Abstract
Purpose
Despite the potential benefits of minimally invasive hysterectomy for uterine cancer, population-level data describing the procedure’s safety in unselected patients are lacking. We examined the use of minimally invasive surgery and the association between the route of the procedure and long-term survival.
Methods
We used the SEER-Medicare database to identify women with stage I-III uterine cancer who underwent hysterectomy from 2006 to 2011. Patients who underwent abdominal hysterectomy were compared with those who had minimally invasive hysterectomy (laparoscopic and robot-assisted). Perioperative morbidity, use of adjuvant therapy, and long-term survival were examined after propensity score balancing.
Results
We identified 6,304 patients, including 4,139 (65.7%) who underwent abdominal hysterectomy and 2,165 (34.3%) who underwent minimally invasive hysterectomy; performance of minimally invasive hysterectomy increased from 9.3% in 2006 to 61.7% in 2011. Robot-assisted procedures accounted for 62.3% of the minimally invasive operations. Compared with women who underwent abdominal hysterectomy, minimally invasive hysterectomy was associated with a lower overall complication rate (22.7% v 39.7%; P < .001), and lower perioperative mortality (0.6% v 1.1%), but these women were more likely to receive adjuvant pelvic radiotherapy (34.3% v 31.3%) and brachytherapy (33.6% v 31.0%; P < .05). The complication rate was higher after robot-assisted hysterectomy compared with laparoscopic hysterectomy (23.7% v 19.5%; P = .03). There was no association between the use of minimally invasive hysterectomy and either overall (HR, 0.89; 95% CI, 0.75 to 1.04) or cancer-specific (HR, 0.83; 95% CI, 0.59 to 1.16) mortality.
Conclusion
Minimally invasive hysterectomy does not appear to compromise long-term survival for women with endometrial cancer.
INTRODUCTION
Surgical management remains the cornerstone of treatment for most women with endometrial cancer. The majority of patients diagnosed with uterine cancer undergo hysterectomy, often in combination with salpingo-oophorectomy and sometimes lymphadenectomy. Traditionally, the procedure was most commonly performed through a midline laparotomy and is associated with substantial perioperative morbidity and mortality.1,2
Laparoscopic hysterectomy was first described in the early 1990s. Compared with laparotomy, laparoscopy has been associated with decreased perioperative morbidity and an earlier return to normal functioning for gynecologic cancer surgery.3 By the mid-2000s, robot-assisted hysterectomy also began to diffuse into clinical practice.4,5 Similar to laparoscopy, robot-assisted hysterectomy appears to have a favorable morbidity profile compared with laparotomy. Despite the potential benefits of these procedures, prior studies have reported that uptake of minimally invasive surgery (MIS) in gynecology has been slow.4,6
In addition to short-term complications, long-term outcomes and survival are an important concern for patients with cancer. In addition to the technical challenges of the surgery, the potential risks of minimally invasive hysterectomy for patients with cancer include metastases to the port sites and disruption of the uterus at the time of surgery. Although a large trial by the Gynecologic Oncology Group was unable to demonstrate that minimally invasive hysterectomy was not inferior to laparotomy, other small studies have suggested that the procedure is safe.7-13
Despite the potential benefits of minimally invasive hysterectomy for uterine cancer, population-level data describing the safety of the procedure in unselected patients are largely lacking. We performed a population-based analysis to compare minimally invasive with abdominal hysterectomy for uterine cancer. We specifically examined trends in the use of MIS, as well as the association between the route of the procedure and long-term survival.
METHODS
Data Source
The SEER-Medicare database was used for analysis.14-16 SEER is a population-based tumor registry developed by the National Cancer Institute (NCI). SEER captures data on time of diagnosis, tumor histology, location, stage, treatment, and survival, as well as demographic and selected census tract-level information. The Medicare database captures data on patients with Medicare Part A (inpatient) and Part B (outpatient), including billed claims, services, and diagnoses. These two files are linked and provide data on initial services and all follow-up care. Exemption from the Columbia University Institutional Review Board was obtained.
Cohort Selection
Women 65 years of age or older with stage I-III uterine cancer who underwent hysterectomy from 2006 to 2011 were included in the analysis. Patients with endometrioid, serous, and carcinosarcoma histology were included. Women who received chemotherapy or radiation prior to hysterectomy, patients enrolled in a non-Medicare health maintenance organization, those receiving Medicare for a reason other than age, and patients with other primary cancers were excluded.
Patients were initially classified as having undergone an abdominal (open) hysterectomy or minimally invasive hysterectomy based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) and Current Procedural Terminology coding. Patients with billing codes for both an abdominal and minimally invasive hysterectomy were categorized as having undergone an abdominal hysterectomy. Billing codes for robot-assisted surgery were introduced in October 2008. Given the availability of this coding, we further stratified women treated between 2009 and 2011 as having undergone either a laparoscopic or robot-assisted hysterectomy. For each subject, we noted the performance of concurrent lymphadenectomy.
Patient Characteristics
Age at the time of diagnosis was stratified into 5-year intervals, and race was recorded as white, black, and other. The marital status of each patient was recorded as married, unmarried, and unknown. An aggregate socioeconomic status (SES) score was calculated from education, poverty level, and income data from the 2000 census tract data, as previously reported by Du et al.17 Patient scores were ranked on a scale of 1 through 5 by use of a formula that incorporated education, poverty, and income weighted equally, with 1 being the lowest value. Comorbid medical diseases were assessed using the Klabunde adaptation of the Charlson comorbidity index (ie, the Klabunde–Charlson index).18,19 Medicare claims were examined for diagnostic codes of the International Classification of Disease, Ninth Revision. Each condition was weighted, and patients were assigned a score that was based on the Klabunde-Charlson index.19 Area of residence was categorized as metropolitan or nonmetropolitan, and the SEER registries were grouped as East, West, and Midwest.
Hospital characteristics analyzed included hospital teaching status (yes or no), NCI Cancer Center designation (yes or no), and hospital bed size (< 200, 200-400, 401-600, > 600). Procedural volume was assessed as mean annual procedural volume, calculated as the mean number of procedures performed per year for years in which a hospital performed at least one operation.20 Separate volume estimates were tabulated for abdominal, minimally invasive, laparoscopic, and robotically assisted hysterectomy. Stage was captured using the American Joint Committee on Cancer staging criteria and converted to the current International Federation of Gynecology and Obstetrics staging system for uterine cancer, and tumor grade was grouped as moderately or poorly differentiated or unknown.
Outcomes
The primary outcome of the analysis was long-term survival. Cancer-specific and overall survival were assessed for each surgical group. In addition, perioperative morbidity and mortality were analyzed. Perioperative complications were categorized based on a previously described system: (1) intraoperative complications (bladder injury, ureteral injury, intestinal injury, vascular injury, and other operative injury); (2) surgical site complications (wound complications, abscess, hemorrhage, bowel obstruction, ileus); and (3) medical complications (venous thromboembolism, myocardial infarction, cardiopulmonary arrest, renal failure, respiratory failure, stroke, bacteremia/sepsis, shock, and pneumonia).4,5 A composite score of any of these complications was also examined.
Transfusion during the hospitalization was measured. Perioperative mortality was defined as death within 30 days of the procedure. Use of adjuvant vaginal brachytherapy, whole pelvic radiation, and chemotherapy during the 6-month period after surgery was noted for each subject.
Statistical Analyses
Frequency distributions between categorical variables were compared using χ2 tests. The association between demographic, clinical, and oncologic characteristics and use of minimally invasive or robot-assisted hysterectomy was estimated using multivariable random effects logistic regression models accounting for hospital-level clustering.
The inverse probability of treatment weighting (IPTW) approach based on propensity score was used to balance the observed confounders between treatments. Separate models were developed to compare the abdominal versus minimally invasive hysterectomy cohort and the laparoscopic versus robot-assisted cohorts.
The propensity score is the predicted probability of treatment, minimally invasive or robot-assisted hysterectomy, in the current analysis.21-23 To calculate the propensity score, we fit a logistic regression model that included all of the clinical, oncologic, and hospital characteristics and two-way interaction terms with a P value of less than .15 for the use of minimally invasive hysterectomy and a P value of less than .05 for the use of robot-assisted hysterectomy to allow model convergence. The predicted probability (the propensity score) was estimated for each patient and ranged from 0 to 1. The weighting assumptions of the IPTW approach assigned patients who underwent the treatment of interest a weight of 1/propensity score and those who did not undergo the treatment of interest a weight of 1/(1-propensity score).21,24 To reduce the bias from extreme weights, a stabilization technique that multiplies the treatment and comparison weights by a constant and a trimming technique that trims the stabilized weights within a specified range (≤ 10) were applied.25
After IPTW, we assessed the balance of measured confounders between treatments via a weighted regression approach, in which each covariate was regressed on the treatment variable. The clinically unimportant differences between treatment groups were determined by a threshold value of less than 0.2 for all coefficients in the weighted regression model.26
After propensity score balancing, morbidity, mortality, and subsequent treatment were compared between groups. The effect of route of hysterectomy on overall mortality and cancer-specific mortality was evaluated using Cox proportional hazards models. Survival was also examined using Kaplan-Meier analyses and compared with log rank tests. All analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided. A P value of less than .05 was considered statistically significant.
RESULTS
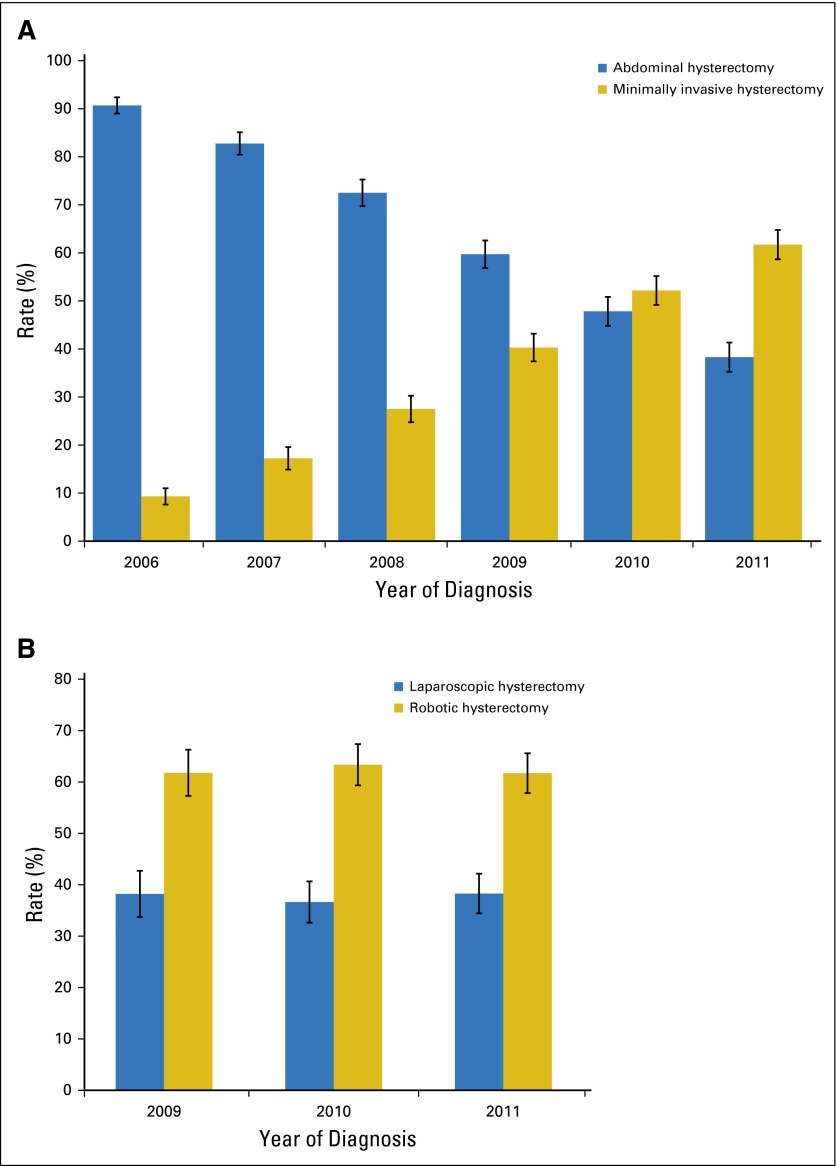
A total of 6,304 patients, including 4,139 (65.7%) women who underwent abdominal hysterectomy and 2,165 (34.3%) who underwent minimally invasive hysterectomy, were identified. Performance of minimally invasive hysterectomy increased from 9.3% (95% CI, 7.6% to 11.0%) in 2006 to 61.7% (95% CI, 58.7% to 64.8%) in 2011 (Fig 1A). Among those women who underwent a minimally invasive operation, robot-assisted hysterectomy accounted for 61.8% (95% CI, 57.3% to 66.3%) of the procedures in 2009, 63.4% (95% CI, 59.3% to 67.4%) of the procedures in 2010, and 61.7% (95% CI, 57.8% to 65.6%) of the procedures in 2011 (Fig 1B).
Fig 1.
(A) Trends in the use of minimally invasive and abdominal hysterectomy (2006-2011; P < .001). (B) Trends in the use of robot-assisted and laparoscopic hysterectomy (2009-2011; P = .94).
Table 1 displays the characteristics of women who underwent abdominal versus minimally invasive (laparoscopic or robot-assisted) hysterectomy. After propensity score balancing, treatment in a more recent year and endometrioid histology remained associated with performance of MIS hysterectomy (P < .05 for both). After propensity score balancing, among women who underwent a minimally invasive hysterectomy, hospital teaching status and treatment at a NCI-designated cancer center remained associated with performance of robot-assisted compared with laparoscopic hysterectomy (P < .05 for both; Table A1, online only).
Table 1.
Clinical and Demographic Characteristics of the Cohort Comparing Minimally Invasive and Abdominal Hysterectomy
| Characteristic | Unadjusted | Inverse Probability of Treatment Weighting* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal Hysterectomy (N = 4,139) | MIS Hysterectomy (N = 2,165) | P† | Abdominal Hysterectomy (N = 4,762) | MIS Hysterectomy (N = 3,100) | P‡ | |||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Year of diagnosis | < .001 | .01 | ||||||||
| 2006 | 1,032 | (24.9) | 106 | (4.9) | 877 | (18.4) | 402 | (13.0) | ||
| 2007 | 826 | (20.0) | 172 | (7.9) | 768 | (16.1) | 495 | (16.0) | ||
| 2008 | 730 | (17.6) | 277 | (12.8) | 789 | (16.6) | 489 | (15.8) | ||
| 2009 | 667 | (16.1) | 450 | (20.8) | 837 | (17.6) | 595 | (19.2) | ||
| 2010 | 508 | (12.3) | 554 | (25.6) | 764 | (16.1) | 569 | (18.4) | ||
| 2011 | 376 | (9.1) | 606 | (28.0) | 727 | (15.3) | 550 | (17.8) | ||
| Age, years | .01 | .91 | ||||||||
| < 65-69 | 1,151 | (27.8) | 648 | (29.9) | 1,353 | (28.4) | 904 | (29.2) | ||
| 70-74 | 1,176 | (28.4) | 6535 | (30.2) | 1,401 | (29.4) | 928 | (29.9) | ||
| 75-79 | 873 | (21.1) | 450 | (20.8) | 986 | (20.7) | 619 | (20.0) | ||
| ≥ 80 | 939 | (22.7) | 414 | (19.1) | 1,022 | (21.5) | 649 | (20.9) | ||
| Race/ethnicity | .001 | .29 | ||||||||
| White | 3,608 | (87.2) | 1,951 | (90.1) | 4,201 | (88.2) | 2,787 | (89.9) | ||
| Black | 288 | (7.0) | 109 | (5.0) | 296 | (6.2) | 167 | (5.4) | ||
| Other | 243 | (5.9) | 105 | (4.9) | 265 | (5.6) | 146 | (4.7) | ||
| Marital status | . | . | .002 | .61 | ||||||
| Married | 1,859 | (44.9) | 1,055 | (48.7) | 2,185 | (45.9) | 1,395 | (45.0) | ||
| Unmarried | 2,148 | (51.9) | 1,026 | (47.4) | 2,411 | (50.6) | 1,610 | (51.9) | ||
| Other | 132 | (3.2) | 84 | (3.9) | 166 | (3.5) | 95 | (3.1) | ||
| Residence | < .001 | .39 | ||||||||
| Metropolitan | 3,381 | (81.7) | 1,920 | (88.7) | 3,984 | (83.7) | 2,633 | (84.9) | ||
| Nonmetropolitan | 758 | (18.3) | 245 | (11.3) | 778 | (16.3) | 467 | (15.1) | ||
| Socioeconomic status | < .001 | .38 | ||||||||
| Lowest (first) quintile | 1,109 | (26.8) | 421 | (19.5) | 1,186 | (24.9) | 710 | (22.9) | ||
| Second quintile | 1,030 | (24.9) | 463 | (21.4) | 1,133 | (23.8) | 725 | (23.4) | ||
| Third quintile | 692 | (16.7) | 358 | (16.5) | 792 | (16.6) | 493 | (15.9) | ||
| Fourth quintile | 918 | (22.2) | 606 | (28.0) | 1,126 | (23.7) | 778 | (25.1) | ||
| Highest (fifth) quintile | 390 | (9.4) | 317 | (14.6) | 525 | (11.0) | 394 | (12.7) | ||
| Registry area | < .001 | .16 | ||||||||
| East | 959 | (23.2) | 566 | (26.1) | 1,164 | (24.5) | 763 | (24.6) | ||
| Midwest | 1,485 | (35.9) | 546 | (25.2) | 1,513 | (31.8) | 889 | (28.7) | ||
| West | 1,695 | (41.0) | 1,053 | (48.6) | 2,084 | (43.8) | 1,447 | (46.7) | ||
| Comorbidity score | < .001 | .76 | ||||||||
| 0 | 2,252 | (54.4) | 1,329 | (61.4) | 2,686 | (56.4) | 1,754 | (56.6) | ||
| 1 | 1,184 | (28.6) | 548 | (25.3) | 1,315 | (27.6) | 879 | (28.4) | ||
| ≥ 2 | 703 | (17.0) | 288 | (13.3) | 761 | (16.0) | 467 | (15.1) | ||
| Grade | < .001 | .43 | ||||||||
| 1 | 1,369 | (33.1) | 841 | (38.9) | 1,665 | (35.0) | 1,165 | (37.6) | ||
| 2 | 1,331 | (32.2) | 660 | (30.5) | 1,521 | (31.9) | 980 | (31.6) | ||
| 3 | 1,008 | (24.4) | 398 | (18.4) | 1,049 | (22.0) | 632 | (20.4) | ||
| Unknown | 431 | (10.4) | 266 | (12.3) | 528 | (11.1) | 324 | (10.5) | ||
| Tumor stage | < .001 | .37 | ||||||||
| IA | 2,037 | (49.2) | 1,193 | (55.1) | 2,410 | (50.6) | 1,670 | (53.9) | ||
| IB | 859 | (20.8) | 424 | (19.6) | 986 | (20.7) | 603 | (19.4) | ||
| INOS | 138 | (3.3) | 69 | (3.2) | 165 | (3.5) | 102 | (3.3) | ||
| II | 436 | (10.5) | 162 | (7.5) | 463 | (9.7) | 258 | (8.3) | ||
| III | 669 | (16.2) | 317 | (14.6) | 737 | (15.5) | 467 | (15.1) | ||
| Lymphadenectomy | .40 | .64 | ||||||||
| No | 1,227 | (29.6) | 620 | (28.6) | 1,405 | (29.5) | 939 | (30.3) | ||
| Yes | 2,912 | (70.4) | 1,545 | (71.4) | 3,357 | (70.5) | 2,161 | (69.7) | ||
| Histology | < .001 | .003 | ||||||||
| Endometrioid | 3,654 | (88.3) | 1,961 | (90.6) | 4,259 | (89.4) | 2,829 | (91.3) | ||
| Carcinosarcoma | 151 | (3.7) | 39 | (1.8) | 133 | (2.8) | 40 | (1.3) | ||
| Serous | 334 | (8.1) | 165 | (7.6) | 370 | (7.8) | 231 | (7.5) | ||
| Teaching hospital | < .001 | .06 | ||||||||
| No | 1,363 | (32.9) | 727 | (33.6) | 1,588 | (33.3) | 1,054 | (34.0) | ||
| Yes | 2,567 | (62.0) | 1,201 | (55.5) | 2,855 | (60.0) | 1,790 | (57.7) | ||
| Unknown | 209 | (5.1) | 237 | (11.0) | 319 | (6.7) | 256 | (8.3) | ||
| NCI center | < .001 | |||||||||
| No | 3,701 | (89.4) | 1,857 | (85.8) | 4,178 | (87.7) | 2,726 | (88.0) | .98 | |
| Yes | 367 | (8.9) | 243 | (11.2) | 475 | (10.0) | 303 | (9.8) | ||
| Unknown | 71 | (1.7) | 65 | (3.0) | 108 | (2.3) | 70 | (2.3) | ||
| Hospital beds | < .001 | .69 | ||||||||
| < 200 | 563 | (13.6) | 182 | (8.4) | 556 | (11.7) | 341 | (11.0) | ||
| 200-400 | 1,182 | (28.6) | 528 | (24.4) | 1,322 | (27.8) | 820 | (26.5) | ||
| 401-600 | 1,486 | (35.9) | 908 | (41.9) | 1,788 | (37.6) | 1,242 | (40.1) | ||
| > 600 | 837 | (20.2) | 480 | (22.2) | 988 | (20.8) | 624 | (20.1) | ||
| Unknown | 71 | (1.7) | 67 | (3.1) | 108 | (2.3) | 72 | (2.3) | ||
| Hospital volume | < .001 | .37 | ||||||||
| Median (IQR) | 4.2 | (2.2-9.7) | 5.7 | (3.0-9.8) | 4.6 | (2.4-9.7) | 5.2 | (2.3-9.7) | ||
Abbreviations: IQR, interquartile range; MIS, minimally invasive surgery; NCI, National Cancer Institute.
Frequency numbers were rounded to integers based on weight.
P values were derived from χ2 tests.
P values were derived from weighted surveylogistic model.
In a multivariable model of the entire cohort, more recent year of diagnosis, higher SES, treatment at a larger hospital, and treatment at an intermediate-volume hospital were associated with minimally invasive hysterectomy (P < .05 for all; Table 2). In contrast, patients treated in the Midwest and who had more comorbidities, higher tumor grade, higher stage, and carcinosarcomas were less likely to undergo a minimally invasive hysterectomy (P < .05 for all). When limited to women who underwent a minimally invasive hysterectomy, patients treated at an NCI-designated cancer center and at larger hospitals were more likely to undergo a robot-assisted procedure, whereas older women were less likely to have a robotically assisted procedure (P < .05 for all).
Table 2.
Multivariable Models of Predictors of Minimally Invasive and Robot-Assisted Hysterectomy
| Predictor | Minimally Invasive Hysterectomy (v Abdominal Hysterectomy) OR (95% CI) | Robot-Assisted Hysterectomy (v Laparoscopic Hysterectomy) OR (95% CI) |
|---|---|---|
| Year of diagnosis | ||
| 2006 | Referent | — |
| 2007 | 2.19 (1.60 to 2.98)* | — |
| 2008 | 4.84 (3.59 to 6.53)* | — |
| 2009 | 10.50 (7.81 to 14.11)* | Referent |
| 2010 | 19.25 (14.08 to 26.33)* | 1.24 (0.82 to 1.87) |
| 2011 | 33.66 (24.60 to 46.06)* | 1.08 (0.73 to 1.58) |
| Age, years | ||
| < 65-69 | Referent | Referent |
| 70-74 | 1.04 (0.86 to 1.26) | 0.80 (0.54 to 1.19) |
| 75-79 | 1.11 (0.90 to 1.38) | 0.77 (0.50 to 1.19) |
| ≥ 80 | 0.91 (0.73 to 1.13) | 0.59 (0.37 to 0.95)† |
| Race/ethnicity | ||
| White | Referent | Referent |
| Black | 0.73 (0.52 to 1.03) | 1.30 (0.66 to 2.57) |
| Other | 0.72 (0.51 to 1.02) | 0.81 (0.40 to 1.65) |
| Marital status | ||
| Married | Referent | Referent |
| Unmarried | 0.90 (0.77 to 1.05) | 1.34 (0.97 to 1.86) |
| Other | 1.07 (0.69 to 1.65) | 1.61 (0.71 to 3.65) |
| Residence | ||
| Metropolitan | Referent | Referent |
| Nonmetropolitan | 0.97 (0.74 to 1.28) | 1.86 (0.93 to 3.70) |
| Socioeconomic status | ||
| Lowest (first) quintile | Referent | Referent |
| Second quintile | 1.19 (0.94 to 1.50) | 0.71 (0.42 to 1.21) |
| Third quintile | 1.31 (1.01 to 1.70)† | 1.04 (0.59 to 1.82) |
| Fourth quintile | 1.47 (1.15 to 1.88)† | 0.81 (0.49 to 1.36) |
| Highest (fifth) quintile | 1.47 (1.09 to 1.98)† | 0.73 (0.40 to 1.31) |
| Registry area | ||
| East | Referent | Referent |
| Midwest | 0.45 (0.25 to 0.82)† | 2.78 (0.90 to 8.62) |
| West | 1.03 (0.58 to 1.85) | 0.43 (0.15 to 1.23) |
| Comorbidity score | ||
| 0 | Referent | Referent |
| 1 | 0.77 (0.65 to 0.92)† | 0.82 (0.57 to 1.18) |
| ≥ 2 | 0.52 (0.42 to 0.65)* | 1.27 (0.80 to 2.03) |
| Grade | ||
| 1 | Referent | Referent |
| 2 | 0.84 (0.70 to 1.02) | 0.90 (0.62 to 1.32) |
| 3 | 0.62 (0.49 to 0.78)* | 1.05 (0.64 to 1.71) |
| Unknown | 0.79 (0.60 to 1.03) | 0.84 (0.50 to 1.42) |
| Tumor stage | ||
| IA | Referent | Referent |
| IB | 0.99 (0.81 to 1.21) | 1.00 (0.66 to 1.50) |
| INOS | 1.23 (0.80 to 1.92) | 0.87 (0.35 to 2.15) |
| II | 0.70 (0.54 to 0.92)† | 1.17 (0.64 to 2.14) |
| III | 0.68 (0.54 to 0.85)† | 1.15 (0.73 to 1.81) |
| Lymphadenectomy | ||
| No | Referent | Referent |
| Yes | 0.89 (0.74 to 1.07) | 2.05 (1.41 to 2.98)† |
| Histology | ||
| Endometrioid | Referent | Referent |
| Carcinosarcoma | 0.34 (0.20 to 0.56)* | 1.07 (0.38 to 3.04) |
| Serous | 1.08 (0.81 to 1.45) | 1.79 (0.94 to 3.39) |
| Teaching hospital | ||
| No | Referent | Referent |
| Yes | 1.03 (0.70 to 1.52) | 1.31 (0.57 to 3.00) |
| Unknown | 1.24 (0.81 to 1.89) | 1.83 (0.75 to 4.47) |
| NCI center | ||
| No | Referent | Referent |
| Yes | 0.93 (0.48 to 1.81) | 5.61 (1.36 to 23.16)† |
| Unknown | NA | NA |
| Hospital beds | ||
| < 200 | Referent | Referent |
| 200-400 | 1.36 (0.82 to 2.25) | 7.29 (2.01 to 26.45)† |
| 401-600 | 2.93 (1.56 to 5.49)† | 3.90 (0.92 to 16.47) |
| > 600 | 4.05 (2.03 to 8.11)* | 8.11 (1.77 to 37.25)† |
| Unknown | NA | NA |
| Hospital volume | ||
| Low | Referent | Referent |
| Intermediate | 1.80 (1.07 to 3.03)† | 1.83 (0.74 to 4.53) |
| High | 1.38 (0.63 to 3.05) | 0.99 (0.29 to 3.38) |
| Unknown | NA | NA |
Abbreviations: NA, Not applicable for the colinearity between hospital factors; OR, odds ratio.
P < .001.
P < .05.
After propensity score balancing, minimally invasive hysterectomy was associated with a lower overall complication rate compared with abdominal hysterectomy (22.7% v 39.7%; odds ratio [OR], 0.46; 95% CI, 0.41 to 0.51) and lower rates of surgical site complications (OR, 0.32; 95% CI, 0.28 to 0.37), medical complications (OR, 0.56; 95% CI, 0.50 to 0.63), transfusions (OR, 0.44; 95% CI, 0.35 to 0.56), and perioperative mortality (OR, 0.57; 95% CI, 0.34 to 0.95). Women who underwent minimally invasive hysterectomy were more likely to receive adjuvant pelvic radiotherapy compared with women who underwent abdominal hysterectomy (34.3% v 31.3%; OR, 1.14; 95% CI, 1.04 to 1.26) and brachytherapy (33.6% v 31.0%; OR, 1.13; 95% CI, 1.03 to 1.24). Receipt of adjuvant chemotherapy was similar between the groups (P = .81)
The overall rate of complications was 23.7% in women who underwent robot-assisted hysterectomy compared with 19.5% after laparoscopic hysterectomy (OR, 1.28; 95% CI, 1.03 to 1.59; Table 3). There were no differences in the rates of intraoperative complications (OR, 0.84; 95% CI, 0.53 to 1.33), surgical site complications (OR, 1.26; 95% CI, 0.92 to 1.72), or transfusions (OR, 0.84; 95% CI, 0.51 to 1.39), whereas the rate of medical complications was higher after robotically assisted hysterectomy (14.1% v 10.0%; OR, 1.48; 95% CI, 1.12 to 1.96). There were no statistically significant differences in the rate of use of adjuvant therapy between laparoscopic and robot-assisted hysterectomy.
Table 3.
Comparison of Morbidity and Mortality for Patients Who Underwent Minimally Invasive Compared With Abdominal Hysterectomy and Robotically Assisted Compared With Laparoscopic Hysterectomy After Adjusting for Observed Confounders Using Inverse Probability of Treatment Weighting
| Inverse Probability of Treatment Weighting | Inverse Probability of Treatment Weighting | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdominal Hysterectomy (N = 4,762) | MIS Hysterectomy (N = 3,100) | P | MIS Versus Abdominal Hysterectomy | Laparoscopic Hysterectomy (N = 821) | Robotic-Assisted Hysterectomy (N = 1,157) | P | Robot-Assisted Versus Laparoscopic Hysterectomy | |||||
| No. | (%) | No. | (%) | OR (95% CI)* | No. | (%) | No. | (%) | OR (95% CI) | |||
| Any complication | 1,861 | (39.1) | 703 | (22.7) | < .001 | 0.46 (0.41 to 0.51) | 160 | (19.5) | 274 | (23.7) | 0.03 | 1.28 (1.03 to 1.59) |
| Intraoperative complications | 289 | (6.1) | 179 | (5.8) | .59 | 0.95 (0.78 to 1.15) | 52 | (6.3) | 60 | (5.2) | 0.29 | 0.84 (0.53 to 1.33) |
| Surgical site complications | 1,102 | (23.1) | 272 | (8.8) | < .001 | 0.32 (0.28 to 0.37) | 67 | (8.2) | 117 | (10.1) | 0.15 | 1.26 (0.92 to 1.72) |
| Medical complications | 1,094 | (23) | 444 | (14.3) | < .001 | 0.56 (0.50 to 0.63) | 82 | (10) | 164 | (14.1) | 0.01 | 1.48 (1.12 to 1.96) |
| Transfusion | 321 | (6.7) | 96 | (3.1) | < .001 | 0.44 (0.35 to 0.56) | 29 | (3.5) | 34 | (3.0) | 0.49 | 0.84 (0.51 to 1.39) |
| Perioperative death | 53 | (1.1) | 20 | (0.6) | .03 | 0.57 (0.34 to 0.95) | * | * | * | * | 0.09 | 0.14 (0.01 to 1.39) |
| Discharge status | < .001 | < .001 | ||||||||||
| Home | 4,193 | (88.1) | 2,812 | (90.7) | 629 | (76.6) | 1,107 | (95.6) | ||||
| Nursing home | 506 | (10.6) | 147 | (4.7) | 46 | (5.6) | 50 | (4.3) | ||||
| Dead | * | * | * | * | * | * | * | * | ||||
| Unknown | * | * | * | * | * | * | * | * | ||||
| Adjuvant therapy | ||||||||||||
| Brachytherapy | 1,475 | (31.0) | 1,042 | (33.6) | .01 | 1.13 (1.03 to 1.24) | 244 | (29.8) | 391 | (33.8) | .06 | 1.21 (0.99 to 1.46) |
| Pelvic radiotherapy | 1,492 | (31.3) | 1,062 | (34.3) | .01 | 1.14 (1.04 to 1.26) | 249 | (30.3) | 395 | (34.1) | .07 | 1.19 (0.99 to 1.48) |
| Chemotherapy | 637 | (13.4) | 421 | (13.6) | .81 | 1.02 (0.89 to 1.16) | 141 | (17.2) | 210 | (18.1) | .58 | 1.07 (0.84 to 1.35) |
Abbreviation: OR, odds ratio.
Suppressed due to small cell size.
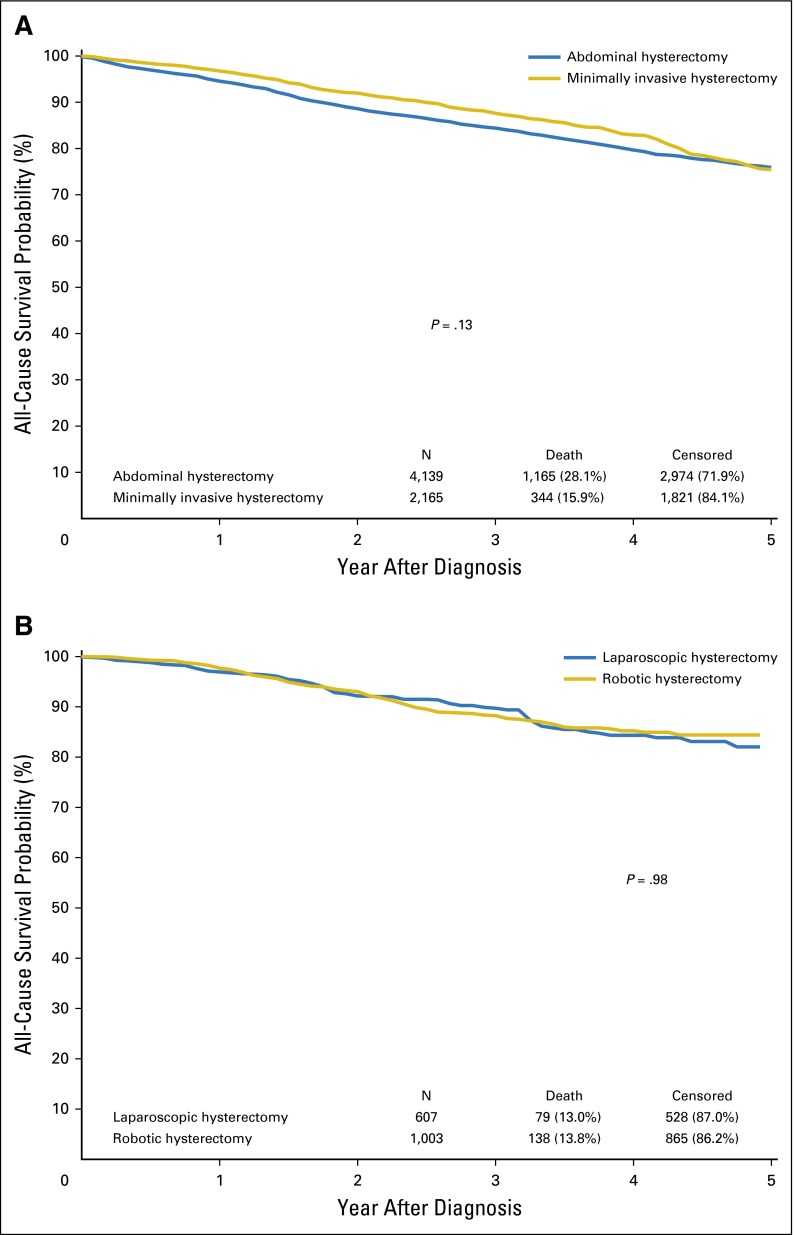
There was no significant association between the use of minimally invasive hysterectomy and either overall (HR, 0.89; 95% CI, 0.75 to 1.04) or cancer-specific (HR, 0.83; 95% CI, 0.59 to 1.16) mortality (Table 4). The results were similar after adjustment for adjuvant therapy and when the cohort was limited to patients with stage I tumors. Similarly, there was no statistically significant difference in either overall (HR, 1.00; 95% CI, 0.72 to 1.41) or cancer-specific (HR, 1.53; 95% CI, 0.76 to 3.05) mortality for robotic compared with laparoscopic hysterectomy or in any of the sensitivity analyses. Likewise, there was no difference in survival in any of the Kaplan-Meier analyses (Fig 2).
Table 4.
Adjusted Mortality Based on Route of Hysterectomy
| Route of hysterectomy | Cancer-Specific Mortality HR (95% CI) | Overall Mortality HR (95% CI) |
|---|---|---|
| Minimally invasive versus abdominal hysterectomy | ||
| Base model | ||
| Abdominal hysterectomy | Referent | Referent |
| Minimally invasive hysterectomy | 0.83 (0.59 to 1.16) | 0.89 (0.75 to 1.04) |
| Adjusted for adjuvant therapy | ||
| Abdominal hysterectomy | Referent | Referent |
| Minimally invasive hysterectomy | 0.81 (0.58 to 1.14) | 0.88 (0.75 to 1.04) |
| Stage I base model | ||
| Abdominal hysterectomy | Referent | Referent |
| Minimally invasive hysterectomy | 1.01 (0.58 to 1.73) | 0.98 (0.78 to 1.21) |
| Stage I adjusted for adjuvant therapy | ||
| Abdominal hysterectomy | Referent | Referent |
| Minimally invasive hysterectomy | 0.97 (0.56 to 1.67) | 1.01 (0.77 to 1.19) |
| Robot-assisted v laparoscopic hysterectomy | ||
| Base model | ||
| Laparoscopic | Referent | Referent |
| Robot-assisted | 1.53 (0.76 to 3.05) | 1.00 (0.72 to 1.41) |
| Adjusted for adjuvant therapy | ||
| Laparoscopic | Referent | Referent |
| Robot-assisted | 1.45 (0.73 to 2.918) | 0.97 (0.69 to 1.37) |
| Stage I base model | ||
| Laparoscopic | Referent | Referent |
| Robot-assisted | 1.47 (0.43 to 5.08) | 0.92 (0.57 to 1.49) |
| Stage I adjusted for adjuvant therapy | ||
| Laparoscopic | Referent | Referent |
| Robot-assisted | 1.38 (0.39 to 4.82) | 0.92 (0.57 to 1.49) |
NOTE. Comparison of propensity score balanced groups and adjusted for adjuvant therapy (radiation, brachytherapy, chemotherapy).
Fig 2.
(A) Kaplan-Meier analysis of overall survival for abdominal versus minimally invasive hysterectomy (P = .13). (B) Kaplan-Meier analysis of overall survival for laparoscopic versus robot-assisted hysterectomy (P = .98).
DISCUSSION
Our findings suggest that long-term survival is similar after minimally invasive and abdominal hysterectomy in women with endometrial cancer. Although minimally invasive hysterectomy is associated with decreased morbidity, the procedure was also associated with increased use of adjuvant radiation compared with abdominal hysterectomy. There was no difference in survival between robot-assisted and laparoscopic hysterectomy; however, robotically assisted hysterectomy was associated with a small, but statistically significant, increased risk of complications.
We noted a marked increase in the use of MIS for uterine cancer between 2006 and 2011. In a previous analysis of the same data set, our group found that only 8.5% of hysterectomies for uterine cancer were performed minimally invasively in 2005.6 In the current study, we found that the use of minimally invasive hysterectomy increased by more than six-fold, from 9% in 2006 to 62% in 2011. In the later years of the study, robot-assisted procedures accounted for approximately two thirds of the minimally invasive operations. During the time frame of the study, robotic technology diffused widely into gynecologic practice and likely contributed to the increased use of minimally invasive techniques for uterine cancer.4
An important benefit of MIS is lower rates of perioperative morbidity. Prior studies comparing laparoscopic hysterectomy with laparotomy for endometrial cancer have consistently shown lower complication rates and shorter hospitalizations.12,27,28 Furthermore, MIS appears to be associated with improved quality of life during the perioperative period.9,29 Our data are consistent with these findings. Compared with laparotomy, women who underwent minimally invasive hysterectomy were 60% less likely to experience a complication.
We noted a slightly higher complication rate for robot-assisted hysterectomy compared with laparoscopic hysterectomy. Importantly, the higher perioperative morbidity rate with robotically assisted surgery was not due to intraoperative injuries or surgical site complications, but rather, postoperative medical complications. Specifically, the rates of respiratory and renal failure as well as bacteremia were higher after robot-assisted surgery. Prior studies have reported substantially longer operative times with robot-assisted hysterectomy, which may in part explain the morbidity profile we identified.30,31 Higher pulmonary morbidity was also reported in a prior study in which the pneumonia rate after robotically assisted hysterectomy was more than double that found after laparoscopy.32 These data may also suggest that some higher risk patients who would previously have undergone laparotomy are undergoing less morbid, MIS with robotic assistance. Although we did not specifically analyze cost, prior studies have suggested that robot-assisted surgery is more costly than laparoscopy.4,5,33
Encouragingly, we found no difference in survival between laparotomy and MIS for endometrial cancer. In the Gynecologic Oncology Group’s LAP2 trial, 2,616 women with apparent stage I-II endometrial cancer were randomly assigned to either laparotomy or laparoscopy. Although the study did not meet the protocol-specified definition of noninferiority, survival was similar between the two arms, and the findings were largely interpreted to indicate that MIS is safe for endometrial cancer.7 Other smaller prospective series have reported similar outcomes comparing laparoscopy and laparotomy.8-13 Although data comparing survival for robot-assisted hysterectomy with other modalities of hysterectomy are more limited, institutional studies have generally reported equivalent outcomes.34-37 Importantly, the 95% CI for MIS compared with abdominal hysterectomy in our primary survival analysis ranged from 0.75 to 1.04, suggesting that MIS hysterectomy is unlikely to be inferior to an abdominal procedure. Furthermore, our findings of equivalent survival across the modalities of hysterectomy were similar in a wide range of sensitivity analyses.
We identified an increased use of adjuvant radiation, both whole pelvic radiation and brachytherapy, in women who underwent minimally invasive hysterectomy. The need for additional cancer-directed therapy has been used as a surrogate for surgical outcomes and quality for other procedures, such as prostatectomy, a disease process that similarly has favorable survival and high cure rates.38 The mechanism underlying the need for increased use of adjuvant therapy after minimally invasive hysterectomy remains unclear, particularly as patients who had a minimally invasive procedure had somewhat more favorable tumor prognostic factors, even after propensity score balancing. Particularly for women with large uteri, manipulation at the time of surgery or disruption or spillage of tumor from the uterine cavity may prompt use of radiation. This phenomenon warrants further investigation and careful monitoring.
Like prior studies, we noted a number of disparities in the allocation of treatment of women with endometrial cancer.39 Minimally invasive hysterectomy was more commonly used in patients with higher SES and in larger hospitals. This is in accord with other studies of hysterectomy for both benign and oncologic indications that have shown significant disparities in the use of newer procedures.4,5 Not surprisingly, women with higher stage tumors and more aggressive histologic subtypes were less likely to undergo a minimally invasive operation.
Although our study benefits from the inclusion of a large sample of patients, we recognize a number of important limitations. First, claims data undercaptures complications. To mitigate this bias, we chose to include only major perioperative complications. Although any undercapture of complications should be balanced across the groups, we recognize that we cannot measure minor complications with the current study design. Second, our analysis is limited to elderly Medicare beneficiaries and may not be generalizable to all women. However, the SEER-Medicare dataset represents a unique resource to examine long-term survival linked to detailed tumor data. Third, given that survival is favorable for early-stage endometrial cancer, our analysis is not powered to detect small differences in survival between groups. As discussed previously, this is an intrinsic limitation of studies of endometrial cancer and highlights the importance of including other outcomes metrics, such as complications and patient-reported outcomes in comparative effectiveness studies. Lastly, as with any study of administrative data, we cannot capture individual patient and physician preferences that undoubtedly influenced treatment decision making.
In sum, these data suggest that the use of minimally invasive hysterectomy for uterine cancer has increased rapidly since 2007 and now accounts for more than 60% of operations for the disease. Importantly, the procedure is associated with long-term survival that is comparable to abdominal hysterectomy and a favorable morbidity profile.
Acknowledgment
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services, Inc; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Table A1.
Clinical and Demographic Characteristics of Women Who Underwent Laparoscopic or Robot-Assisted Hysterectomy
| Characteristic | Unadjusted | Inverse Probability of Treatment Weighting* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Laparoscopic Hysterectomy (N = 607) | Robot-Assisted Hysterectomy (N = 1,003) | P† | Laparoscopic Hysterectomy (N = 821) | Robot-Assisted Hysterectomy (N = 1,157) | P‡ | |||||
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | |||
| Year of diagnosis | .82 | .61 | ||||||||
| 2009 | 172 | (28.3) | 278 | (27.7) | 220 | (26.8) | 318 | (27.5) | ||
| 2010 | 203 | (33.4) | 351 | (35.0) | 264 | (32.2) | 400 | (34.6) | ||
| 2011 | 232 | (38.2) | 374 | (37.3) | 337 | (41.0) | 439 | (38.0) | ||
| Age, years | .31 | .83 | ||||||||
| < 65-69 | 167 | (27.5) | 312 | (31.1) | 238 | (28.9) | 353 | (30.5) | ||
| 70-74 | 192 | (31.6) | 298 | (29.7) | 268 | (32.6) | 345 | (29.9) | ||
| 75-79 | 122 | (20.1) | 211 | (21.0) | 164 | (20.0) | 245 | (21.2) | ||
| ≥ 80 | 126 | (20.8) | 182 | (18.2) | 152 | (18.5) | 214 | (18.5) | ||
| Race/ethnicity | .44 | .56 | ||||||||
| White | 544 | (89.6) | 897 | (89.4) | 740 | (90.1) | 1,021 | (88.2) | ||
| Black | 28 | (4.6) | 58 | (5.8) | 45 | (5.5) | 72 | (6.2) | ||
| Other | 35 | (5.8) | 48 | (4.8) | 37 | (4.5) | 65 | (5.6) | ||
| Marital status | .21 | .70 | ||||||||
| Married | 308 | (50.7) | 465 | (46.4) | 418 | (51.0) | 559 | (48.3) | ||
| Unmarried | 274 | (45.1) | 488 | (48.7) | 371 | (45.2) | 548 | (47.3) | ||
| Other | 25 | (4.1) | 50 | (5.0) | 32 | (3.9) | 51 | (4.4) | ||
| Residence | < .001 | .43 | ||||||||
| Metropolitan | 565 | (93.1) | 857 | (85.4) | 741 | (90.2) | 1,020 | (88.1) | ||
| Nonmetropolitan | 42 | (6.9) | 146 | (14.6) | 80 | (9.8) | 137 | (11.9) | ||
| Socioeconomic status | .001 | .59 | ||||||||
| Lowest (first) quintile | 110 | (18.1) | 207 | (20.6) | 141 | (17.2) | 223 | (19.3) | ||
| Second quintile | 120 | (19.8) | 219 | (21.8) | 180 | (21.9) | 238 | (20.6) | ||
| Third quintile | 83 | (13.7) | 173 | (17.3) | 118 | (14.4) | 192 | (16.6) | ||
| Fourth quintile | 175 | (28.8) | 289 | (28.8) | 253 | (30.8) | 349 | (30.2) | ||
| Highest (fifth) quintile | 119 | (19.6) | 115 | (11.5) | 129 | (15.7) | 154 | (13.3) | ||
| Registry area | < .001 | .17 | ||||||||
| East | 153 | (25.2) | 273 | (27.2) | 224 | (27.3) | 314 | (27.1) | ||
| Midwest | 125 | (20.6) | 319 | (31.8) | 190 | (23.1) | 330 | (28.5) | ||
| West | 329 | (54.2) | 411 | (41.0) | 407 | (49.6) | 513 | (44.4) | ||
| Comorbidity score | .49 | .59 | ||||||||
| 0 | 360 | (59.3) | 605 | (60.3) | 518 | (63.0) | 696 | (60.1) | ||
| 1 | 164 | (27) | 247 | (24.6) | 201 | (24.5) | 296 | (25.6) | ||
| ≥ 2 | 83 | (13.7) | 151 | (15.1) | 102 | (12.5) | 166 | (14.3) | ||
| Grade | .04 | .83 | ||||||||
| 1 | 245 | (40.4) | 346 | (34.5) | 300 | (36.5) | 418 | (36.1) | ||
| 2 | 184 | (30.3) | 297 | (29.6) | 241 | (29.4) | 343 | (29.7) | ||
| 3 | 102 | (16.8) | 207 | (20.6) | 175 | (21.3) | 228 | (19.7) | ||
| Unknown | 76 | (12.5) | 153 | (15.3) | 105 | (12.8) | 168 | (14.6) | ||
| Tumor stage | .40 | .51 | ||||||||
| IA | 338 | (55.7) | 532 | (53.0) | 462 | (56.2) | 631 | (54.5) | ||
| IB | 119 | (19.6) | 188 | (18.7) | 169 | (20.6) | 211 | (18.2) | ||
| INOS | 21 | (3.5) | 33 | (3.3) | 19 | (2.3) | 37 | (3.2) | ||
| II | 47 | (7.7) | 77 | (7.7) | 60 | (7.3) | 88 | (7.6) | ||
| III | 82 | (13.5) | 173 | (17.3) | 111 | (13.5) | 191 | (16.5) | ||
| Lymphadenectomy | < .001 | .41 | ||||||||
| No | 220 | (36.2) | 231 | (23.0) | 236 | (28.7) | 306 | (26.4) | ||
| Yes | 387 | (63.8) | 772 | (77.0) | 586 | (71.3) | 852 | (73.6) | ||
| Histology | .10 | .32 | ||||||||
| Endometrioid | 557 | (91.8) | 887 | (88.4) | 752 | (91.6) | 1,030 | (89.0) | ||
| Carcinosarcoma | 12 | (2.0) | 25 | (2.5) | 13 | (1.6) | 27 | (2.3) | ||
| Serous | 38 | (6.3) | 91 | (9.1) | 56 | (6.9) | 100 | (8.7) | ||
| Teaching hospital | < .001 | .0037 | ||||||||
| No | 233 | (38.4) | 275 | (27.4) | 283 | (34.4) | 347 | (30.0) | ||
| Yes | 298 | (49.1) | 594 | (59.2) | 453 | (55.1) | 620 | (53.6) | ||
| Unknown | 76 | (12.5) | 134 | (13.4) | 86 | (10.4) | 190 | (16.4) | ||
| NCI center | < .001 | .024 | ||||||||
| No | 547 | (90.1) | 813 | (81.1) | 728 | (88.6) | 957 | (82.7) | ||
| Yes | 41 | (6.8) | 152 | (15.2) | 72 | (8.8) | 146 | (12.6) | ||
| Unknown | 19 | (3.1) | 38 | (3.8) | 21 | (2.6) | 54 | (4.7) | ||
| Hospital beds | < .001 | .43 | ||||||||
| < 200 | 55 | (9.1) | 49 | (4.9) | 45 | (5.5) | 61 | (5.3) | ||
| 200-400 | 129 | (21.3) | 269 | (26.8) | 218 | (26.5) | 286 | (24.7) | ||
| 401-600 | 295 | (48.6) | 370 | (36.9) | 350 | (42.6) | 472 | (40.8) | ||
| > 600 | 107 | (17.6) | 277 | (27.6) | 185 | (22.6) | 284 | (24.5) | ||
| Unknown | 21 | (3.5) | 38 | (3.8) | 24 | (2.9) | 54 | (4.7) | ||
| Hospital volume | ||||||||||
| Median (IQR) | 5.8 | (3-10.0) | 5.5 | (3.3-9.7) | .01 | 6 | (3.3-10.0) | 5.7 | (3.3-9.7) | .78 |
Frequency numbers were rounded to integers based on weight.
P values were derived from χ2 tests.
P values were derived from weighted surveylogistic model.
Footnotes
J.D.W. (NCI R01CA169121-01A1) and D.L.H. (NCI R01CA134964) are recipients of grants from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Jason D. Wright, Dawn L. Hershman
Financial support: Jason D. Wright
Administrative support: Jason D. Wright
Collection and assembly of data: Jason D. Wright, Dawn L. Hershman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comparative Effectiveness of Minimally Invasive Hysterectomy for Endometrial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Jason D. Wright
No relationship to disclose
William M. Burke
No relationship to disclose
Ana I. Tergas
Consulting or Advisory Role: Helomics
June Y. Hou
No relationship to disclose
Yongmei Huang
No relationship to disclose
Jim C. Hu
No relationship to disclose
Grace Clark Hillyer
No relationship to disclose
Cande V. Ananth
No relationship to disclose
Alfred I. Neugut
Stock or Other Ownership: Stemline Therapeutics
Consulting or Advisory Role: Pfizer, Teva Pharmaceuticals Industry, Otsuka, United Biosource Cooperation, EHE International
Dawn L. Hershman
No relationship to disclose
REFERENCES
- 1.Mourits MJ, Bijen CB, Arts HJ, et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: A randomised trial. Lancet Oncol. 2010;11:763–771. doi: 10.1016/S1470-2045(10)70143-1. [DOI] [PubMed] [Google Scholar]
- 2.Wright JD, Barrena Medel NI, Sehouli J, et al. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–1360. doi: 10.1016/S0140-6736(12)60442-5. [DOI] [PubMed] [Google Scholar]
- 3.Childers JM, Surwit EA. Combined laparoscopic and vaginal surgery for the management of two cases of stage I endometrial cancer. Gynecol Oncol. 1992;45:46–51. doi: 10.1016/0090-8258(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 4.Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA. 2013;309:689–698. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Burke WM, Wilde ET, et al. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clin Oncol. 2012 doi: 10.1200/JCO.2011.36.7508. 30:783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright JD, Neugut AI, Wilde ET, et al. Use and benefits of laparoscopic hysterectomy for stage I endometrial cancer among medicare beneficiaries. J Oncol Pract. 2012;8:e89–e99. doi: 10.1200/JOP.2011.000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalogiannidis I, Lambrechts S, Amant F, Neven P, Van Gorp T, Vergote I. Laparoscopy-assisted vaginal hysterectomy compared with abdominal hysterectomy in clinical stage I endometrial cancer: Safety, recurrence, and long-term outcome. Am J Obstet Gynecol 196:248.e1-e8, 2007. [DOI] [PubMed]
- 9.Janda M, Gebski V, Brand A, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): A randomised trial. Lancet Oncol. 2010;11:772–780. doi: 10.1016/S1470-2045(10)70145-5. [DOI] [PubMed] [Google Scholar]
- 10.Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic hysterectomy versus abdominal hysterectomy with lymphadenectomy for early-stage endometrial cancer: A prospective randomized study. Gynecol Oncol. 2009;112:126–133. doi: 10.1016/j.ygyno.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Zullo F, Palomba S, Falbo A, et al. Laparoscopic surgery vs laparotomy for early stage endometrial cancer: Long-term data of a randomized controlled trial. Am J Obstet Gynecol 200:296.e1-e9, 2009. [DOI] [PubMed]
- 12.Galaal K, Bryant A, Fisher AD, et al. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2012;9:CD006655. doi: 10.1002/14651858.CD006655.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Tozzi R, Malur S, Koehler C, et al. Laparoscopy versus laparotomy in endometrial cancer: First analysis of survival of a randomized prospective study. J Minim Invasive Gynecol. 2005;12:130–136. doi: 10.1016/j.jmig.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Wright J, Doan T, McBride R, et al. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98:1197–1203. doi: 10.1038/sj.bjc.6604298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JD, Herzog TJ, Neugut AI, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol. 2012;120:871–881. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 16.Wright JD, Neugut AI, Wilde ET, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29:3408–3418. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Sax FL, MacKenzie CR, et al. Assessing illness severity: Does clinical judgment work? J Chronic Dis. 1986;39:439–452. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709–716. doi: 10.1097/AOG.0b013e318248f7a8. [DOI] [PubMed] [Google Scholar]
- 21.Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–1793. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmila MR, Birkmeyer NJ, Arbabi S, et al. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–945. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 23.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: Effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA. 2012;307:1611–1620. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder VS, Stuart EA, Anthony JC. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods. 2010;15:234–249. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross ME, Kreider AR, Huang YS, et al. Propensity score methods for analyzing observational data like randomized experiments: Challenges and solutions for rare outcomes and exposures. Am J Epidemiol. 2015;181:989–995. doi: 10.1093/aje/kwu469. [DOI] [PubMed] [Google Scholar]
- 27.Obermair A, Janda M, Baker J, et al. Improved surgical safety after laparoscopic compared to open surgery for apparent early stage endometrial cancer: Results from a randomised controlled trial. Eur J Cancer. 2012;48:1147–1153. doi: 10.1016/j.ejca.2012.02.055. [DOI] [PubMed] [Google Scholar]
- 28.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kornblith AB, Huang HQ, Walker JL, et al. Quality of life of patients with endometrial cancer undergoing laparoscopic international federation of gynecology and obstetrics staging compared with laparotomy: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:5337–5342. doi: 10.1200/JCO.2009.22.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paraiso MF, Ridgeway B, Park AJ, et al. A randomized trial comparing conventional and robotically assisted total laparoscopic hysterectomy. Am J Obstet Gynecol 208:368.e1-e7, 2013. [DOI] [PubMed]
- 31.Sarlos D, Kots L, Stevanovic N, et al. Robotic compared with conventional laparoscopic hysterectomy: A randomized controlled trial. Obstet Gynecol. 2012;120:604–611. doi: 10.1097/AOG.0b013e318265b61a. [DOI] [PubMed] [Google Scholar]
- 32.Rosero EB, Kho KA, Joshi GP, et al. Comparison of robotic and laparoscopic hysterectomy for benign gynecologic disease. Obstet Gynecol. 2013;122:778–786. doi: 10.1097/AOG.0b013e3182a4ee4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright JD, Ananth CV, Tergas AI, et al. An economic analysis of robotically assisted hysterectomy. Obstet Gynecol. 2014;123:1038–1048. doi: 10.1097/AOG.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coronado PJ, Herraiz MA, Magrina JF, et al. Comparison of perioperative outcomes and cost of robotic-assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2012;165:289–294. doi: 10.1016/j.ejogrb.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Doo DW, Guntupalli SR, Corr BR, et al. Comparative surgical outcomes for endometrial cancer patients 65 years old or older staged with robotics or laparotomy. Ann Surg Oncol. 2015;22:3687–3694. doi: 10.1245/s10434-015-4428-0. [DOI] [PubMed] [Google Scholar]
- 36.Lavoue V, Zeng X, Lau S, et al. Impact of robotics on the outcome of elderly patients with endometrial cancer. Gynecol Oncol. 2014;133:556–562. doi: 10.1016/j.ygyno.2014.03.572. [DOI] [PubMed] [Google Scholar]
- 37.Park HK, Helenowski IB, Berry E, et al. A comparison of survival and recurrence outcomes in patients with endometrial cancer undergoing robotic versus open surgery. J Minim Invasive Gynecol. 2015;22:961–967. doi: 10.1016/j.jmig.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Hu JC, Gandaglia G, Karakiewicz PI, et al. Comparative effectiveness of robot-assisted versus open radical prostatectomy cancer control. Eur Urol. 2014;66:666–672. doi: 10.1016/j.eururo.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Wright JD, Fiorelli J, Schiff PB, et al. Racial disparities for uterine corpus tumors: Changes in clinical characteristics and treatment over time. Cancer. 2009;115:1276–1285. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]