumor; RT, radiation therapy; SES, socioeconomic status; VP, ventriculoperitoneal; XRT, photon radiation therapy.
Twenty-seven patients were excluded from the model because of missing data on covariates.
Beta = slope.
Data were missing for tumor location (n = 2), tumor diameter (n = 7), performance score (n = 15), SES (n = 1), and race (n = 3).
Other histology category includes craniopharyngioma (n = 13), choroid plexus carcinoma (n = 4), meningioma (n = 2), ganglioneuroblastoma (n = 1), neuronal glial (n = 1), epidermoid (n = 1), and undetermined (n = 1).
║Performance score was obtained from the Lansky/Karnofsky rating at the first clinic visit after diagnosis.
Other race category includes Asian (n = 8) and American Indian (n = 2).
Because the overall effect for race/ethnicity was not significant, the significant result for the black versus white contrast is not interpreted as a significant effect.
Fig 1.
Bold lines represent the regression lines of IQ change over time since RT for each RT group, on the basis of average values for all variables included in the model (Table 2). Fine lines represent unadjusted change in IQ over time for individual patients. IQ, intelligence quotient; PBRT, proton beam radiation therapy; RT, radiation therapy; XRT, photon radiation therapy.
In our second model (Table 3), we examined change in IQ over time by RT group (PBRT v XRT) for patients who received CSI. Statistically significant IQ decline was not found in either group (PBRT: P = .203; XRT: P = .060), and IQ slopes did not differ between PBRT and XRT groups (−0.8 v −0.9 points per year, respectively; P = .890). IQ scores were persistently lower in the XRT group by 12.5 points on average compared with the PBRT group (P = .004). Lower IQ scores were also significantly associated with lower Lansky/Karnofsky performance scores after diagnosis (P = .001). A significant association between race or ethnicity and IQ was identified (P < .001), with lower IQ scores among black patients and higher IQ scores among the other race or ethnicity category compared with white patients. The type of IQ test administered was again associated with IQ, with higher Woodcock-Johnson scores compared with Leiter scores (P < .001). No other significant associations were identified in this model. History of PF boost did not account for a significant amount of variance when added to this model, and the exclusion of patients who received a PF boost did not change the overall results of this model (data not shown).
Table 3.
Linear Mixed Effects Model of IQ Change Over Time by RT Group for CSI Only (n = 69)*
| Parameter | Beta† | 95% CI | P |
|---|---|---|---|
| Time since RT, years | −0.8 | −1.9 to 0.4 | .203 |
| RT group (XRT) × time since RT, years | −0.1 | −1.6 to 1.4 | .890 |
| RT group (XRT) | −12.5 | −21.1 to −3.9 | .004 |
| Age at RT, years | −0.5 | −1.6 to 0.6 | .381 |
| Total RT dose to tumor, Gy | 0.0 | −1.0 to 1.1 | .964 |
| VP shunt | −5.0 | −12.9 to 2.9 | .217 |
| Tumor location‡ | 2.7 | −9.9 to 15.2 | .680 |
| Tumor diameter, cm‡ | −1.6 | −4.4 to 1.1 | .251 |
| Histology | .608 | ||
| Glioma | — | ||
| Medulloblastoma/PNET | 0.8 | −34.3 to 35.8 | .966 |
| Germ cell tumor | 1.9 | −31.8 to 35.7 | .911 |
| Other§ | 14.8 | −22.9 to 52.4 | .441 |
| Performance score ≤ 80‡║ | −14.4 | −22.7 to −6.0 | .001 |
| SES‡ | −16.1 | −46.4 to 14.1 | .296 |
| Race/ethnicity‡ | < .001 | ||
| White | — | ||
| Black | −16.0 | −26.2 to −5.8 | .002 |
| Hispanic/Latino | 0.3 | −9.2 to 9.9 | .946 |
| Other¶ | 16.5 | 3.7 to 29.2 | .011 |
| IQ test type | < .001 | ||
| Leiter | — | ||
| Woodcock-Johnson | 13.4 | 6.1 to 20.7 | < .001 |
| Wechsler | 3.9 | −2.3 to 10.0 | .218 |
Abbreviations: —, reference group; CSI, craniospinal irradiation; IQ, intelligence quotient; PNET, primitive neuroectodermal tumor; RT, radiation therapy; SES, socioeconomic status; VP, ventriculoperitoneal; XRT, photon radiation therapy.
Of the patients who underwent CSI, 13 were excluded from the model because of missing data on covariates.
Beta = slope.
Data were missing for tumor location (n = 1), tumor diameter (n = 3), performance score (n = 7), SES (n = 1), and race/ethnicity (n = 2).
Other histology category includes choroid plexus carcinoma (n = 3), ganglioneuroblastoma (n = 1), epidermoid (n = 1), and undetermined (n = 1).
║Performance score was obtained from the Lansky/Karnofsky rating at the first clinic visit after diagnosis.
Other race category includes Asian (n = 5) and American Indian (n = 1).
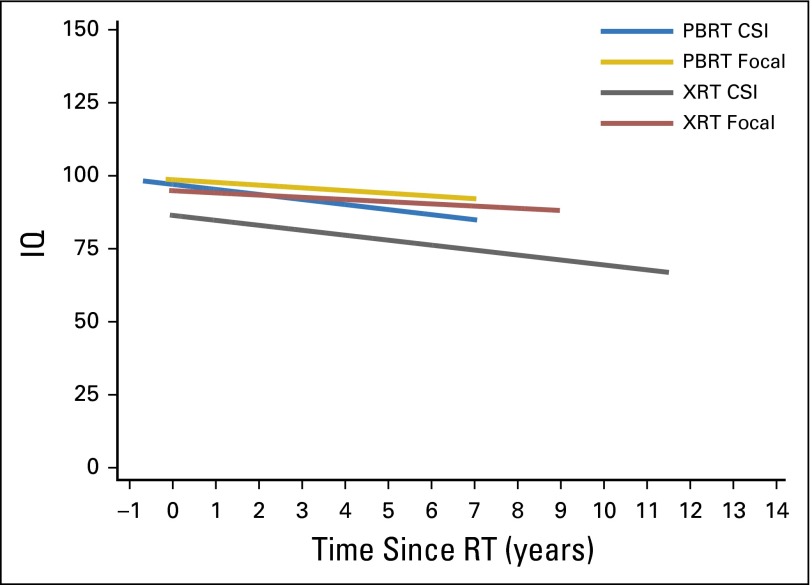
Our final model (Table 4) compared IQ change over time between PBRT and XRT groups for patients who received focal RT. Statistically significant IQ decline was not found in the PBRT group (95% CI, −2.0 to 0.8; P = .401). In contrast, a significant IQ decline of 1.6 points per year on average was observed in the XRT group (95% CI, −3.0 to −0.2; P = .026). Still, a significant difference in IQ slopes was not detected between PBRT and XRT groups (−0.6 v −1.6 points per year, respectively; P = .342). Lower SES was associated with lower IQ scores (P = .001). IQ scores derived from the Leiter International Performance Scale were lower than Wechsler scores (P = .031). No other significant associations were identified in this model. Figure 2 presents regression lines of change in IQ over time since RT by RT group and by CSI/focal RT history, on the basis of average values for all variables included in these models.
Table 4.
Linear Mixed Effects Model of IQ Change Over Time by RT Group for Focal RT Only (n = 54)*
| Parameter | Beta† | 95% CI | P |
|---|---|---|---|
| Time since RT, years | −0.6 | −2.0 to 0.8 | .401 |
| RT group (XRT) × time since RT, years | −1.0 | −3.0 to 1.0 | .342 |
| RT group (XRT) | −3.0 | −12.6 to 6.6 | .541 |
| Age at RT, years | −0.3 | −1.5 to 0.9 | .602 |
| Total RT dose to tumor, Gy | −0.5 | −1.6 to 0.6 | .360 |
| VP shunt | −1.5 | −10.3 to 7.2 | .730 |
| Tumor location‡ | 3.0 | −9.4 to 15.4 | .640 |
| Tumor diameter, cm‡ | −3.0 | −6.0 to 0.0 | .051 |
| Histology | .323 | ||
| Glioma | — | ||
| Medulloblastoma/PNET | −12.0 | −31.9 to 8.0 | .241 |
| Ependymoma | 6.4 | −10.0 to 22.8 | .446 |
| Germ cell tumor | −12.6 | −31.8 to 6.6 | .199 |
| Other§ | 0.3 | −10.6 to 11.2 | .955 |
| Performance score ≤ 80‡║ | 2.4 | −9.5 to 14.2 | .697 |
| SES‡ | −79.2 | −125.7 to −32.8 | .001 |
| Race/ethnicity‡ | .065 | ||
| White | — | ||
| Black | −1.5 | −14.4 to 11.5 | .825 |
| Hispanic/Latino | −1.6 | −13.6 to 10.3 | .789 |
| Other¶ | −26.5 | −45.9 to −7.1 | .007# |
| IQ test type | |||
| Leiter | — | ||
| Wechsler | −15.1 | −28.9 to −1.3 | .031 |
Abbreviations: —, reference group; IQ, intelligence quotient; PNET, primitive neuroectodermal tumor; RT, radiation therapy; SES, socioeconomic status; VP, ventriculoperitoneal; XRT, photon radiation therapy.
Fourteen focal patients were excluded from the model because of missing data on covariates.
Beta = slope.
Data were missing for tumor location (n = 1), tumor diameter (n = 4), performance score (n = 8), and race/ethnicity (n = 1).
Other histology category includes craniopharyngioma (n = 13), choroid plexus carcinoma (n = 1), meningioma (n = 2), and neuronal glial (n = 1).
║Performance score was obtained from the Lansky/Karnofsky rating at the first clinic visit after diagnosis.
Other race category includes Asian (n = 3) and American Indian (n = 1).
Because the overall effect for race/ethnicity was not significant, the significant result for the other versus white contrast is not interpreted as a significant effect.
Fig 2.
Regression lines of IQ change over time since RT for each RT group and by CSI/focal RT history, on the basis of average values for all variables included in the model (Tables 3 and 4). CSI, craniospinal irradiation; IQ, intelligence quotient; PBRT, proton beam radiation therapy; RT, radiation therapy; XRT, photon radiation therapy.
DISCUSSION
In the largest report of neurocognitive outcomes after treatment of pediatric brain tumors with PBRT, significant IQ decline was not identified in patients treated with PBRT. In contrast, IQ decline was observed in the XRT group, consistent with the preponderance of reports in the cognitive late-effects literature.1-3 Still, a significant difference in the change in IQ over time was not identified between the PBRT and XRT groups. As such, this study does not provide clear evidence that PBRT results in clinically meaningful sparing of global IQ significantly exceeding that of modern XRT protocols.
There are several reasonable explanations for the failure to detect a difference in IQ slopes between groups. First, our study may have lacked the power to detect an actual significant difference. The unadjusted difference in slopes was −0.3 IQ points per year (PBRT slope, −0.9; XRT slope, −1.2). Our current sample size only provided 11% power to detect a significant difference of this magnitude. A difference in slopes of 1.5 IQ points per year would be required to achieve 80% power with this sample size. Still, it is difficult to ascribe clinical meaningfulness to a difference in IQ change as small as that observed in this sample. Second, global IQ may not be a sensitive enough measure to detect neurocognitive change in patients treated with contemporary RT methods. Possibly, PBRT better preserves functioning in domains known to be particularly RT sensitive (ie, processing speed, executive functioning), but the magnitude of the sparing was masked by our exclusive examination of global IQ scores. Third, patients in the PBRT group more recently were off treatment and, thus, had a shorter follow-up interval available for study. The trajectories in Figure 1 seem to trend toward divergence over time; however, our sample may not have provided enough long-term follow-up data in both groups to achieve a significant difference. Finally, the groups may be equivalent in terms of the amount of IQ change resulting after RT. The neurocognitive sparing expected with PBRT may not be as substantial as previously postulated. Alternatively, modern XRT protocols may be so successful at limiting exposure to healthy surrounding brain tissue that patients treated since 2002 are not experiencing the extent of neurocognitive decline reported in previous studies,1-3 a possibility supported by the 1.1 points per year decline observed in the XRT group in our sample. Importantly, a recent report demonstrated the importance of boost volume in neurocognitive decline.25 Only 35% of our CSI-treated XRT cohort received a PF boost.
Notably, XRT was associated with persistently lower IQ versus PBRT in the total sample and the CSI subsample. Because this was not a randomized clinical trial, it cannot be assumed RT group alone (PBRT v XRT) resulted in the clinically meaningful nine-point difference observed between groups. Our models adjusted for all demographic and medical variables we identified as differing significantly between groups. Pre-RT differences may have existed between groups that we did not reliably identify. Furthermore, not all patients received true baseline evaluations, making it impossible to fully appreciate the neurocognitive profiles of both groups at the time of RT. Alternatively, PBRT may result in less early neurotoxicity, having a lasting effect on IQ and resulting in the persistent difference in IQ observed between groups. We are examining this possibility in a current, prospective multisite study.
The IQ difference between RT groups seems driven entirely by the CSI-treated subsample, in which the XRT group exhibited persistently lower IQ compared with the PBRT group. Yet, the change in IQ over time was similar between groups. The relative vulnerability of the XRT CSI subgroup is most apparent in Figure 2, in which the IQ trajectory of that subgroup remains well below the trajectories of all other RT subgroups. It seems the possible tissue-sparing benefit of PBRT is most apparent in the context of CSI (rather than focal). The reduced PBRT dose deposited beyond the boundaries of the TB boost may spare specific structures (ie, posterior hippocampus) from reaching a critical level of RT exposure that would result in clinically meaningful IQ change. The benefit of boost volume reduction on IQ has been demonstrated in an XRT sample.25 However, the lack of an IQ slope difference suggests the differential damage caused by XRT CSI occurs early and without a larger associated decline over time, unless the power to detect a true difference in slope was too low with this sample size. Alternatively, there may have been other unidentified systematic differences between groups in the CSI subsample not included in our modeling that better explain the lower IQ in the XRT group.
In contrast to CSI findings, no differences in IQ were found between PBRT and XRT groups in the focal RT subsample. Possibly, the heterogeneity of this subgroup (and, thus, differences in the extent and location of RT across patients) masked RT-related differences between groups. Furthermore, measurement of more RT-sensitive neurocognitive domains may have resulted in detectible differences. Alternatively, advanced XRT techniques may reduce RT exposure to surrounding healthy brain tissue to an extent comparable to the sparing expected with PBRT, making clinical outcomes indistinguishable between groups. Regarding the nonsignificant difference in IQ slopes between groups, it remains possible that a statistical difference was not detected because of the moderate sample size in this subanalysis. The unadjusted difference in slopes was −1.0 IQ points per year, which is clinically notable, if not statistically significant.
Study limitations must be considered. First, patients were not randomized to RT groups because of practical and ethical barriers preventing such randomized controlled trials at our institution and elsewhere. We attempted to reduce treatment selection bias in our sample by restricting our XRT cohort to patients treated before the availability of PBRT and took great care to adjust for relevant demographic and treatment variables that differed systematically between groups. Second, our power to detect a difference in IQ slope between groups was limited by our relatively small sample size. Third, our retrospective data collection presents methodological challenges, with 26.8% of eligible patients having no available IQ data. Furthermore, IQ scores were derived from three different tests (and different versions of those tests) across patients and, for a minority of patients, different tests across time points. Despite high correlations across tests,21,22 replication of our findings is needed using a consistent assessment battery across patients and time points to determine whether measurement issues impacted our ability to identify differences between groups over time. Finally, patients included in our sample received neurocognitive testing at different points in time after RT and with varying frequency, and the PBRT group had fewer IQ evaluations and shorter follow-up than the XRT group.
Despite these limitations, this study remains the largest comparison to date of IQ scores between pediatric patients with brain tumors treated with PBRT versus XRT. Overall, our findings suggest PBRT is not associated with significant IQ decline or impairment in survivors of pediatric brain tumors. The relative neurocognitive benefits of PBRT compared with XRT remain uncertain, but were not large or definitive in this sample. Until we acquire more long-term data, we will not fully understand the impact of PBRT on the neurocognitive trajectories of these patients. Currently, we are collecting data with larger samples and later follow-up, and shifting toward examining outcome differences in specific neurocognitive and functional domains on the basis of radiation doses received by specific brain regions rather than strictly the PBRT versus XRT IQ comparisons of the current study. Despite improvements in RT technology and regardless of the RT modality used, neuropsychological monitoring remains essential to the comprehensive care of patients with pediatric brain tumors and survivors after RT.
Appendix
Table A1.
Univariate Regressions With IQ Adjusted for Time Since RT
| Parameter | Beta* | 95% CI | P |
|---|---|---|---|
| RT group (XRT) | −12.0 | −17.3 to −6.7 | < .001 |
| Sex, male | −1.5 | −7.0 to 4.1 | .609 |
| Race/ethnicity† | .019 | ||
| White | — | ||
| Black | −9.6 | −17.9 to −1.3 | .023 |
| Hispanic/Latino | −8.4 | −14.6 to −2.2 | .008 |
| Other‡ | −0.3 | −11.4 to 10.8 | .959 |
| Age at RT, years | 0.7 | 0.1 to 1.4 | .029 |
| Tumor location, supratentorial† | 6.2 | 0.8 to 11.7 | .024 |
| Histology | .047 | ||
| Glioma | — | ||
| Medulloblastoma/PNET | −8.1 | −15.6 to −0.5 | .036 |
| Ependymoma | −0.4 | −10.5 to 9.8 | .945 |
| Germ cell tumor | 2.2 | −7.5 to 11.9 | .653 |
| Other§ | 0.2 | −9.1 to 9.5 | .969 |
| Tumor diameter, cm† | −2.3 | −4.1 to −0.5 | .011 |
| Craniotomy | 0.6 | −9.0 to 10.2 | .901 |
| VP shunt | −9.3 | −14.8 to −3.9 | .001 |
| Total RT dose to tumor, Gy | −0.4 | −1.0 to 0.2 | .192 |
| CSI | −5.0 | −10.5 to 0.5 | .073 |
| CSI dose ≥ 30.6 Gy | −1.7 | −9.6 to 6.1 | .664 |
| Posterior fossa boost | −15.0 | −25.8 to −4.1 | .007 |
| IQ test type | .001 | ||
| Leiter | — | ||
| Woodcock-Johnson | 9.9 | 4.0 to 15.8 | .001 |
| Wechsler | 2.9 | −1.7 to 7.5 | .211 |
| Performance score ≤ 80† | −9.6 | −15.2 to −3.9 | .001 |
| SES† | −34.4 | −58.0 to −10.8 | .004 |
| Time since RT, years | −1.1 | −1.6 to −0.6 | < .001 |
Abbreviations: —, reference group; CI, confidence interval; CSI, craniospinal irradiation; IQ, intelligence quotient; PNET, primitive neuroectodermal tumor; RT, radiation therapy; SES, socioeconomic status; VP, ventriculoperitoneal; XRT, photon radiation therapy.
Beta = slope.
Data were missing for race (n = 3), tumor location (n = 2), tumor diameter (n = 7), performance score (n = 15), and SES (n = 1).
Other race category includes Asian (n = 8) and American Indian (n = 2).
Other histology category includes craniopharyngioma (n = 13), choroid plexus carcinoma (n = 4), meningioma (n = 2), ganglioneuroblastoma (n = 1), neuronal glial (n = 1), epidermoid (n = 1), and undetermined (n = 1).
Footnotes
See accompanying editorial on page 1024
This work was supported, in part, by the Texas Children’s Hospital Pediatric Pilot Research Fund and by the National Cancer Institute Grants K07CA157923 and R01CA187202 (principal investigator: L.S.K.).
Presented in part at the 49th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013; the 16th International Symposium on Pediatric Neuro-Oncology, Singapore, June 28-July 2; and the 56th Annual Meeting of the American Society for Radiation Oncology, San Francisco, CA, September 14–17, 2014.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Lisa S. Kahalley, M. Douglas Ris, David R. Grosshans, M. Fatih Okcu, Arnold C. Paulino, Murali Chintagumpala, Bartlett D. Moore, Danielle Guffey, Charles G. Minard, Anita Mahajan
Provision of study materials or patients: M. Fatih Okcu, Murali Chintagumpala
Collection and assembly of data: Lisa S. Kahalley, M. Douglas Ris, David R. Grosshans, Heather S. Stancel, Anita Mahajan
Data analysis and interpretation: Lisa S. Kahalley, M. Douglas Ris, David R. Grosshans, M. Fatih Okcu, Arnold C. Paulino, Murali Chintagumpala, Bartlett D. Moore, Danielle Guffey, Charles G. Minard, Anita Mahajan
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparing Intelligence Quotient Change after Treatment with Proton Versus Photon Radiation Therapy for Pediatric Brain Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Lisa S. Kahalley
No relationship to disclose
M. Douglas Ris
No relationship to disclose
David R. Grosshans
No relationship to disclose
M. Fatih Okcu
No relationship to disclose
Arnold C. Paulino
Employment: MD Anderson Cancer Center
Patents, Royalties, Other Intellectual Property: Royalty from Elsevier for textbook
Murali Chintagumpala
No relationship to disclose
Bartlett D. Moore
No relationship to disclose
Danielle Guffey
No relationship to disclose
Charles G. Minard
No relationship to disclose
Heather H. Stancel
No relationship to disclose
Anita Mahajan
Consulting or Advisory Role: Bristol-Myers Squibb (I), Exilixis (I), Novartis (I), Pfizer (I)
Research Funding: Exilixis (I), Pfizer (I), Novartis (I)
Patents, Royalties, Other Intellectual Property: Springer
Travel, Accommodations, Expenses: Elekta, IBA
REFERENCES
- 1.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: A longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 2.Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children’s Cancer Group study. J Clin Oncol. 2001;19:3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 3.Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17:548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 4.Merchant TE, Conklin HM, Wu S, et al. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: Prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 6.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman KE, Yock TI. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24:1387–1396. doi: 10.1177/0883073809342275. [DOI] [PubMed] [Google Scholar]
- 8.Kun LE, Beltran C. Radiation therapy for children: Evolving technologies in the era of ALARA. Pediatr Radiol. 2009;39:S65–S70. doi: 10.1007/s00247-008-1098-0. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 9.Yock TI, Tarbell NJ. Technology insight: Proton beam radiotherapy for treatment in pediatric brain tumors. Nat Clin Pract Oncol. 2004;1:97–103. doi: 10.1038/ncponc0090. [DOI] [PubMed] [Google Scholar]
- 10.Merchant TE. Proton beam therapy in pediatric oncology. Cancer J. 2009;15:298–305. doi: 10.1097/PPO.0b013e3181b6d4b7. [DOI] [PubMed] [Google Scholar]
- 11.Wilson VC, McDonough J, Tochner Z. Proton beam irradiation in pediatric oncology: An overview. J Pediatr Hematol Oncol. 2005;27:444–448. doi: 10.1097/01.mph.0000174030.55485.54. [DOI] [PubMed] [Google Scholar]
- 12.Kirsch DG, Tarbell NJ. New technologies in radiation therapy for pediatric brain tumors: The rationale for proton radiation therapy. Pediatr Blood Cancer. 2004;42:461–464. doi: 10.1002/pbc.10471. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez RB, Sethi R, Depauw N, et al. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: Outcomes for very young children treated with upfront chemotherapy. Int J Radiat Oncol Biol Phys. 2013;87:120–126. doi: 10.1016/j.ijrobp.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: Clinical outcomes for 70 patients. Neuro-oncol. 2013;15:1552–1559. doi: 10.1093/neuonc/not121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberger BA, Pulsifer MB, Ebb DH, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89:1060–1068. doi: 10.1016/j.ijrobp.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 16.Roid GH, Miller LJ, Pomplun M, et al. Leiter International Performance Scale. (ed. 3). Wood Dale, IL,: Stoelting Company; 2013. [Google Scholar]
- 17.Wechsler D. Wechsler Adult Intelligence Scale. (ed. 4). San Antonio, TX, NCS: Pearson; 2008. [Google Scholar]
- 18.Wechsler D. Wechsler Intelligence Scale for Children. (ed. 4). San Antonio, TX,: The Psychological Corporation; 2003. [Google Scholar]
- 19.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. (ed. 3). San Antonio, TX: NCS Pearson; 2002. [Google Scholar]
- 20.Woodcock RW, McGrew KS, Mather N: Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL,: The Riverside Publishing Company; 2001. [Google Scholar]
- 21.McGrew KS, Woodcock RW: Technical Manual Woodcock-Johnson III. Itasca, IL,: The Riverside Publishing Company; 2001. [Google Scholar]
- 22.Roid GH, Miller LJ: Leiter International Performance Scale-Revised: Examiner’s Manual. Wood Dale, IL, Stoelting: Company; 1997. [Google Scholar]
- 23.Lansky SB, List MA, Lansky LL, et al. The measurement of performance in childhood cancer patients. Cancer. 1987;60:1651–1656. doi: 10.1002/1097-0142(19871001)60:7<1651::aid-cncr2820600738>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York, NY,: Columbia University Press; 1949. The clinical evaluation of chemotherapeutic agents in cancer, , p 191. [Google Scholar]
- 25.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32:1760–1768. doi: 10.1200/JCO.2013.52.3290. [DOI] [PubMed] [Google Scholar]