Abstract
Purpose
Progressive malignancy is the leading cause of death after allogeneic hematopoietic stem-cell transplantation (alloHSCT). After alloHSCT, B-cell malignancies often are treated with unmanipulated donor lymphocyte infusions (DLIs) from the transplant donor. DLIs frequently are not effective at eradicating malignancy and often cause graft-versus-host disease, a potentially lethal immune response against normal recipient tissues.
Methods
We conducted a clinical trial of allogeneic T cells genetically engineered to express a chimeric antigen receptor (CAR) targeting the B-cell antigen CD19. Patients with B-cell malignancies that had progressed after alloHSCT received a single infusion of CAR T cells. No chemotherapy or other therapies were administered. The T cells were obtained from each recipient’s alloHSCT donor.
Results
Eight of 20 treated patients obtained remission, which included six complete remissions (CRs) and two partial remissions. The response rate was highest for acute lymphoblastic leukemia, with four of five patients obtaining minimal residual disease–negative CR. Responses also occurred in chronic lymphocytic leukemia and lymphoma. The longest ongoing CR was more than 30 months in a patient with chronic lymphocytic leukemia. New-onset acute graft-versus-host disease after CAR T-cell infusion developed in none of the patients. Toxicities included fever, tachycardia, and hypotension. Peak blood CAR T-cell levels were higher in patients who obtained remissions than in those who did not. Programmed cell death protein-1 expression was significantly elevated on CAR T cells after infusion. Presence of blood B cells before CAR T-cell infusion was associated with higher postinfusion CAR T-cell levels.
Conclusion
Allogeneic anti-CD19 CAR T cells can effectively treat B-cell malignancies that progress after alloHSCT. The findings point toward a future when antigen-specific T-cell therapies will play a central role in alloHSCT.
INTRODUCTION
Allogeneic hematopoietic stem-cell transplantation (alloHSCT) cures some patients with advanced B-cell malignancies; however, many patients never enter complete remission (CR) after alloHSCT, and many who do enter CR have a relapse.1,2 Progressive malignancy is the leading cause of death after alloHSCT.3 Patients with relapsing acute lymphoblastic leukemia (ALL) after alloHSCT have a median survival of 5.5 months,4 and diffuse large–B-cell lymphoma (DLBCL) that persists despite alloHSCT also carries a poor prognosis.5,6
Donor lymphocyte infusions (DLIs) of unmanipulated allogeneic lymphocytes from the transplant donor are commonly used to treat B-cell malignancies after alloHSCT.2,6 Remissions after DLIs are more likely with indolent lymphomas and chronic lymphocytic leukemia (CLL) than with ALL and DLBCL.2,6,7 ALL is particularly resistant to DLIs, with reported CR rates of 0% to 20%.6-8 Graft-versus-host disease (GVHD) is damage to normal recipient tissues caused by allogeneic immune responses after alloHSCT.2 Clinically significant acute GVHD (aGVHD) develops in approximately one-third of patients who receive DLIs, and GVHD is the main cause of the 6% to 11% treatment-related mortality rate from DLIs.6,9 Improved treatments for B-cell malignancies that progress after alloHSCT are needed.
Chimeric antigen receptors (CARs) are fusion proteins that contain an antigen recognition moiety and a T-cell activation domain.10-13 T cells genetically modified to express anti-CD19 CARs (CAR19) have specific cytotoxic effects against CD19+ target cells.14-16 Autologous CAR19 T cells have produced remissions in previously treated patients with B-cell malignancies.11,17-23 Preliminary results with donor-derived CAR19 T cells have been reported.24,25 We report mature clinical results and an extensive immunologic analysis of 20 patients who received alloHSCT treated on a clinical trial of donor-derived allogeneic CAR19 T cells.
METHODS
Study Design and Participants
This phase I dose escalation trial was carried out by the Experimental Transplantation and Immunology Branch of the US National Cancer Institute. Patients were treated between August 2010 and February 2015. The study protocol was approved by the National Cancer Institute institutional review board and by the US Food and Drug Administration. Patients gave informed consent in compliance with the Declaration of Helsinki.
We enrolled adult patients with measurable CD19+ B-cell malignancies. Patients had previously undergone HLA-matched sibling (sibling) or unrelated donor (URD) alloHSCT. URD cells were acquired through the National Marrow Donor Program. Uniform CD19 expression on malignant cells by immunohistochemistry or flow cytometry was required. Except for patients with ALL or DLBCL, at least one prior DLI was required for enrollment. Immunosuppressive drugs, which included systemic corticosteroids above physiologic dosing, were not allowed within 4 weeks before CAR19 T-cell infusion. Chemotherapy and antibody therapies were required to be stopped by 2 weeks before CAR19 T-cell infusion, and staging was always carried out more than 2 weeks after the last therapy before CAR T-cell infusions. Patients with evidence of aGVHD of greater than grade I26 and patients with evidence of chronic GVHD greater than mild global score were excluded.27 An Eastern Cooperative Oncology Group performance status of 2 or less and essentially normal major organ function were required.
Cell Production and Administration
The CAR used in this work included a murine single-chain variable fragment antigen-recognition domain, a CD28 costimulatory domain, and a CD3ζ T-cell activation domain.14 Peripheral blood mononuclear cells used to produce CAR T cells were provided by the patient’s transplant donor. Cell culture and gamma-retroviral transductions were performed as described in the Data Supplement.20,24 CAR T-cell production took 8 days. Enzyme-linked immunosorbent assays; quantitative polymerase chain reaction; and flow cytometry, which included a CAR-specific antibody provided by L. Cooper,28 are described in the Data Supplement.
Patients received a single dose of CAR T cells; no other treatments were administered on the protocol. Separate dose escalations were conducted for recipients of URD transplants and those of sibling transplants (Data Supplement). Each dose level included a range of possible cell doses. Dose escalation followed a standard 3 + 3 design.
Toxicity Grading and Response Staging
Toxicity was graded by the Common Terminology Criteria for Adverse Events version 4.02. In general, grade 4 toxicities attributable to CAR19 T cells were considered dose-limiting toxicities, with the exception of pre-existing cytopenias and B-cell depletion.
Malignancy response assessments for lymphoma and CLL were as previously specified.29,30 Criteria for a CR for ALL were 5% lymphoblasts or less on bone marrow aspirate, no extramedullary leukemia, blood platelet count of 100,000/μL or more, and blood neutrophil count of 1,000/μL or more. Minimal residual disease (MRD)–negative CR required less than 0.01% ALL by multicolor flow cytometry of bone marrow.
RESULTS
Patient Characteristics
Twenty patients with B-cell malignancies were treated (Table 1). Six patients provided informed consent for the protocol but did not receive the CAR T-cell infusion (Data Supplement). Preliminary results of the first 10 patients have been previously reported.24 The protocol treatment consisted of a single infusion of CAR19 T cells obtained from the allogeneic transplant donor. Thirteen sibling and seven URD recipients were treated (Table 1). Patient 5 had received three prior alloHSCTs. Patients 7 and 15 each received two prior alloHSCTs; all other patients had received one prior alloHSCT. Patients had received a median of four post-transplant lines of therapy before enrollment; at least 2 months elapsed between the most recent standard DLI and CAR19 T-cell infusion (Data Supplement). A median of 100% of recipient’s endogenous T cells were of donor origin at the time of study enrollment (Data Supplement). All patients had measurable malignancy at the time of CAR19 T-cell infusion. An important contrast between this study and most prior CAR T-cell studies was that chemotherapy was not administered before CAR19 T-cell infusion, so most patients were not depleted of endogenous T cells and natural killer cells at the time of CAR19 T-cell infusion (Table 1).
Table 1.
Patient Characteristics
| Patient No. | Age (Years) | Sex | Type of Transplant | Malignancy | Malignancy Status Immediately Before CAR T-Cell Infusion | Blood B-Lymphocyte Count Immediately Before CAR T-Cell Infusion (Cells/μL)* | Blood NK-Cell Count Immediately Before CAR T-Cell Infusion (Cells/μL)† | Blood T-Cell Count Immediately Before CAR T-Cell Infusion (Cells/μL)‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | URD 10/10 HLA match | CLL | PD | 286 | 81 | 3,873 |
| 2 | 44 | M | Sibling | DLBCL | PD | 7 | 53 | 394 |
| 3 | 55 | M | Sibling | CLL | SD | 4 | 42 | 1,954 |
| 4 | 49 | M | Sibling | DLBCL | SD | 0 | 177 | 323 |
| 5 | 44 | F | URD 10/10 HLA match | CLL | PD | 319 | 192 | 3,362 |
| 6 | 48 | M | Sibling | MCL | SD | 1 | 97 | 1,176 |
| 7 | 48 | M | URD 10/10 HLA match | CLL | PD | 1,805 | 164 | 1,981 |
| 8 | 63 | F | Sibling | MCL | PR | 1 | 86 | 354 |
| 9 | 57 | M | URD 10/10 HLA match | MCL | SD | 0 | 169 | 479 |
| 10 | 50 | M | Sibling | MCL | PD | 83 | 101 | 712 |
| 11 | 59 | F | URD 9/10 HLA match | CLL | PD | 3,372 | 275 | 1,216 |
| 12 | 68 | F | Sibling | ALL Ph+ | Relapse | 0 | 38 | 184 |
| 13 | 61 | F | Sibling | MCL | PR | 1 | 133 | 2,024 |
| 14 | 32 | M | Sibling | ALL Ph-neg | PD | 1 | 148 | 370 |
| 15 | 20 | F | Sibling | ALL Ph-neg | PD | 127 | 79 | 867 |
| 16 | 25 | F | Sibling | ALL Ph-neg | PD | 0 | 15 | 291 |
| 17 | 46 | F | Sibling | DLBCL | PD | 2 | 26 | 2,171 |
| 18 | 54 | M | Sibling | DLBCL | PD | 325 | 209 | 809 |
| 19 | 47 | F | URD 10/10 HLA match | FL transformed to DLBCL | PD | 0 | 64 | 22 |
| 20 | 25 | M | URD 9/10 HLA match | ALL Ph-neg | Relapse | 1,109 | 269 | 1,766 |
Abbreviations: ALL Ph+, Philadelphia chromosome-positive acute lymphoblastic leukemia; ALL Ph-neg, Philadelphia chromosome-negative acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large–B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; NK, natural killer; PD, progressive disease; PR, partial remission; SD, stable disease; Sibling, HLA-matched sibling donor; URD, unrelated donor.
Normal range of blood B-lymphocyte (CD19+) count is 81 to 493/μL.
Normal range of blood NK cells (CD3−, CD56+) is 109 to 607/μL.
Normal range of blood T (CD3+) cells is 615 to 2,348/μL.
Cell Characteristics, Response Rates, and Toxicity
A median of 67.3% of all infused T cells expressed CAR19, and the median CD8:CD4 ratio of the infused CAR-positive T cells was 1.2 (Table 2). Most of the toxicities the patients experienced (eg, fever, tachycardia, hypotension) have been observed on other trials of CAR T cells and are consistent with cytokine release syndrome as described.19-22,31 Grade 3 and 4 toxicities for each patient are listed in Table 2. An increase in serum creatine kinase occurred in two patients; increased creatine kinase was associated with muscle pain in both and weakness in one. A complete lack of new-onset aGVHD after CAR19 T-cell infusion occurred, despite 14 of the 20 patients having had a history of GVHD at some time after their most recent alloHSCT before enrollment on the CAR19 T-cell trial. GVHD occurred in only two patients on the trial. Mild chronic ocular GVHD developed in patient 5 approximately 2 years after CAR19 T-cell infusion, which was long after all CAR19 T cells had disappeared from her blood. Patient 17 had slowly worsening chronic GVHD still meeting criteria for mild27 involvement before her CAR19 T-cell infusion; the GVHD continued to slowly worsen after her CAR19 T-cell infusion. Cell doses were progressively increased, with different dose levels for sibling and URD recipients; maximum tolerated doses were not reached. Overall, eight of the 20 patients obtained either CR or partial remission (PR; Table 2).
Table 2.
Infused Cell Characteristics and Patient Outcomes
| Patient No. | Total T Cells Infused/kg* | Infused T Cells Expressing Anti-CD19 CAR† (%) | Anti-CD19 CAR-Expressing T Cells Infused/kg† | CD8:CD4 Ratio of Infused CAR-Positive T Cells | Malignancy Response at Last Follow-Up (Interval From Infusion to Last Follow-Up in Months) | Grade 3 and 4 Toxicities‡ |
|---|---|---|---|---|---|---|
| 1 | 1 × 106 | 39.7 | 0.4 × 106 | 1.7 | SD (3) | Tumor lysis syndrome, fatigue, cardiac ventricular dysfunction, fever, tachycardia, troponin increase, anemia, neutropenia |
| 2 | 2 × 106 | 36.4 | 0.7 × 106 | 1.1 | SD (1) | None |
| 3 | 4 × 106 | 60.8 | 2.4 × 106 | 1.0 | PD | Pneumonitis, hypoxia, dyspnea, fever, hypophosphatemia |
| 4 | 4 × 106 | 55.3 | 2.2 × 106 | 3.5 | SD (31+) | None |
| 5 | 1.5 × 106 | 64.5 | 1.0 × 106 | 1.2 | CR (30+) | Hypotension |
| 6 | 7 × 106 | 65.8 | 4.6 × 106 | 2.6 | SD (3) | None |
| 7 | 1 × 106 | 68.9 | 0.7 × 106 | 0.6 | PD | None |
| 8 | 7 × 106 | 55.5 | 3.9 × 106 | 0.7 | SD (24+) | None |
| 9 | 4 × 106 | 54.6 | 2.2 × 106 | 1.4 | PR (3) | None |
| 10 | 10 × 106 | 78.4 | 7.8 × 106 | 1.1 | SD (2) | Hypotension, headache |
| 11 | 5 × 106 | 62.4 | 3.1 × 106 | 5.1 | PR (18+) | Fever, anemia, thrombocytopenia, neutropenia, hypophosphatemia |
| 12 | 7 × 106 | 74.2 | 5.2 × 106 | 0.9 | MRD-negative CR (16+) | Fever, hypophosphatemia, hypokalemia, diarrhea, headache, neutropenia |
| 13 | 10 × 106 | 71.4 | 7.1 × 106 | 0.2 | SD (9) | None |
| 14 | 10 × 106 | 69.7 | 7.0 × 106 | 1.8 | MRD-negative CR (5) | Fever, sinus tachycardia, hypotension, anemia, thrombocytopenia, neutropenia |
| 15 | 10 × 106 | 68.8 | 6.9 × 106 | 1.3 | MRD-negative CR (3) | Sinus tachycardia, hypotension, hypoxia, anemia, thrombocytopenia, febrile neutropenia, left ventricular systolic dysfunction, alkaline phosphatase increased, ALT increased, AST increased, total bilirubin increased, CPK increased, hypophosphatemia, myositis, nausea, aPTT prolonged |
| 16 | 7 × 106 | 79.7 | 5.6 × 106 | 1.2 | PD | Fever, sinus tachycardia, hypotension, hypoxia, increased bilirubin, hypophosphatemia, headache, nausea |
| 17 | 10 × 106 | 81.5 | 8.2 × 106 | 0.5 | CR (6+) | Sinus tachycardia, anemia, neutropenia, AST increased, ALT increased |
| 18 | 10 × 106 | 30.8 | 3.1 × 106 | 0.6 | SD (2) | None |
| 19 | 5 × 106 | 86.3 | 4.3 × 106 | 0.3 | PD | Fever, sinus tachycardia, hypotension, bilirubin increased, thrombocytopenia, neutropenia, sinus bradycardia, pain, hypophosphatemia, death from acidosis due to progressive lymphoma, hypercalcemia from lymphoma |
| 20 | 5 × 106 | 84.8 | 4.2 × 106 | 3.1 | MRD-negative CR (3+)§ | Fever, hypotension, dyspnea, CPK increased, AST elevated, hyponatremia, hypophosphatemia, thrombocytopenia, neutropenia, aPTT prolonged, colitis (likely infectious) |
Abbreviations: aPTT, activated partial thromboplastin time; CAR, chimeric antigen receptor; CPK, creatine phosphokinase; CR, complete remission; MRD, minimal residual disease; PD, progressive disease; PR, partial remission; SD, stable disease.
Total T cells refers to the total number of T cells administered, which includes CAR-expressing and CAR-negative T cells.
The number of anti-CD19 CAR-expressing T cells administered was determined by flow cytometry staining for the CAR as described in Methods.
All grade 3 and 4 toxicities during the 4 weeks after the CAR T-cell infusion are listed.
Patient 20 underwent a second alloHSCT 3.5 months after CAR19 T-cell infusion while in MRD-negative CR.
Rapid Elimination of CLL Cells After CAR19 T-Cell Infusion
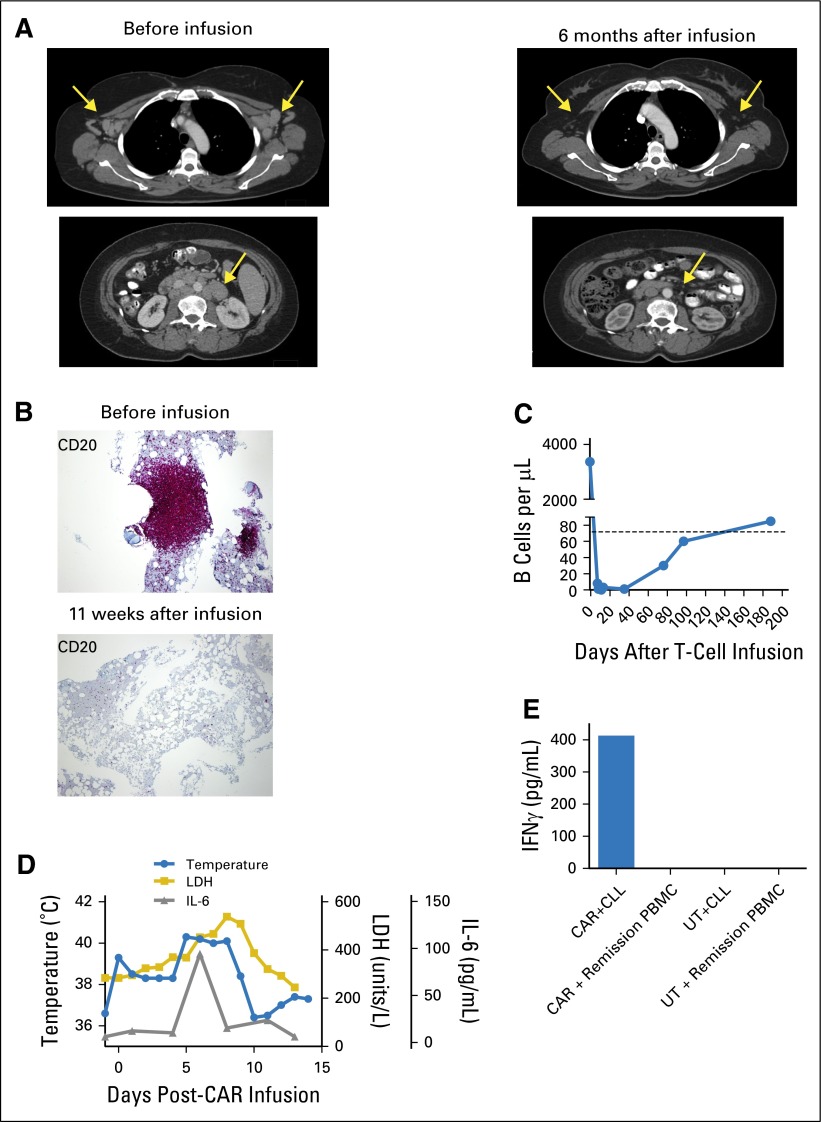
The course of patient 11 illustrates many common findings in patients who receive CAR19 T cells. Patient 11 had CLL that persisted despite a URD transplant and four standard DLIs. Her last standard DLI of 5 × 107 CD3+ T cells/kg was administered 4 months before her CAR19 T-cell infusion and was followed by progressive CLL. At the time of the CAR T-cell infusion, her blood B-lymphocyte count was elevated due to CLL; blood T-cell and natural killer cell counts were normal (Table 1). After infusion of 5 × 106 CAR19 T cells/kg, which was a T-cell dose 10-fold lower than her last standard DLI dose, a rapid elimination of CLL cells from the lymph nodes, blood, and bone marrow occurred (Fig 1A-1C). Blood B lymphocytes, which were predominantly CLL cells, dropped from 3,372 to 0/μL over the 11 days after CAR19 T-cell infusion (Fig 1C). Such rapid responses are not characteristic of the antimalignancy responses that occur after standard DLIs, which usually occur over several weeks.7 During the time that CLL was being eliminated, Patient 11 experienced fevers, and her serum lactate dehydrogenase and interleukin-6 levels increased (Fig 1D). After a 9-day in vitro culture period with CLL cells from patient 11, CAR-transduced donor T cells but not untransduced donor T cells recognized the CLL cells as demonstrated by specific interferon gamma production (Fig 1E).
Fig 1.
Rapid reduction of chronic lymphocytic leukemia (CLL) in lymph nodes, bone marrow, and blood after infusion of unrelated donor anti-CD19 chimeric antigen receptor (CAR19) T cells. (A) Computerized axial tomography scans demonstrate elimination of adenopathy (arrows) after CAR19 T-cell infusion. (B) CD20 immunohistochemical staining of bone marrow demonstrates eradication of bone marrow CLL (violet mass) after CAR19 T-cell infusion. (C) Before CAR19 T-cell infusion, patient 11 had a blood CD19+ B-cell count of 3,372 cells/μL (normal B-cell count, 81 to 493/μL). The B-cell count was elevated because of a large burden of CD19+ CLL cells. On day 11 after CAR19 T-cells infusion, the blood B-cell count was 0 cells/μL, which indicates a complete elimination of both CLL and normal B cells from the blood. Polyclonal B cells recovered to normal levels by 188 days after CAR19 T-cell infusion. (D) During the first 10 days after CAR19 T-cell infusion, serum lactate dehydrogenase (LDH), serum interleukin-6 (IL-6), and temperature were elevated. (E) Specific recognition of CLL cells by CAR19 T cells was demonstrated in vitro. T cells from patient 11’s unrelated donor were either transduced with the CAR19 vector or left untransduced. Both CAR19 T cells and untransduced donor T cells were then cultured in vitro with the patient’s CLL cells for 9 days to simulate in vivo exposure to CLL cells. After this culture period, the CAR19 T cells (CAR) recognized the patient’s CLL as shown by interferon γ (IFNγ) production in an enzyme-linked immunosorbent assay, whereas the untransduced T cells (UT) failed to recognize the CLL. Neither the CAR19 nor the untransduced donor T cells produced IFNγ in response to remission peripheral blood mononuclear cells (PBMCs) obtained from patient 11 after CAR19 T-cell infusion when CLL cells and normal B cells were absent from the blood.
Allogeneic CAR19 T Cells Were Highly Effective at Inducing Remission of ALL
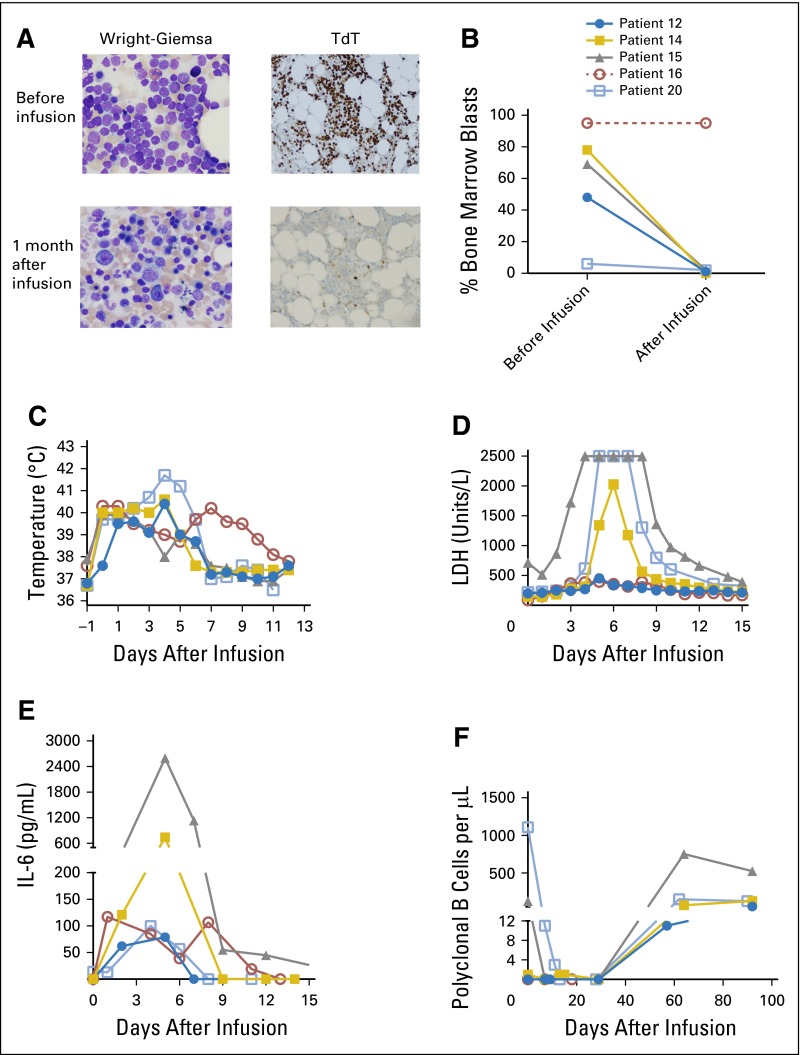
Four of five patients with ALL with elevated bone marrow blast counts of up to 78% obtained MRD-negative CR, with subsequent recovery of normal hematopoiesis after CAR19 T-cell infusion (Fig 2A and 2B). All the patients with ALL experienced fevers and elevated serum lactate dehydrogenase and interleukin-6 levels in the first 2 weeks after CAR19 T-cell infusion (Fig 2C-2E). All four patients with ALL who obtained CR had recovery of normal polyclonal B cells (Fig 2F).
Fig 2.
Allogeneic anti-CD19 chimeric antigen receptor (CAR19) T cells are highly effective against acute lymphoblastic leukemia (ALL). (A) Patient 14 obtained a minimal residual disease–negative complete remission and had reconstitution of normal hematopoiesis after CAR19 T-cell infusion. Wright-Giemsa and terminal deoxynucleotidyl transferase (TdT) stains are shown. (B) The bone marrow blast percentages from before CAR19 T-cell infusion and 1 month after the infusion are shown. Blast percentages were determined morphologically on bone marrow aspirates. ALL in patient 16 did not respond to the CAR T-cell infusion as determined by peripheral blood counts, and no follow-up bone marrow biopsy was performed, so this patient’s postinfusion bone marrow blast percentage is reported as 95%, which was the same as that before infusion. Because no postinfusion bone marrow biopsy was performed on patient 16, the line for this patient is dashed. The symbols for each patient are defined in (B). The same symbols are used for each patient in (C), (D), (E), and (F). (C) Fever developed in all the patients with ALL after infusion of CAR19 T cells. The maximum daily temperature of each patient is shown. (D) Serum lactase dehydrogenase (LDH) levels increased in all patients with ALL during the time when they were experiencing clinical toxicity. The maximum measurable serum LDH concentration was 2,500 units/L, and patients 15 and 20 both had serum LDH concentrations of greater than 2,500 units/L on multiple days. (E) Serum interleukin-6 (IL-6) levels increased after CAR19 T-cell infusion. (F) In patients 15 and 20, normal polyclonal B cells were eradicated after CAR19 T-cell infusion. The other patients with ALL already had very low blood B-cell levels before CAR19 T-cell infusion. Recovery of polyclonal B cells was detected in four of five patients with ALL. Patient 16 did not recover polyclonal B cells by 29 days after infusion, and after that time, was not evaluable for B-cell recovery.
Rapid Remission of DLBCL After CAR19 T-Cell Infusion
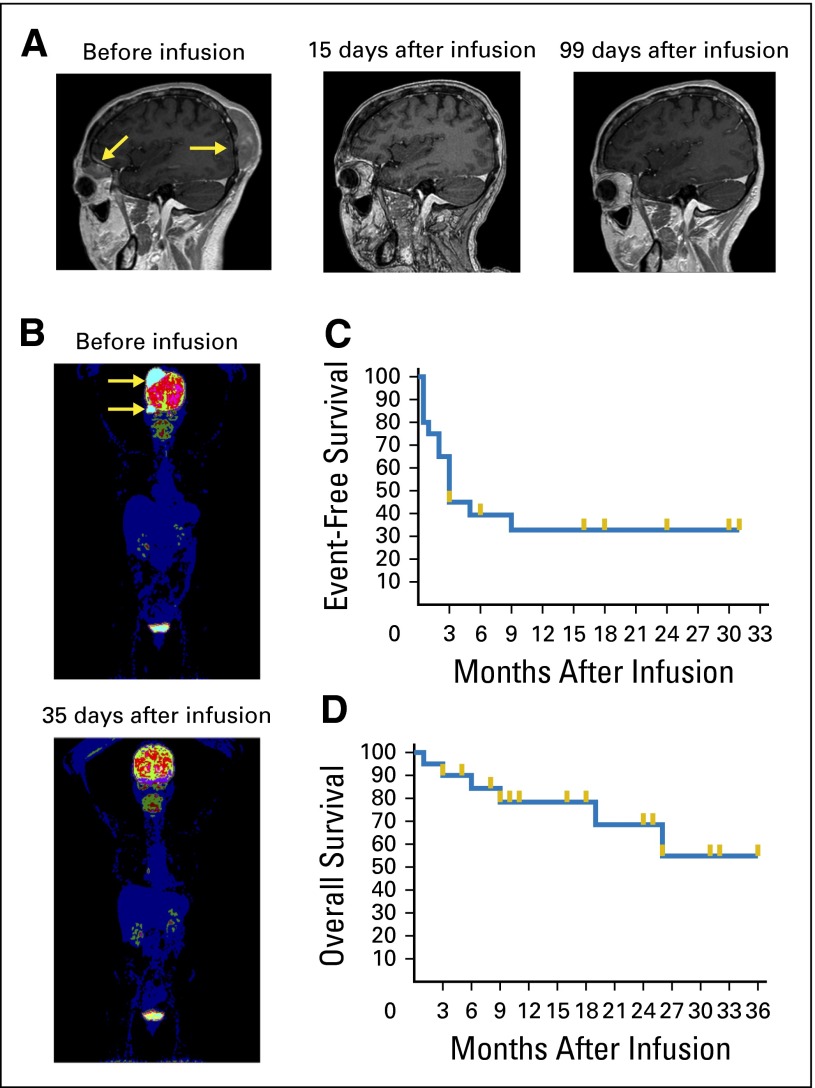
Patient 17 had DLBCL that persisted after alloHSCT. After infusion of donor-derived CAR19 T cells, regression of large lymphoma masses occurred with striking rapidity (Fig 3A and 3B); a large head mass that was easily palpable before the CAR T-cell infusion was not palpable 5 days after the infusion. The 6-month event-free survival of all 20 treated patients was 39% (Fig 3C); the overall survival is shown in Figure 3D.
Fig 3.
Rapid eradication of diffuse large–B-cell lymphoma after allogeneic CAR19 T-cell infusion. (A) Magnetic resonance images show rapid complete elimination of lymphoma masses (arrows). Images are from before treatment, 15 days after anti-CD19 chimeric antigen receptor (CAR19) T-cell infusion, and 99 days after CAR19 T-cell infusion. The remission continued over 6 months after infusion. (B) Positron emission tomography scan shows metabolically active lymphoma before CAR19 T-cell infusion (arrows). Thirty-five days after the CAR19 T-cell infusion, the scan showed no evidence of lymphoma. (C) The event-free survival of all 20 patients treated on the study is shown. Events were defined as progression of malignancy, receipt of any antimalignancy therapy after CAR19 T-cell infusion, or death from any cause. (D) The overall survival of all 20 patients treated on the study is shown. For (C) and (D), survival fractions were calculated by Kaplan-Meier method, and gold lines indicate censored patients.
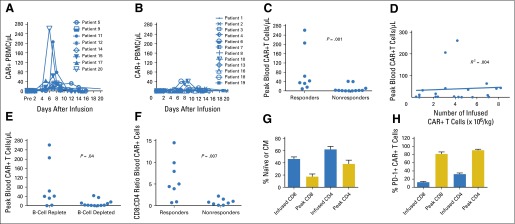
Patients Obtaining Remission Had Higher Peak Blood CAR19 Levels
CAR19 T cells were measured by quantitative polymerase chain reaction (Fig 4A and 4B). CAR T cells in the blood followed one of two patterns: They were undetectable or detectable at very-low levels or they rapidly rose to a peak within 2 weeks after infusion followed by a rapid decline. Patients who did not obtain an antimalignancy response of CR or PR were more likely to have low or undetectable blood CAR T-cell levels (Fig 4B). The peak levels of blood CAR19 T cells were significantly higher in patients with an antimalignancy response of CR or PR compared with patients with stable disease or progressive disease (Fig 4C). Of note, peak blood CAR19 T-cell levels were not predicted by the number of CAR19 T cells infused (Fig 4D), which indicates that factors other than T-cell dose must contribute to the determination of peak CAR19 T-cell numbers. Because CAR19 T cells proliferate when exposed to CD19+ target cells,14 we hypothesized that exposure to CD19+ cells in vivo increases proliferation and persistence of CAR19 T cells. In agreement with this hypothesis, peak blood CAR T-cell levels were higher in patients with blood B-lymphocyte counts above the lower limit of normal immediately before infusion of CAR19 T cells than in those with below-normal blood B-lymphocyte counts immediately before CAR19 T-cell infusion (Fig 4E).
Fig 4.
High peak blood levels of anti-CD19 chimeric antigen receptor (CAR19) T cells were associated with remissions of malignancy. (A) The absolute numbers of blood T cells that contained the CAR gene were assessed by quantitative polymerase chain reaction (qPCR) before infusion and at multiple time points after infusion. Results for patients who obtained a response of either a complete remission (CR) or a partial remission (PR) are shown. (B) The absolute numbers of T cells that contained the CAR gene were assessed by qPCR for all patients with malignancies that did not respond to CAR19 T cells. Lack of response was defined as a response of stable disease (SD) or progressive disease (PD). (C) The peak numbers of blood CAR-positive (CAR+) T cells were higher in responders (patients obtaining CR or PR) than in nonresponders (patients with SD or PD). CAR+ T cells were measured by qPCR. P = .001 by Mann-Whitney test. (D) Linear regression analysis of the number of infused CAR+ T cells per kilogram for each patient versus the peak number of blood CAR+ T cells for each patient was performed. Blood CAR+ T cells were measured by qPCR. The peak CAR+ T-cell level was not predicted by the number of infused CAR+ T cells (R2 = 0.004). (E) Patients with blood B-lymphocyte counts above the lower limit of normal before CAR19 T-cell infusion (B-cell replete) had higher peak blood CAR+ T-cell levels than patients with low blood B-lymphocyte counts before CAR19 T-cell infusion (B-cell depleted). CAR+ T cells were measured by qPCR. The normal range for blood B lymphocytes is 81 to 493/mL. B lymphocytes were defined as CD19+ lymphocytes, which included both normal lymphocytes and chronic lymphocytic leukemia lymphocytes. The median blood B-cell count for B-cell–replete patients was 322/μL. The median blood B-cell count for B-cell–depleted patients was 1/μL. The groups were compared by Mann-Whitney test. For (C), (D), and (E), analysis was performed on all 20 treated patients. (F) Flow cytometry staining with a monoclonal antibody specific for CAR19 was performed. Cells were also stained for CD3, CD4, and CD8. The CD8:CD4 ratios of CD3+ CAR+ cells were calculated. Flow cytometry was performed on peripheral blood mononuclear cells (PBMCs) obtained 5 to 14 days after CAR T-cell infusion during the time of each patient’s peak CAR19 blood level. The fraction of CAR19 T cells that were CD8+ was higher for patients with responses of CR or PR (responders) than for patients with outcomes of SD or PD (nonresponders). The groups were compared by Mann-Whitney test. (G) Flow cytometry was performed on a sample of the infused T cells or on PBMCs from the time of each patient’s peak CAR19 T-cell level between 5 and 14 days after infusion. For this analysis, naïve T cells were defined as cells with a CD45RA+ C-C chemokine receptor type 7–positive (CCR7+) phenotype, and central memory (CM) T cells were defined as cells with a CD45RA− CCR7+ phenotype. A substantial fraction of the CAR+ T cells had a naïve or CM phenotype at the time of infusion. For both CD8+ and CD4+ T cells, the fraction of CAR-expressing T cells with a naïve or CM phenotype decreased between the time of infusion and the time of peak blood levels of CAR19 T cells. The mean and SEM are shown for each category. The Wilcoxon matched pairs signed rank test was used to compare the fraction of naïve plus CM cells among the infused CAR-expressing T cells to the fraction of naïve plus CM cells among CAR-expressing T cells from the time of peak blood CAR T-cell levels (for both CD8+ and CD4+ T cells, P < .001). (H) Flow cytometry to detect programmed cell death protein-1 (PD-1) was performed on a sample of the infused T cells or on PBMCs from the time of peak CAR19 levels. The fraction of both CD8+ and CD4+ CAR+ T cells expressing PD-1 increased between the time of infusion and the time of peak blood CAR19 T-cell levels. The mean and SEM are shown for each category. By the Wilcoxon matched pairs signed rank test, P < .001 for both the CD8 and the CD4 comparisons. For (F), (G), and (H), analyses were performed on all 16 patients with detectable blood CAR19 T cells and available blood samples.
Characterization of Blood CAR19 T Cells
The median ratio of CD8+ to CD4+ CAR19 T cells in the blood during the time of peak in vivo CAR19 T-cell levels was 1.3. The CD8:CD4 CAR19 T-cell ratio was higher in patients who obtained either CR or PR after infusion than in those who did not obtain a remission (Fig 4F). Based on expression of CD45RA and C-C chemokine receptor type 7 (CCR7), T cells can be divided into four subsets: naïve, central memory (CM), effector memory, and effector memory RA.32 Naïve and CM T cells are less differentiated and have greater proliferative capacity than the other two T-cell subsets.32 A large fraction of the infused CAR19 cells had phenotypes of less-differentiated naïve and CM T cells (Fig 4G). After infusion, the fraction of CAR19 T cells with naïve or CM phenotypes decreased as the fraction of T cells with more-differentiated effector memory and effector memory RA phenotypes increased (Fig 4G; Data Supplement). The programmed cell death protein-1 (PD-1) is an inhibitory receptor expressed on T cells.33 A dramatic increase in the fraction of CAR19 T cells that expressed PD-1 occurred between the time of infusion and the time of peak CAR19 blood levels (Fig 4H). As well, PD-1 expression was higher on blood CAR19-expressing T cells than on blood CAR19-negative T cells (Data Supplement).
DISCUSSION
The graft-versus-malignancy (GVM) effect of HLA-matched alloHSCT and standard DLIs is caused mainly by donor lymphocyte targeting of allogeneic antigens that differ between the donor and recipient.34,35 These allogeneic immune responses do not specifically target malignant cells and often cause GVHD, which damages normal recipient tissues.2,9 We aimed to enhance the GVM potency of allogeneic T cells without worsening GVHD by genetically engineering allogeneic T cells to specifically target the B-cell antigen CD19. We have demonstrated remissions of B-cell malignancies in patients who did not obtain remissions after standard DLIs, and patients receiving CAR19 T cells obtained CR of ALL and DLBC, malignancies that generally have low CR rates after standard DLIs.5-8
In contrast to standard DLIs,6,9 new-onset aGVHD after infusion of CAR19 T cells developed in none of the patients. One factor that might explain this lack of GVHD is the limited persistence of the CAR19 T cells because aGVHD takes a median of 4 weeks to develop after standard DLIs,7,9 whereas the persistence of CAR19 T cells was generally limited to fewer than 4 weeks (Fig 4A and 4B). T-cell doses administered on this trial ranged from 106 to 107/kg; these modest doses are another possible explanation for the lack of GVHD. With standard DLIs, a threshold dose of 107 T cells/kg has been suggested, above which clinically significant GVHD becomes more likely in HLA-matched sibling alloHSCT.6,36 Genetic modification of T cells to specifically target CD19 makes each CAR19 T cell a more potent killer of malignancy compared with unmanipulated T cells. The increased antimalignancy potency of CAR19 T cells allowed small doses of T cells to eradicate malignancy without causing GVHD; therefore, this work demonstrates a solution to the central problem of alloHSCT, the separation of GVM from GVHD.
Substantial evidence indicates that depletion of recipient lymphocytes enhances the antitumor activity of adoptively transferred T cells.37,38 Because patients on our protocol did not receive chemotherapy before CAR T-cell infusion, most were not depleted of lymphocytes at the time of the infusion; therefore, their remissions demonstrated that prior lymphocyte depletion is not an absolute requirement for the antimalignancy activity of CAR19 T cells. In other studies, administration of autologous CAR19 T cells after chemotherapy achieved higher response rates for CLL and lymphoma than those reported here.20,22 This higher response rate could be attributable to increased T-cell activity in patients with depleted lymphocytes, with a possible contribution by direct antimalignancy activity of chemotherapy. Lymphocyte-depleting chemotherapy was not included in the current protocol due to concern that introduction of CAR T cells into a recipient with depleted lymphocytes might cause severe GVHD. Lack of chemotherapy in this protocol allowed a clear interpretation of antimalignancy responses of CAR19 T cells without the confounding direct antimalignancy activity of chemotherapy.
Use of normal donor T cells, which contained large percentages of naïve and CM T cells (Fig 4G), might have contributed to the ability of allogeneic CAR19 T cells to cause remissions of malignancy in the absence of recipient lymphocyte depletion. Allogeneic responses, when CAR T cells target allogeneic antigens through their natural HLA-restricted T-cell receptors, might have made CAR19 T cells less dependent on recipient lymphocyte depletion by contributing to cell proliferation and antimalignancy activity of the CAR19 T cells.
Although allogeneic T-cell responses might contribute to the persistence and antimalignancy activity of CAR19 T cells, several observations from this study indicate that allogeneic responses were not the main cause of the remissions. GVHD is strongly linked to GVM after standard DLIs,6,9 but new-onset aGVHD developed in none of the patients receiving CAR19 T cells. Many of the patients who obtained remission after CAR19 T-cell infusions did not obtain remission after standard DLIs that contained higher T-cell doses, which demonstrates that in some cases, CAR19 T-cell infusion is superior to standard DLI at eradicating malignancy. In many patients, onset of remission occurred within 10 days after CAR19 T-cell infusion. This was not consistent with a major contribution from allogeneic T-cell response because most remissions caused by standard DLIs occur over several weeks.7 Finally, there was no statistically significant difference in peak blood CAR19 T-cell levels between sibling and URD donor-recipient pairs, despite a generally greater allogeneic antigen disparity with URD donor-recipient pairs compared with sibling donor-recipient pairs (Data Supplement).9 In other trials, patients with ALL after alloHSCT were treated with T cells collected from the patients with ALL, with results similar to the current study.21,39 These findings show that in some patients, T cells can be collected from patients after allogeneic transplant to generate CAR19 T cells.
The finding that remission was more likely in patients with higher peak blood levels of CAR19 T cells (Fig 4C) indicates that increasing the peak blood levels of CAR19 T cells in vivo is an important goal for future research. Patients with normal or above-normal blood B-lymphocyte levels had higher peak blood CAR19 T-cell levels (Fig 4E), and we observed that many patients with a low malignancy burden and low normal blood B-cell level did not obtain remission of malignancy after CAR19 T-cell infusion. These observations indicate that endogenous CD19+ cells might have enhanced proliferation of CAR19 T cells and suggest that CD19+ cellular vaccines might enhance CAR19 T-cell proliferation in patients with low levels of endogenous CD19+ cells. Another possible avenue of improvement is by administering PD-1 antagonists after CAR19 T-cell infusion because of the high levels of PD-1 expression on CAR19 T cells at the time of peak blood CAR19 T-cell levels (Fig 4H).40
We envision a promising future when CAR T-cell therapy will be commonly used in transplant regimens to specifically target malignancy-associated antigens. CAR T cells could be administered as planned infusions along with or soon after stem-cell infusions. Genetically targeted T cells will be an integral part of allogeneic transplant protocols to separate GVM from GVHD.
Supplementary Material
Acknowledgment
We thank Monica Cho for assistance with processing clinical specimens. We also thank the attending physicians, fellows, nurses, and other staff of the Clinical Center, National Institutes of Health, intensive care unit for expert patient care.
Footnotes
Supported by intramural funding of the Center for Cancer Research, National Cancer Institute. This project has been funded in part with federal funds from the National Cancer Institute, under contract HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer N. Brudno, Daniel H. Fowler, Steven Z. Pavletic, Dennis D. Hickstein, Steven A. Rosenberg, Ronald E. Gress, James N. Kochenderfer
Administrative support: Steven A. Rosenberg
Provision of study materials or patients: Robert P.T. Somerville, Daniel H. Fowler, Andre Goy, Steven A. Rosenberg
Collection and assembly of data: Jennifer N. Brudno, Robert P.T. Somerville, Jeremy J. Rose, Tangying L. Lu, Alexander T. Iwamoto, Andre Goy, Brenna G. Hansen, Bazetta Blacklock-Schuver, Ronald E. Gress, James N. Kochenderfer
Data analysis and interpretation: Jennifer N. Brudno, Robert P.T. Somerville, Victoria Shi, David C. Halverson, Juan C. Gea-Banacloche, Steven A. Feldman, Roger Kurlander, Irina Maric, Brenna G. Hansen, Jennifer S. Wilder, Frances T. Hakim, James N. Kochenderfer
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jennifer N. Brudno
No relationship to disclose
Robert P.T. Somerville
No relationship to disclose
Victoria Shi
No relationship to disclose
Jeremy J. Rose
No relationship to disclose
David C. Halverson
No relationship to disclose
Daniel H. Fowler
No relationship to disclose
Juan C. Gea-Banacloche
No relationship to disclose
Steven Z. Pavletic
No relationship to disclose
Dennis D. Hickstein
No relationship to disclose
Tangying L. Lu
No relationship to disclose
Steven A. Feldman
No relationship to disclose
Alexander T. Iwamoto
No relationship to disclose
Roger Kurlander
No relationship to disclose
Irina Maric
No relationship to disclose
Andre Goy
Consulting or Advisory Role: Celgene, Takeda, Pharmacyclics, Johnson & Johnson, Acerta Pharma, Infinity Pharmaceuticals
Speakers’ Bureau: Takeda, Johnson & Johnson, Pharmacyclics
Brenna G. Hansen
No relationship to disclose
Jennifer S. Wilder
No relationship to disclose
Bazetta Blacklock-Schuver
No relationship to disclose
Frances T. Hakim
No relationship to disclose
Steven A. Rosenberg
Research Funding: Kite Pharma (Inst), Lion Biotechnologies (Inst)
Ronald E. Gress
No relationship to disclose
James N. Kochenderfer
Research Funding: Bluebird Bio
Patents, Royalties, Other Intellectual Property: Two patents pending on chimeric antigen receptor T-cell technology
REFERENCES
- 1.Khouri IF, Champlin RE. Nonmyeloablative allogeneic stem cell transplantation for non-Hodgkin lymphoma. Cancer J. 2012;18:457–462. doi: 10.1097/PPO.0b013e31826b124c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavletic SZ, Kumar S, Mohty M, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Epidemiology and Natural History of Relapse Following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:871–890. doi: 10.1016/j.bbmt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brink MR, Porter DL, Giralt S, et al. Relapse after allogeneic hematopoietic cell therapy. Biol Blood Marrow Transplant. 2010;16:S138–S145. doi: 10.1016/j.bbmt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spyridonidis A, Labopin M, Schmid C, et al. Immunotherapy Subcommittee of Acute Leukemia Working Party Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. doi: 10.1038/leu.2011.351. [DOI] [PubMed] [Google Scholar]
- 5.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 6.Roddie C, Peggs KS. Donor lymphocyte infusion following allogeneic hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2011;11:473–487. doi: 10.1517/14712598.2011.554811. [DOI] [PubMed] [Google Scholar]
- 7.Kolb H-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 8.Kolb HJ, Schattenberg A, Goldman JM, et al. European Group for Blood and Marrow Transplantation Working Party Chronic Leukemia Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 9.Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: Presentation and management. Best Pract Res Clin Haematol. 2008;21:205–222. doi: 10.1016/j.beha.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus A, Eshhar Z. Allogeneic chimeric antigen receptor-modified cells for adoptive cell therapy of cancer. Expert Opin Biol Ther. 2014;14:947–954. doi: 10.1517/14712598.2014.900540. [DOI] [PubMed] [Google Scholar]
- 11.Ghorashian S, Pule M, Amrolia P. CD19 chimeric antigen receptor T cell therapy for haematological malignancies. Br J Haematol. 2015;169:463–478. doi: 10.1111/bjh.13340. [DOI] [PubMed] [Google Scholar]
- 12.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [Comment: Nat Med 9:257-258, 2003] [DOI] [PubMed] [Google Scholar]
- 16.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 17.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz CRY, Micklethwaite KP, Savoldo B, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 27.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Jena B, Maiti S, Huls H, et al. Chimeric antigen receptor (CAR)-specific monoclonal antibody to detect CD19-specific T cells in clinical trials. PLoS One. 2013;8:e57838. doi: 10.1371/journal.pone.0057838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Pfistner B, Juweid ME, et al. International Harmonization Project on Lymphoma Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 30.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [Erratum: Blood 126:1048, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 33.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 34.Vincent K, Roy DC, Perreault C. Next-generation leukemia immunotherapy. Blood. 2011;118:2951–2959. doi: 10.1182/blood-2011-04-350868. [DOI] [PubMed] [Google Scholar]
- 35.Warren EH, Deeg HJ. Dissecting graft-versus-leukemia from graft-versus-host-disease using novel strategies. Tissue Antigens. 2013;81:183–193. doi: 10.1111/tan.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackinnon S, Papadopoulos EB, Carabasi MH, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: Separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 37.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochenderfer JN, Yu Z, Frasheri D, et al. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.